Exogenous Melatonin Application Accelerated the Healing Process of Oriental Melon Grafted onto Squash by Promoting Lignin Accumulation
Abstract
1. Introduction
2. Results
2.1. Identification of Members of the CmGH9B Gene Family Members
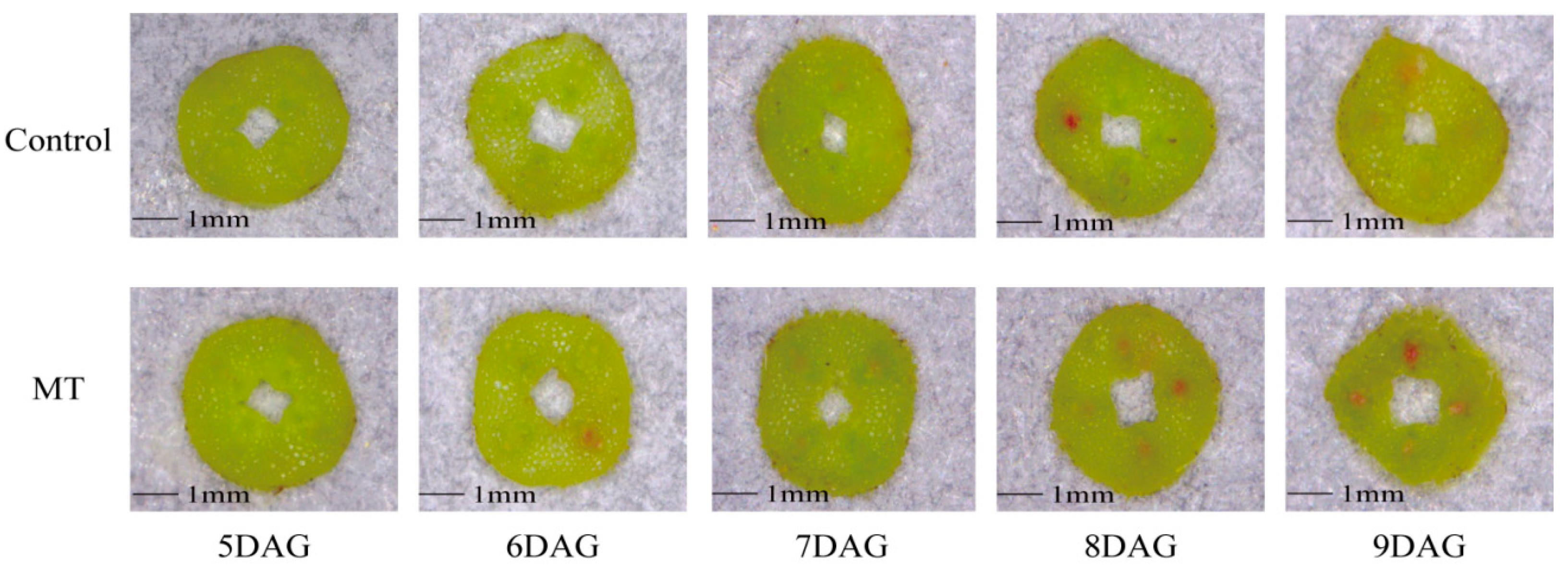
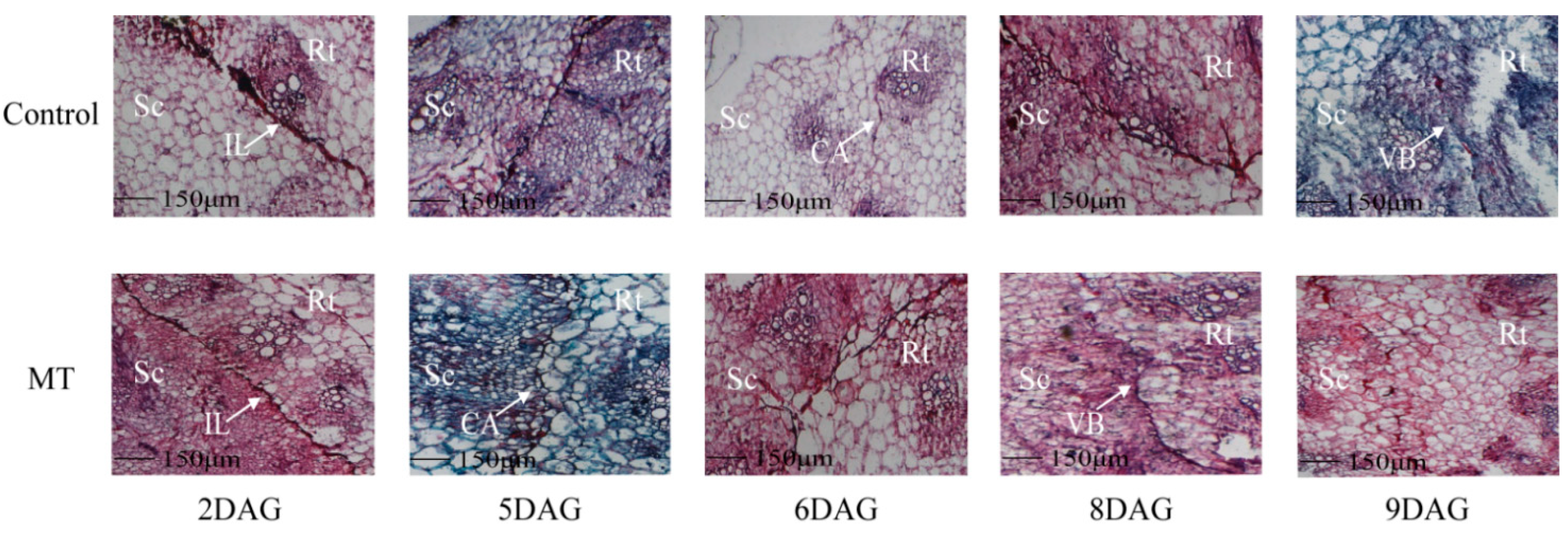
2.2. Historic Observation of the Graft Union Healing Process of the Oriental Melon Scion Grafted onto Squash Rootstock by the Exogenous MT Treatment
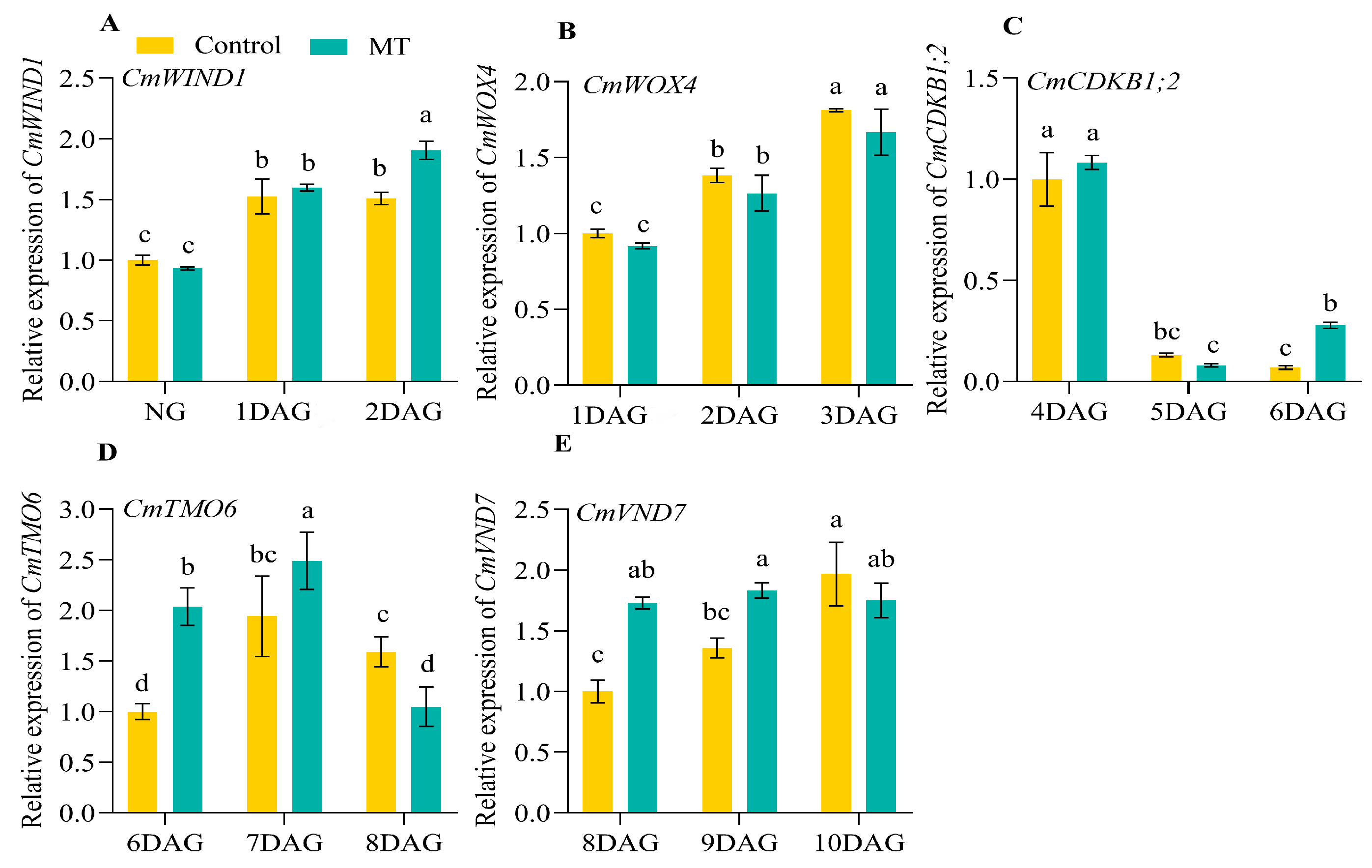
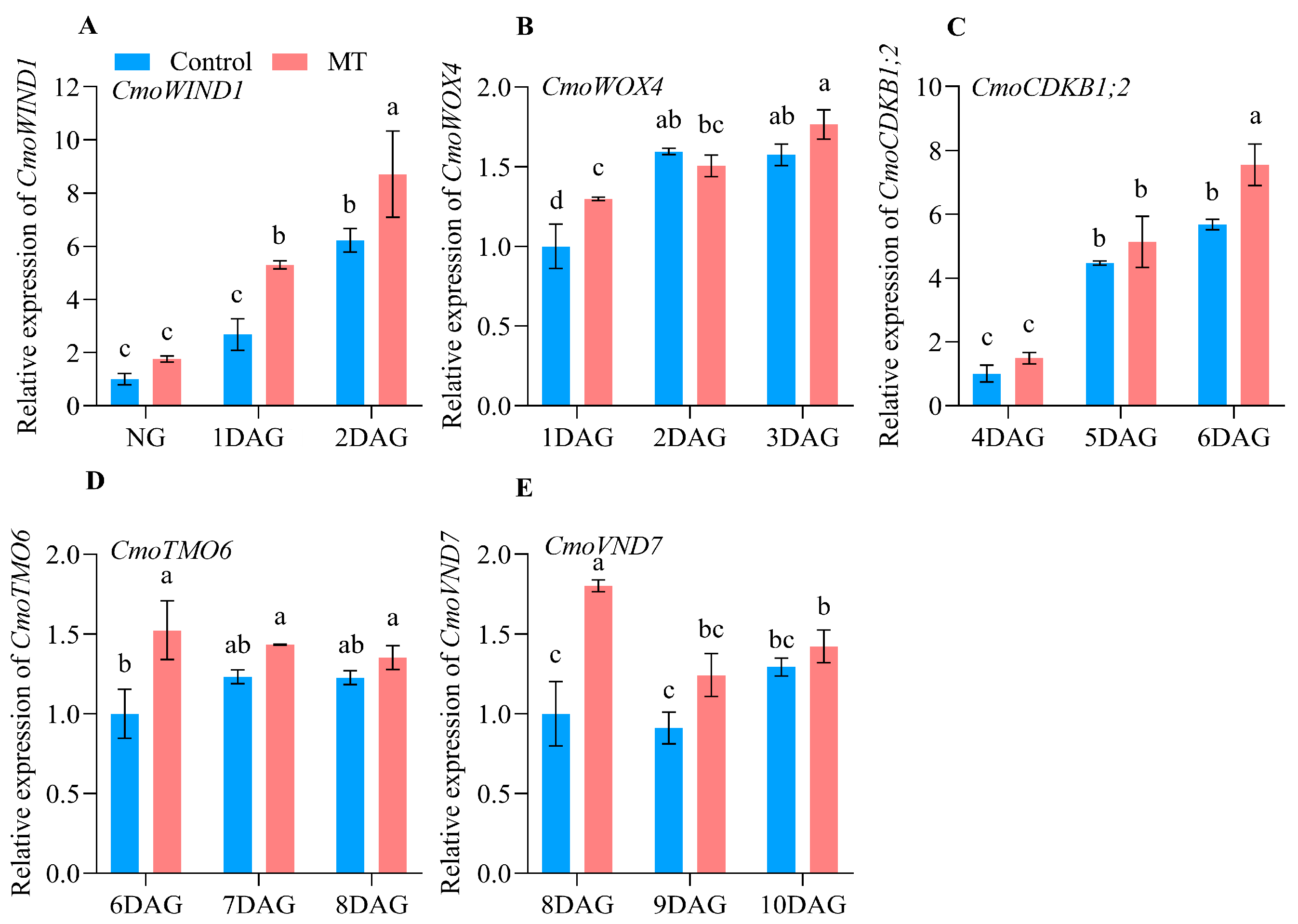
2.3. Expression Profiles of the Genes Related to Graft Union Healing of the Oriental Melon Scion Grafted onto Squash Rootstock by the Exogenous MT Treatment
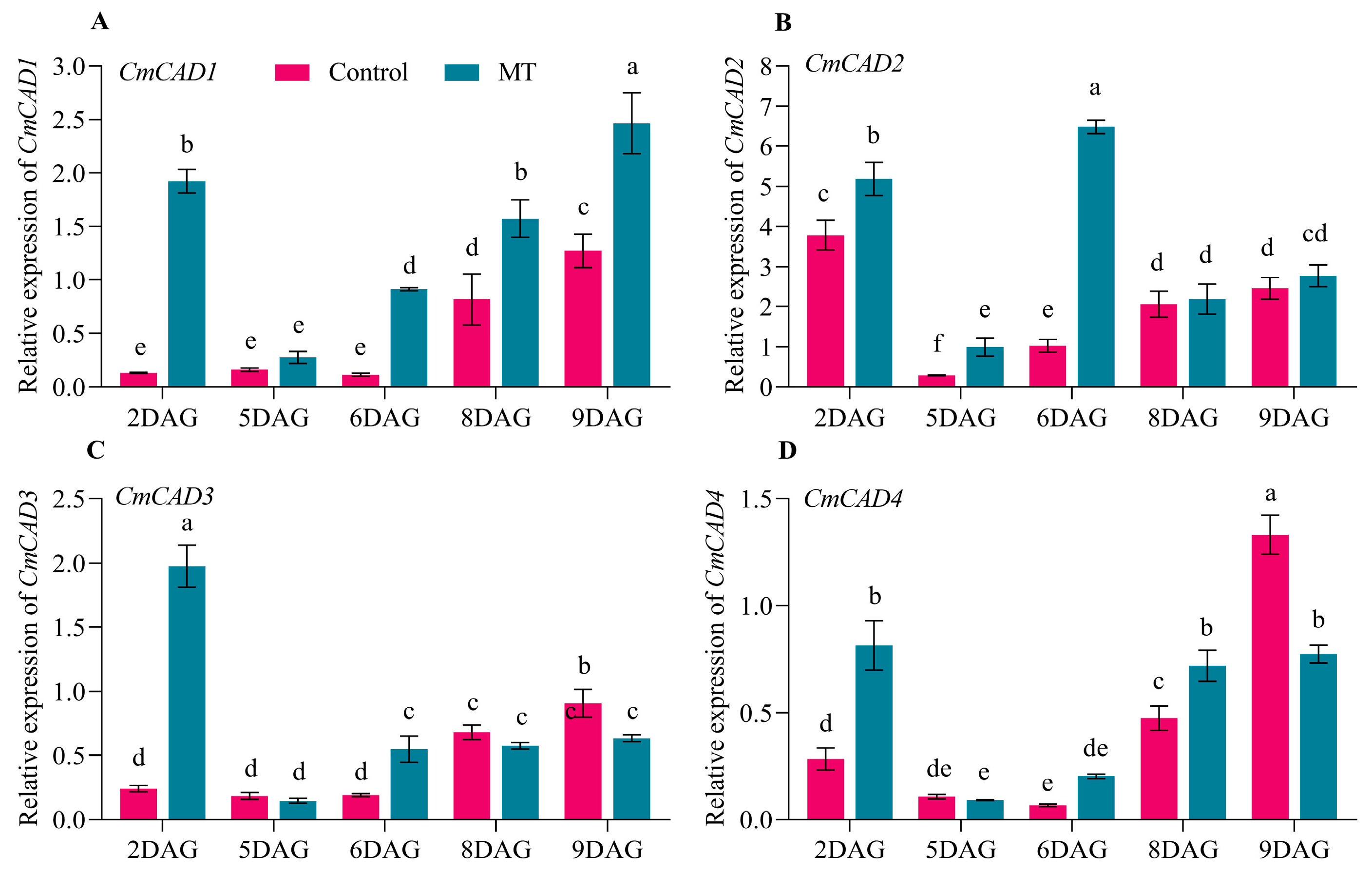
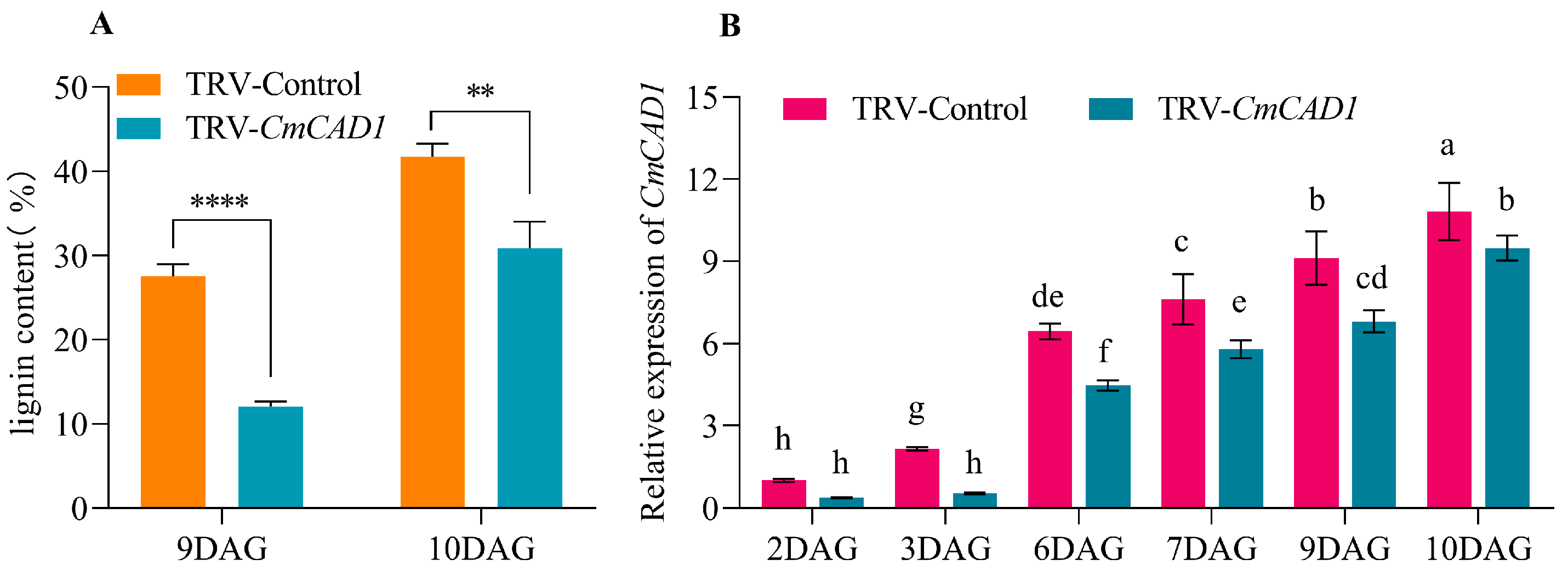
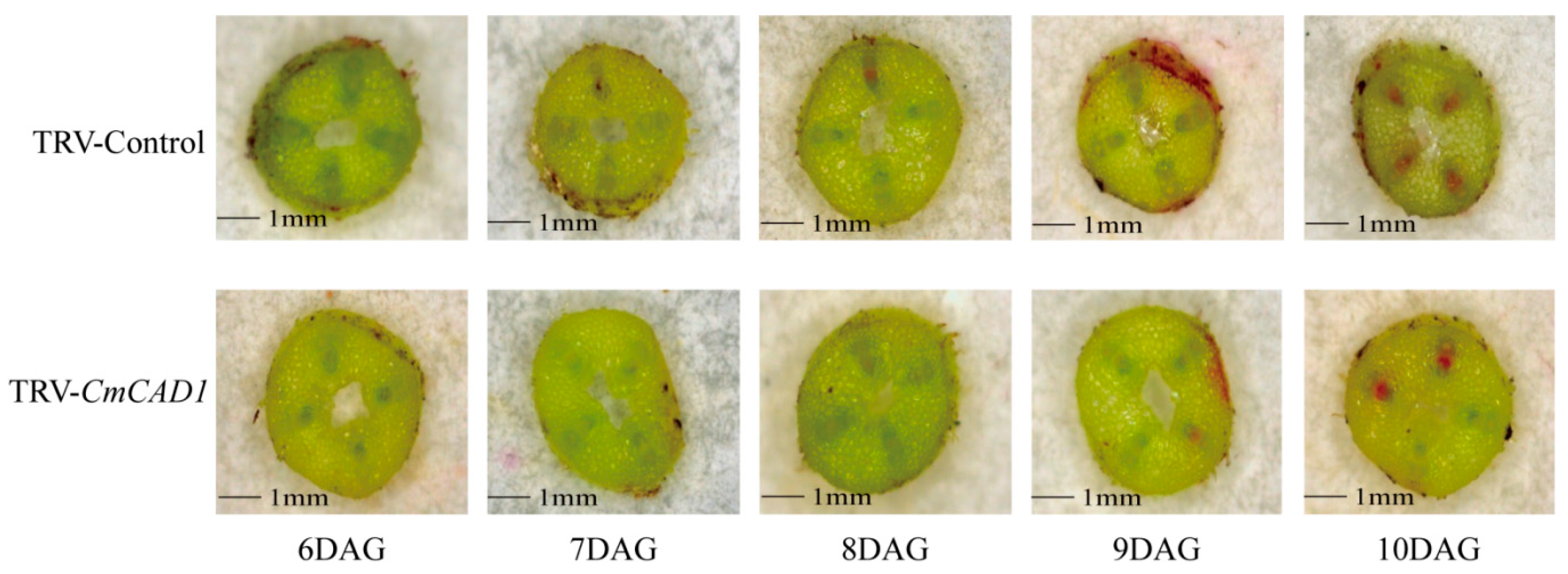
2.4. Expression Profiles and Functions of CmCADs during the Graft Healing Process of Oriental Melon Scion Grafted onto Squash Rootstock by the Exogenous MT Treatment
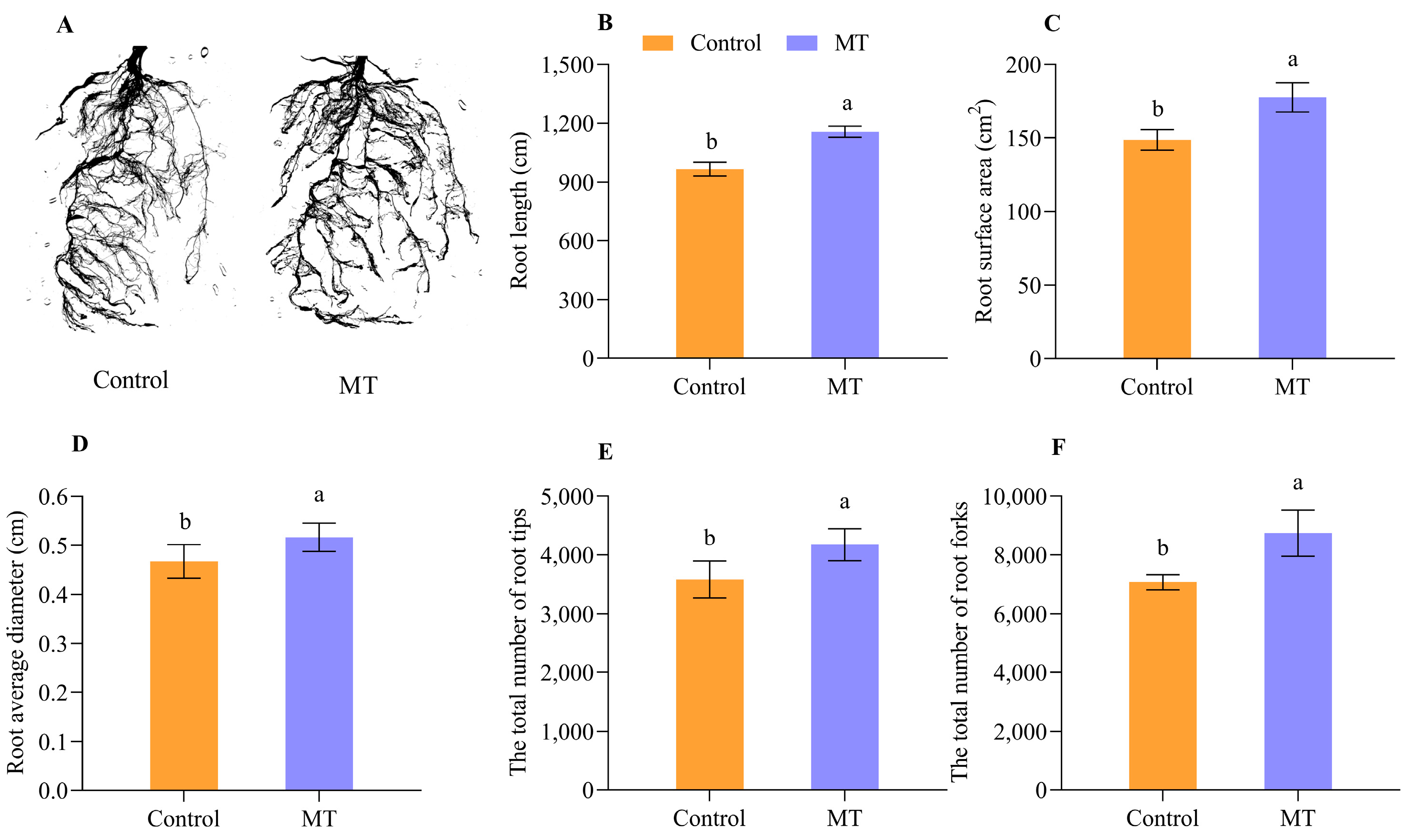
2.5. Effects of the Exogenous MT Treatment on the Grafted Seedling Root Growth of Oriental Melon Scion Grafted onto Squash Rootstock
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Determination of Lignin Content and Enzyme Activities
4.3. Histological Section Observation
4.4. Quantitative Real-Time PCR (qRT-PCR)
4.5. Virus-Induced Gene Silencing (VIGS) Assay
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Louws, F.J.; Rivard, C.L.; Kubota, C. Grafting fruiting vegetables to manage soilborne pathogens, foliar pathogens, arthropods and weeds. Sci. Hortic. 2010, 127, 127–146. [Google Scholar] [CrossRef]
- Lu, X.; Liu, W.; Wang, T.; Zhang, J.; Li, X.; Zhang, W. Systemic Long-Distance Signaling and Communication Between Rootstock and Scion in Grafted Vegetables. Front. Plant Sci. 2020, 11, 460. [Google Scholar] [CrossRef] [PubMed]
- Moore, R. Studies of vegetative compatibility-incompatibility in higher plants. IV. The development of tensile strength in a compatible and an incompatible graft. Protoplasma 1983, 115, 114–121. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Reiter, R.J. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 2007, 42, 28–42. [Google Scholar] [CrossRef]
- Fazal, H.; Abbasi, B.H.; Ahmad, N.; Ali, M. Exogenous melatonin trigger biomass accumulation and production of stress enzymes during callogenesis in medicinally important Prunella vulgaris L. (Selfheal). Physiol. Mol. Biol. Plants 2018, 24, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Yang, K.; Zhang, Y.; Li, Z. Can antioxidant’s reactive oxygen species (ROS) scavenging capacity contribute to aged seed recovery? Contrasting effect of melatonin, ascorbate and glutathione on germination ability of aged maize seeds. Free Radic. Res. 2017, 51, 765–771. [Google Scholar] [CrossRef]
- Chen, Q.; Qi, W.B.; Reiter, R.J.; Wei, W.; Wang, B.M. Exogenously applied melatonin stimulates root growth and raises endogenous indoleacetic acid in roots of etiolated seedlings of Brassica juncea. J. Plant Physiol. 2009, 166, 324–328. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, T.; Liu, G.; Hu, M.; Yun, Z.; Duan, X.; Cai, K.; Jiang, G. Inhibition of downy blight and enhancement of resistance in litchi fruit by postharvest application of melatonin. Food Chem. 2021, 347, 129009. [Google Scholar] [CrossRef]
- Shi, H.; Tan, D.X.; Reiter, R.J.; Ye, T.; Yang, F.; Chan, Z. Melatonin induces class A1 heat-shock factors (HSFA1s) and their possible involvement of thermotolerance in Arabidopsis. J. Pineal Res. 2015, 58, 335–342. [Google Scholar] [CrossRef]
- Wang, P.; Sun, X.; Li, C.; Wei, Z.; Liang, D.; Ma, F. Long-term exogenous application of melatonin delays drought-induced leaf senescence in apple. J. Pineal Res. 2013, 54, 292–302. [Google Scholar] [CrossRef]
- Uchendu, E.E.; Shukla, M.R.; Reed, B.M.; Saxena, P.K. Melatonin enhances the recovery of cryopreserved shoot tips of American elm (Ulmus americana L.). J. Pineal Res. 2013, 55, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Wang, P.; Li, M.; Ke, X.; Li, C.; Liang, D.; Wu, S.; Ma, X.; Li, C.; Zou, Y.; et al. Exogenous melatonin improves Malus resistance to Marssonina apple blotch. J. Pineal Res. 2013, 54, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Qi, L.W.; Wang, W.M.; Saxena, P.K.; Liu, C.Z. Melatonin improves the survival of cryopreserved callus of Rhodiola crenulata. J. Pineal Res. 2011, 50, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.Q.; Luan, Y.T.; Shi, W.B.; Tang, Y.H.; Huang, X.Q.; Tao, J. Melatonin enhances stem strength by increasing lignin content and secondary cell wall thickness in herbaceous peony. J. Exp. Bot. 2022, 73, 5974–5991. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.N.; Zhao, F.A.; Yang, X.J.; Li, W.; Xie, D.Y.; Tang, Z.J.; Lv, S.P.; Nie, L.H.; Sun, Y.; Wang, M.M.; et al. Lignin synthesis related genes with potential significance in the response of upland cotton to Fusarium wilt identified by transcriptome profiling. Trop. Plant Biol. 2021, 14, 106–119. [Google Scholar] [CrossRef]
- Han, M.H.; Yang, N.; Wan, Q.W.; Teng, R.M.; Duan, A.Q.; Wang, Y.H.; Zhuang, J. Exogenous melatonin positively regulates lignin biosynthesis in Camellia sinensis. Int. J. Biol. Macromol. 2021, 179, 485–499. [Google Scholar] [CrossRef]
- Moura, J.C.M.S.; Bonine, C.A.V.; Viana, J.D.O.F.; Dornelas, M.C.; Mazzafera, P. Abiotic and biotic stresses and changes in the lignin content and composition in plants. J. Integr. Plant Biol. 2010, 52, 360–376. [Google Scholar] [CrossRef] [PubMed]
- Derikvand, M.M.; Sierra, J.B.; Ruel, K.; Pollet, B.; Do, C.T.; Thévenin, J.; Buffard, D.; Jouanin, L.; Lapierre, C. Redirection of the phenylpropanoid pathway to feruloyl malate in Arabidopsis mutants deficient for cinnamoyl-CoA reductase 1. Planta 2008, 227, 943–956. [Google Scholar] [CrossRef]
- Rest, B.V.D.; Rochange, S.F. Down-regulation of cinnamoyl-CoA reductase in tomato (Solanum lycopersicum L.) induces dramatic changes in soluble phenolic pools. J. Exp. Bot. 2006, 57, 1399–1411. [Google Scholar] [CrossRef]
- Tripathi, S.C.; Sayre, K.D.; Kaul, J.N.; Narang, R.S. Growth and morphology of spring wheat (Triticum aestivum L.) culms and their association with lodging: Effects of genotypes, N levels and ethephon. Field Crops Res. 2003, 84, 271–290. [Google Scholar] [CrossRef]
- Xu, C.Q.; Zhang, Y.; Zhao, M.Z.; Liu, Y.; Xu, X.; Li, T.L. Transcriptomic analysis of melon/squash graft junction reveals molecular mechanisms potentially underlying the graft union development. Peer J. 2021, 9, e12569. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhao, C.; Hu, F.; Qin, Y.; Wang, X.; Hu, G. Transcriptome changes between compatible and in compatible graft combination of Litchi chinensis by digital gene expression profile. Sci. Rep. 2017, 7, 3954. [Google Scholar] [CrossRef] [PubMed]
- Cookson, S.J.; Clemente Moreno, M.J.; Hevin, C.; Nyamba Mendome, L.Z.; Delrot, S.; Trossat-Magnin, C.; Ollat, N. Graft union formation in grapevine induces transcriptional changes related to cell wall modification, wounding, hormone signaling, and secondary metabolism. J. Exp. Bot. 2013, 64, 2997–3008. [Google Scholar] [CrossRef] [PubMed]
- Melnyk, C.W.; Meyerowitz, E.M. Plant grafting. Curr. Biol. 2015, 25, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Ikeuchi, M.; Sugimoto, K.; Iwase, A. Plant callus: Mechanisms of induction and repression. Plant Cell 2013, 25, 3159–3173. [Google Scholar] [CrossRef]
- Tanaka, W.; Hirano, H.Y. Antagonistic action of TILLERS ABSENT1 and FLORAL ORGAN NUMBER2 regulates stem cell maintenance during axillary meristem development in rice. New Phytol. 2020, 2, 974–984. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiao, Y.; Jiao, H.; Zhao, H.; Zhu, Y.X. Two-Step Functional Innovation of the Stem-Cell Factors WUS/WOX5 during Plant Evolution. Mol. Biol. Evol. 2017, 34, 640–653. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, Y.; Kondo, Y.; Fukuda, H. TDIF peptide signaling regulates vascular stem cell proliferation via the WOX4 homeobox gene in Arabidopsis. Plant Cell 2010, 22, 2618–2629. [Google Scholar] [CrossRef]
- Haecker, A.; Gross-Hardt, R.; Geiges, B.; Sarkar, A.; Breuninger, H.; Herrmann, M.; Laux, T. Expression dynamics of WOX genes mark cell fate decisions during early embryonic patterning in Arabidopsis thaliana. Development 2004, 131, 657–668. [Google Scholar] [CrossRef]
- Thomas, H.; Van den Broeck, L.; Spurney, R.; Sozzani, R.; Frank, M. Gene regulatory networks for compatible versus incompatible grafts identify a role for SlWOX4 during junction formation. Plant Cell 2022, 34, 535–556. [Google Scholar] [CrossRef]
- Zhang, A.; Matsuoka, K.; Kareem, A.; Robert, M.; Roszak, P.; Blob, B.; Anchal Bisht, A.; De Veylder, L.; Voiniciuc, C.; Asahina, M.; et al. Cell-wall damage activates DOF transcription factors to promote wound healing and tissue regeneration in Arabidopsis thaliana. Curr. Biol. 2022, 32, 1883–1894. [Google Scholar] [CrossRef]
- Tan, T.T.; Endo, H.; Sano, R.; Kurata, T.; Yamaguchi, M.; Ohtani, M.; Demura, T. Transcription Factors VND1-VND3 Contribute to Cotyledon Xylem Vessel Formation. Plant Physiol. 2018, 176, 773–789. [Google Scholar] [CrossRef]
- Kubo, M.; Udagawa, M.; Nishikubo, N.; Horiguchi, G.; Yamaguchi, M.; Ito, J.; Tetsuro, M.; Hiroo, F.; Taku, D. Transcription switches for protoxylem and metaxylem vessel formation. Genes. Dev. 2005, 19, 1855–1860. [Google Scholar] [CrossRef]
- Cass, C.L.; Peraldi, A.; Dowd, P.F.; Mottiar, Y.; Santoro, N.; Karlen, S.D.; Bukhman, Y.V.; Foster, C.E.; Thrower, N.; Bruno, L.C.; et al. Effects of PHENYLALANINE AMMONIA LYASE (PAL) knockdown on cell wall composition, biomass digestibility, and biotic and abiotic stress responses in Brachypodium. J. Exp. Bot. 2015, 66, 4317–4335. [Google Scholar] [CrossRef] [PubMed]
- La Camera, S.; Gouzerh, G.; Dhondt, S.; Hoffmann, L.; Fritig, B.; Legrand, M.; Heitz, T. Metabolic reprogramming in plant innate immunity: The contributions of phenylpropanoid and oxylipin pathways. Immunol. Rev. 2004, 198, 267–284. [Google Scholar] [CrossRef]
- Li, C.; He, Q.; Zhang, F.; Yu, J.; Li, C.; Zhao, T.; Zhang, Y.; Xie, Q.; Su, B.; Mei, L.; et al. Melatonin enhances cotton immunity to Verticillium wilt via manipulating lignin and gossypol biosynthesis. Plant J. 2019, 100, 784–800. [Google Scholar] [CrossRef]
- Qu, G.F.; Wu, W.N.; Ba, L.J.; Ma, C.; Ji, N.; Cao, S. Melatonin Enhances the Postharvest Disease Resistance of Buleberries Fruit by Modulaing the Jasmonic Acid Signaling Pathway and Phenylpropanoid Metabolites. Front. Chem. 2022, 10, 957581. [Google Scholar] [CrossRef] [PubMed]
- Pina, A.; Cookson, S.J.; Calatayud, A.; Trinchera, A.; Errea, P. Physiological and molecular mechanisms underlying graft compatibility. In Vegetable Grafting Principles and Practices; Colla, G., Perez Alfocea, F., Schwarz, D., Eds.; CABI: Wallingford, UK, 2017. [Google Scholar]
- Yang, Z.J.; Feng, J.L.; Chen, H. Study on the Anatomical Structures in Development of the Nurse Seed Grafted Union of Camellia oleifera. Plant Sci. J. 2013, 31, 313–320. [Google Scholar] [CrossRef]
- Liu, W.; Jiang, Y.; Jin, Y.Z.; Wang, C.H.; Yang, J.; Qi, H.Y. Drought-induced ABA, H2O2 and JA positively regulate CmCAD genes and lignin synthesis in melon stems. BMC Plant Biol. 2021, 21, 83. [Google Scholar] [CrossRef] [PubMed]
- Bishopp, A.; Help, H.; El-Showk, S.; Weijers, D.; Scheres, B.; Friml, J.; Benková, E.; Mähönen, A.P.; Helariuttam, Y. A mutually inhibitory interaction between auxin and cytokinin specifies vascular pattern in roots. Curr. Biol. 2011, 21, 917–926. [Google Scholar] [CrossRef]
- Mauriat, M.; Moritz, T. Analyses of GA20ox- and GID1-over-expressing aspen suggest that gibberellins play two distinct roles in wood formation. Plant J. 2009, 58, 989–1003. [Google Scholar] [CrossRef] [PubMed]
- Nieminen, K.; Immanen, J.; Laxell, M.; Kauppinen, L.; Tarkowski, P.; Dolezal, K.; Tähtiharju, S.; Elo, A.; Decourteix, M.; Ljung, K. Cytokinin signaling regulates cambial development in poplar. Proc. Natl. Acad. Sci. USA 2008, 105, 20032–20037. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.Q.; Wu, F.; Guo, J.Y.; Hou, S.A.; Wu, X.F.; Xin, Y. Transcriptomic analysis and physiological characteristics of exogenous naphthylacetic acid application to regulate the healing process of oriental melon grafted onto squash. Peer J. 2022, 10, e13980. [Google Scholar] [CrossRef] [PubMed]
- Iwase, A.; Mitsuda, N.; Koyama, T.; Hiratsu, K.; Kojima, M.; Arai, T.; Inoue, Y.; Seki, M.; Sakakibara, H.; Sugimoto, K.; et al. The AP2/ERF transcription factor WIND1 controls cell dedifferentiation in Arabidopsis. Curr. Biol. 2011, 21, 508–514. [Google Scholar] [CrossRef]
- Zhang, J.; Eswaran, G.; Alonso-Serra, J.; Kucukoglu, M.; Xiang, J.L.; Yang, W.B.; Elo, A.; Nieminen, K.; Damén, T.; Joung, J.G.; et al. Transcriptional regulatory framework for vascular cambium development in Arabidopsis roots. Nat. Plants 2019, 5, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Van Leene, J.; Hollunder, J.; Eeckhout, D.; Persiau, G.; Van De Slijke, E.; Stals, H.; Van Isterdale, G.; Verkest, A.; Neirynck, S.; Buffel, Y.; et al. Targeted interactomics reveals a complex core cell cycle machinery in Arabidopsis thaliana. Mol. Syst. Biol. 2010, 6, 397. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.R.; Perkins-Veazie, P.; Sakata, Y.; Lopez-Galarza, S.; Maroto, J.V.; Lee, S.G.; Huh, Y.C.; Sun, Z.Y.; Migule, A.; King, S.R.; et al. Cucurbit grafting. Crit. Rev. Plant Sci. 2008, 27, 50–74. [Google Scholar] [CrossRef]
- Zhang, L.B.; Wang, G.; Chang, J.M.; Liu, J.S.; Cai, J.H.; Rao, X.W.; Zhang, L.J.; Zhong, J.J.; Xie, J.H.; Zhu, S.J. Effects of 1-MCP and ethylene on expression of three CAD genes and lignification in stems of harvested Tsai Tai (Brassica chinensis). Food Chem. 2010, 123, 32–40. [Google Scholar] [CrossRef]
- Takshak, S.; Agrawal, S.B. Secondary metabolites and phenylpropanoid pathway enzymes as influenced under supplemental ultraviolet-B radiation in Withania somnifera Dunal, an indigenous medicinal plant. J. Photochem. Photobiol. B Biol. 2014, 140, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Tu, K.; Cheng, L.; Tu, S.C.; Wang, M.; Xu, H.R.; Zhan, G. Wound-induced H2O2 and resistance to Botrytis cinerea decline with the ripening of apple fruit. Postharvest Biol. Technol. 2011, 62, 64–70. [Google Scholar] [CrossRef]
- Ribeiro, L.M.; Nery, L.A.; Vieira, L.M.; Mercadante-Simões, M.O. Histological study of micrografting in passionfruit. Plant Cell Tissue Organ Cult. (PCTOC) 2015, 123, 173–181. [Google Scholar] [CrossRef]
- O’Brien, T.P.; Feder, N.; McCully, M.E. Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 1964, 59, 368–373. [Google Scholar] [CrossRef]
- Hou, S.A.; Zhu, Y.L.; Wu, X.F.; Xin, Y.; Guo, J.Y.; Wu, F.; Yu, H.Q.; Sun, Z.Q.; Xu, C.Q. Scion-to-Rootstock Mobile Transcription Factor CmHY5 Positively Modulates the Nitrate Uptake Capacity of Melon Scion Grafted on Squash Rootstock. Int. J. Mol. Sci. 2023, 24, 162. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Wu, Y.; Zhang, C.; Fu, Y.; Liu, Z.; Zhang, X. Simultaneous silencing of two different Arabidopsis genes with a novel virus-induced gene silencing vector. Plant Methods 2021, 17, 6. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Guo, J.; Wu, F.; Yu, H.; Min, J.; Zhao, Y.; Tan, C.; Liu, Y.; Xu, C. Exogenous Melatonin Application Accelerated the Healing Process of Oriental Melon Grafted onto Squash by Promoting Lignin Accumulation. Int. J. Mol. Sci. 2024, 25, 3690. https://doi.org/10.3390/ijms25073690
Zhu Y, Guo J, Wu F, Yu H, Min J, Zhao Y, Tan C, Liu Y, Xu C. Exogenous Melatonin Application Accelerated the Healing Process of Oriental Melon Grafted onto Squash by Promoting Lignin Accumulation. International Journal of Molecular Sciences. 2024; 25(7):3690. https://doi.org/10.3390/ijms25073690
Chicago/Turabian StyleZhu, Yulei, Jieying Guo, Fang Wu, Hanqi Yu, Jiahuan Min, Yingtong Zhao, Changhua Tan, Yuanwei Liu, and Chuanqiang Xu. 2024. "Exogenous Melatonin Application Accelerated the Healing Process of Oriental Melon Grafted onto Squash by Promoting Lignin Accumulation" International Journal of Molecular Sciences 25, no. 7: 3690. https://doi.org/10.3390/ijms25073690
APA StyleZhu, Y., Guo, J., Wu, F., Yu, H., Min, J., Zhao, Y., Tan, C., Liu, Y., & Xu, C. (2024). Exogenous Melatonin Application Accelerated the Healing Process of Oriental Melon Grafted onto Squash by Promoting Lignin Accumulation. International Journal of Molecular Sciences, 25(7), 3690. https://doi.org/10.3390/ijms25073690





