Synthesis and Crystal Structure of the Europium(II) Hydride Oxide Iodide Eu5H2O2I4 Showing Blue-Green Luminescence
Abstract
:1. Introduction
2. Results and Discussion
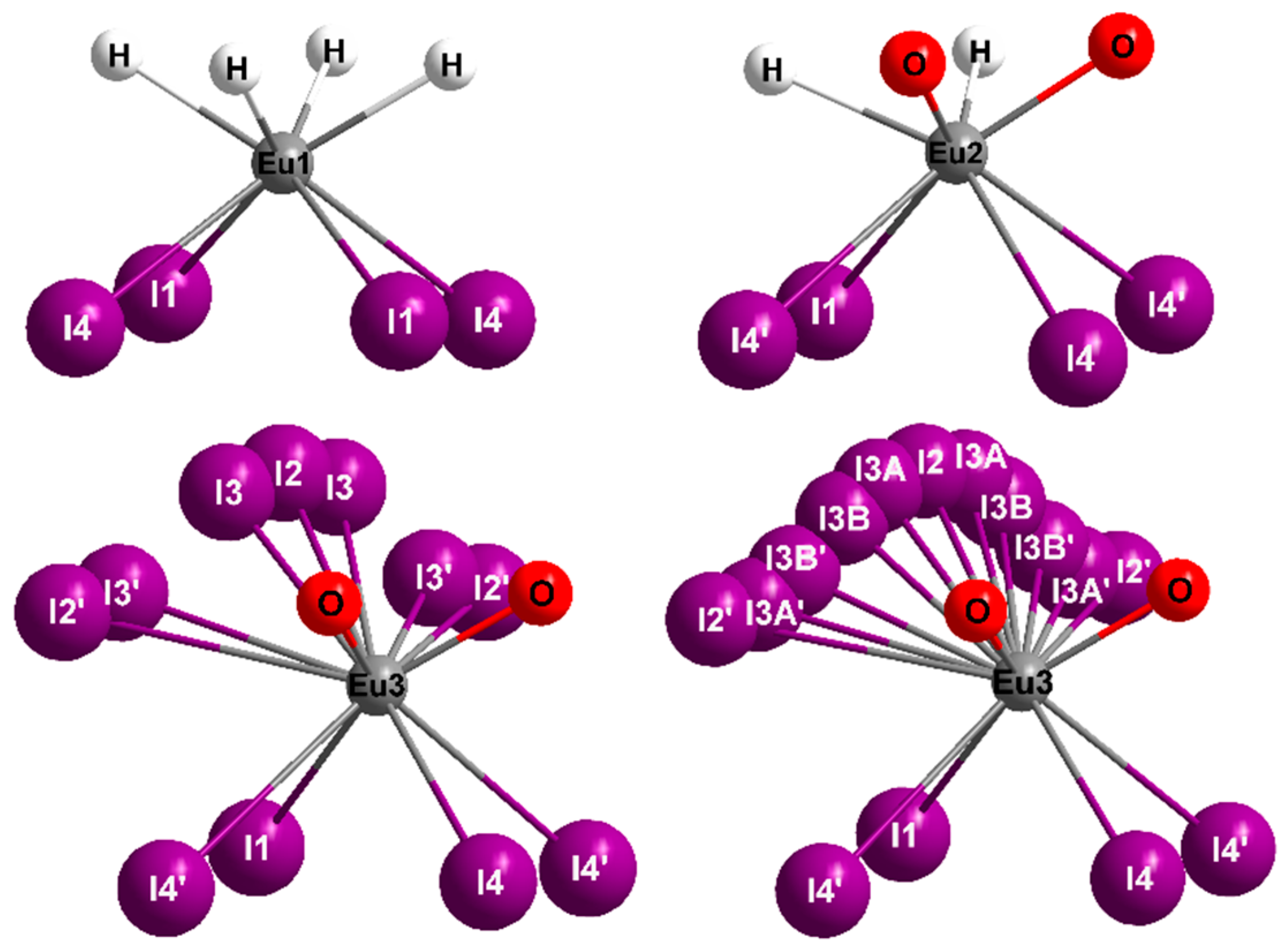
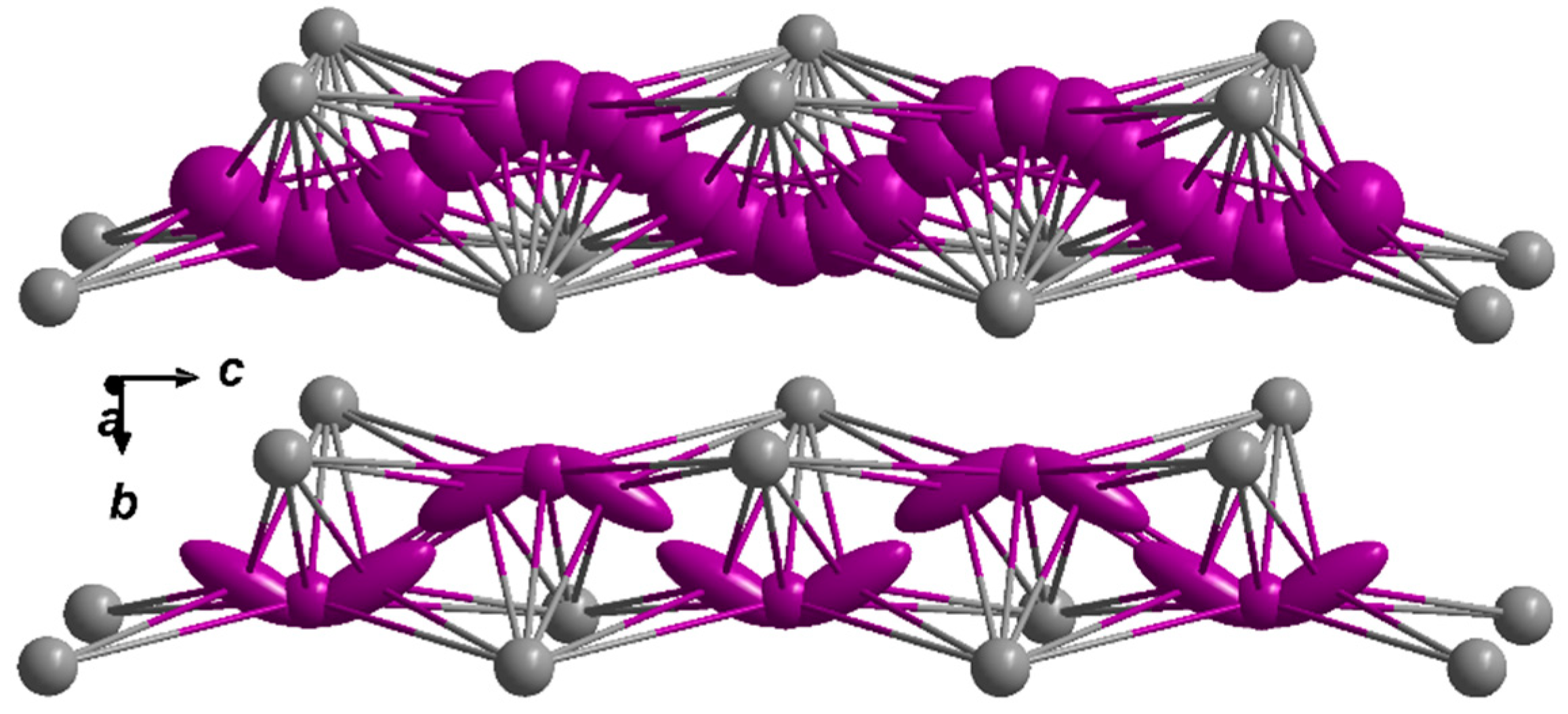
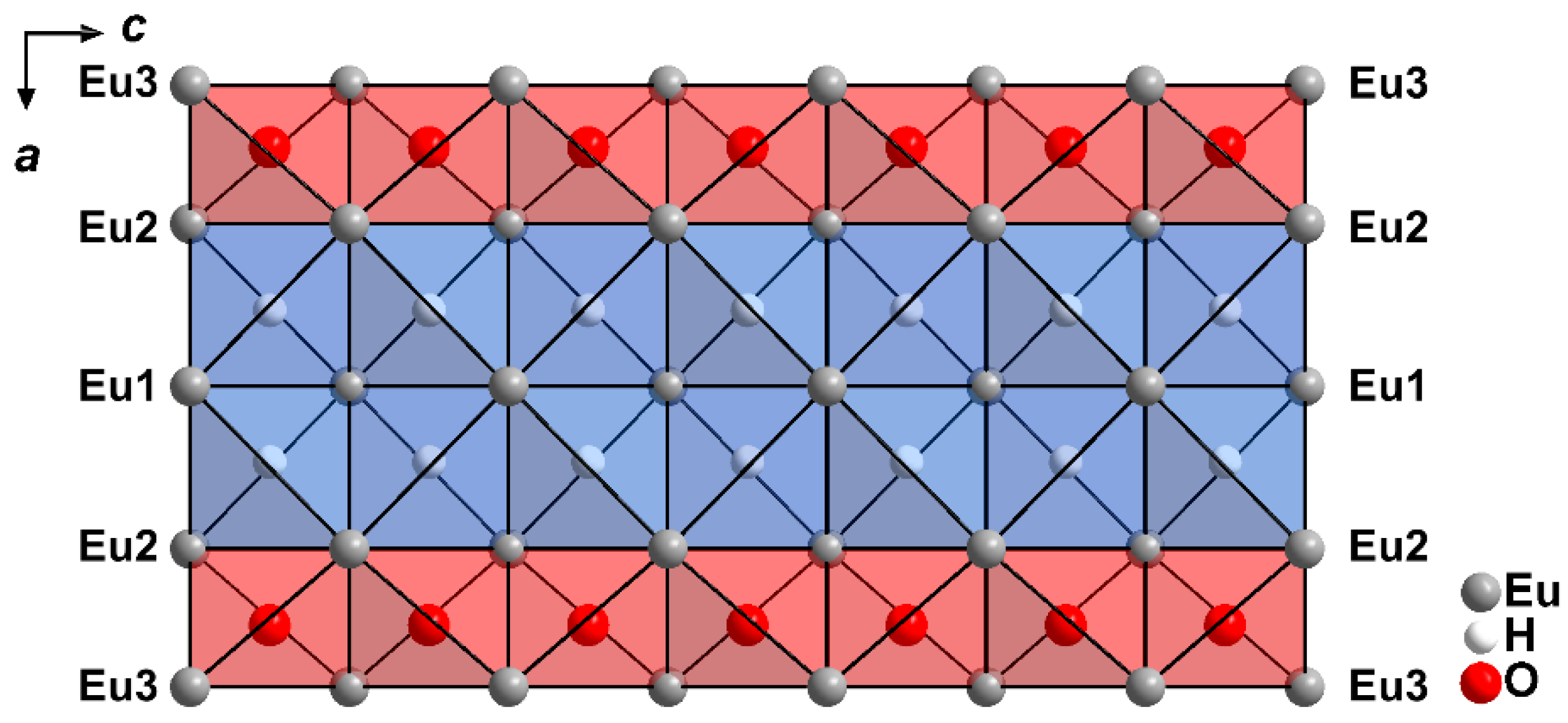
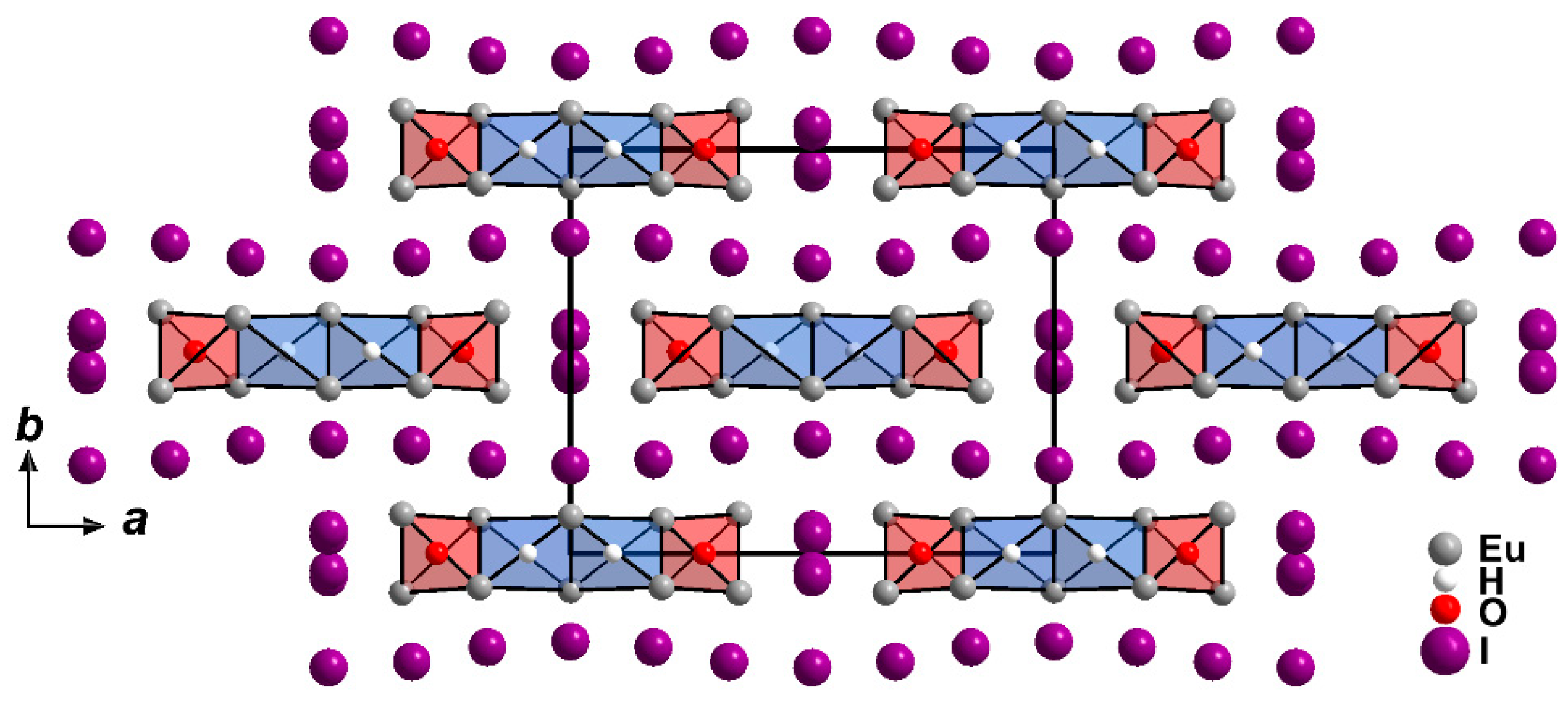
2.1. Crystal Structure
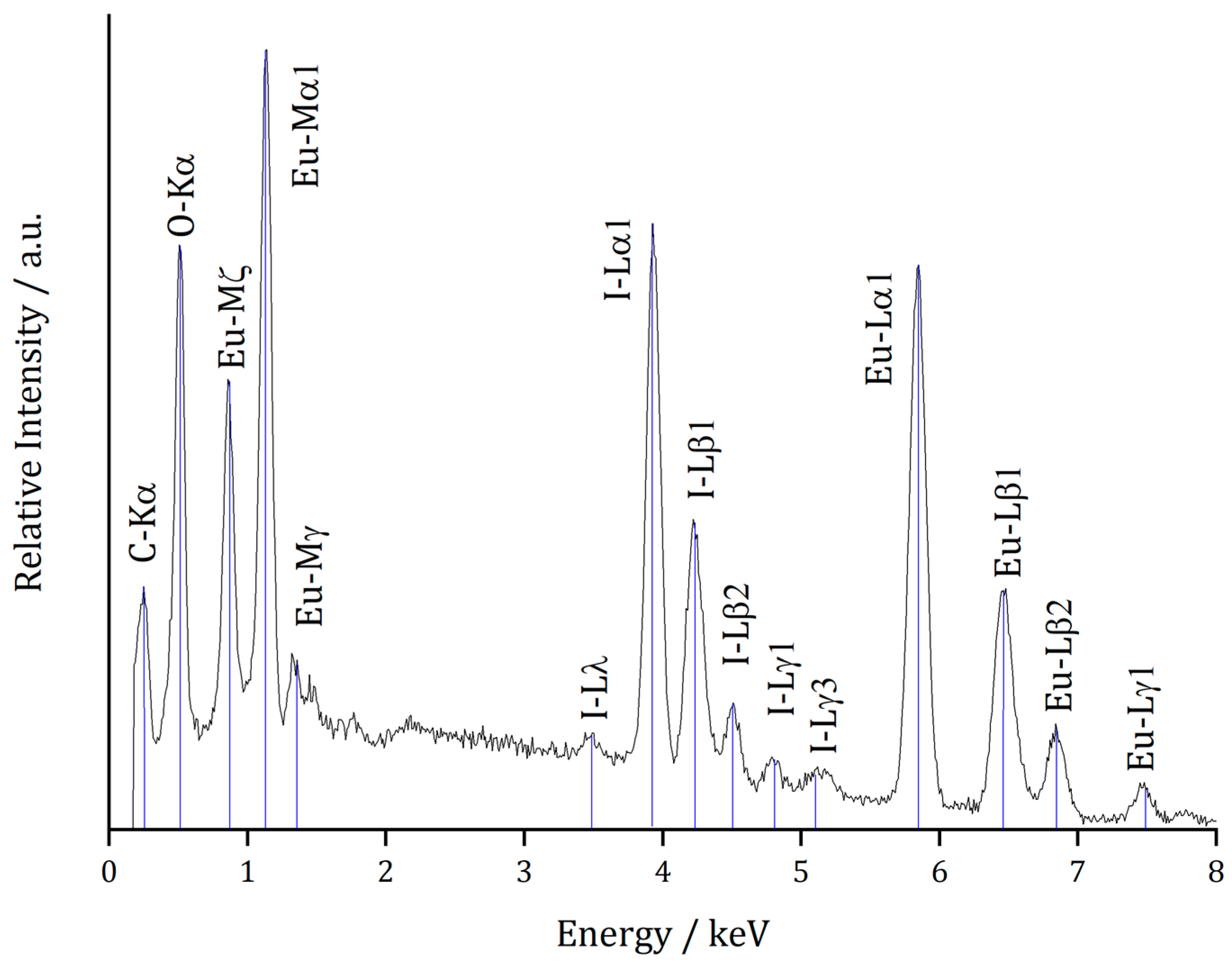
2.2. Microprobe Analyses
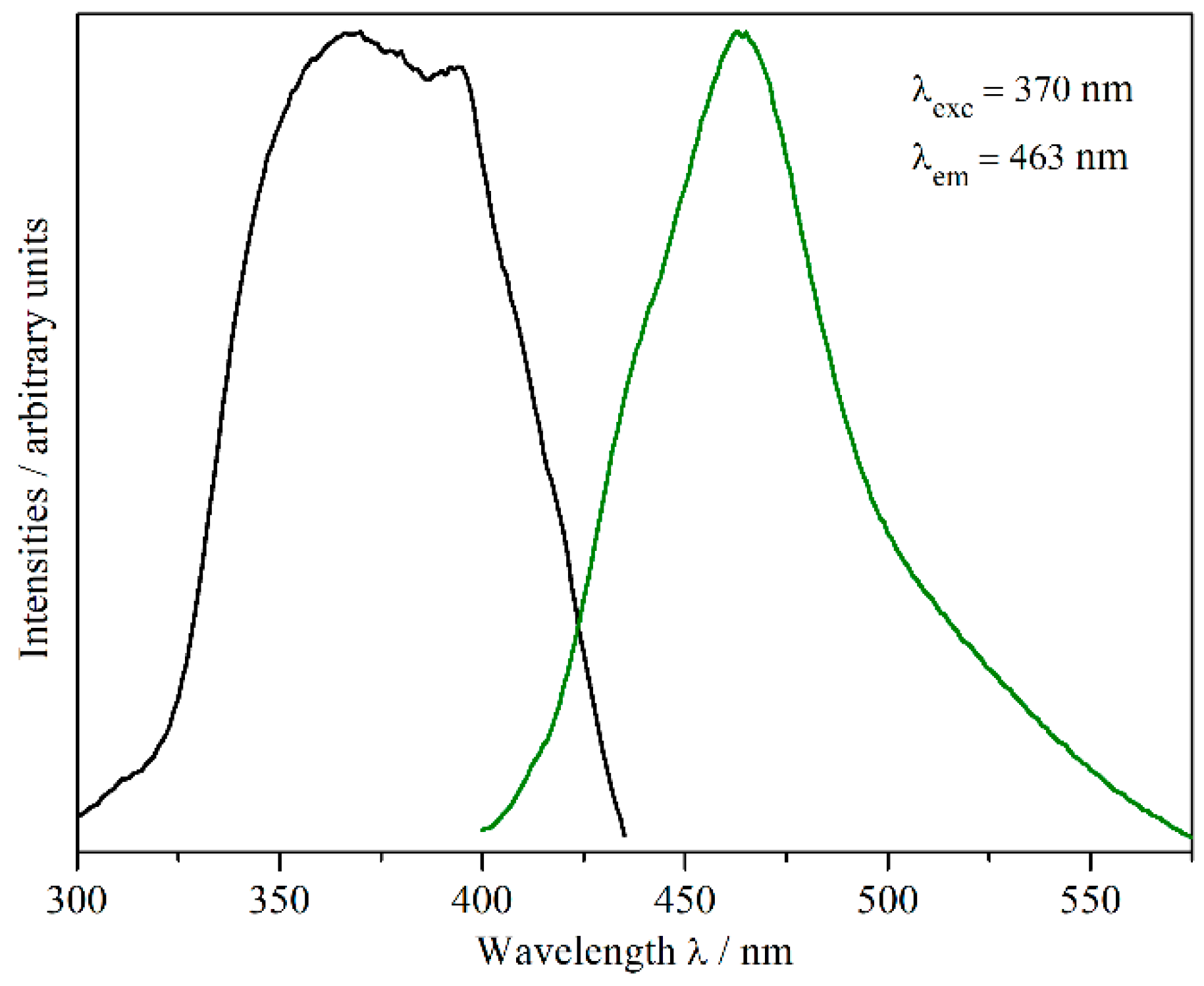
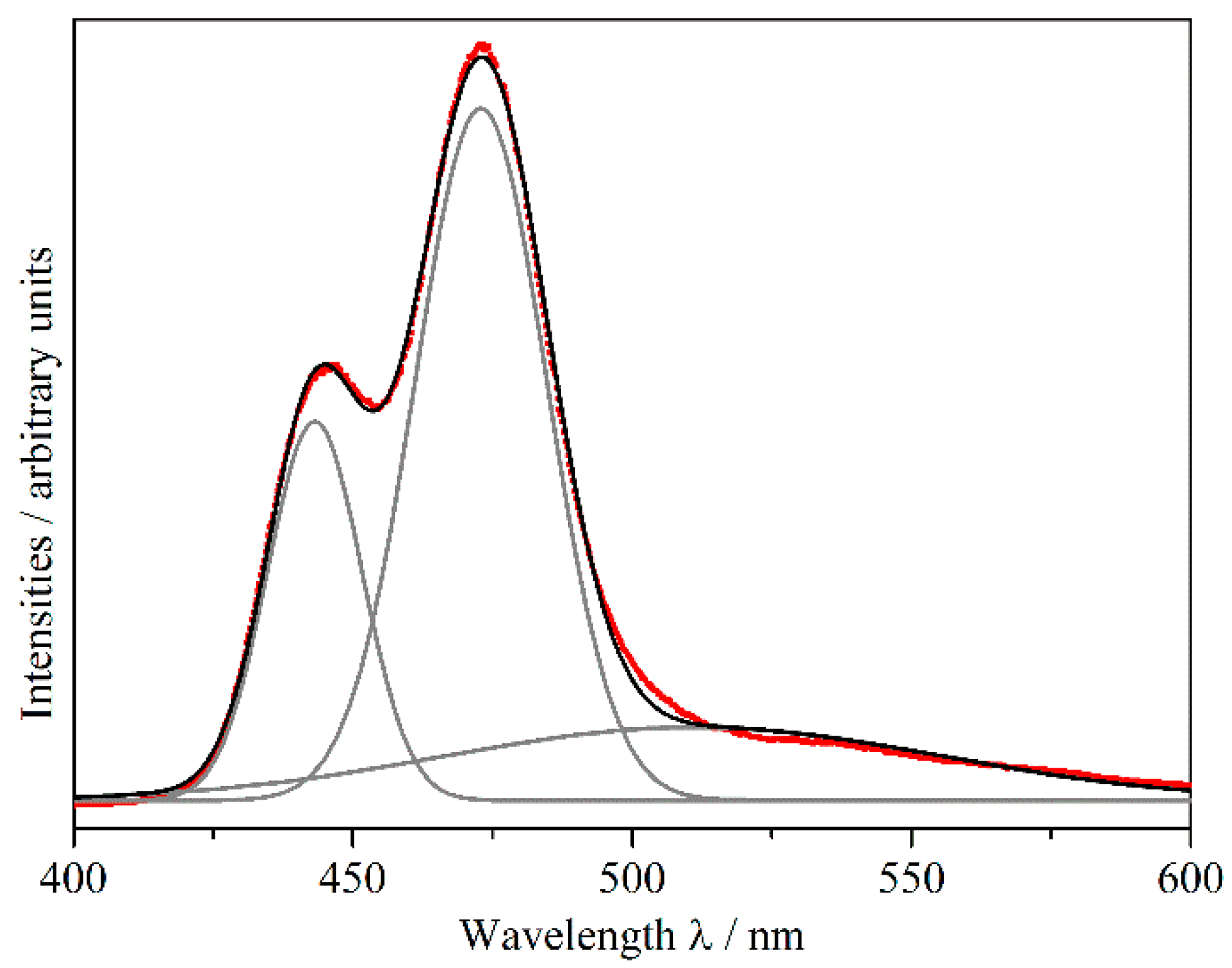
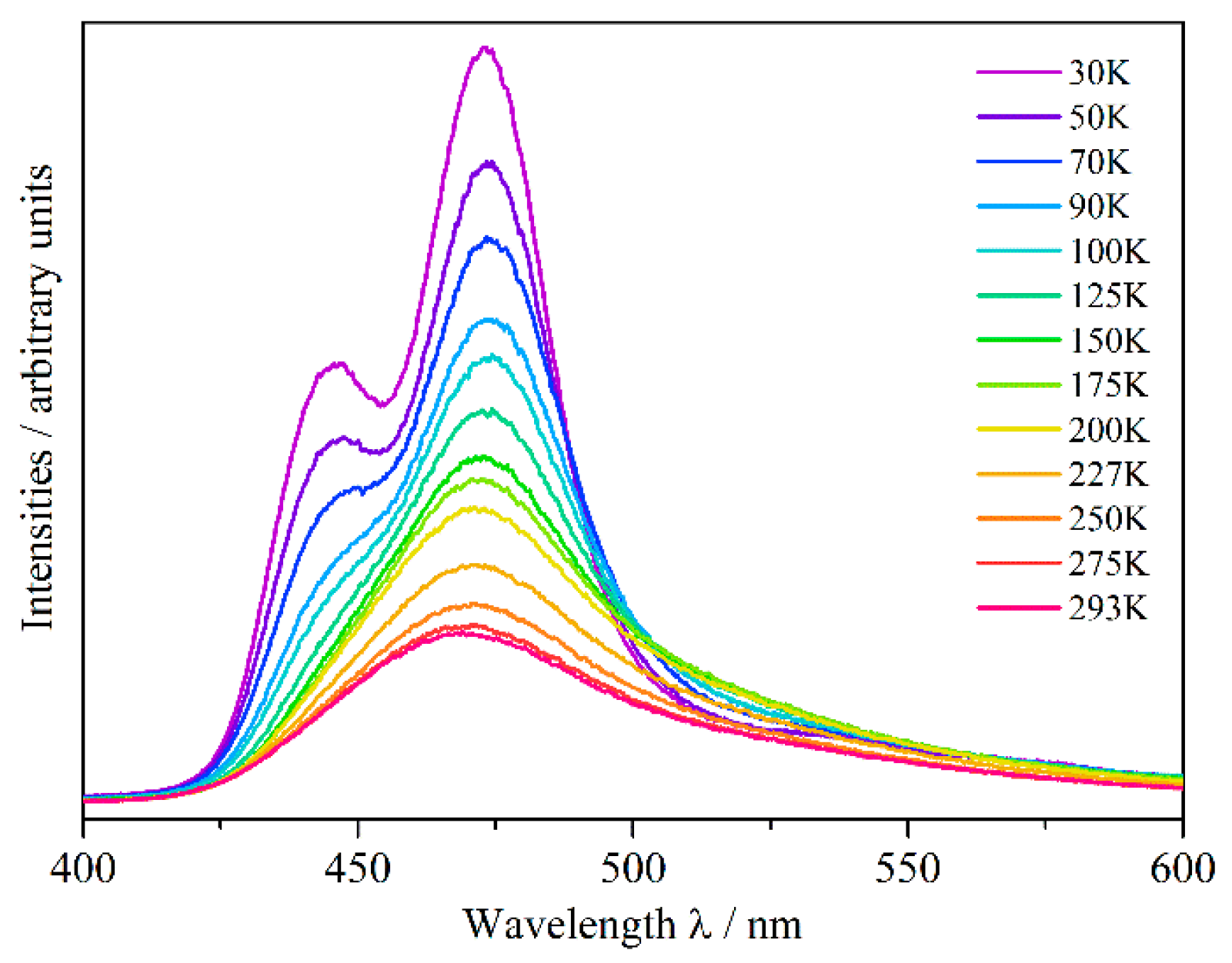
2.3. Luminescence
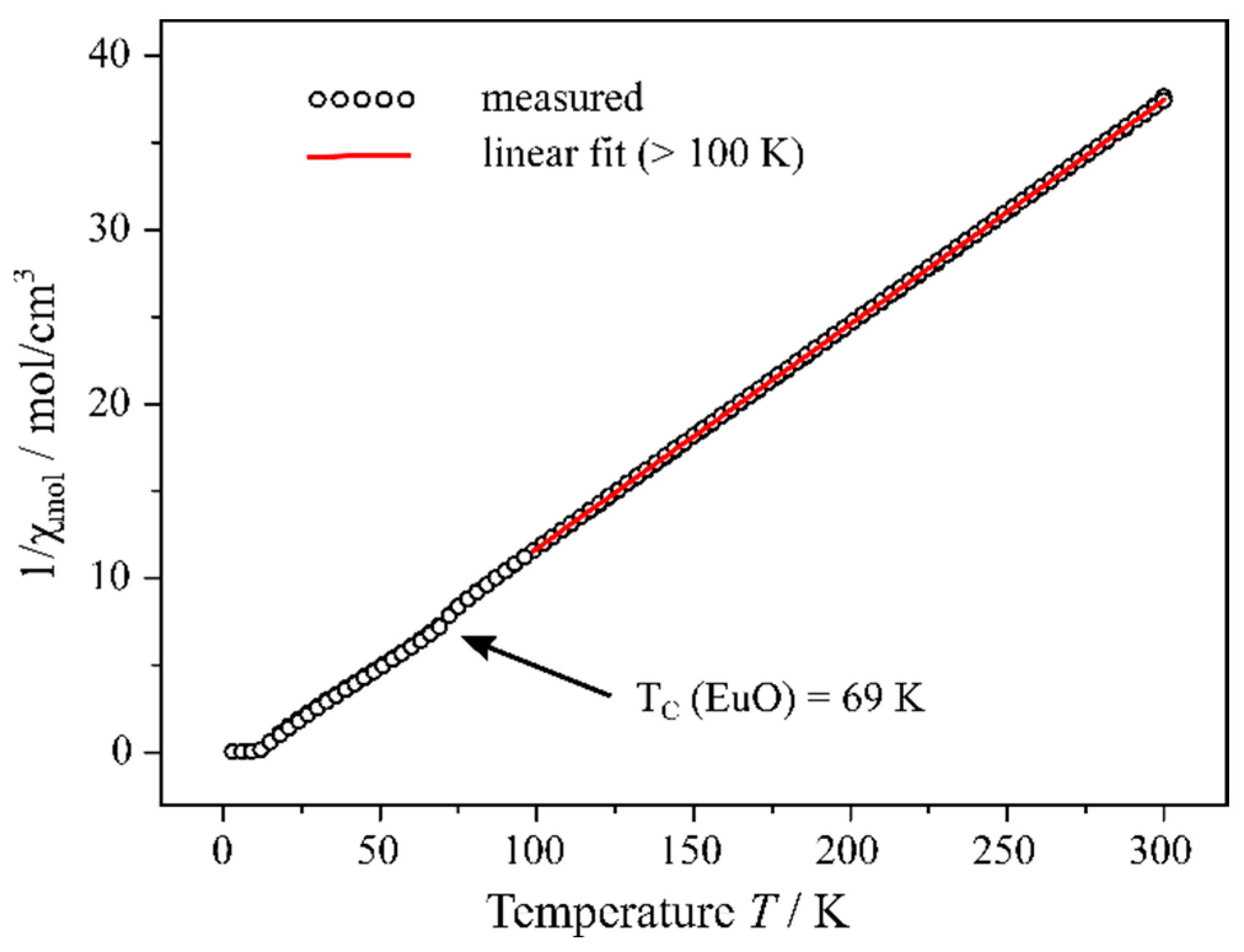
2.4. Magnetism
3. Experimental Procedure
3.1. Motivation
3.2. Synthesis
3.3. X-ray Diffraction
3.4. Microprobe
3.5. Luminescence
3.6. Magnetism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carter, F.L. Certain Mixed Hydride-Chalcogenide Rare Earth-Compounds. Met. Soc. Conf. 1962, 15, 245–261. [Google Scholar]
- Brice, J.F.; Moreau, A. Synthese et conductivite anionique des hydruro-oxydes de lanthane de formule LaHO, LaH1+2xO1−x et LaH1+yO1−x (y <2x). Ann. Chim. Sci. Mater. 1982, 7, 623–634. [Google Scholar]
- Malaman, B.; Brice, J.F. Etude structurale de l’hydruro-oxyde LaHO par diffraction des rayons X et par diffraction des neutrons. J. Solid State Chem. 1984, 53, 44–54. [Google Scholar] [CrossRef]
- Widerøe, M.; Fjellvåg, H.; Norby, T.; Poulsen, F.W.; Berg, R.W. NdHO, a novel oxyhydride. J. Solid State Chem. 2011, 184, 1890–1894. [Google Scholar] [CrossRef]
- Zapp, N.; Kohlmann, H. The lanthanide hydride oxides SmHO and HoHO. Z. Naturforsch. 2018, 73b, 535–538. [Google Scholar] [CrossRef]
- Yamashita, H.; Broux, T.; Kobayashi, Y.; Takeiri, F.; Ubukata, H.; Zhu, T.; Hayward, M.A.; Fujii, K.; Yashima, M.; Shitara, K.; et al. Chemical pressure-induced anion order–disorder transition in LnHO enabled by hydride size flexibility. J. Am. Chem. Soc. 2018, 140, 11170–11173. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, H. Neuartige Hydrid-Oxide der Seltenen Erden: Ln2LiHO3 mit Ln = La, Ce, Pr und Nd. Ph.D. Thesis, Universität Karlsruhe, Karlsruhe, Germany, 1991. [Google Scholar]
- Kobayashi, G.; Hinuma, Y.; Matsuoka, S.; Watanabe, A.; Iqbal, M.; Hirayama, M.; Yonemura, M.; Kamiyama, T.; Tanaka, I.; Kanno, R. Pure H− conduction in oxyhydrides. Science 2016, 351, 1314–1317. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Kobayashi, G.; Matsui, N.; Yonemura, M.; Kubota, A.; Suzuki, K.; Hirayama, M.; Kanno, R. Ambient pressure synthesis and H− conductivity of LaSrLiH2O2. Electrochem. 2017, 85, 88–92. [Google Scholar] [CrossRef]
- Fjellvåg, Ø.S.; Armstrong, J.; Sjåstad, A.O. Thermal and Structural Aspects of the Hydride-Conducting Oxyhydride La2LiHO3 Obtained via a Halide Flux Method. Inorg. Chem. 2017, 56, 11123–11128. [Google Scholar] [CrossRef]
- Fjellvåg, Ø.S.; Armstrong, J.; Vajeeston, P.; Sjåstad, A.O. New Insights into Hydride Bonding, Dynamics, and Migration in La2LiHO3 Oxyhydride. J. Phys. Chem. Lett. 2018, 9, 353–358. [Google Scholar] [CrossRef]
- Kageyama, H.; Hayashi, K.; Maeda, K.; Attfield, P.J.; Hiroi, Z.; Rondinelli, J.M.; Poeppelmeier, K.R. Expanding frontiers in materials chemistry and physics with multiple anions. Nat. Commun. 2018, 9, 772. [Google Scholar] [CrossRef]
- Kunkel, N.; Wylezich, T. Recent Advances in Rare Earth-Doped Hydrides. Z. Anorg. Allg. Chem. 2019, 645, 137–145. [Google Scholar] [CrossRef]
- Rudolph, D.; Enseling, D.; Jüstel, T.; Schleid, T. Crystal Structure and Luminescence Properties of the First Hydride Oxide Chloride with Divalent Europium: LiEu2HOCl2. Z. Anorg. Allg. Chem. 2017, 643, 1525–1530. [Google Scholar] [CrossRef]
- Kunkel, N.; Meijerink, A.; Kohlmann, H. Variation of the EuII Emission Wavelength by Substitution of Fluoride by Hydride in Fluorite-Type Compounds EuHxF2–x (0.20 ≤ x ≤ 0.67). Inorg. Chem. 2014, 53, 4800–4802. [Google Scholar] [CrossRef]
- Kunkel, N.; Kohlmann, H. Ionic mixed hydride fluoride compounds: Stabilities predicted by DFT, synthesis, and luminescence of divalent europium. J. Phys. Chem. C 2016, 120, 10506–10511. [Google Scholar] [CrossRef]
- Kunkel, N.; Rudolph, D.; Meijerink, A.; Rommel, S.; Weihrich, R.; Kohlmann, H.; Schleid, T. Green luminescence of divalent europium in the hydride chloride EuHCl. Z. Anorg. Allg. Chem. 2015, 641, 1220–1224. [Google Scholar] [CrossRef]
- Rudolph, D. Strukturelle und Spektroskopische Untersuchungen an Gemischtanionischen Hydriden und Oxidhalogeniden der Seltenerdmetalle. Ph.D. Thesis, Universität Stuttgart, Stuttgart, Germany, 2018. [Google Scholar]
- Rudolph, D.; Wylezich, T.; Sontakke, A.D.; Meijerink, A.; Goldner, P.; Netzsch, P.; Höppe, H.A.; Kunkel, N.; Schleid, T. Synthesis and optical properties of the Eu2+-doped alkaline-earth metal hydride chlorides AE7H12Cl2 (AE= Ca and Sr). J. Lumin. 2019, 209, 150–155. [Google Scholar] [CrossRef]
- Ueda, J.; Matsuishi, S.; Tokunaga, T.; Tanabe, S. Preparation, electronic structure of gadolinium oxyhydride and low-energy 5d excitation band for green luminescence of doped Tb3+ ions. J. Mater. Chem. C 2018, 6, 7541–7548. [Google Scholar] [CrossRef]
- Höppe, H.A. Recent developments in the field of inorganic phosphors. Angew. Chem. Int. Ed. 2009, 48, 3572–3582. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Xiao, F.; Pan, X.Y.; Ma, Y.Y.; Zhang, Q.Y. Phosphors in phosphor-converted white light-emitting diodes: Recent advances in materials, techniques and properties. Mater. Sci. Eng. 2010, R71, 1–34. [Google Scholar] [CrossRef]
- Pust, P.; Schmidt, P.J.; Schnick, W. A revolution in lighting. Nat. Mater. 2015, 15, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Wylezich, T.; Welinski, S.; Hoelzel, M.; Goldner, P.; Kunkel, N. Lanthanide luminescence as a local probe in mixed anionic hydrides – a case study on Eu2+-doped RbMgHxF3−x and KMgHxF3−x. J. Mater. Chem. C 2018, 6, 13006–13012. [Google Scholar] [CrossRef]
- Brites, C.D.S.; Millán, A.; Carlos, L.D. Handbook on the Physics and Chemistry of Rare Earths, Volume 49, Chapter 281 – Lanthanides in Luminescent Thermometry; Bünzli, J.-C., Pecharsky, V.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 339–427. [Google Scholar]
- Wang, X.; Liu, Q.; Bu, Y.; Liu, C.-S.; Liu, T.; Yan, X. Optical temperature sensing of rare-earth ion doped phosphors. RSC Adv. 2015, 5, 86219–86236. [Google Scholar] [CrossRef]
- Chen, D.; Wang, Z.; Zhou, Y.; Huang, P.; Ji, Z. Tb3+/Eu3+: YF3 nanophase embedded glass ceramics: Structural characterization, tunable luminescence and temperature sensing behavior. J. Alloys Compd. 2015, 646, 339–344. [Google Scholar] [CrossRef]
- Dong, H.; Sun, L.-D.; Yan, C.-H. Energy transfer in lanthanide upconversion studies for extended optical applications. Chem. Soc. Rev. 2015, 44, 1608–1634. [Google Scholar] [CrossRef]
- Wang, X.; Xu, T.; Cai, P.; Vu, T.; Seo, H.J. Controlled synthesis, multicolor luminescence, and optical thermometer of bifunctional NaYbF4:Nd3+ @ NaYF4:Yb3+ active-core/active-shell colloidal nanoparticles. J. Alloys Compd. 2017, 691, 530–536. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Yu, J.; Bu, Y.; Yan, X. Modifying phase, shape and optical thermometry of NaGdF4:2%Er3+ phosphors through Ca2+ doping. Opt. Expr. 2018, 26, 21950–21959. [Google Scholar] [CrossRef]
- Reckeweg, O.; DiSalvo, F.J. Alkaline Earth Metal-Hydride-Iodide Compounds: Syntheses and Crystal Structures of Sr2H3I and Ba5H2I3.9(2)O2. Z. Naturforsch. 2011, 66b, 21–26. [Google Scholar]
- Beck, H.P.; Limmer, A. Zur Kenntnis von Hydridhalogeniden MHX der Seltenen Erden Eu, Yb und Sm (X = Cl, Br, I). Z. Naturforsch. 1982, 37b, 574–578. [Google Scholar] [CrossRef]
- Reckeweg, O.; Weber, F.A.; Blaschkowski, B.; Schleid, T. Eu2H3Br and Eu2O2S: A Structural Comparison of Two Ternary Mixed-Anion Europium Compounds. Z. Anorg. Allg. Chem. 2014, 640, 2354. [Google Scholar]
- Rudolph, D.; Schleid, T. Eu2H3I: A New Hydride Iodide with Divalent Europium. Z. Anorg. Allg. Chem. 2016, 642, 1036. [Google Scholar]
- Rudolph, D.; Bohem, M.E.; Schleid, T. Structural Comparison of the Matlockite-Type Pair EuFBr and EuHBr. Z. Kristallogr. 2017, 37, 106. [Google Scholar]
- Liao, W.; Dronskowski, R. Europium(II) oxyiodide. Acta Crystallogr. 2004, C60, 23–24. [Google Scholar]
- Rudolph, D.; Schleid, T. Die Europium(II)-Oxidhalogenide Eu2OBr2 und Eu2OI2. Z. Naturforsch. 2017, 72b, 795–799. [Google Scholar] [CrossRef]
- Bertolotti, F.; Shishkina, A.V.; Forni, A.; Gervasio, G.; Stash, A.; Tsirelson, V.G. Intermolecular bonding features in solid iodine. Cryst. Growth Des. 2014, 14, 3587–3595. [Google Scholar] [CrossRef]
- Dorenbos, P. Energy of the first 4f7→4f65d transition of Eu2+ in inorganic compounds. J. Lumin. 2003, 104, 239–260. [Google Scholar] [CrossRef]
- Ronda, C.R. Luminescence; Ronda, C.R., Ed.; Wiley-VCH: Weinheim, Germany, 2008; pp. 27–28. [Google Scholar]
- Kunkel, N.; Meijerink, A.; Kohlmann, H. Bright yellow and green Eu(II) luminescence and vibronic fine structures in LiSrH3, LiBaH3 and their corresponding deuterides. Phys. Chem. Chem. Phys. 2014, 16, 4807–4813. [Google Scholar] [CrossRef] [PubMed]
- Linn, D.E., Jr.; Gibbins, S.G. Solution Spectroscopic and Chemical Properties of the Complex Hydride [FeH6]4–. Inorg. Chem. 1997, 36, 3461–3465. [Google Scholar] [CrossRef]
- Hsu, D.; Skinner, J.L. Nonperturbative theory of temperature-dependent optical dephasing in crystals. I. Acoustic or optical phonons. J. Chem. Phys. 1984, 81, 5471–5479. [Google Scholar] [CrossRef]
- Mikhailik, V.B.; Kraus, H.; Wahl, D.; Itoh, M.; Koike, M.; Bailiff, I.K. One- and two-photon excited luminescence and band-gap assignment in CaWO4. Phys. Rev. B 2004, 69, 205110. [Google Scholar] [CrossRef]
- Kim, J.S.; Park, Y.H.; Kim, S.M.; Choi, J.C.; Park, H.L. Temperature-dependent emission spectra of M2SiO4:Eu2+ (M = Ca, Sr, Ba) phosphors for green and greenish white LEDs. Solid State Commun. 2005, 133, 445–448. [Google Scholar] [CrossRef]
- Kunkel, N.; Kohlmann, H.; Sayede, A.; Springborg, M. Alkaline-Earth Metal Hydrides as Novel Host Lattices for EuII Luminescence. Inorg. Chem. 2011, 50, 5873–5875. [Google Scholar] [CrossRef] [PubMed]
- Wood, D.L.; Ferguson, J.; Knox, K.; Dillon, J.F., Jr. Crystal-Field Spectra of d3,7 Ions. III. Spectrum of Cr3+ in Various Octahedral Crystal Fields. J. Chem. Phys. 1963, 39, 890–898. [Google Scholar] [CrossRef]
- Orchard, A.F. Magnetochemistry; Oxford University Press: Oxford, UK, 2003; pp. 98–99. [Google Scholar]
- Kornblit, A.; Ahlers, G. Heat capacity of EuO near the Curie temperature. Phys. Rev. B 1975, 11, 2678–2688. [Google Scholar] [CrossRef]
- Otwinowski, Z.; Minor, W. Methods in Enzymology, Vol. 276, Macromolecular Crystallography, Part A; Carter, C.W., Sweet, R.M., Eds.; Academic Press: New York, NY, USA, 1997; pp. 307–326. [Google Scholar]
- Sheldrick, G.M. Programs for Crystal Structure Determination; Universität Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. SHELX-97. Acta Crystallogr. 2007, A64, 112–122. [Google Scholar]


| Chemical formula | Eu5H2O2I4 | |
| mol−1 | 1301.41 | |
| Crystal system | orthorhombic | |
| Space group | Cmcm (no. 63) | |
| Measuring temperature, T/K | 100 (2) | 293 (2) |
| a/pm | 1636.97 (9) | 1642.51 (9) |
| b/pm | 1369.54 (8) | 1374.23 (8) |
| c/pm | 604.36 (4) | 606.58 (4) |
| mol−1 | 203.98 (2) | 206.15 (2) |
| Number of formula units, Z | 4 | |
| Number of measured reflections | 2931 | 1618 |
| Number of independent reflections | 1645 | 921 |
| wR2 | 0.092 | 0.106 |
| R1 | 0.042 | 0.041 |
| Goodness of Fit | 1.090 | 1.068 |
| CSD number | 434116 | 434115 |
| Atom | Site | s. o. f. | x/a | y/b | z/c |
|---|---|---|---|---|---|
| Eu1 | 4c | 1 | 0 | 0.90892 (4) | 1/4 |
| Eu2 | 8g | 1 | 0.18814 (2) | 0.08380 (3) | 1/4 |
| Eu3 | 8g | 1 | 0.15203 (2) | 0.40322 (3) | 1/4 |
| H | 8e | 1 | 0.090 (7) | 0 | 0 |
| O | 8e | 1 | 0.2762 (3) | 0 | 0 |
| I1 | 4c | 1 | 0 | 0.21783 (6) | 1/4 |
| I2 | 4c | 0.232 (15) | 0 | 0.5589 (4) | 1/4 |
| I3 | 8f | 0.377 (9) | 0 | 0.5436 (4) | 0.1192 (15) |
| I4 | 8g | 1 | 0.32809 (3) | 0.26778 (4) | 1/4 |
| Eu1 | 4c | 1 | 0 | 0.90947 (6) | 1/4 |
| Eu2 | 8g | 1 | 0.18819 (4) | 0.08329 (4) | 1/4 |
| Eu3 | 8g | 1 | 0.15234 (4) | 0.40378 (4) | 1/4 |
| H | 8e | 1 | 0.089 (9) | 0 | 0 |
| O | 8e | 1 | 0.2766 (5) | 0 | 0 |
| I1 | 4c | 1 | 0 | 0.21795 (9) | 1/4 |
| I2 | 4c | 0.284 (11) | 0 | 0.5601 (5) | 1/4 |
| I3A | 8f | 0.212 (8) | 0 | 0.5503 (4) | 0.1422 (11) |
| I3B | 8f | 0.150 (7) | 0 | 0.5234 (7) | 0.0577 (16) |
| I4 | 8g | 1 | 0.32802 (5) | 0.26771 (7) | 1/4 |
| Atom | U11 | U22 | U33 | U23 | U13 | U12 | Ueq |
|---|---|---|---|---|---|---|---|
| Eu1 | 62 (2) | 25 (2) | 133 (3) | 0 | 0 | 0 | 73 (1) |
| Eu2 | 43 (2) | 8 (2) | 98 (2) | 0 | 0 | 7 (1) | 50 (1) |
| Eu3 | 31 (2) | 31 (2) | 14 (1) | 0 | 0 | −11 (1) | 67 (1) |
| H | – | – | – | – | – | – | 191 (b) |
| O | 67 (22) | 38 (22) | 125 (26) | −7 (20) | 0 | 76 (10) | 76 (10) |
| I1 | 45 (3) | 100 (3) | 99 (3) | 0 | 0 | 0 | 81 (2) |
| I2 | 130 (18) | 176 (20) | 196 (52) | 0 | 0 | 0 | 167 (21) |
| I3 | 142 (9) | 391 (17) | 888 (51) | 417 (27) | 0 | 0 | 474 (21) |
| I4 | 49 (2) | 58 (2) | 120 (2) | 0 | 0 | −29 (2) | 76 (1) |
| Eu1 | 131 (5) | 123 (4) | 262 (5) | 0 | 0 | 0 | 172 (3) |
| Eu2 | 113 (4) | 87 (4) | 224 (4) | 0 | 0 | 7 (2) | 141 (2) |
| Eu3 | 117 (4) | 133 (4) | 294 (4) | 0 | 0 | −32 (2) | 181 (3) |
| H | – | – | – | – | – | – | 516 (b) |
| O | 210 (40) | 160 (40) | 260 (40) | −60 (30) | 0 | 0 | 206 (18) |
| I1 | 167 (6) | 218 (6) | 262 (6) | 0 | 0 | 0 | 216 (3) |
| I2 | – | – | – | – | – | – | 394 (25) (c) |
| I3A | – | – | – | – | – | – | 267 (20) (c) |
| I3B | – | – | – | – | – | – | 402 (29) (c) |
| I4 | 160 (4) | 186 (5) | 328 (5) | 0 | 0 | −53 (3) | 225 (3) |
| Ion | Emission Line (Standard) | Content/wt.-% | Normalized Content/at.-% |
|---|---|---|---|
| Eu2+ | Lα (Eu[PO4]) | 39.8 (2) | 41.7 (6) |
| I− | Lα (KI) | 26.3 (2) | 33.1 (4) |
| O2− | – | 5.9 (4) | 25.2 (2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudolph, D.; Wylezich, T.; Netzsch, P.; Blaschkowski, B.; Höppe, H.A.; Goldner, P.; Kunkel, N.; Hoslauer, J.-L.; Schleid, T. Synthesis and Crystal Structure of the Europium(II) Hydride Oxide Iodide Eu5H2O2I4 Showing Blue-Green Luminescence. Int. J. Mol. Sci. 2023, 24, 14969. https://doi.org/10.3390/ijms241914969
Rudolph D, Wylezich T, Netzsch P, Blaschkowski B, Höppe HA, Goldner P, Kunkel N, Hoslauer J-L, Schleid T. Synthesis and Crystal Structure of the Europium(II) Hydride Oxide Iodide Eu5H2O2I4 Showing Blue-Green Luminescence. International Journal of Molecular Sciences. 2023; 24(19):14969. https://doi.org/10.3390/ijms241914969
Chicago/Turabian StyleRudolph, Daniel, Thomas Wylezich, Philip Netzsch, Björn Blaschkowski, Henning A. Höppe, Philippe Goldner, Nathalie Kunkel, Jean-Louis Hoslauer, and Thomas Schleid. 2023. "Synthesis and Crystal Structure of the Europium(II) Hydride Oxide Iodide Eu5H2O2I4 Showing Blue-Green Luminescence" International Journal of Molecular Sciences 24, no. 19: 14969. https://doi.org/10.3390/ijms241914969
APA StyleRudolph, D., Wylezich, T., Netzsch, P., Blaschkowski, B., Höppe, H. A., Goldner, P., Kunkel, N., Hoslauer, J.-L., & Schleid, T. (2023). Synthesis and Crystal Structure of the Europium(II) Hydride Oxide Iodide Eu5H2O2I4 Showing Blue-Green Luminescence. International Journal of Molecular Sciences, 24(19), 14969. https://doi.org/10.3390/ijms241914969








