Retinal Neurodegeneration in an Intraocular Pressure Fluctuation Rat Model
Abstract
1. Introduction
2. Results
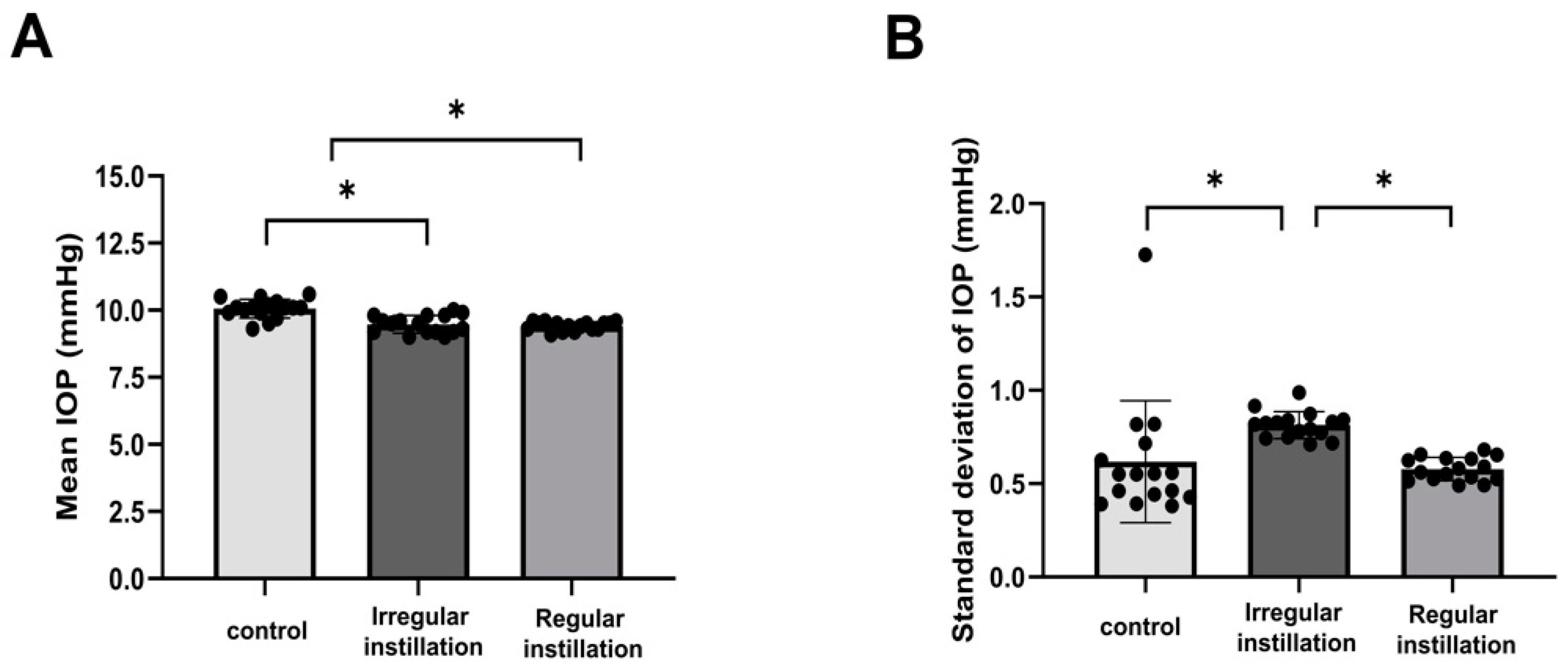
2.1. IOP Fluctuation Induced by Irregular Instillation of IOP-Lowering Eyedrops
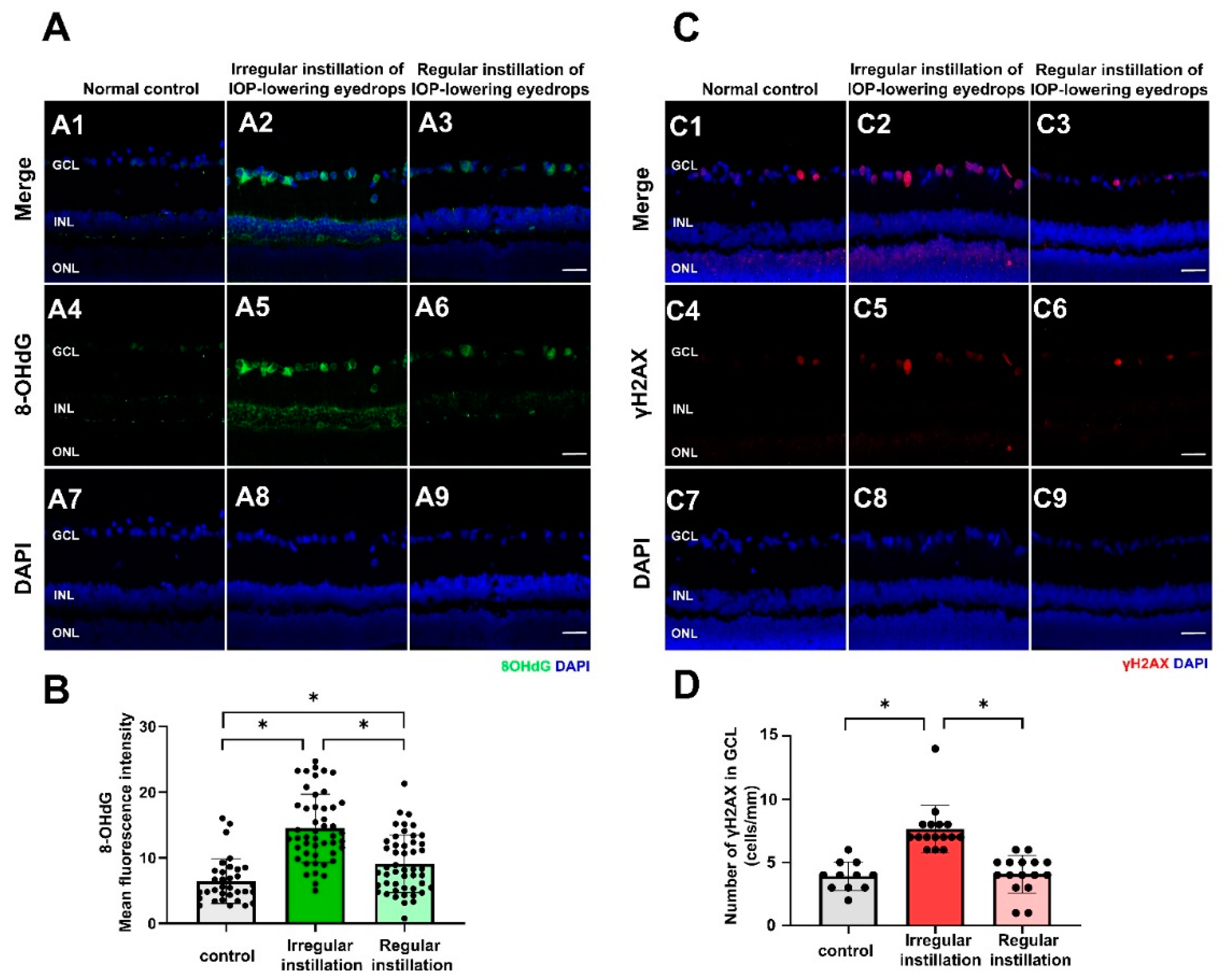
2.2. Oxidative Stress and DNA Damage
2.3. Mitochondrial Protein for Oxidative Phosphorylation
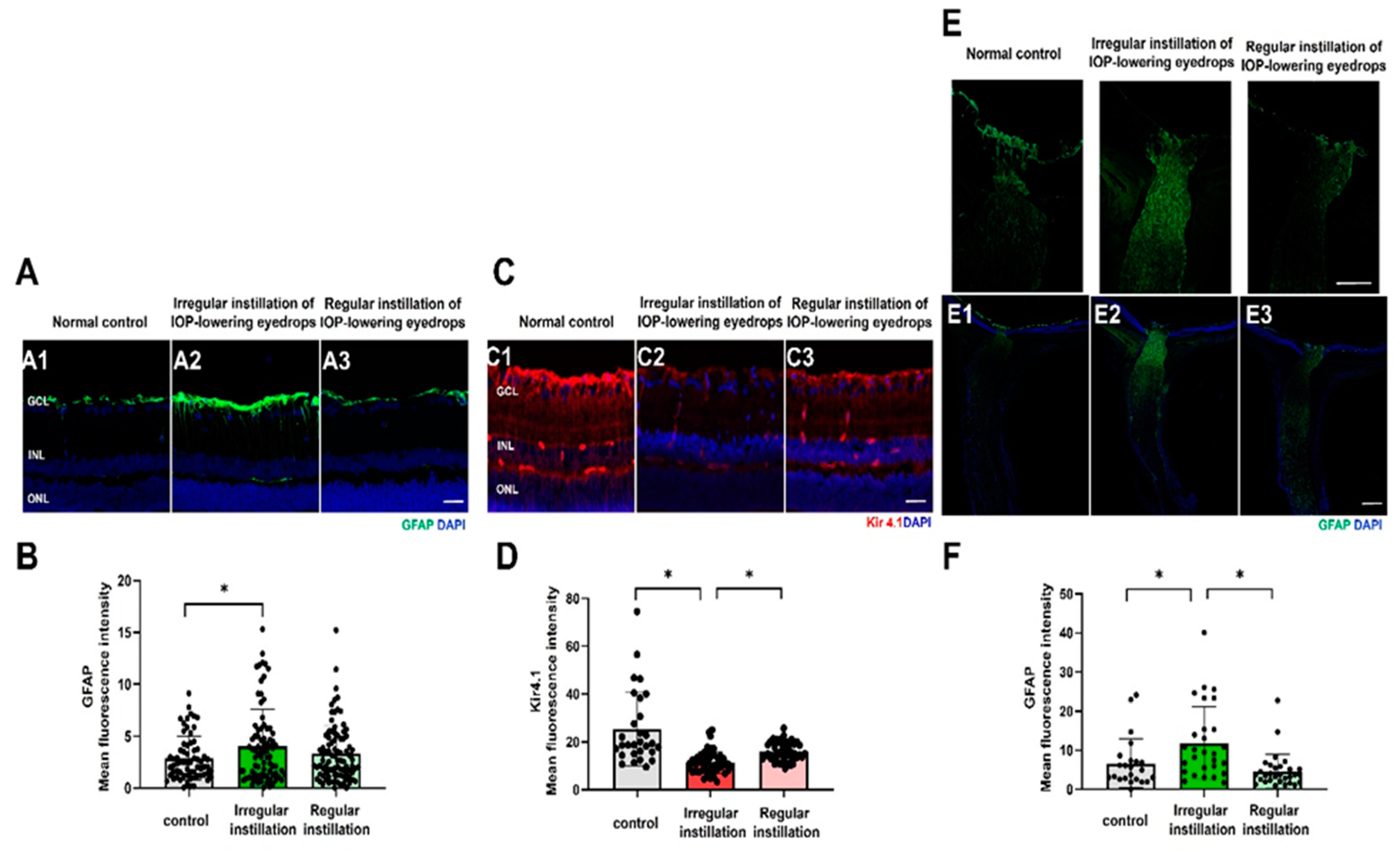
2.4. Macrogliosis
2.5. Microgliosis
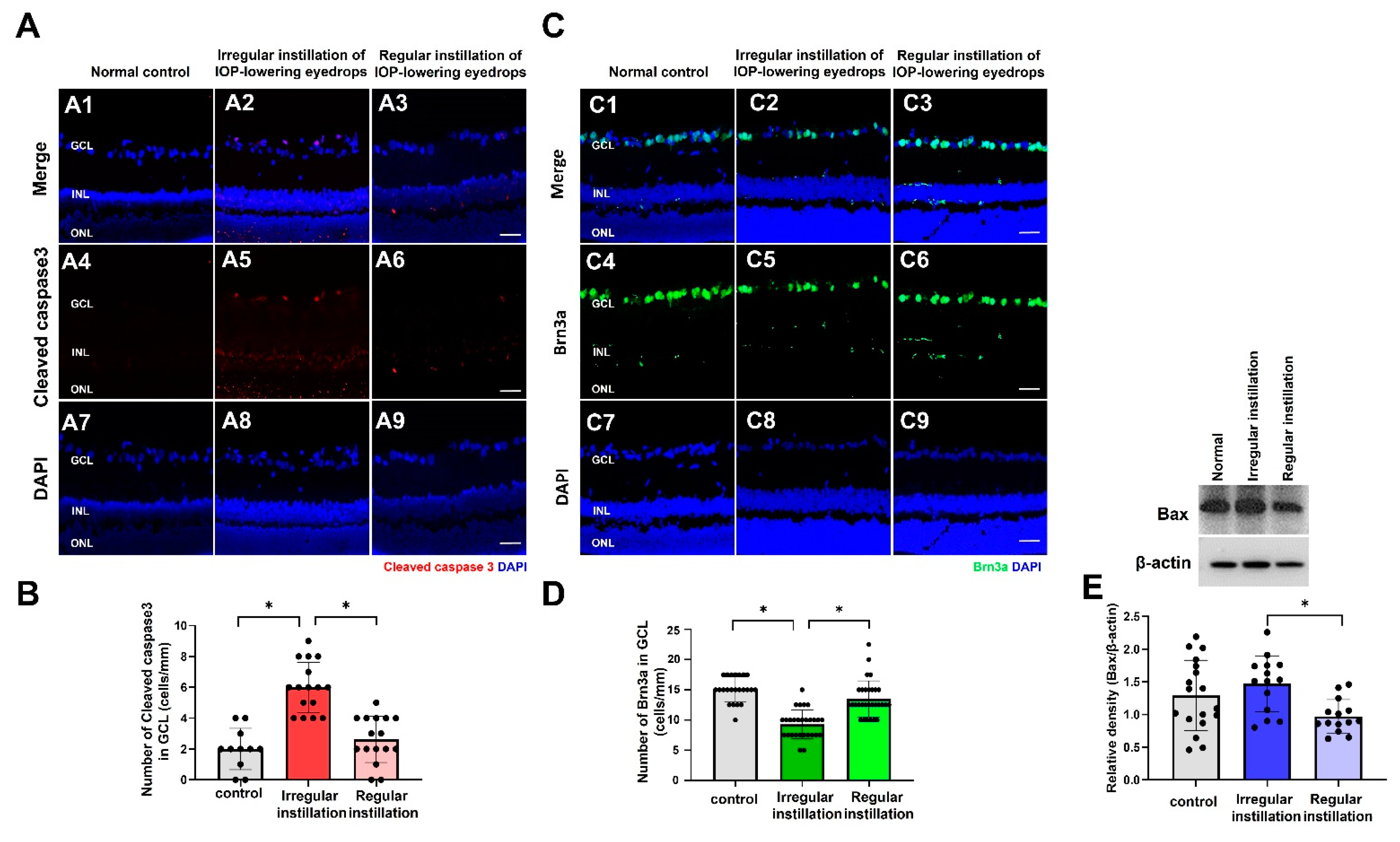
2.6. Degeneration of RGCs
2.7. Ultrastructural Findings of the Optic Nerve
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Allocation of Groups and Drug Treatments
4.3. Measurement of IOP
4.4. Immunofluorescence Staining
4.5. Measurement of Reactive Oxygen Species
4.6. Mitochondrial Isolation
4.7. Western Blot Analysis
4.8. Ultrastructural Examination
4.9. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Resnikoff, S.; Pascolini, D.; Etya’ale, D.; Kocur, I.; Pararajasegaram, R.; Pokharel, G.P.; Mariotti, S.P. Global data on visual impairment in the year 2002. Bull. World Health Organ. 2004, 82, 844–851. [Google Scholar] [PubMed]
- Ernest, P.J.; Schouten, J.S.; Beckers, H.J.; Hendrikse, F.; Prins, M.H.; Webers, C.A. An evidence-based review of prognostic factors for glaucomatous visual field progression. Ophthalmology 2013, 120, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Caprioli, J. Intraocular Pressure Fluctuation: Is It Important? J. Ophthalmic. Vis. Res. 2018, 13, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Sacca, S.C.; Rolando, M.; Marletta, A.; Macri, A.; Cerqueti, P.; Ciurlo, G. Fluctuations of intraocular pressure during the day in open-angle glaucoma, normal-tension glaucoma and normal subjects. Ophthalmologica 1998, 212, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Agnifili, L.; Mastropasqua, R.; Frezzotti, P.; Fasanella, V.; Motolese, I.; Pedrotti, E.; Di Iorio, A.; Mattei, P.A.; Motolese, E.; Mastropasqua, L. Circadian intraocular pressure patterns in healthy subjects, primary open angle and normal tension glaucoma patients with a contact lens sensor. Acta Ophthalmol. 2015, 93, e14–e21. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.; Lee, J.Y.; Jeong, D.W.; Kim, S.; Han, S.; Kook, M.S. Relationship between Nocturnal Intraocular Pressure Elevation and Diurnal Intraocular Pressure Level in Normal-Tension Glaucoma Patients. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5271–5279. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tojo, N.; Abe, S.; Ishida, M.; Yagou, T.; Hayashi, A. The Fluctuation of Intraocular Pressure Measured by a Contact Lens Sensor in Normal-Tension Glaucoma Patients and Nonglaucoma Subjects. J. Glaucoma 2017, 26, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.W.; Kim, J.S.; Lee, S.Y.; Ha, A.; Lee, J.; Park, Y.J.; Kim, Y.K.; Jeoung, J.W.; Park, K.H. Twenty-four-Hour Intraocular Pressure-Related Patterns from Contact Lens Sensors in Normal-Tension Glaucoma and Healthy Eyes: The Exploring Nyctohemeral Intraocular pressure related pattern for Glaucoma Management (ENIGMA) Study. Ophthalmology 2020, 127, 1487–1497. [Google Scholar] [CrossRef]
- Nouri-Mahdavi, K.; Hoffman, D.; Coleman, A.L.; Liu, G.; Li, G.; Gaasterland, D.; Caprioli, J.; Advanced Glaucoma Intervention Study. Predictive factors for glaucomatous visual field progression in the Advanced Glaucoma Intervention Study. Ophthalmology 2004, 111, 1627–1635. [Google Scholar] [CrossRef]
- Bengtsson, B.; Leske, M.C.; Hyman, L.; Heijl, A.; Early Manifest Glaucoma Trial Group. Fluctuation of intraocular pressure and glaucoma progression in the early manifest glaucoma trial. Ophthalmology 2007, 114, 205–209. [Google Scholar] [CrossRef]
- Medeiros, F.A.; Weinreb, R.N.; Zangwill, L.M.; Alencar, L.M.; Sample, P.A.; Vasile, C.; Bowd, C. Long-term intraocular pressure fluctuations and risk of conversion from ocular hypertension to glaucoma. Ophthalmology 2008, 115, 934–940. [Google Scholar] [CrossRef] [PubMed]
- De Moraes, C.G.; Jasien, J.V.; Simon-Zoula, S.; Liebmann, J.M.; Ritch, R. Visual Field Change and 24-Hour IOP-Related Profile with a Contact Lens Sensor in Treated Glaucoma Patients. Ophthalmology 2016, 123, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Caprioli, J.; Coleman, A.L. Intraocular pressure fluctuation a risk factor for visual field progression at low intraocular pressures in the advanced glaucoma intervention study. Ophthalmology 2008, 115, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Lee, J.R.; Lee, Y.; Lee, K.S.; Na, J.H.; Han, S.; Kook, M.S. Relationship between 24-hour mean ocular perfusion pressure fluctuation and rate of paracentral visual field progression in normal-tension glaucoma. Investig. Ophthalmol Vis. Sci. 2013, 54, 6150–6157. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sakata, R.; Yoshitomi, T.; Iwase, A.; Matsumoto, C.; Higashide, T.; Shirakashi, M.; Aihara, M.; Sugiyama, K.; Araie, M.; Lower Normal Pressure Glaucoma Study Members in Japan Glaucoma Society. Factors Associated with Progression of Japanese Open-Angle Glaucoma with Lower Normal Intraocular Pressure. Ophthalmology 2019, 126, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.U.; Ha, A.; Kim, D.W.; Jeoung, J.W.; Park, K.H.; Kim, Y.K. Risk factors for disease progression in low-teens normal-tension glaucoma. Br. J. Ophthalmol. 2020, 104, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Na, K.S.; Lee, N.Y.; Park, S.H.; Park, C.K. Autonomic dysfunction in normal tension glaucoma: The short-term heart rate variability analysis. J. Glaucoma 2010, 19, 377–381. [Google Scholar] [CrossRef]
- Kurysheva, N.I.; Ryabova, T.Y.; Shlapak, V.N. Heart rate variability: The comparison between high tension and normal tension glaucoma. EPMA J. 2018, 9, 35–45. [Google Scholar] [CrossRef]
- Wolff, H.A.; Rodel, R.M.; Gunawan, B.; Overbeck, T.; Herrmann, M.K.; Hennies, S.; Hille, A.; Vorwerk, H.; Matthias, C.; Hess, C.F.; et al. Nasopharyngeal carcinoma in adults: Treatment results after long-term follow-up with special reference to adjuvant interferon-beta in undifferentiated carcinomas. J. Cancer Res. Clin. Oncol. 2010, 136, 89–97. [Google Scholar] [CrossRef][Green Version]
- Mudumbai, R.C. Clinical update on normal tension glaucoma. Semin. Ophthalmol. 2013, 28, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarieh, M.; Flammer, J. New insights in the pathogenesis and treatment of normal tension glaucoma. Curr. Opin. Pharmacol. 2013, 13, 43–49. [Google Scholar] [CrossRef]
- Joos, K.M.; Li, C.; Sappington, R.M. Morphometric changes in the rat optic nerve following short-term intermittent elevations in intraocular pressure. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6431–6440. [Google Scholar] [CrossRef] [PubMed]
- Kuo, L.J.; Yang, L.X. Gamma-H2AX—A novel biomarker for DNA double-strand breaks. In Vivo 2008, 22, 305–309. [Google Scholar]
- Liu, Q.; Ju, W.K.; Crowston, J.G.; Xie, F.; Perry, G.; Smith, M.A.; Lindsey, J.D.; Weinreb, R.N. Oxidative stress is an early event in hydrostatic pressure induced retinal ganglion cell damage. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4580–4589. [Google Scholar] [CrossRef]
- Moreno, M.C.; Campanelli, J.; Sande, P.; Sanez, D.A.; Keller Sarmiento, M.I.; Rosenstein, R.E. Retinal oxidative stress induced by high intraocular pressure. Free Radic. Biol. Med. 2004, 37, 803–812. [Google Scholar] [CrossRef]
- Guo, Z.Z.; Chang, K.; Wei, X. Intraocular pressure fluctuation and the risk of glaucomatous damage deterioration: A Meta-analysis. Int. J. Ophthalmol. 2019, 12, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Chrysostomou, V.; Rezania, F.; Trounce, I.A.; Crowston, J.G. Oxidative stress and mitochondrial dysfunction in glaucoma. Curr. Opin. Pharmacol. 2013, 13, 12–15. [Google Scholar] [CrossRef]
- Tezel, G. Oxidative stress in glaucomatous neurodegeneration: Mechanisms and consequences. Prog. Retin. Eye Res. 2006, 25, 490–513. [Google Scholar] [CrossRef]
- Bringmann, A.; Wiedemann, P. Muller glial cells in retinal disease. Ophthalmologica 2012, 227, 1–19. [Google Scholar] [CrossRef]
- Xue, L.P.; Lu, J.; Cao, Q.; Hu, S.; Ding, P.; Ling, E.A. Muller glial cells express nestin coupled with glial fibrillary acidic protein in experimentally induced glaucoma in the rat retina. Neuroscience 2006, 139, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Bringmann, A.; Iandiev, I.; Pannicke, T.; Wurm, A.; Hollborn, M.; Wiedemann, P.; Osborne, N.N.; Reichenbach, A. Cellular signaling and factors involved in Muller cell gliosis: Neuroprotective and detrimental effects. Prog. Retin. Eye Res. 2009, 28, 423–451. [Google Scholar] [CrossRef] [PubMed]
- Beverley, K.M.; Pattnaik, B.R. Inward rectifier potassium (Kir) channels in the retina: Living our vision. Am. J. Physiol. Cell Physiol. 2022, 323, C772–C782. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Miao, Y.; Dong, L.D.; Chen, J.; Mo, X.F.; Jiang, S.X.; Sun, X.H.; Yang, X.L.; Wang, Z. Group I mGluR-mediated inhibition of Kir channels contributes to retinal Müller cell gliosis in a rat chronic ocular hypertension model. J. Neurosci. 2012, 32, 12744–12755. [Google Scholar] [CrossRef] [PubMed]
- Bolz, S.; Schuettauf, F.; Fries, J.E.; Thaler, S.; Reichenbach, A.; Pannicke, T. K+ currents fail to change in reactive retinal glial cells in a mouse model of glaucoma. Graefes Arch. Clin. Exp. Ophthalmol. 2008, 246, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.A.; Roux, A.L.; Wareham, L.K.; Sappington, R.M. Pressure-dependent modulation of inward-rectifying K+ channels: Implications for cation homeostasis and K+ dynamics in glaucoma. Am. J. Physiol. Cell Physiol. 2019, 317, C375–c389. [Google Scholar] [CrossRef]
- Miao, Y.; Zhao, G.L.; Cheng, S.; Wang, Z.; Yang, X.L. Activation of retinal glial cells contributes to the degeneration of ganglion cells in experimental glaucoma. Prog. Retin. Eye Res. 2023, 93, 101169. [Google Scholar] [CrossRef]
- Tehrani, S.; Johnson, E.C.; Cepurna, W.O.; Morrison, J.C. Astrocyte processes label for filamentous actin and reorient early within the optic nerve head in a rat glaucoma model. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6945–6952. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, S.; Davis, L.; Cepurna, W.O.; Choe, T.E.; Lozano, D.C.; Monfared, A.; Cooper, L.; Cheng, J.; Johnson, E.C.; Morrison, J.C. Astrocyte Structural and Molecular Response to Elevated Intraocular Pressure Occurs Rapidly and Precedes Axonal Tubulin Rearrangement within the Optic Nerve Head in a Rat Model. PLoS ONE 2016, 11, e0167364. [Google Scholar] [CrossRef]
- Maddineni, P.; Kasetti, R.B.; Patel, P.D.; Millar, J.C.; Kiehlbauch, C.; Clark, A.F.; Zode, G.S. CNS axonal degeneration and transport deficits at the optic nerve head precede structural and functional loss of retinal ganglion cells in a mouse model of glaucoma. Mol. Neurodegene. 2020, 15, 48. [Google Scholar] [CrossRef]
- Lam, T.T.; Kwong, J.M.; Tso, M.O. Early glial responses after acute elevated intraocular pressure in rats. Investig. Ophthalmol. Vis. Sci. 2003, 44, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, M.R. The optic nerve head in glaucoma: Role of astrocytes in tissue remodeling. Prog. Retin. Eye Res. 2000, 19, 297–321. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, A.I.; de Hoz, R.; Fernandez-Albarral, J.A.; Salobrar-Garcia, E.; Rojas, B.; Valiente-Soriano, F.J.; Aviles-Trigueros, M.; Villegas-Perez, M.P.; Vidal-Sanz, M.; Trivino, A.; et al. Time course of bilateral microglial activation in a mouse model of laser-induced glaucoma. Sci. Rep. 2020, 10, 4890. [Google Scholar] [CrossRef] [PubMed]
- Gallego, B.I.; Salazar, J.J.; de Hoz, R.; Rojas, B.; Ramirez, A.I.; Salinas-Navarro, M.; Ortin-Martinez, A.; Valiente-Soriano, F.J.; Aviles-Trigueros, M.; Villegas-Perez, M.P.; et al. IOP induces upregulation of GFAP and MHC-II and microglia reactivity in mice retina contralateral to experimental glaucoma. J. Neuroinflamm. 2012, 9, 92. [Google Scholar] [CrossRef] [PubMed]
- Guo, R. Minocycline Protects against the Degeneration of Retinal Neurons in Mice. J. Explor. Res. Pharmacol. 2020, 5, 61–72. [Google Scholar] [CrossRef]
- Gómez Morillas, A.; Besson, V.C.; Lerouet, D. Microglia and Neuroinflammation: What Place for P2RY12? Int. J. Mol. Sci. 2021, 22, 1636. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhao, G.L.; Xu, M.X.; Zhou, H.; Li, F.; Miao, Y.; Lei, B.; Yang, X.L.; Wang, Z. Interplay between Müller cells and microglia aggravates retinal inflammatory response in experimental glaucoma. J. Neuroinflamm. 2021, 18, 303. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, N.; Liu, Q.; Zajadacz, J.; Franze, K.; Reichenbach, A. Retinal glial (Muller) cells: Sensing and responding to tissue stretch. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1683–1690. [Google Scholar] [CrossRef]
- Choi, H.J.; Sun, D.; Jakobs, T.C. Astrocytes in the optic nerve head express putative mechanosensitive channels. Mol. Vis. 2015, 21, 749–766. [Google Scholar]
- Bringmann, A.; Grosche, A.; Pannicke, T.; Reichenbach, A. GABA and Glutamate Uptake and Metabolism in Retinal Glial (Muller) Cells. Front. Endocrinol. 2013, 4, 48. [Google Scholar] [CrossRef]
- Sedlak, L.; Zych, M.; Wojnar, W.; Wygledowska-Promienska, D. Effect of Topical Prostaglandin F2alpha Analogs on Selected Oxidative Stress Parameters in the Tear Film. Medicina 2019, 55, 366. [Google Scholar] [CrossRef] [PubMed]
- Basu, S. Bioactive eicosanoids: Role of prostaglandin F2α and F2-isoprostanes in inflammation and oxidative stress related pathology. Mol. Cells 2010, 30, 383–391. [Google Scholar] [CrossRef]
- Cheng, J.W.; Cai, J.P.; Wei, R.L. Meta-analysis of medical intervention for normal tension glaucoma. Ophthalmology 2009, 116, 1243–1249. [Google Scholar] [CrossRef]
- Symes, R.J.; Mikelberg, F.S. Normal tension glaucoma management: A survey of contemporary practice. Can. J. Ophthalmol. 2017, 52, 361–365. [Google Scholar] [CrossRef]
- Jung, K.I.; Woo, J.E.; Park, C.K. Intraocular pressure fluctuation and neurodegeneration in the diabetic rat retina. Br. J. Pharmacol. 2020, 177, 3046–3059. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.H.; Bui, B.V.; Vingrys, A.J. Clinical and experimental links between diabetes and glaucoma. Clin. Exp. Optom. 2011, 94, 4–23. [Google Scholar] [CrossRef]
- Quaranta, L.; Gandolfo, F.; Turano, R.; Rovida, F.; Pizzolante, T.; Musig, A.; Gandolfo, E. Effects of topical hypotensive drugs on circadian IOP, blood pressure, and calculated diastolic ocular perfusion pressure in patients with glaucoma. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2917–2923. [Google Scholar] [CrossRef] [PubMed]
- Pang, I.H.; Clark, A.F. Inducible rodent models of glaucoma. Prog. Retin. Eye Res. 2020, 75, 100799. [Google Scholar] [CrossRef]
- Newman-Casey, P.A.; Niziol, L.M.; Gillespie, B.W.; Janz, N.K.; Lichter, P.R.; Musch, D.C. The Association between Medication Adherence and Visual Field Progression in the Collaborative Initial Glaucoma Treatment Study. Ophthalmology 2020, 127, 477–483. [Google Scholar] [CrossRef]
- Rossi, G.C.; Pasinetti, G.M.; Scudeller, L.; Radaelli, R.; Bianchi, P.E. Do adherence rates and glaucomatous visual field progression correlate? Eur. J. Ophthalmol. 2011, 21, 410–414. [Google Scholar] [CrossRef]
- Sasaki, M.; Ozawa, Y.; Kurihara, T.; Kubota, S.; Yuki, K.; Noda, K.; Kobayashi, S.; Ishida, S.; Tsubota, K. Neurodegenerative influence of oxidative stress in the retina of a murine model of diabetes. Diabetologia 2010, 53, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Laspas, P.; Zhutdieva, M.B.; Brochhausen, C.; Musayeva, A.; Zadeh, J.K.; Pfeiffer, N.; Xia, N.; Li, H.; Wess, J.; Gericke, A. The M(1) muscarinic acetylcholine receptor subtype is important for retinal neuron survival in aging mice. Sci. Rep. 2019, 9, 5222. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.-S.; Park, C.K.; Jung, K.I. Retinal Neurodegeneration in an Intraocular Pressure Fluctuation Rat Model. Int. J. Mol. Sci. 2024, 25, 3689. https://doi.org/10.3390/ijms25073689
Han J-S, Park CK, Jung KI. Retinal Neurodegeneration in an Intraocular Pressure Fluctuation Rat Model. International Journal of Molecular Sciences. 2024; 25(7):3689. https://doi.org/10.3390/ijms25073689
Chicago/Turabian StyleHan, Jeong-Sun, Chan Kee Park, and Kyoung In Jung. 2024. "Retinal Neurodegeneration in an Intraocular Pressure Fluctuation Rat Model" International Journal of Molecular Sciences 25, no. 7: 3689. https://doi.org/10.3390/ijms25073689
APA StyleHan, J.-S., Park, C. K., & Jung, K. I. (2024). Retinal Neurodegeneration in an Intraocular Pressure Fluctuation Rat Model. International Journal of Molecular Sciences, 25(7), 3689. https://doi.org/10.3390/ijms25073689





