Confinement of a Styryl Dye into Nanoporous Aluminophosphates: Channels vs. Cavities
Abstract
1. Introduction
2. Results and Discussion
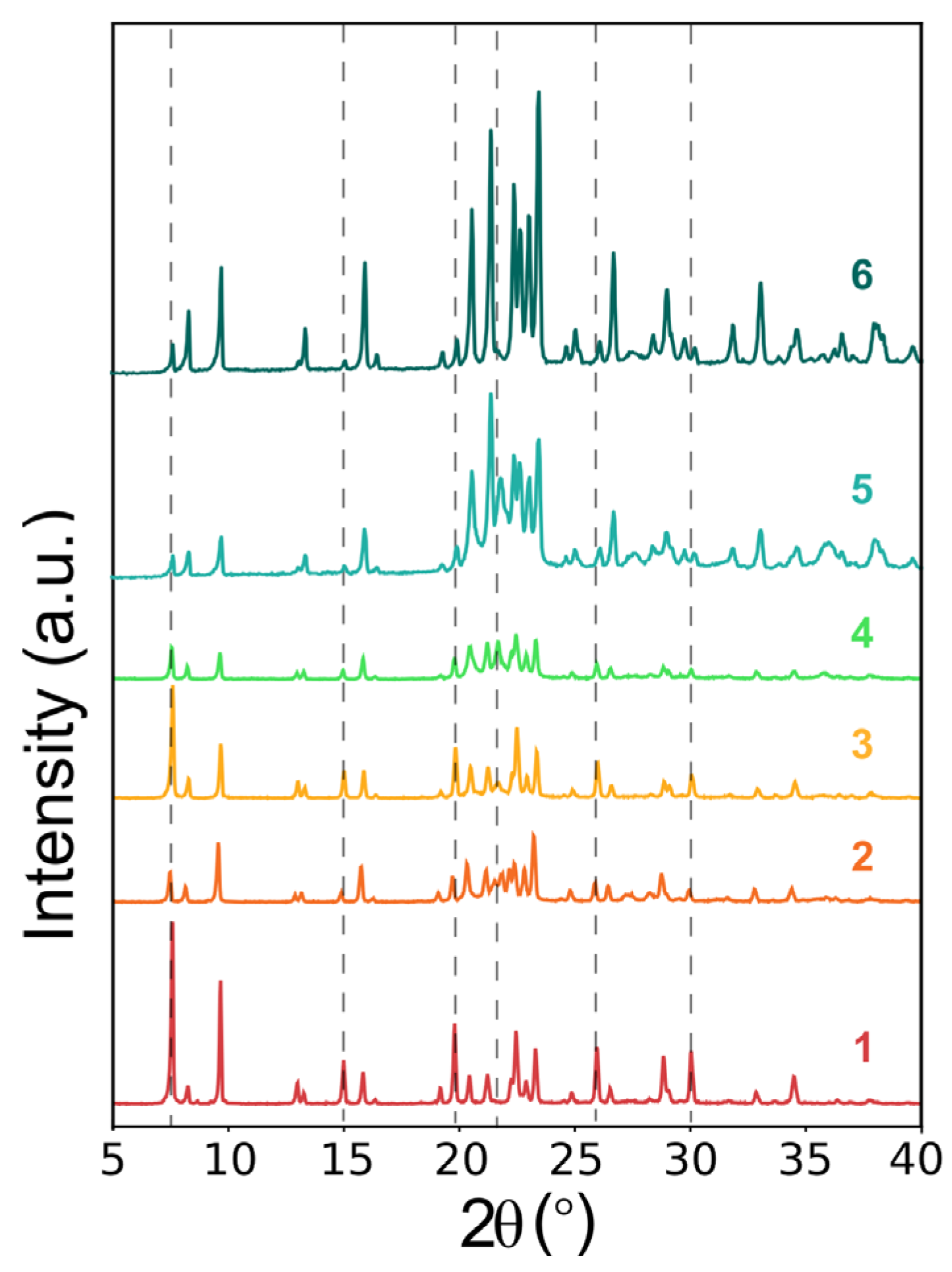
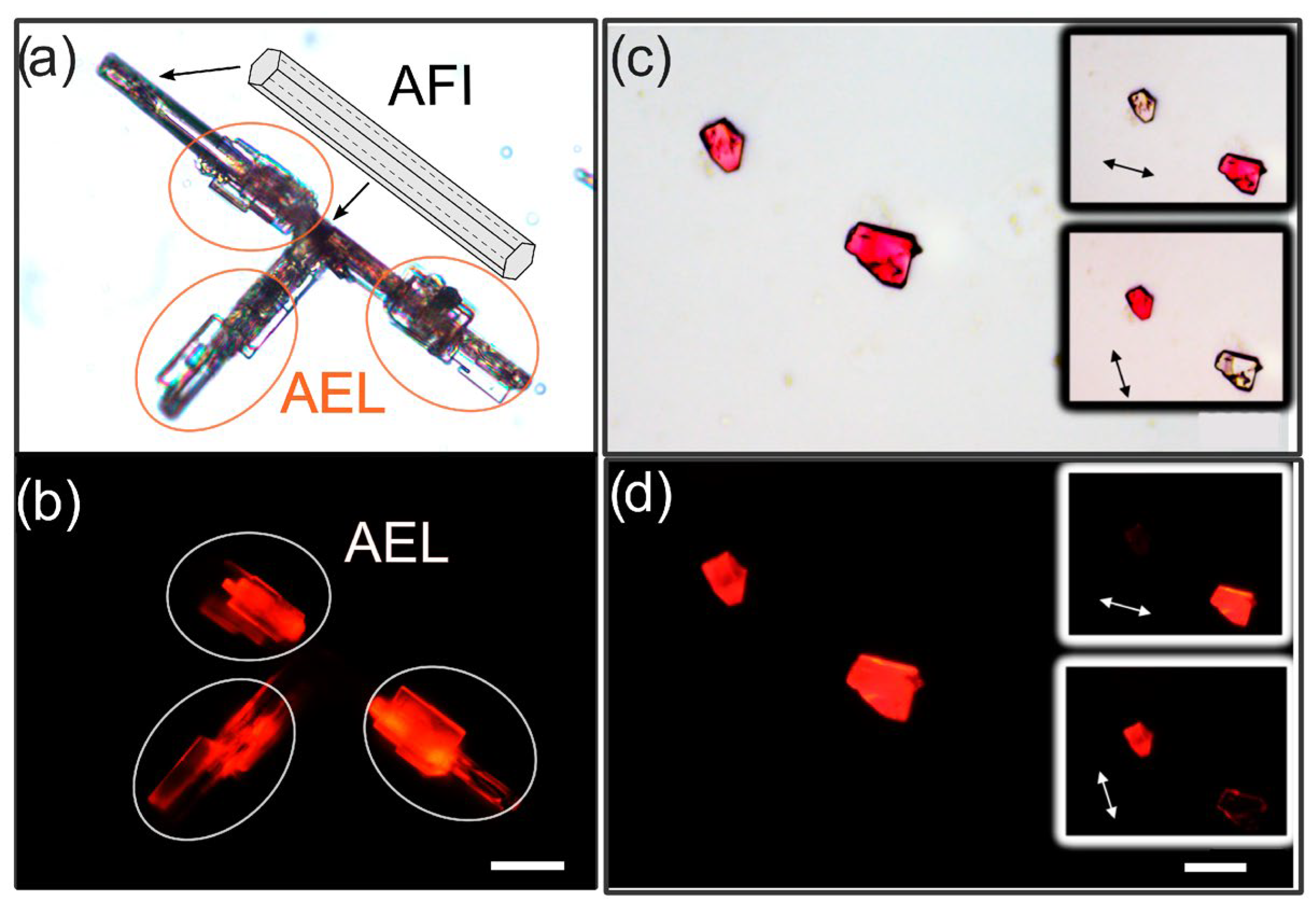
2.1. Synthesis of 4-DASPI@MgAPO-CHA System
2.2. Synthesis of 4-DASPI@MgAPO-AEL System
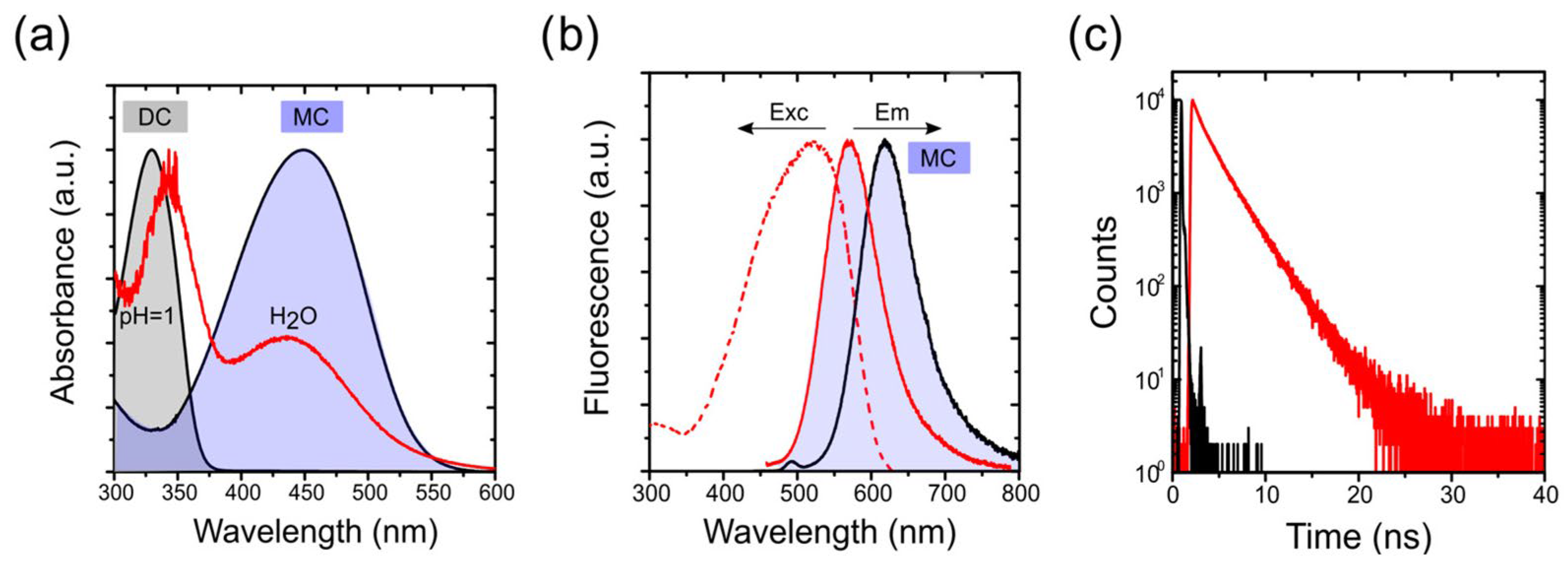
2.3. Photophysics of 4-DASPI Dye in Solution
2.4. Photophysics of 4-DASPI@MgAPO-CHA
2.5. Photophysics of 4-DASPI@MgAPO-AEL
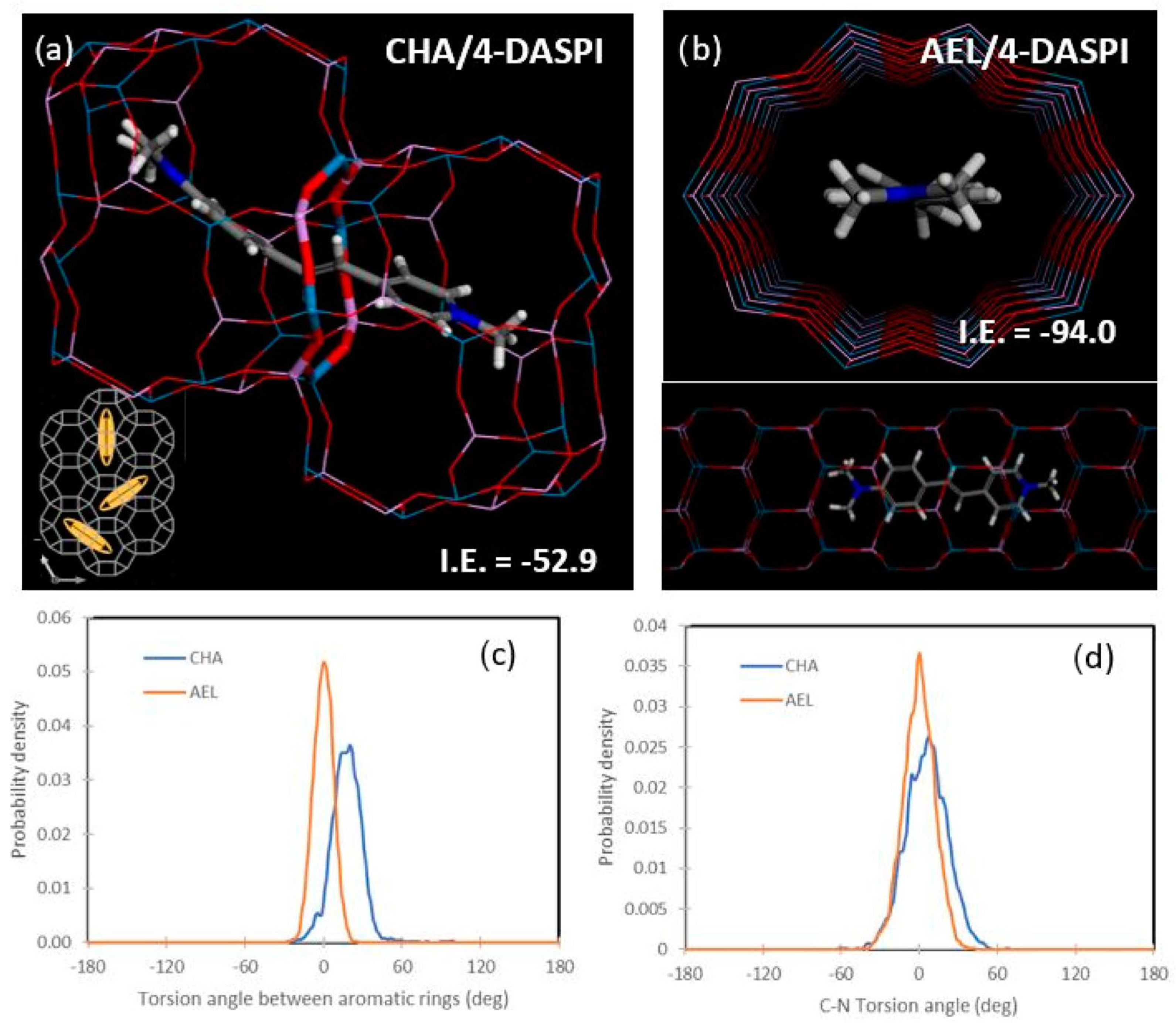
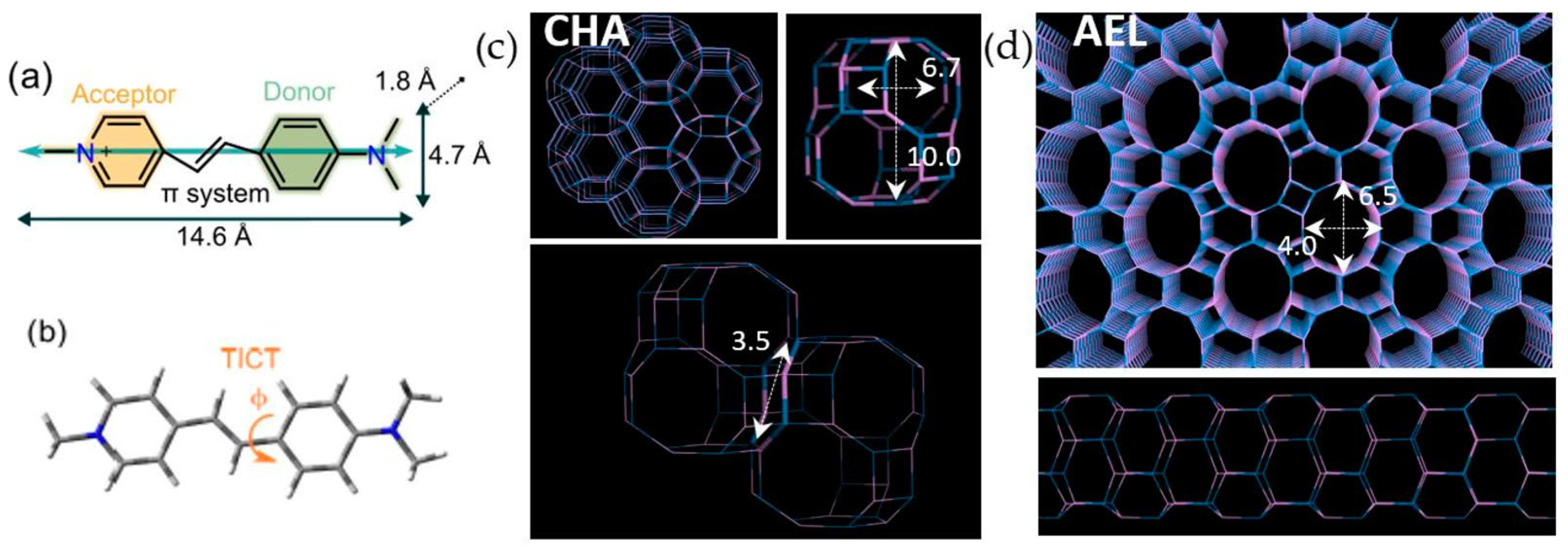
2.6. Molecular Simulations
3. Materials and Methods
3.1. Synthesis of Hybrid Materials
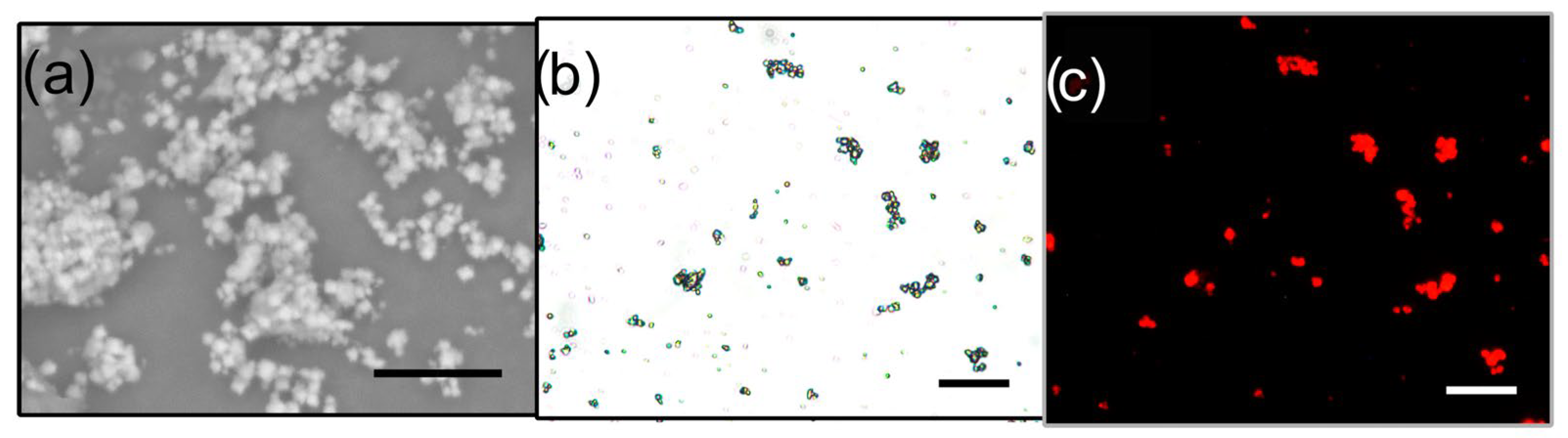
3.2. Characterization Techniques
3.3. Quantification of DASPI Loading into MgCHA and MgAEL
3.4. Photophysical Characterization
3.5. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wuskell, J.P.; Boudreau, D.; De Wei, M.; Jin, L.; Engl, R.; Chebolu, R.; Bullen, A.; Hoffacker, K.D.; Kerimo, J.; Cohen, L.B.; et al. Synthesis, Spectra, Delivery and Potentiometric Responses of New Styryl Dyes with Extended Spectral Ranges. J. Neurosci. Methods 2006, 151, 200–215. [Google Scholar] [CrossRef]
- Safir Filho, M.; Fiorucci, S.; Martin, A.R.; Benhida, R. Design, Synthesis and Photophysical Studies of Styryl-Based Push–Pull Fluorophores with Remarkable Solvatofluorochromism. New J. Chem. 2017, 41, 13760–13772. [Google Scholar] [CrossRef]
- Du, Y.; Liu, X.; Zhu, S. Near-Infrared-II Cyanine/Polymethine Dyes, Current State and Perspective. Front. Chem. 2021, 9, 718709. [Google Scholar] [CrossRef]
- Kothavale, S.; Sekar, N. Methoxy Supported, Deep Red Emitting Mono, Bis and Tris Triphenylamine-Isophorone Based Styryl Colorants: Synthesis, Photophysical Properties, ICT, TICT Emission and Viscosity Sensitivity. Dye. Pigment. 2017, 136, 116–130. [Google Scholar] [CrossRef]
- Abeywickrama, C.S.; Wijesinghe, K.J.; Stahelin, R.V.; Pang, Y. Bright Red-Emitting Highly Reliable Styryl Probe with Large Stokes Shift for Visualizing Mitochondria in Live Cells under Wash-Free Conditions. Sens. Actuators B Chem. 2019, 285, 76–83. [Google Scholar] [CrossRef]
- Dahal, D.; Ojha, K.R.; Pokhrel, S.; Paruchuri, S.; Konopka, M.; Liu, Q.; Pang, Y. NIR-Emitting Styryl Dyes with Large Stokes’ Shifts for Imaging Application: From Cellular Plasma Membrane, Mitochondria to Zebrafish Neuromast. Dye. Pigment. 2021, 194, 109629. [Google Scholar] [CrossRef]
- Li, B.; Tong, R.; Zhu, R.; Meng, F.; Tian, H.; Qian, S. The Ultrafast Dynamics and Nonlinear Optical Properties of Tribranched Styryl Derivatives Based on 1,3,5-Triazine. J. Phys. Chem. B 2005, 109, 10705–10710. [Google Scholar] [CrossRef]
- Huang, J.Y.; Lewis, A.; Loew, L. Nonlinear Optical Properties of Potential Sensitive Styryl Dyes. Biophys. J. 1988, 53, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Millard, A.C.; Jin, L.; Wuskell, J.P.; Lewis, A.; Loew, L.M. Sensitivity of Second Harmonic Generation from Styryl Dyes to Transmembrane Potential. Biophys. J. 2004, 86, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Supabowornsathit, K.; Faikhruea, K.; Ditmangklo, B.; Jaroenchuensiri, T.; Wongsuwan, S.; Junpra-Ob, S.; Choopara, I.; Palaga, T.; Aonbangkhen, C.; Somboonna, N. Dicationic Styryl Dyes for Colorimetric and Fluorescent Detection of Nucleic Acids. Sci. Rep. 2022, 12, 14250. [Google Scholar] [CrossRef] [PubMed]
- Deligeorgiev, T.; Vasilev, A.; Kaloyanova, S.; Vaquero, J.J. Styryl Dyes—Synthesis and Applications during the Last 15 Years. Color. Technol. 2010, 126, 55–80. [Google Scholar] [CrossRef]
- Wickramasinghe, N.I.; Corbin, B.; Kanakarathna, D.Y.; Pang, Y.; Abeywickrama, C.S.; Wijesinghe, K.J. Bright NIR-Emitting Styryl Pyridinium Dyes with Large Stokes’ Shift for Sensing Applications. Biosensors 2023, 13, 799. [Google Scholar] [CrossRef]
- Cao, X.; Tolbert, R.W.; McHale, J.L.; Edwards, W.D. Theoretical Study of Solvent Effects on the Intramolecular Charge Transfer of a Hemicyanine Dye. J. Phys. Chem. A 1998, 102, 2739–2748. [Google Scholar] [CrossRef]
- Mártire, D.; Massad, W.; Montejano, H.; Gonzalez, M.; Caregnato, P.; Villata, L.; García, N. Properties of Singlet-and Triplet-Excited States of Hemicyanine Dyes. Chem. Pap. 2014, 68, 1137–1140. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, M. Volume Increment Effect on the Photoisomerization of Hemicyanine Dyes in Oligo(ethylene glycol)s. J. Phys. Chem. A 2013, 117, 12878–12883. [Google Scholar] [CrossRef] [PubMed]
- Schmidtke, J.; Stille, W.; Finkelmann, H.; Kim, S.T. Laser Emission in a Dye Doped Cholesteric Polymer Network. Adv. Mater. 2002, 14, 746–749. [Google Scholar] [CrossRef]
- Xu, B.; Gao, Z.; Wei, Y.; Liu, Y.; Sun, X.; Zhang, W.; Wang, X.; Wang, Z.; Meng, X. Dynamically Wavelength-Tunable Random Lasers Based on Metal–Organic Framework Particles. Nanoscale 2020, 12, 4833–4838. [Google Scholar] [CrossRef] [PubMed]
- Udayan, S.; MM, R.S.; Sebastian, M.; Nampoori, V.P.N.; Thomas, S. Two Photon Induced Amplified Spontaneous Emission at Low Threshold from Styryl 7 Dye Incorporated DNA Template. Opt. Mater. 2018, 86, 492–497. [Google Scholar] [CrossRef]
- Yu, J.; Cui, Y.; Wu, C.; Yang, Y.; Wang, Z.; O’Keeffe, M.; Chen, B.; Qian, G. Second-Order Nonlinear Optical Activity Induced by Ordered Dipolar Chromophores Confined in the Pores of an Anionic Metal-Organic Framework. Angew. Chem. 2012, 124, 10694–10697. [Google Scholar] [CrossRef]
- Sola-Llano, R.; Martínez-Martínez, V.; Fujita, Y.; Gómez-Hortigüela, L.; Alfayate, A.; Uji-i, H.; Fron, E.; Pérez-Pariente, J.; López-Arbeloa, I. Formation of a Nonlinear Optical Host–Guest Hybrid Material by Tight Confinement of LDS 722 into Aluminophosphate 1D Nanochannels. Chem. A Eur. J. 2016, 22, 15700–15711. [Google Scholar] [CrossRef]
- IZA International Zeolite Association. Available online: https://europe.iza-structure.org/IZA-SC/ftc_table.php (accessed on 8 February 2024).
- Sola-Llano, R.; Fujita, Y.; Gómez-Hortigüela, L.; Alfayate, A.; Uji-I, H.; Fron, E.; Toyouchi, S.; Pérez-Pariente, J.; López-Arbeloa, I.; Martínez-Martínez, V. One-Directional Antenna Systems: Energy Transfer from Monomers to J-Aggregates within 1D Nanoporous Aluminophosphates. ACS Photonics 2018, 5, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Sola-Llano, R.; Gartzia-Rivero, L.; Oliden-Sanchez, A.; Bañuelos, J.; Arbeloa, I.L.; Martínez-Martínez, V. Dye Encapsulation into One-Dimensional Zeolitic Materials for Optical Applications. In Chemistry of Silica and Zeolite-Based Materials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 229–248. [Google Scholar]
- Martínez, V.; García Salas, R.; Gómez-Hortigüela Sainz, L.; Pérez Pariente, J.; López-Arbeloa, Í. Modulating Dye Aggregation by Incorporation into 1D-MgAPO Nanochannels. Chem. A Eur. J. 2013, 19, 9859–9865. [Google Scholar] [CrossRef] [PubMed]
- Sola-Llano, R.; Oliden-Sánchez, A.; Alfayate, A.; Gómez-Hortigüela, L.; Pérez-Pariente, J.; Arbeloa, T.; Hofkens, J.; Fron, E.; Martínez-Martínez, V. White Light Emission by Simultaneous One Pot Encapsulation of Dyes into One-Dimensional Channelled Aluminophosphate. Nanomaterials 2020, 10, 1173. [Google Scholar] [CrossRef] [PubMed]
- PetteráLillerud, K. Two Members of the ABC-D6R Family of Zeolites: Zeolite Phi and Linde D. J. Chem. Soc. Faraday Trans. 1994, 90, 1547–1551. [Google Scholar]
- Turrina, A.; Garcia, R.; Watts, A.E.; Greer, H.F.; Bradley, J.; Zhou, W.; Cox, P.A.; Shannon, M.D.; Mayoral, A.; Casci, J.L.; et al. STA-20: An ABC-6 Zeotype Structure Prepared by Co-Templating and Solved via a Hypothetical Structure Database and STEM-ADF Imaging. Chem. Mater. 2017, 29, 2180–2190. [Google Scholar] [CrossRef]
- Wilson, S.T.; Broach, R.W.; Blackwell, C.S.; Bateman, C.A.; McGuire, N.K.; Kirchner, R.M. Synthesis, Characterization and Structure of SAPO-56, a Member of the ABC Double-Six-Ring Family of Materials with Stacking Sequence AABBCCBB. Microporous Mesoporous Mater. 1999, 28, 125–137. [Google Scholar] [CrossRef]
- McCusker, L.B.; Olson, D.H.; Baerlocher, C. Atlas of Zeolite Framework Types; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Dent, L.S.; Smith, J. V Crystal Structure of Chabazite, a Molecular Sieve. Nature 1958, 181, 1794–1796. [Google Scholar] [CrossRef]
- Saxton, C.G.; Kruth, A.; Castro, M.; Wright, P.A.; Howe, R.F. Xenon Adsorption in Synthetic Chabazite Zeolites. Microporous Mesoporous Mater. 2010, 129, 68–73. [Google Scholar] [CrossRef]
- García Salas, R.; Pérez Pariente, J.; Gómez Hortigüela, L.; Martínez Martínez, V.; López Arbeloa, I. Hybrid Photoactive Material, Method for Obtaining Same and Use of the Material. WO2014020225 A1, 2 February 2014. [Google Scholar]
- Chen, Y.; Fu, L.; Xu, X.; Ling Li, I.; Ruan, S.; Jian, D.; Zhai, J. Host-Guest Interaction between Acridine Orange Molecules and AFI or CHA Zeolite Crystals. In Proceedings of the Second International Conference on Photonics and Optical Engineering, Xi’an, China, 14–17 October 2016; SPIE: Bellingham, WA, USA, 2017; pp. 785–792. [Google Scholar]
- Hoppe, R.; Schulz-Ekloff, G.; Wöhrle, D.; Shpiro, E.S.; Tkachenko, O.P. Xps Investigation of Methylene Blue Incorporated into Faujasites and AIPO Family Molecular Sieves. Zeolites 1993, 13, 222–228. [Google Scholar] [CrossRef]
- Shim, T.; Lee, M.H.; Kim, D.; Ouchi, Y. Comparison of Photophysical Properties of the Hemicyanine Dyes in Ionic and Nonionic Solvents. J. Phys. Chem. B 2008, 112, 1906–1912. [Google Scholar] [CrossRef]
- Jee, A.Y.; Bae, E.; Lee, M. Internal Motion of an Electronically Excited Molecule in Viscoelastic Media. J. Chem. Phys. 2010, 133, 014507. [Google Scholar] [CrossRef]
- Jee, A.Y.; Lee, M. Excited-State Dynamics of a Hemicyanine Dye in Polymer Blends. ChemPhysChem 2010, 11, 793–795. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, G.; Wang, J.; Sun, S.; Zhang, Z. The Mechanisms of Large Stokes Shift and Fluorescence Quantum Yields in Anilino Substituted Rhodamine Analogue: TICT and PICT. Comput. Theor. Chem. 2016, 1095, 44–53. [Google Scholar] [CrossRef]
- Brunel, D.; Dumur, F. Recent Advances in Organic Dyes and Fluorophores Comprising a 1,2,3-Triazole Moiety. New J. Chem. 2020, 44, 3546–3561. [Google Scholar] [CrossRef]
- Tavares, T.K.; Almeida, G.; Variani, Y.M.; Bernardo-Gusmao, K.; Vinaches, P.; Alcântara, A.C.S.; Paiva, A.E.M.; Gómez-Hortigüela, L.; Rojas, A. Structure-Directing Study of 1-Methylimidazolium-Based Dication with Tetramethylene as Spacer Length in the Synthesis of Microporous Silicoaluminophosphates. New J. Chem. 2021, 45, 7185–7195. [Google Scholar] [CrossRef]
- Concepción, P.; Nieto, J.M.L.; Mifsud, A.; Pérez-Pariente, J. Preparation and Characterization of Mg-Containing AFI and Chabazite-Type Materials. Zeolites 1996, 16, 56–64. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-Consistent Molecular Orbital Methods. XX. A Basis Set for Correlated Wave Functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Frisch, M.J.; Pople, J.A.; Binkley, J.S. Self-Consistent Molecular Orbital Methods 25. Supplementary Functions for Gaussian Basis Sets. J. Chem. Phys. 1984, 80, 3265–3269. [Google Scholar] [CrossRef]
- Dauber-Osguthorpe, P.; Roberts, V.A.; Osguthorpe, D.J.; Wolff, J.; Genest, M.; Hagler, A.T. Structure and Energetics of Ligand Binding to Proteins: Escherichia coli Dihydrofolate Reductase-trimethoprim, a Drug-receptor System. Proteins Struct. Funct. Bioinform. 1988, 4, 31–47. [Google Scholar] [CrossRef] [PubMed]

| Sample 4-DASPI@MgAPO-AEL | Gel Composition | Dye Uptake | X-ray | ||||
|---|---|---|---|---|---|---|---|
| x (MgO) | y (EBA) | P2O5 | z (DASPI) | mmol/100 g | % | Phase | |
| 1 | 0.20 | 1.00 | 1.00 | 0.024 | 8.8 | 93 | AFI(↑) + AEL(↑) |
| 2 | 0.20 | 0.75 | 1.00 | 0.024 | 3.1 | 27 | AEL(↑) + AFI(↓) |
| 3 | 0.10 | 1.00 | 1.00 | 0.024 | 4.8 | 41 | AFI(↑) + AEL(↑) |
| 4 | 0.10 | 0.75 | 1.00 | 0.024 | 3.8 | 26 | AEL(↑) + AFI(↓) |
| 5 | 0.20 | 0.75 | 1.00 | 0.008 | 1.7 1 | 26 | AEL(↑) + AFI(↓↓) + try |
| 6 | 0.20 | 0.75 | 1.20 | 0.008 | 2.3 | 34 | AEL(↑) + AFI(↓↓↓) |
| 4-DASPI@ | λab (nm) | λfl (nm) | ϕfl | τ (ns) |
|---|---|---|---|---|
| H2O | 329.0 D/449.5 M | 618.5 a | <0.01 | <0.01 |
| MgAPO-CHA | 343.0 D/437.0 M | 583.0 a | 0.33 | 2.73 |
| MgAPO-AEL-1 | * | 610.0 a | 0.10 | 0.13 (20%) 3.07 (80%) |
| MgAPO-AEL-6 | * | 613.0 a | 0.41 | 3.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliden-Sánchez, A.; Sola-Llano, R.; Pérez-Pariente, J.; Gómez-Hortigüela, L.; Martínez-Martínez, V. Confinement of a Styryl Dye into Nanoporous Aluminophosphates: Channels vs. Cavities. Int. J. Mol. Sci. 2024, 25, 3577. https://doi.org/10.3390/ijms25073577
Oliden-Sánchez A, Sola-Llano R, Pérez-Pariente J, Gómez-Hortigüela L, Martínez-Martínez V. Confinement of a Styryl Dye into Nanoporous Aluminophosphates: Channels vs. Cavities. International Journal of Molecular Sciences. 2024; 25(7):3577. https://doi.org/10.3390/ijms25073577
Chicago/Turabian StyleOliden-Sánchez, Ainhoa, Rebeca Sola-Llano, Joaquín Pérez-Pariente, Luis Gómez-Hortigüela, and Virginia Martínez-Martínez. 2024. "Confinement of a Styryl Dye into Nanoporous Aluminophosphates: Channels vs. Cavities" International Journal of Molecular Sciences 25, no. 7: 3577. https://doi.org/10.3390/ijms25073577
APA StyleOliden-Sánchez, A., Sola-Llano, R., Pérez-Pariente, J., Gómez-Hortigüela, L., & Martínez-Martínez, V. (2024). Confinement of a Styryl Dye into Nanoporous Aluminophosphates: Channels vs. Cavities. International Journal of Molecular Sciences, 25(7), 3577. https://doi.org/10.3390/ijms25073577







