A Breakthrough in the Treatment of Necrobiosis Lipoidica? Update on Treatment, Etiopathogenesis, Diagnosis, and Clinical Presentation
Abstract
1. Introduction
2. Etiopathogenesis
3. Clinical Features
4. Diagnosis
5. Differential Diagnosis
6. Treatment Standards and New Therapeutic Options
6.1. Ustekinumab
6.2. Secukinumab
6.3. JAK Inhibitors
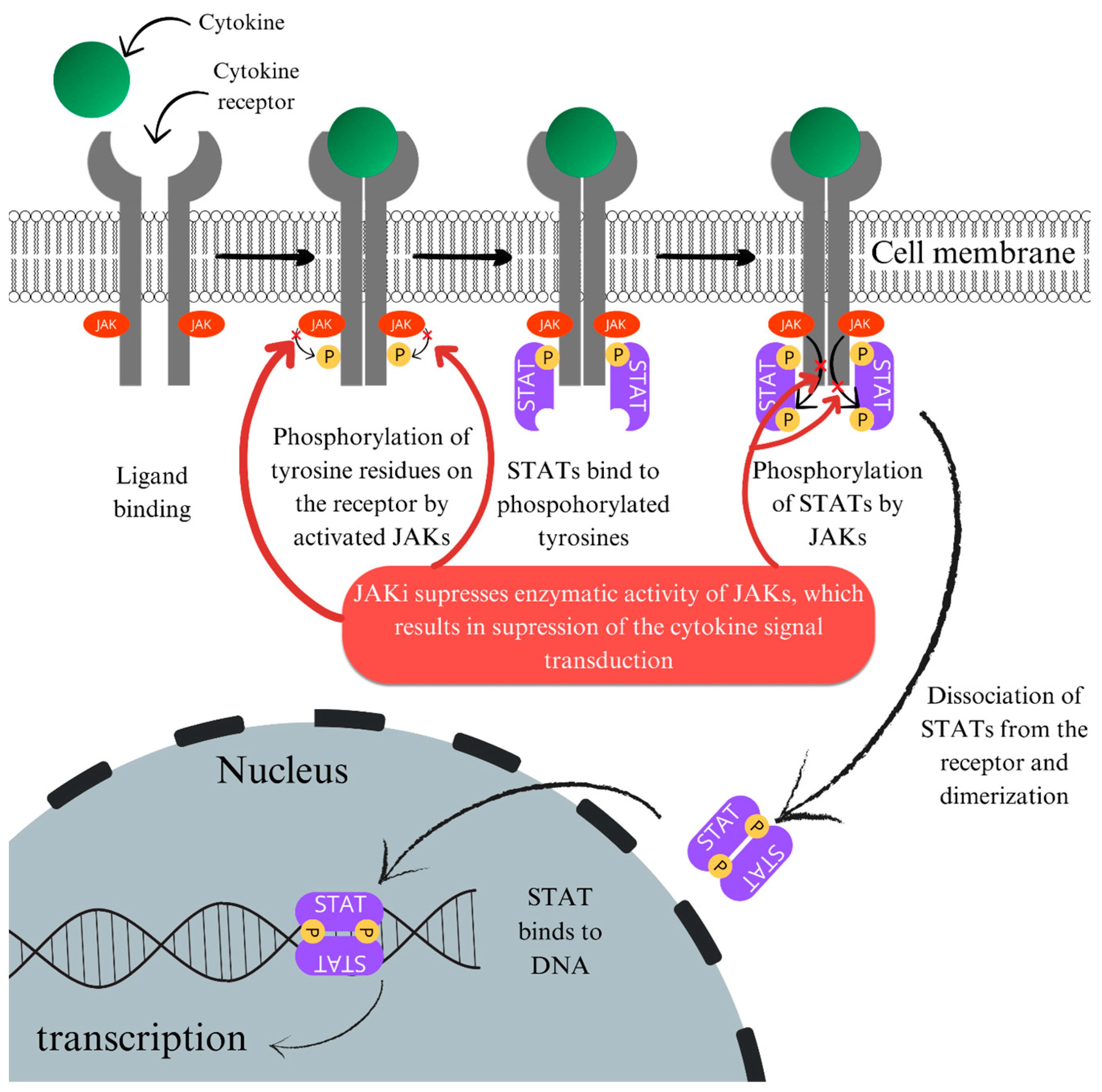
6.3.1. Potential Mechanism of Action of JAK Inhibitors in NL
6.3.2. Side Effects of JAK Inhibitors
6.3.3. Clinical Reports
6.3.4. Ruxolitinib
6.3.5. Tofacitinib
6.3.6. Baricitinib
6.3.7. Abrocitinib
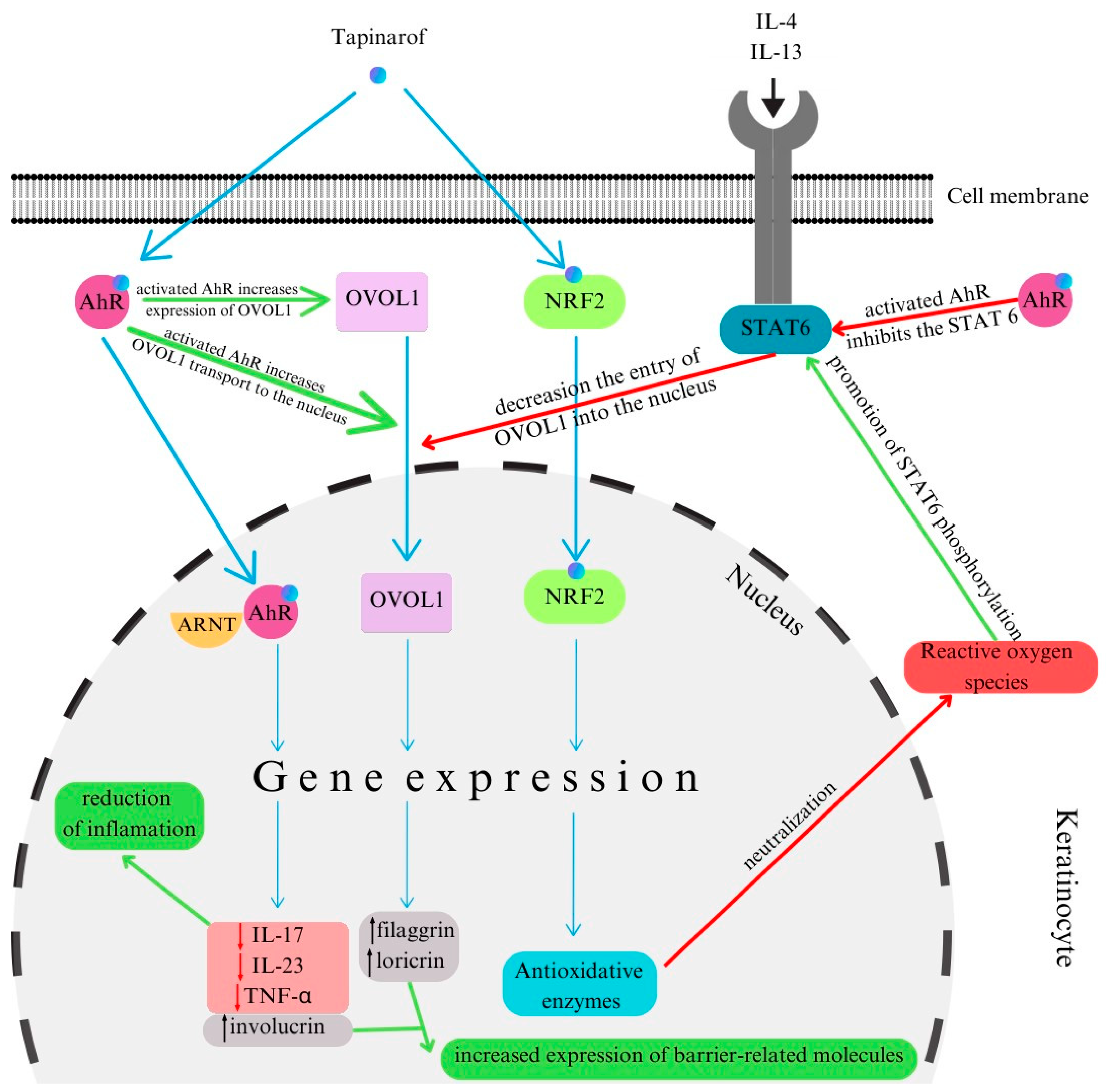
6.4. Tapinarof
6.5. Other Methods
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Muller, S.A.; Winkelmann, R.K. Necrobiosis lipoidica diabeticorum histopathologic study of 98 cases. Arch. Dermatol. 1966, 94, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.S.; Shabihkhani, M.; Hogeling, M. Pediatric necrobiosis lipoidica: Case report and review of the literature. Dermatol. Online J. 2021, 27, 4. [Google Scholar] [CrossRef] [PubMed]
- Özkur, E.; Hasçiçek, S.; Altunay, İ. Atypical presentation of necrobiosis lipoidica in a pediatric patient. Pediatr. Dermatol. 2019, 36, e31–e33. [Google Scholar] [CrossRef]
- Bonura, C.; Frontino, G.; Rigamonti, A.; Battaglino, R.; Favalli, V.; Ferro, G.; Rubino, C.; Del Barba, P.; Pesapane, F.; Nazzaro, G.; et al. Necrobiosis Lipoidica Diabeticorum: A pediatric case report. Dermatoendocrinol 2014, 6, e27790. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Peyrí, J.; Moreno, A.; Marcoval, J. Necrobiosis lipoidica. Semin. Cutan. Med. Surg. 2007, 26, 87–89. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.M.; Barrett, T.L. Collagenolytic (necrobiotic) granulomas: Part II—The ‘red’ granulomas. J. Cutan. Pathol. 2004, 31, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Körber, A.; Dissemond, J. Necrobiosis lipoidica diabeticorum. Can. Med. Assoc. J. 2007, 177, 1498. [Google Scholar] [CrossRef]
- Goldsmith, W.N. Necrobiosis Lipoidica. Proc. R. Soc. Med. 1935, 28, 363–364. [Google Scholar] [CrossRef]
- Sibbald, C.; Reid, S.; Alavi, A. Necrobiosis Lipoidica. Dermatol. Clin. 2015, 33, 343–360. [Google Scholar] [CrossRef]
- Jockenhöfer, F.; Kröger, K.; Klode, J.; Renner, R.; Erfurt-Berge, C.; Dissemond, J. Cofactors and comorbidities of necrobiosis lipoidica: Analysis of the German DRG data from 2012. J. Dtsch. Dermatol. Ges. 2016, 14, 277–284. [Google Scholar] [CrossRef]
- Heite, H.J.; Scharwenka, H.X. [Erythema elevatum diutinum, granuloma annulare, necrobiosis lipoidica & granulomatosis disciformis Gottron-Miescher; a comparative study on their incidence]. Arch. Klin. Exp. Dermatol. 1959, 208, 260–290. [Google Scholar] [CrossRef] [PubMed]
- Muller, S.A.; Winkelmann, R.K. Necrobiosis lipoidica diabeticorum. A clinical and pathological investigation of 171 cases. Arch. Dermatol. 1966, 93, 272–281. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, E.A.; Kennedy, U.; Nolan, J.J.; Young, M.M.; Rogers, S.; Barnes, L. Necrobiosis lipoidica: Only a minority of patients have diabetes mellitus. Br. J. Dermatol. 1999, 140, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Glovaci, D.; Fan, W.; Wong, N.D. Epidemiology of Diabetes Mellitus and Cardiovascular Disease. Curr. Cardiol. Rep. 2019, 21, 21. [Google Scholar] [CrossRef] [PubMed]
- Holland, C.; Givens, V.; Smoller, B.R. Expression of the human erythrocyte glucose transporter Glut-1 in areas of sclerotic collagen in necrobiosis lipoidica. J. Cutan. Pathol. 2001, 28, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Oikarinen, A.; Mörtenhumer, M.; Kallioinen, M.; Savolainen, E.R. Necrobiosis lipoidica: Ultrastructural and biochemical demonstration of a collagen defect. J. Investig. Dermatol. 1987, 88, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Mistry, B.D.; Alavi, A.; Ali, S.; Mistry, N. A systematic review of the relationship between glycemic control and necrobiosis lipoidica diabeticorum in patients with diabetes mellitus. Int. J. Dermatol. 2017, 56, 1319–1327. [Google Scholar] [CrossRef] [PubMed]
- Erfurt-Berge, C.; Seitz, A.T.; Rehse, C.; Wollina, U.; Schwede, K.; Renner, R. Update on clinical and laboratory features in necrobiosis lipoidica: A retrospective multicentre study of 52 patients. Eur. J. Dermatol. 2012, 22, 770–775. [Google Scholar] [CrossRef]
- Boateng, B.; Hiller, D.; Albrecht, H.P.; Hornstein, O.P. [Cutaneous microcirculation in pretibial necrobiosis lipoidica. Comparative laser Doppler flowmetry and oxygen partial pressure determinations in patients and healthy probands]. Hautarzt 1993, 44, 581–586. [Google Scholar]
- Ngo, B.; Wigington, G.; Hayes, K.; Huerter, C.; Hillman, B.; Adler, M.; Rendell, M. Skin blood flow in necrobiosis lipoidica diabeticorum. Int. J. Dermatol. 2008, 47, 354–358. [Google Scholar] [CrossRef]
- Markey, A.C.; Tidman, M.J.; Rowe, P.H.; Missen, G.A.; Macdonald, D.M. Aggressive ulcerative necrobiosis lipoidica associated with venous insufficiency, giant-cell phlebitis and arteritis. Clin. Exp. Dermatol. 1988, 13, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Ullman, S.; Dahl, M.V. Necrobiosis lipoidica. An immunofluorescence study. Arch. Dermatol. 1977, 113, 1671–1673. [Google Scholar] [CrossRef]
- Lause, M.; Kamboj, A.; Fernandez Faith, E. Dermatologic manifestations of endocrine disorders. Transl. Pediatr. 2017, 6, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Seremet, S.; Gurel, M.S. Miscellaneous skin disease and the metabolic syndrome. Clin. Dermatol. 2018, 36, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Bungau, A.F.; Radu, A.F.; Bungau, S.G.; Vesa, C.M.; Tit, D.M.; Endres, L.M. Oxidative stress and metabolic syndrome in acne vulgaris: Pathogenetic connections and potential role of dietary supplements and phytochemicals. Biomed. Pharmacother. 2023, 164, 115003. [Google Scholar] [CrossRef] [PubMed]
- Ashcroft, G.S.; Jeong, M.J.; Ashworth, J.J.; Hardman, M.; Jin, W.; Moutsopoulos, N.; Wild, T.; McCartney-Francis, N.; Sim, D.; McGrady, G.; et al. Tumor necrosis factor-alpha (TNF-α) is a therapeutic target for impaired cutaneous wound healing. Wound Repair. Regen. 2012, 20, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Miele, C.; Formisano, P.; Condorelli, G.; Caruso, M.; Oriente, F.; Andreozzi, F.; Tocchetti, C.G.; Riccardi, G.; Beguinot, F. Abnormal glucose transport and GLUT1 cell-surface content in fibroblasts and skeletal muscle from NIDDM and obese subjects. Diabetologia 1997, 40, 421–429. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hiraki, Y.; Rosen, O.M.; Birnbaum, M.J. Growth factors rapidly induce expression of the glucose transporter gene. J. Biol. Chem. 1988, 263, 13655–13662. [Google Scholar] [CrossRef]
- Sandhu, V.K.; Alavi, A. The role of anti-tumour necrosis factor in wound healing: A case report of refractory ulcerated necrobiosis lipoidica treated with adalimumab and review of the literature. SAGE Open Med. Case Rep. 2019, 7, 2050313X19881594. [Google Scholar] [CrossRef]
- Ho, K.K.; O’Loughlin, S.; Powell, F.C. Familial non-diabetic necrobiosis lipoidica. Australas. J. Dermatol. 1992, 33, 31–34. [Google Scholar] [CrossRef]
- Seviour, P.W.; Elkeles, R.S. Necrobiosis lipoidica in two diabetic sisters. Clin. Exp. Dermatol. 1985, 10, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Shimanovich, I.; Erdmann, H.; Grabbe, J.; Zillikens, D.; Rose, C. Necrobiosis lipoidica in monozygotic twins. Arch. Dermatol. 2008, 144, 119–120. [Google Scholar] [CrossRef] [PubMed]
- Soler, N.G.; McConnachie, P.R. HLA antigens and necrobiosis lipoidica diabeticorum—A comparison between insulin-dependent diabetics with and without necrobiosis. Postgrad. Med. J. 1983, 59, 759–762. [Google Scholar] [CrossRef] [PubMed]
- Patel, G.K.; Mills, C.M. A prospective open study of topical psoralen-UV-A therapy for Necrobiosis lipoidica. Arch. Dermatol. 2001, 137, 1658–1660. [Google Scholar] [PubMed]
- Hines, A.; Alavi, A.; Davis, M.D.P. Cutaneous Manifestations of Diabetes. Med. Clin. N. Am. 2021, 105, 681–697. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, G.M.; Novelli, M.; Hartog, M.; Kennedy, C.T. Necrobiosis lipoidica—Involvement of atypical sites. Clin. Exp. Dermatol. 1993, 18, 543–544. [Google Scholar] [CrossRef] [PubMed]
- Alhameedy, M.M. Necrobiosis Lipoidica: Atypical Presentation in a Diabetic Girl. Case Rep. Dermatol. 2021, 13, 547–552. [Google Scholar] [CrossRef]
- Scaramuzza, A.; Macedoni, M.; Tadini, G.L.; De Angelis, L.; Redaelli, F.; Gazzarri, A.; Comaschi, V.; Giani, E.; Zuccotti, G.V. Necrobiosis lipoidica diabeticorum. Case Rep. Pediatr. 2012, 2012, 152602. [Google Scholar] [CrossRef]
- Ito, H.; Imamura, S. Scald-Induced Necrobiosis Lipoidica in a Patient with Diabetes Mellitus and Psoriasis. Case Rep. Dermatol. 2016, 8, 1–4. [Google Scholar] [CrossRef]
- Sizmaz, S.; Pelit, A.; Bolat, F.; Tuncer, I.; Akova, Y.A. Periorbital necrobiosis lipoidica diabeticorum: Case report. Int. Ophthalmol. 2008, 28, 307–309. [Google Scholar] [CrossRef]
- Helander, I.; Niemi, K.M.; Tyrkkö, J. Atypical necrobiosis lipoidica of the face. Acta Derm. Venereol. 1978, 58, 276–277. [Google Scholar] [CrossRef] [PubMed]
- Mackey, J.P. Necrobiosis lipoidica diabeticorum involving scalp and face. Br. J. Dermatol. 1975, 93, 729–730. [Google Scholar] [CrossRef] [PubMed]
- Boyd, A.S.; Neldner, K.H. The isomorphic response of Koebner. Int. J. Dermatol. 1990, 29, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Acebo, E.; Gardeazábal, J.; Marcellán, M.; Lasa, O.; Burgos, J.J.; Díaz-Pérez, J.L. [Necrobiosis lipoidica over appendectomy scar in a patient with morphea of the breast]. Actas Dermosifiliogr. 2006, 97, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Absil, G.; Collins, P.; El Hayderi, L.; Nikkels, A.F. Necrobiosis Lipoidica following Breast Reduction. Plast. Reconstr. Surg. Glob. Open 2021, 9, e3788. [Google Scholar] [CrossRef] [PubMed]
- Vion, B.; Burri, G.; Ramelet, A.A. Necrobiosis lipoidica and silicotic granulomas on Muller’s phlebectomy scars. Dermatology 1997, 194, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Tokura, Y.; Mizushima, Y.; Hata, M.; Takigawa, M. Necrobiosis lipoidica of the glans penis. J. Am. Acad. Dermatol. 2003, 49, 921–924. [Google Scholar] [CrossRef]
- Alonso, M.L.; Riós, J.C.; González-Beato, M.J.; Herranz, P. Necrobiosis lipoidica of the glans penis. Acta Derm. Venereol. 2011, 91, 105–106. [Google Scholar] [CrossRef]
- Sawada, Y.; Mori, T.; Nakashima, D.; Nakamura, M.; Tokura, Y. Necrobiosis lipoidica of the scrotum. Eur. J. Dermatol. 2011, 21, 98–99. [Google Scholar] [CrossRef]
- Lepe, K.; Riley, C.A.; Salazar, F.J. StatPearls. In Necrobiosis Lipoidica; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Lowitt, M.H.; Dover, J.S. Necrobiosis lipoidica. J. Am. Acad. Dermatol. 1991, 25, 735–748. [Google Scholar] [CrossRef]
- Shrestha, S.; Spierings, N.; Marahatta, S. Necrobiosis lipoidica: A case report with dermoscopic review. Clin. Case Rep. 2021, 9, 1171–1174. [Google Scholar] [CrossRef] [PubMed]
- Bakos, R.M.; Cartell, A.; Bakos, L. Dermatoscopy of early-onset necrobiosis lipoidica. J. Am. Acad. Dermatol. 2012, 66, e143–e144. [Google Scholar] [CrossRef] [PubMed]
- Pellicano, R.; Caldarola, G.; Filabozzi, P.; Zalaudek, I. Dermoscopy of necrobiosis lipoidica and granuloma annulare. Dermatology 2013, 226, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Lallas, A.; Zaballos, P.; Zalaudek, I.; Apalla, Z.; Gourhant, J.Y.; Longo, C.; Moscarella, E.; Tiodorovic-Zivkovic, D.; Argenziano, G. Dermoscopic patterns of granuloma annulare and necrobiosis lipoidica. Clin. Exp. Dermatol. 2013, 38, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Conde-Montero, E.; Avilés-Izquierdo, J.A.; Mendoza-Cembranos, M.D.; Parra-Blanco, V. Dermoscopy of necrobiosis lipoidica. Actas Dermosifiliogr. 2013, 104, 534–537. [Google Scholar] [CrossRef] [PubMed]
- Terziroli Beretta-Piccoli, B.; Mainetti, C.; Peeters, M.A.; Laffitte, E. Cutaneous Granulomatosis: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2018, 54, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Hawryluk, E.B.; Izikson, L.; English, J.C. Non-infectious granulomatous diseases of the skin and their associated systemic diseases: An evidence-based update to important clinical questions. Am. J. Clin. Dermatol. 2010, 11, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Izikson, L.; English, J.C. Noninfectious granulomatous diseases: An update. Adv. Dermatol. 2006, 22, 31–53. [Google Scholar] [CrossRef]
- Miola, A.C.; Marques, M.E.A.; Miot, H.A. Necrobiotic xanthogranuloma (without paraproteinemia) in a patient with Pelger-Huët anomaly. Int. J. Dermatol. 2019, 58, E93–E94. [Google Scholar] [CrossRef]
- Errichetti, E.; Stinco, G. Dermoscopy in General Dermatology: A Practical Overview. Dermatol. Ther. (Heidelb.) 2016, 6, 471–507. [Google Scholar] [CrossRef]
- Erfurt-Berge, C.; Heusinger, V.; Reinboldt-Jockenhöfer, F.; Dissemond, J.; Renner, R. Comorbidity and Therapeutic Approaches in Patients with Necrobiosis Lipoidica. Dermatology 2022, 238, 148–155. [Google Scholar] [CrossRef]
- Burns, E.; Ukoha, U.; Chan, A. Necrobiosis lipoidica with rapid response to doxycycline. Pediatr. Dermatol. 2020, 37, 981–982. [Google Scholar] [CrossRef] [PubMed]
- Hengge, U.R.; Ruzicka, T.; Schwartz, R.A.; Cork, M.J. Adverse effects of topical glucocorticosteroids. J. Am. Acad. Dermatol. 2006, 54, 1–15, quiz 16–18. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.; Patel, V.; Berth-Jones, J. Systemic corticosteroids for the outpatient treatment of necrobiosis lipoidica in a diabetic patient. J. Dermatolog Treat. 2007, 18, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Schiefer-Niederkorn, A.; Sadoghi, B.; Binder, B. Necrobiosis lipoidica in childhood: A review of literature with emphasis on therapy. J. Dtsch. Dermatol. Ges. 2023, 21, 1120–1129. [Google Scholar] [CrossRef] [PubMed]
- Pitarch, G.; Giner, F. Periorbital necrobiosis lipoidica. Actas Dermosifiliogr. 2013, 104, 636–638. [Google Scholar] [CrossRef]
- Koura-Nishiura, A.; Yoneda, K.; Nakai, K.; Demitsu, T.; Kubota, Y. Clearance of atypical facial necrobiosis lipoidica with tacrolimus ointment. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 383–385. [Google Scholar] [CrossRef]
- Clayton, T.H.; Harrison, P.V. Successful treatment of chronic ulcerated necrobiosis lipoidica with 0.1% topical tacrolimus ointment. Br. J. Dermatol. 2005, 152, 581–582. [Google Scholar] [CrossRef]
- Ginocchio, L.; Draghi, L.; Darvishian, F.; Ross, F.L. Refractory Ulcerated Necrobiosis Lipoidica: Closure of a Difficult Wound with Topical Tacrolimus. Adv. Skin. Wound Care 2017, 30, 469–472. [Google Scholar] [CrossRef]
- Rajabi-Estarabadi, A.; Aickara, D.J.; Hirsch, M.; Williams, N.M.; Maranda, E.L.; Van Badiavas, E. Laser and light therapies for the treatment of necrobiosis lipoidica. Lasers Med. Sci. 2021, 36, 497–506. [Google Scholar] [CrossRef]
- Peckruhn, M.; Tittelbach, J.; Elsner, P. Update: Treatment of necrobiosis lipoidica. J. Dtsch. Dermatol. Ges. 2017, 15, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Reid, S.D.; Ladizinski, B.; Lee, K.; Baibergenova, A.; Alavi, A. Update on necrobiosis lipoidica: A review of etiology, diagnosis, and treatment options. J. Am. Acad. Dermatol. 2013, 69, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Nihal, A.; Caplan, A.S.; Rosenbach, M.; Damsky, W.; Mangold, A.R.; Shields, B.E. Treatment options for necrobiosis lipoidica: A systematic review. Int. J. Dermatol. 2023, 62, 1529–1537. [Google Scholar] [CrossRef]
- Ehlers, S. Tumor necrosis factor and its blockade in granulomatous infections: Differential modes of action of infliximab and etanercept? Clin. Infect. Dis. 2005, 41 (Suppl. S3), S199–S203. [Google Scholar] [CrossRef] [PubMed]
- Hassoun, L.A.; Sivamani, R.K.; Sharon, V.R.; Silverstein, M.A.; Burrall, B.A.; Tartar, D.M. Ustekinumab to target granulomatous dermatitis in recalcitrant ulcerative necrobiosis lipoidica: Case report and proposed mechanism. Dermatol. Online J. 2017, 23, 18. [Google Scholar] [CrossRef]
- Pourang, A.; Sivamani, R.K. Treatment-resistant ulcerative necrobiosis lipoidica in a diabetic patient responsive to ustekinumab. Dermatol. Online J. 2019, 25, 11. [Google Scholar] [CrossRef]
- Gibson, R.S.; Salian, P.; Beckles, A.; Stavert, R.; Tahan, S.; Kimball, A.B.; Porter, M.L. Treatment of necrobiosis lipoidica with secukinumab (Cosentyx): A case series. Int. J. Dermatol. 2023, 62, 1198–1201. [Google Scholar] [CrossRef]
- Lee, J.J.; English, J.C. Improvement in Ulcerative Necrobiosis Lipoidica After Janus Kinase-Inhibitor Therapy for Polycythemia Vera. JAMA Dermatol. 2018, 154, 733–734. [Google Scholar] [CrossRef]
- Beatty, P.; Killion, L.; Blake, C.; Kelly, G.; Tobin, A. Ulcerating necrobiosis lipoidica successfully treated with ustekinumab. Australas. J. Dermatol. 2021, 62, e473–e474. [Google Scholar] [CrossRef]
- McPhie, M.L.; Swales, W.C.; Gooderham, M.J. Improvement of granulomatous skin conditions with tofacitinib in three patients: A case report. SAGE Open Med. Case Rep. 2021, 9, 2050313X211039477. [Google Scholar] [CrossRef]
- Benson, J.M.; Peritt, D.; Scallon, B.J.; Heavner, G.A.; Shealy, D.J.; Giles-Komar, J.M.; Mascelli, M.A. Discovery and mechanism of ustekinumab: A human monoclonal antibody targeting interleukin-12 and interleukin-23 for treatment of immune-mediated disorders. MAbs 2011, 3, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Oiso, N.; Itoh, T.; Sato, M.; Matsuo, K.; Nakayama, T.; Satou, T.; Kawada, A. Necrobiosis lipoidica with infiltration of Th17 cells into vascular lesions. J. Dermatol. 2014, 41, 459–461. [Google Scholar] [CrossRef] [PubMed]
- Baeten, D.; Sieper, J.; Braun, J.; Baraliakos, X.; Dougados, M.; Emery, P.; Deodhar, A.; Porter, B.; Martin, R.; Andersson, M.; et al. Secukinumab, an Interleukin-17A Inhibitor, in Ankylosing Spondylitis. N. Engl. J. Med. 2015, 373, 2534–2548. [Google Scholar] [CrossRef]
- Wakusawa, C.; Fujimura, T.; Kambayashi, Y.; Furudate, S.; Hashimoto, A.; Aiba, S. Pigmented necrobiosis lipoidica accompanied by insulin-dependent diabetes mellitus induces CD163+ proinflammatory macrophages and interleukin-17-producing cells. Acta Derm. Venereol. 2013, 93, 475–476. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, A.B.; Deodhar, A.; Mcinnes, I.B.; Baraliakos, X.; Reich, K.; Schreiber, S.; Bao, W.; Marfo, K.; Richards, H.B.; Pricop, L.; et al. Long-term Safety of Secukinumab Over Five Years in Patients with Moderate-to-severe Plaque Psoriasis, Psoriatic Arthritis and Ankylosing Spondylitis: Update on Integrated Pooled Clinical Trial and Post-marketing Surveillance Data. Acta Derm. Venereol. 2022, 102, adv00698. [Google Scholar] [CrossRef] [PubMed]
- Kisseleva, T.; Bhattacharya, S.; Braunstein, J.; Schindler, C.W. Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene 2002, 285, 1–24. [Google Scholar] [CrossRef]
- Roskoski, R. Janus kinase (JAK) inhibitors in the treatment of inflammatory and neoplastic diseases. Pharmacol. Res. 2016, 111, 784–803. [Google Scholar] [CrossRef]
- Grunewald, J.; Eklund, A. Role of CD4+ T cells in sarcoidosis. Proc. Am. Thorac. Soc. 2007, 4, 461–464. [Google Scholar] [CrossRef]
- Hilhorst, M.; Shirai, T.; Berry, G.; Goronzy, J.J.; Weyand, C.M. T cell-macrophage interactions and granuloma formation in vasculitis. Front. Immunol. 2014, 5, 432. [Google Scholar] [CrossRef]
- Wang, A.; Singh, K.; Ibrahim, W.; King, B.; Damsky, W. The Promise of JAK Inhibitors for Treatment of Sarcoidosis and Other Inflammatory Disorders with Macrophage Activation: A Review of the Literature. Yale J. Biol. Med. 2020, 93, 187–195. [Google Scholar]
- Damsky, W.; Singh, K.; Galan, A.; King, B. Treatment of necrobiosis lipoidica with combination Janus kinase inhibition and intralesional corticosteroid. JAAD Case Rep. 2020, 6, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.J.; Banerjee, S.; Raychaudhuri, S.; Raychaudhuri, S.P. JAK-STAT inhibitors in Immune mediated diseases: An Overview. Indian. J. Dermatol. Venereol. Leprol. 2023, 89, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Sepriano, A.; Kerschbaumer, A.; Smolen, J.S.; van der Heijde, D.; Dougados, M.; van Vollenhoven, R.; McInnes, I.B.; Bijlsma, J.W.; Burmester, G.R.; de Wit, M.; et al. Safety of synthetic and biological DMARDs: A systematic literature review informing the 2019 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis 2020, 79, 760–770. [Google Scholar] [CrossRef]
- Desai, R.J.; Pawar, A.; Weinblatt, M.E.; Kim, S.C. Comparative Risk of Venous Thromboembolism in Rheumatoid Arthritis Patients Receiving Tofacitinib Versus Those Receiving Tumor Necrosis Factor Inhibitors: An Observational Cohort Study. Arthritis Rheumatol. 2019, 71, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Wollenhaupt, J.; Lee, E.B.; Curtis, J.R.; Silverfield, J.; Terry, K.; Soma, K.; Mojcik, C.; DeMasi, R.; Strengholt, S.; Kwok, K.; et al. Safety and efficacy of tofacitinib for up to 9.5 years in the treatment of rheumatoid arthritis: Final results of a global, open-label, long-term extension study. Arthritis Res. Ther. 2019, 21, 89. [Google Scholar] [CrossRef] [PubMed]
- Nugent, S.; Coromilas, A.J.; English, J.C.; Rosenbach, M. Improvement of necrobiosis lipoidica with topical ruxolitinib cream after prior nonresponse to compounded topical tofacitinib cream. JAAD Case Rep. 2022, 29, 25–26. [Google Scholar] [CrossRef] [PubMed]
- Janßen, S.; Jansen, T.M. Ulcerated necrobiosis lipoidica successfully treated with tofacitinib. Int. J. Dermatol. 2022, 61, 739–741. [Google Scholar] [CrossRef] [PubMed]
- Erfurt-Berge, C.; Sticherling, M. Successful treatment of ulcerative necrobiosis lipoidica with janus kinase inhibitor. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e331–e333. [Google Scholar] [CrossRef]
- Barbet-Massin, M.A.; Rigalleau, V.; Blanco, P.; Mohammedi, K.; Poupon, P.; Belin, E.; Poursac, N.; Cadart, O.; Blanco, L. Remission of necrobiosis lipoidica diabeticorum with a JAK1/2 inhibitor: A case report. Diabetes Metab. 2021, 47, 101143. [Google Scholar] [CrossRef]
- Arnet, L.; Erfurt-Berge, C. Effect of abrocitinib in a patient with extensive necrobiosis lipoidica. J. Eur. Acad. Dermatol. Venereol. 2023, 37, e1208–e1210. [Google Scholar] [CrossRef]
- Furue, M.; Hashimoto-Hachiya, A.; Tsuji, G. Aryl Hydrocarbon Receptor in Atopic Dermatitis and Psoriasis. Int. J. Mol. Sci. 2019, 20, 5424. [Google Scholar] [CrossRef] [PubMed]
- Furue, M. Regulation of Filaggrin, Loricrin, and Involucrin by IL-4, IL-13, IL-17A, IL-22, AHR, and NRF2: Pathogenic Implications in Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 5382. [Google Scholar] [CrossRef] [PubMed]
- Bissonnette, R.; Stein Gold, L.; Rubenstein, D.S.; Tallman, A.M.; Armstrong, A. Tapinarof in the treatment of psoriasis: A review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent. J. Am. Acad. Dermatol. 2021, 84, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Furue, M. Regulation of Skin Barrier Function via Competition between AHR Axis versus IL-13/IL-4-JAK-STAT6/STAT3 Axis: Pathogenic and Therapeutic Implications in Atopic Dermatitis. J. Clin. Med. 2020, 9, 3741. [Google Scholar] [CrossRef] [PubMed]
- Palomares, S.J.; Farberg, A.S. Nonulcerated Necrobiosis Lipoidica Successfully Treated with Tapinarof: A Case Report. Clin. Cosmet. Investig. Dermatol. 2023, 16, 1373–1376. [Google Scholar] [CrossRef] [PubMed]
- Bissonnette, R.; Vasist, L.S.; Bullman, J.N.; Collingwood, T.; Chen, G.; Maeda-Chubachi, T. Systemic Pharmacokinetics, Safety, and Preliminary Efficacy of Topical AhR Agonist Tapinarof: Results of a Phase 1 Study. Clin. Pharmacol. Drug Dev. 2018, 7, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Strober, B.; Stein Gold, L.; Bissonnette, R.; Armstrong, A.W.; Kircik, L.; Tyring, S.K.; Piscitelli, S.C.; Brown, P.M.; Rubenstein, D.S.; Tallman, A.M.; et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: Results from the PSOARING 3 trial. J. Am. Acad. Dermatol. 2022, 87, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Baccarani, A.; De Maria, F.; Pappalardo, M.; Pedone, A.; De Santis, G. Necrobiosis Lipoidica Affecting the Leg: What Is the Best Treatment in a Patient with Very High Aesthetic Demand? Plast. Reconstr. Surg. Glob. Open 2020, 8, e3000. [Google Scholar] [CrossRef]
- Fulgencio-Barbarin, J.; Conde Montero, E. Sequential punch grafting for treatment of ulcerative necrobiosis lipoidica. J. Tissue Viability 2022, 31, 560–561. [Google Scholar] [CrossRef]
- Pimenta, R.; Roda, Â.; Freitas, J.P. Ulcerated Necrobiosis Lipoidica. Acta Med. Port. 2018, 31, 440. [Google Scholar] [CrossRef]
- Abdat, R.; Cohen, S.R.; Deverapalli, S.; Hoot, J.; Yang, F.C. Use of fractionated microneedle radiofrequency for necrobiosis lipoidica. Lasers Med. Sci. 2021, 36, 1337–1339. [Google Scholar] [CrossRef]
- Motolese, A.; Vignati, F.; Antelmi, A.; Saturni, V. Effectiveness of platelet-rich plasma in healing necrobiosis lipoidica diabeticorum ulcers. Clin. Exp. Dermatol. 2015, 40, 39–41. [Google Scholar] [CrossRef]
| Trait | Observations | Possible Explanation |
|---|---|---|
| Vessel morphology | Initially, the linearly curved (comma-shaped) vessels become wavy and then tree-branched. | In early lesions, the comma-shaped vessels are due to vasodilatation in the papillary layer of the dermis as there are no epidermal changes. As the lesion progresses, the epidermis atrophies, resulting in the appearance of dilated vessels located in the deep layers of the dermis, which appear as linear–wavy and then tree-like branching. |
| Distribution of vessels | Vessels are evenly distributed. | - |
| Background | The vessels can be seen against a background of evenly spaced yellow areas without structure and whitish linear streaks. | Yellow areas without structure represent skin granulomas, while white linear streaks represent fibrosis. |
| Pigment network | In advanced lesions, brown pigmented networks may be visible. | This change is caused by the stimulation of melanocytes at the dermal–epidermal border. This phenomenon is nonspecific and common to many inflammatory skin lesions. |
| Disease | Clinical Description | Histological Description | Dermatoscopic Description |
|---|---|---|---|
| Necrobiosis lipoidica | Red papules or nodules, transform into round, oval plaques on the pretibial surface of the lower extremities; they enlarge peripherally, leaving a yellowish-brown atrophy in the central part with dilated small vessels. | Interstitial and palisade granulomas are visible. Lesions are layered and mixed with degenerated collagen. No increase in central mucin is observed. Plasma cells, multinucleated cells, and loss of elastic tissue are present. | Tree-shaped vessels of equal diameter without branching into finer capillaries. White linear streaks present. Lesions on a yellow-white background. |
| Granuloma annulare | Skin-colored papules, merging into ring-shaped foci. Various varieties possible. | Palisade granulomas with necrotic core of collagen and mucin can be seen. A lymphocytic infiltrate is present. | No visible vessels. Background in various shades of red, without structures on the periphery. |
| Sarcoidosis | Reddish-brown, round or ring-shaped plaque or nodular foci. Various variations possible. | In the specific type, there is a dermal infiltration of non-necrotic epithelioid granulomas. In the nonspecific type, the lesion is reactive. The epithelioid infiltration of histiocytes is present. | Vessels shorter and less branched than in NL. White reticulate streaks present. Orange globules in the background. Millia-like cysts also visible. |
| Necrobiotic xanthogranuloma | Hardened erythematous and yellowish plaques that may develop into scars, ulcers, and telangiectasias. The most common localization is on the face. | Widespread vitreous necrosis with foci of xanthogranulomatous infiltration in the reticular layer of the dermis into the subcutaneous fat. Cholesterol fissures with histiocytic infiltration with giant cells are visible. | Red-yellow area with irregular telangiectasias. |
| Localized scleroderma | Early lesions are erythematous. As the lesions progress, they become sclerotic and are surrounded by a “lilac” ring, and the center of the lesions is whitish or ivory in color. | In early lesions, the inflammatory margin shows an inflammatory infiltrate composed mainly of large numbers of lymphocytes and plasma cells. Sclerotic lesions show collagen fibers extending into the reticular layer of the dermis. | Whitish bundles of fibrosis that often cross linear branching vessels. Pigment network-like structures are also often visible. |
| References | Sex/Age | Ulceration | Earlier Treatment | Biological Treatment | Response to Biological Treatment | Adverse Events |
|---|---|---|---|---|---|---|
| Beatty et al. [80] | 24/F | + | topical clobetasol propionate, intralesional triamcinolone, doxycycline | Ustekinumab 45 mg week 0, week 4 and every 12 weeks thereafter | after 4 weeks: re-granulation of the ulcerated plaque after 12 weeks: almost complete re-granulation | none |
| Pourang et al. [77] | 29/F | + | topical, intralesional, and systemic steroids, topical tacrolimus, oral antibiotics, and antifungals, hydroxychloroquine, pentoxifylline | Ustekinumab 90 mg every 2 months | after a few months: lesions improved | cellulitis |
| earlier: adalimumab | new plaques have appeared (treatment was discontinued due to adverse effects) | abdominal rash, urticarial plaques at the injection site, new plaques | ||||
| Hassoun et al. [76] | 42/F | + | pentoxifylline, cyclosporine, mycophenolate mofetil | Ustekinumab 45 mg every 9 weeks | significant reduction in pain and pruritus, absence of recurrent ulcerations | none |
| earlier: infliximab | healing of ulcerations (treatment was discontinued due to adverse effects) | anaphylactoid reaction | ||||
| earlier: adalimumab | no response | none | ||||
| earlier: etanercept | etanercept was effective for 6 months, until loss of efficacy, recurrence of ulcerations | none | ||||
| McPhie et al. [81] | 71/M | + | topical and intralesional corticosteroids, cephalexin, hydroxychloroquine, acitretin, pentoxifylline | ustekinumab 90 mg every 8 weeks | no response | not mentioned |
| Gibson et al. [78] | 45/F | + | none | secukinumab s.c. 300 mg weekly for 5 weeks then every 4 weeks (total: 24 weeks) | moderate improvement (about 50%) | toothache, dry socket reported; extraction of broken tooth, peritonsillar cellulitis |
| earlier: adalimumab s.c 40 mg weekly (for several months) | no response | not mentioned | ||||
| 30/F | - | topical corticosteroid | secukinumab s.c. 300 mg weekly for 5 weeks then every 4 weeks (total: 24 weeks) | very significant clearance (about 90%) | none | |
| 38/F | + | topical corticosteroid, intralesional triamcinolone | secukinumab s.c. 300 mg weekly for 5 weeks then every 4 weeks (total: 24 weeks) | marked improvement (about 75%) | shortness of breath, possible anxiety attack reported at week 1 | |
| 56/F | + | phototherapy, clobetasol and other corticosteroids, hydroxychloroquine | secukinumab s.c. 300 mg weekly for 5 weeks then every 4 weeks (total: 16 weeks) | slight improvement (about 25%) | swollen feet at week 1, improvement at week 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naumowicz, M.; Modzelewski, S.; Macko, A.; Łuniewski, B.; Baran, A.; Flisiak, I. A Breakthrough in the Treatment of Necrobiosis Lipoidica? Update on Treatment, Etiopathogenesis, Diagnosis, and Clinical Presentation. Int. J. Mol. Sci. 2024, 25, 3482. https://doi.org/10.3390/ijms25063482
Naumowicz M, Modzelewski S, Macko A, Łuniewski B, Baran A, Flisiak I. A Breakthrough in the Treatment of Necrobiosis Lipoidica? Update on Treatment, Etiopathogenesis, Diagnosis, and Clinical Presentation. International Journal of Molecular Sciences. 2024; 25(6):3482. https://doi.org/10.3390/ijms25063482
Chicago/Turabian StyleNaumowicz, Maciej, Stefan Modzelewski, Angelika Macko, Bartosz Łuniewski, Anna Baran, and Iwona Flisiak. 2024. "A Breakthrough in the Treatment of Necrobiosis Lipoidica? Update on Treatment, Etiopathogenesis, Diagnosis, and Clinical Presentation" International Journal of Molecular Sciences 25, no. 6: 3482. https://doi.org/10.3390/ijms25063482
APA StyleNaumowicz, M., Modzelewski, S., Macko, A., Łuniewski, B., Baran, A., & Flisiak, I. (2024). A Breakthrough in the Treatment of Necrobiosis Lipoidica? Update on Treatment, Etiopathogenesis, Diagnosis, and Clinical Presentation. International Journal of Molecular Sciences, 25(6), 3482. https://doi.org/10.3390/ijms25063482











