Preparation of Bispecific IgY-scFvs Inhibition Adherences of Enterotoxigenic Escherichia coli (K88 and F18) to Porcine IPEC-J2 Cell
Abstract
1. Introduction
2. Results
2.1. Fimbrial Typing of ETEC Isolates
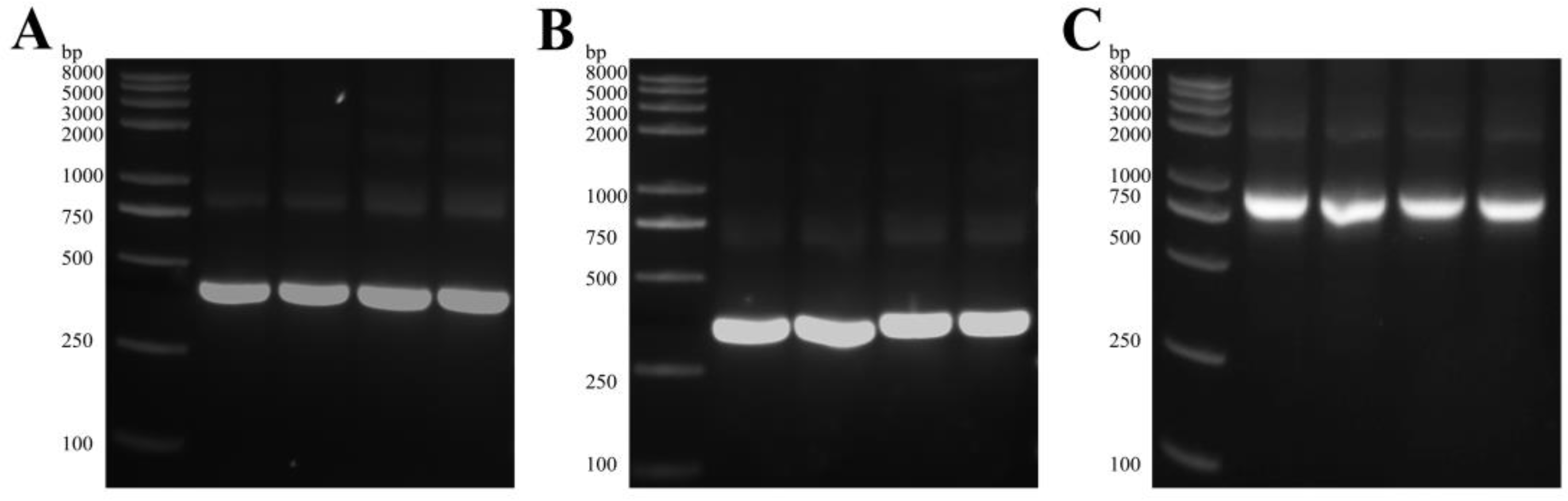
2.2. Construction of the ScFv Library against ETEC
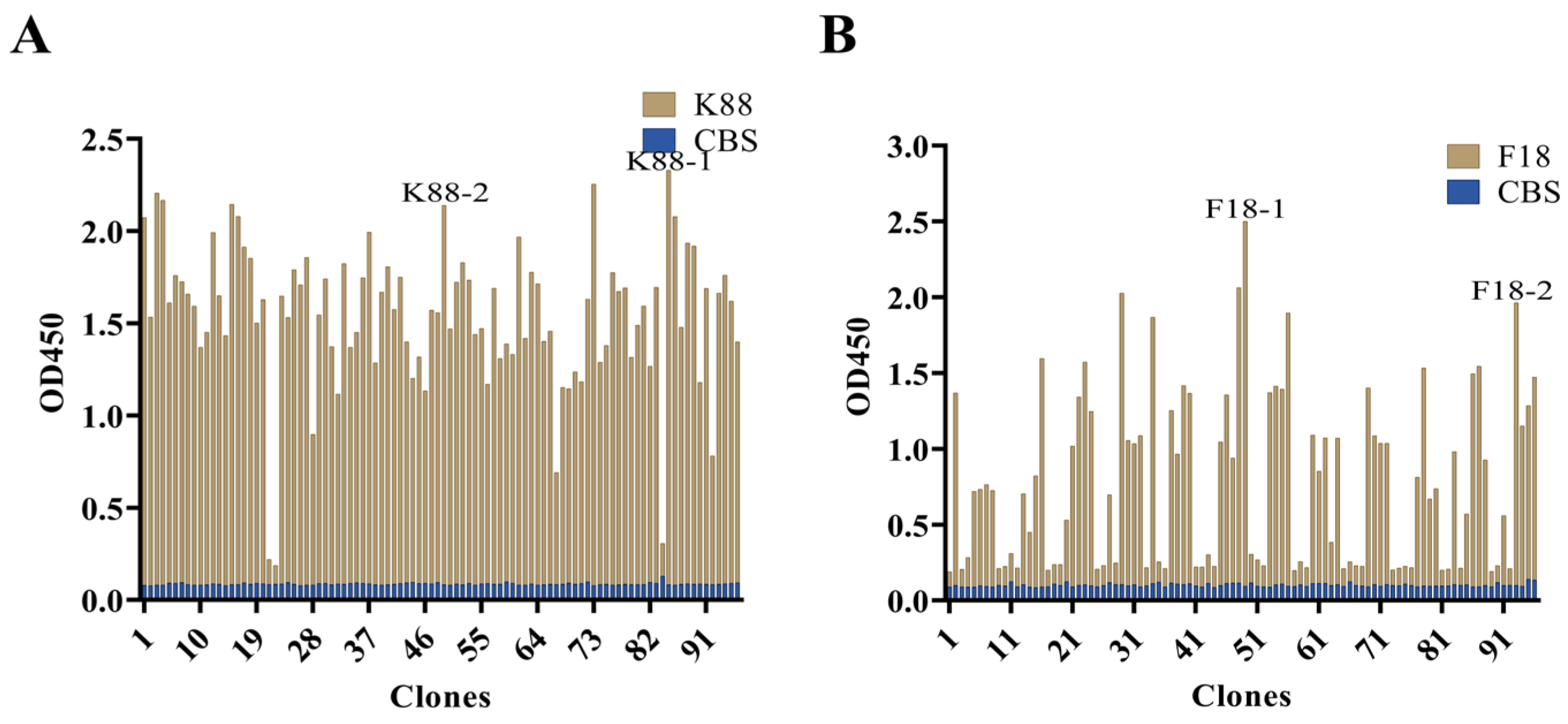
2.3. Bio-Screening and Sequencing of ScFv against ETEC
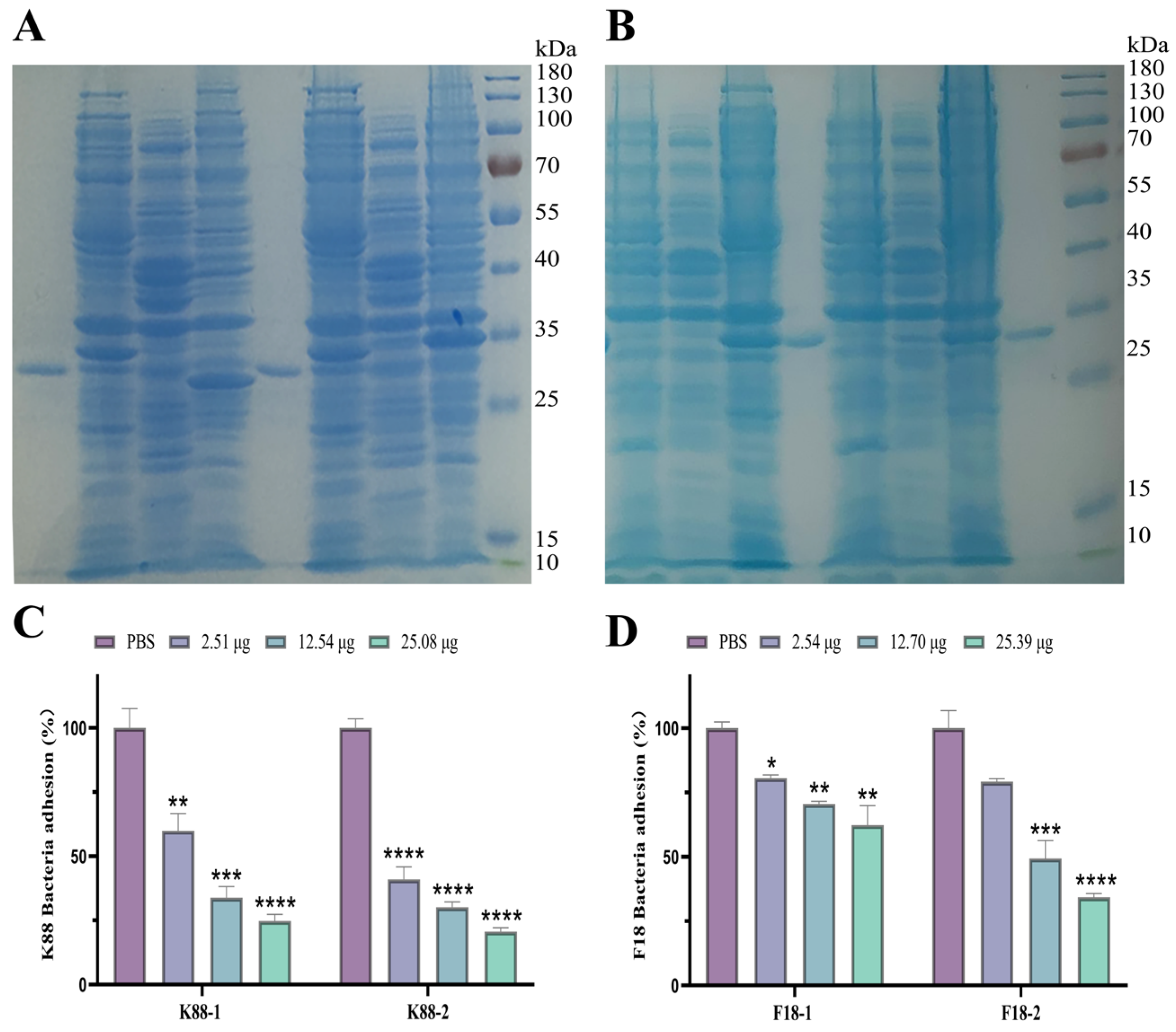
2.4. Evaluation of scFv against K88 and F18
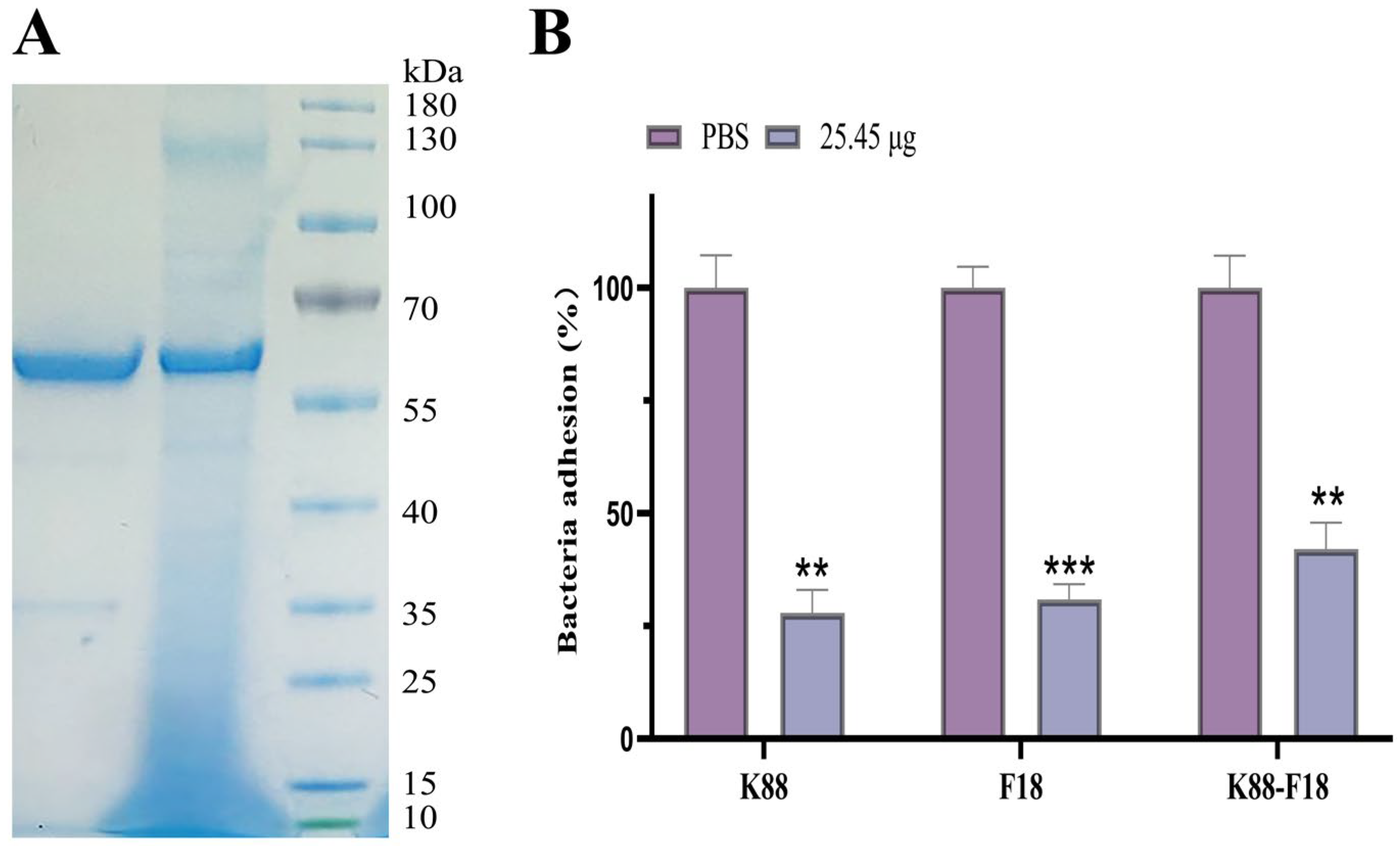
2.5. Evaluation of Bispecific scFv Inhibition Adherence ETEC K88 and F18 to Porcine IPEC-J2 Cells
3. Discussion
4. Materials and Methods
4.1. Preparation of ETEC K88 and F18 Antigens
4.2. Chicken Immunization
4.3. Extraction of IgY
4.4. Determination of the Specific IgY Titer
4.5. Construction of the Library and Selection of Anti-ETEC K88 and F18 scFvs
4.6. Phage ELISA
4.7. Expression and Purification of the Specific scFv in E. coli
4.8. Specific scFv Adherence Inhibition Assay
4.9. Construction of Bispecific scFv
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Castro, J.; Barros, M.M.; Araujo, D.; Campos, A.M.; Oliveira, R.; Silva, S.; Almeida, C. Swine enteric colibacillosis: Current treatment avenues and future directions. Front. Vet. Sci. 2022, 9, 981207. [Google Scholar] [CrossRef]
- Frydendahl, K. Prevalence of serogroups and virulence genes in Escherichia coli associated with postweaning diarrhoea and edema disease in pigs and a comparison of diagnostic approaches. Vet. Microbiol. 2002, 85, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Gao, S.; Jiao, X.; Liu, X.F. Prevalence of serogroups and virulence factors of Escherichia coli strains isolated from pigs with postweaning diarrhoea in eastern China. Vet. Microbiol. 2004, 103, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhao, M.; Ruesch, L.; Omot, A.; Francis, D. Prevalence of virulence genes in Escherichia coli strains recently isolated from young pigs with diarrhea in the US. Vet. Microbiol. 2007, 123, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Dubreuil, J.D.; Isaacson, R.E.; Schifferli, D.M. Animal Enterotoxigenic Escherichia coli. EcoSal Plus 2016, 7, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.; Pang, S.; Wu, W.; Jiang, B.; Zhang, W.; Liu, S.; Wang, X.; Pan, Z.; Zhu, G. A multivalent vaccine candidate targeting enterotoxigenic Escherichia coli fimbriae for broadly protecting against porcine post-weaning diarrhea. Vet. Res. 2020, 51, 93. [Google Scholar] [CrossRef] [PubMed]
- Qadri, F.; Svennerholm, A.M.; Faruque, A.S.; Sack, R.B. Enterotoxigenic Escherichia coli in developing countries: Epidemiology, microbiology, clinical features, treatment, and prevention. Clin. Microbiol. Rev. 2005, 18, 465–483. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.; Bai, Y.; Li, Y.; Huang, Y.; Li, L.; Wang, G.; Qu, Y.; Wang, J.; Yu, L.Y.; Hou, X. Changes in Gut Microbiota by the Lactobacillus casei Anchoring the K88 Fimbrial Protein Prevented Newborn Piglets From Clinical Diarrhea. Front. Cell Infect. Microbiol. 2022, 12, 842007. [Google Scholar] [CrossRef]
- Duan, Q.; Chen, D.; Yu, B.; Huang, Z.; Luo, Y.; Zheng, P.; Mao, X.; Yu, J.; Luo, J.; Yan, H.; et al. Effect of sialyllactose on growth performance and intestinal epithelium functions in weaned pigs challenged by enterotoxigenic Escherichia coli. J. Anim. Sci. Biotechnol. 2022, 13, 30. [Google Scholar] [CrossRef]
- Fairbrother, J.M.; Nadeau, E.; Gyles, C.L. Escherichia coli in postweaning diarrhea in pigs: An update on bacterial types, pathogenesis, and prevention strategies. Anim. Health Res. Rev. 2005, 6, 17–39. [Google Scholar] [CrossRef]
- Amezcua, R.F.R.; Dewey, C.E.; Gyles, C.; Fairbrother, J.M. Presentation of postweaning Escherichia coli diarrhea in southern Ontario, prevalence of hemolytic E. coli serogroups involved, and their antimicrobial resistance patterns. Can. J. Vet. Res. 2002, 66, 73–78. [Google Scholar]
- Barton, M.D. Antibiotic use in animal feed and its impact on human health. Nutr. Res. Rev. 2000, 13, 279–299. [Google Scholar] [CrossRef]
- Gao, J.; Duan, X.; Li, X.; Cao, H.; Wang, Y.; Zheng, S.J. Emerging of a highly pathogenic and multi-drug resistant strain of Escherichia coli causing an outbreak of colibacillosis in chickens. Infect. Genet. Evol. 2018, 65, 392–398. [Google Scholar] [CrossRef]
- Perez de la Lastra, J.M.; Baca-Gonzalez, V.; Asensio-Calavia, P.; Gonzalez-Acosta, S.; Morales-delaNuez, A. Can Immunization of Hens Provide Oral-Based Therapeutics against COVID-19? Vaccines 2020, 8, 486. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Li, J.; Li, Y.; Wang, L.; Wang, Q.; Fang, L.; Ding, X.; Huang, P.; Yin, J.; et al. Protective effect of chicken egg yolk immunoglobulins (IgY) against enterotoxigenic Escherichia coli K88 adhesion in weaned piglets. BMC Vet. Res. 2019, 15, 234. [Google Scholar] [CrossRef]
- Chalghoumi, R.; Marcq, C.; Thewis, A.; Portetelle, D.; Beckers, Y. Effects of feed supplementation with specific hen egg yolk antibody (immunoglobin Y) on Salmonella species cecal colonization and growth performances of challenged broiler chickens. Poult. Sci. 2009, 88, 2081–2092. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Jeon, Y.S.; Park, C.K.; Kim, S.; Lee, D.S.; Lee, C. Immunoprophylactic effect of chicken egg yolk antibody (IgY) against a recombinant S1 domain of the porcine epidemic diarrhea virus spike protein in piglets. Arch. Virol. 2015, 160, 2197–2207. [Google Scholar] [CrossRef]
- Schade, R.; Calzado, E.G.; Sarmiento, R.; Chacana, P.A.; Porankiewicz-Asplund, J.; Terzolo, H.R. Chicken egg yolk antibodies (IgY-technology): A review of progress in production and use in research and human and veterinary medicine. Altern. Lab. Anim. 2005, 33, 129–154. [Google Scholar] [CrossRef] [PubMed]
- Holliger, P.; Hudson, P.J. Engineered antibody fragments and the rise of single domains. Nat. Biotechnol. 2005, 23, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Fercher, C.; Keshvari, S.; McGuckin, M.A.; Barnard, R.T. Evolution of the magic bullet: Single chain antibody fragments for the targeted delivery of immunomodulatory proteins. Exp. Biol. Med. 2018, 243, 166–183. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.Y.; George, S.; Brozel, V.; Moxley, R.; Francis, D.; Kaushik, R.S. Porcine intestinal epithelial cell lines as a new in vitro model for studying adherence and pathogenesis of enterotoxigenic Escherichia coli. Vet. Microbiol. 2008, 130, 191–197. [Google Scholar] [CrossRef]
- Lu, H.; Luo, H.; Shi, P.; Huang, H.; Meng, K.; Yang, P.; Yao, B. Erratum to: A novel thermophilic endo-beta-1,4-mannanase from Aspergillus nidulans XZ3: Functional roles of carbohydrate-binding module and Thr/Ser-rich linker region. Appl. Microbiol. Biotechnol. 2015, 99, 1023. [Google Scholar] [CrossRef]
- Royle, K.E.; Polizzi, K. A streamlined cloning workflow minimising the time-to-strain pipeline for Pichia pastoris. Sci. Rep. 2017, 7, 15817. [Google Scholar] [CrossRef]
- Kotlan, B.; Glassy, M.C. Antibody phage display: Overview of a powerful technology that has quickly translated to the clinic. Methods Mol. Biol. 2009, 562, 1–15. [Google Scholar]
- Galan, A.; Comor, L.; Horvatic, A.; Kules, J.; Guillemin, N.; Mrljak, V.; Bhide, M. Library-based display technologies: Where do we stand? Mol. Biosyst. 2016, 12, 2342–2358. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Xu, L.; Li, B.; Zhong, F.; Liu, X.; Zhang, X. Canine Parvovirus is diagnosed and neutralized by chicken IgY-scFv generated against the virus capsid protein. Vet. Res. 2020, 51, 110. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, Y.; Wang, X.; Li, Y.; Wang, L.; Li, X. Construction and characterization of a highly reactive chicken-derived single-chain variable fragment (scFv) antibody against Staphylococcus aureus developed with the T7 phage display system. Int. Immunopharmacol. 2016, 35, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Wu, R.; Zhou, T.; Liu, X.; Zhu, J.; Zhang, X. Specific anti-SARS-CoV-2 S1 IgY-scFv is a promising tool for recognition of the virus. AMB Express 2022, 12, 18. [Google Scholar] [CrossRef] [PubMed]
- de Sousa-Pereira, P.; Woof, J.M. IgA: Structure, Function, and Developability. Antibodies 2019, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Ohno, S.; Mori, N.; Matsunaga, T. Antigen-binding specificities of antibodies are primarily determined by seven residues of VH. Proc. Natl. Acad. Sci. USA 1985, 82, 2945–2949. [Google Scholar] [CrossRef] [PubMed]
- Baker, K.K.; Levine, M.M.; Morison, J.; Phillips, A.; Barry, E.M. CfaE tip mutations in enterotoxigenic Escherichia coli CFA/I fimbriae define critical human intestinal binding sites. Cell Microbiol. 2009, 11, 742–754. [Google Scholar] [CrossRef]
- Jin, L.Z.; Baidoo, S.K.; Marquardt, R.R.; Frohlich, A.A. In vitro inhibition of adhesion of enterotoxigenic Escherichia coli K88 to piglet intestinal mucus by egg-yolk antibodies. FEMS Immunol. Med. Microbiol. 1998, 21, 313–321. [Google Scholar] [CrossRef]
- Zelenovic, N.; Filipovic, L.; Popovic, M. Recent Developments in Bioprocessing of Recombinant Antibody Fragments. Biochemistry 2023, 88, 1191–1204. [Google Scholar] [CrossRef]
- Gupta, S.K.; Shukla, P. Sophisticated Cloning, Fermentation, and Purification Technologies for an Enhanced Therapeutic Protein Production: A Review. Front. Pharmacol. 2017, 8, 419. [Google Scholar] [CrossRef]
- Han, S.; Yu, H.; Yang, F.; Qiao, S.; He, P. Effect of dietary supplementation with hyperimmunized hen egg yolk powder on diarrhoea incidence and intestinal health of weaned pigs. Food Agric. Immunol. 2019, 30, 333–348. [Google Scholar] [CrossRef]
- He, J.; Tao, X.; Wang, K.; Ding, G.; Li, J.; Li, Q.X.; Gee, S.J.; Hammock, B.D.; Xu, T. One-step immunoassay for the insecticide carbaryl using a chicken single-chain variable fragment (scFv) fused to alkaline phosphatase. Anal. Biochem. 2019, 572, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, S.; Miao, Z.; Liu, Y.; Liu, X.; Xiao, Z.X.; Cao, Y. AbRSA: A robust tool for antibody numbering. Protein Sci. 2019, 28, 1524–1531. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Yang, Y.; Liu, A.; Lei, S.; He, P. Preparation of Bispecific IgY-scFvs Inhibition Adherences of Enterotoxigenic Escherichia coli (K88 and F18) to Porcine IPEC-J2 Cell. Int. J. Mol. Sci. 2024, 25, 3638. https://doi.org/10.3390/ijms25073638
Yang L, Yang Y, Liu A, Lei S, He P. Preparation of Bispecific IgY-scFvs Inhibition Adherences of Enterotoxigenic Escherichia coli (K88 and F18) to Porcine IPEC-J2 Cell. International Journal of Molecular Sciences. 2024; 25(7):3638. https://doi.org/10.3390/ijms25073638
Chicago/Turabian StyleYang, Luqing, Yuanhe Yang, Anguo Liu, Siqi Lei, and Pingli He. 2024. "Preparation of Bispecific IgY-scFvs Inhibition Adherences of Enterotoxigenic Escherichia coli (K88 and F18) to Porcine IPEC-J2 Cell" International Journal of Molecular Sciences 25, no. 7: 3638. https://doi.org/10.3390/ijms25073638
APA StyleYang, L., Yang, Y., Liu, A., Lei, S., & He, P. (2024). Preparation of Bispecific IgY-scFvs Inhibition Adherences of Enterotoxigenic Escherichia coli (K88 and F18) to Porcine IPEC-J2 Cell. International Journal of Molecular Sciences, 25(7), 3638. https://doi.org/10.3390/ijms25073638





