1. Introduction
When animals undergo temperatures that exceed their physiological tolerance and compensatory capacity, heat stress (HS) occurs [
1]. The testis is a temperature-sensitive organ that needs to be maintained 2–7 °C below core body temperature to ensure normal spermatogenesis [
2]. Therefore, the testis is more susceptible to HS than other tissues. There is plenty of evidence that HS results in defects in testicular development, the arrest of spermatogenesis, worsened semen quality, and infertility in males [
3,
4,
5]. Sertoli cells serve as primary supportive cells within the seminiferous epithelium, offering vital nutrients and assistance to spermatogenic cells. The adjacent Sertoli cells near the basement membrane form a special cellular junction, the blood–testis barrier (BTB). The BTB is constituted by tight junctions, adherens junctions (also known as basal ectoplasmic specialization), gap junctions and desmosomes [
6,
7,
8,
9,
10]. The function of BTB is to restrict substances, such as nutrients, vital molecules and harmful toxicants, into the apical compartment, providing a unique microenvironment for post-meiotic spermatid development. An increase in testicular temperature triggers ultrastructural damage to the BTB and the reversible disruption of the function of Sertoli cells. For example, after the heat treatment of Sertoli cells, a notable decrease occurs in both mRNA and protein levels of the tight junction-related proteins Occludin and ZO-1, leading to the disruption of the BTB structure and an increase in BTB permeability [
11]. Heat-induced cell junction disruption can be rescued by androgen receptor overexpression, which acts upstream to regulate the reversible change in the BTB by rescuing the expression of ZO-1, N-Cadherin and E-Cadherin [
12].
Stress granules (SGs), ribonucleoprotein (RNP) granules, manifest in cells as a response to stressors like chemical injury, oxidative stress and viral incursion [
13]. SGs form from mRNAs stalled in translation initiation and are composed of multiple constituents, including translationally stalled mRNAs, translation initiation factors, RNA-binding proteins and non-RNA-binding proteins [
14]. However, the molecular composition and regulatory dynamics of SGs vary with cellular context or stressor type [
15]. For example, G3BP1 and G3BP2 serve core functions in facilitating SG assembly within mammalian cells when subjected to oxidative stress, while they are not required during osmotic stress [
16,
17]. Moreover, Gtr1, Rps1b, and Hgh1 promote SG formation during glucose starvation while repressing SG formation during heat shock in yeast [
18]. And it should be noted that the way in which SG assembly affects gene expression and participates in cellular physiology remains to be determined.
A growing body of studies has revealed that persistent aberrant SG formation or mutations in nucleators contribute to neurodegenerative diseases, including amyotrophic lateral sclerosis and Alzheimer’s disease [
19,
20,
21], while the implication and function of SGs in spermatogenesis have been seldom elucidated. Kim et al. report that DAZL acts as a specific translational regulator and localizes to SGs during HS, curbing germ cell apoptosis by sequestering signaling molecules such as RACK1 [
22]. Another testis-specific protein, MAGE-B2, has been proven to enhance the cellular stress threshold and protect this highly thermosensitive germline from heat stress by suppressing G3BP1 translation and SG assembly in germ cells [
23]. However, insights from these studies about SG function in the male reproductive system remain limited. Gaps remain in our understanding of SG components, regulation and function in germline cells and somatic cells (e.g., Sertoli cells) during spermatogenesis.
Thus, this study aims to investigate the implication and function of SGs in Sertoli cells by elaborating their formation, composition and effects on BTB integrity and BTB protein expression during HS. We found that inhibition of SG formation with an inhibitor or siRNAs resulted in the impairment of the BTB and a reduction in BTB-related proteins. Moreover, we found that UBAP2 promoted SG formation by maintaining the expression of the SG nucleator G3BP1 and the deficiency of UBAP2 further exacerbated the decrease in BTB-related proteins. These results firstly demonstrate the role of SGs in maintaining BTB functions and identified a new component that is essential for SG formation in Sertoli cells upon heat stress.
3. Discussion
Although many studies have identified an association between heat exposures and low fertility in males, few studies have focused on the function of SGs in spermatogenesis. It is reported that SGs can protect germ cells because the percentage of apoptotic germ cells in the testis increases significantly in the absence of DAZL and SG formation [
22]. But in another study, the testis-specific protein MAGE-B2 increases cellular stress tolerance through the suppression of SG assembly by translationally inhibiting the SG nucleator G3BP [
23]. Whether SGs play a positive or negative role in spermatogenesis remains difficult to determine. Our results have shown that the suppression of SG assembly aggravates BTB damage and decreases BTB protein expression, and have also emphasized the role of SGs in Sertoli cells during the maintenance of the functional BTB.
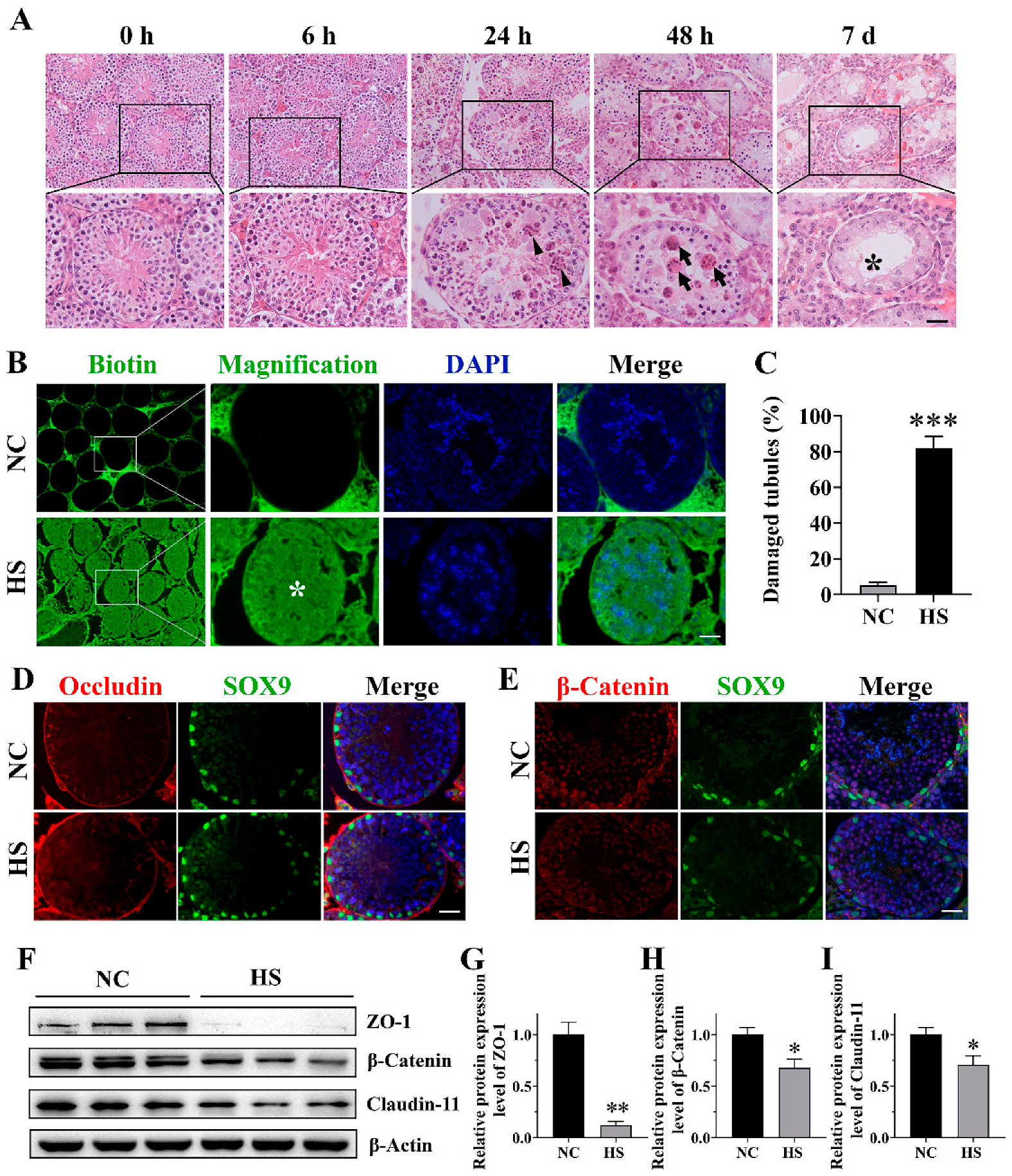
Sertoli cells, directly in contact with germ cells, provide the microenvironment and nourishment necessary for spermatogenesis. Testicular injury is mild just after experiencing acute HS, but can become more severe in the following recovery period, as shown by the appearance of multinuclear giant cells after 48 h and Sertoli-cell-only-like seminiferous tubules after 7 d in the mice in our experiments. This result agrees with a previous study suggesting that the testes experience a lasting negative effect after a single period of mild HS. The testes of mice were stressed at 42 °C for 30 min, and a histological examination revealed multinucleated giant cells, germ cell depletion and collapsed seminiferous epithelium in the testes at 24 h, 48 h and 7 d after HS, respectively. It was also suggested that TUNEL-positive cells might burst at 24 h after heat stress [
24]. However, the changes in the BTB were not examined in that study. As a supplement, our study proved that HS caused the breakdown of the BTB and disrupted the expression of the BTB-related proteins Claudin-11, ZO-1 and β-Catenin in the testis.
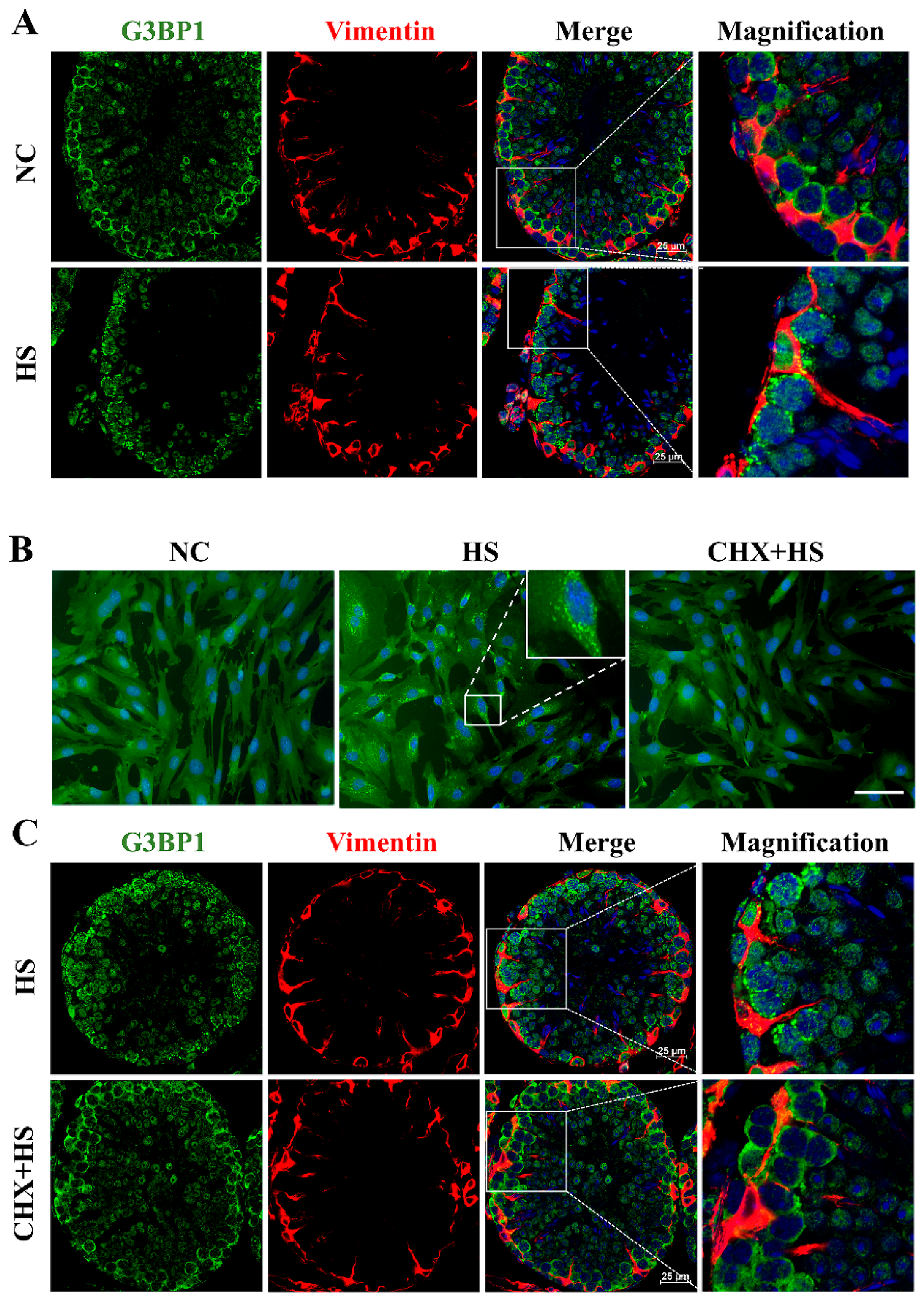
Eukaryotic cells exhibit stereotypical responses to various stressors, aiding their survival under extreme conditions and during their subsequent recovery [
25]. While SG assembly is a universal process in response to different cellular stresses [
26], previous research has demonstrated HS-induced SG assembly primarily in male germ cells and early spermatocytes indicated by the staining of TIA-1 [
22]. As an extension, our study proved that both Sertoli cells and germ cells form obvious SGs after treatment at 43 °C for 30 min. Since Sertoli cells are responsible for nourishing spermatogenic cells and have no fixed morphology, their boundaries are difficult to determine. It is difficult to detect whether Sertoli cells generate SGs in response to HS. We therefore used Vimentin, a cytosolic marker of Sertoli cells [
27], to determine Sertoli cell boundaries and confirmed SG formation during HS.
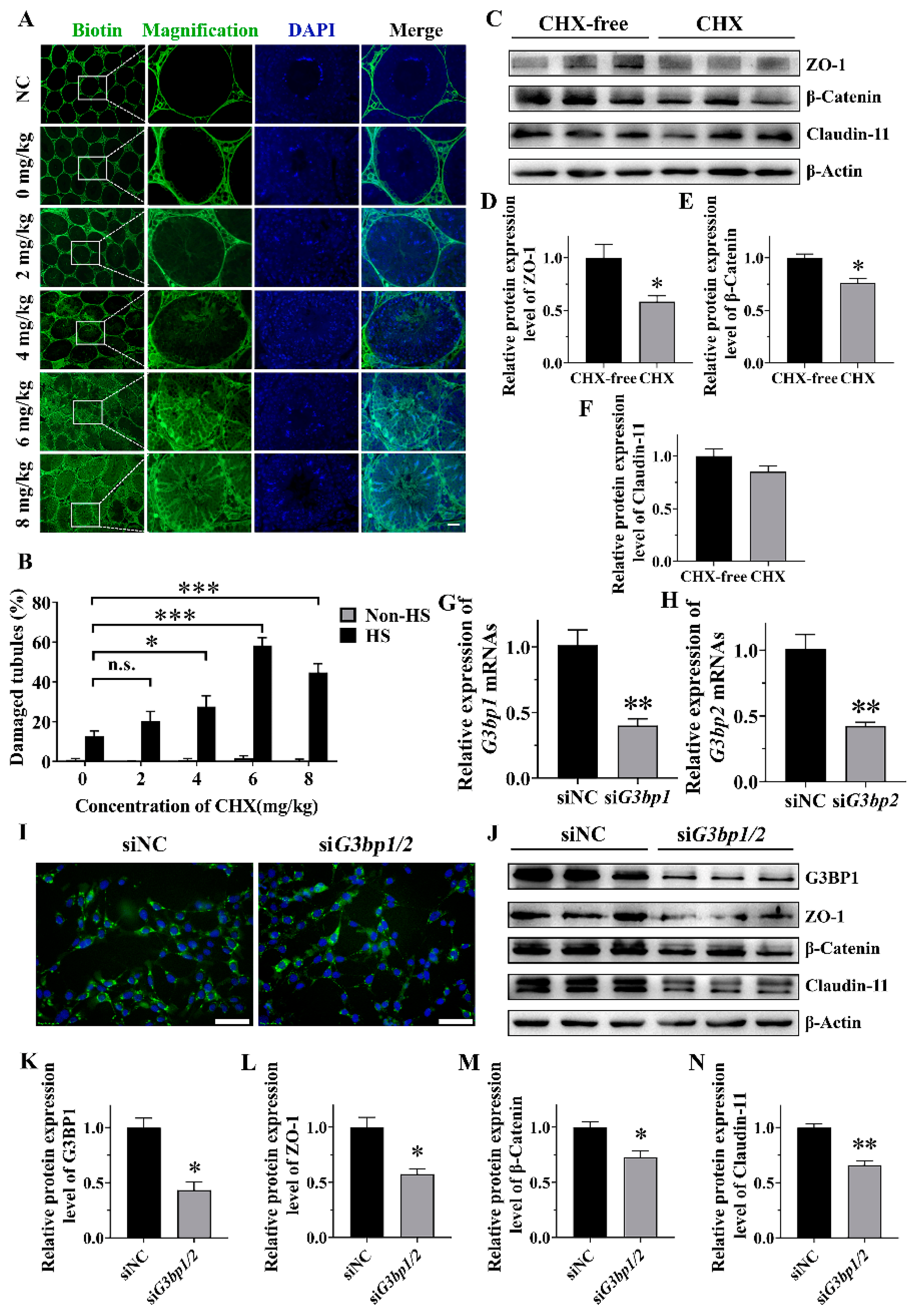
In order to investigate the effects of SGs on the BTB integrity and BTB-related protein expression under HS, CHX was used as an inhibitor to suppress SG formation in vivo. CHX can inhibit SG assembly by interrupting mRNA–polysome interaction and is frequently used as an inhibitor of SG assembly in vitro [
28,
29,
30]. The present results also suggest it can inhibit the formation of cellular SGs in vivo. To support this finding, we used another method to inhibit the formation of SGs in vitro: double interference of
G3bp1 and
G3bp2. G3BP1 and G3BP2 have been identified as playing a central role within the core SG network. A study examined the importance of 36 proteins in the core SG network, using CRISPR/Cas9 to knock out one or both isoforms of their genes in the U2OS cell line. The result suggested that the cells only with the double knockout of
G3bp1 and
G3bp2 are devoid of SGs [
31]. In our study, G3BP1/2 in TM4 cells was knocked down by siRNA and resulted in a reduction in the SG-positive cells from 82% to 65% during HS. Therefore, we suppressed SG assembly both in vivo and in vitro, and proved the protective function of SGs in Sertoli cells during the maintenance of the functional BTB.
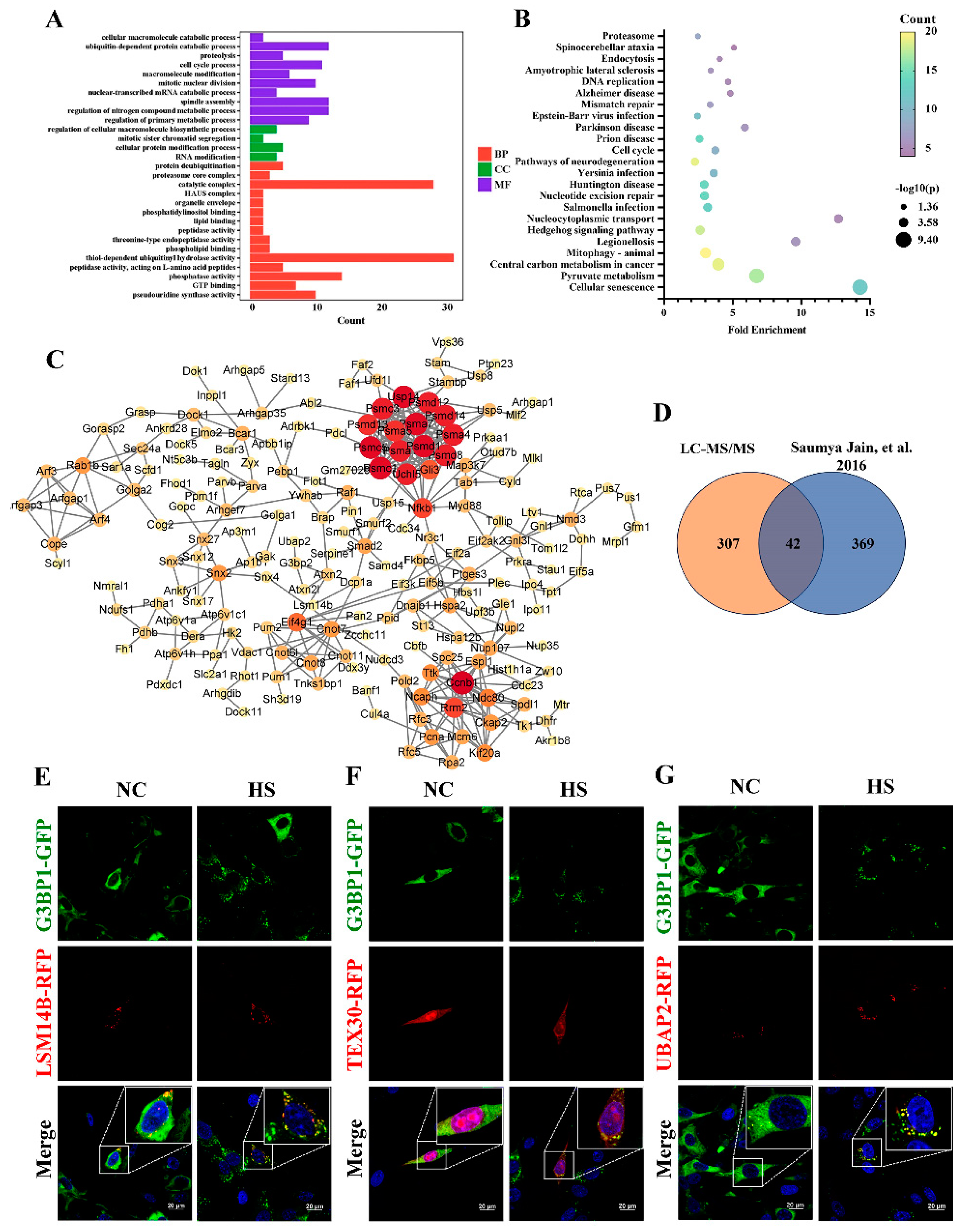
For the purification of SGs, we established a TM4 cell line stably expressing G3BP1-GFP and isolated SGs according to the method described by Wheeler et al. [
32,
33]. LC-MS/MS identified 349 possible core proteins, as assessed with a
p < 0.05 compared to the unstressed control. The statistical analysis showed that at least 42 of the identified proteins overlap with those found in a previous study, many of which have been identified as SG proteins [
14]. Based on previous studies, ubiquitination and the proteasome play critical roles in the assembly of SGs, which are pivotal for the restoration of cellular functions following HS [
34,
35]. In this study, some enriched proteins related to ubiquitination and the proteasome highly expressed specifically in the testes (as shown in a public database) were found in the SGs. Meanwhile, novel components within SGs in Sertoli cells were identified, including UBAP2, TEX30 and LSM14B, which were highly expressed in the testes and had not been reported to exist within SGs previously.
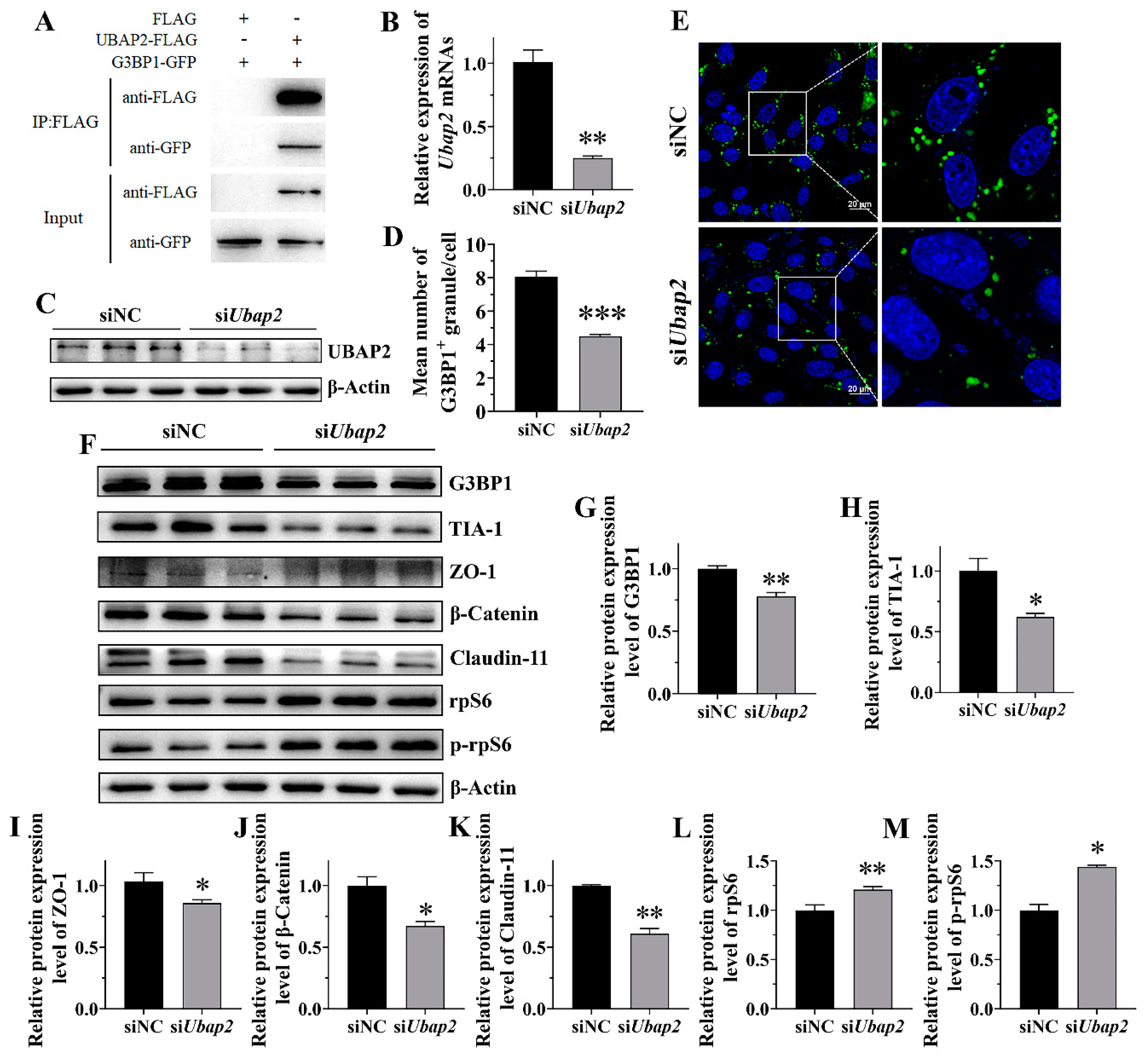
SG assembly is triggered by liquid–liquid phase separation arising from interactions distributed unevenly within the protein–RNA interaction network. The molecular switch of this network, G3BP1, triggers RNA-dependent LLPS when concentrations of intracellular free RNA rise [
31]. Other G3BP1-binding factors further regulate SG assembly by strengthening or weakening the core network of the SGs through positive or negative cooperativity. In this study, the knockdown of UBAP2 resulted in a significant reduction both in the quantity of SGs and in the expression of G3BP1 during HS, while it did not affect the expression of G3BP1 in normal conditions. Therefore, we proposed that UBAP2 probably acts upstream of G3BP1 to maintain SG assembly in Sertoli cells in response to HS.
The knockdown of UBAP2 also further suppressed the expression of Claudin-11, ZO-1 and β-Catenin during HS. This can be explained by the decreased expression of rpS6 and rpS6 phosphorylation. As a downstream member of the mTORC1 signaling cascade, the enhanced expression of rps6 would inhibit BTB-related expression in Sertoli cells and in turn impair BTB integrity [
36,
37].
The assembly and removal of SGs from the male reproductive system appears to be important for male fecundity. One possible regulator is an SG-associated factor, SERBP1, which acts as a regulator of heat-induced SG elimination and regulates 26S proteasome activity and G3BP1 ubiquitination in germ cell survival against HS [
38]. Similarly, the important role of SGs in the maintenance of the BTB and Sertoli cell function was confirmed in this study. SGs are important for Sertoli cells since they provide a homeostatic environment resistant to HS for spermatogenesis. This suggests that further exploration of SGs in the male reproductive system is warranted. In addition, because of the critical cellular functions of mRNPs and the possible links to stress-related diseases, the role of SGs in testicular inflammation and disease is also of interest. Whether some testicular diseases and long-term inflammation can induce the formation of SGs and the effect of the clearance and maintenance of these SGs on male fertility is an interesting topic and worthy of further investigation. At present, there are still many gaps and controversies in our understanding of the mechanism of the SG system in male reproduction. And a lot of work remains to be done, such as the expansion of the SG pool within the testis and the demonstration of the role of these markers more fully in vivo.
In summary, our study demonstrates the role of SGs in maintaining BTB functions during HS and identifies UBAP2 as a functional component in heat-induced SGs in Sertoli cells. Our findings not only help to expand our understanding of SGs in male reproduction and the molecular mechanism of low fertility in males in summer but also potentially provide guidance for the development of clinical drugs protecting against HS.
4. Materials and Methods
4.1. Animals
All the ICR mice aged 8 weeks were purchased from Spf (Sipeifu, Beijing, China) Biotechnology Company. Mice were maintained under standard conditions with free access to food and water in a cycle of 12 h light and 12 h dark. The entire study was reviewed and approved by the China Agricultural University Institutional Animal Care (permission No. AW52503202-1-1) and Use Committee and performed in accordance with the committee’s guidelines. All efforts were made to minimize animal suffering.
4.2. Heat Stress of Mice
Before the experiment, 8-week-old male mice were randomly divided into control group and experimental group, with 3 mice in each group. Mice were anesthetized and subjected to HS. The lower 1/3 of the bodies (hind legs, tail, and scrotum) were immersed in a 43 °C water bath for 30 min. For SG detection, testes were removed from the mice immediately and fixed in 4% paraformaldehyde. For testicular seminiferous tubules morphology and BTB integrity, mice were maintained under standard conditions for recovery with different times before testis collection. Standard room conditions were maintained in the non-HS control group.
For CHX treatment, a dosing solution of 10 μg/μL CHX in saline was prepared before treatment. For inhibition of SG formation, mice were injected with different doses of CHX (mentioned in Figure captions) via the tail vein. After half an hour, mice were anesthetized and subjected to HS. Each group contained three mice. Negative controls received PBS injections at room temperature.
4.3. Cell Culture
The 293T cells were grown in DMEM (11965092, Gibco, New York, NY, USA) supplemented with 10% (v/v) fetal bovine serum (C04001, Vivacell, Shanghai, China), 1% (v/v) MEM NEAA (11140076, Gibco, New York, NY, USA), 1 mM sodium pyruvate (11360070, Gibco, New York, NY, USA), 100 U/mL penicillin and 100 μg/mL streptomycin (15140122, Gibco, New York, NY, USA) in a 37 °C incubator with 5% CO2. TM4 cells were grown in DMEM/F12 (11330032, Gibco, New York, NY, USA) supplemented with 10% (v/v) fetal bovine serum (C04001, Vivacell, Shanghai, China), 100 U/mL penicillin and 100 μg/mL streptomycin (15140122, Gibco, New York, NY, USA) in a 37 °C incubator with 5% CO2. Cells were allowed to adhere and to reach 50–70% confluency for HS, siRNA and vector transfection, as described below.
4.4. Heat Stress of TM4 Cells
TM4 cells were allowed to adhere and to reach 70% confluency for experiments. Cells were cultured under HS at 43 °C for 30 min, followed by maintenance at 37 °C for 7 h. The above procedure was repeated three times.
For the CHX treatment, cells were replaced with fresh medium containing 20 μg/mL CHX and cultured for 30 min before 43 °C HS for 30 min. Subsequently, cells were replaced with fresh medium to resume culture for 7 h. The above procedure was repeated three times. Cells were collected in liquid nitrogen. Standard conditions with CHX treatment were maintained for the control group.
For the siRNA treatment, after transfection for 48 h, cells were cultured under HS at 43 °C for 30 min, followed by maintenance at 37 °C for 7 h. The above procedure was repeated three times. After the indicated time, the cells were harvested for the subsequent experiments.
For all cell experiments, three biological replicates were assayed.
4.5. BTB Permeability Assay
The integrity of BTB was assessed using EZ-Link SulfoNHS-LC-Biotin (21335, Thermo Fisher Scientific, Waltham, MA, USA) as previously described [
39]. Biotin was dissolved in PBS containing 1 mM calcium chloride at a concentration of 10 mg/mL. After mice anesthesia and testis HS, the biotin solution was injected into the interstitial area under the tunica albuginea before 30 min incubation (25 μL per testis). Subsequently, the testes were fixed with Bouin’s fixative for subsequent paraffin embedding and section. The sections were stained with FITC-streptavidin (1:200; 4800-30-14, Thermo Fisher Scientific, Waltham, MA, USA) and DAPI (P0131, Beyotime, Shanghai, China). Testicular sections were photographed with a fluorescence microscope. The FITC signal of tracer in the seminiferous tubules was used to indicate BTB damage and imaged using a fluorescence microscope (ScopeA1, ZEISS, Oberkochen, Germany). Three random sections of a whole cross-section from each mouse were examined. The final data were collected from three mice in each group. The proportion of damaged seminiferous tubules to the total seminiferous tubules in the cross-section was used for statistically analysis.
4.6. Plasmid Constructs and Transfections
For lentiviral plasmid construction, the cDNA of mouse G3BP1 was cloned into the BsrGI and SaII sites of the pLenti-CMV-GFP plasmid (17448, Addgene, Watertown, MA, USA). HEK293FT cells were seeded on 15 cm plates at approximately 50% confluence and left overnight, and 25 μg of total plasmid (pLenti-CMV-GFP:pSPAX2:pMD2.G = 3:2:1) was co-transfected with Lipofectamine 2000 (11668500, Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s protocol. Transfected cells were cultured for 48 h under standard conditions and the supernatant was collected. Lentivirus was obtained by centrifugation at 45,000 rpm for 2 h at 4 °C and concentrated using PBS (30 μL per 15 cm dish).
TM4 cells grown to 70% confluency were used for lentivirus transfection. A total of 30 μL of concentrated lentivirus per 3.5cm dish was added. Infected cells were cultured for 48 h under standard conditions. Positive monoclonal G3BP1-GFP TM4 cells were selected using fresh medium containing Puromycin (final concentration 1 μg/mL) and identified by PCR.
Candidate proteins from proteomics were amplified and cloned into the AscI and PacI sites of pDsRed-C1. G3BP1-GFP TM4 cells were seeded on 3.5 cm plates at approximately 50% confluence and left overnight and 2 μg of total plasmid was transfected with Lipofectamine 2000 according to the manufacturer’s protocol. The empty vector was used as control. 48 h after transfection, the transfected cells were subjected to HS (43 °C for 30 min) for colocalization of testis. For the siRNA transfection, all the siRNAs were obtained from Suzhou Genepharma Co., Ltd. (Genepharma, Suzhou, China) and transfected into TM4 using GP-RNA Mate (G04006, Genepharma, Suzhou, China) according to the manufacturer’s instructions. All the primers and siRNA sequences are listed in
Supplementary Table S4.
4.7. SG Isolation
SG isolation was adapted from previous paper [
32,
33]. TM4 cells expressing G3BP1-GFP with 80–90% confluency were suitable for experiments. Cell media were replaced with fresh media after 1 h and then treated at 43 °C for 30 min. Standard culture conditions were maintained as the control group (SG-negative). Cells were collected in falcon tube after centrifuging at 1500×
g for 3 min. The pellets were flash-frozen in liquid N
2 and placed on ice for 5 min before subsequent isolation.
The pellets were re-suspended in SG lysis buffer (50 mM TrisHCl pH 7.4, 100 mM KOAc, 2 mM MgOAc, 0.5 mM DTT, 50 μg/mL Heparin, 0.5% NP40, protease inhibitor (11836170001, Sigma Aldrich, St Louis, MO, USA), 1 U/μL of recombinant RNase inhibitor (2313A, Takara, Dalian, China)). Syringe with 25 gauge 5/8 needle on ice was used to accelerate lysis. The lysates were centrifuged at 1000× g for 5 min at 4 °C to remove cell debris. The supernatant was then spun at 18,000× g for 20 min at 4 °C to collect SG cores. SG core pellets re-suspended in 1 mL SG lysis buffer were spun at 18,000× g for 20 min at 4 °C again and re-suspended in 300 μL SG lysis buffer. The supernatant was spun at 850× g for 2 min at 4 °C. At this stage, the processing supernatant contained the SG-core-enriched fraction.
The SG-enriched fraction was pre-cleared twice by rotating with 30 μL equilibrated dynabeads (10002D, Thermo Fisher Scientific, Waltham, MA, USA) at 4 °C for 30 min. SG-enriched fraction was rotated with 20 μL of anti-GFP antibody at 4 °C for 1 h after removing dynabeads. The SG-enriched fraction was precipitated by spinning at 18,000× g for 20 min at 4 °C and re-suspended in 500 μL SG lysis buffer and 60 μL equilibrated Protein A Dynabeads. The mixture was rotated for 3 h at 4 °C. Then, dynabeads were collected and washed using three different wash buffers for 5 min each (wash buffer 1: 20 mM Tris HCl pH 8.0, 200 mM NaCl, 1 U/μL of recombinant RNase inhibitor; wash buffer 2: 20 mM Tris HCl pH 8.0, 500 mM NaCl, 1 U/μL of recombinant RNase inhibitor; wash buffer 3: SG lysis buffer, 2M Urea, 1 U/μL of recombinant RNase inhibitor).
The beads were mixed with 2× loading buffer and incubated at 95 °C for 10 min. Then, these samples were used for proteome sequencing or Western blotting.
4.8. Western Blot
Whole cells and tissues were lysed in cell lysis buffer (P0013, Beyotime, Shanghai, China) with a protease and phosphatase inhibitor cocktail (P1045, Beyotime, Shanghai, China) on ice. Centrifugation was performed at 12,000× g for 15 min to remove cell debris, followed by quantification using the BCA Protein Assay Kit (P0012, Beyotime, Shanghai, China).
For Western blotting, proteins were separated on sodium dodecyl sulfate polyacrylamide gel electrophoresis gels and electrotransferred to PVDF membranes (1620177, Bio-rad, Hercules, CA, USA). The membranes were incubated with primary antibodies overnight at 4 °C after blocking with 5% skimmed milk in TBST for 1 h. Then, the membranes were incubated with HRP-conjugated secondary antibodies for 1 h at room temperature after washing. The proteins were visualized using Western ECL substrate (1705060, Bio-rad, Hercules, CA, USA) on the Tanon 5200 (5200, Tanon, Shanghai, China) chemiluminescence imaging system. Image analysis software (ImageJ, v1.8.0.345) was used to quantify the band density. The band density of each protein was corrected with β-Actin as the relative expression, which was used for statistical analysis.
Antibodies against GFP (#66002-1-lg), G3BP1 (#66486-1-lg), rpS6 (#66886-1-lg) and phospho-rpS6 (Ser236/236, #29223-1-AP) were from Proteintech (Proteintech, Wuhan, China). Antibodies against Claudin-11 (#HX13363) were from Huaxingbio (Huaxingbio, Beijing, China). Antibodies against TIA-1 (#sc-166247) were from Santa Cruz (Santa Cruz Biotechnology, Shanghai, China). Antibodies against β-Catenin (#BE3365) and FLAG (#BE7003) were from Easybio (Easybio, Beijing, China). Antibodies against ZO-1 (#33-9100) were from Thermo Fisher (Thermo Fisher Scientific, Waltham, MA, USA). Antibodies against β-Actin (#ab49900) were from Abcam (Abcam, Cambridge, Britain). Antibodies against UBAP2 (#PK59381) were from Abmart (Abmart, Shanghai, China).
4.9. RT-qPCR
Total RNA was extracted using TRIzol Reagent (T9424, Sigma Aldrich, St Louis, MO, USA) and reverse transcribed into cDNA using Fastking RT kit (KR116-02, Tiangen, Beijing, China) according to the manufacturer’s instructions. The specific quantitative primers are listed in
Supplementary Table S4. A set of cDNA, 2× SuperReal PreMix Plus SYBR Green (FP205, Tiangen, Beijing, China) and forward and reverse primers were mixed and subjected to RT-qPCR performed on CFX96 Touch Deep Well Real-Time PCR Detection System (CFX96, Bio-rad, Hercules, CA, USA). The program was as follows: 95 °C for 15 min, followed by 40 cycles of 95 °C for 10 s, 60 °C for 20 s and 72 °C for 30 s. The reaction was performed in a CFX96™ Real-Time PCR machine (CFX96, Bio-rad, Hercules, CA, USA). The standard curve was used to determine PCR efficiency. The dissociation curve of amplified products was used to evaluate product specificity. Relative expression levels were normalized to β-Actin using the 2
−ΔΔCt method. For each gene, three technical replicates and three biological replicates were assayed.
4.10. Immunofluorescence
Testes were fixed in 4% paraformaldehyde at 4 °C for 48 h. The fixed samples were embedded in paraffin and cut into 5 μm sections for immunostaining after being dewaxed and rehydrated. After subjecting to antigen retrieval with 0.01 M sodium citrate buffer, testicular sections were blocked with 5% normal goat serum at room temperature for 1 h. Then, sections were incubated with primary antibodies at 4 °C overnight, followed by PBST washing and secondary antibody incubation. Before microscopic imaging, slides were mounted with antifade mounting medium with DAPI (H1200, Vector Laboratories, Burlingame, CA, USA). Cells grown in slides were fixed in 4% paraformaldehyde for 15 min and permeabilized with 0.5% Triton X-100 in PBS for 20 min. The process of blocking and antibody incubation was consistent with the tissue section. The signal was imaged using a fluorescence microscope (ScopeA1, ZEISS, Oberkochen, Germany).
Antibodies against G3BP1 (#66486-1-lg) were from Proteintech (Proteintech, Wuhan, China). Antibodies against β-Catenin (#BE3365) were from Easybio (Easybio, Beijing, China). Antibodies against Occludin (#33-1500) were from Thermo Fisher (Thermo Fisher Scientific, Waltham, MA, USA). Antibodies against SOX9 (T55400) were from Abmart (Abmart, Shanghai, China). Antibodies against Vimentin (#5741S) were from Cell Signaling Technology(Cell Signaling Technology, Danvers, MA, USA).
4.11. Statistical Analysis
Experiments were independently replicated three times, and all data were reported as the mean ± SEM. T-test was used to analyze the significant difference between the treatment and control groups (two groups). Multiple comparison was assessed using one-way ANOVA with Dunnett’s post hoc test using SPSS 24.0 software. For all statistical analyses, * = p ≤ 0.05, ** = p ≤ 0.01, *** = p ≤ 0.001, n.s. = not significant.