Anti-Cancer Mechanisms of Diarylpentanoid MS17 (1,5-Bis(2-hydroxyphenyl)-1,4-pentadiene-3-one) in Human Colon Cancer Cells: A Proteomics Approach
Abstract
1. Introduction
2. Results
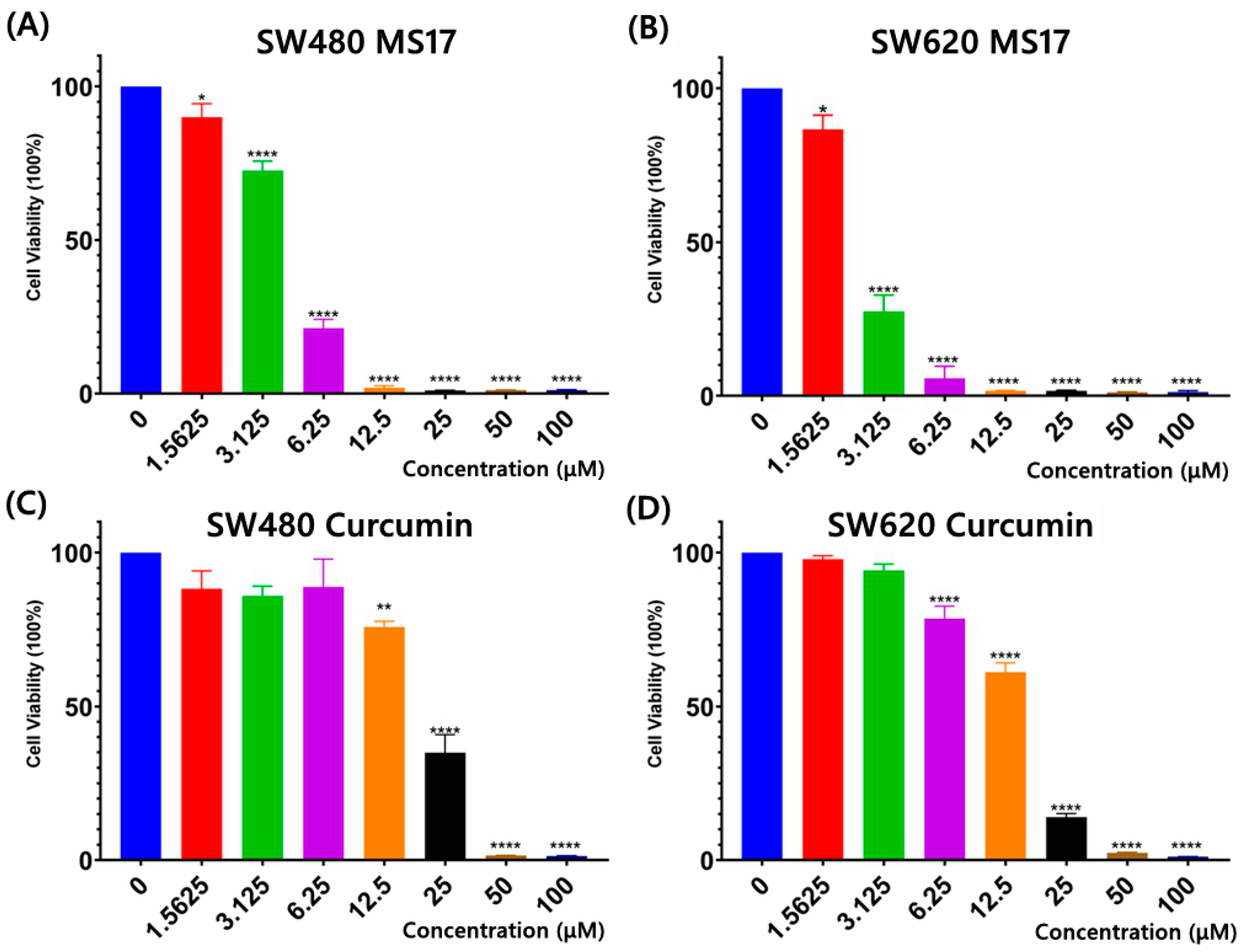
2.1. Cytotoxicity and Selective Index of MS17 and Curcumin in SW480 and SW620 Colon Cancer Cells
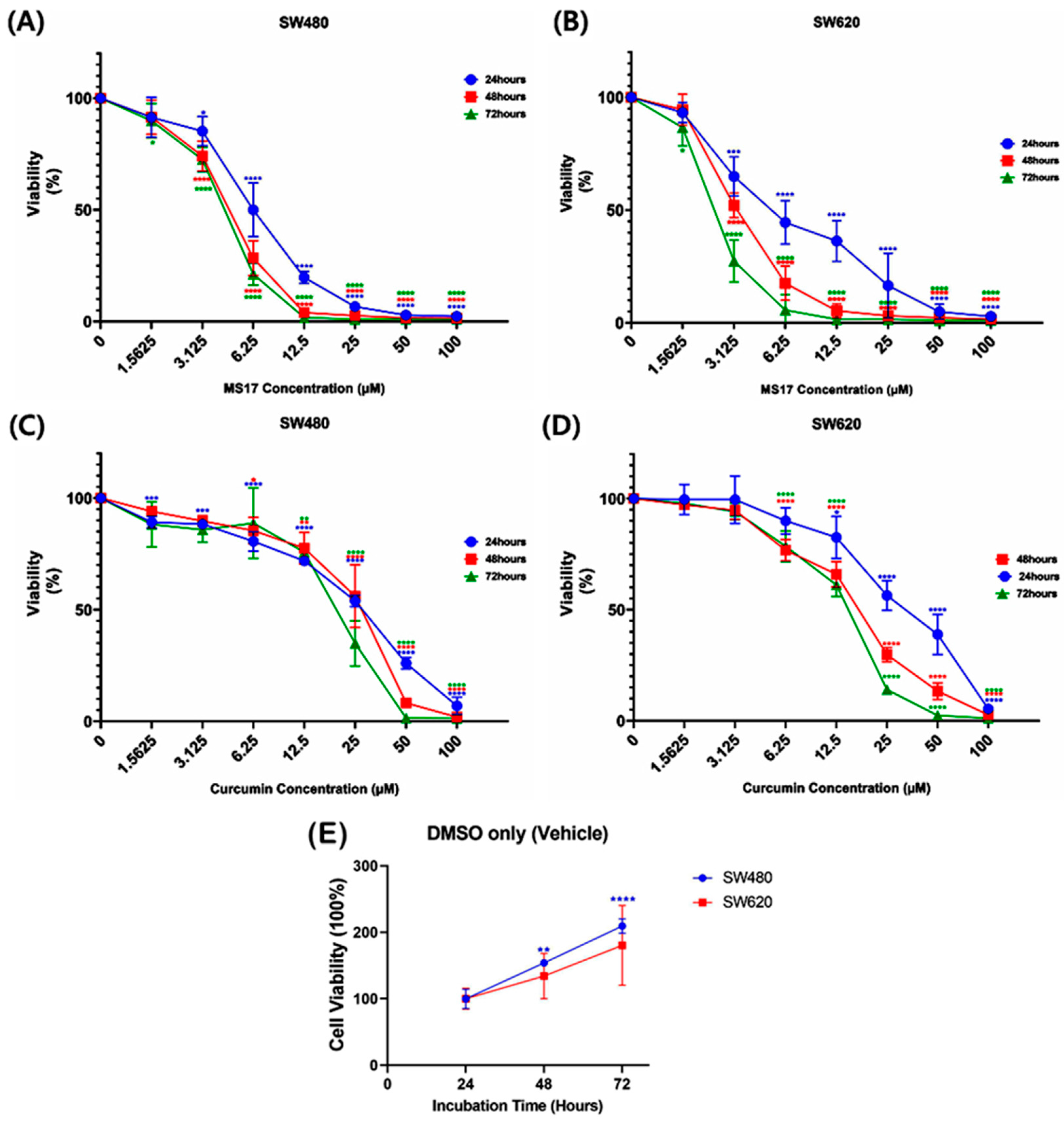
2.2. Anti-Proliferative Effect of MS17 in SW480 and SW620 Colon Cancer Cells
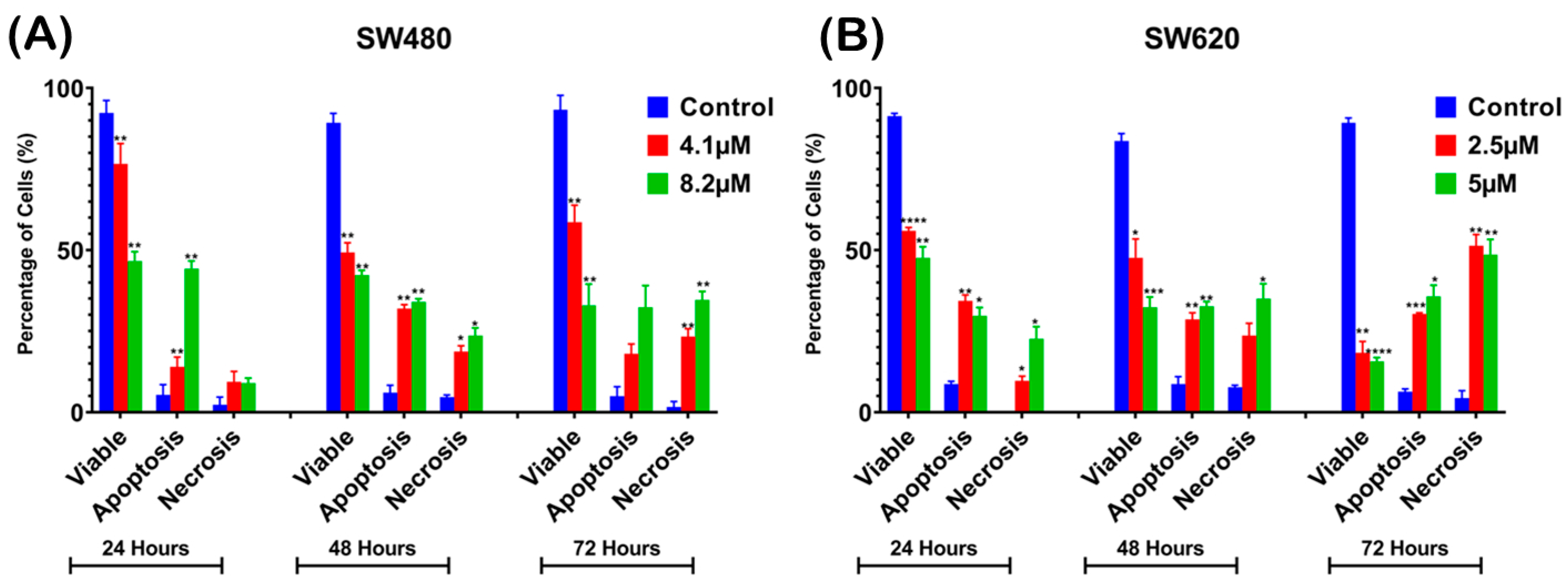
2.3. Induction of Apoptosis by MS17 Treatment in SW480 and SW620 Colon Cancer Cells
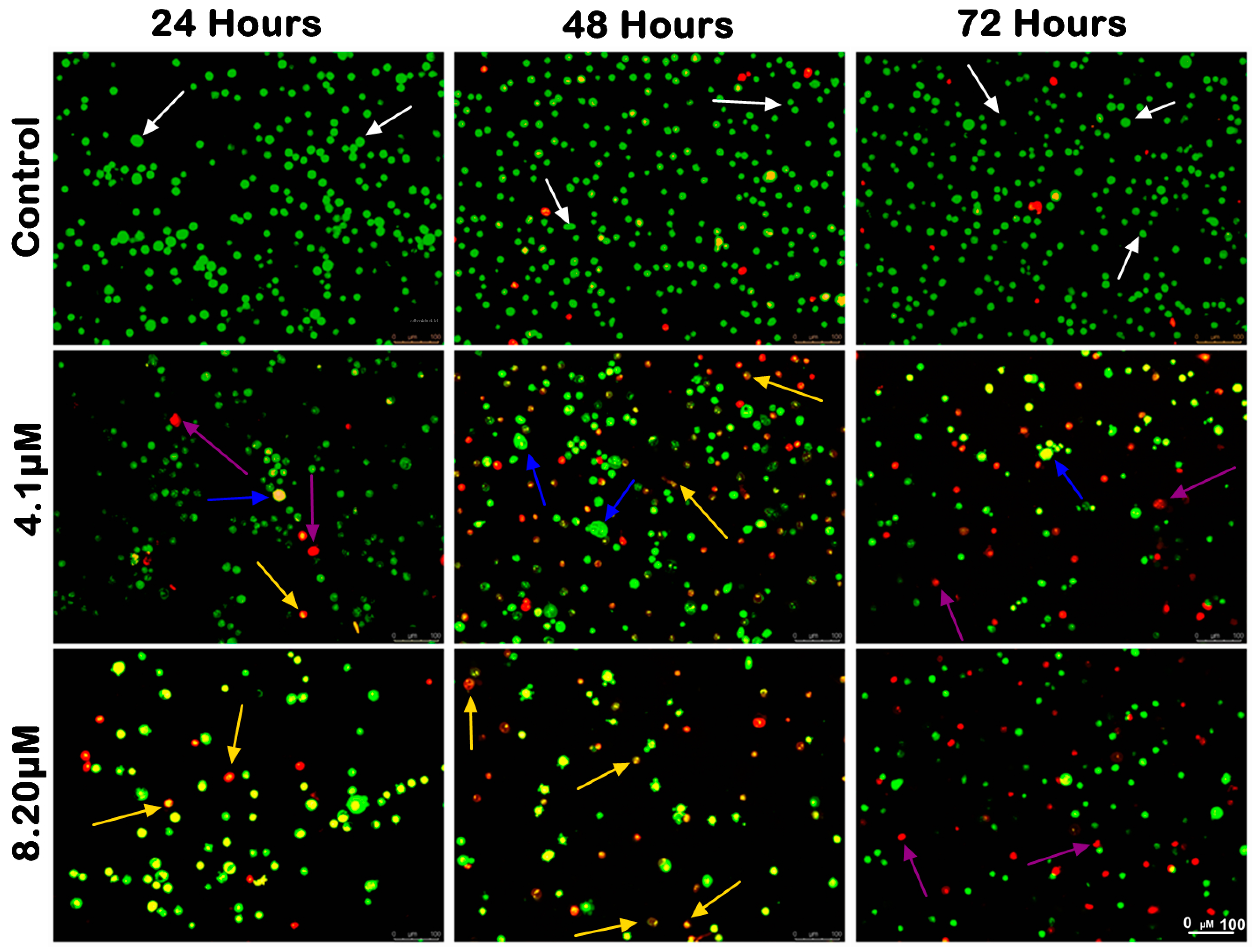
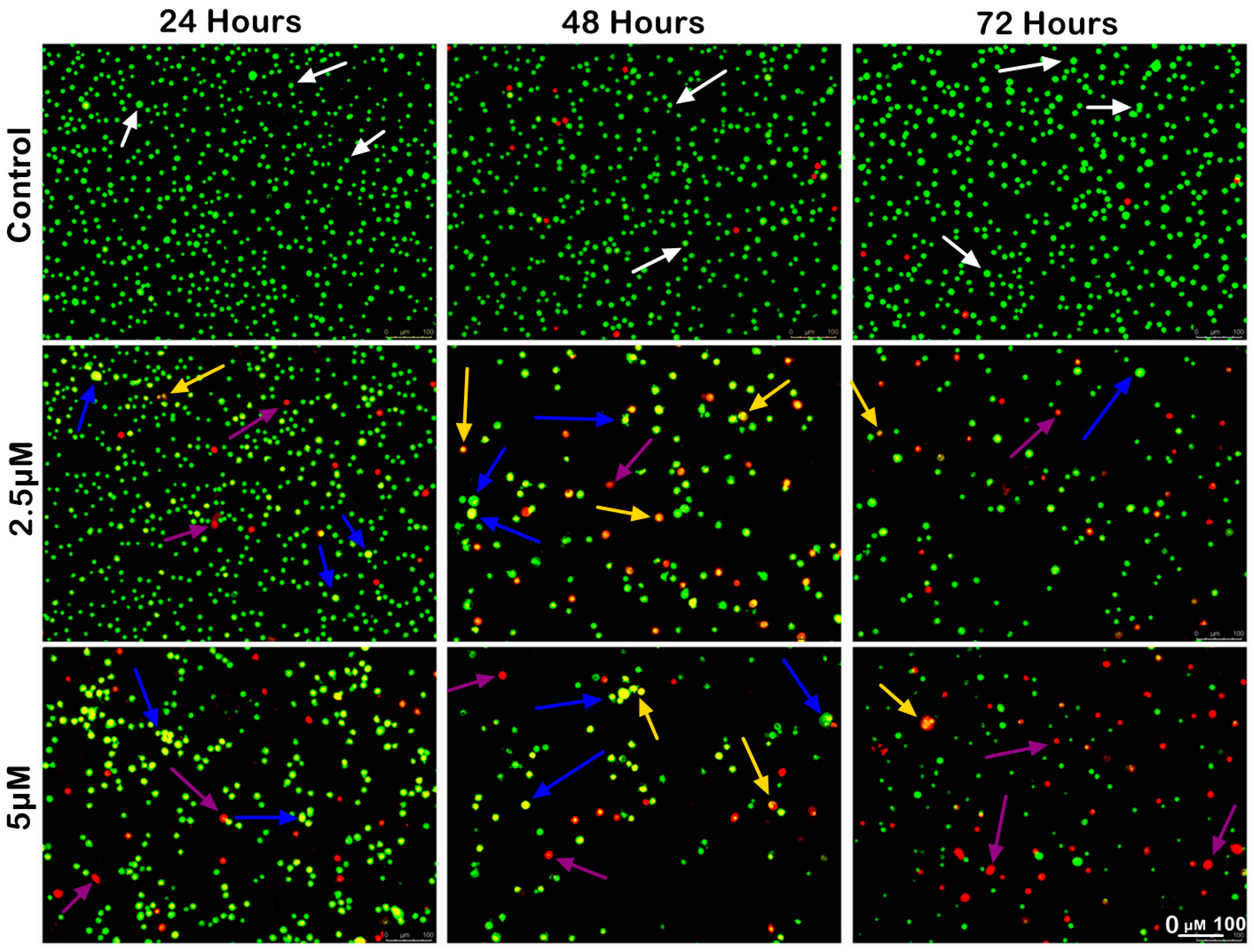
2.3.1. Morphological Observation and Quantitative Analysis of MS17-Treated Colon Cancer Cells
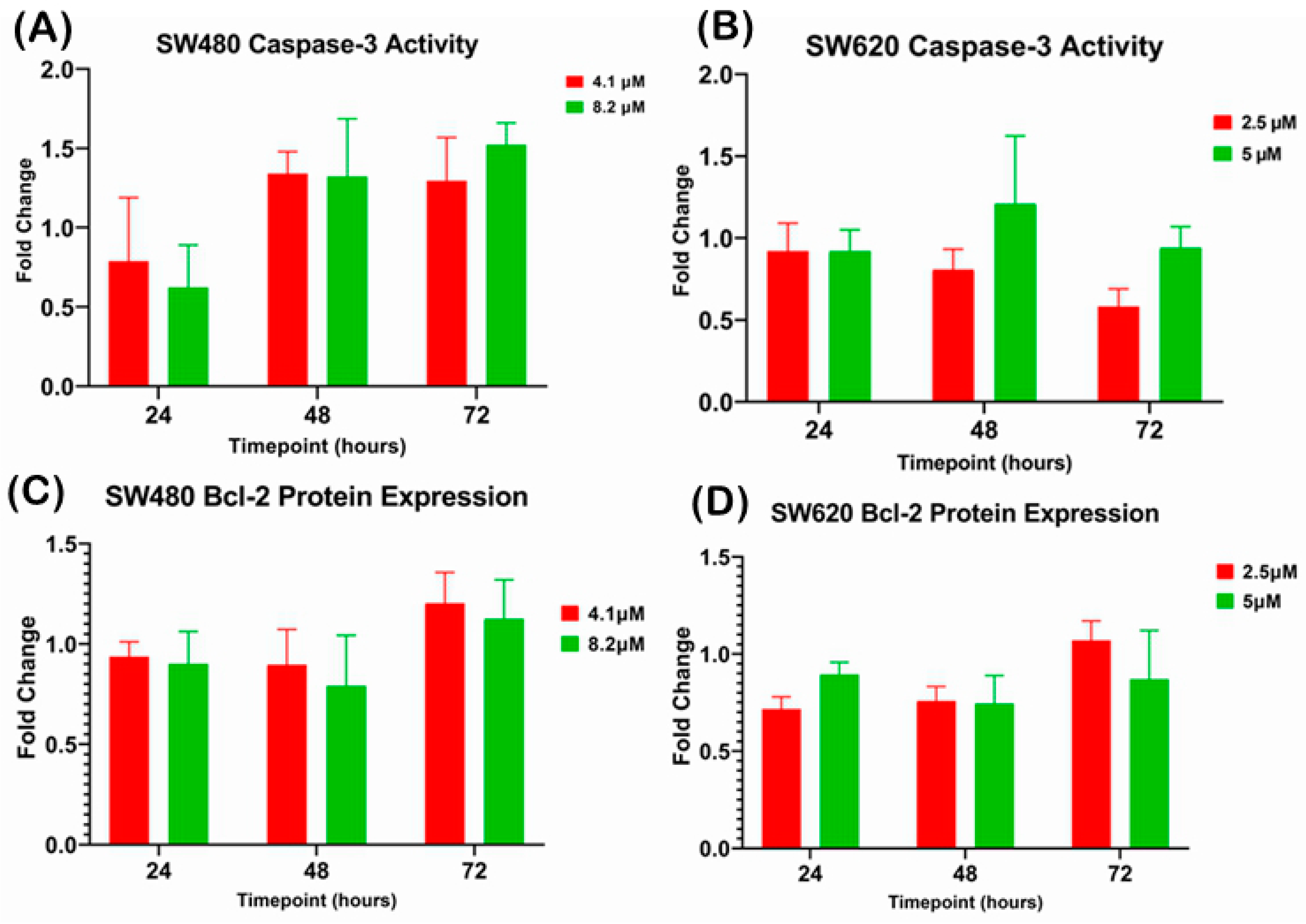
2.3.2. Quantification of Caspase-3 Activity and Bcl-2 Protein Expression upon MS17 Treatment in SW480 and SW620 Colon Cancer Cells
2.4. Proteomic Profiling of MS17-Treated SW480 and SW620 Cells via Shotgun Proteomic Approach
2.4.1. Protein Identification and Classification
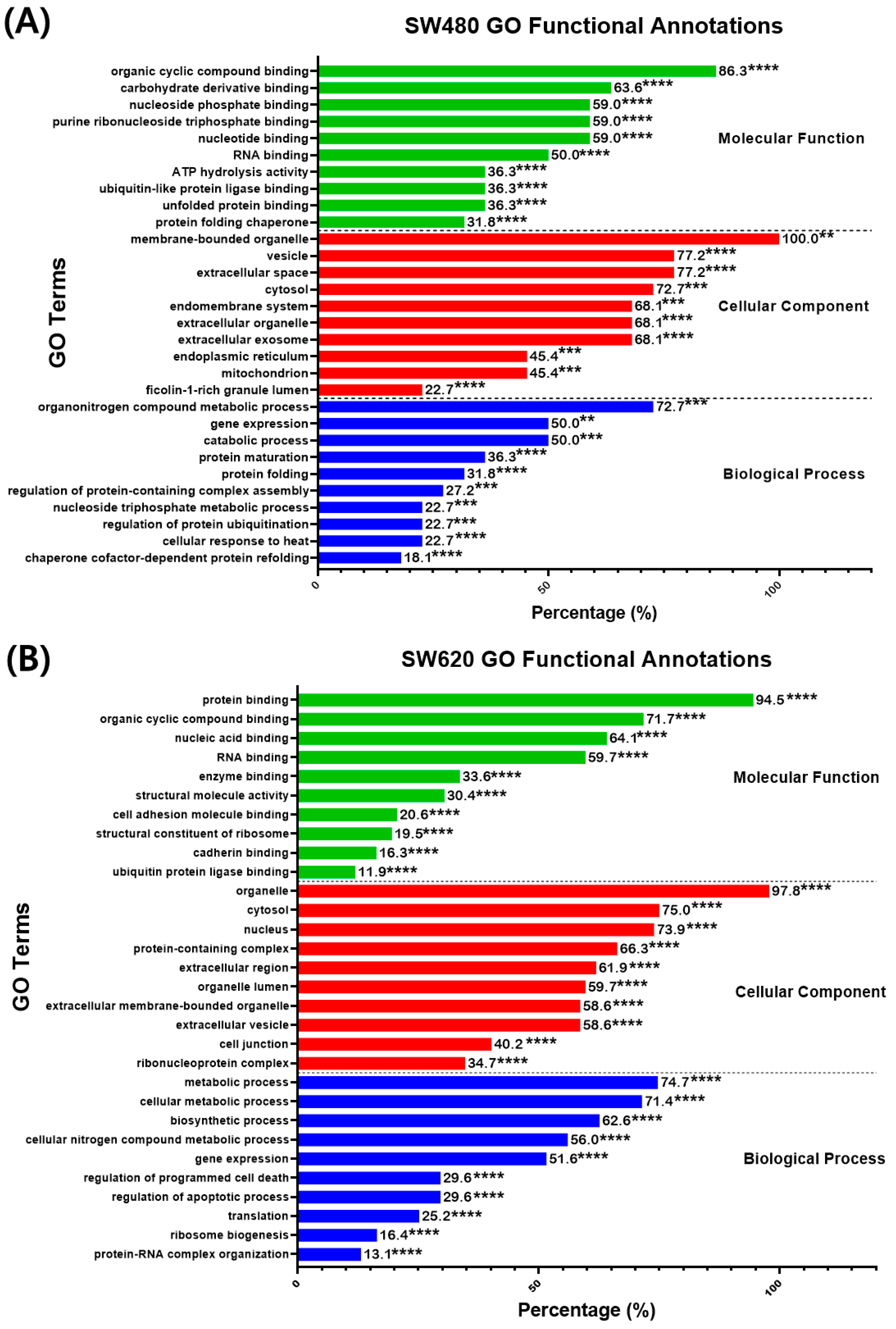
2.4.2. Gene Ontology: Functional Annotations
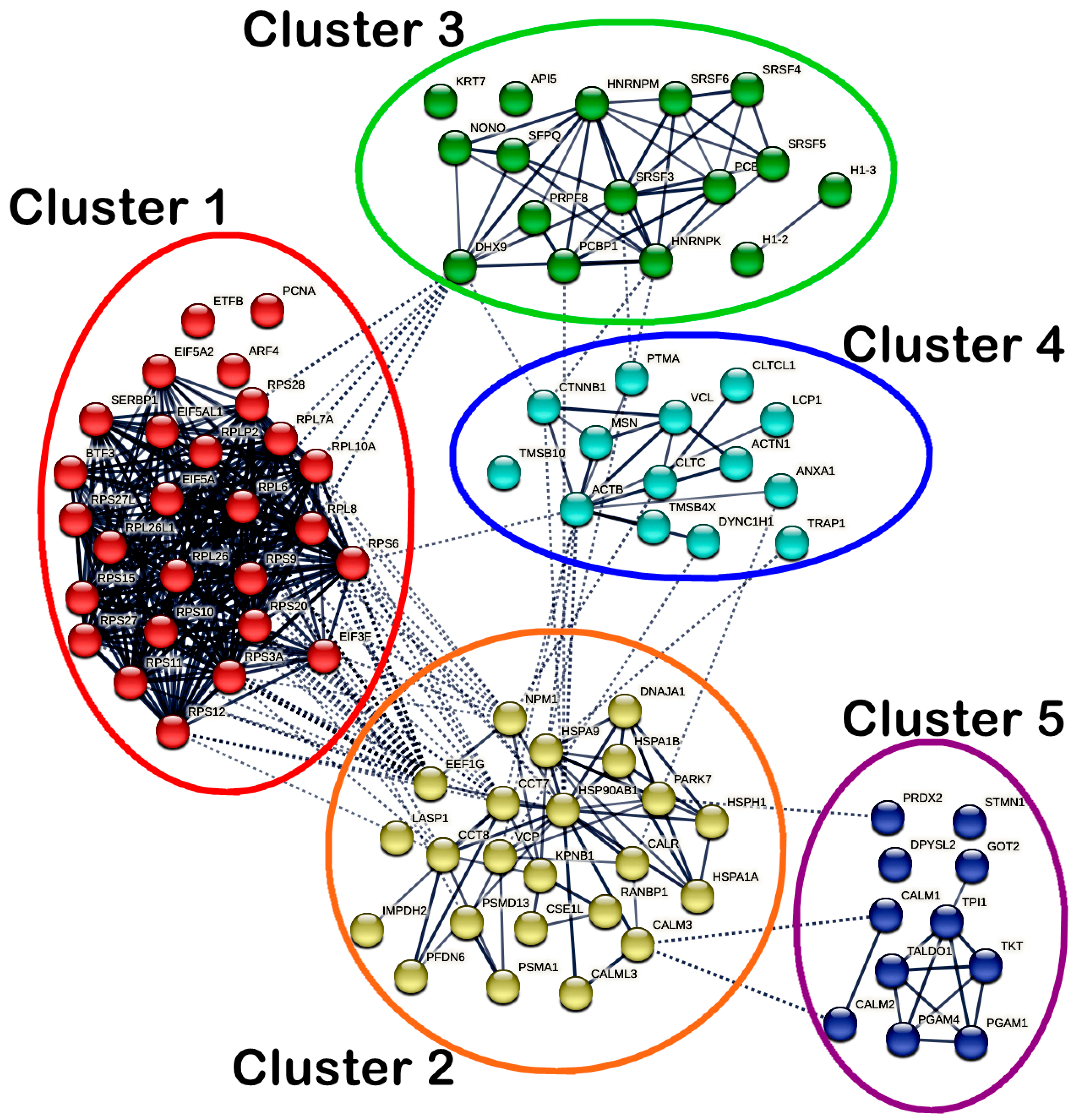
2.4.3. Protein–Protein Interaction (PPI) Network
2.4.4. Reactome Pathway Analysis
3. Discussion
4. Materials and Methods
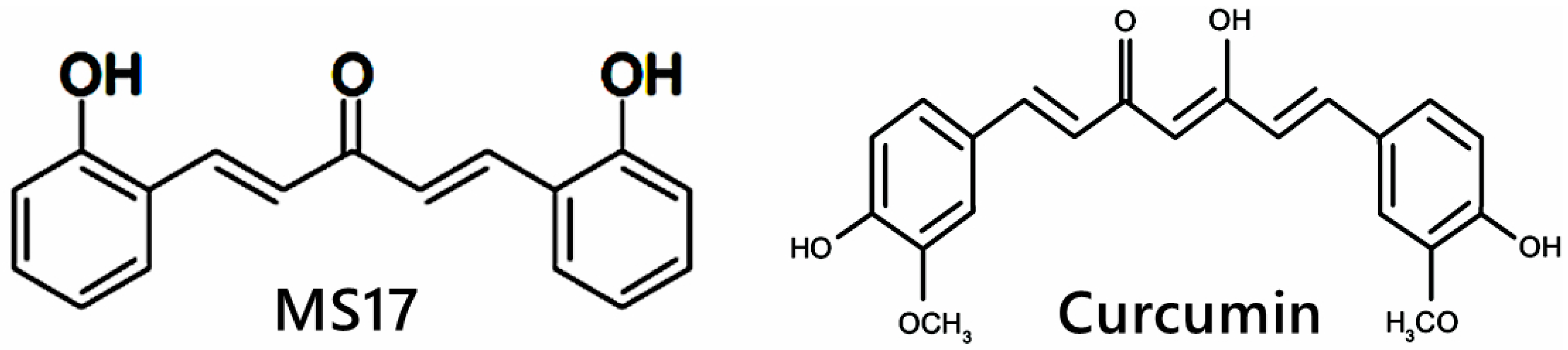
4.1. Preparation of Curcumin Analogue (MS17) and Curcumin
4.2. Cell Culture and Maintenance
4.3. Cytotoxicity and Anti-Proliferative Assays of MS17 and Curcumin
4.4. Induction of Apoptosis with MS17
4.5. Morphological Observation and Quantitative Analysis of Apoptotic Cells by Acridine Orange–Propidium Iodide Double-Staining Technique Using Fluorescence Microscope
4.6. Measurement of Caspase-3 Activity in MS17-Treated SW480 and SW620 Colon Cancer Cells
4.7. Quantification of Bcl-2 Protein Concentration in MS17-Treated SW480 and SW620 Colon Cancer Cells
4.8. MS17 Treatment for Proteomic Analysis
4.9. In-Solution Digestion and Cleanup
4.10. Liquid Chromatography–Tandem Mass Spectrometry (LCMS/MS) Analysis
4.11. Total Protein Identification and Label-Free Quantification
4.12. Bioinformatic Analysis
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F.; Bsc, M.F.B.; Me, J.F.; Soerjomataram, M.I.; et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.R.A.; Ismail, I.; Suan, M.A.M.; Ahmad, F.; Khazim, W.K.W.; Othman, Z.; Said, R.M.; Tan, W.L.; Rahmah, S.; Mohammed, N.S.; et al. Incidence and mortality rates of colorectal cancer in Malaysia. Epidemiol. Health 2016, 38, e2016007. [Google Scholar] [CrossRef]
- Ramzi, N.H.; Chahil, J.K.; Lye, S.H.; Munretnam, K.; Sahadevappa, K.I.; Velapasamy, S.; Hashim, N.A.N.; Cheah, S.K.; Lim, G.C.C.; Hussein, H.; et al. Role of genetic & environment risk factors in the aetiology of colorectal cancer in Malaysia. Indian J. Med. Res. 2014, 139, 873–882. [Google Scholar] [PubMed]
- Veettil, S.K.; Lim, K.G.; Chaiyakunapruk, N.; Ching, S.M.; Abu Hassan, M.R. Colorectal cancer in Malaysia: Its burden and implications for a multiethnic country. Asian J. Surg. 2017, 40, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Wolpin, B.M.; Meyerhardt, J.A.; Mamon, H.J.; Mayer, R.J. Adjuvant Treatment of Colorectal Cancer. CA Cancer J. Clin. 2007, 57, 168–185. [Google Scholar] [CrossRef]
- McQuade, M.R.; Stojanovska, V.; Bornstein, C.J.; Nurgali, K. Colorectal Cancer Chemotherapy: The Evolution of Treatment and New Approaches. Curr. Med. Chem. 2017, 24, 1537–1557. [Google Scholar] [CrossRef] [PubMed]
- Vodenkova, S.; Buchler, T.; Cervena, K.; Veskrnova, V.; Vodicka, P.; Vymetalkova, V. 5-fluorouracil and other fluoropyrimidines in colorectal cancer: Past, present and future. Pharmacol. Ther. 2020, 206, 107447. [Google Scholar] [CrossRef]
- Wang, M.-T.; Jiang, H.; Boral, D.; Nie, D. Cancer Stem Cells in Resistance to Cytotoxic Drugs: Implications in Chemotherapy. In Molecular Mechanisms of Tumor Cell Resistance to Chemotherapy: Targeted Therapies to Reverse Resistance; Bonavida, B., Ed.; Springer: New York, NY, USA, 2013; pp. 151–161. [Google Scholar]
- To, K.K.W.; Wu, M.; Tong, C.W.S.; Yan, W. Drug transporters in the development of multidrug resistance in colorectal cancer. In Drug Resistance in Colorectal Cancer: Molecular Mechanisms and Therapeutic Strategies; Cho, C.H., Hu, T., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 35–55. [Google Scholar]
- Phipps, A.I.; Limburg, P.J.; Baron, J.A.; Burnett-Hartman, A.N.; Weisenberger, D.J.; Laird, P.W.; Sinicrope, F.A.; Rosty, C.; Buchanan, D.D.; Potter, J.D.; et al. Association Between Molecular Subtypes of Colorectal Cancer and Patient Survival. Gastroenterology 2015, 148, 77–87.e2. [Google Scholar] [CrossRef]
- Wu, J.B.; Li, X.J.; Liu, H.; Liu, Y.J.; Liu, X.P. Association of KRAS, NRAS, BRAF and PIK3CA gene mutations with clinicopathological features, prognosis and ring finger protein 215 expression in patients with colorectal cancer. Biomed. Rep. 2023, 19, 104. [Google Scholar] [CrossRef]
- Włodarczyk, M.; Włodarczyk, J.; Siwiński, P.; Sobolewska-Włodarczyk, A.; Fichna, J. Genetic Molecular Subtypes in Optimizing Personalized Therapy for Metastatic Colorectal Cancer. Curr. Drug Targets 2018, 19, 1731–1737. [Google Scholar] [CrossRef]
- Feng, F.; Sun, H.; Zhao, Z.; Sun, C.; Zhao, Y.; Lin, H.; Yang, J.; Xiao, Y.; Wang, W.; Wu, D. Identification of APC Mutation as a Potential Predictor for Immunotherapy in Colorectal Cancer. J. Oncol. 2022, 2022, 6567998. [Google Scholar] [CrossRef] [PubMed]
- Lestari, M.L.A.D.; Indrayanto, G. Chapter Three—Curcumin. In Profiles of Drug Substances, Excipients and Related Methodology; Brittain, H.G., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 113–204. [Google Scholar]
- Mosieniak, G.; Adamowicz, M.; Alster, O.; Jaskowiak, H.; Szczepankiewicz, A.A.; Wilczynski, G.M.; Ciechomska, I.A.; Sikora, E. Curcumin induces permanent growth arrest of human colon cancer cells: Link between senescence and autophagy. Mech. Ageing Dev. 2012, 133, 444–455. [Google Scholar] [CrossRef]
- Lim, T.G.; Lee, S.Y.; Huang, Z.; Lim, D.Y.; Chen, H.; Jung, S.K.; Bode, A.M.; Lee, K.W.; Dong, Z. Curcumin Suppresses Proliferation of Colon Cancer Cells by Targeting CDK2Curcumin Inhibits CDK2 to Suppress Colon Cancer Cell Growth. Cancer Prev. Res. 2014, 7, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.I.; Othman, I.; Abas, F.; Lajis, N.H.; Naidu, R. Mechanism of Apoptosis Induced by Curcumin in Colorectal Cancer. Int. J. Mol. Sci. 2019, 20, 2454. [Google Scholar] [CrossRef]
- Reyhaneh, M.-M.; Seyed Mahdi, H.; Soodabeh, S.; Amir, A.; Majid, K. Curcumin Effects on the Wnt Signaling Pathway in Colorectal Cancer Stem Cells. Basic Clin. Cancer Res. 2018, 10, 33–48. [Google Scholar]
- Weng, W.; Goel, A. Curcumin and colorectal cancer: An update and current perspective on this natural medicine. Semin. Cancer Biol. 2022, 80, 73–86. [Google Scholar] [CrossRef]
- Villota, H.; Röthlisberger, S.; Pedroza-Díaz, J. Modulation of the Canonical Wnt Signaling Pathway by Dietary Polyphenols, an Opportunity for Colorectal Cancer Chemoprevention and Treatment. Nutr. Cancer 2022, 74, 384–404. [Google Scholar] [CrossRef]
- Toden, S.; Okugawa, Y.; Buhrmann, C.; Nattamai, D.; Anguiano, E.; Baldwin, N.; Shakibaei, M.; Boland, C.R.; Goel, A. Novel evidence for curcumin and boswellic acid–induced chemoprevention through regulation of miR-34a and miR-27a in colorectal cancer. Cancer Prev. Res. 2015, 8, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Mudduluru, G.; George-William, J.N.; Muppala, S.; Asangani, I.A.; Kumarswamy, R.; Nelson, L.D.; Allgayer, H. Curcumin regulates miR-21 expression and inhibits invasion and metastasis in colorectal cancer. Biosci. Rep. 2011, 31, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Gandhy, S.U.; Kim, K.; Larsen, L.; Rosengren, R.J.; Safe, S. Curcumin and synthetic analogs induce reactive oxygen species and decreases specificity protein (Sp) transcription factors by targeting microRNAs. BMC Cancer 2012, 12, 564. [Google Scholar] [CrossRef]
- Shakibaei, M.; Mobasheri, A.; Lueders, C.; Busch, F.; Shayan, P.; Goel, A. Curcumin enhances the effect of chemotherapy against colorectal cancer cells by inhibition of NF-κB and Src protein kinase signaling pathways. PLoS ONE 2013, 8, e57218. [Google Scholar] [CrossRef]
- Howells, L.M.; Iwuji, C.O.O.; Irving, G.R.B.; Barber, S.; Walter, H.; Sidat, Z.; Griffin-Teall, N.; Singh, R.; Foreman, N.; Patel, S.R.; et al. Curcumin Combined with FOLFOX Chemotherapy Is Safe and Tolerable in Patients with Metastatic Colorectal Cancer in a Randomized Phase IIa Trial. J. Nutr. 2019, 149, 1133–1139. [Google Scholar] [CrossRef]
- Fan, W.-H.; Wang, F.-C.; Jin, Z.; Zhu, L.; Zhang, J.-X. Curcumin Synergizes with Cisplatin to Inhibit Colon Cancer through Targeting the MicroRNA-137-Glutaminase Axis. Curr. Med. Sci. 2021, 42, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Abdul Satar, N.; Ismail, M.N.; Yahaya, B.H. Synergistic Roles of Curcumin in Sensitising the Cisplatin Effect on a Cancer Stem Cell-Like Population Derived from Non-Small Cell Lung Cancer Cell Lines. Molecules 2021, 26, 1056. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhai, Y.; Heng, X.; Che, F.Y.; Chen, W.; Sun, D.; Zhai, G. Oral bioavailability of curcumin: Problems and advancements. J. Drug Target. 2016, 24, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Harsha, C.; Banik, K.; Vikkurthi, R.; Sailo, B.L.; Bordoloi, D.; Gupta, S.C.; Aggarwal, B.B. Is curcumin bioavailability a problem in humans: Lessons from clinical trials. Expert Opin. Drug Metab. Toxicol. 2019, 15, 705–733. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, K.; Rasoulpoor, S.; Daneshkhah, A.; Abolfathi, S.; Salari, N.; Mohammadi, M.; Shabani, S. Clinical effects of curcumin in enhancing cancer therapy: A systematic review. BMC Cancer 2020, 20, 791. [Google Scholar] [CrossRef] [PubMed]
- Alven, S.; Aderibigbe, B.A. Efficacy of Polymer-Based Nanocarriers for Co-Delivery of Curcumin and Selected Anticancer Drugs. Nanomaterials 2020, 10, 1556. [Google Scholar] [CrossRef]
- Anthwal, A.; Thakur, B.K.; Rawat, M.S.M.; Rawat, D.S.; Tyagi, A.K.; Aggarwal, B.B. Synthesis, characterization and in vitro anticancer activity of C-5 curcumin analogues with potential to inhibit TNF-α-induced NF-κB activation. BioMed Res. Int. 2014, 2014, 524161. [Google Scholar] [CrossRef]
- Arshad, L.; Haque, M.A.; Bukhari, S.N.A.; Jantan, I. An overview of structure–activity relationship studies of curcumin analogs as antioxidant and anti-inflammatory agents. Future Med. Chem. 2017, 9, 605–626. [Google Scholar] [CrossRef] [PubMed]
- Cavaleri, F. Presenting a New Standard Drug Model for Turmeric and Its Prized Extract, Curcumin. Int. J. Inflamm. 2018, 2018, 5023429. [Google Scholar] [CrossRef] [PubMed]
- Chainoglou, E.; Hadjipavlou-Litina, D. Curcumin analogues and derivatives with anti-proliferative and anti-inflammatory activity: Structural characteristics and molecular targets. Expert Opin. Drug Discov. 2019, 14, 821–842. [Google Scholar] [CrossRef] [PubMed]
- Charan, T.R.; Bhutto, M.A.; Bhutto, M.A.; Tunio, A.A.; Khuhro, G.M.; Khaskheli, S.A.; Mughal, A.A. “Nanomaterials of curcumin-hyaluronic acid”: Their various methods of formulations, clinical and therapeutic applications, present gap, and future directions. Future J. Pharm. Sci. 2021, 7, 126. [Google Scholar] [CrossRef]
- Ciochina, R.; Savella, C.; Cote, B.; Chang, D.; Rao, D. Synthesis and Characterization of New Curcumin Derivatives as Potential Chemotherapeutic and Antioxidant Agents. Drug Dev. Res. 2014, 75, 88–96. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, N.A.; Noronha, M.A.; Kurnik, I.S.; Câmara, M.C.; Vieira, J.M.; Abrunhosa, L.; Martins, J.T.; Alves, T.F.; Tundisi, L.L.; Ataide, J.A.; et al. Curcumin encapsulation in nanostructures for cancer therapy: A 10-year overview. Int. J. Pharm. 2021, 604, 120534. [Google Scholar] [CrossRef]
- Kabir, T.; Rahman, H.; Akter, R.; Behl, T.; Kaushik, D.; Mittal, V.; Pandey, P.; Akhtar, M.F.; Saleem, A.; Albadrani, G.M.; et al. Potential Role of Curcumin and Its Nanoformulations to Treat Various Types of Cancers. Biomolecules 2021, 11, 392. [Google Scholar] [CrossRef]
- Karthikeyan, A.; Senthil, N.; Min, T. Nanocurcumin: A Promising Candidate for Therapeutic Applications. Front. Pharmacol. 2020, 11, 487. [Google Scholar] [CrossRef]
- Gupta, A.P.; Khan, S.; Manzoor, M.M.; Yadav, A.K.; Sharma, G.; Anand, R.; Gupta, S. Chapter 10—Anticancer Curcumin: Natural Analogues and Structure-Activity Relationship. In Studies in Natural Products Chemistry; Atta ur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 355–401. [Google Scholar]
- Shahriari, M.; Kesharwani, P.; Johnston, T.P.; Sahebkar, A. Anticancer potential of curcumin-cyclodextrin complexes and their pharmacokinetic properties. Int. J. Pharm. 2023, 631, 122474. [Google Scholar] [CrossRef]
- Nag, A.; Chakraborty, P.; Natarajan, G.; Baksi, A.; Mudedla, S.K.; Subramanian, V.; Pradeep, T. Bent Keto Form of Curcumin, Preferential Stabilization of Enol by Piperine, and Isomers of Curcumin∩Cyclodextrin Complexes: Insights from Ion Mobility Mass Spectrometry. Anal. Chem. 2018, 90, 8776–8784. [Google Scholar] [CrossRef]
- Alizadeh, N.; Malakzadeh, S. Changes in chemical stability and bioactivities of curcumin by forming inclusion complexes of beta- and Gama-cyclodextrins. J. Polym. Res. 2020, 27, 42. [Google Scholar] [CrossRef]
- Khudhayer Oglah, M.; Fakri Mustafa, Y. Curcumin analogs: Synthesis and biological activities. Med. Chem. Res. 2020, 29, 479–486. [Google Scholar] [CrossRef]
- Tabanelli, R.; Brogi, S.; Calderone, V. Improving Curcumin Bioavailability: Current strategies and future perspectives. Pharmaceutics 2021, 13, 1715. [Google Scholar] [CrossRef]
- Joshi, P.; Verma, K.; Kumar Semwal, D.; Dwivedi, J.; Sharma, S. Mechanism insights of curcumin and its analogues in cancer: An update. Phytother. Res. 2023, 37, 5435–5463. [Google Scholar] [CrossRef] [PubMed]
- Clariano, M.; Marques, V.; Vaz, J.; Awam, S.; Afonso, M.B.; Perry, M.J.; Rodrigues, C.M.P. Monocarbonyl Analogs of Curcumin with Potential to Treat Colorectal Cancer. Chem. Biodivers. 2023, 20, e202300222. [Google Scholar] [CrossRef] [PubMed]
- Padhye, S.; Yang, H.; Jamadar, A.; Cui, Q.C.; Chavan, D.; Dominiak, K.; McKinney, J.; Banerjee, S.; Dou, Q.P.; Sarkar, F.H. New difluoro Knoevenagel condensates of curcumin, their Schiff bases and copper complexes as proteasome inhibitors and apoptosis inducers in cancer cells. Pharm. Res. 2009, 26, 1874–1880. [Google Scholar] [CrossRef] [PubMed]
- Huber, I.; Zupkó, I.; Gyovai, A.; Horváth, P.; Kiss, E.; Gulyás-Fekete, G.; Schmidt, J.; Perjési, P. A novel cluster of C5-curcuminoids: Design, synthesis, in vitro antiproliferative activity and DNA binding of bis(arylidene)-4-cyclanone derivatives based on 4-hydroxycyclohexanone scaffold. Res. Chem. Intermed. 2019, 45, 4711–4735. [Google Scholar] [CrossRef]
- Citalingam, K.; Abas, F.; Lajis, N.H.; Othman, I.; Naidu, R. Anti-proliferative effect and induction of apoptosis in androgen-independent human prostate cancer cells by 1,5-bis(2-hydroxyphenyl)-1,4-pentadiene-3-one. Molecules 2015, 20, 3406–3430. [Google Scholar] [CrossRef] [PubMed]
- Nagaraju, G.P.; Benton, L.; Bethi, S.R.; Shoji, M.; El-Rayes, B.F. Curcumin analogs: Their roles in pancreatic cancer growth and metastasis. Int. J. Cancer 2019, 145, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Noureddin, S.A.; El-Shishtawy, R.M.; Al-Footy, K.O. Curcumin analogues and their hybrid molecules as multifunctional drugs. Eur. J. Med. Chem. 2019, 182, 111631. [Google Scholar] [CrossRef]
- Friedman, L.; Lin, L.; Ball, S.; Bekaii-Saab, T.; Fuchs, J.; Li, P.-K.; Li, C.; Lin, J. Curcumin analogues exhibit enhanced growth suppressive activity in human pancreatic cancer cells. Anti-Cancer Drugs 2009, 20, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.L.; Zhong, D.; Zhou, W.; Malik, S.; Liotta, D.; Snyder, J.P.; Hamel, E.; Giannakakou, P. EF24, a novel curcumin analog, disrupts the microtubule cytoskeleton and inhibits HIF-1. Cell Cycle 2008, 7, 2409–2417. [Google Scholar] [CrossRef] [PubMed]
- Adeluola, A.; Zulfiker, A.H.M.; Brazeau, D.; Amin, A. Perspectives for synthetic curcumins in chemoprevention and treatment of cancer: An update with promising analogues. Eur. J. Pharmacol. 2021, 906, 174266. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.S.; Chen, L.Z.; Zhou, H.P.; Liu, X.H.; Chen, F.H. Diarylpentadienone derivatives (curcumin analogues): Synthesis and anti-inflammatory activity. Bioorganic Med. Chem. Lett. 2017, 27, 1803–1807. [Google Scholar] [CrossRef] [PubMed]
- Amalraj, A.; Pius, A.; Gopi, S.; Gopi, S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—A review. J. Tradit. Complement. Med. 2017, 7, 205–233. [Google Scholar] [CrossRef] [PubMed]
- Novais, P.; Silva, P.M.A.; Moreira, J.; Palmeira, A.; Amorim, I.; Pinto, M.; Cidade, H.; Bousbaa, H. BP-M345, a New Diarylpentanoid with Promising Antimitotic Activity. Molecules 2021, 26, 7139. [Google Scholar] [CrossRef]
- Qudjani, E.; Iman, M.; Davood, A.; Ramandi, M.F.; Shafiee, A. Design and Synthesis of Curcumin-like Diarylpentanoid Analogues as Potential Anticancer Agents. Recent Pat. Anticancer Drug Discov. 2016, 11, 342–351. [Google Scholar] [CrossRef]
- Wan Mohd Tajuddin, W.N.B.; Abas, F.; Othman, I.; Naidu, R. Molecular Mechanisms of Antiproliferative and Apoptosis Activity by 1,5-Bis(4-Hydroxy-3-Methoxyphenyl)1,4-Pentadiene-3-one (MS13) on Human Non-Small Cell Lung Cancer Cells. Int. J. Mol. Sci. 2021, 22, 7424. [Google Scholar] [CrossRef]
- Abd Wahab, N.A.; Abas, F.; Othman, I.; Naidu, R. Diarylpentanoid (1,5-bis(4-hydroxy-3-methoxyphenyl)-1,4-pentadiene-3-one) (MS13) Exhibits Anti-proliferative, Apoptosis Induction and Anti-migration Properties on Androgen-independent Human Prostate Cancer by Targeting Cell Cycle-Apoptosis and PI3K Signalling Pathways. Front. Pharmacol. 2021, 12, 707335. [Google Scholar]
- Ismail, N.I.; Othman, I.; Abas, F.; Lajis, N.H.; Naidu, R. The Curcumin Analogue, MS13 (1,5-Bis(4-hydroxy-3- methoxyphenyl)-1,4-pentadiene-3-one), Inhibits Cell Proliferation and Induces Apoptosis in Primary and Metastatic Human Colon Cancer Cells. Molecules 2020, 25, 3798. [Google Scholar] [CrossRef]
- He, G.; Feng, C.; Vinothkumar, R.; Chen, W.; Dai, X.; Chen, X.; Ye, Q.; Qiu, C.; Zhou, H.; Wang, Y.; et al. Curcumin analog EF24 induces apoptosis via ROS-dependent mitochondrial dysfunction in human colorectal cancer cells. Cancer Chemother. Pharmacol. 2016, 78, 1151–1161. [Google Scholar] [CrossRef]
- Kanwar, S.S.; Yu, Y.; Nautiyal, J.; Patel, B.B.; Padhye, S.; Sarkar, F.H.; Majumdar, A.P.N. Difluorinated-Curcumin (CDF): A Novel Curcumin Analog is a Potent Inhibitor of Colon Cancer Stem-like Cells. Pharm. Res. 2011, 28, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Paulraj, F.; Abas, F.; Lajis, N.H.; Othman, I.; Hassan, S.S.; Naidu, R. The Curcumin Analogue 1,5-Bis(2-hydroxyphenyl)-1,4-pentadiene-3-one Induces Apoptosis and Downregulates E6 and E7 Oncogene Expression in HPV16 and HPV18-Infected Cervical Cancer Cells. Molecules 2015, 20, 11830–11860. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Liu, Y.; Li, H.; Li, P.-K.; Fuchs, J.; Shibata, H.; Iwabuchi, Y.; Lin, J. Targeting colon cancer stem cells using a new curcumin analogue, GO-Y030. Br. J. Cancer 2011, 105, 212–220. [Google Scholar] [CrossRef]
- Citalingam, K.; Abas, F.; Lajis, N.; Othman, I.; Naidu, R. Identification of commonly regulated protein targets and molecular pathways in PC-3 and DU145 androgen-independent human prostate cancer cells treated with the curcumin analogue 1,5-bis(2-hydroxyphenyl)-1,4-pentadiene-3-one. Asian Pac. J. Trop. Biomed. 2018, 8, 436–445. [Google Scholar]
- Lee, K.-H.; Aziz, F.H.A.; Syahida, A.; Abas, F.; Shaari, K.; Israf, D.A.; Lajis, N.H. Synthesis and biological evaluation of curcumin-like diarylpentanoid analogues for anti-inflammatory, antioxidant and anti-tyrosinase activities. Eur. J. Med. Chem. 2009, 44, 3195–3200. [Google Scholar] [CrossRef]
- Cen, L.; Hutzen, B.; Ball, S.; DeAngelis, S.; Chen, C.-L.; Fuchs, J.R.; Li, C.; Li, P.-K.; Lin, J. New structural analogues of curcumin exhibit potent growth suppressive activity in human colorectal carcinoma cells. BMC Cancer 2009, 9, 99. [Google Scholar] [CrossRef]
- Jitoe-Masuda, A.; Fujimoto, A.; Masuda, T. Curcumin: From Chemistry to Chemistry-Based Functions. Curr. Pharm. Des. 2013, 19, 2084–2092. [Google Scholar] [PubMed]
- Indira Priyadarsini, K. Chemical and structural features influencing the biological activity of curcumin. Curr. Pharm. Des. 2013, 19, 2093–2100. [Google Scholar]
- Melcher, R.; Steinlein, C.; Feichtinger, W.; Müller, C.; Menzel, T.; Lührs, H.; Scheppach, W.; Schmid, M. Spectral karyotyping of the human colon cancer cell lines SW480 and SW620. Cytogenet. Cell Genet. 2000, 88, 145–152. [Google Scholar] [CrossRef]
- Hewitt, R.E.; McMarlin, A.; Kleiner, D.; Wersto, R.; Martin, P.; Tsoskas, M.; Stamp, G.W.; Stetler-Stevenson, W.G. Validation of a model of colon cancer progression. J. Pathol. 2000, 192, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Yang, W.; Liu, Z.; Wu, G. Characterization of microRNA expression in primary human colon adenocarcinoma cells (SW480) and their lymph node metastatic derivatives (SW620). OncoTargets Ther. 2018, 11, 4701–4709. [Google Scholar] [CrossRef]
- Schønberg, S.A.; Lundemo, A.G.; Fladvad, T.; Holmgren, K.; Bremseth, H.; Nilsen, A.; Gederaas, O.; Tvedt, K.E.; Egeberg, K.W.; Krokan, H.E. Closely related colon cancer cell lines display different sensitivity to polyunsaturated fatty acids, accumulate different lipid classes and downregulate sterol regulatory element-binding protein1. FEBS J. 2006, 273, 2749–2765. [Google Scholar] [CrossRef]
- Bauer, K.M.; Lambert, P.A.; Hummon, A.B. Comparative label-free LC-MS/MS analysis of colorectal adenocarcinoma and metastatic cells treated with 5-fluorouracil. Proteomics 2012, 12, 1928–1937. [Google Scholar] [CrossRef]
- Fhaner, C.J.; Liu, S.; Ji, H.; Simpson, R.J.; Reid, G.E. Comprehensive Lipidome Profiling of Isogenic Primary and Metastatic Colon Adenocarcinoma Cell Lines. Anal. Chem. 2012, 84, 8917–8926. [Google Scholar] [CrossRef] [PubMed]
- McCool, E.N.; Xu, T.; Chen, W.; Beller, N.C.; Nolan, S.M.; Hummon, A.B.; Liu, X.; Sun, L. Deep top-down proteomics revealed significant proteoform-level differences between metastatic and nonmetastatic colorectal cancer cells. Sci. Adv. 2022, 8, eabq6348. [Google Scholar] [CrossRef]
- Kubens, B.S.; Zänker, K.S. Differences in the migration capacity of primary human colon carcinoma cells (SW480) and their lymph node metastatic derivatives (SW620). Cancer Lett. 1998, 131, 55–64. [Google Scholar] [CrossRef]
- Siekmann, W.; Tina, E.; Koskela von Sydow, A.; Gupta, A. Effect of lidocaine and ropivacaine on primary (SW480) and metastatic (SW620) colon cancer cell lines. Oncol. Lett. 2019, 18, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Abdulrehman, G.; Xv, K.; Li, Y.; Kang, L. Effects of meta-tetrahydroxyphenylchlorin photodynamic therapy on isogenic colorectal cancer SW480 and SW620 cells with different metastatic potentials. Lasers Med. Sci. 2018, 33, 1581–1590. [Google Scholar] [CrossRef]
- Cecilia Subauste, M.; Kupriyanova, T.A.; Conn, E.M.; Ardi, V.C.; Quigley, J.P.; Deryugina, E.I. Evaluation of metastatic and angiogenic potentials of human colon carcinoma cells in chick embryo model systems. Clin. Exp. Metastasis 2009, 26, 1033–1047. [Google Scholar] [CrossRef]
- Rashmi, R.; Santhosh Kumar, T.R.; Karunagaran, D. Human colon cancer cells differ in their sensitivity to curcumin-induced apoptosis and heat shock protects them by inhibiting the release of apoptosis-inducing factor and caspases. FEBS Lett. 2003, 538, 19–24. [Google Scholar] [CrossRef]
- Sato, T.; Higuchi, Y.; Shibagaki, Y.; Hattori, S. Phosphoproteomic Analysis Identifies Signaling Pathways Regulated by Curcumin in Human Colon Cancer Cells. Anticancer Res. 2017, 37, 4789–4798. [Google Scholar]
- Hussar, P. Apoptosis Regulators Bcl-2 and Caspase-3. Encyclopedia 2022, 2, 1624–1636. [Google Scholar] [CrossRef]
- Kirsch, D.G.; Doseff, A.; Chau, B.N.; Lim, D.-S.; de Souza-Pinto, N.C.; Hansford, R.; Kastan, M.B.; Lazebnik, Y.A.; Hardwick, J.M. Caspase-3-dependent Cleavage of Bcl-2 Promotes Release of Cytochrome, C. J. Biol. Chem. 1999, 274, 21155–21161. [Google Scholar] [CrossRef]
- Galluzzi, L.; López-Soto, A.; Kumar, S.; Kroemer, G. Caspases Connect Cell-Death Signaling to Organismal Homeostasis. Immunity 2016, 44, 221–231. [Google Scholar] [CrossRef]
- Van Opdenbosch, N.; Lamkanfi, M. Caspases in Cell Death, Inflammation, and Disease. Immunity 2019, 50, 1352–1364. [Google Scholar] [CrossRef]
- Nirmala, J.G.; Lopus, M. Cell death mechanisms in eukaryotes. Cell Biol. Toxicol. 2020, 36, 145–164. [Google Scholar] [CrossRef]
- Watson, J.L.; Hill, R.; Yaffe, P.B.; Greenshields, A.; Walsh, M.; Lee, P.W.; Giacomantonio, C.A.; Hoskin, D.W. Curcumin causes superoxide anion production and p53-independent apoptosis in human colon cancer cells. Cancer Lett. 2010, 297, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Mao, Y.B.; Cai, Q.F.; Yao, L.M.; Ouyang, G.L.; Bao, S.D. Curcumin induces human HT-29 colon adenocarcinoma cell apoptosis by activating p53 and regulating apoptosis-related protein expression. Braz. J. Med. Biol. Res. 2005, 38, 1791–1798. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.-D.; Chen, X.-J.; Hu, Y.-H.; Yu, Z.-J.; Wang, D.; Liu, J.-Z. Curcumin Inhibits Proliferation and Induces Apoptosis of Human Colorectal Cancer Cells by Activating the Mitochondria Apoptotic Pathway. Phytother. Res. 2013, 27, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Su, C.-C.; Lin, J.-G.; Li, T.-M.; Chung, J.G.; Yang, J.S.; Ip, S.-W.; Lin, W.-C.; Chen, G.-W. Curcumin-induced Apoptosis of Human Colon Cancer Colo 205 Cells through the Production of ROS, Ca2+ and the Activation of Caspase-3. Anticancer. Res. 2006, 26, 4379–4389. [Google Scholar] [PubMed]
- Weng, Q.; Fu, L.; Chen, G.; Hui, J.; Song, J.; Feng, J.; Shi, D.; Cai, Y.; Ji, J.; Liang, G. Design, synthesis, and anticancer evaluation of long-chain alkoxylated mono-carbonyl analogues of curcumin. Eur. J. Med. Chem. 2015, 103, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, D.; May, R.; Sureban, S.M.; Lee, K.B.; George, R.; Kuppusamy, P.; Ramanujam, R.P.; Hideg, K.; Dieckgraefe, B.K.; Houchen, C.W.; et al. Diphenyl Difluoroketone: A Curcumin Derivative with Potent In vivo Anticancer Activity. Cancer Res. 2008, 68, 1962–1969. [Google Scholar] [CrossRef]
- Yang, S.-J.; Lee, S.A.; Park, M.-G.; Kim, J.-S.; Yu, S.-K.; Kim, C.S.; Kim, J.-S.; Kim, S.-G.; Oh, J.-S.; Kim, H.-J.; et al. Induction of apoptosis by diphenyldifluoroketone in osteogenic sarcoma cells is associated with activation of caspases. Oncol. Rep. 2014, 31, 2286–2292. [Google Scholar] [CrossRef]
- Lee, Y.Q.; Rajadurai, P.; Abas, F.; Othman, I.; Naidu, R. Proteomic Analysis on Anti-Proliferative and Apoptosis Effects of Curcumin Analog, 1,5-bis(4-Hydroxy-3-Methyoxyphenyl)-1,4-Pentadiene-3-One-Treated Human Glioblastoma and Neuroblastoma Cells. Front. Mol. Biosci. 2021, 8, 645856. [Google Scholar] [CrossRef]
- Rual, J.-F.; Venkatesan, K.; Hao, T.; Hirozane-Kishikawa, T.; Dricot, A.; Li, N.; Berriz, G.F.; Gibbons, F.D.; Dreze, M.; Ayivi-Guedehoussou, N.; et al. Towards a proteome-scale map of the human protein–protein interaction network. Nature 2005, 437, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Raman, K. Construction and analysis of protein–protein interaction networks. Autom. Exp. 2010, 2, 2–11. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein–protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2022, 51, D638–D646. [Google Scholar] [CrossRef]
- Hu, C.; Yang, J.; Qi, Z.; Wu, H.; Wang, B.; Zou, F.; Mei, H.; Liu, J.; Wang, W.; Liu, Q. Heat shock proteins: Biological functions, pathological roles, and therapeutic opportunities. MedComm 2022, 3, e161. [Google Scholar] [CrossRef]
- Arya, R.; Mallik, M.; Lakhotia, S.C. Heat shock genes—Integrating cell survival and death. J. Biosci. 2007, 32, 595–610. [Google Scholar] [CrossRef]
- Mayer, M.P.; Bukau, B. Hsp70 chaperones: Cellular functions and molecular mechanism. Cell. Mol. Life Sci. 2005, 62, 670. [Google Scholar] [CrossRef]
- Wegele, H.; Müller, L.; Buchner, J. Hsp70 and Hsp90—A Relay Team for Protein Folding. Reviews of Physiology, Biochemistry and Pharmacology; Springer: Berlin/Heidelberg, Germany, 2004; pp. 1–44. [Google Scholar]
- Teiten, M.-H.; Reuter, S.; Schmucker, S.; Dicato, M.; Diederich, M. Induction of heat shock response by curcumin in human leukemia cells. Cancer Lett. 2009, 279, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.D.; Shen, Y.Q.; Zhao, X.H.; Guo, L.J.; Yu, Z.J.; Wang, D.; Liu, L.M.; Liu, J.Z. Curcumin Combined with Oxaliplatin Effectively Suppress Colorectal Carcinoma in vivo Through Inducing Apoptosis. Phytother. Res. 2015, 29, 357–365. [Google Scholar] [CrossRef]
- Rak, S.; Čimbora-Zovko, T.; Gajski, G.; Dubravčić, K.; Domijan, A.-M.; Delaš, I.; Garaj-Vrhovac, V.; Batinić, D.; Sorić, J.; Osmak, M. Carboplatin resistant human laryngeal carcinoma cells are cross resistant to curcumin due to reduced curcumin accumulation. Toxicol. In Vitro 2013, 27, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-W.; Yu, S.-L.; Chen, J.J.W.; Li, H.-N.; Lin, Y.-C.; Yao, P.-L.; Chou, H.-Y.; Chien, C.-T.; Chen, W.-J.; Lee, Y.-T.; et al. Anti-Invasive Gene Expression Profile of Curcumin in Lung Adenocarcinoma Based on a High Throughput Microarray Analysis. Mol. Pharmacol. 2004, 65, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Dunsmore, K.E.; Chen, P.G.; Wong, H.R. Curcumin, a medicinal herbal compound capable of inducing the heat shock response. Crit. Care Med. 2001, 29, 2199–2204. [Google Scholar] [CrossRef]
- Szebeni, G.J.; Balázs, Á.; Madarász, I.; Pócz, G.; Ayaydin, F.; Kanizsai, I.; Fajka-Boja, R.; Alföldi, R.; Hackler, L., Jr.; Puskás, L.G. Achiral Mannich-Base Curcumin Analogs Induce Unfolded Protein Response and Mitochondrial Membrane Depolarization in PANC-1 Cells. Int. J. Mol. Sci. 2017, 18, 2105. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R. The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacol. Res. 2014, 79, 34–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z. ErbB Receptors and Cancer. In ErbB Receptor Signaling: Methods and Protocols; Wang, Z., Ed.; Springer: New York, NY, USA, 2017; pp. 3–35. [Google Scholar]
- Wang, N.; Cao, Y.; Si, C.; Shao, P.; Su, G.; Wang, K.; Bao, J.; Yang, L. Emerging Role of ERBB2 in Targeted Therapy for Metastatic Colorectal Cancer: Signaling Pathways to Therapeutic Strategies. Cancers 2022, 14, 5160. [Google Scholar] [CrossRef]
- Nowak, J.A. HER2 in Colorectal Carcinoma: Are We There yet? Surg. Pathol. Clin. 2020, 13, 485–502. [Google Scholar] [CrossRef]
- Loree, J.M.; Bailey, A.M.; Johnson, A.M.; Yu, Y.; Wu, W.; Bristow, C.A.; Davis, J.S.; Shaw, K.R.; Broaddus, R.; Banks, K.C.; et al. Molecular Landscape of ERBB2/ERBB3 Mutated Colorectal Cancer. JNCI J. Natl. Cancer Inst. 2018, 110, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Citri, A.; Gan, J.; Mosesson, Y.; Vereb, G.; Szollosi, J.; Yarden, Y. Hsp90 restrains ErbB-2/HER2 signalling by limiting heterodimer formation. EMBO Rep. 2004, 5, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Segers, V.F.M.; Dugaucquier, L.; Feyen, E.; Shakeri, H.; De Keulenaer, G.W. The role of ErbB4 in cancer. Cell. Oncol. 2020, 43, 335–352. [Google Scholar] [CrossRef]
- Williams, C.S.; Bernard, J.K.; Beckler, M.D.; Almohazey, D.; Washington, M.K.; Smith, J.J.; Frey, M.R. ERBB4 is over-expressed in human colon cancer and enhances cellular transformation. Carcinogenesis 2015, 36, 710–718. [Google Scholar] [CrossRef]
- Frey, M.R.; Edelblum, K.L.; Mullane, M.T.; Liang, D.; Polk, D.B. The ErbB4 Growth Factor Receptor Is Required for Colon Epithelial Cell Survival in the Presence of TNF. Gastroenterology 2009, 136, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Lin, W.; Long, Y.; Yang, Y.; Zhang, H.; Wu, K.; Chu, Q. Notch signaling pathway: Architecture, disease, and therapeutics. Signal Transduct. Target. Ther. 2022, 7, 95. [Google Scholar] [CrossRef]
- Reedijk, M.; Odorcic, S.; Zhang, H.; Chetty, R.; Tennert, C.; Dickson, B.C.; Lockwood, G.; Gallinger, S.; Egan, S.E. Activation of Notch signaling in human colon adenocarcinoma. Int. J. Oncol. 2008, 33, 1223–1229. [Google Scholar]
- Tyagi, A.; Sharma, A.K.; Damodaran, C. A Review on Notch Signaling and Colorectal Cancer. Cells 2020, 9, 1549. [Google Scholar] [CrossRef]
- Rajendran, D.T.; Subramaniyan, B.; Ganeshan, M. Role of Notch Signaling in Colorectal Cancer. In Role of Transcription Factors in Gastrointestinal Malignancies; Nagaraju, G.P., Bramhachari, P.V., Eds.; Springer: Singapore, 2017; pp. 307–314. [Google Scholar]
- Subramaniam, D.; Ponnurangam, S.; Ramamoorthy, P.; Standing, D.; Battafarano, R.J.; Anant, S.; Sharma, P. Curcumin Induces Cell Death in Esophageal Cancer Cells through Modulating Notch Signaling. PLoS ONE 2012, 7, e30590. [Google Scholar] [CrossRef]
- Sha, J.; Li, J.; Wang, W.; Pan, L.; Cheng, J.; Li, L.; Zhao, H.; Lin, W. Curcumin induces G0/G1 arrest and apoptosis in hormone independent prostate cancer DU-145 cells by down regulating Notch signaling. Biomed. Pharmacother. 2016, 84, 177–184. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, S.; Shen, H.; Chen, W.; Xu, H.; Chen, X.; Sun, D.; Zhong, S.; Zhao, J.; Tang, J. Curcumin inhibits cancer progression through regulating expression of microRNAs. Tumor Biol. 2017, 39, 1010428317691680. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Ma, D.; Zhang, L.; Si, M.; Yin, H.; Li, J. Curcumin inhibits proliferation and invasion of osteosarcoma cells through inactivation of Notch-1 signaling. FEBS J. 2012, 279, 2247–2259. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-C.; Yang, Z.-X.; Zhou, J.-S.; Zhang, H.-T.; Huang, Q.-K.; Dang, L.-L.; Liu, G.-X.; Tao, K.-S. Curcumin regulates hepatoma cell proliferation and apoptosis through the Notch signaling pathway. Int. J. Clin. Exp. Med. 2014, 7, 714–718. [Google Scholar] [PubMed]
- Leslie, N.R.; Kriplani, N.; Hermida, M.A.; Alvarez-Garcia, V.; Wise, H.M. The PTEN protein: Cellular localization and post-translational regulation. Biochem. Soc. Trans. 2016, 44, 273–278. [Google Scholar] [CrossRef]
- Milella, M.; Falcone, I.; Conciatori, F.; Cesta Incani, U.; Del Curatolo, A.; Inzerilli, N.; Nuzzo, C.M.; Vaccaro, V.; Vari, S.; Cognetti, F.; et al. PTEN: Multiple Functions in Human Malignant Tumors. Front. Oncol. 2015, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Serebriiskii, I.G.; Pavlov, V.; Tricarico, R.; Andrianov, G.; Nicolas, E.; Parker, M.I.; Newberg, J.; Frampton, G.; Meyer, J.E.; Golemis, E.A. Comprehensive characterization of PTEN mutational profile in a series of 34,129 colorectal cancers. Nat. Commun. 2022, 13, 1618. [Google Scholar] [CrossRef]
- Abbas Momtazi, A.; Sahebkar, A. Difluorinated Curcumin: A Promising Curcumin Analogue with Improved Anti-Tumor Activity and Pharmacokinetic Profile. Curr. Pharm. Des. 2016, 22, 4386–4397. [Google Scholar] [CrossRef]
- Roy, S.; Yu, Y.; Padhye, S.B.; Sarkar, F.H.; Majumdar, A.P.N. Difluorinated-Curcumin (CDF) Restores PTEN Expression in Colon Cancer Cells by Down-Regulating miR-21. PLoS ONE 2013, 8, e68543. [Google Scholar] [CrossRef]
- Dandawate, P.R.; Vyas, A.; Ahmad, A.; Banerjee, S.; Deshpande, J.; Swamy, K.V.; Jamadar, A.; Dumhe-Klaire, A.C.; Padhye, S.; Sarkar, F.H. Inclusion Complex of Novel Curcumin Analogue CDF and β-Cyclodextrin (1:2) and Its Enhanced In Vivo Anticancer Activity Against Pancreatic Cancer. Pharm. Res. 2012, 29, 1775–1786. [Google Scholar] [CrossRef]
- Saini, A.K.; Kumar, V. Chapter 2—Ribosome structure. In Emerging Concepts in Ribosome Structure, Biogenesis, and Function; Kumar, V., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 15–31. [Google Scholar]
- Kumar, J.; Kumar, V. Chapter 4—Ribosome proteins—Their balanced production. In Emerging Concepts in Ribosome Structure, Biogenesis, and Function; Kumar, V., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 47–87. [Google Scholar]
- Baker, R.T.; Board, P.G. The human ubiquitin-52 amino acid fusion protein gene shares several structural features with mammalian ribosomal protein genes. Nucleic Acids Res. 1991, 19, 1035–1040. [Google Scholar] [CrossRef]
- Barnard, G.F.; Mori, M.; Staniunas, R.J.; Begum, N.A.; Bao, S.; Puder, M.; Cobb, J.; Redman, K.L.; Steele, G.D.; Chen, L.B. Ubiquitin fusion proteins are overexpressed in colon cancer but not in gastric cancer. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 1995, 1272, 147–153. [Google Scholar] [CrossRef]
- Luo, J.; Zhao, H.; Chen, L.; Liu, M. Multifaceted functions of RPS27a: An unconventional ribosomal protein. J. Cell. Physiol. 2023, 238, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, E.S.; Tasini, F.; Crinelli, R.; Ceccarini, C.; Magnani, M.; Bianchi, M. The Ubiquitin Gene Expression Pattern and Sensitivity to UBB and UBC Knockdown Differentiate Primary 23132/87 and Metastatic MKN45 Gastric Cancer Cells. Int. J. Mol. Sci. 2020, 21, 5435. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.J.; Sun, L.J. Nonproteolytic Functions of Ubiquitin in Cell Signaling. Mol. Cell 2009, 33, 275–286. [Google Scholar] [CrossRef]
- Demasi, M.; da Cunha, F.M. The physiological role of the free 20S proteasome in protein degradation: A critical review. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2018, 1862, 2948–2954. [Google Scholar] [CrossRef] [PubMed]
- Sahu, I.; Glickman, M.H. Proteasome in action: Substrate degradation by the 26S proteasome. Biochem. Soc. Trans. 2021, 49, 629–644. [Google Scholar] [CrossRef] [PubMed]
- Budenholzer, L.; Cheng, C.L.; Li, Y.; Hochstrasser, M. Proteasome Structure and Assembly. J. Mol. Biol. 2017, 429, 3500–3524. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Liu, Z.; Yang, Q. The role of ubiquitination and deubiquitination in cancer metabolism. Mol. Cancer 2020, 19, 146. [Google Scholar] [CrossRef]
- Deng, L.; Meng, T.; Chen, L.; Wei, W.; Wang, P. The role of ubiquitination in tumorigenesis and targeted drug discovery. Signal Transduct. Target. Ther. 2020, 5, 11. [Google Scholar] [CrossRef]
- Bhattacharjee, P.; Mazumdar, M.; Guha, D.; Sa, G. Ubiquitin–Proteasome System in the Hallmarks of Cancer. In Role of Proteases in Cellular Dysfunction; Dhalla, N.S., Chakraborti, S., Eds.; Springer: New York, NY, USA, 2014; pp. 159–186. [Google Scholar]
- Kitahara, O.; Furukawa, Y.; Tanaka, T.; Kihara, C.; Ono, K.; Yanagawa, R.; E Nita, M.; Takagi, T.; Nakamura, Y.; Tsunoda, T. Alterations of gene expression during colorectal carcinogenesis revealed by cDNA microarrays after laser-capture microdissection of tumor tissues and normal epithelia. Cancer Res. 2001, 61, 3544–3549. [Google Scholar]
- Lin, Y.-M.; Furukawa, Y.; Tsunoda, T.; Yue, C.-T.; Yang, K.-C.; Nakamura, Y. Molecular diagnosis of colorectal tumors by expression profiles of 50 genes expressed differentially in adenomas and carcinomas. Oncogene 2002, 21, 4120–4128. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.J.; Lai, M.D. Identification of differentially expressed genes in normal mucosa, adenoma and adenocarcinoma of colon by SSH. World J. Gastroenterol. 2001, 7, 726–731. [Google Scholar] [CrossRef]
- Chester, K.A.; Robson, L.; Begent, R.H.; Talbot, I.C.; Pringle, J.H.; Primrose, L.; Macpherson, A.J.; Boxer, G.; Southall, P.; Malcolm, A.D. Identification of a human ribosomal protein mRNA with increased expression in colorectal tumours. Biochim. Biophys. Acta (BBA)-Gene Struct. Expr. 1989, 1009, 297–300. [Google Scholar] [CrossRef]
- Wang, Y.; Cheong, D.; Chan, S.; Hooi, S.C. Ribosomal protein L7a gene is up-regulated but not fused to the tyrosine kinase receptor as chimeric trk oncogene in human colorectal carcinoma. Int. J. Oncol. 2000, 16, 757–762. [Google Scholar] [CrossRef]
- Iizumi, Y.; Oishi, M.; Taniguchi, T.; Goi, W.; Sowa, Y.; Sakai, T. The flavonoid apigenin downregulates CDK1 by directly targeting ribosomal protein S9. PLoS ONE 2013, 8, e73219. [Google Scholar] [CrossRef]
- Huang, C.J.; Yang, S.H.; Lee, C.L.; Cheng, Y.C.; Tai, S.Y.; Chien, C.C. Ribosomal protein S27-like in colorectal cancer: A candidate for predicting prognoses. PLoS ONE 2013, 8, e67043. [Google Scholar] [CrossRef]
- Nieminen, T.T.; O’donohue, M.-F.; Wu, Y.; Lohi, H.; Scherer, S.W.; Paterson, A.D.; Ellonen, P.; Abdel-Rahman, W.M.; Valo, S.; Mecklin, J.-P.; et al. Germline Mutation of RPS20, Encoding a Ribosomal Protein, Causes Predisposition to Hereditary Nonpolyposis Colorectal Carcinoma Without DNA Mismatch Repair Deficiency. Gastroenterology 2014, 147, 595–598.e5. [Google Scholar] [CrossRef]
- Broderick, P.; Dobbins, S.E.; Chubb, D.; Kinnersley, B.; Dunlop, M.G.; Tomlinson, I.; Houlston, R.S. Validation of Recently Proposed Colorectal Cancer Susceptibility Gene Variants in an Analysis of Families and Patients—A Systematic Review. Gastroenterology 2017, 152, 75–77.e4. [Google Scholar] [CrossRef] [PubMed]
- González-González, M.; Sayagués, J.M.; Muñoz-Bellvís, L.; Pedreira, C.E.; de Campos, M.L.R.; García, J.; Alcázar, J.A.; Braz, P.F.; Galves, B.L.; González, L.M.; et al. Tracking the Antibody Immunome in Sporadic Colorectal Cancer by Using Antigen Self-Assembled Protein Arrays. Cancers 2021, 13, 2718. [Google Scholar] [CrossRef]
- Kim, T.-H.; Leslie, P.; Zhang, Y. Ribosomal proteins as unrevealed caretakers for cellular stress and genomic instability. Oncotarget 2014, 5, 860–871. [Google Scholar] [CrossRef] [PubMed]
- Coppolino, M.G.; Woodside, M.J.; Demaurex, N.; Grinstein, S.; St-Arnaud, R.; Dedhar, S. Calreticulin is essential for integrin-mediated calcium signalling and cell adhesion. Nature 1997, 386, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M.; Groenendyk, J.; Szabo, E.; Gold, L.I.; Opas, M. Calreticulin, a multi-process calcium-buffering chaperone of the endoplasmic reticulum. Biochem. J. 2009, 417, 651–666. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Zuppini, A.; Arnaudeau, S.; Lynch, J.; Ahsan, I.; Krause, R.; Papp, S.; De Smedt, H.; Parys, J.B.; Müller-Esterl, W.; et al. Functional specialization of calreticulin domains. J. Cell Biol. 2001, 154, 961–972. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Chen, H.; Xie, P.; Ma, R.; He, J.; Zhang, H. Bioinformatics analysis for the role of CALR in human cancers. PLoS ONE 2021, 16, e0261254. [Google Scholar] [CrossRef] [PubMed]
- Papp, S.; Fadel, M.P.; Kim, H.; McCulloch, C.A.; Opas, M. Calreticulin Affects Fibronectin-based Cell-Substratum Adhesion via the Regulation of c-Src Activity. J. Biol. Chem. 2007, 282, 16585–16598. [Google Scholar] [CrossRef]
- Alfonso, P.; Nunez, A.; Madoz-Gurpide, J.; Lombardia, L.; Sanchez, L.; Casal, J.I. Proteomic expression analysis of colorectal cancer by two-dimensional differential gel electrophoresis. Proteomics 2005, 5, 2602–2611. [Google Scholar] [CrossRef]
- Vougas, K.; Gaitanarou, E.; Marinos, E.; Kittas, C.; Voloudakis-Baltatzis, I.E. Two-dimensional electrophoresis and immunohistochemical study of calreticulin in colorectal adenocarcinoma and mirror biopsies. Off. J. Balk. Union Oncol. 2008, 13, 101–107. [Google Scholar]
- Kaida, A.; Yamamoto, S.; Parrales, A.; Young, E.D.; Ranjan, A.; Alalem, M.A.; Morita, K.-I.; Oikawa, Y.; Harada, H.; Ikeda, T.; et al. DNAJA1 promotes cancer metastasis through interaction with mutant p53. Oncogene 2021, 40, 5013–5025. [Google Scholar] [CrossRef]
- Mattoo, R.U.H.; Sharma, S.K.; Priya, S.; Finka, A.; Goloubinoff, P. Hsp110 Is a Bona Fide Chaperone Using ATP to Unfold Stable Misfolded Polypeptides and Reciprocally Collaborate with Hsp70 to Solubilize Protein Aggregates. J. Biol. Chem. 2013, 288, 21399–21411. [Google Scholar] [CrossRef]
- Javid, H.; Hashemian, P.; Yazdani, S.; Sharbaf Mashhad, A.; Karimi-Shahri, M. The role of heat shock proteins in metastatic colorectal cancer: A review. J. Cell. Biochem. 2022, 123, 1704–1735. [Google Scholar] [CrossRef]
- Haase, M.; Fitze, G. HSP90AB1: Helping the good and the bad. Gene 2016, 575 Pt 1, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Whitesell, L.; Lindquist, S.L. HSP90 and the chaperoning of cancer. Nat. Rev. Cancer 2005, 5, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Zhao, F.; Li, H.; Li, L.; Yang, Y.; Liu, F. HSP90 mediates the connection of multiple programmed cell death in diseases. Cell Death Dis. 2022, 13, 929. [Google Scholar] [CrossRef] [PubMed]
- Streicher, J.M. The Role of Heat Shock Proteins in Regulating Receptor Signal Transduction. Mol. Pharmacol. 2019, 95, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Tutar, Y.; Naureen, H.; Farooqi, A.A. Chapter 13—Heat shock proteins in tumor progression and metastasis. In Unraveling the Complexities of Metastasis; Farooqi, A.A., Qureshi, M.Z., Sabitaliyevich, U.Y., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 187–201. [Google Scholar]
- Yu, N.; Kakunda, M.; Pham, V.; Lill, J.R.; Du, P.; Wongchenko, M.; Yan, Y.; Firestein, R.; Huang, X. HSP105 Recruits Protein Phosphatase 2A To Dephosphorylate β-Catenin. Mol. Cell. Biol. 2015, 35, 1390–1400. [Google Scholar] [CrossRef] [PubMed]
- Berthenet, K.; Bokhari, A.; Lagrange, A.; Marcion, G.; Boudesco, C.; Causse, S.; De Thonel, A.; Svrcek, M.; Goloudina, A.R.; Dumont, S.; et al. HSP110 promotes colorectal cancer growth through STAT3 activation. Oncogene 2017, 36, 2328–2336. [Google Scholar] [CrossRef]
- Wang, W.; Wei, J.; Zhang, H.; Zheng, X.; Zhou, H.; Luo, Y.; Yang, J.; Deng, Q.; Huang, S.; Fu, Z. PRDX2 promotes the proliferation of colorectal cancer cells by increasing the ubiquitinated degradation of p53. Cell Death Dis. 2021, 12, 605. [Google Scholar] [CrossRef]
- Lu, W.; Fu, Z.; Wang, H.; Feng, J.; Wei, J.; Guo, J. Peroxiredoxin 2 knockdown by RNA interference inhibits the growth of colorectal cancer cells by downregulating Wnt/beta-catenin signaling. Cancer Lett. 2014, 343, 190–199. [Google Scholar] [CrossRef]
- Lu, W.; Fu, Z.; Wang, H.; Feng, J.; Wei, J.; Guo, J. Peroxiredoxin 2 is upregulated in colorectal cancer and contributes to colorectal cancer cells’ survival by protecting cells from oxidative stress. Mol. Cell. Biochem. 2014, 387, 261–270. [Google Scholar] [CrossRef]
- Feng, J.; Fu, Z.; Guo, J.; Lu, W.; Wen, K.; Chen, W.; Wang, H.; Wei, J.; Zhang, S. Overexpression of peroxiredoxin 2 inhibits TGF-β1-induced epithelial-mesenchymal transition and cell migration in colorectal cancer. Mol. Med. Rep. 2014, 10, 867–873. [Google Scholar] [CrossRef][Green Version]
- Zhao, J.; Lin, X.; Meng, D.; Zeng, L.; Zhuang, R.; Huang, S.; Lv, W.; Hu, J. Nrf2 Mediates Metabolic Reprogramming in Non-Small Cell Lung Cancer. Front. Oncol. 2020, 10, 578315. [Google Scholar] [CrossRef] [PubMed]
- Mitsuishi, Y.; Taguchi, K.; Kawatani, Y.; Shibata, T.; Nukiwa, T.; Aburatani, H.; Yamamoto, M.; Motohashi, H. Nrf2 Redirects Glucose and Glutamine into Anabolic Pathways in Metabolic Reprogramming. Cancer Cell 2012, 22, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Cai, G.; Zhou, B.; Li, D.; Zhao, A.; Xie, G.; Li, H.; Cai, S.; Xie, D.; Huang, C.; et al. A distinct metabolic signature of human colorectal cancer with prognostic potential. Clin. Cancer Res. 2014, 20, 2136–2146. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhao, X.; Yong, H.; Xu, J.; Qu, P.; Qiao, S.; Hou, P.; Li, Z.; Chu, S.; Zheng, J.; et al. Transketolase promotes colorectal cancer metastasis through regulating AKT phosphorylation. Cell Death Dis. 2022, 13, 99. [Google Scholar] [CrossRef]
- Zhang, D.D. Mechanistic Studies of the Nrf2-Keap1 Signaling Pathway. Drug Metab. Rev. 2006, 38, 769–789. [Google Scholar] [CrossRef]
- Taguchi, K.; Motohashi, H.; Yamamoto, M. Molecular mechanisms of the Keap1–Nrf2 pathway in stress response and cancer evolution. Genes Cells 2011, 16, 123–140. [Google Scholar] [CrossRef]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell. Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef] [PubMed]
- Song, M.-Y.; Lee, D.-Y.; Chun, K.-S.; Kim, E.-H. The Role of NRF2/KEAP1 Signaling Pathway in Cancer Metabolism. Int. J. Mol. Sci. 2021, 22, 4376. [Google Scholar] [CrossRef]
- Wang, B.; Liu, K.; Lin, H.Y.; Bellam, N.; Ling, S.; Lin, W.C. 14-3-3Tau regulates ubiquitin-independent proteasomal degradation of p21, a novel mechanism of p21 downregulation in breast cancer. Mol. Cell Biol. 2010, 30, 1508–1527. [Google Scholar] [CrossRef]
- Kästle, M.; Grune, T. Chapter 4—Interactions of the Proteasomal System with Chaperones: Protein Triage and Protein Quality control. In Progress in Molecular Biology and Translational Science; Grune, T., Ed.; Academic Press: Cambridge, MA, USA, 2012; pp. 113–160. [Google Scholar]
- Berthold, J.; Schenková, K.; Ramos, S.; Miura, Y.; Furukawa, M.; Aspenström, P.; Rivero, F. Characterization of RhoBTB-dependent Cul3 ubiquitin ligase complexes—Evidence for an autoregulatory mechanism. Exp. Cell Res. 2008, 314, 3453–3465. [Google Scholar] [CrossRef]
- Wang, X.; Dong, L.; Cheng, J.; Verdine, G.L.; Lin, A.; Chu, Q. Targeted β-catenin ubiquitination and degradation by multifunctional stapled peptides. J. Pept. Sci. 2022, 28, e3389. [Google Scholar] [CrossRef]
- Patel, J.; Tripathi, E. Targeting the Ubiquitin Machinery for Cancer Therapeutics. In Drug Repurposing for Emerging Infectious Diseases and Cancer; Sobti, R.C., Lal, S.K., Goyal, R.K., Eds.; Springer: Singapore, 2023; pp. 181–201. [Google Scholar]
- Ma, L.; Li, X.; Zhao, X.; Sun, H.; Kong, F.; Li, Y.; Sui, Y.; Xu, F. Oxaliplatin promotes siMAD2L2induced apoptosis in colon cancer cells. Mol. Med. Rep. 2021, 24, 629. [Google Scholar] [CrossRef]
- Koohini, Z.; Koohini, Z.; Teimourian, S. Slit/Robo Signaling Pathway in Cancer, a New Stand Point for Cancer Treatment. Pathol. Oncol. Res. 2019, 25, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Liang, G.; Xiao, Y.; Qin, T.; Chen, X.; Wu, E.; Ma, Q.; Wang, Z. Targeting the SLIT/ROBO pathway in tumor progression: Molecular mechanisms and therapeutic perspectives. Ther. Adv. Med. Oncol. 2019, 11, 1758835919855238. [Google Scholar] [CrossRef]
- Kong, R.; Yi, F.; Wen, P.; Liu, J.; Chen, X.; Ren, J.; Li, X.; Shang, Y.; Nie, Y.; Wu, K.; et al. Myo9b is a key player in SLIT/ROBO-mediated lung tumor suppression. J. Clin. Investig. 2015, 125, 4407–4420. [Google Scholar] [CrossRef]
- Wang, B.; Xiao, Y.; Ding, B.-B.; Zhang, N.; Yuan, X.-B.; Gui, L.; Qian, K.-X.; Duan, S.; Chen, Z.; Rao, Y.; et al. Induction of tumor angiogenesis by Slit-Robo signaling and inhibition of cancer growth by blocking Robo activity. Cancer Cell 2003, 4, 19–29. [Google Scholar] [CrossRef]
- Zhou, W.J.; Geng, Z.H.; Chi, S.; Zhang, W.; Niu, X.F.; Lan, S.J.; Ma, L.; Yang, X.; Wang, L.J.; Ding, Y.Q.; et al. Slit-Robo signaling induces malignant transformation through Hakai-mediated E-cadherin degradation during colorectal epithelial cell carcinogenesis. Cell Res. 2011, 21, 609–626. [Google Scholar] [CrossRef]
- Dickinson, R.E.; Fegan, K.S.; Ren, X.; Hillier, S.G.; Duncan, W.C. Glucocorticoid Regulation of SLIT/ROBO Tumour Suppressor Genes in the Ovarian Surface Epithelium and Ovarian Cancer Cells. PLoS ONE 2011, 6, e27792. [Google Scholar] [CrossRef]
- Prasad, A.; Fernandis, A.Z.; Rao, Y.; Ganju, R.K. Slit Protein-mediated Inhibition of CXCR4-induced Chemotactic and Chemoinvasive Signaling Pathways in Breast Cancer Cells. J. Biol. Chem. 2004, 279, 9115–9124. [Google Scholar] [CrossRef]
- Wang, Z.; Hou, Y.; Guo, X.; van der Voet, M.; Boxem, M.; Dixon, J.E.; Chisholm, A.D.; Jin, Y. The EBAX-type Cullin-RING E3 Ligase and Hsp90 Guard the Protein Quality of the SAX-3/Robo Receptor in Developing Neurons. Neuron 2013, 79, 903–916. [Google Scholar] [CrossRef]
- Blanco, A.; Blanco, G. Amino Acid Metabolism. In Medical Biochemistry; Blanco, A., Blanco, G., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 367–399. [Google Scholar]
- Pavlova, N.N.; Thompson, C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef]
- Blanco, A.; Blanco, G. Integration and Regulation of Metabolism. In Medical Biochemistry; Blanco, A., Blanco, G., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 425–445. [Google Scholar]
- Kerk, S.A.; Lin, L.; Myers, A.L.; Sutton, D.J.; Andren, A.; Sajjakulnukit, P.; Zhang, L.; Zhang, Y.; Jiménez, J.A.; Nelson, B.S.; et al. Metabolic requirement for GOT2 in pancreatic cancer depends on environmental context. eLife 2022, 11, e73245. [Google Scholar] [CrossRef]
- Birsoy, K.; Wang, T.; Chen, W.W.; Freinkman, E.; Abu-Remaileh, M.; Sabatini, D.M. An Essential Role of the Mitochondrial Electron Transport Chain in Cell Proliferation Is to Enable Aspartate Synthesis. Cell 2015, 162, 540–551. [Google Scholar] [CrossRef]
- Kerk, S.A.; Garcia-Bermudez, J.; Birsoy, K.; Sherman, M.H.; Shah, Y.M.; Lyssiotis, C.A. Spotlight on GOT2 in Cancer Metabolism. Onco. Targets Ther. 2023, 16, 695–702. [Google Scholar] [CrossRef]
- Du, F.; Chen, J.; Liu, H.; Cai, Y.; Cao, T.; Han, W.; Yi, X.; Qian, M.; Tian, D.; Nie, Y.; et al. SOX12 promotes colorectal cancer cell proliferation and metastasis by regulating asparagine synthesis. Cell Death Dis. 2019, 10, 239. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Tang, M.; Peng, B.; Lu, X.; Yang, Q.; Zhu, Q.; Hou, T.; Li, M.; Liu, C.; et al. Destabilization of linker histone H1.2 is essential for ATM activation and DNA damage repair. Cell Res. 2018, 28, 756–770. [Google Scholar] [CrossRef]
- Okamura, H.; Yoshida, K.; Amorim, B.R.; Haneji, T. Histone H1.2 is translocated to mitochondria and associates with bak in bleomycin-induced apoptotic cells. J. Cell. Biochem. 2008, 103, 1488–1496. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Y.; Xie, Y.; Yang, D.; Sun, Y.; Yuan, Y.; Chen, H.; Zhang, Y.; Huang, K.; Zheng, L. Histone H1.2 promotes hepatocarcinogenesis by regulating signal transducer and activator of transcription 3 signaling. Cancer Sci. 2022, 113, 1679–1692. [Google Scholar] [CrossRef]
- Konishi, A.; Shimizu, S.; Hirota, J.; Takao, T.; Fan, Y.; Matsuoka, Y.; Zhang, L.; Yoneda, Y.; Fujii, Y.; Skoultchi, A.I.; et al. Involvement of Histone H1.2 in Apoptosis Induced by DNA Double-Strand Breaks. Cell 2003, 114, 673–688. [Google Scholar] [CrossRef]
- Schnetler, R.; Fanucchi, S.; Moldoveanu, T.; Koorsen, G. Linker Histone H1.2 Directly Activates BAK through the K/RVVKP Motif on the C-Terminal Domain. Biochemistry 2020, 59, 3332–3346. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.; Jia, J.; Cao, X.; Zhou, P.-K.; Gao, S. Molecular and Cellular Functions of the Linker Histone H1.2. Front. Cell Dev. Biol. 2022, 9, 773195. [Google Scholar] [CrossRef] [PubMed]
- Medrzycki, M.; Zhang, Y.; Zhang, W.; Cao, K.; Pan, C.; Lailler, N.; McDonald, J.F.; Bouhassira, E.E.; Fan, Y. Histone H1.3 Suppresses H19 Noncoding RNA Expression and Cell Growth of Ovarian Cancer Cells. Cancer Res. 2014, 74, 6463–6473. [Google Scholar] [CrossRef]
- Armeev, G.A.; Kniazeva, A.S.; Komarova, G.A.; Kirpichnikov, M.P.; Shaytan, A.K. Histone dynamics mediate DNA unwrapping and sliding in nucleosomes. Nat. Commun. 2021, 12, 2387. [Google Scholar] [CrossRef]
- Hsu, C.-L.; Chong, S.Y.; Lin, C.-Y.; Kao, C.-F. Histone dynamics during DNA replication stress. J. Biomed. Sci. 2021, 28, 48. [Google Scholar] [CrossRef]
- Prendergast, L.; Reinberg, D. The missing linker: Emerging trends for H1 variant-specific functions. Genes Dev. 2021, 35, 40–58. [Google Scholar] [CrossRef] [PubMed]
- Healton, S.E.; Pinto, H.D.; Mishra, L.N.; Hamilton, G.A.; Wheat, J.C.; Swist-Rosowska, K.; Shukeir, N.; Dou, Y.; Steidl, U.; Jenuwein, T.; et al. H1 linker histones silence repetitive elements by promoting both histone H3K9 methylation and chromatin compaction. Proc. Natl. Acad. Sci. USA 2020, 117, 14251–14258. [Google Scholar] [CrossRef] [PubMed]
- Viéitez, C.; Martínez-Cebrián, G.; Solé, C.; Böttcher, R.; Potel, C.M.; Savitski, M.M.; Onnebo, S.; Fabregat, M.; Shilatifard, A.; Posas, F.; et al. A genetic analysis reveals novel histone residues required for transcriptional reprogramming upon stress. Nucleic Acids Res. 2020, 48, 3455–3475. [Google Scholar] [CrossRef] [PubMed]
- Aricthota, S.; Rana, P.P.; Haldar, D. Histone acetylation dynamics in repair of DNA double-strand breaks. Front. Genet. 2022, 13, 926577. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.J.; Kon, T.; Knight, P.J.; Sutoh, K.; Burgess, S.A. Functions and mechanics of dynein motor proteins. Nat. Rev. Mol. Cell Biol. 2013, 14, 713–726. [Google Scholar] [CrossRef]
- Xiang, X.; Qiu, R.; Yao, X.; Arst, H.N.; Peñalva, M.A., Jr.; Zhang, J. Cytoplasmic dynein and early endosome transport. Cell Mol. Life Sci. 2015, 72, 3267–3280. [Google Scholar] [CrossRef] [PubMed]
- Garrett, C.A.; Barri, M.; Kuta, A.; Soura, V.; Deng, W.; Fisher, E.M.; Schiavo, G.; Hafezparast, M. DYNC1H1 mutation alters transport kinetics and ERK1/2-cFos signalling in a mouse model of distal spinal muscular atrophy. Brain 2014, 137, 1883–1893. [Google Scholar] [CrossRef] [PubMed]
- Jeger, J.L. Endosomes, lysosomes, and the role of endosomal and lysosomal biogenesis in cancer development. Mol. Biol. Rep. 2020, 47, 9801–9810. [Google Scholar] [CrossRef] [PubMed]
- Granger, E.; McNee, G.; Allan, V.; Woodman, P. The role of the cytoskeleton and molecular motors in endosomal dynamics. Semin. Cell Dev. Biol. 2014, 31, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Scott, C.C.; Vacca, F.; Gruenberg, J. Endosome maturation, transport and functions. Semin. Cell Dev. Biol. 2014, 31, 2–10. [Google Scholar]
- Ahlstedt, B.A.; Ganji, R.; Raman, M. The functional importance of VCP to maintaining cellular protein homeostasis. Biochem. Soc. Trans. 2022, 50, 1457–1469. [Google Scholar] [CrossRef]
- van den Boom, J.; Meyer, H. VCP/p97-Mediated Unfolding as a Principle in Protein Homeostasis and Signaling. Mol. Cell 2018, 69, 182–194. [Google Scholar] [CrossRef]
- Song, C.; Wang, Q.; Song, C.; Rogers, T.J. Valosin-containing protein (VCP/p97) is capable of unfolding polyubiquitinated proteins through its ATPase domains. Biochem. Biophys. Res. Commun. 2015, 463, 453–457. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hemion, C.; Flammer, J.; Neutzner, A. Quality control of oxidatively damaged mitochondrial proteins is mediated by p97 and the proteasome. Free. Radic. Biol. Med. 2014, 75, 121–128. [Google Scholar] [CrossRef]
- Antoine-Bertrand, J.; Ghogha, A.; Luangrath, V.; Bedford, F.K.; Lamarche-Vane, N. The activation of ezrin-radixin-moesin proteins is regulated by netrin-1 through Src kinase and RhoA/Rho kinase activities and mediates netrin-1-induced axon outgrowth. Mol. Biol. Cell. 2011, 22, 3734–3746. [Google Scholar] [CrossRef]
- Çelik, H.; Bulut, G.; Han, J.; Graham, G.T.; Minas, T.Z.; Conn, E.J.; Hong, S.-H.; Pauly, G.T.; Hayran, M.; Li, X.; et al. Ezrin Inhibition Up-regulates Stress Response Gene Expression. J. Biol. Chem. 2016, 291, 13257–13270. [Google Scholar] [CrossRef]
- Mintz, C.D.; Carcea, I.; McNickle, D.G.; Dickson, T.C.; Ge, Y.; Salton, S.R.; Benson, D.L. ERM proteins regulate growth cone responses to, Sema3A. J. Comp. Neurol. 2008, 510, 351–366. [Google Scholar] [CrossRef]
- Bagci, H.; Sriskandarajah, N.; Robert, A.; Boulais, J.; Elkholi, I.E.; Tran, V.; Lin, Z.Y.; Thibault, M.P.; Dubé, N.; Faubert, D.; et al. Mapping the proximity interaction network of the Rho-family GTPases reveals signalling pathways and regulatory mechanisms. Nat. Cell Biol. 2020, 22, 120–134. [Google Scholar] [CrossRef]
- Gallo, G. Semaphorin 3A inhibits ERM protein phosphorylation in growth cone filopodia through inactivation of PI3K. Dev. Neurobiol. 2008, 68, 926–933. [Google Scholar] [CrossRef]
- Riento, K.; Ridley, A.J. ROCKs: Multifunctional kinases in cell behaviour. Nat. Rev. Mol. Cell Biol. 2003, 4, 446–456. [Google Scholar] [CrossRef]
- Lee, P.J.; Yang, S.; Sun, Y.; Guo, J.U. Regulation of nonsense-mediated mRNA decay in neural development and disease. J. Mol. Cell Biol. 2021, 13, 269–281. [Google Scholar] [CrossRef]
- Villa, N.; Fraser, C.S. Mechanism of Translation in Eukaryotes. In Translation and Its Regulation in Cancer Biology and Medicine; Parsyan, A., Ed.; Springer: Dordrecht, The Netherlands, 2014; pp. 7–37. [Google Scholar]
- Negrutskii, B.S.; Shalak, V.F.; Novosylna, O.V.; Porubleva, L.V.; Lozhko, D.M.; El’skaya, A.V. The eEF1 family of mammalian translation elongation factors. BBA Adv. 2023, 3, 100067. [Google Scholar] [CrossRef]
- Hassan, M.K.; Kumar, D.; Naik, M.; Dixit, M. The expression profile and prognostic significance of eukaryotic translation elongation factors in different cancers. PLoS ONE 2018, 13, e0191377. [Google Scholar] [CrossRef]
- Chi, K.; Jones, D.V.; Frazier, M.L. Expression of an elongation factor 1 gamma-related sequence in adenocarcinomas of the colon. Gastroenterology 1992, 103, 98–102. [Google Scholar] [CrossRef]
- Colak, D.; Ji, S.J.; Porse, B.T.; Jaffrey, S.R. Regulation of axon guidance by compartmentalized nonsense-mediated mRNA decay. Cell 2013, 153, 1252–1265. [Google Scholar] [CrossRef]
- Kadlec, J.; Izaurralde, E.; Cusack, S. The structural basis for the interaction between nonsense-mediated mRNA decay factors UPF2 and UPF3. Nat. Struct. Mol. Biol. 2004, 11, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Friocourt, F.; Chédotal, A. The Robo3 receptor, a key player in the development, evolution, and function of commissural systems. Dev. Neurobiol. 2017, 77, 876–890. [Google Scholar] [CrossRef] [PubMed]
- Farabaugh, P.J. Translational Control and Fidelity. In Encyclopedia of Microbiology, 3rd ed.; Schaechter, M., Ed.; Academic Press: Oxford, UK, 2009; pp. 517–528. [Google Scholar]
- Segev, N.; Gerst, J.E. Specialized Ribosomes and Specific Ribosomal Protein Paralogs Control Translation of Mitochondrial Proteins. J. Cell Biol. 2018, 217, 117. [Google Scholar] [CrossRef]
- Zhou, X.; Liao, W.J.; Liao, J.M.; Liao, P.; Lu, H. Ribosomal proteins: Functions beyond the ribosome. J. Mol. Cell Biol. 2015, 7, 92–104. [Google Scholar] [CrossRef]
- Kumari, A. Chapter 24—Translation. In Sweet Biochemistry, 2nd ed.; Kumari, A., Ed.; Academic Press: Cambridge, MA, USA, 2023; pp. 151–157. [Google Scholar]
- Puria, R.; Rohilla, S.; Kaur, S. Chapter 9—Translation—Process and control. In Emerging Concepts in Ribosome Structure, Biogenesis, and Function; Kumar, V., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 183–211. [Google Scholar]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef]
- Bharadwaj, U.; Kasembeli, M.M.; Robinson, P.; Tweardy, D.J. Targeting Janus Kinases and Signal Transducer and Activator of Transcription 3 to Treat Inflammation, Fibrosis, and Cancer: Rationale, Progress, and Caution. Pharmacol. Rev. 2020, 72, 486–526. [Google Scholar] [CrossRef]
- Abd El-Fattah, E.E.; Zakaria, A.Y. Targeting HSP47 and HSP70: Promising therapeutic approaches in liver fibrosis management. J. Transl. Med. 2022, 20, 544. [Google Scholar] [CrossRef]
- Morris, R.; Kershaw, N.J.; Babon, J.J. The molecular details of cytokine signaling via the JAK/STAT pathway. Protein Sci. 2018, 27, 1984–2009. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Cortopassi, G. Mitochondrial Hspa9/Mortalin regulates erythroid differentiation via iron-sulfur cluster assembly. Mitochondrion 2016, 26, 94–103. [Google Scholar] [CrossRef]
- Alfadhel, M.; Nashabat, M.; Ali, Q.A.; Hundallah, K. Mitochondrial iron-sulfur cluster biogenesis from molecular understanding to clinical disease. Neurosci. J. 2017, 22, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Seif, F.; Khoshmirsafa, M.; Aazami, H.; Mohsenzadegan, M.; Sedighi, G.; Bahar, M. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun. Signal. 2017, 15, 23. [Google Scholar] [CrossRef]
- Ross, S.H.; Rollings, C.; Anderson, K.E.; Hawkins, P.T.; Stephens, L.R.; Cantrell, D.A. Phosphoproteomic Analyses of Interleukin 2 Signaling Reveal Integrated JAK Kinase-Dependent and -Independent Networks in CD8+ T Cells. Immunity 2016, 45, 685–700. [Google Scholar] [CrossRef]
- Zareifard, A.; Beaudry, F.; Ndiaye, K. Janus Kinase 3 phosphorylation and the JAK/STAT pathway are positively modulated by follicle-stimulating hormone (FSH) in bovine granulosa cells. BMC Mol. Cell Biol. 2023, 24, 21. [Google Scholar] [CrossRef]
- Koide, N.; Kasamatsu, A.; Endo-Sakamoto, Y.; Ishida, S.; Shimizu, T.; Kimura, Y.; Miyamoto, I.; Yoshimura, S.; Shiiba, M.; Tanzawa, H.; et al. Evidence for Critical Role of Lymphocyte Cytosolic Protein 1 in Oral Cancer. Sci. Rep. 2017, 7, 43379. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.J.; Snowden, J.A.; Zeidler, M.P.; Danson, S.J. The role of JAK/STAT signalling in the pathogenesis, prognosis and treatment of solid tumours. Br. J. Cancer 2015, 113, 365–371. [Google Scholar] [CrossRef]
- Cen, K.; Lu, C. Prognostic and Immune Infiltration Analysis of Transaldolase 1 (TALDO1) in Hepatocellular Carcinoma. Int. J. Gen. Med. 2023, 16, 5779–5788. [Google Scholar] [CrossRef]
- Alfarsi, L.H.; El Ansari, R.; Craze, M.L.; Mohammed, O.J.; Masisi, B.K.; Ellis, I.O.; Rakha, E.A.; Green, A.R. SLC1A5 co-expression with TALDO1 associates with endocrine therapy failure in estrogen receptor-positive breast cancer. Breast Cancer Res. Treat. 2021, 189, 317–331. [Google Scholar] [CrossRef]
- Moriyama, T.; Tanaka, S.; Nakayama, Y.; Fukumoto, M.; Tsujimura, K.; Yamada, K.; Bamba, T.; Yoneda, Y.; Fukusaki, E.; Oka, M. Two isoforms of TALDO1 generated by alternative translational initiation show differential nucleocytoplasmic distribution to regulate the global metabolic network. Sci. Rep. 2016, 6, 34648. [Google Scholar] [CrossRef]
- Popiołkiewicz, J.; Polkowski, K.; Skierski, J.S.; Mazurek, A.P. In vitro toxicity evaluation in the development of new anticancer drugs—Genistein glycosides. Cancer Lett. 2005, 229, 67–75. [Google Scholar] [CrossRef]
- Nordin, N.; Fadaeinasab, M.; Mohan, S.; Hashim, N.M.; Othman, R.; Karimian, H.; Iman, V.; Ramli, N.; Ali, H.M.; Majid, N.A. Pulchrin A, a new natural coumarin derivative of Enicosanthellum pulchrum, induces apoptosis in ovarian cancer cells via intrinsic pathway. PLoS ONE 2016, 11, e0154023. [Google Scholar] [CrossRef]

| Pathway Identifier | Pathway Name | Entities FDR | Submitted Entities Found | Protein Class | Cluster |
|---|---|---|---|---|---|
| R-HSA-2262752 | Cellular responses to stress | 1.23 × 10−14 | HSP90AA1, HSPA5, HSPA1B, HSPA1A | Chaperone | Cluster 1 |
| RPL7A, RPS27A, UBA52 | Ribosomal protein | Cluster 2 | |||
| UBB, UBC | Ubiquitin–protein ligase | ||||
| R-HSA-8948747 | Regulation of PTEN localization | 1.35 × 10−6 | RPS27A, UBA52 | Ribosomal protein | Cluster 2 |
| UBB, UBC | Ubiquitin–protein ligase | ||||
| R-HSA-1253288 | Downregulation of ERBB4 signaling | 1.55 × 10−6 | RPS27A, UBA52 | Ribosomal protein | Cluster 2 |
| R-HSA-1643713 | Signaling by EGFR in Cancer | 1.60 × 10−6 | HSP90AA1 | Chaperone | Cluster 1 |
| RPS27A, UBA52 | Ribosomal protein | Cluster 2 | |||
| UBB, UBC | Ubiquitin–protein ligase | ||||
| R-HSA-8863795 | Downregulation of ERBB2 signaling | 2.06 × 10−6 | HSP90AA1 | Chaperone | Cluster 1 |
| RPS27A, UBA52 | Ribosomal protein | Cluster 2 | |||
| UBB, UBC | Ubiquitin–protein ligase | ||||
| R-HSA-2691230 | Signaling by NOTCH1 HD Domain mutants in Cancer | 2.41 × 10−6 | RPS27A, UBA52 | Ribosomal protein | Cluster 2 |
| UBB, UBC | Ubiquitin–protein ligase |
| Pathway Identifier | Pathway Name | Entities FDR | Submitted Entities Found | Protein Class | Cluster |
|---|---|---|---|---|---|
| R-HSA-2262752 | Cellular responses to stress | 2.78 × 10−15 | RPS9, RPS6, RPS27L, RPS3A, RPL10A, RPL8, RPL6, RPS15, RPL7A, RPS28, RPS27, RPLP2, RPS20, RPL26, RPS11, RPS10, RPL26L1, RPS12 | Ribosomal protein | Cluster 1 |
| CALR, DNAJA1, HSP90AB1, HSPA1A, HSPA1B, HSPA9, HSPH1 | Chaperone | Cluster 2 | |||
| VCP | Transport protein | ||||
| PSMA1, PSMD13, | Ubiquitin–protein ligase | ||||
| H1-3, H1-2, | Chromatin-regulatory | Cluster 3 | |||
| MSN | Cytoskeletal protein | Cluster 4 | |||
| DYNC1H1 | DNA binding protein | ||||
| PRDX2, TALDO1, TKT | Metabolic enzyme | Cluster 5 | |||
| R-HSA-156842 | Eukaryotic Translation Elongation | 2.78 × 10−15 | RPS9, RPS6, RPS27L, RPS3A, RPL10A, RPL8, RPL6, RPS15, RPL7A, RPS28, RPS27, RPLP2, RPS20, RPL26, RPS11, RPS10, RPL26L1, RPS12 | Ribosomal protein | Cluster 1 |
| EEF1G | RNA-binding protein | Cluster 2 | |||
| R-HSA-927802 | Nonsense-Mediated Decay (NMD) | 2.78 × 10−15 | RPS9, RPS6, RPS27L, RPS3A, RPL10A, RPL8, RPL6, RPS15, RPL7A, RPS28, RPS27, RPLP2, RPS20, RPL26, RPS11, RPS10, RPL26L1, RPS12 | Ribosomal protein | Cluster 1 |
| R-HSA-9010553 | Regulation of expression of SLITs and ROBOs | 2.78 × 10−15 | RPS9, RPS6, RPS27L, RPS3A, RPL10A, RPL8, RPL6, RPS15, RPL7A, RPS28, RPS27, RPLP2, RPS20, RPL26, RPS11, RPS10, RPL26L1, RPS12 | Ribosomal protein | Cluster 1 |
| PSMA1, PSMD13 | Ubiquitin–protein ligase | Cluster 2 | |||
| R-HSA-71291 | Metabolism of amino acids and derivatives | 4.78 × 10−8 | RPS9, RPS6, RPS27L, RPS3A, RPL10A, RPL8, RPL6, RPS15, RPL7A, RPS28, RPS27, RPLP2, RPS20, RPL26, RPS11, RPS10, RPL26L1, RPS12 | Ribosomal protein | Cluster 1 |
| GOT2 | Metabolic enzyme | Cluster 5 | |||
| PSMA1, PSMD13 | Ubiquitin–protein ligase | Cluster 2 | |||
| R-HSA-8950505 | Gene and protein expression by JAK-STAT signaling after Interleukin-12 stimulation | 2.27 × 10−6 | HSPA9 | Chaperone | Cluster 2 |
| LCP1, MSN | Cytoskeletal protein | Cluster 4 | |||
| TALDO1 | Metabolic enzyme | Cluster 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hon, K.W.; Zainal Abidin, S.A.; Abas, F.; Othman, I.; Naidu, R. Anti-Cancer Mechanisms of Diarylpentanoid MS17 (1,5-Bis(2-hydroxyphenyl)-1,4-pentadiene-3-one) in Human Colon Cancer Cells: A Proteomics Approach. Int. J. Mol. Sci. 2024, 25, 3503. https://doi.org/10.3390/ijms25063503
Hon KW, Zainal Abidin SA, Abas F, Othman I, Naidu R. Anti-Cancer Mechanisms of Diarylpentanoid MS17 (1,5-Bis(2-hydroxyphenyl)-1,4-pentadiene-3-one) in Human Colon Cancer Cells: A Proteomics Approach. International Journal of Molecular Sciences. 2024; 25(6):3503. https://doi.org/10.3390/ijms25063503
Chicago/Turabian StyleHon, Kha Wai, Syafiq Asnawi Zainal Abidin, Faridah Abas, Iekhsan Othman, and Rakesh Naidu. 2024. "Anti-Cancer Mechanisms of Diarylpentanoid MS17 (1,5-Bis(2-hydroxyphenyl)-1,4-pentadiene-3-one) in Human Colon Cancer Cells: A Proteomics Approach" International Journal of Molecular Sciences 25, no. 6: 3503. https://doi.org/10.3390/ijms25063503
APA StyleHon, K. W., Zainal Abidin, S. A., Abas, F., Othman, I., & Naidu, R. (2024). Anti-Cancer Mechanisms of Diarylpentanoid MS17 (1,5-Bis(2-hydroxyphenyl)-1,4-pentadiene-3-one) in Human Colon Cancer Cells: A Proteomics Approach. International Journal of Molecular Sciences, 25(6), 3503. https://doi.org/10.3390/ijms25063503






