Possible Role of Cannabis in the Management of Neuroinflammation in Patients with Post-COVID Condition
Abstract
1. Introduction
2. Materials and Methods
3. Generalities of the PCC
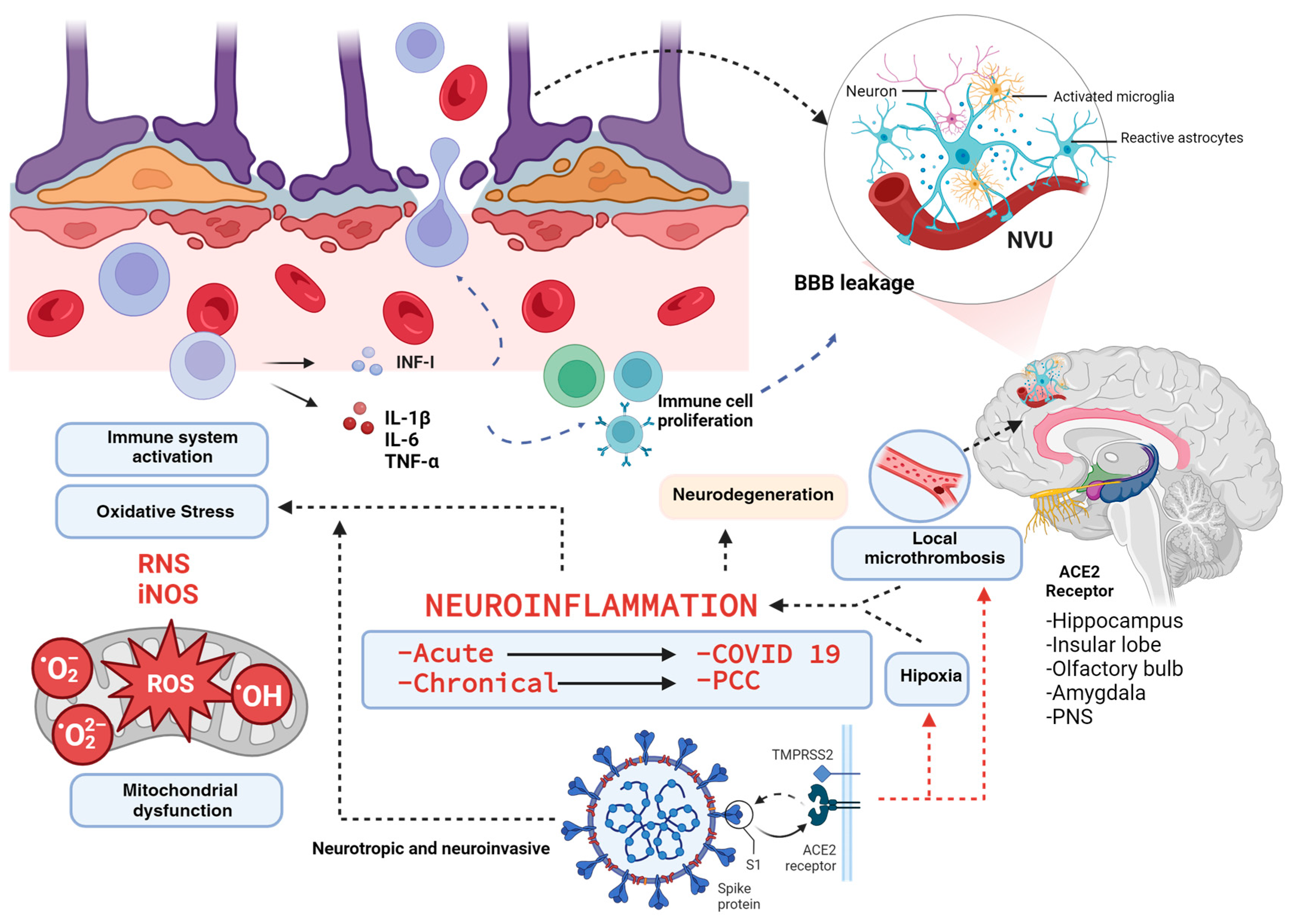
4. Neuroinflammation and Oxidative Stress in the PCC
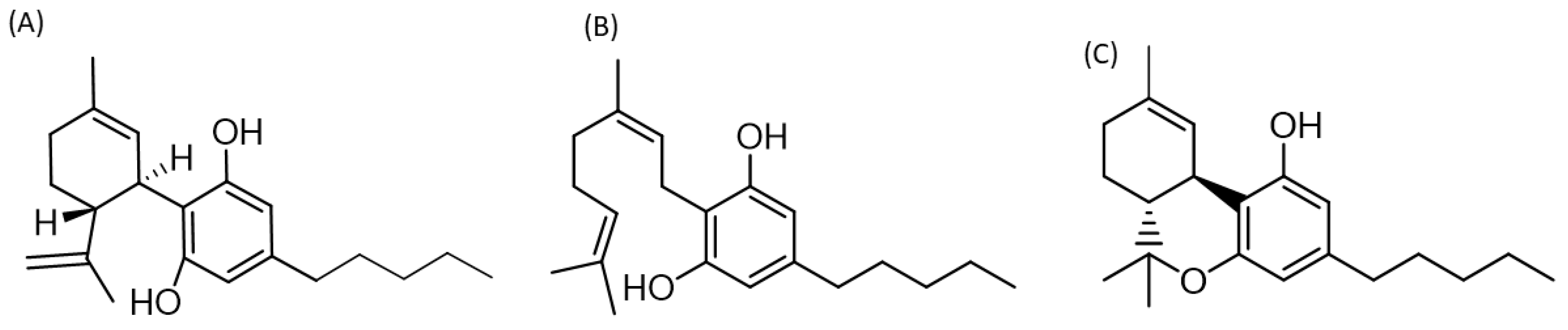
5. Role of Cannabis Compounds in Neurological and Systemic Inflammatory Diseases
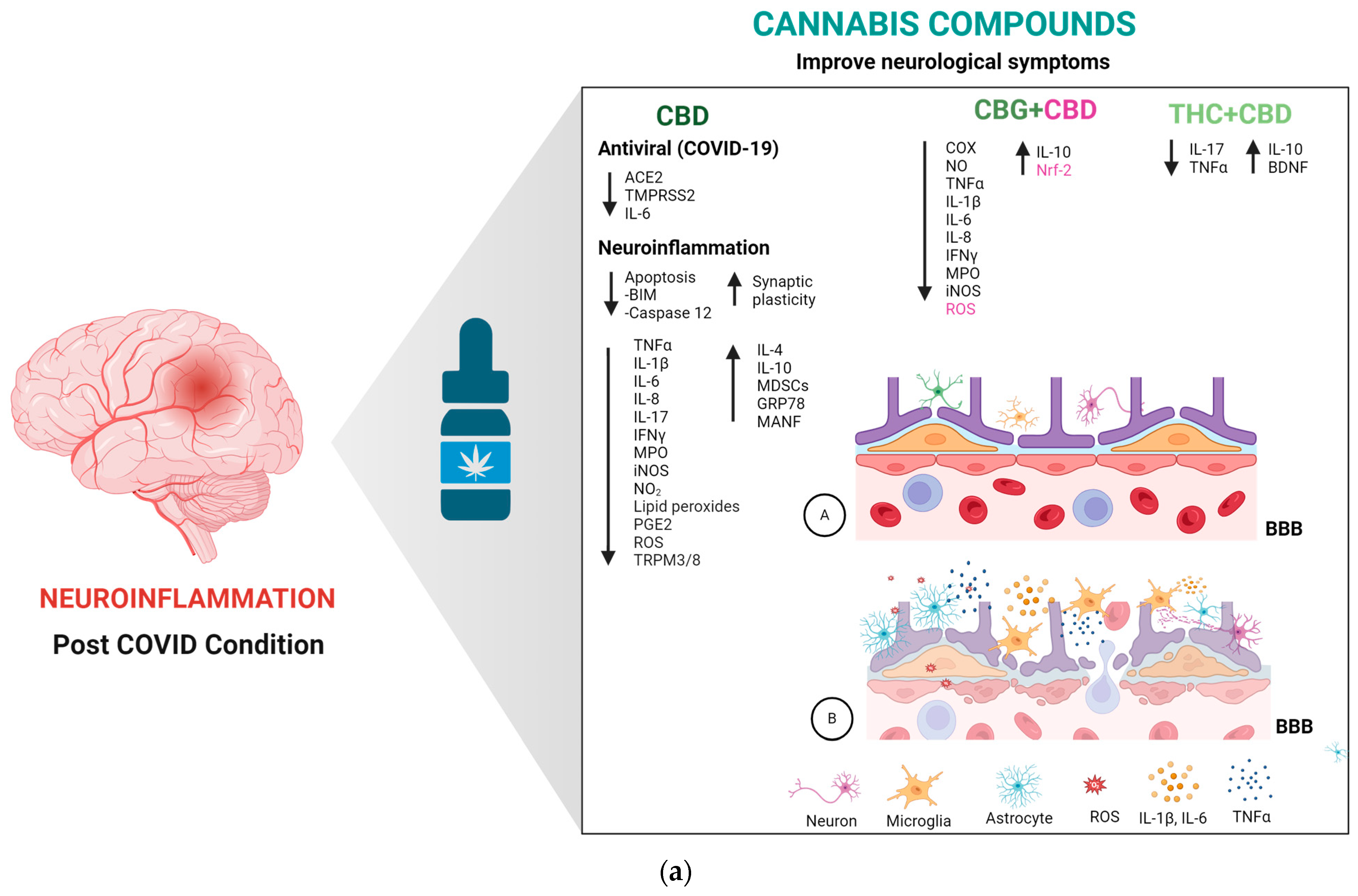
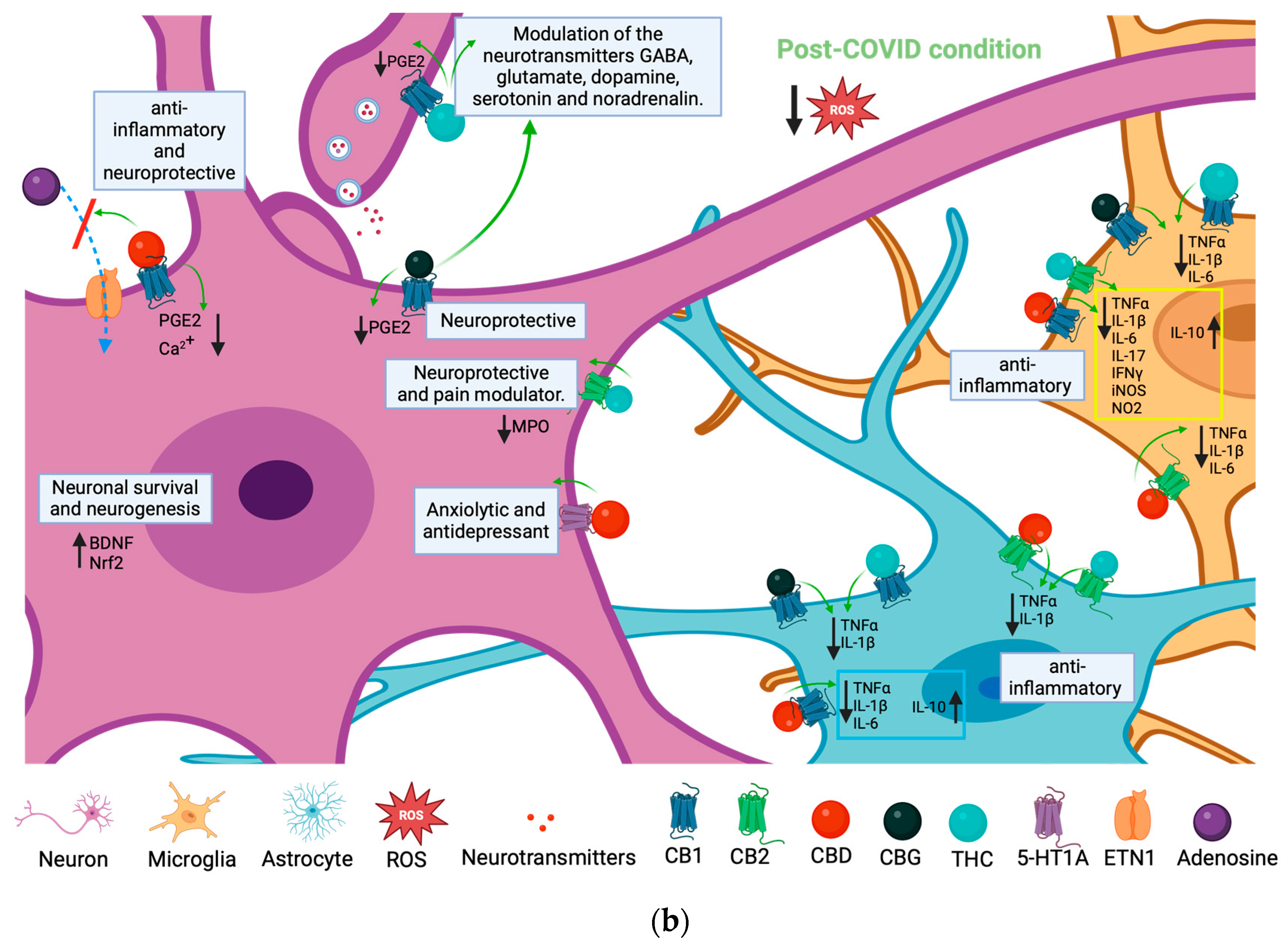
6. Possible Therapeutic Effects of Cannabis Compounds on PCC Neuroinflammation
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pierce, J.D.; Shen, Q.; Cintron, S.A.; Hiebert, J.B. Post-COVID-19 syndrome. Nurs. Res. 2022, 71, 164–174. [Google Scholar] [CrossRef]
- Stefanou, M.I.; Palaiodimou, L.; Bakola, E.; Smyrnis, N.; Papadopoulou, M.; Paraskevas, G.P. Neurological manifestations of the persistent COVID syndrome: A narrative review. Ther. Adv. Chronic. Dis. 2022, 13, 204062232210768. [Google Scholar] [CrossRef]
- Greenhalgh, T.; Knight, M.; A’Court, C.; Buxton, M.; Husain, L. Management of post-acute COVID-19 in primary care. BMJ 2020, 370, m3026. [Google Scholar] [CrossRef]
- Levinson, R.; Elbaz, M.; Ben-Ami, R.; Shasha, D.; Levinson, T.; Choshen, G.; Petrov, K.; Gadoth, A.; Paran, Y. Time course of anosmia and dysgeusia in patients with mild SARS-CoV-2 infection. Infect. Dis. 2020, 52, 600–602. [Google Scholar] [CrossRef]
- Gain, C.; Song, S.; Angtuaco, T.; Satta, S.; Kelesidis, T. The role of oxidative stress in the pathogenesis of infections with coronaviruses. Front. Microbiol. 2023, 13, 1111930. [Google Scholar] [CrossRef]
- Georgieva, E.; Ananiev, J.; Yovchev, Y.; Arabadzhiev, G.; Abrashev, H.; Abrasheva, D.; Atanasov, V.; Kostandieva, R.; Mitev, M.; Petkova-Parlapanska, K.; et al. COVID-19 Complications: Oxidative Stress, Inflammation, and Mitochondrial and Endothelial Dysfunction. Int. J. Mol. Sci. 2023, 24, 14876. [Google Scholar] [CrossRef]
- Akanchise, T.; Angelova, A. Potential of Nano-Antioxidants and Nanomedicine for Recovery from Neurological Disorders Linked to Long COVID Syndrome. Antioxidants 2023, 12, 393. [Google Scholar] [CrossRef]
- Akanchise, T.; Angelova, A. Ginkgo Biloba and Long COVID: In Vivo and In Vitro Models for the Evaluation of Nanotherapeutic Efficacy. Pharmaceutics 2023, 15, 1562. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, H.; Teschler, M.; Mooren, F.C.; Schmitz, B. Altered tissue oxygenation in patients with post COVID-19 syndrome. Microvasc. Res. 2023, 148, 104551. [Google Scholar] [CrossRef] [PubMed]
- Herder, V.; Dee, K.; Wojtus, J.K.; Epifano, I.; Goldfarb, D.; Rozario, C.; Gu, Q.; Da Silva Filipe, A.; Nomikou, K.; Nichols, J.; et al. Elevated temperature inhibits SARS-CoV-2 replication in respiratory epithelium independently of IFN-mediated innate immune defenses. PLoS Biol. 2021, 19, e3001065. [Google Scholar] [CrossRef] [PubMed]
- Al-Hakeim, H.K.; Al-Rubaye, H.T.; Al-Hadrawi, D.S.; Almulla, A.F.; Maes, M. Chronic post-viral fatigue and affective symptoms of prolonged COVID are associated with oxidative damage, decreased antioxidant defenses, and inflammation: A proof of concept and mechanism study. Mol. Psychiatry 2023, 28, 564–578. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, S.; Kolishetti, N.; Arias, A.Y.; Vashist, A.; Nair, M. Cannabidiol for neurodegenerative disorders: A comprehensive review. Front. Pharmacol. 2022, 13, 989717. [Google Scholar] [CrossRef] [PubMed]
- Salha, M.; Adenusi, H.; Dupuis, J.H.; Bodo, E.; Botta, B.; McKenzie, I.; Yada, R.Y.; Farrar, D.H.; Magolan, J.; Tian, K.V.; et al. Bioactivity of the cannabigerol cannabinoid and its analogues—The role of 3-dimensional conformation. Org. Biomol. Chem. 2023, 21, 4683–4693. [Google Scholar] [CrossRef] [PubMed]
- Yong, S.J.; Liu, S. Proposed subtypes of post-COVID-19 syndrome (or long-COVID) and their respective potential therapies. Rev. Med. Virol. 2022, 32, e2315. [Google Scholar] [CrossRef]
- Fernández-de-Las-Peñas, C. Long COVID: Current definition. Infection 2022, 50, 285–286. [Google Scholar] [CrossRef]
- COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19. London: National Institute for Health and Care Excellence (NICE); 2020 Dec 18. (NICE Guideline, No. 188). Available online: https://www.ncbi.nlm.nih.gov/books/NBK567261/ (accessed on 27 November 2023).
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V. WHO Clinical Case Definition Working Group on Post-COVID-19 Condition. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2022, 22, e102–e107. [Google Scholar] [CrossRef]
- Munblit, D.; Nicholson, T.; Akrami, A.; Apfelbacher, C.; Chen, J.; De Groote, W.; Diaz, J.V.; Gorst, S.L.; Harman, N.; Kokorina, A.; et al. A core outcome set for post-COVID-19 condition in adults for use in clinical practice and research: An international Delphi consensus study. Lancet Respir. Med. 2022, 10, 715–724. [Google Scholar] [CrossRef]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 long-term effects of COVID-19: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 16144. [Google Scholar] [CrossRef]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. eClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef]
- Yong, S.J. Long COVID or post-COVID-19 syndrome: Putative pathophysiology, risk factors, and treatments. Infect. Dis. 2021, 53, 737–754. [Google Scholar] [CrossRef]
- Brightling, C.E.; Evans, R.A. Long COVID: Which symptoms can be attributed to SARS-CoV-2 infection? Lancet 2022, 400, 411–413. [Google Scholar] [CrossRef]
- Bandala, C.; Cortes-Altamirano, J.L.; Reyes-Long, S.; Lara-Padilla, E.; Ilizaliturri-Flores, I.; Alfaro-Rodríguez, A. Putative mechanism of neurological damage in COVID-19 infection. Acta Neurobiol. Exp. 2021, 81, 69–79. [Google Scholar] [CrossRef]
- Castanares-Zapatero, D.P.; Chalon, L.; Kohn, M.; Dauvrin, J.; Detollenaere, C.; Maertens de Noordhout, C.; Primus-de Jong, C.; Cleemput, I.; Van den Heede, K. Pathophysiology and mechanism of long COVID: A comprehensive review. Ann. Med. 2022, 54, 1473–1487. [Google Scholar] [CrossRef]
- Lu, Y.; Li, X.; Geng, D.; Mei, N.; Wu, P.Y.; Huang, C.C.; Jia, T.; Zhao, Y.; Wang, D.; Xiao, A.; et al. Cerebral Micro-Structural Changes in COVID-19 Patients—An MRI-based 3-month Follow-up Study. eClinicalMedicine 2020, 25, 100484. [Google Scholar] [CrossRef]
- Qin, Y.; Wu, J.; Chen, T.; Li, J.; Zhang, G.; Wu, D.; Zhou, Y.; Zheng, N.; Cai, A.; Ning, Q.; et al. Long-term microstructure and cerebral blood flow changes in patients recovered from COVID-19 without neurological man-ifestations. J. Clin. Investig. 2021, 131, e147329. [Google Scholar] [CrossRef] [PubMed]
- Cau, R.; Faa, G.; Nardi, V.; Balestrieri, A.; Puig, J.; Suri, J.S.; SanFilippo, R.; Saba, L. Long-COVID diagnosis: From diagnostic to advanced AI-driven models. Eur. J. Radiol. 2022, 148, 110164. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas-Rodríguez, N.; Bandala, C.; Vanoye-Carlo, A.; Ignacio-Mejía, I.; Gómez-Manzo, S.; Hernández-Cruz, E.Y.; Pedraza-Chaverri, J.; Carmona-Aparicio, L.; Hernández-Ochoa, B. Use of Antioxidants for the Neuro-Therapeutic Management of COVID-19. Antioxidants 2021, 10, 971. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, F.A.A.; Palmeira, D.C.C.; Rocha-Filho, P.A.S. Headache and pleocytosis in CSF associated with COVID-19: Case report. Neurol. Sci. 2020, 41, 3021–3022. [Google Scholar] [CrossRef]
- Ellul, M.A.; Benjamin, L.; Singh, B.; Lant, S.; Michael, B.D.; Easton, A. Neurological associations of COVID-19. Lancet Neurol. 2020, 19, 767–783. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, D.T.; Montemurro, N.; Petrella, G.; Siciliano, G.; Ceravolo, R.; Perrini, P. Exploring the clinical association between neurological symptoms and COVID-19 pandemic outbreak: A systematic review of current literature. J. Neurol. 2020, 268, 1561–1569. [Google Scholar] [CrossRef] [PubMed]
- Leng, A.; Shah, M.; Ahmad, S.A.; Premraj, L.; Wildi, K.; Li Bassi, G.; Pardo, C.A.; Choi, A.; Cho, S.M. Pathogenesis Underlying Neurological Manifestations of Long COVID Syndrome and Potential Therapeutics. Cells 2023, 12, 816. [Google Scholar] [CrossRef] [PubMed]
- Saucier, J.; Comeau, D.; Robichaud, G.A.; Chamard-Witkowski, L. Reactive gliosis and neuroinflammation: Prime suspects in the pathophysiology of post-acute neuroCOVID-19 syndrome. Front. Neurol. 2023, 14, 1221266. [Google Scholar] [CrossRef] [PubMed]
- Robinson-Agramonte, M.L.A.; Sánchez, T.S.; García, E.N.; Barrera, O.R.S.; Siniscalco, D. COVID-19 and the Nervous System from a Cuban Experience. Behav. Sci. 2023, 13, 776. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.L.; Benjamin, L.; Lunn, M.P.; Bharucha, T.; Zandi, M.S.; Hoskote, C.; McNamara, P.; Manji, H. Pathophysiology, diagnosis, and management of neuroinflammation in COVID-19. BMJ 2023, 382, e073923. [Google Scholar] [CrossRef] [PubMed]
- Reiss, A.B.; Greene, C.; Dayaramani, C.; Rauchman, S.H.; Stecker, M.M.; De Leon, J.; Pinkhasov, A. Long COVID, the Brain, Nerves, and Cognitive Function. Neurol. Int. 2023, 15, 821–841. [Google Scholar] [CrossRef]
- Woo, M.S.; Shafiq, M.; Fitzek, A.; Dottermusch, M.; Altmeppen, H.; Mohammadi, B.; Mayer, C.; Bal, L.C.; Raich, L.; Matschke, J.; et al. Vagus nerve inflammation contributes to dysautonomia in COVID-19. Acta Neuropathol. 2023, 146, 387–394. [Google Scholar] [CrossRef]
- Möller, M.; Borg, K.; Janson, C.; Lerm, M.; Normark, J.; Niward, K. Cognitive dysfunction in post-COVID-19 condition: Mechanisms, management, and rehabilitation. J. Intern. Med. 2023, 294, 563–581. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.; Zhang, Z.; Wang, Z.; Li, H. Cognitive impairment after long COVID-19: Current evidence and perspectives. Front. Neurol. 2023, 14, 1239182. [Google Scholar] [CrossRef]
- Vlaicu, S.I.; Tatomir, A.; Cuevas, J.; Rus, V.; Rus, H. COVID, complement, and the brain. Front. Immunol. 2023, 14, 1216457. [Google Scholar] [CrossRef]
- Freidin, M.B.; Cheetham, N.; Duncan, E.L.; Steves, C.J.; Doores, K.J.; Malim, M.H.; Rossi, N.; Lord, J.M.; Franks, P.W.; Borsini, A.; et al. Long-COVID fatigue is not predicted by pre-pandemic plasma IL-6 levels in mild COVID-19. Inflamm. Res. 2023, 72, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Pablos, M.; Paiva, B.; Zabaleta, A. Epstein-Barr virus-acquired immunodeficiency in myalgic encephalomyelitis-Is it present in long COVID? J. Transl. Med. 2023, 21, 633. [Google Scholar] [CrossRef] [PubMed]
- Meyerson, W.U.; Hoyle, R.H. Pre-pandemic activity on a myalgic encephalomyelitis/chronic fatigue syndrome support forum is highly associated with later activity on a long COVID support forum for a variety of reasons: A mixed methods study. PLoS ONE 2023, 18, e0291173. [Google Scholar] [CrossRef]
- AlMuhaissen, S.; Abu Libdeh, A.; ElKhatib, Y.; Alshayeb, R.; Jaara, A.; Bardaweel, S.K. Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and COVID-19: Is there a connection? Curr. Med. Res. Opin. 2023, 39, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Blazhenets, G.; Schröter, N.; Bormann, T.; Thurow, J.; Wagner, D.; Frings, L. Slow but evident recovery from neocortical dysfunction and cognitive impairment in a series of chronic COVID-19 patients. J. Nucl. Med. 2021, 62, 910–915. [Google Scholar] [CrossRef]
- Guedj, E.; Campion, J.Y.; Dudouet, P.; Kaphan, E.; Bregeon, F.; Tissot-Dupont, H. 18F-FDG brain PET hypometabolism in patients with long COVID. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2823–2833. [Google Scholar] [CrossRef]
- Almutairi, M.M.; Sivandzade, F.; Albekairi, T.H.; Alqahtani, F.; Cucullo, L. Neuroinflammation and its impact on the pathogenesis of COVID-19. Front. Med. 2021, 8, 745789. [Google Scholar] [CrossRef]
- Yang, Q.Q.; Zhou, J.W. Neuroinflammation in the central nervous system: Symphony of glial cells. Glia 2019, 67, 1017–1035. [Google Scholar] [CrossRef]
- Sivandzade, F.; Alqahtani, F.; Cucullo, L. Traumatic brain injury and blood-brain barrier (BBB): Underlying pathophysiological mechanisms and the influence of smoking as a premorbid condition. Int. J. Mol. Sci. 2020, 21, 2721. [Google Scholar] [CrossRef]
- Ong, W.Y.; Go, M.L.; Wang, D.Y.; Cheah, I.K.M.; Halliwell, B. Effects of antimalarial drugs on neuroinflammation: Potential use for treatment of neurological complications related to COVID-19. Mol. Neurobiol. 2021, 58, 106–117. [Google Scholar] [CrossRef]
- Zabetakis, I.; Lordan, R.; Norton, C.; Tsoupras, A. COVID-19: The inflammation link and the role of nutrition in potential mitigation. Nutrients 2020, 12, 1466. [Google Scholar] [CrossRef]
- Jakhmola, S.; Indari, O.; Chatterjee, S.; Jha, H.C. SARS-CoV-2, an Underestimated Pathogen of the Nervous System. SN Compr. Clin. Med. 2020, 2, 2137–2146. [Google Scholar] [CrossRef]
- Gebo, K.A.; Heath, S.L.; Fukuta, Y.; Zhu, X.; Baksh, S.; Abraham, A.G.; Habtehyimer, F.; Shade, D.; Ruff, J.; Ram, M.; et al. CSSC-004 Consortium. Early antibody treatment, inflammation, and risk of post-COVID conditions. mBio 2023, 14, e0061823. [Google Scholar] [CrossRef]
- Matschke, J.; Lütgehetmann, M.; Hagel, C.; Sperhake, J.P.; Schröder, A.S.; Edler, C. Neuropathology of patients with COVID-19 in Germany: A post-mortem case series. Lancet Neurol. 2020, 19, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Kuba, K.; Imai, Y.; Rao, S.; Gao, H.; Guo, F.; Guan, B. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005, 11, 875–879. [Google Scholar] [CrossRef]
- Tan, P.H.; Ji, J.; Hsing, C.H.; Tan, R.; Ji, R.R. Emerging functions of type I interferons in neuroinflammation, neurological diseases, and long COVID. Int. J. Mol. Sci. 2022, 23, 14394. [Google Scholar] [CrossRef] [PubMed]
- Chernyak, B.V.; Popova, E.N.; Prikhodko, A.S.; Grebenchikov, O.A.; Zinovkina, L.A.; Zinovkin, R.A. COVID-19 and Oxidative Stress. Biochemistry 2020, 85, 1543–1553. [Google Scholar] [CrossRef]
- Wieczfinska, J.; Kleniewska, P.; Pawliczak, R. Mechanisms related to oxidative stress in SARS-CoV-2 infections. Oxid Med. Cell Longev. 2022, 2022, 5589089. [Google Scholar] [CrossRef] [PubMed]
- Semerdzhiev, S.A.; Fakhree, M.A.A.; Segers-Nolten, I.; Blum, C.; Claessens, M.M.A.E. Interactions between SARS-CoV-2 N-Protein and α-Synuclein Accelerate Amyloid Formation. ACS Chem. Neurosci. 2022, 13, 143–150. [Google Scholar] [CrossRef]
- Melrose, J.; Smith, M.M. Natural and Semi-Synthetic Flavonoid Anti-SARS-CoV-2 Agents for the Treatment of Long COVID-19 Disease and Neurodegenerative Disorders of Cognitive Decline. Front. Biosci. 2022, 14, 27. [Google Scholar] [CrossRef]
- Quintero-Fabián, S.; Bandala, C.; Pichardo-Macías, L.A.; Contreras-García, I.J.; Gómez-Manzo, S.; Hernández-Ochoa, B.; Martínez-Orozco, J.A.; Ignacio-Mejía, I.; Cárdenas-Rodríguez, N. Vitamin D and its Possible Relationship to Neuroprotection in COVID-19: Evidence in the Literature. Curr. Top Med. Chem. 2022, 22, 1346–1368. [Google Scholar] [CrossRef]
- Chen, T.B.; Chang, C.M.; Yang, C.C.; Tsai, I.J.; Wei, C.Y.; Yang, H.W.; Yang, C.P. Neuroimmunological Effect of Vitamin D on Neuropsychiatric Long COVID Syndrome: A Review. Nutrients 2023, 15, 3802. [Google Scholar] [CrossRef]
- Pirro, M.; Ferri, L.; Piccioni, L.; Bellucci, A.M.; Bartolucci, F.; Russo, A.; Piga, A.; Ciaramaglia, P.L.; Lucangeli, M.; Russo, A.M.; et al. What Is the Role of Palmitoylethanolamide Co-Ultramicronized with Luteolin on the Symptomatology Reported by Patients Suffering from Long COVID? A Retrospective Analysis Performed by a Group of General Practitioners in a Real-Life Setting. Nutrients 2023, 15, 3701. [Google Scholar] [CrossRef]
- Di Stadio, A.; Bernitsas, E.; La Mantia, I.; Brenner, M.J.; Ralli, M.; Vaira, L.A.; Colizza, A.; Cavaliere, C.; Laudani, M.; Frohman, T.C.; et al. Targeting Neuroinflammation to Alleviate Chronic Olfactory Dysfunction in Long COVID: A Role for Investigating Disease-Modifying Therapy (DMT)? Life 2023, 13, 226. [Google Scholar] [CrossRef]
- Di Stadio, A.; Gallina, S.; Cocuzza, S.; De Luca, P.; Ingrassia, A.; Oliva, S.; Sireci, F.; Camaioni, A.; Ferreli, F.; Mercante, G.; et al. Treatment of COVID-19 olfactory dysfunction with olfactory training, palmitoylethanolamide with luteolin, or combined therapy: A blinded controlled multicenter randomized trial. Eur. Arch. Otorhinolaryngol. 2023, 280, 4949–4961. [Google Scholar] [CrossRef] [PubMed]
- Versace, V.; Ortelli, P.; Dezi, S.; Ferrazzoli, D.; Alibardi, A.; Bonini, I.; Engl, M.; Maestri, R.; Assogna, M.; Ajello, V.; et al. Co-ultramicronized palmitoylethanolamide/luteolin normalizes GABAB-ergic activity and cortical plasticity in long COVID-19 syndrome. Clin. Neurophysiol. 2023, 145, 81–88. [Google Scholar] [CrossRef]
- Cash, A.; Kaufman, D.L. Oxaloacetate Treatment For Mental And Physical Fatigue In Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) and Long-COVID fatigue patients: A non-randomized controlled clinical trial. J. Transl. Med. 2022, 20, 295. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Cholevas, C.; Polyzoidis, K.; Politis, A. Long-COVID syndrome-associated brain fog and chemofog: Luteolin to the rescue. Biofactors 2021, 47, 232–241. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Shi, M.M.; Monsel, A.; Dai, C.X.; Dong, X.; Shen, H.; Li, S.K.; Chang, J.; Xu, C.L.; Li, P.; et al. Nebulized exosomes derived from allogenic adipose tissue mesenchymal stromal cells in patients with severe COVID-19: A pilot study. Stem Cell Res. Ther. 2022, 13, 220. [Google Scholar] [CrossRef] [PubMed]
- Saeedi-Boroujeni, A.; Nashibi, R.; Ghadiri, A.A.; Nakajima, M.; Salmanzadeh, S.; Mahmoudian-Sani, M.R.; Hanafi, M.G.; Sharhani, A.; Khodadadi, A. Tranilast as an Adjunctive Therapy in Hospitalized Patients with Severe COVID-19: A Randomized Controlled Trial. Arch. Med. Res. 2022, 53, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, K.; Dedeepiya, V.D.; Suryaprakash, V.; Rao, K.S.; Ikewaki, N.; Sonoda, T.; Levy, G.A.; Iwasaki, M.; Senthilkumar, R.; Preethy, S.; et al. Beneficial effects of novel aureobasidium pullulans strains produced beta-1, 3-1, 6 glucans on interleukin-6 and D-dimer levels in COVID-19 patients; results of a randomized multiple-arm pilot clinical study. Biomed. Pharmacother. 2022, 145, 112243. [Google Scholar] [CrossRef] [PubMed]
- Bellucci, G.; Albanese, A.; Rizzi, C.; Rinaldi, V.; Salvetti, M.; Ristori, G. The value of Interferon β in multiple sclerosis and novel opportunities for its anti-viral activity: A narrative literature review. Front. Immunol. 2023, 14, 1161849. [Google Scholar] [CrossRef]
- Pintori, N.; Caria, F.; De Luca, M.A.; Miliano, C. THC and CBD: Villain versus Hero? Insights into Adolescent Exposure. Int. J. Mol. Sci. 2023, 24, 5251. [Google Scholar] [CrossRef]
- Henshaw, F.R.; Dewsbury, L.S.; Lim, C.K.; Steiner, G.Z. The Effects of Cannabinoids on Pro- and Anti-Inflammatory Cytokines: A Systematic Review of In Vivo Studies. Cannabis Cannabinoid Res. 2021, 6, 177–195. [Google Scholar] [CrossRef]
- Van Rensburg, R.; Pillay-Fuentes, L.V.; Blockman, M.; Moodley, K.; Wilmshurst, J.M.; Decloedt, E.H. Medical cannabis: What practitioners need to know. S. Afr. Med. J. 2020, 110, 192–196. [Google Scholar] [CrossRef]
- Krzyżewska, A.; Baranowska-Kuczko, M.; Jastrząb, A.; Kasacka, I.; Kozłowska, H. Cannabidiol Improves Antioxidant Capacity and Reduces Inflammation in the Lungs of Rats with Monocrotaline-Induced Pulmonary Hypertension. Molecules 2022, 27, 3327. [Google Scholar] [CrossRef]
- Burstein, S. Cannabidiol (CBD) and its analogs: A review of their effects on inflammation. Bioorganic. Med. Chem. 2015, 23, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Jean-Gilles, L.; Braitch, M.; Latif, M.L.; Aram, J.; Fahey, A.J.; Edwards, L.J.; Robins, R.A.; Tanasescu, R.; Tighe, P.J.; Gran, B.; et al. Effects of pro-inflammatory cytokines on cannabinoid CB1 and CB2 receptors in immune cells. Acta Physiol. 2015, 214, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, M.; Mukhopadhyay, P.; Haskó, G.; Liaudet, L.; Mackie, K.; Pacher, P. Cannabinoid-1 receptor activation induces reactive oxygen species-dependent and -independent mitogen-activated protein kinase activation and cell death in human coronary artery endothelial cells. Br. J. Pharmacol. 2010, 160, 688–700. [Google Scholar] [CrossRef]
- Lu, H.C.; Mackie, K. Review of the Endocannabinoid System. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6, 607–615. [Google Scholar] [CrossRef]
- Barichello, T.; Ceretta, R.A.; Generoso, J.S.; Moreira, A.P.; Simões, L.R.; Comim, C.M.; Quevedo, J.; Vilela, M.C.; Zuardi, A.W.; Crippa, J.A.; et al. Cannabidiol reduces host immune response and prevents cognitive impairments in Wistar rats submitted to pneumococcal meningitis. Eur. J. Pharmacol. 2012, 697, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Tanikawa, T.; Kitamura, M.; Hayashi, Y.; Tomida, N.; Uwaya, A.; Isami, F.; Yokogawa, T.; Inoue, Y. Anti-inflammatory Effect of a Combination of Cannabidiol and Morinda citrifolia Extract on Lipopolysaccharide-stimulated RAW264 Macrophages. In Vivo 2023, 37, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Sonego, A.B.; Prado, D.S.; Vale, G.T.; Sepulveda-Diaz, J.E.; Cunha, T.M.; Tirapelli, C.R.; Del Bel, E.A.; Raisman-Vozari, R.; Guimarães, F.S. Cannabidiol prevents haloperidol-induced vacuos chewing movements and inflammatory changes in mice via PPARγ receptors. Brain Behav. Immun. 2018, 74, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Elliott, D.M.; Singh, N.; Nagarkatti, M.; Nagarkatti, P.S. Cannabidiol Attenuates Experimental Autoimmune Encephalomyelitis Model of Multiple Sclerosis Through Induction of Myeloid-Derived Suppressor Cells. Front. Immunol. 2018, 9, 1782. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.; Trovato, A.E.; Comelli, F.; Giagnoni, G.; Colleoni, M. The non-psychoactive cannabis constituent cannabidiol is an orally effective therapeutic agent in rat chronic inflammatory and neuropathic pain. Eur. J. Pharmacol. 2007, 556, 75–83. [Google Scholar] [CrossRef]
- Patel, V.; Abu-Hijleh, F.; Rigg, N.; Mishra, R. Cannabidiol Protects Striatal Neurons by Attenuating Endoplasmic Reticulum Stress. Cannabis Cannabinoid Res. 2023, 8, 299–308. [Google Scholar] [CrossRef]
- Gallily, R.; Yekhtin, Z.; Hanuš, L.O. Overcoming the bell-shaped dose-response of cannabidiol by using cannabis extract enriched in cannabidiol. Pharmacol. Pharm. 2015, 6, 75–85. [Google Scholar] [CrossRef]
- Giuliano, C.; Francavilla, M.; Ongari, G.; Petese, A.; Ghezzi, C.; Rossini, N.; Blandini, F.; Cerri, S. Neuroprotective and Symptomatic Effects of Cannabidiol in an Animal Model of Parkinson’s Disease. Int. J. Mol. Sci. 2021, 22, 8920. [Google Scholar] [CrossRef]
- Leo, A.; Russo, E.; Elia, M. Cannabidiol and epilepsy: Rationale and therapeutic potential. Pharmacol. Res. 2016, 107, 85–92. [Google Scholar] [CrossRef]
- Dopkins, N.; Miranda, K.; Wilson, K.; Holloman, B.L.; Nagarkatti, P.; Nagarkatti, M. Effects of Orally Administered Cannabidiol on Neuroinflammation and Intestinal Inflammation in the Attenuation of Experimental Autoimmune Encephalomyelitis. J. Neuroimmune Pharmacol. 2022, 17, 15–32. [Google Scholar] [CrossRef]
- Meyer, E.; Rieder, P.; Gobbo, D.; Candido, G.; Scheller, A.; de Oliveira, R.M.W.; Kirchhoff, F. Cannabidiol Exerts a Neuroprotective and Glia-Balancing Effect in the Subacute Phase of Stroke. Int. J. Mol. Sci. 2022, 23, 12886. [Google Scholar] [CrossRef]
- Verrico, C.D.; Wesson, S.; Konduri, V.; Hofferek, C.J.; Vazquez-Perez, J.; Blair, E.; Dunner, K., Jr.; Salimpour, P.; Decker, W.K.; Halpert, M.M. A randomized, double-blind, placebo-controlled study of daily cannabidiol for the treatment of canine osteoarthritis pain. Pain 2020, 161, 2191–2202. [Google Scholar] [CrossRef]
- Napimoga, M.H.; Benatti, B.B.; Lima, F.O.; Alves, P.M.; Campos, A.C.; Pena-Dos-Santos, D.R.; Severino, F.P.; Cunha, F.Q.; Guimarães, F.S. Cannabidiol decreases bone resorption by inhibiting RANK/RANKL expression and pro-inflammatory cytokines during experimental periodontitis in rats. Int. Immunopharmacol. 2009, 9, 216–222. [Google Scholar] [CrossRef]
- Soares, R.Z.; Vuolo, F.; Dall’Igna, D.M.; Michels, M.; Crippa, J.A.; Hallak, J.E.; Zuardi, A.W.; Dal-Pizzol, F. Evaluation of the role of the cannabidiol system in an animal model of ischemia/reperfusion kidney injury. Rev. Bras. Ter. Intensiv. 2015, 27, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mukhopadhyay, P.; Cao, Z.; Wang, H.; Feng, D.; Haskó, G.; Mechoulam, R.; Gao, B.; Pacher, P. Cannabidiol attenuates alcohol-induced liver steatosis, metabolic dysregulation, inflammation and neutrophil-mediated injury. Sci. Rep. 2017, 7, 12064. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Wang, H.; Yang, J.; Dai, Z.; Yang, R.; Meng, S.; Li, Y.; Lin, X. Effects of O-1602 and CBD on TNBS-induced colonic disturbances. Neurogastroenterol. Motil. 2020, 32, e13756. [Google Scholar] [CrossRef] [PubMed]
- Weiss, L.; Zeira, M.; Reich, S.; Har-Noy, M.; Mechoulam, R.; Slavin, S.; Gallily, R. Cannabidiol lowers incidence of diabetes in non-obese diabetic mice. Autoimmunity 2006, 39, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, F.; Aviello, G.; Romano, B.; Orlando, P.; Capasso, R.; Maiello, F.; Guadagno, F.; Petrosino, S.; Capasso, F.; Di Marzo, V.; et al. Cannabidiol, a safe and non-psychotropic ingredient of the marijuana plant Cannabis sativa, is protective in a murine model of colitis. J. Mol. Med. 2009, 87, 1111–1121. [Google Scholar] [CrossRef]
- Li, K.; Feng, J.Y.; Li, Y.Y.; Yuece, B.; Lin, X.H.; Yu, L.Y.; Li, Y.N.; Feng, Y.J.; Storr, M. Anti-inflammatory role of cannabidiol and O-1602 in cerulein-induced acute pancreatitis in mice. Pancreas 2013, 42, 123–129. [Google Scholar] [CrossRef]
- Di Marzo, V.; Piscitelli, F. The Endocannabinoid System and its Modulation by Phytocannabinoids. Neurotherapeutics 2015, 12, 692–698. [Google Scholar] [CrossRef]
- Kathmann, M.; Flau, K.; Redmer, A.; Trankle, C.; Schlicker, E. Cannabidiol is an allosteric modulator at mu-and delta-opioid receptors. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2006, 372, 354–361. [Google Scholar] [CrossRef]
- Zamberletti, E.; Gabaglio, M.; Grilli, M.; Prini, P.; Catanese, A.; Pittaluga, A.; Marchi, M.; Rubino, T.; Parolaro, D. Long-term hippocampal glutamate synapse and astrocyte dysfunctions underlying the altered phenotype induced by adolescent THC treatment in male rats. Pharmacol. Res. 2016, 111, 459–470. [Google Scholar] [CrossRef]
- Borgonetti, V.; Anceschi, L.; Brighenti, V.; Corsi, L.; Governa, P.; Manetti, F.; Pellati, F.; Galeotti, N. Cannabidiol-rich non-psychotropic Cannabis sativa L. oils attenuate peripheral neuropathy symptoms by regulation of CB2-mediated microglial neuroinflammation. Phytother. Res. 2023, 37, 1924–1937. [Google Scholar] [CrossRef] [PubMed]
- Gugliandolo, A.; Blando, S.; Salamone, S.; Caprioglio, D.; Pollastro, F.; Mazzon, E.; Chiricosta, L. Δ8-THC Protects against Amyloid Beta Toxicity Modulating ER Stress In Vitro: A Transcriptomic Analysis. Int. J. Mol. Sci. 2023, 24, 6598. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.; Henn, V.; Dittrich, A.; Hofmann, A. Delta-9-tetrahydrocannabinol shows antispastic and analgesic effects in a single case double-blind trial. Eur. Arch. Psychiatry Clin. Neurosci. 1990, 240, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.; Gunasekaran, N.; Hancock, D.P.; Denyer, G.S.; Meng, L.; Radford, J.L.; McGregor, I.S.; Arnold, J.C. The major plant-derived cannabinoid Δ(9)-tetrahydrocannabinol promotes hypertrophy and macrophage infiltration in adipose tissue. Horm. Metab. Res. 2012, 44, 105–113. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, R.; Lu, H.; Zhang, X. Systemic administration with tetrahydrocannabinol causes retinal damage in BALB/c mice. Hum. Exp. Toxicol. 2020, 39, 290–300. [Google Scholar] [CrossRef]
- Yazici, Z.M.C.; Bilge, B.; Bolkent, S. Anti-inflammatory potential of delta-9-tetrahydrocannabinol in hyperinsulinemia: An experimental study. Mol. Biol. Rep. 2022, 49, 11891–11899. [Google Scholar] [CrossRef]
- Al-Ghezi, Z.Z.; Busbee, P.B.; Alghetaa, H.; Nagarkatti, P.S.; Nagarkatti, M. Combination of cannabinoids, delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD), mitigates experimental autoimmune encephalomyelitis (EAE) by altering the gut microbiome. Brain Behav. Immun. 2019, 82, 25–35. [Google Scholar] [CrossRef]
- Zhou, T.; Ahmad, T.K.; Alrushaid, S.; Pozdirca, M.; Ethans, K.; Intrater, H.; Le, T.; Burczynski, F.; Kong, J.; Namaka, M. Therapeutic impact of orally administered cannabinoid oil extracts in an experimental autoimmune encephalomyelitis animal model of multiple sclerosis. Biochem. Biophys. Res. Commun. 2019, 516, 373–380. [Google Scholar] [CrossRef]
- Britch, S.C.; Goodman, A.G.; Wiley, J.L.; Pondelick, A.M.; Craft, R.M. Antinociceptive and Immune Effects of Delta-9-Tetrahydrocannabinol or Cannabidiol in Male Versus Female Rats with Persistent Inflammatory Pain. J. Pharmacol. Exp. Ther. 2020, 373, 416–428. [Google Scholar] [CrossRef]
- Al-Ghezi, Z.Z.; Miranda, K.; Nagarkatti, M.; Nagarkatti, P.S. Combination of Cannabinoids, Δ9- Tetrahydrocannabinol and Cannabidiol, Ameliorates Experimental Multiple Sclerosis by Suppressing Neuroinflammation Through Regulation of miRNA-Mediated Signaling Pathways. Front. Immunol. 2019, 10, 1921. [Google Scholar] [CrossRef]
- Calapai, F.; Cardia, L.; Esposito, E.; Ammendolia, I.; Mondello, C.; Lo Giudice, R.; Gangemi, S.; Calapai, G.; Mannucci, C. Pharmacological Aspects and Biological Effects of Cannabigerol and Its Synthetic Derivatives. Evid Based Complement. Altern. Med. 2022, 2022, 3336516. [Google Scholar] [CrossRef] [PubMed]
- Perez, E.; Fernandez, J.R.; Fitzgerald, C.; Rouzard, K.; Tamura, M.; Savile, C. In Vitro and Clinical Evaluation of Cannabigerol (CBG) Produced via Yeast Biosynthesis: A Cannabinoid with a Broad Range of Anti-Inflammatory and Skin Health-Boosting Properties. Molecules 2022, 27, 491. [Google Scholar] [CrossRef] [PubMed]
- Fleisher-Berkovich, S.; Ventura, Y.; Amoyal, M.; Dahan, A.; Feinshtein, V.; Alfahel, L.; Israelson, A.; Bernstein, N.; Gorelick, J.; Ben-Shabat, S. Therapeutic Potential of Phytocannabinoid Cannabigerol for Multiple Sclerosis: Modulation of Microglial Activation In Vitro and In Vivo. Biomolecules 2023, 13, 376. [Google Scholar] [CrossRef]
- Granja, A.G.; Carrillo-Salinas, F.; Pagani, A.; Gómez-Cañas, M.; Negri, R.; Navarrete, C.; Mecha, M.; Mestre, L.; Fiebich, B.L.; Cantarero, I.; et al. A cannabigerol quinone alleviates neuroinflammation in a chronic model of multiple sclerosis. J. Neuroimmune Pharmacol. 2012, 7, 1002–1016. [Google Scholar] [CrossRef]
- Aguareles, J.; Paraíso-Luna, J.; Palomares, B.; Bajo-Grañeras, R.; Navarrete, C.; Ruiz-Calvo, A.; García-Rincón, D.; García-Taboada, E.; Guzmán, M.; Muñoz, E.; et al. Oral administration of the cannabigerol derivative VCE-003.2 promotes subventricular zone neurogenesis and protects against mutant huntingtin-induced neurodegeneration. Transl. Neurodegener. 2019, 8, 9. [Google Scholar] [CrossRef]
- Díaz-Alonso, J.; Paraíso-Luna, J.; Navarrete, C.; Del Río, C.; Cantarero, I.; Palomares, B.; Aguareles, J.; Fernández-Ruiz, J.; Bellido, M.L.; Pollastro, F.; et al. VCE-003.2, a novel cannabigerol derivative, enhances neuronal progenitor cell survival and alleviates symptomatology in murine models of Huntington’s disease. Sci. Rep. 2016, 6, 29789. [Google Scholar] [CrossRef] [PubMed]
- García, C.; Gómez-Cañas, M.; Burgaz, S.; Palomares, B.; Gómez-Gálvez, Y.; Palomo-Garo, C.; Campo, S.; Ferrer-Hernández, J.; Pavicic, C.; Navarrete, C.; et al. Benefits of VCE-003.2, a cannabigerol quinone derivative, against inflammation-driven neuronal deterioration in experimental Parkinson’s disease: Possible involvement of different binding sites at the PPARγ receptor. J. Neuroinflamm. 2018, 15, 19. [Google Scholar] [CrossRef]
- Burgaz, S.; García, C.; Gómez-Cañas, M.; Muñoz, E.; Fernández-Ruiz, J. Development of An Oral Treatment with the PPAR-γ-Acting Cannabinoid VCE-003.2 Against the Inflammation-Driven Neuronal Deterioration in Experimental Parkinson’s Disease. Molecules 2019, 24, 2702. [Google Scholar] [CrossRef]
- Mammana, S.; Cavalli, E.; Gugliandolo, A.; Silvestro, S.; Pollastro, F.; Bramanti, P.; Mazzon, E. Could the Combination of Two Non-Psychotropic Cannabinoids Counteract Neuroinflammation? Effectiveness of Cannabidiol Associated with Cannabigerol. Medicina 2019, 55, 747. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, F.; Fasolino, I.; Romano, B.; Capasso, R.; Maiello, F.; Coppola, D.; Orlando, P.; Battista, G.; Pagano, E.; Di Marzo, V.; et al. Beneficial effect of the non-psychotropic plant cannabinoid cannabigerol on experimental inflammatory bowel disease. Biochem. Pharmacol. 2013, 85, 1306–1316. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.; Mirabella, M.; De Fino, C.; Bianco, A.; Lucchini, M.; Losavio, F.; Sabino, A.; Nociti, V. Sativex® effects on promoter methylation and on CNR1/CNR2 expression in peripheral blood mononuclear cells of progressive multiple sclerosis patients. J. Neurol. Sci. 2017, 379, 298–303. [Google Scholar] [CrossRef]
- Zheng, T.; BouSaba, J.; Taylor, A.; Dilmaghani, S.; Busciglio, I.; Carlson, P.; Torres, M.; Ryks, M.; Burton, D.; Harmsen, W.S.; et al. A Randomized, Controlled Trial of Efficacy and Safety of Cannabidiol in Idiopathic and Diabetic Gastroparesis. Clin. Gastroenterol. Hepatol. 2023, 21, 3405–3414. [Google Scholar] [CrossRef] [PubMed]
- Couch, D.G.; Cook, H.; Ortori, C.; Barrett, D.; Lund, J.N.; O’Sullivan, S.E. Palmitoylethanolamide and Cannabidiol Prevent Inflammation-induced Hyperpermeability of the Human Gut In Vitro and In Vivo-A Randomized, Placebo-controlled, Double-blind Controlled Trial. Inflamm. Bowel Dis. 2019, 25, 1006–1018. [Google Scholar] [CrossRef]
- Werth, V.P.; Hejazi, E.; Pena, S.M.; Haber, J.; Zeidi, M.; Reddy, N.; Okawa, J.; Feng, R.; Bashir, M.M.; Gebre, K.; et al. Safety and Efficacy of Lenabasum, a Cannabinoid Receptor Type 2 Agonist, in Patients with Dermatomyositis with Refractory Skin Disease: A Randomized Clinical Trial. J. Investig. Dermatol. 2022, 142, 2651–2659. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, Y.; Tan, Y.; Liu, W.; Ouaddi, S.; McCoy, J.; Kovacevic, M.; Situm, M.; Stanimirovic, A.; Li, M.; et al. Novel cannabidiol aspartame combination treatment (JW-100) significantly reduces ISGA score in atopic dermatitis: Results from a randomized double-blinded placebo-controlled interventional study. J. Cosmet Dermatol. 2022, 21, 1647–1650. [Google Scholar] [CrossRef]
- Chmiel, J.F.; Flume, P.; Downey, D.G.; Dozor, A.J.; Colombo, C.; Mazurek, H.; Sapiejka, E.; Rachel, M.; Constantine, S.; Conley, B.; et al. Safety and efficacy of lenabasum in a phase 2 randomized, placebo-controlled trial in adults with cystic fibrosis. J. Cyst. Fibros. 2021, 20, 78–85. [Google Scholar] [CrossRef]
- Spiera, R.; Hummers, L.; Chung, L.; Frech, T.M.; Domsic, R.; Hsu, V.; Furst, D.E.; Gordon, J.; Mayes, M.; Simms, R.; et al. Safety and Efficacy of Lenabasum in a Phase II, Randomized, Placebo-Controlled Trial in Adults with Systemic Sclerosis. Arthritis Rheumatol. 2020, 72, 1350–1360. [Google Scholar] [CrossRef]
- Hobbs, J.M.; Vazquez, A.R.; Remijan, N.D.; Trotter, R.E.; McMillan, T.V.; Freedman, K.E.; Wei, Y.; Woelfel, K.A.; Arnold, O.R.; Wolfe, L.M.; et al. Evaluation of pharmacokinetics and acute anti-inflammatory potential of two oral cannabidiol preparations in healthy adults. Phytother. Res. 2020, 34, 1696–1703. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Pesce, M.; Seguella, L.; Sanseverino, W.; Lu, J.; Corpetti, C.; Sarnelli, G. The potential of cannabidiol in the COVID-19 pandemic. Br. J. Pharmacol. 2020, 177, 4967–4970. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.C.; Yang, D.; Nicolaescu, V.; Best, T.J.; Gula, H.; Saxena, D.; Gabbard, J.D.; Chen, S.N.; Ohtsuki, T.; Friesen, J.B.; et al. Cannabidiol inhibits SARS-CoV-2 replication through induction of the host ER stress and innate immune responses. Sci. Adv. 2022, 8, eabi6110. [Google Scholar] [CrossRef]
- Young, T.P.; Erickson, J.S.; Hattan, S.L.; Guzy, S.; Hershkowitz, F.; Steward, M.D. A Single-Blind, Randomized, Placebo Controlled Study to Evaluate the Benefits and Safety of Endourage Targeted Wellness Formula C Sublingual +Drops in People with Post-Acute Coronavirus Disease 2019 Syndrome. Cannabis Cannabinoid Res. 2022, 9, 282–292. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine (U.S.). Feasibility of Cannabidiol for the Treatment of Long COVID. Identifier: NCT04997395. Available online: https://clinicaltrials.gov (accessed on 27 November 2023).
- National Library of Medicine (U.S.). Double-Blind Randomized Placebo-Controlled Trial of a Proprietary Full Hemp Flower Formulation for Long COVID. Identifier: NCT05467904. Available online: https://clinicaltrials.gov (accessed on 27 November 2023).
- National Library of Medicine (U.S.). (CBDRA60) to Prevent or Reduce Symptoms of COVID-19 and Prevention of Post-Acute Sequelae of SARS-CoV-2 Infection PASC. Identifier: NCT04777981. Available online: https://clinicaltrials.gov (accessed on 27 November 2023).
- Niloy, N.; Hediyal, T.A.; Vichitra, C.; Sonali, S.; Chidambaram, S.B.; Gorantla, V.R.; Mahalakshmi, A.M. Effect of Cannabis on Memory Consolidation, Learning and Retrieval and Its Current Legal Status in India: A Review. Biomolecules 2023, 13, 162. [Google Scholar] [CrossRef] [PubMed]
- Castillo, A.; Tolón, M.R.; Fernández-Ruiz, J.; Romero, J.; Martinez-Orgado, J. The neuroprotective effect of cannabidiol in an in vitro model of newborn hypoxic-ischemic brain damage in mice is mediated by CB(2) and adenosine receptors. Neurobiol. Dis. 2010, 37, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.C.; Fogaça, M.V.; Sonego, A.B.; Guimarães, F.S. Cannabidiol, neuroprotection and neuropsychiatric disorders. Pharmacol. Res. 2016, 112, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, A.F.L.; Campos, R.M.P.; Isaac, A.R.; Paes-Colli, Y.; Carvalho, V.M.; Sampaio, L.S.; de Melo Reis, R.A. Long-Term Treatment with Cannabidiol-Enriched Cannabis Extract Induces Synaptic Changes in the Adolescent Rat Hippocampus. Int. J. Mol. Sci. 2023, 24, 11775. [Google Scholar] [CrossRef] [PubMed]
- Weinbroum, A.A. Rehabilitating Effect of Marijuana on Post-COVID-19 Pain Syndrome. Arch. Clin. Med. Case Rep. 2022, 6, 105–109. [Google Scholar] [CrossRef]
- Sarkar, I.; Sen, G.; Bhattacharya, M.; Bhattacharyya, S.; Sen, A. Insilico inquest reveals the efficacy of Cannabis in the treatment of post-COVID-19 related neurodegeneration. J. Biomol. Struct. Dyn. 2022, 40, 8030–8039. [Google Scholar] [CrossRef]
- Palit, P.; Chattopadhyay, D.; Thomas, S.; Kundu, A.; Kim, H.S.; Rezaei, N. Phytopharmaceuticals mediated Furin and TMPRSS2 receptor blocking: Can it be a potential therapeutic option for COVID-19? Phytomedicine 2021, 85, 153396. [Google Scholar] [CrossRef]
- Koyama, S.; Heinbockel, T. Chapter 22—Possible use of beta-caryophyllene as an agent to facilitate the recovery from COVID-19-induced tissue and organ damage. In Neurobiology and Physiology of the Endocannabinoid, 1st ed.; Patel, V.B., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 297–308. ISBN 9780323908788. [Google Scholar]
- Cinar, R.; Iyer, M.R.; Kunos, G. Dual inhibition of CB1 receptors and iNOS, as a potential novel approach to the pharmacological management of acute and long COVID-19. Br. J. Pharmacol. 2022, 179, 2121–2127. [Google Scholar] [CrossRef]
- Kazama, I. Brain Leukocytes as the Potential Therapeutic Target for Post-COVID-19 Brain Fog. Neurochem. Res. 2023, 48, 2345–2349. [Google Scholar] [CrossRef]
- Giudice, E.D.; Rinaldi, L.; Passarotto, M.; Facchinetti, F.; D’Arrigo, A.; Guiotto, A.; Carbonare, M.D.; Battistin, L.; Leon, A. Cannabidiol, unlike synthetic cannabinoids, triggers activation of RBL-2H3 mast cells. J. Leukoc Biol. 2007, 81, 1512–1522. [Google Scholar] [CrossRef]
- Hegde, V.L.; Singh, U.P.; Nagarkatti, P.S.; Nagarkatti, M. Critical Role of Mast Cells and Peroxisome Proliferator-Activated Receptor γ in the Induction of Myeloid-Derived Suppressor Cells by Marijuana Cannabidiol In Vivo. J. Immunol. 2015, 194, 5211–5222. [Google Scholar] [CrossRef]
- Wang, X.; Lin, C.; Jin, S.; Wang, Y.; Peng, Y.; Wang, X. Cannabidiol alleviates neuroinflammation and attenuates neuropathic pain via targeting FKBP5. Brain Behav. Immun. 2023, 111, 365–375. [Google Scholar] [CrossRef]
- Sasso, E.M.; Muraki, K.; Eaton-Fitch, N.; Smith, P.; Lesslar, O.L.; Deed, G.; Marshall-Gradisnik, S. Transient receptor potential melastatin 3 dysfunction in post COVID-19 condition and myalgic encephalomyelitis/chronic fatigue syndrome patients. Mol. Med. 2022, 28, 98. [Google Scholar] [CrossRef] [PubMed]
- De Petrocellis, L.; Ligresti, A.; Schiano Moriello, A.; Iappelli, M.; Verde, R.; Stott, C.G.; Cristino, L.; Orlando, P.; Di Marzo, V. Non-THC cannabinoids inhibit prostate carcinoma growth in vitro and in vivo: Pro-apoptotic effects and underlying mechanisms. Br. J. Pharmacol. 2013, 168, 79–102. [Google Scholar] [CrossRef]
- Ercegovac, M.; Asanin, M.; Savic-Radojevic, A.; Ranin, J.; Matic, M.; Djukic, T.; Coric, V.; Jerotic, D.; Todorovic, N.; Milosevic, I.; et al. Antioxidant Genetic Profile Modifies Probability of Developing Neurological Sequelae in Long-COVID. Antioxidants 2022, 11, 954. [Google Scholar] [CrossRef] [PubMed]
- Akinloye, D.I.; Metibemu, D.S.; Shittu, M.T.; Lawal, M.A.; Olatunji, F.O.; Oyediran, M.A.; Akinloye, O.A. Cannabis sativa demonstrates anti-hepatocellular carcinoma potentials in animal model: In silico and in vivo studies of the involvement of Akt. J. Cannabis Res. 2023, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C. Could SARS-CoV-2 Spike Protein Be Responsible for Long-COVID Syndrome? Mol. Neurobiol. 2022, 59, 1850–1861. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Zhang, J.; Schiavon, C.R.; He, M.; Chen, L.; Shen, H.; Zhang, Y.; Yin, Q.; Cho, Y.; Andrade, L.; et al. SARS-CoV-2 Spike Protein Impairs Endothelial Function via Downregulation of ACE 2. Circ. Res. 2021, 128, 1323–1326. [Google Scholar] [CrossRef]
- Raghavan, S.; Kenchappa, D.B.; Leo, M.D. SARS-CoV-2 Spike Protein Induces Degradation of Junctional Proteins That Maintain Endothelial Barrier Integrity. Front. Cardiovasc. Med. 2021, 8, 687783. [Google Scholar] [CrossRef]
- Kim, E.S.; Jeon, M.T.; Kim, K.S.; Lee, S.; Kim, S.; Kim, D.G. Spike Proteins of SARS-CoV-2 Induce Pathological Changes in Molecular Delivery and Metabolic Function in the Brain Endothelial Cells. Viruses 2021, 13, 2021. [Google Scholar] [CrossRef] [PubMed]
- Rhea, E.M.; Logsdon, A.F.; Hansen, K.M.; Williams, L.M.; Reed, M.J.; Baumann, K.K.; Holden, S.J.; Raber, J.; Banks, W.A.; Erickson, M.A. The S1 protein of SARS-CoV-2 crosses the blood-brain barrier in mice. Nat. Neurosci. 2021, 24, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Paladino, L.; Vitale, A.M.; Caruso Bavisotto, C.; Conway de Macario, E.; Cappello, F.; Macario, A.J.L.; Gammazza, A.M. The Role of Molecular Chaperones in Virus Infection and Implications for Understanding and Treating COVID-19. J. Clin. Med. 2020, 9, 3518. [Google Scholar] [CrossRef] [PubMed]
- van Breemen, R.B.; Muchiri, R.N.; Bates, T.A.; Weinstein, J.B.; Leier, H.C.; Farley, S.; Tafesse, F.G. Cannabinoids Block Cellular Entry of SARS-CoV-2 and the Emerging Variants. J. Nat. Prod. 2022, 85, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Binetti, J.; Real, M.; Renzulli, M.; Bertran, L.; Riesco, D.; Perpiñan, C.; Mohedano, A.; Segundo, R.S.; Ortiz, M.; Porras, J.A.; et al. Clinical and Biomarker Profile Responses to Rehabilitation Treatment in Patients with Long COVID Characterized by Chronic Fatigue. Viruses 2023, 15, 1452. [Google Scholar] [CrossRef] [PubMed]
- Ghali, A.; Lacombe, V.; Ravaiau, C.; Delattre, E.; Ghali, M.; Urbanski, G.; Lavigne, C. The relevance of pacing strategies in managing symptoms of post-COVID-19 syndrome. J. Transl. Med. 2023, 21, 375. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.; Sawant, H.B.; Flannery, T.; Tarrant, R.; Shardha, J.; Bannister, R.; Ross, D.; Halpin, S.; Greenwood, D.C.; Sivan, M. Effect of using a structured pacing protocol on post-exertional symptom exacerbation and health status in a longitudinal cohort with the post-COVID-19 syndrome. J. Med. Virol. 2023, 95, e28373. [Google Scholar] [CrossRef]
- Müller, L.; Di Benedetto, S. Aged brain and neuroimmune responses to COVID-19: Post-acute sequelae and modulatory effects of behavioral and nutritional interventions. Immun. Ageing 2023, 20, 17. [Google Scholar] [CrossRef] [PubMed]
- Tirelli, U.; Franzini, M.; Valdenassi, L.; Pisconti, S.; Taibi, R.; Torrisi, C.; Pandolfi, S.; Chirumbolo, S. Fatigue in post-acute sequelae of SARS-CoV2 (PASC) treated with oxygen-ozone autohemotherapy—Preliminary results on 100 patients. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 5871–5875. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cárdenas-Rodríguez, N.; Ignacio-Mejía, I.; Correa-Basurto, J.; Carrasco-Vargas, H.; Vargas-Hernández, M.A.; Albores-Méndez, E.M.; Mayen-Quinto, R.D.; De La Paz-Valente, R.; Bandala, C. Possible Role of Cannabis in the Management of Neuroinflammation in Patients with Post-COVID Condition. Int. J. Mol. Sci. 2024, 25, 3805. https://doi.org/10.3390/ijms25073805
Cárdenas-Rodríguez N, Ignacio-Mejía I, Correa-Basurto J, Carrasco-Vargas H, Vargas-Hernández MA, Albores-Méndez EM, Mayen-Quinto RD, De La Paz-Valente R, Bandala C. Possible Role of Cannabis in the Management of Neuroinflammation in Patients with Post-COVID Condition. International Journal of Molecular Sciences. 2024; 25(7):3805. https://doi.org/10.3390/ijms25073805
Chicago/Turabian StyleCárdenas-Rodríguez, Noemi, Iván Ignacio-Mejía, Jose Correa-Basurto, Humberto Carrasco-Vargas, Marco Antonio Vargas-Hernández, Exal Manuel Albores-Méndez, Rodolfo David Mayen-Quinto, Reynita De La Paz-Valente, and Cindy Bandala. 2024. "Possible Role of Cannabis in the Management of Neuroinflammation in Patients with Post-COVID Condition" International Journal of Molecular Sciences 25, no. 7: 3805. https://doi.org/10.3390/ijms25073805
APA StyleCárdenas-Rodríguez, N., Ignacio-Mejía, I., Correa-Basurto, J., Carrasco-Vargas, H., Vargas-Hernández, M. A., Albores-Méndez, E. M., Mayen-Quinto, R. D., De La Paz-Valente, R., & Bandala, C. (2024). Possible Role of Cannabis in the Management of Neuroinflammation in Patients with Post-COVID Condition. International Journal of Molecular Sciences, 25(7), 3805. https://doi.org/10.3390/ijms25073805







