Biodiesel Production by Methanolysis of Rapeseed Oil—Influence of SiO2/Al2O3 Ratio in BEA Zeolite Structure on Physicochemical and Catalytic Properties of Zeolite Systems with Alkaline Earth Oxides (MgO, CaO, SrO)
Abstract
1. Introduction
2. Results and Discussion
2.1. Transesterification of Vegetable Oil with Methanol Reaction
2.2. Specific Surface Area Measurements of the Catalytic Materials—Brunauer–Emmett–Teller
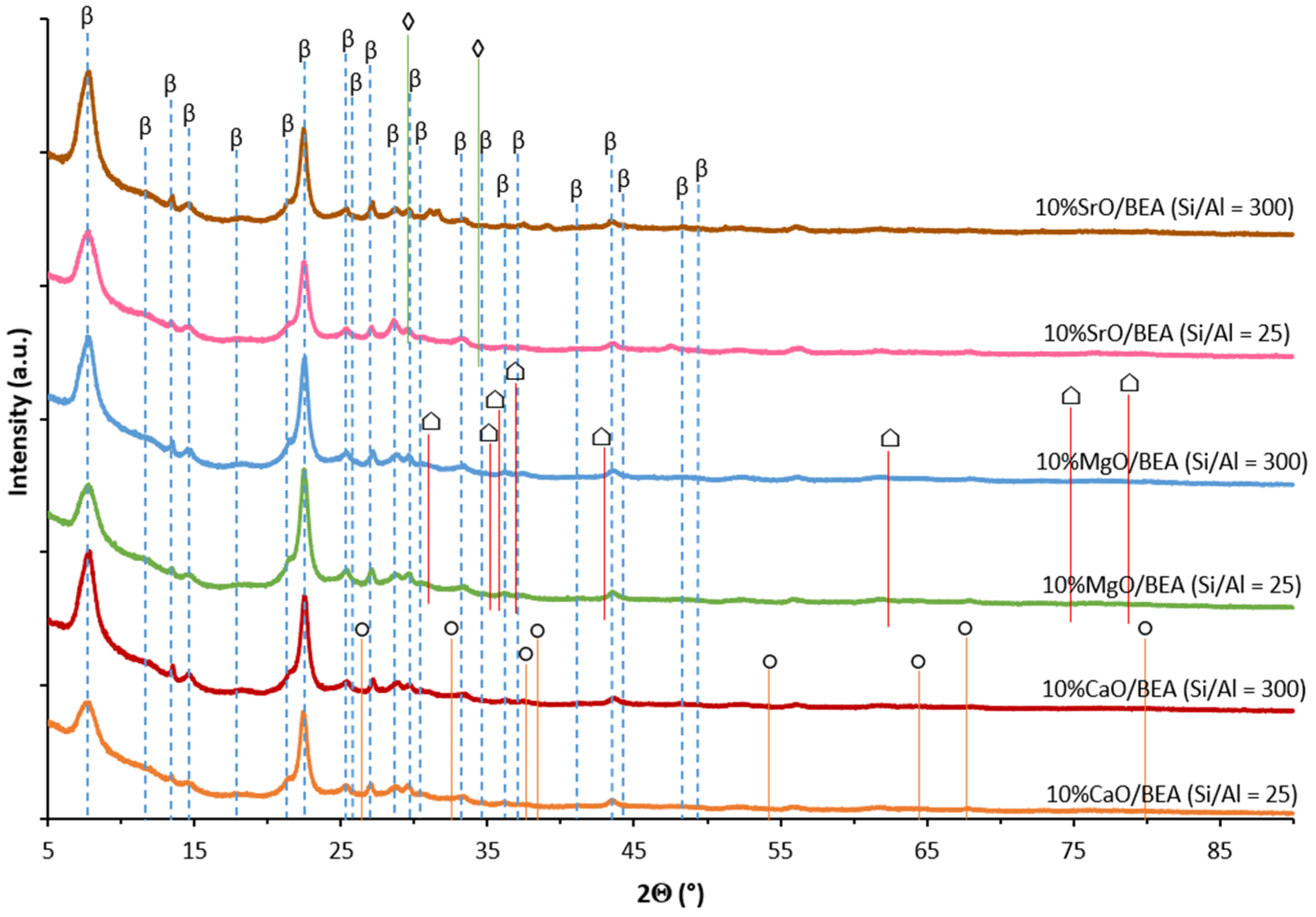
2.3. Phase Composition Studies of Catalysts
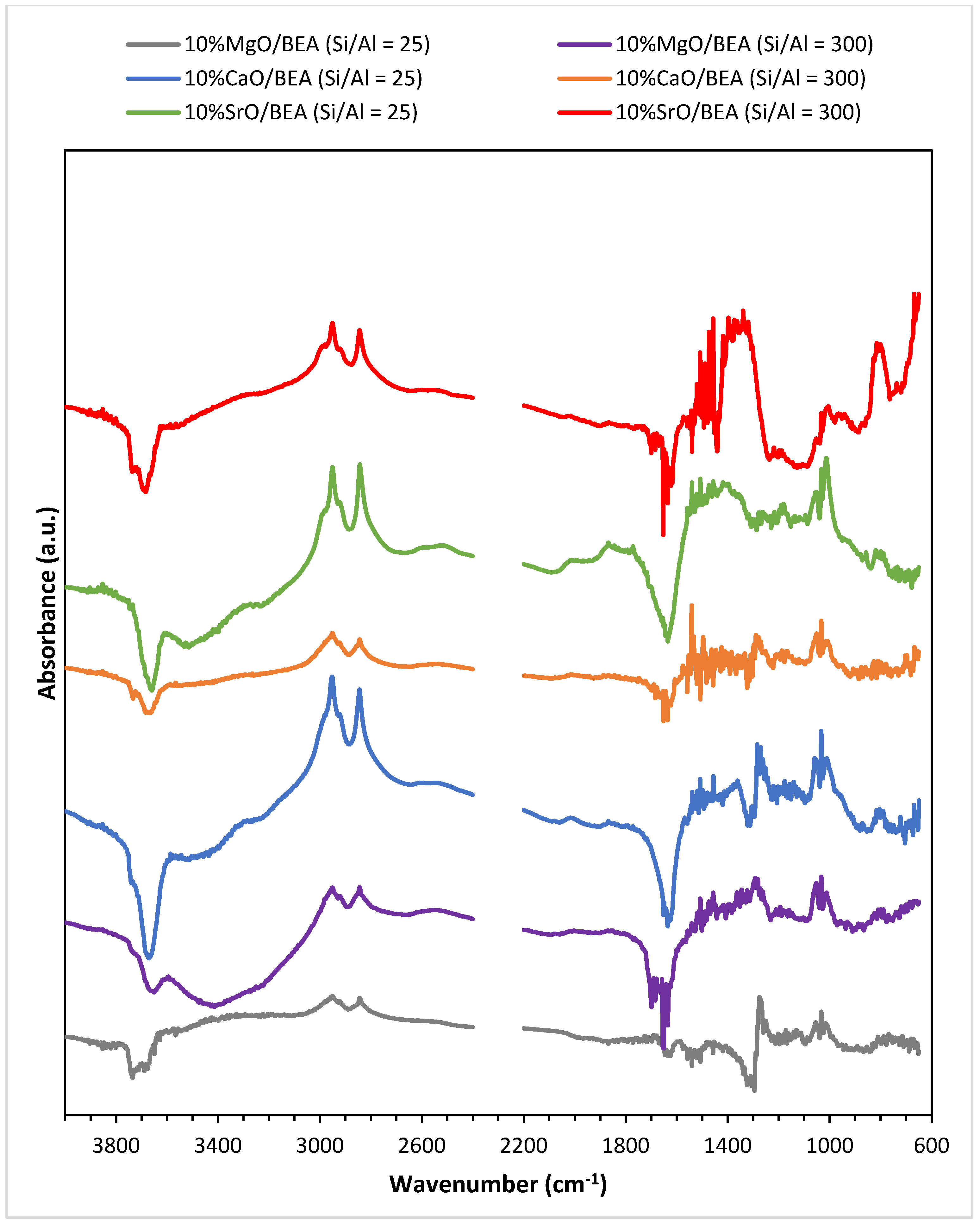
2.4. Basic Properties of the Synthesized Catalyst Systems
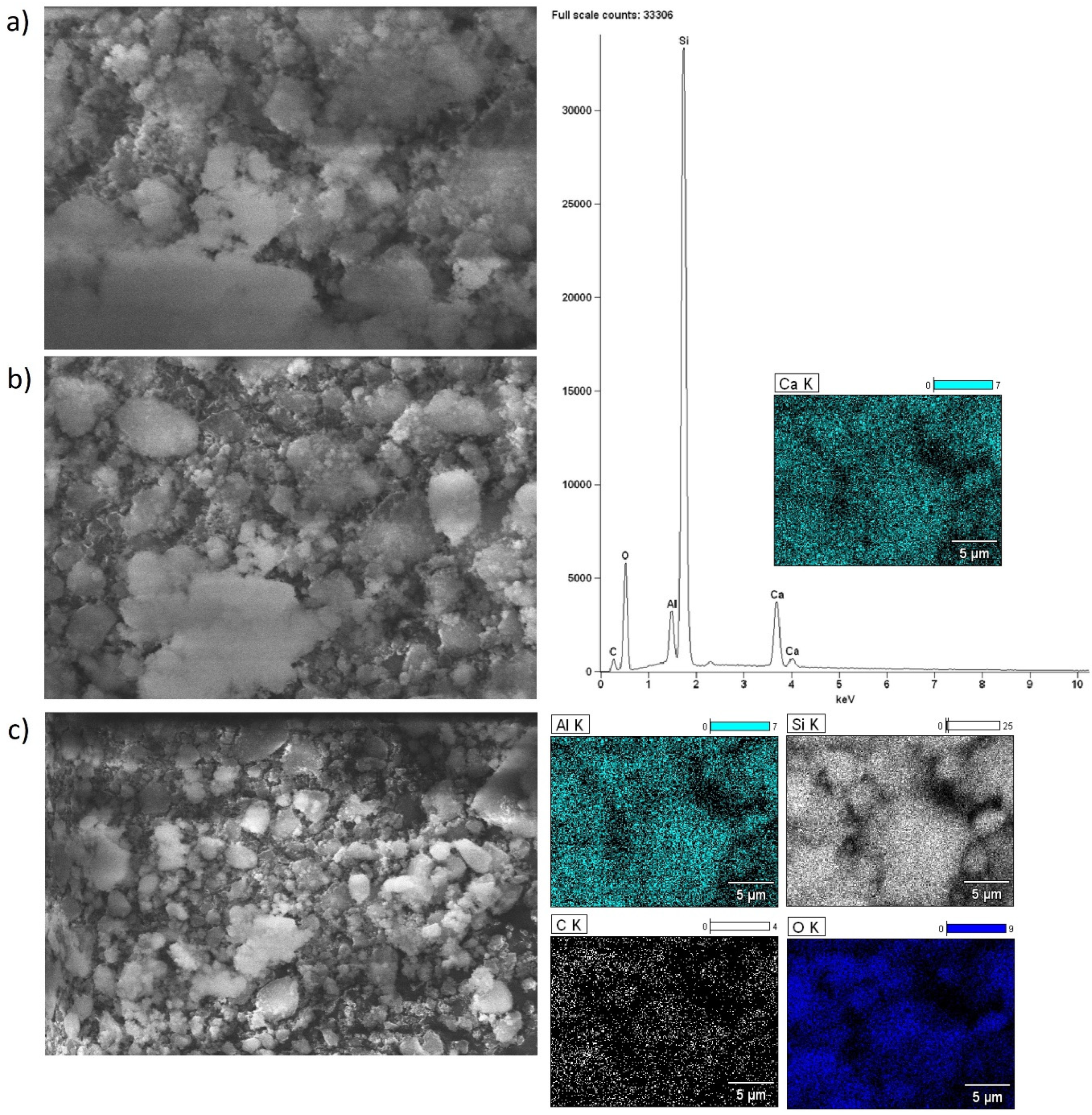
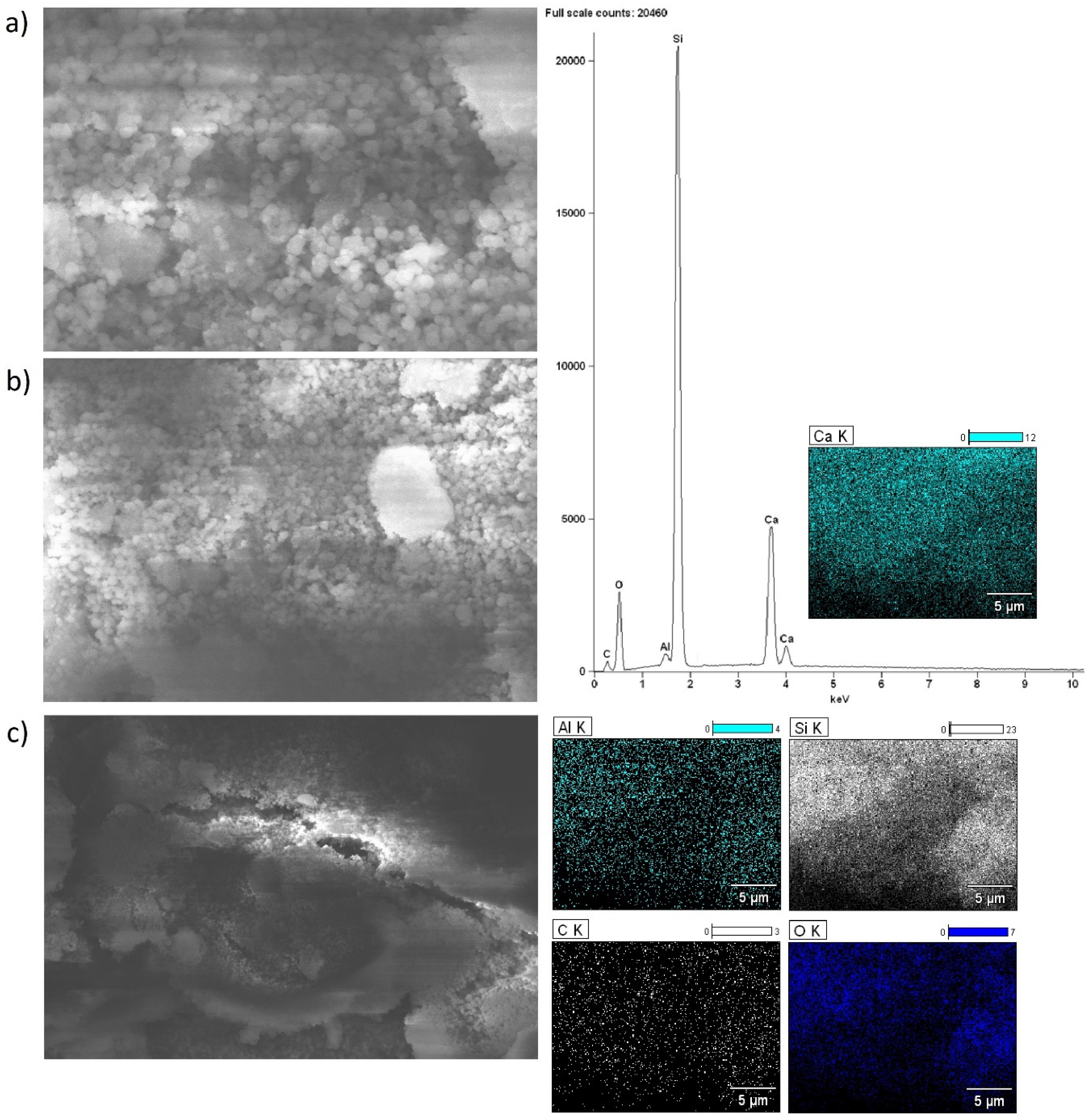
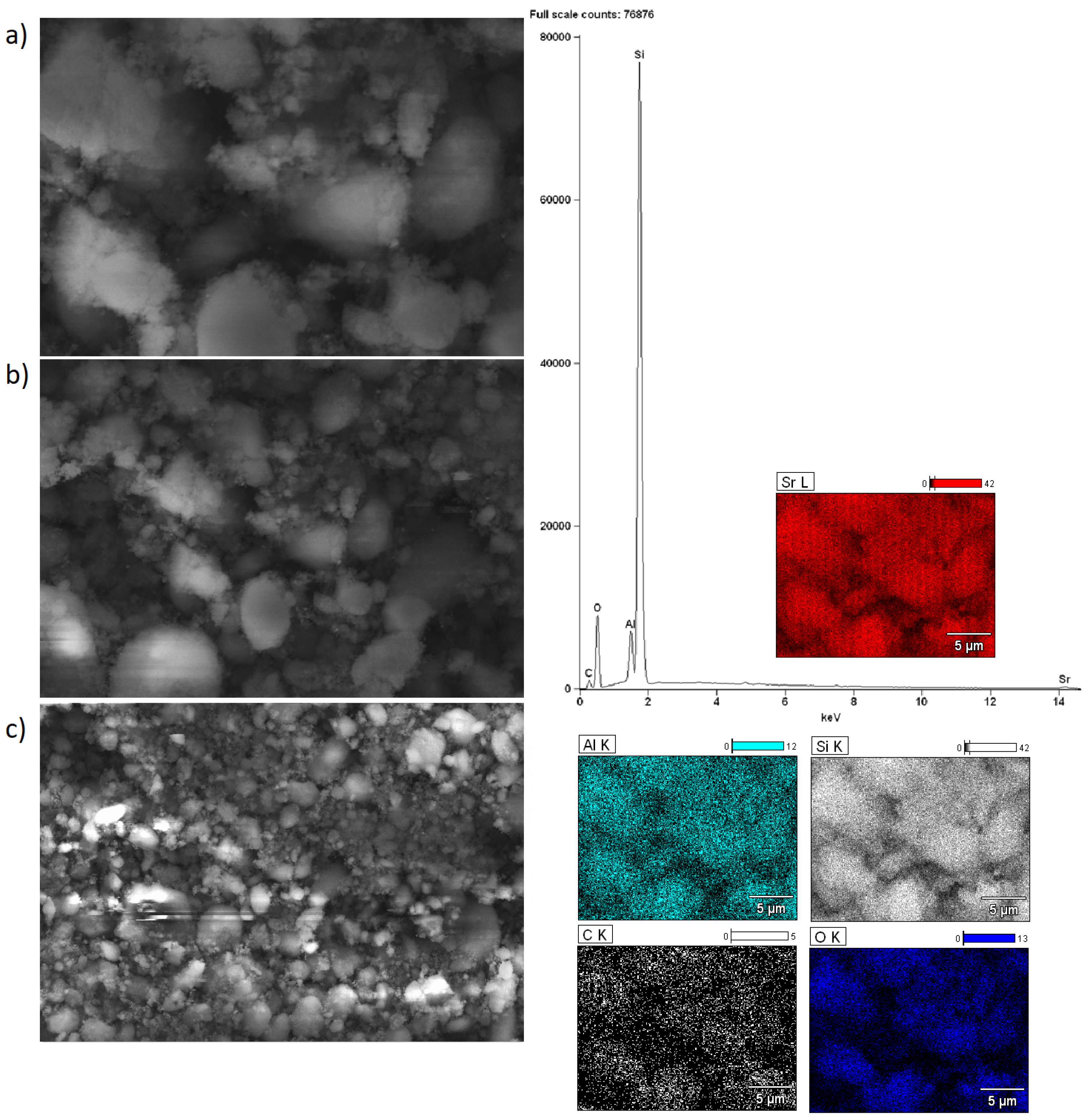
2.5. Morphology of the Investigated Catalysts
2.6. Sorption Properties of Investigated Catalysts in Relation to Methanol
3. Materials and Methods
3.1. Preparation of the Catalytic Materials
3.2. Characterization of the Catalytic Material
3.3. Catalytic Activity Measurements in Transesterification of the Vegetable Oil with Methanol
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ngomade, S.B.L.; Tchuifon, R.D.T.; Tagne, R.F.T.; Ngueteu, M.L.T.; Patai, H.M.; Nche, G.N.-A.; Anagho, S.G. Optimization by Response Surface Methodology of Biodiesel Production from Podocarpus falcatus Oil as a Cameroonian Novel Nonedible Feedstock. J. Chem. 2022, 2022, 3786602. [Google Scholar] [CrossRef]
- Kadapure, S.A.; Kirti, P.; Singh, S.; Kokatnur, S.; Hiremath, N.; Variar, A.; Shaikh, S.; Chittaragi, R. Studies on process optimization of biodiesel production from waste cooking and palm oil. Int. J. Sustain. Eng. 2018, 11, 167–172. [Google Scholar] [CrossRef]
- Jayakumar, M.; Karmegam, N.; Gundupalli, M.P.; Bizuneh Gebeyehu, K.; Tessema Asfaw, B.; Chang, S.W.; Ravindran, B.; Kumar Awasthi, M. Heterogeneous base catalysts: Synthesis and application for biodiesel production—A review. Bioresour. Technol. 2021, 331, 125054. [Google Scholar] [CrossRef]
- Shaah, M.A.; Hossain, M.S.; Allafi, F.; Ab Kadir, M.O.; Ahmad, M.I. Biodiesel production from candlenut oil using a non-catalytic supercritical methanol transesterification process: Optimization, kinetics, and thermodynamic studies. RSC Adv. 2022, 12, 9845–9861. [Google Scholar] [CrossRef]
- Sahar, J.; Farooq, M.; Ramli, A.; Naeem, A.; Khattak, N.S.; Ghazi, Z.A. Highly efficient heteropoly acid decorated SnO2@Co-ZIF nanocatalyst for sustainable biodiesel production from Nannorrhops ritchiana seeds oil. Renew. Energy 2022, 198, 306–318. [Google Scholar] [CrossRef]
- Khan, K.; Ul-Haq, N.; Rahman, W.U.; Ali, M.; Rashid, U.; Ul-Haq, A.; Jamil, F.; Ahmed, A.; Ahmed, F.; Moser, B.R.; et al. Comprehensive Comparison of Hetero-Homogeneous Catalysts for Fatty Acid Methyl Ester Production from Non-Edible Jatropha curcas Oil. Catalysts 2021, 11, 1420. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, V.; Nerella, V.K.V. Experimental Analysis of Exhaust Emissions from Transit Buses Fuelled with Biodiesel. Open Environ. Eng. J. 2009, 2, 81–96. [Google Scholar] [CrossRef][Green Version]
- Yusuff, A.S.; Popoola, L.T.; Adeniyi, D.O.; Olutoye, M.A. Coal fly ash supported ZnO catalyzed transesterification of Jatropha curcas oil: Optimization by response surface methodology. Energy Convers. Manag. X 2022, 16, 100302. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, Y.; Liu, H.; Yan, T.; Zhang, Z. Preparation of nano-CaO and catalyzing tri-component coupling transesterification to produce biodiesel. Inorg. Nano-Met. Chem. 2020, 50, 501–507. [Google Scholar] [CrossRef]
- Hossain, M.; Muntaha, N.; Osman Goni, L.K.M.; Jamal, M.S.; Gafur, M.A.; Islam, D.; Fakhruddin, A.N.M. Triglyceride Conversion of Waste Frying Oil up to 98.46% Using Low Concentration K+/CaO Catalysts Derived from Eggshells. ACS Omega 2021, 6, 35679–35691. [Google Scholar] [CrossRef]
- Lokman, I.M.; Rashid, U.; Yunus, R.; Taufiq-Yap, Y.H. Carbohydrate-derived Solid Acid Catalysts for Biodiesel Production from Low-Cost Feedstocks: A Review. Catal. Rev. 2014, 56, 187–219. [Google Scholar] [CrossRef]
- Abdulkareem-Alsultan, G.; Asikin-Mijan, N.; Lee, H.V.; Rashid, U.; Islam, A.; Taufiq-Yap, Y.H. A Review on Thermal Conversion of Plant Oil (Edible and Inedible) into Green Fuel Using Carbon-Based Nanocatalyst. Catalysts 2019, 9, 350. [Google Scholar] [CrossRef]
- Pan, H.; Xia, Q.; Wang, Y.; Shen, Z.; Huang, H.; Ge, Z.; Li, X.; He, J.; Wang, X.; Li, L.; et al. Recent advances in biodiesel production using functional carbon materials as acid/base catalysts. Fuel Process. Technol. 2022, 237, 107421. [Google Scholar] [CrossRef]
- Long, F.; Liu, W.; Jiang, X.; Zhai, Q.; Cao, X.; Jiang, J.; Xu, J. State-of-the-art technologies for biofuel production from triglycerides: A review. Renew. Sustain. Energy Rev. 2021, 148, 111269. [Google Scholar] [CrossRef]
- Makareviciene, V.; Skorupskaite, V.; Levisauskas, D.; Andruleviciute, V.; Kazancev, K. The Optimization of Biodiesel Fuel Production from Microalgae Oil Using Response Surface Methodology. Int. J. Green Energy 2014, 11, 527–541. [Google Scholar] [CrossRef]
- Rahman, M.W.; Mondal, A.K.; Hasan, M.S.; Sultana, M. Biodiesel production from a non-edible source of royna (Aphanamixis polystachya) oil. Energy Sustain. Soc. 2022, 12, 33. [Google Scholar] [CrossRef]
- Singh, R.; Kumar, A.; Chandra Sharma, Y. Biodiesel Production from Microalgal Oil Using Barium–Calcium–Zinc Mixed Oxide Base Catalyst: Optimization and Kinetic Studies. Energy Fuels 2019, 33, 1175–1184. [Google Scholar] [CrossRef]
- Huang, S.; Cui, Z.; Zhu, R.; Chen, C.; Song, S.; Song, J.; Wang, M.; Tan, T. Design and development of a new static mixing bioreactor for enzymatic bioprocess: Application in biodiesel production. Renew. Energy 2022, 197, 922–931. [Google Scholar] [CrossRef]
- Patil, P.D.; Gude, V.G.; Deng, S. Biodiesel Production from Jatropha Curcas, Waste Cooking, and Camelina Sativa Oils. Ind. Eng. Chem. Res. 2009, 48, 10850–10856. [Google Scholar] [CrossRef]
- Gao, X.; Chen, C.; Zhang, W.; Hong, Y.; Wang, C.; Wu, G. Sulfated TiO2 supported molybdenum-based catalysts for transesterification of Jatropha seed oil: Effect of molybdenum species and acidity properties. Renew. Energy 2022, 191, 357–369. [Google Scholar] [CrossRef]
- Lee, H.V.; Juan, J.C.; Binti Abdullah, N.F.; Nizah Mf, R.; Taufiq-Yap, Y.H. Heterogeneous base catalysts for edible palm and non-edible Jatropha-based biodiesel production. Chem. Cent. J. 2014, 8, 30. [Google Scholar] [CrossRef]
- Keera, S.T.; El Sabagh, S.M.; Taman, A.R. Transesterification of vegetable oil to biodiesel fuel using alkaline catalyst. Fuel 2011, 90, 42–47. [Google Scholar] [CrossRef]
- Li, H.; Liu, F.; Ma, X.; Wu, Z.; Li, Y.; Zhang, L.; Zhou, S.; Helian, Y. Catalytic performance of strontium oxide supported by MIL–100(Fe) derivate as transesterification catalyst for biodiesel production. Energy Convers. Manag. 2019, 180, 401–410. [Google Scholar] [CrossRef]
- Jamil, F.; Al-Haj, L.; Al-Muhtaseb, A.a.H.; Al-Hinai, M.A.; Baawain, M.; Rashid, U.; Ahmad, M.N.M. Current scenario of catalysts for biodiesel production: A critical review. Rev. Chem. Eng. 2018, 34, 267–297. [Google Scholar] [CrossRef]
- Kusdiana, D.; Saka, S. Effects of water on biodiesel fuel production by supercritical methanol treatment. Bioresour. Technol. 2004, 91, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Hanna, M.A. Biodiesel production: A review. Bioresour. Technol. 1999, 70, 1–15. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Nguyen, M.-L.; Su, C.-H.; Ong, H.C.; Juan, H.-Y.; Wu, S.-J. Bio-Derived Catalysts: A Current Trend of Catalysts Used in Biodiesel Production. Catalysts 2021, 11, 812. [Google Scholar] [CrossRef]
- de Oliveira, D.; Di Luccio, M.; Faccio, C.; Dalla Rosa, C.; Bender, J.P.; Lipke, N.; Amroginski, C.; Dariva, C.; de Oliveira, J.V. Optimization of alkaline transesterification of soybean oil and castor oil for biodiesel production. Appl. Biochem. Biotechnol. 2005, 122, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Shan, R.; Zhao, C.; Lv, P.; Yuan, H.; Yao, J. Catalytic applications of calcium rich waste materials for biodiesel: Current state and perspectives. Energy Convers. Manag. 2016, 127, 273–283. [Google Scholar] [CrossRef]
- Muninathan, K.; Venkata Ramanan, M.; Baskar, G.; Rajendran, N. Biodiesel Production from Sapindus trifoliatus oil using Mg doped CaO Heterogeneous Nanocatalyst. Energy Sources Part A Recovery Util. Environ. Eff. 2022, 44, 6099–6110. [Google Scholar] [CrossRef]
- Konwar, L.J.; Das, R.; Thakur, A.J.; Salminen, E.; Mäki-Arvela, P.; Kumar, N.; Mikkola, J.-P.; Deka, D. Biodiesel production from acid oils using sulfonated carbon catalyst derived from oil-cake waste. J. Mol. Catal. A Chem. 2014, 388–389, 167–176. [Google Scholar] [CrossRef]
- Idrissou, Y.; Mazari, T.; Benadji, S.; Hamdi, M.; Rabia, C. Homogeneous and heterogeneous sunflower oil methanolysis over 12-tungstophosphoric, sulfuric and boric acids. React. Kinet. Mech. Catal. 2016, 119, 291–304. [Google Scholar] [CrossRef]
- Jitputti, J.; Kitiyanan, B.; Rangsunvigit, P.; Bunyakiat, K.; Attanatho, L.; Jenvanitpanjakul, P. Transesterification of crude palm kernel oil and crude coconut oil by different solid catalysts. Chem. Eng. J. 2006, 116, 61–66. [Google Scholar] [CrossRef]
- Anbalagan, A.; Thiyagaragan, S.; Kumaragurubaran, J.; Arumugam, A. Synthesis of sulfonated mesoporous carbon from Calophyllum inophyllum oil cake as heterogeneous transesterification catalyst for biodiesel production: A process optimization study. Energy Sources Part A Recovery Util. Environ. Eff. 2021, 1–17. [Google Scholar] [CrossRef]
- Kawashima, A.; Matsubara, K.; Honda, K. Development of heterogeneous base catalysts for biodiesel production. Bioresour. Technol. 2008, 99, 3439–3443. [Google Scholar] [CrossRef] [PubMed]
- Faruque, M.O.; Razzak, S.A.; Hossain, M.M. Application of Heterogeneous Catalysts for Biodiesel Production from Microalgal Oil—A Review. Catalysts 2020, 10, 1025. [Google Scholar] [CrossRef]
- Hamzah, N.; Edlina, N.; Hafiza, E.; Tajuddin, N.A. MgAl Mixed Oxide Derived Alkali-free Hydrotalcite for Transesterification of Waste Cooking Oil to Biodiesel. ASM Sci. J. 2020, 13, 1–7. [Google Scholar] [CrossRef]
- Sulaiman, N.F.; Ramly, N.I.; Abd Mubin, M.H.; Lee, S.L. Transition metal oxide (NiO, CuO, ZnO)-doped calcium oxide catalysts derived from eggshells for the transesterification of refined waste cooking oil. RSC Adv. 2021, 11, 21781–21795. [Google Scholar] [CrossRef]
- Calero, J.; Luna, D.; Sancho, E.D.; Luna, C.; Bautista, F.M.; Romero, A.A.; Posadillo, A.; Verdugo, C. Development of a new biodiesel that integrates glycerol, by using CaO as heterogeneous catalyst, in the partial methanolysis of sunflower oil. Fuel 2014, 122, 94–102. [Google Scholar] [CrossRef]
- Mijangos, G.E.; Cuautli, C.; Romero-Ibarra, I.C.; Vazquez-Arenas, J.; Santolalla-Vargas, C.E.; Santes, V.; Castañeda-Galván, A.A.; Pfeiffer, H. Experimental and theoretical analysis revealing the underlying chemistry accounting for the heterogeneous transesterification reaction in Na2SiO3 and Li2SiO3 catalysts. Renew. Energy 2022, 184, 845–856. [Google Scholar] [CrossRef]
- Lee, J.H.; Jeon, H.; Park, J.T.; Kim, J.H. Synthesis of hierarchical flower-shaped hollow MgO microspheres via ethylene-glycol-mediated process as a base heterogeneous catalyst for transesterification for biodiesel production. Biomass Bioenergy 2020, 142, 105788. [Google Scholar] [CrossRef]
- Palitsakun, S.; Koonkuer, K.; Topool, B.; Seubsai, A.; Sudsakorn, K. Transesterification of Jatropha oil to biodiesel using SrO catalysts modified with CaO from waste eggshell. Catal. Commun. 2021, 149, 106233. [Google Scholar] [CrossRef]
- Laskar, I.B.; Rajkumari, K.; Gupta, R.; Chatterjee, S.; Paul, B.; Rokhum, S.L. Waste snail shell derived heterogeneous catalyst for biodiesel production by the transesterification of soybean oil. RSC Adv. 2018, 8, 20131–20142. [Google Scholar] [CrossRef]
- Mansir, N.; Teo, S.H.; Rashid, U.; Taufiq-Yap, Y.H. Efficient waste Gallus domesticus shell derived calcium-based catalyst for biodiesel production. Fuel 2018, 211, 67–75. [Google Scholar] [CrossRef]
- Koberg, M.; Abu-Much, R.; Gedanken, A. Optimization of bio-diesel production from soybean and wastes of cooked oil: Combining dielectric microwave irradiation and a SrO catalyst. Bioresour. Technol. 2011, 102, 1073–1078. [Google Scholar] [CrossRef]
- Liu, X.; He, H.; Wang, Y.; Zhu, S. Transesterification of soybean oil to biodiesel using SrO as a solid base catalyst. Catal. Commun. 2007, 8, 1107–1111. [Google Scholar] [CrossRef]
- Martins, A.; Nunes, N.; Carvalho, A.P.; Martins, L.M.D.R.S. Zeolites and Related Materials as Catalyst Supports for Hydrocarbon Oxidation Reactions. Catalysts 2022, 12, 154. [Google Scholar] [CrossRef]
- Almeida, A.; Ribeiro, R.P.P.L.; Mota, J.P.B.; Grande, C. Extrusion and Characterization of High Si/Al Ratio ZSM-5 Using Silica Binder. Energies 2020, 13, 1201. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Shu, Q.; Yang, B.; Yuan, H.; Qing, S.; Zhu, G. Synthesis of biodiesel from soybean oil and methanol catalyzed by zeolite beta modified with La3+. Catal. Commun. 2007, 8, 2159–2165. [Google Scholar] [CrossRef]
- Alshahidy, B.A.; Abbas, A.S. Comparative Study on the Catalytic Performance of a 13X Zeolite and its Dealuminated Derivative for Biodiesel Production. Bull. Chem. React. Eng. Catal. 2021, 16, 763–772. [Google Scholar] [CrossRef]
- Shang, N.-C.; Liu, R.-Z.; Chen, Y.-H.; Chang, C.-Y.; Lin, R.-H. Characterization of fatty acid methyl esters in biodiesel using high-performance liquid chromatography. J. Taiwan Inst. Chem. Eng. 2012, 43, 354–359. [Google Scholar] [CrossRef]
- Lawan, I.; Garba, Z.N.; Zhou, W.; Zhang, M.; Yuan, Z. Synergies between the microwave reactor and CaO/zeolite catalyst in waste lard biodiesel production. Renew. Energy 2020, 145, 2550–2560. [Google Scholar] [CrossRef]
- Luz Martinez, S.; Romero, R.; López, J.C.; Romero, A.; Sanchez Mendieta, V.; Natividad, R. Preparation and characterization of CaO nanoparticles/NaX zeolite catalysts for the transesterification of sunflower oil. Ind. Eng. Chem. Res. 2011, 50, 2665–2670. [Google Scholar] [CrossRef]
- Carrero, A.; Vicente, G.; Rodríguez, R.; Linares, M.; del Peso, G.L. Hierarchical zeolites as catalysts for biodiesel production from Nannochloropsis microalga oil. Catal. Today 2011, 167, 148–153. [Google Scholar] [CrossRef]
- Kiss, A.A.; Dimian, A.C.; Rothenberg, G. Solid Acid Catalysts for Biodiesel Production—Towards Sustainable Energy. Adv. Synth. Catal. 2006, 348, 75–81. [Google Scholar] [CrossRef]
- Szkudlarek, Ł.; Chałupka, K.; Maniukiewicz, W.; Albińska, J.; Szynkowska-Jóźwik, M.I.; Mierczyński, P. The influence of si/al ratio on the physicochemical and catalytic properties of mgo/zsm-5 catalyst in transesterification reaction of rapeseed oil. Catalysts 2021, 11, 1260. [Google Scholar] [CrossRef]
- Atchimarungsri, T.; Gao, X.; Wang, K.; Ma, Q.; Zhang, J.; Fan, S.; He, F.; Tian, J.; Reubroycharoen, P.; Zhao, T. Selective Conversion of Glycerol to Methanol over CaO-Modified HZSM-5 Zeolite. Molecules 2022, 27, 7221. [Google Scholar] [CrossRef]
- Lani, N.S.; Ngadi, N. Highly efficient CaO–ZSM-5 zeolite/Fe3O4 as a magnetic acid–base catalyst upon biodiesel production from used cooking oil. Appl. Nanosci. 2022, 12, 3755–3769. [Google Scholar] [CrossRef]
- Akbar, S. Characterization of Beta Zeolites by X-ray Diffraction, Scanning Electron Microscope, and Refractive Index Techniques. J. Chem. Soc. Pak. 2010, 32, 592–598. [Google Scholar]
- Mierczynski, P.; Stępińska, N.; Mosinska, M.; Chalupka, K.; Albinska, J.; Maniukiewicz, W.; Rogowski, J.; Nowosielska, M.; Szynkowska, M.I. Hydrogen Production via the Oxy-Steam Reforming of LNG or Methane on Ni Catalysts. Catalysts 2020, 10, 346. [Google Scholar] [CrossRef]
- Taufiq-Yap, Y.H.; Lee, H.V.; Yunus, R.; Juan, J.C. Transesterification of non-edible Jatropha curcas oil to biodiesel using binary Ca–Mg mixed oxide catalyst: Effect of stoichiometric composition. Chem. Eng. J. 2011, 178, 342–347. [Google Scholar] [CrossRef]
- Di Serio, M.; Ledda, M.; Cozzolino, M.; Minutillo, G.; Tesser, R.; Santacesaria, E. Transesterification of Soybean Oil to Biodiesel by Using Heterogeneous Basic Catalysts. Ind. Eng. Chem. Res. 2006, 45, 3009–3014. [Google Scholar] [CrossRef]
- Mohamad, M.; Ngadi, N.; Wong, S.; Yahya, N.Y.; Inuwa, I.M.; Lani, N.S. Synthesis and Characterization of CaO-TiO2 for Transesterification of Vegetable Palm Oil. Int. J. Eng. 2018, 31, 1326–1333. [Google Scholar]
- Albuquerque, M.C.G.; Santamaría-González, J.; Mérida-Robles, J.M.; Moreno-Tost, R.; Rodríguez-Castellón, E.; Jiménez-López, A.; Azevedo, D.C.S.; Cavalcante, C.L.; Maireles-Torres, P. MgM (M=Al and Ca) oxides as basic catalysts in transesterification processes. Appl. Catal. A Gen. 2008, 347, 162–168. [Google Scholar] [CrossRef]
- Deshmane, V.G.; Adewuyi, Y.G. Synthesis and kinetics of biodiesel formation via calcium methoxide base catalyzed transesterification reaction in the absence and presence of ultrasound. Fuel 2013, 107, 474–482. [Google Scholar] [CrossRef]
- Gomes, G.J.; Zalazar, M.F.; Lindino, C.A.; Scremin, F.R.; Bittencourt, P.R.S.; Costa, M.B.; Peruchena, N.M. Adsorption of acetic acid and methanol on H-Beta zeolite: An experimental and theoretical study. Microporous Mesoporous Mater. 2017, 252, 17–28. [Google Scholar] [CrossRef]
- Lønstad Bleken, B.-T.; Mino, L.; Giordanino, F.; Beato, P.; Svelle, S.; Lillerud, K.P.; Bordiga, S. Probing the surface of nanosheet H-ZSM-5 with FTIR spectroscopy. Phys. Chem. Chem. Phys. 2013, 15, 13363–13370. [Google Scholar] [CrossRef]
- Forester, T.R.; Howe, R.F. In situ FTIR studies of methanol and dimethyl ether in ZSM-5. J. Am. Chem. Soc. 1987, 109, 5076–5082. [Google Scholar] [CrossRef]
- Hadjiivanov, K.; Saussey, J.; Freysz, J.L.; Lavalley, J.C. FT-IR study of NO + O2 co-adsorption on H-ZSM-5: Re-assignment of the 2133 cm−1 band to NO+ species. Catal. Lett. 1998, 52, 103–108. [Google Scholar] [CrossRef]
- Rep, M.; Palomares, A.E.; Eder-Mirth, G.; van Ommen, J.G.; Rösch, N.; Lercher, J.A. Interaction of Methanol with Alkali Metal Exchanged Molecular Sieves. 1. IR Spectroscopic Study. J. Phys. Chem. B 2000, 104, 8624–8630. [Google Scholar] [CrossRef]
- Schenkel, R.; Jentys, A.; Parker, S.F.; Lercher, J.A. Investigation of the Adsorption of Methanol on Alkali Metal Cation Exchanged Zeolite X by Inelastic Neutron Scattering. J. Phys. Chem. B 2004, 108, 7902–7910. [Google Scholar] [CrossRef]


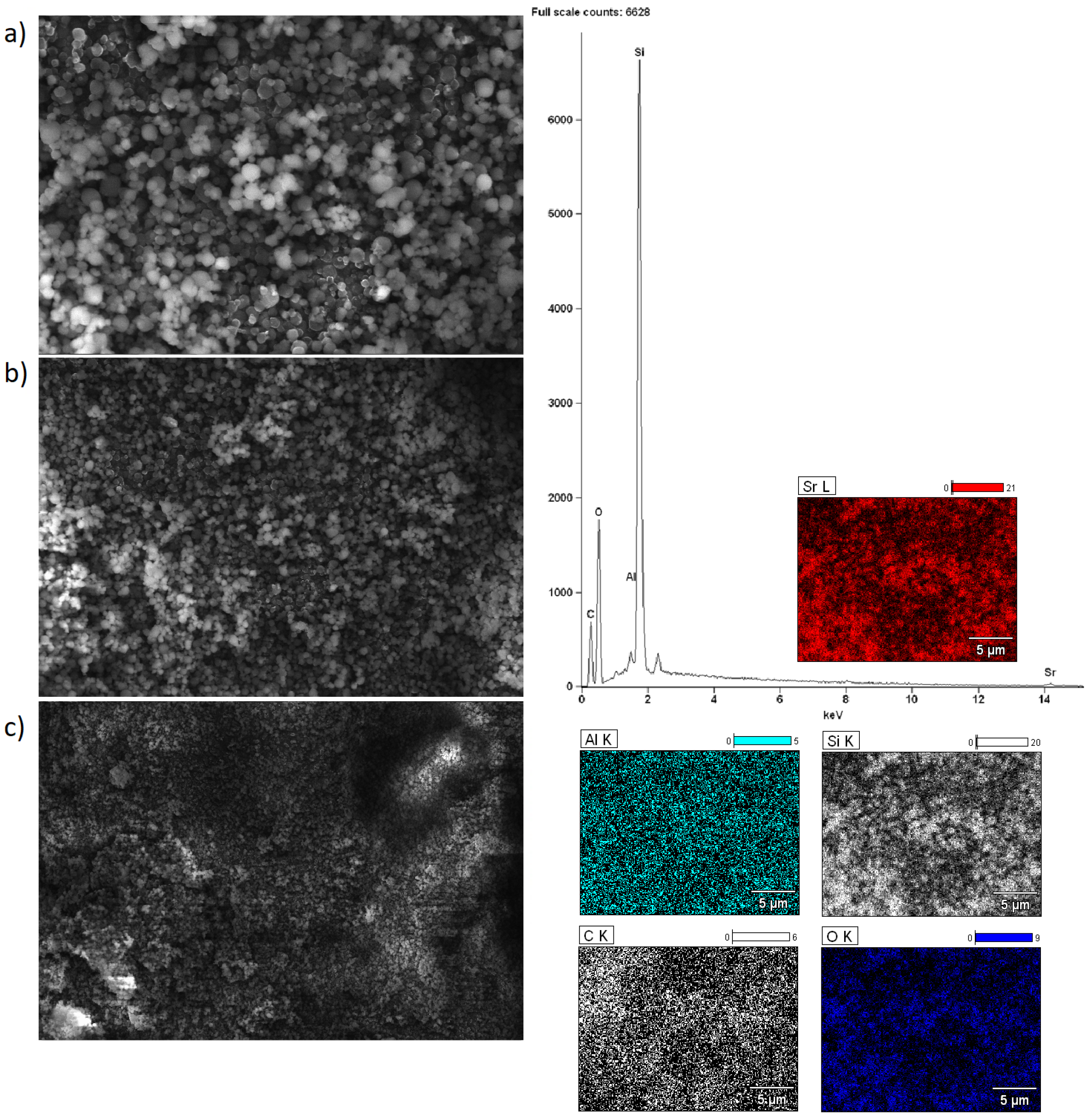
| Catalyst | Reaction Temperature [°C] | Reaction Time [h] | Molar Ratio Methanol/Oil | Calcination Temperature [°C] | Catalyst Weight [g] | Triglycerides Conversion [%] | FAME Yield [%] |
|---|---|---|---|---|---|---|---|
| BEA (Si/Al = 25) | 180 | 2 | 9:1 | 500 | 0.5 | 33.9 | 3.0 |
| 220 | 68.7 | 30.0 | |||||
| BEA (Si/Al = 300) | 180 | 2 | 9:1 | 500 | 0.5 | 17.5 | 3.1 |
| 220 | 52.5 | 25.0 | |||||
| 10% MgO/BEA (Si/Al = 25) | 180 | 2 | 9:1 | 500 | 0.5 | 66.8 | 46.0 |
| 220 | 69.5 | 55.7 | |||||
| 10% MgO/BEA (Si/Al = 300) | 180 | 2 | 9:1 | 500 | 0.5 | 63.0 | 29.0 |
| 220 | 59.7 | 74.4 | |||||
| 10% CaO/BEA (Si/Al = 25) | 180 | 2 | 9:1 | 500 | 0.5 | 90.4 | 78.2 |
| 220 | 90.2 | 92.6 | |||||
| 10% CaO/BEA (Si/Al = 300) | 180 | 2 | 9:1 | 500 | 0.5 | 84.2 | 64.0 |
| 220 | 90.5 | 94.6 | |||||
| 10% SrO/BEA (Si/Al = 25) | 180 | 2 | 9:1 | 600 | 0.5 | 80.0 | 74.8 |
| 220 | 74.4 | 89.6 | |||||
| 10% SrO/BEA (Si/Al = 300) | 180 | 2 | 9:1 | 600 | 0.5 | 71.4 | 65.4 |
| 220 | 87.8 | 98.5 |
| Catalyst | BET Surface Area (m2/g) | t-Plot Micropore Area (m2/g) | t-Plot External Surface Area (m2/g) | Pore Volume (cm3/g) | t-Plot Micropore Volume (cm3/g) | Average Pore Size (nm) |
|---|---|---|---|---|---|---|
| 10% MgO/BEA (Si/Al = 25) | 305 | 191 | 113 | 0.576 | 0.098 | 9.85 |
| 10% MgO/BEA (Si/Al = 300) | 314 | 253 | 62 | 0.083 | 0.130 | 3.28 |
| 10% CaO/BEA (Si/Al = 25) | 346 | 227 | 119 | 0.557 | 0.117 | 8.99 |
| 10% CaO/BEA (Si/Al = 300) | 326 | 262 | 64 | 0.091 | 0.135 | 3.23 |
| 10% SrO/BEA (Si/Al = 25) | 401 | 249 | 152 | 0.681 | 0.129 | 8.27 |
| 10% SrO/BEA (Si/Al = 300) | 404 | 289 | 115 | 0.114 | 0.149 | 2.45 |
| Catalyst | Average Size of Oxide Crystallites [nm] |
|---|---|
| 10% MgO/BEA (Si/Al = 25) | MgO = 12 |
| 10% MgO/BEA (Si/Al = 300) | MgO = 9 |
| 10% CaO/BEA (Si/Al = 25) | CaO = 16 |
| 10% CaO/BEA (Si/Al = 300) | CaO = 14 |
| 10% SrO/BEA (Si/Al = 25) | SrO = 9 |
| 10% SrO/BEA (Si/Al = 300) | SrO = 13 |
| Catalytic Systems | Weak Centers [mmol/g] 100–300 °C | Medium Centers [mmol/g] 300–450 ° C | Strong Centers [mmol/g] 450–600 ° C | Total Basicity [mmol/g] 100–600 ° C |
|---|---|---|---|---|
| 10% MgO/BEA (Si/Al = 25) | 0.10 | 0.28 | 0.64 | 1.03 |
| 10% MgO/BEA (Si/Al = 300) | 0.55 | 0.49 | 0.61 | 1.65 |
| 10% CaO/BEA (Si/Al = 25) | 0.24 | 0.41 | 0.58 | 1.22 |
| 10% CaO/BEA (Si/Al = 300) | 0.57 | 0.69 | 0.67 | 1.93 |
| 10% SrO/BEA (Si/Al = 25) | 0.15 | 0.38 | 0.57 | 1.10 |
| 10% SrO/BEA (Si/Al = 300) | 0.21 | 0.37 | 0.40 | 0.99 |
| Mobile Phase Gradient | Flow Rate [mL∙min−1] | ||
|---|---|---|---|
| Time [min] | Solvent A (%) | Solvent B (%) | |
| 0 | 100 | 0 | 0.9 |
| 20 | 100 | 0 | 0.9 |
| 45 | 0 | 100 | 0.9 |
| 70 | 0 | 100 | 0.9 |
| 75 | 100 | 0 | 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szkudlarek, Ł.; Chałupka-Śpiewak, K.; Maniukiewicz, W.; Nowosielska, M.; Szynkowska-Jóźwik, M.I.; Mierczyński, P. Biodiesel Production by Methanolysis of Rapeseed Oil—Influence of SiO2/Al2O3 Ratio in BEA Zeolite Structure on Physicochemical and Catalytic Properties of Zeolite Systems with Alkaline Earth Oxides (MgO, CaO, SrO). Int. J. Mol. Sci. 2024, 25, 3570. https://doi.org/10.3390/ijms25073570
Szkudlarek Ł, Chałupka-Śpiewak K, Maniukiewicz W, Nowosielska M, Szynkowska-Jóźwik MI, Mierczyński P. Biodiesel Production by Methanolysis of Rapeseed Oil—Influence of SiO2/Al2O3 Ratio in BEA Zeolite Structure on Physicochemical and Catalytic Properties of Zeolite Systems with Alkaline Earth Oxides (MgO, CaO, SrO). International Journal of Molecular Sciences. 2024; 25(7):3570. https://doi.org/10.3390/ijms25073570
Chicago/Turabian StyleSzkudlarek, Łukasz, Karolina Chałupka-Śpiewak, Waldemar Maniukiewicz, Magdalena Nowosielska, Małgorzata Iwona Szynkowska-Jóźwik, and Paweł Mierczyński. 2024. "Biodiesel Production by Methanolysis of Rapeseed Oil—Influence of SiO2/Al2O3 Ratio in BEA Zeolite Structure on Physicochemical and Catalytic Properties of Zeolite Systems with Alkaline Earth Oxides (MgO, CaO, SrO)" International Journal of Molecular Sciences 25, no. 7: 3570. https://doi.org/10.3390/ijms25073570
APA StyleSzkudlarek, Ł., Chałupka-Śpiewak, K., Maniukiewicz, W., Nowosielska, M., Szynkowska-Jóźwik, M. I., & Mierczyński, P. (2024). Biodiesel Production by Methanolysis of Rapeseed Oil—Influence of SiO2/Al2O3 Ratio in BEA Zeolite Structure on Physicochemical and Catalytic Properties of Zeolite Systems with Alkaline Earth Oxides (MgO, CaO, SrO). International Journal of Molecular Sciences, 25(7), 3570. https://doi.org/10.3390/ijms25073570






