A Potential Role of Interleukin-5 in the Pathogenesis and Progression of Amyotrophic Lateral Sclerosis: A New Molecular Perspective
Abstract
1. Introduction
2. Results
2.1. Demographic and Clinical Data
2.1.1. Participant Characteristics
2.1.2. Cinical Assessments
2.2. Correlation of Cytokine Profile with Clinical and Demographical Characteristics
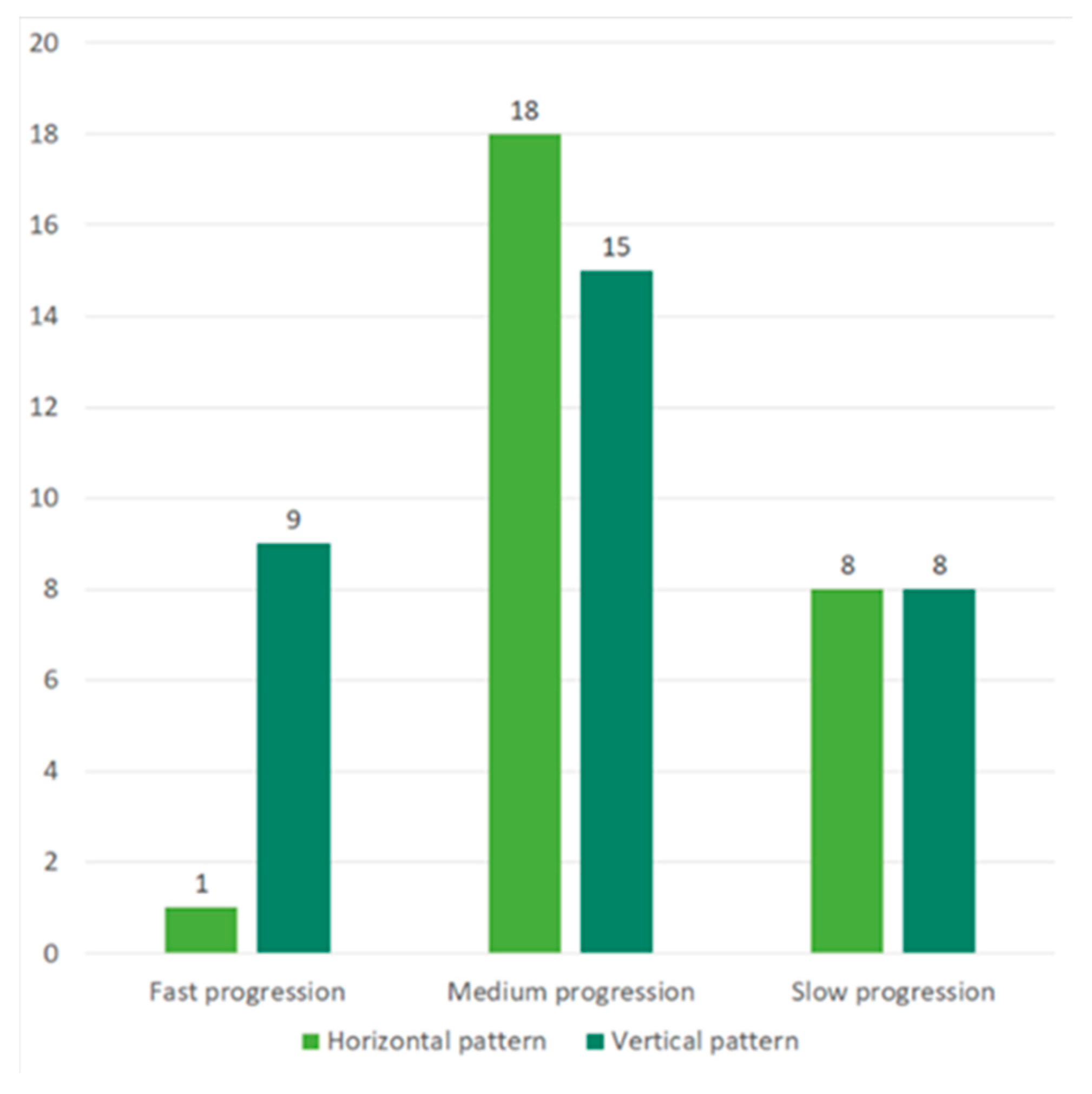
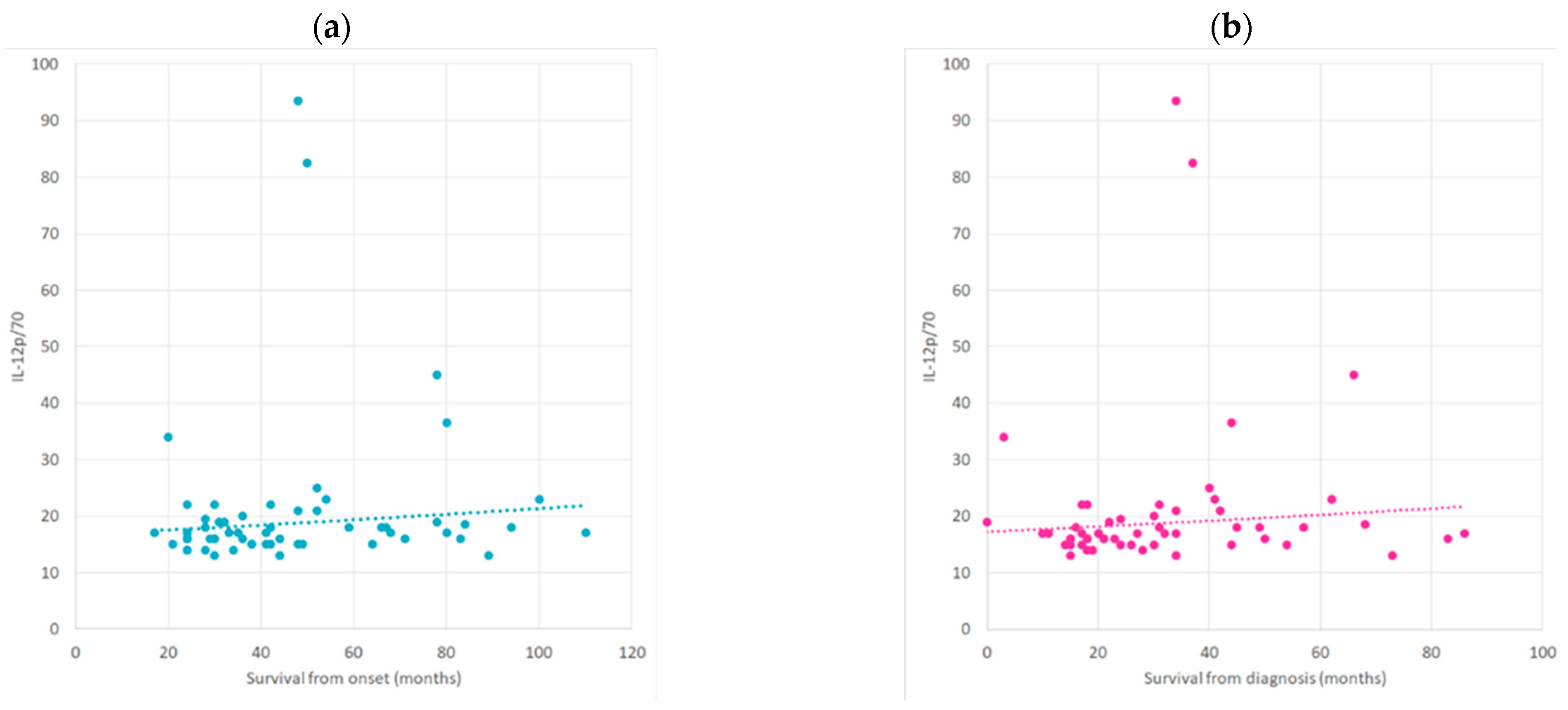
2.3. Correlation between Cytokine Profile concerning ALS Progression Rate and Survival Time
3. Discussion
4. Materials and Methods
4.1. Participants and Assessment
4.2. Preparation of Biological Samples and Cytokine Assay
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mitchell, J.D.; Borasio, G.D. Amyotrophic lateral sclerosis. Lancet 2007, 369, 2031–2041. [Google Scholar] [CrossRef] [PubMed]
- Philips, T.; Robberecht, W. Neuroinflammation in amyotrophic lateral sclerosis: Role of glial activation in motor neuron disease. Lancet Neurol. 2011, 10, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Mejzini, R.; Flynn, L.L.; Pitout, I.L.; Fletcher, S.; Wilton, S.D.; Akkari, P.A. ALS Genetics, Mechanisms, and Therapeutics: Where Are We Now? Front. Neurosci. 2019, 13, 1310. [Google Scholar] [CrossRef] [PubMed]
- McCombe, P.A.; Lee, J.D.; Woodruff, T.M.; Henderson, R.D. The Peripheral Immune System and Amyotrophic Lateral Sclerosis. Front. Neurol. 2020, 11, 279. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, R.M. How neuroinflammation contributes to neurodegeneration. Science 2016, 353, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Beers, D.R.; Appel, S.H. Immune dysregulation in amyotrophic lateral sclerosis: Mechanisms and emerging therapies. Lancet Neurol. 2019, 18, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Bluestone, J.A.; Mackay, C.R.; O’Shea, J.J.; Stockinger, B. The functional plasticity of T cell subsets. Nat. Rev. Immunol. 2009, 9, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Evans, F.L.; Dittmer, M.; de la Fuente, A.G.; Fitzgerald, D.C. Protective and Regenerative Roles of T Cells in Central Nervous System Disorders. Front. Immunol. 2019, 10, 2171. [Google Scholar] [CrossRef] [PubMed]
- Caza, T.; Landas, S. Functional and Phenotypic Plasticity of CD4(+) T Cell Subsets. Biomed Res. Int. 2015, 2015, 521957. [Google Scholar] [CrossRef] [PubMed]
- Beers, D.R.; Henkel, J.S.; Zhao, W.; Wang, J.; Huang, A.; Wen, S.; Liao, B.; Appel, S.H. Endogenous regulatory T lymphocytes ameliorate amyotrophic lateral sclerosis in mice and correlate with disease progression in patients with amyotrophic lateral sclerosis. Brain 2011, 134 Pt 5, 1293–1314. [Google Scholar] [CrossRef]
- Sheean, R.K.; McKay, F.C.; Cretney, E.; Bye, C.R.; Perera, N.D.; Tomas, D.; Weston, R.A.; Scheller, K.J.; Djouma, E.; Menon, P.; et al. Association of Regulatory T-Cell Expansion With Progression of Amyotrophic Lateral Sclerosis: A Study of Humans and a Transgenic Mouse Model. JAMA Neurol. 2018, 75, 681–689. [Google Scholar] [CrossRef]
- Prinz, M.; Priller, J. The role of peripheral immune cells in the CNS in steady state and disease. Nat. Neurosci. 2017, 20, 136–144. [Google Scholar] [CrossRef]
- Williams, G.P.; Schonhoff, A.M.; Jurkuvenaite, A.; Gallups, N.J.; Standaert, D.G.; Harms, A.S. CD4 T cells mediate brain inflammation and neurodegeneration in a mouse model of Parkinson’s disease. Brain 2021, 144, 2047–2059. [Google Scholar] [CrossRef]
- Sommer, A.; Winner, B.; Prots, I. The Trojan horse—Neuroinflammatory impact of T cells in neurodegenerative diseases. Mol. Neurodegener. 2017, 12, 78. [Google Scholar] [CrossRef]
- Kölliker-Frers, R.; Udovin, L.; Otero-Losada, M.; Kobiec, T.; Herrera, M.I.; Palacios, J.; Razzitte, G.; Capani, F. Neuroinflammation: An Integrating Overview of Reactive-Neuroimmune Cell Interactions in Health and Disease. Mediators Inflamm. 2021, 2021, 9999146. [Google Scholar] [CrossRef]
- Moreno-Martinez, L.; Calvo, A.C.; Muñoz, M.J.; Osta, R. Are Circulating Cytokines Reliable Biomarkers for Amyotrophic Lateral Sclerosis? Int. J. Mol. Sci. 2019, 20, 2759. [Google Scholar] [CrossRef]
- Zhao, W.; Xie, W.; Xiao, Q.; Beers, D.R.; Appel, S.H. Protective effects of an anti-inflammatory cytokine, interleukin-4, on motoneuron toxicity induced by activated microglia. J. Neurochem. 2006, 99, 1176–1187. [Google Scholar] [CrossRef]
- Jin, M.; Günther, R.; Akgün, K.; Hermann, A.; Ziemssen, T. Peripheral proinflammatory Th1/Th17 immune cell shift is linked to disease severity in amyotrophic lateral sclerosis. Sci. Rep. 2020, 10, 5941. [Google Scholar] [CrossRef]
- Pinilla, G.; Kumar, A.; Floaters, M.K.; Pardo, C.A.; Rothstein, J.; Ilieva, H. Increased synthesis of pro-inflammatory cytokines in C9ORF72 patients. Amyotroph. Lateral Scler. Frontotemporal. Degener. 2021, 22, 517–527. [Google Scholar] [CrossRef]
- Fu, J.; Huang, Y.; Bao, T.; Liu, C.; Liu, X.; Chen, X. The role of Th17 cells/IL-17A in AD, PD, ALS and the strategic therapy targeting on IL-17A. J. Neuroinflamm. 2022, 19, 98. [Google Scholar] [CrossRef]
- Italiani, P.; Carlesi, C.; Giungato, P.; Puxeddu, I.; Borroni, B.; Bossù, P.; Migliorini, P.; Siciliano, G.; Boraschi, D. Evaluating the levels of interleukin-1 family cytokines in sporadic amyotrophic lateral sclerosis. J. Neuroinflamm. 2014, 11, 94. [Google Scholar] [CrossRef]
- González, H.; Pacheco, R. T-cell-mediated regulation of neuroinflammation involved in neurodegenerative diseases. J. Neuroinflamm. 2014, 11, 201. [Google Scholar] [CrossRef]
- Martynova, E.; Rizvanov, A.; Urbanowicz, R.A.; Khaiboullina, S. Inflammasome Contribution to the Activation of Th1, Th2, and Th17 Immune Responses. Front. Microbiol. 2022, 13, 851835. [Google Scholar] [CrossRef]
- Tang, Y.; Le, W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 1181–1194. [Google Scholar] [CrossRef]
- Cragnolini, A.B.; Lampitella, G.; Virtuoso, A.; Viscovo, I.; Panetsos, F.; Papa, M.; Cirillo, G. Regional brain susceptibility to neurodegeneration: What is the role of glial cells? Neural Regen. Res. 2020, 15, 838–842. [Google Scholar] [CrossRef]
- Guo, S.; Wang, H.; Yin, Y. Microglia Polarization From M1 to M2 in Neurodegenerative Diseases. Front. Aging Neurosci. 2022, 14, 815347. [Google Scholar] [CrossRef]
- Cherry, J.D.; Olschowka, J.A.; O’Banion, M.K. Neuroinflammation and M2 microglia: The good, the bad, and the inflamed. J. Neuroinflamm. 2014, 11, 98. [Google Scholar] [CrossRef]
- Peña-Altamira, E.; Prati, F.; Massenzio, F.; Virgili, M.; Contestabile, A.; Bolognesi, M.L.; Monti, B. Changing paradigm to target microglia in neurodegenerative diseases: From anti-inflammatory strategy to active immunomodulation. Expert. Opin. Ther. Targets 2016, 20, 627–640. [Google Scholar] [CrossRef]
- De Marchi, F.; Munitic, I.; Amedei, A.; Berry, J.D.; Feldman, E.L.; Aronica, E.; Nardo, G.; Van Weehaeghe, D.; Niccolai, E.; Prtenjaca, N.; et al. Interplay between immunity and amyotrophic lateral sclerosis: Clinical impact. Neurosci. Biobehav. Rev. 2021, 127, 958–978. [Google Scholar] [CrossRef]
- McCombe, P.A.; Henderson, R.D. The Role of immune and inflammatory mechanisms in ALS. Curr. Mol. Med. 2011, 11, 246–254. [Google Scholar] [CrossRef]
- Cirulli, E.T.; Lasseigne, B.N.; Petrovski, S.; Sapp, P.C.; Dion, P.A.; Leblond, C.S.; Couthouis, J.; Lu, Y.F.; Wang, Q.; Krueger, B.J.; et al. Exome sequencing in amyotrophic lateral sclerosis identifies risk genes and pathways. Science 2015, 347, 1436–1441. [Google Scholar] [CrossRef] [PubMed]
- Lehnardt, S.; Massillon, L.; Follett, P.; Jensen, F.E.; Ratan, R.; Rosenberg, P.A.; Volpe, J.J.; Vartanian, T. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc. Natl. Acad. Sci. USA 2003, 100, 8514–8519. [Google Scholar] [CrossRef] [PubMed]
- Masrori, P.; Beckers, J.; Gossye, H.; Van Damme, P. The role of inflammation in neurodegeneration: Novel insights into the role of the immune system in C9orf72 HRE-mediated ALS/FTD. Mol. Neurodegener. 2022, 17, 22. [Google Scholar] [CrossRef] [PubMed]
- Thonhoff, J.R.; Beers, D.R.; Zhao, W.; Pleitez, M.; Simpson, E.P.; Berry, J.D.; Cudkowicz, M.E.; Appel, S.H. Expanded autologous regulatory T-lymphocyte infusions in ALS: A phase I, first-in-human study. Neurol. Neuroimmunol. Neuroinflamm. 2018, 5, e465. [Google Scholar] [CrossRef] [PubMed]
- Amici, S.A.; Dong, J.; Guerau-de-Arellano, M. Molecular Mechanisms Modulating the Phenotype of Macrophages and Microglia. Front. Immunol. 2017, 8, 1520. [Google Scholar] [CrossRef]
- Motataianu, A.; Barcutean, L.; Balasa, R. Neuroimmunity in amyotrophic lateral sclerosis: Focus on microglia. Amyotroph. Lateral. Scler. Frontotemporal. Degener. 2020, 21, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Carata, E.; Muci, M.; Di Giulio, S.; Mariano, S.; Panzarini, E. Looking to the Future of the Role of Macrophages and Extracellular Vesicles in Neuroinflammation in ALS. Int. J. Mol. Sci. 2023, 24, 11251. [Google Scholar] [CrossRef] [PubMed]
- Béland, L.C.; Markovinovic, A.; Jakovac, H.; De Marchi, F.; Bilic, E.; Mazzini, L.; Kriz, J.; Munitic, I. Immunity in amyotrophic lateral sclerosis: Blurred lines between excessive inflammation and inefficient immune responses. Brain Commun. 2020, 2, fcaa124. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Huo, Y.; Bai, J.; Wang, H.; Wang, H.; Yang, F.; Cui, F.; Song, H.; Huang, X. Inflammatory Cytokine Levels in Patients with Sporadic Amyotrophic Lateral Sclerosis. Neurodegener. Dis. 2021, 21, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Tortelli, R.; Zecca, C.; Piccininni, M.; Benmahamed, S.; Dell’Abate, M.T.; Barulli, M.R.; Capozzo, R.; Battista, P.; Logroscino, G. Plasma Inflammatory Cytokines Are Elevated in ALS. Front. Neurol. 2020, 11, 552295. [Google Scholar] [CrossRef] [PubMed]
- Poloni, M.; Facchetti, D.; Mai, R.; Micheli, A.; Agnoletti, L.; Francolini, G.; Mora, G.; Camana, C.; Mazzini, L.; Bachetti, T. Circulating levels of tumour necrosis factor-alpha and its soluble receptors are increased in the blood of patients with amyotrophic lateral sclerosis. Neurosci. Lett. 2000, 287, 211–214. [Google Scholar] [CrossRef]
- Peters, S.; Zitzelsperger, E.; Kuespert, S.; Iberl, S.; Heydn, R.; Johannesen, S.; Petri, S.; Aigner, L.; Thal, D.R.; Hermann, A.; et al. The TGF-β System As a Potential Pathogenic Player in Disease Modulation of Amyotrophic Lateral Sclerosis. Front. Neurol. 2017, 8, 669. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.H.; Allen, K.; Oei, F.; Leoni, E.; Kuhle, J.; Tree, T.; Fratta, P.; Sharma, N.; Sidle, K.; Howard, R.; et al. Systemic inflammatory response and neuromuscular involvement in amyotrophic lateral sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2016, 3, e244. [Google Scholar] [CrossRef] [PubMed]
- Prado, L.G.R.; Rocha, N.P.; de Souza, L.C.; Bicalho, I.C.S.; Gomez, R.S.; Vidigal-Lopes, M.; Braz, N.F.T.; Vieira, É.L.M.; Teixeira, A.L. Longitudinal assessment of clinical and inflammatory markers in patients with amyotrophic lateral sclerosis. J. Neurol. Sci. 2018, 394, 69–74. [Google Scholar] [CrossRef]
- Huang, F.; Zhu, Y.; Hsiao-Nakamoto, J.; Tang, X.; Dugas, J.C.; Moscovitch-Lopatin, M.; Glass, J.D.; Brown, R.H., Jr.; Ladha, S.S.; Lacomis, D.; et al. Longitudinal biomarkers in amyotrophic lateral sclerosis. Ann. Clin. Transl. Neurol. 2020, 7, 1103–1116. [Google Scholar] [CrossRef]
- Pronto-Laborinho, A.; Pinto, S.; Gromicho, M.; Pereira, M.; Swash, M.; de Carvalho, M. Interleukin-6 and amyotrophic lateral sclerosis. J. Neurol. Sci. 2019, 398, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xia, K.; Chen, L.; Fan, D. Increased Interleukin-6 Levels in the Astrocyte-Derived Exosomes of Sporadic Amyotrophic Lateral Sclerosis Patients. Front. Neurosci. 2019, 13, 574. [Google Scholar] [CrossRef]
- Lin, C.Y.; Pfluger, C.M.; Henderson, R.D.; McCombe, P.A. Reduced levels of interleukin 33 and increased levels of soluble ST2 in subjects with amyotrophic lateral sclerosis. J. Neuroimmunol. 2012, 249, 93–95. [Google Scholar] [CrossRef]
- Cipollina, G.; Davari Serej, A.; Di Nolfi, G.; Gazzano, A.; Marsala, A.; Spatafora, M.G.; Peviani, M. Heterogeneity of Neuroinflammatory Responses in Amyotrophic Lateral Sclerosis: A Challenge or an Opportunity? Int. J. Mol. Sci. 2020, 21, 7923. [Google Scholar] [CrossRef] [PubMed]
- Fossiez, F.; Djossou, O.; Chomarat, P.; Flores-Romo, L.; Ait-Yahia, S.; Maat, C.; Pin, J.J.; Garrone, P.; Garcia, E.; Saeland, S.; et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J. Exp. Med. 1996, 183, 2593–2603. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Painter, S.L.; Fanslow, W.C.; Ulrich, D.; Macduff, B.M.; Spriggs, M.K.; Armitage, R.J. Human IL-17: A novel cytokine derived from T cells. J. Immunol. 1995, 155, 5483–5486. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, X.; Zhong, Y. Interleukin-17A: The Key Cytokine in Neurodegenerative Diseases. Front. Aging Neurosci. 2020, 12, 566922. [Google Scholar] [CrossRef] [PubMed]
- Saresella, M.; Piancone, F.; Tortorella, P.; Marventano, I.; Gatti, A.; Caputo, D.; Lunetta, C.; Corbo, M.; Rovaris, M.; Clerici, M. T helper-17 activation dominates the immunologic milieu of both amyotrophic lateral sclerosis and progressive multiple sclerosis. Clin. Immunol. 2013, 148, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Rentzos, M.; Rombos, A.; Nikolaou, C.; Zoga, M.; Zouvelou, V.; Dimitrakopoulos, A.; Alexakis, T.; Tsoutsou, A.; Samakovli, A.; Michalopoulou, M.; et al. Interleukin-17 and interleukin-23 are elevated in serum and cerebrospinal fluid of patients with ALS: A reflection of Th17 cells activation? Acta Neurol. Scand. 2010, 122, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef] [PubMed]
- Rentzos, M.; Rombos, A.; Nikolaou, C.; Zoga, M.; Zouvelou, V.; Dimitrakopoulos, A.; Alexakis, T.; Tsoutsou, A.; Samakovli, A.; Michalopoulou, M.; et al. Interleukin-15 and interleukin-12 are elevated in serum and cerebrospinal fluid of patients with amyotrophic lateral sclerosis. Eur. Neurol. 2010, 63, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gao, L.; Zang, D. Elevated Levels of IFN-γ in CSF and Serum of Patients with Amyotrophic Lateral Sclerosis. PLoS ONE 2015, 10, e0136937. [Google Scholar] [CrossRef] [PubMed]
- Maranzano, A.; Verde, F.; Colombo, E.; Poletti, B.; Doretti, A.; Bonetti, R.; Gagliardi, D.; Meneri, M.; Maderna, L.; Messina, S.; et al. Regional spreading pattern is associated with clinical phenotype in amyotrophic lateral sclerosis. Brain 2023, 146, 4105–4116. [Google Scholar] [CrossRef]
- Turner, M.R.; Brockington, A.; Scaber, J.; Hollinger, H.; Marsden, R.; Shaw, P.J.; Talbot, K. Pattern of spread and prognosis in lower limb-onset ALS. Amyotroph. Lateral. Scler. 2010, 11, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, C.J. Interleukin-5, eosinophils, and disease. Blood 1992, 79, 3101–3109. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.F.; Eglinton, J.M.; Lyons, A.B.; Tapley, P.M.; To, L.B.; Park, L.S.; Clark, S.C.; Vadas, M.A. Human interleukin-3 inhibits the binding of granulocyte-macrophage colony-stimulating factor and interleukin-5 to basophils and strongly enhances their functional activity. J. Cell Physiol. 1990, 145, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekharappa, S.C.; Rebelsky, M.S.; Firak, T.A.; Le Beau, M.M.; Westbrook, C.A. A long-range restriction map of the interleukin-4 and interleukin-5 linkage group on chromosome 5. Genomics 1990, 6, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, B.H.; Martinson, M.E.; Webb, G.C.; Young, I.G. Molecular organization of the cytokine gene cluster, involving the human IL-3, IL-4, IL-5, and GM-CSF genes, on human chromosome 5. Blood 1989, 73, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Hoppenot, D.; Malakauskas, K.; Lavinskiene, S.; Sakalauskas, R. p-STAT6, PU.1, and NF-κB are involved in allergen-induced late-phase airway inflammation in asthma patients. BMC Pulm. Med. 2015, 15, 122. [Google Scholar] [CrossRef] [PubMed]
- Hogan, S.P.; Koskinen, A.; Matthaei, K.I.; Young, I.G.; Foster, P.S. Interleukin-5-producing CD4+ T cells play a pivotal role in aeroallergen-induced eosinophilia, bronchial hyperreactivity, and lung damage in mice. Am. J. Respir. Crit. Care Med. 1998, 157, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Luckheeram, R.V.; Zhou, R.; Verma, A.D.; Xia, B. CD4+T cells: Differentiation and functions. Clin. Dev. Immunol. 2012, 2012, 925135. [Google Scholar] [CrossRef] [PubMed]
- Beers, D.R.; Henkel, J.S.; Zhao, W.; Wang, J.; Appel, S.H. CD4+ T cells support glial neuroprotection, slow disease progression, and modify glial morphology in an animal model of inherited ALS. Proc. Natl. Acad. Sci. USA 2008, 105, 15558–15563. [Google Scholar] [CrossRef] [PubMed]
- Ringheim, G.E. Mitogenic effects of interleukin-5 on microglia. Neurosci. Lett. 1995, 201, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Liva, S.M.; de Vellis, J. IL-5 induces proliferation and activation of microglia via an unknown receptor. Neurochem. Res. 2001, 26, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, H.D.; Kirsch, M. JAK2-STAT3 signaling: A novel function and a novel mechanism. JAKSTAT 2012, 1, 191–193. [Google Scholar] [CrossRef]
- Buckingham, S.D.; Jones, A.K.; Brown, L.A.; Sattelle, D.B. Nicotinic acetylcholine receptor signalling: Roles in Alzheimer’s disease and amyloid neuroprotection. Pharmacol. Rev. 2009, 61, 39–61. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, A.M.; Nagai, A.; Wakabayashi, K.; Narantuya, D.; Kobayashi, S.; Yamaguchi, S.; Kim, S.U. Mesenchymal stem cell transplantation modulates neuroinflammation in focal cerebral ischemia: Contribution of fractalkine and IL-5. Neurobiol. Dis. 2011, 41, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, C.; Li, D.; Zheng, Y.; Wang, J. IL-5 blocks apoptosis and tau hyperphosphorylation induced by Aβ25-35 peptide in PC12 cells. J. Physiol. Biochem. 2017, 73, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Ehrhart, J.; Smith, A.J.; Kuzmin-Nichols, N.; Zesiewicz, T.A.; Jahan, I.; Shytle, R.D.; Kim, S.H.; Sanberg, C.D.; Vu, T.H.; Gooch, C.L.; et al. Humoral factors in ALS patients during disease progression. J. Neuroinflamm. 2015, 12, 127. [Google Scholar] [CrossRef] [PubMed]
- Kolbeck, R.; Koike, M.; Kawasaki, K.; Iwashita, K.; Spitalny, G.L.; Hanai, N.; Kiener, P.A.; Gossage, D.; Molfino, N.A.; Coyle, A.J. Monoclonal antibody therapy directed against interleukin-5 receptor α: MEDI-563. In New Drugs and Targets for Asthma and COPD; Hansel, T.T., Barnes, P.J., Eds.; Karger Publishers: Basel, Switzerland, 2010; Volume 39, pp. 109–114. [Google Scholar]
- Wiesemann, E.; Klatt, J.; Wenzel, C.; Heidenreich, F.; Windhagen, A. Correlation of serum IL-13 and IL-5 levels with clinical response to Glatiramer acetate in patients with multiple sclerosis. Clin. Exp. Immunol. 2003, 133, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Akdis, M.; Verhagen, J.; Taylor, A.; Karamloo, F.; Karagiannidis, C.; Crameri, R.; Thunberg, S.; Deniz, G.; Valenta, R.; Fiebig, H.; et al. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J. Exp. Med. 2004, 199, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.R.; Miller, R.G.; Swash, M.; Munsat, T.L.; World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph. Lateral. Scler. Other Motor. Neuron. Disord. 2000, 1, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Cedarbaum, J.M.; Stambler, N.; Malta, E.; Fuller, C.; Hilt, D.; Thurmond, B.; Nakanishi, A. The ALSFRS-R: A revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J. Neurol. Sci. 1999, 169, 13–21. [Google Scholar] [CrossRef]
- Kimura, F.; Fujimura, C.; Ishida, S.; Nakajima, H.; Furutama, D.; Uehara, H.; Shinoda, K.; Sugino, M.; Hanafusa, T. Progression rate of ALSFRS-R at time of diagnosis predicts survival time in ALS. Neurology 2006, 66, 265–267. [Google Scholar] [CrossRef]
- Labra, J.; Menon, P.; Byth, K.; Morrison, S.; Vucic, S. Rate of disease progression: A prognostic biomarker in ALS. J. Neurol. Neurosurg. Psychiatry 2016, 87, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Ravits, J.; Appel, S.; Baloh, R.H.; Barohn, R.; Brooks, B.R.; Elman, L.; Floeter, M.K.; Henderson, C.; Lomen-Hoerth, C.; Macklis, J.D.; et al. Deciphering amyotrophic lateral sclerosis: What phenotype, neuropathology and genetics are telling us about pathogenesis. Amyotroph. Lateral. Scler. Frontotemporal. Degener. 2013, 14 (Suppl. S1), 5–18. [Google Scholar] [CrossRef] [PubMed]
- Roche, J.C.; Rojas-Garcia, R.; Scott, K.M.; Scotton, W.; Ellis, C.E.; Burman, R.; Wijesekera, L.; Turner, M.R.; Leigh, P.N.; Shaw, C.E.; et al. A proposed staging system for amyotrophic lateral sclerosis. Brain 2012, 135 Pt 3, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Milella, G.; Zoccolella, S.; Urso, D.; Nigro, S.; Tamburrino, L.; Gnoni, V.; Filardi, M.; Logroscino, G. Different patterns of spreading direction and motor neurons involvement in a cohort of limb-onset amyotrophic lateral sclerosis patients from Southern Italy: Potential implication on disease course or progression? Brain Behav. 2023, 13, e2899. [Google Scholar] [CrossRef] [PubMed]
- Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: Executive summary. Expert Panel on the Identification, Evaluation, and Treatment of Overweight in Adults. Am. J. Clin. Nutr. 1998, 68, 899–917. [Google Scholar] [CrossRef] [PubMed]
- IBM Corp. Released 2019. IBM SPSS Statistics for Windows, Version 26.0; IBM Corp: Armonk, NY, USA, 2019. [Google Scholar]
- Microsoft Corporation, Inc. Released 2018. Microsoft Office 2019; Microsoft Corporation, Inc.: Redmond, WA, USA, 2018. [Google Scholar]




| Demographic and Clinical Data | ALS Cases | Controls |
|---|---|---|
| Number of patients n | 59 | 40 |
| Gender of patients n (%) | ||
| Females | 22 (37.3%) | 18 (45.0%) |
| Males | 37 (62.7%) | 22 (55.0%) |
| Age years (mean ± standard deviation) | 57.28 ± 9.79 | 56.45 ± 7.95 |
| ALSFRS-R points (mean ± standard deviation) | 38.05 ± 6.40 | |
| Progression rate of ALSFRS-R | ||
| Progression rate < 0.47%—Fast | 40.0% | |
| Progression rate 0.47–1.11%—Moderate | 27.3% | |
| Progression rate > 1.11%—Slow | 32.7% | |
| ALS type n (%) | ||
| Bulbar-onset | 11 (18.6%) | |
| Spinal-onset | 48 (81.4%) | |
| ALS phenotype % | ||
| Flail arm | 18.64% | |
| Flail leg | 16.94% | |
| Bulbar | 3.38% | |
| Typical (LMN and UMN involvement) | 61.01% | |
| King’s staging % | ||
| 2A | 8 | |
| 2B | 46 | |
| 3 | 44 | |
| 4A | 2 | |
| Clinical progression pattern % | ||
| HSP | 42% | |
| Cervical to cervical contralateral | 16% | |
| Lumbar to lumbar contralateral | 26% | |
| VSP | 58% | |
| Cervical–lumbar | 5% | |
| Lumbar–cervical | 17% | |
| Bulbar–cervical | 19% | |
| Bulbar–lumbar | 2% | |
| Cervical–bulbar | 10% | |
| Lumbar–bulbar | 5% | |
| Survival months | ||
| From onset (mean ± standard deviation) | 46.85 ± 22.93 | |
| From diagnosis (mean ± standard deviation) | 32.86 ± 19.69 | |
| Beck’s Depression Inventory points (mean ± standard deviation) | 15.53 ± 9.21 | |
| Frontal Assessment Battery points (mean ± standard deviation) | 13.73 ± 2.06 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moțățăianu, A.; Andone, S.; Stoian, A.; Bălașa, R.; Huțanu, A.; Sărmășan, E. A Potential Role of Interleukin-5 in the Pathogenesis and Progression of Amyotrophic Lateral Sclerosis: A New Molecular Perspective. Int. J. Mol. Sci. 2024, 25, 3782. https://doi.org/10.3390/ijms25073782
Moțățăianu A, Andone S, Stoian A, Bălașa R, Huțanu A, Sărmășan E. A Potential Role of Interleukin-5 in the Pathogenesis and Progression of Amyotrophic Lateral Sclerosis: A New Molecular Perspective. International Journal of Molecular Sciences. 2024; 25(7):3782. https://doi.org/10.3390/ijms25073782
Chicago/Turabian StyleMoțățăianu, Anca, Sebastian Andone, Adina Stoian, Rodica Bălașa, Adina Huțanu, and Emanuela Sărmășan. 2024. "A Potential Role of Interleukin-5 in the Pathogenesis and Progression of Amyotrophic Lateral Sclerosis: A New Molecular Perspective" International Journal of Molecular Sciences 25, no. 7: 3782. https://doi.org/10.3390/ijms25073782
APA StyleMoțățăianu, A., Andone, S., Stoian, A., Bălașa, R., Huțanu, A., & Sărmășan, E. (2024). A Potential Role of Interleukin-5 in the Pathogenesis and Progression of Amyotrophic Lateral Sclerosis: A New Molecular Perspective. International Journal of Molecular Sciences, 25(7), 3782. https://doi.org/10.3390/ijms25073782







