Atlas of Tumor and Tumor Microenvironment Cells of Lymphovascular Space Invasion (LVSI) in High-Grade Serous Endometrial Adenocarcinoma: A Case Study
Abstract
1. Introduction
2. Patient Information and Consent
2.1. Pathology
2.2. Genomics
2.3. Tissue Collection at the Time of Surgery
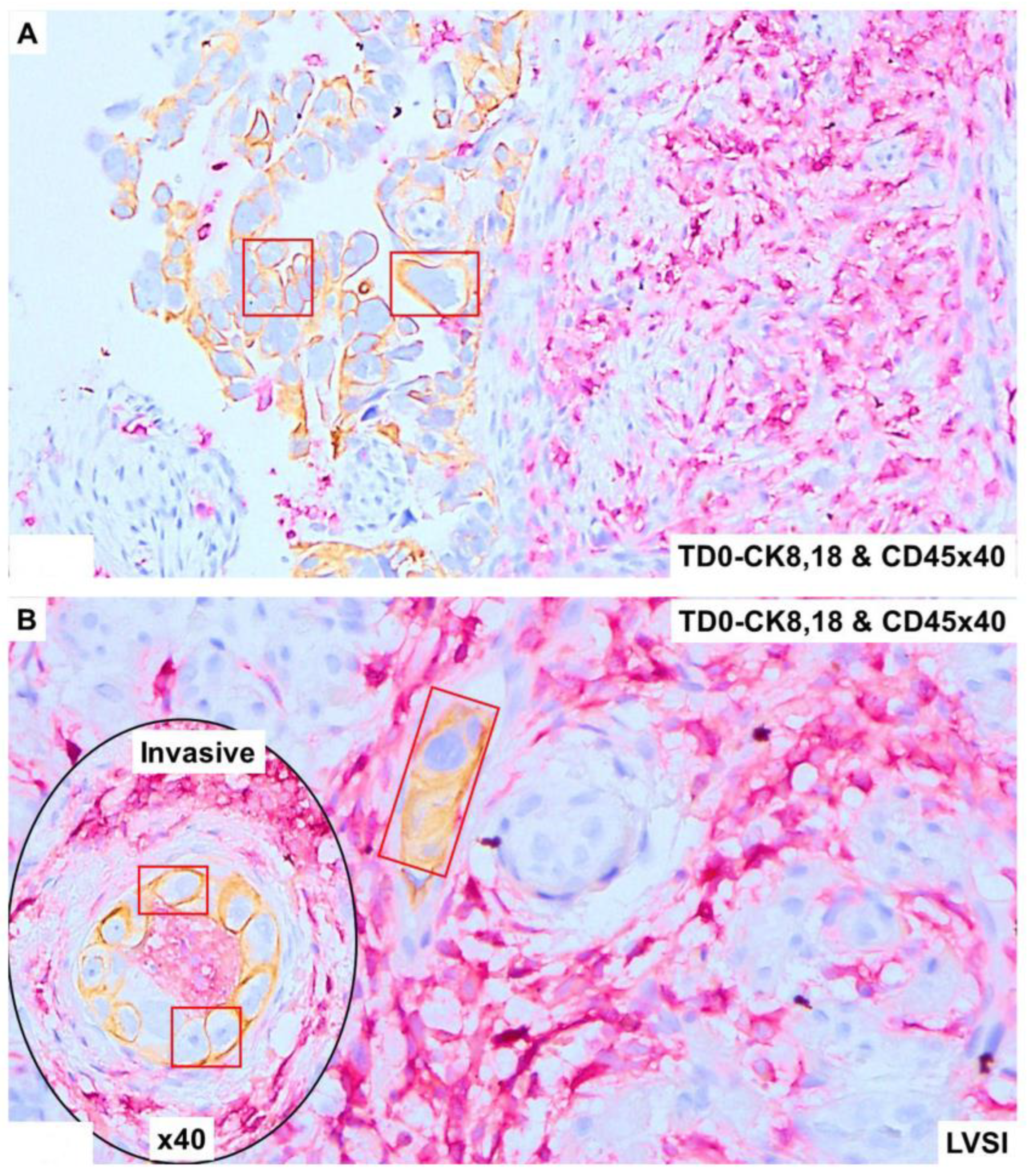
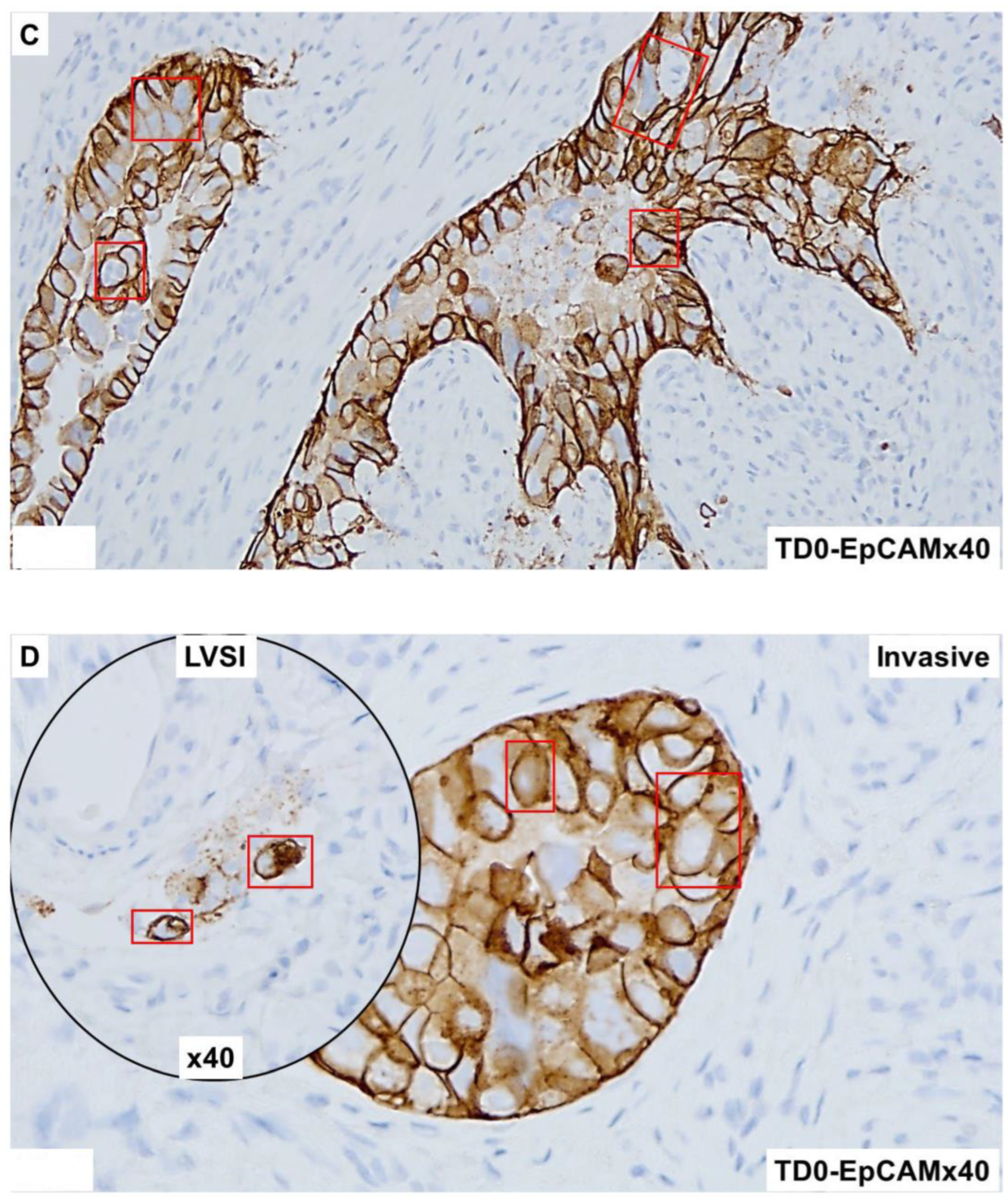
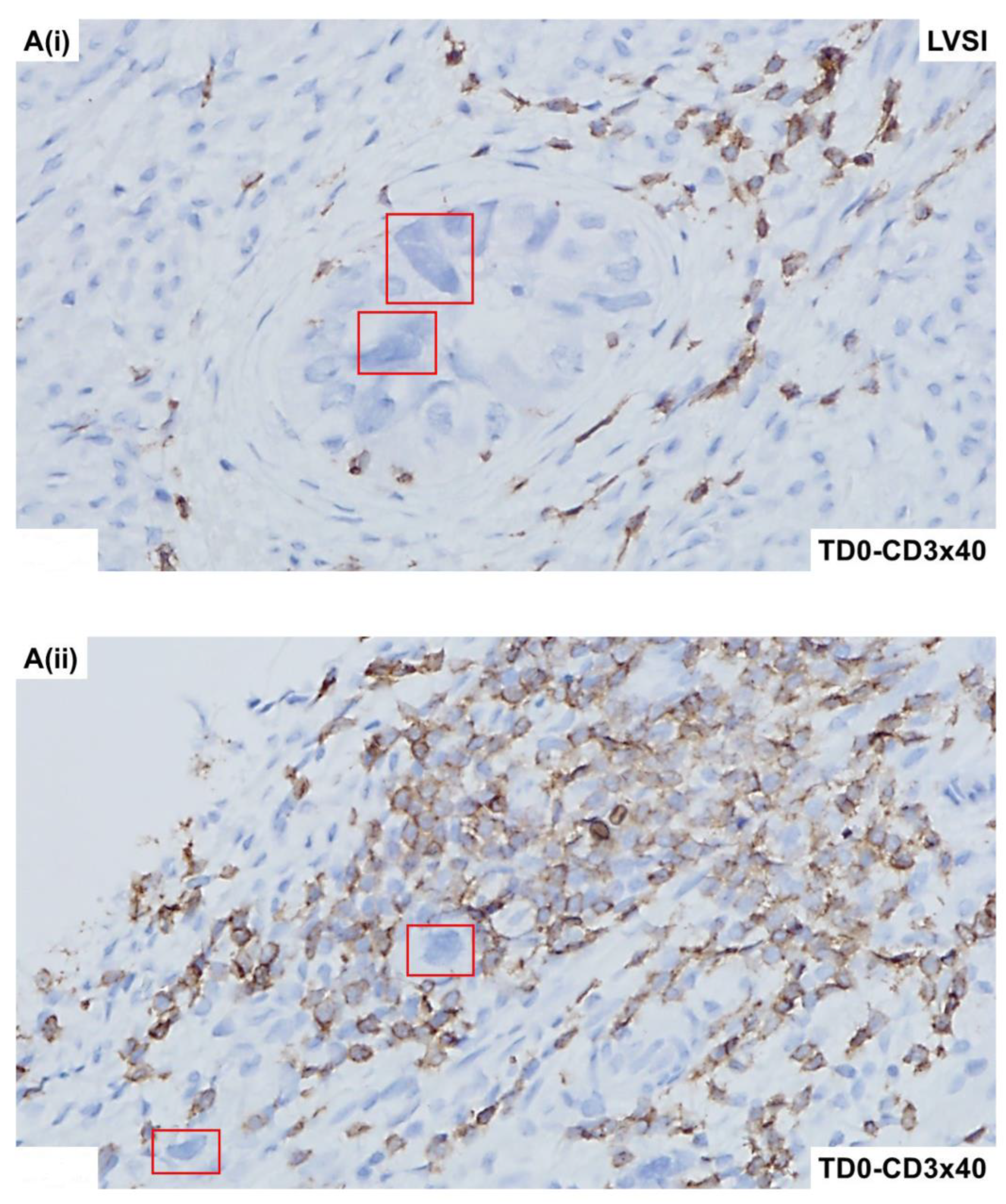
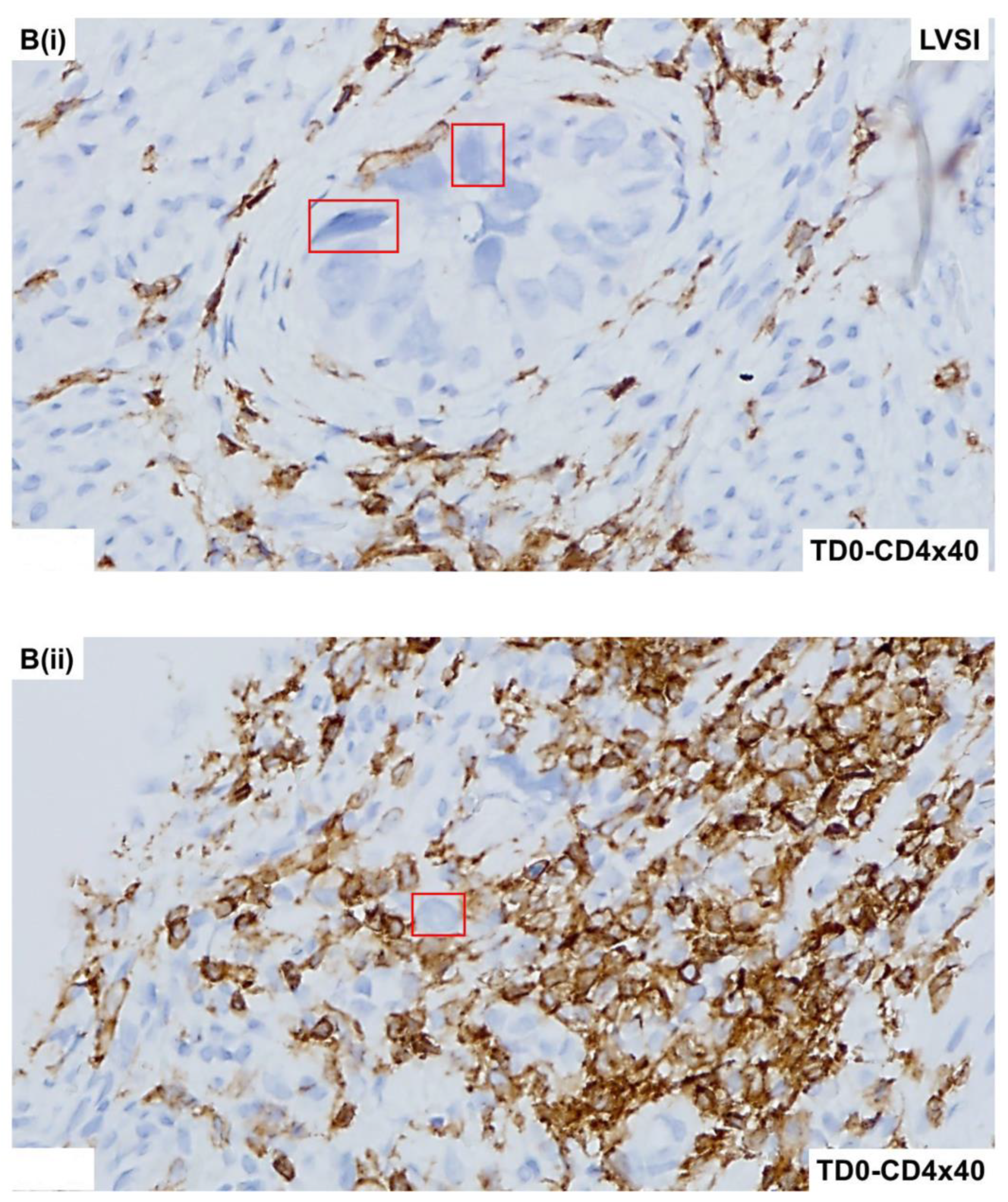
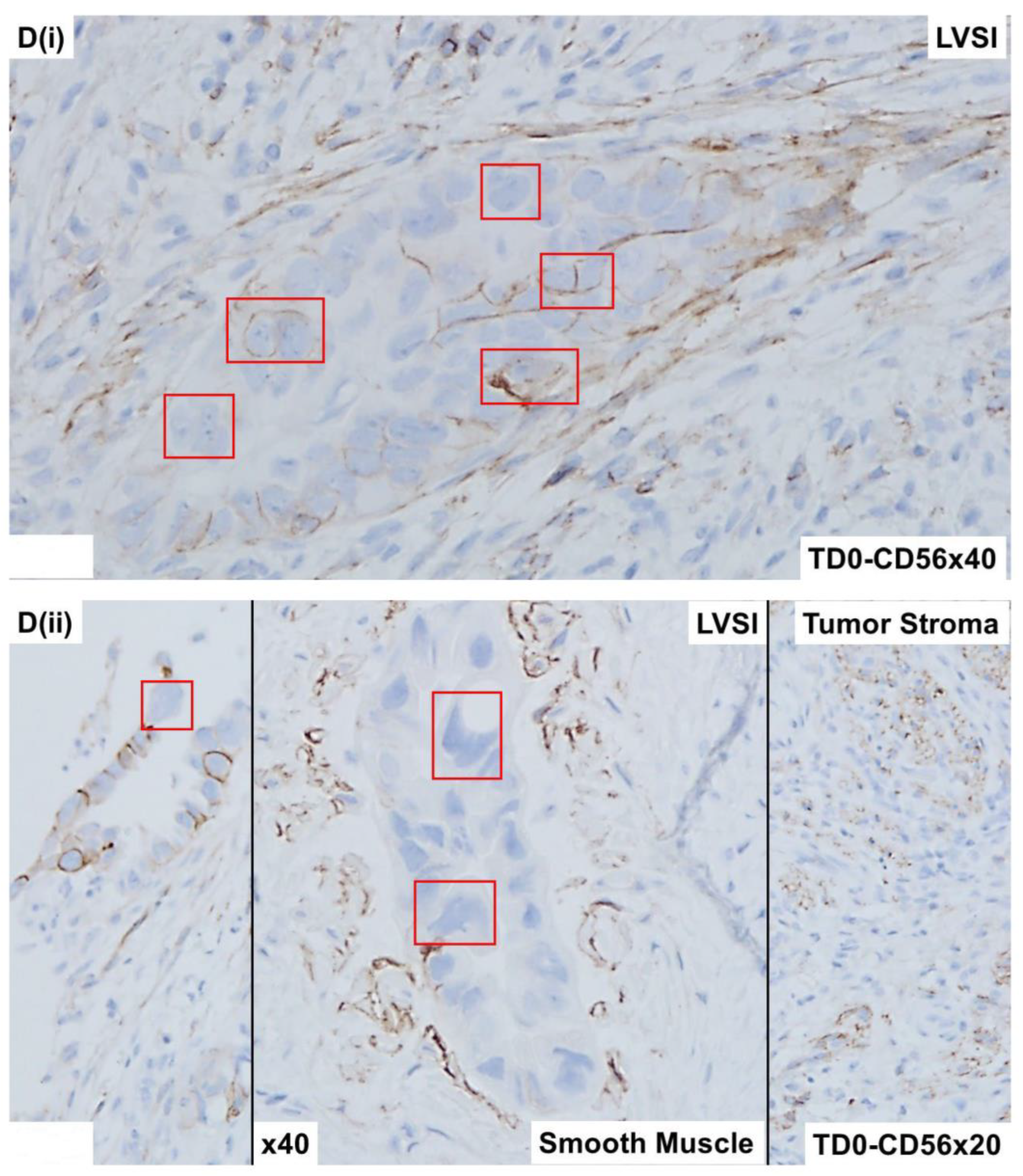
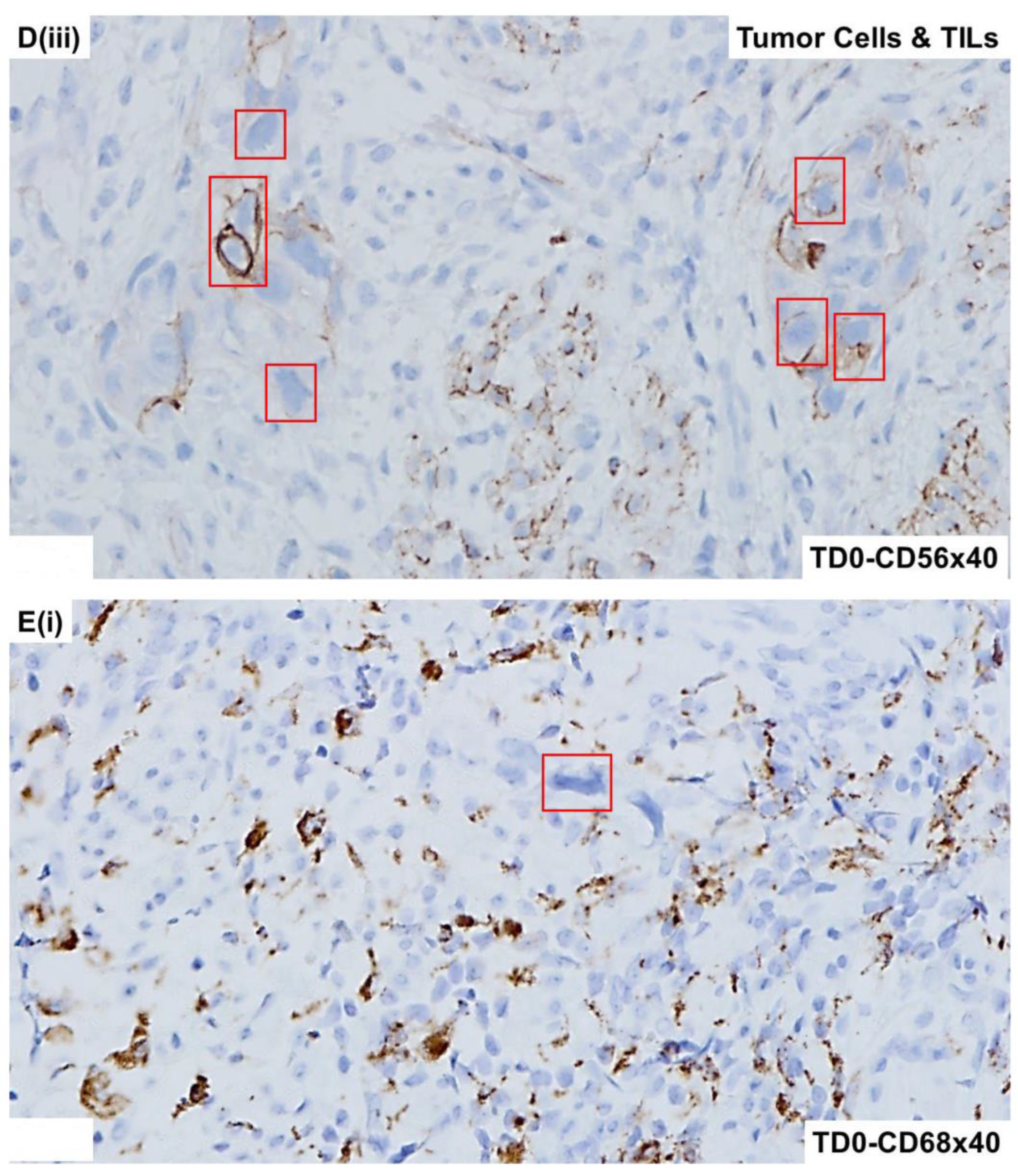
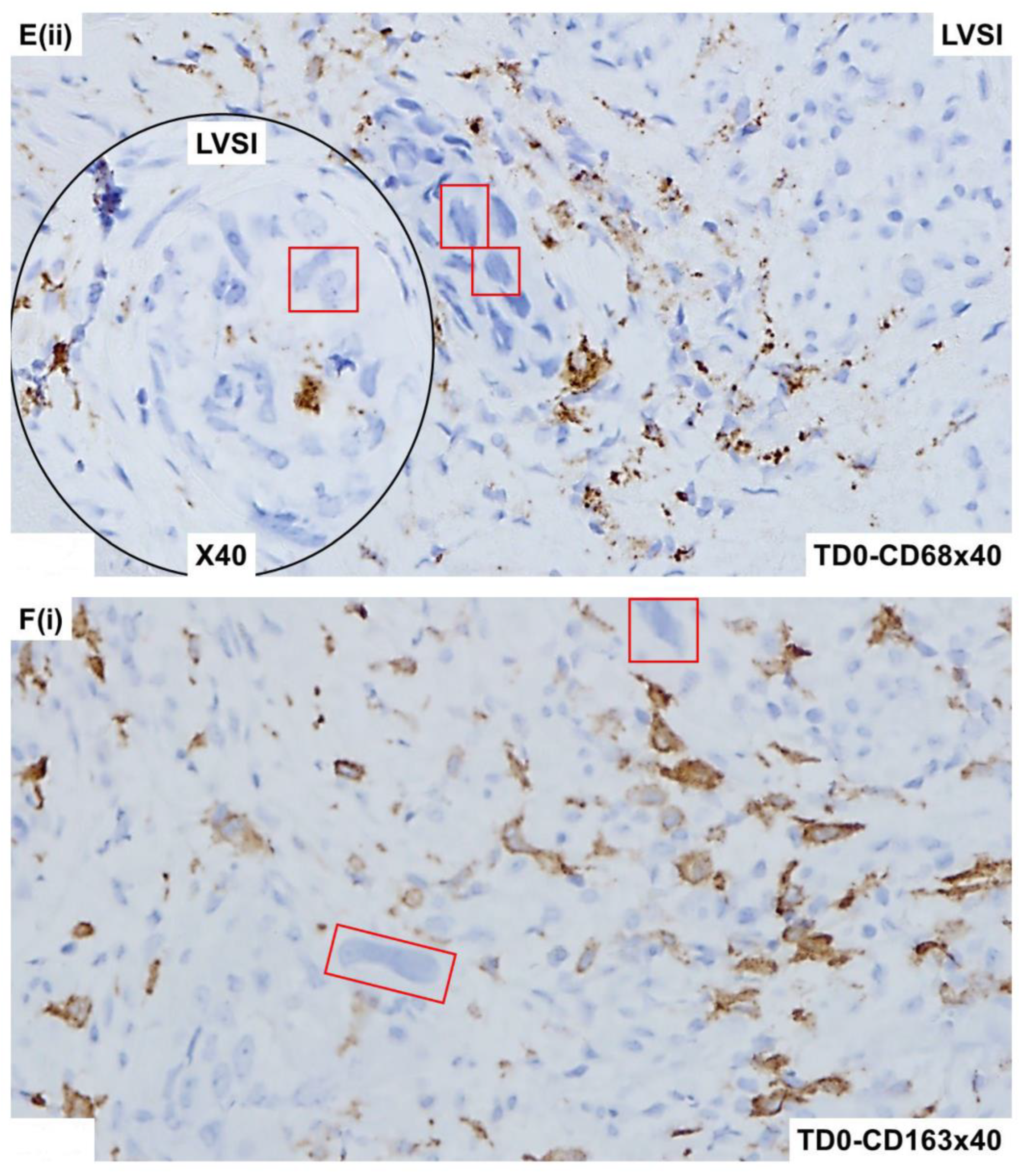
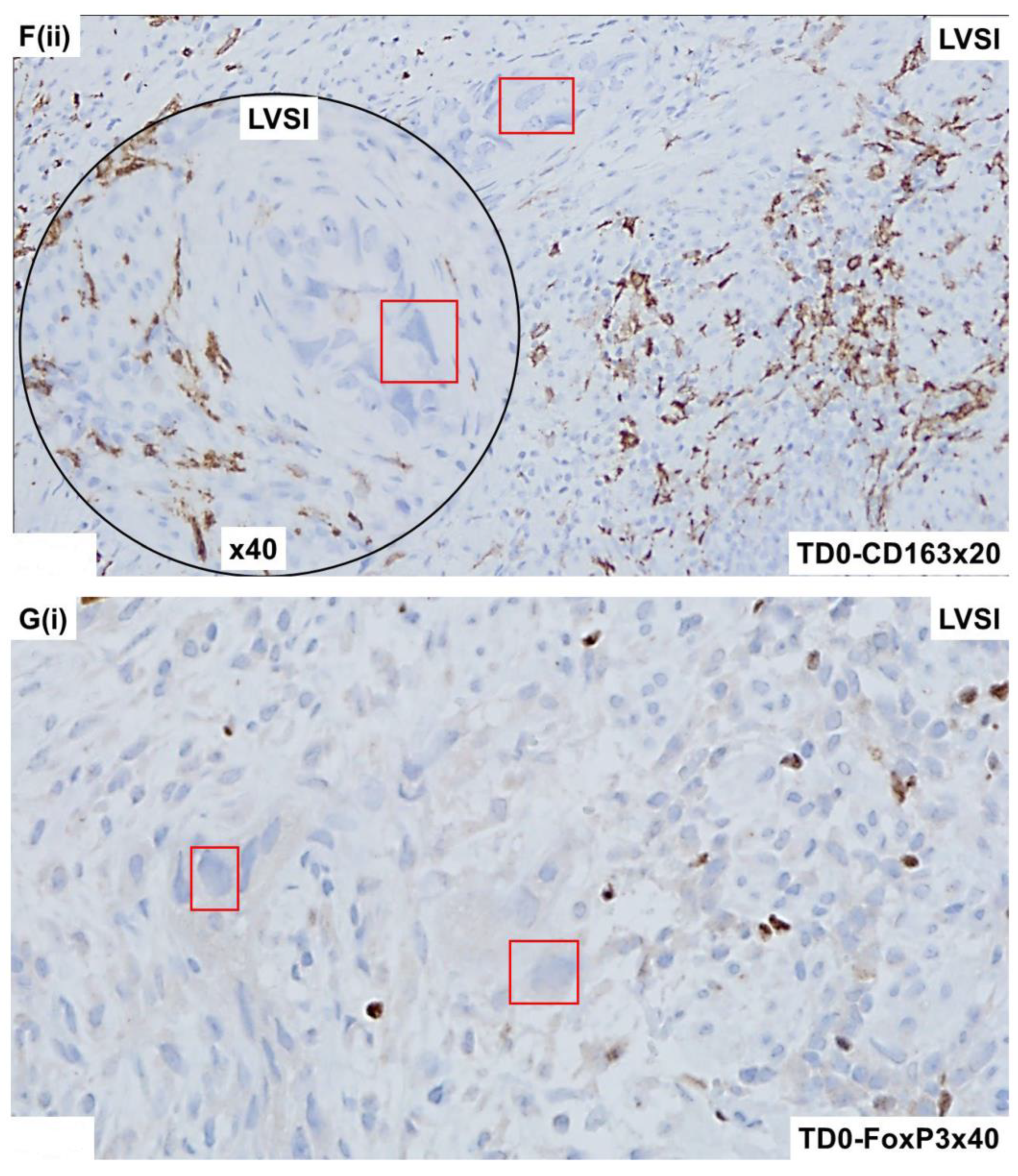
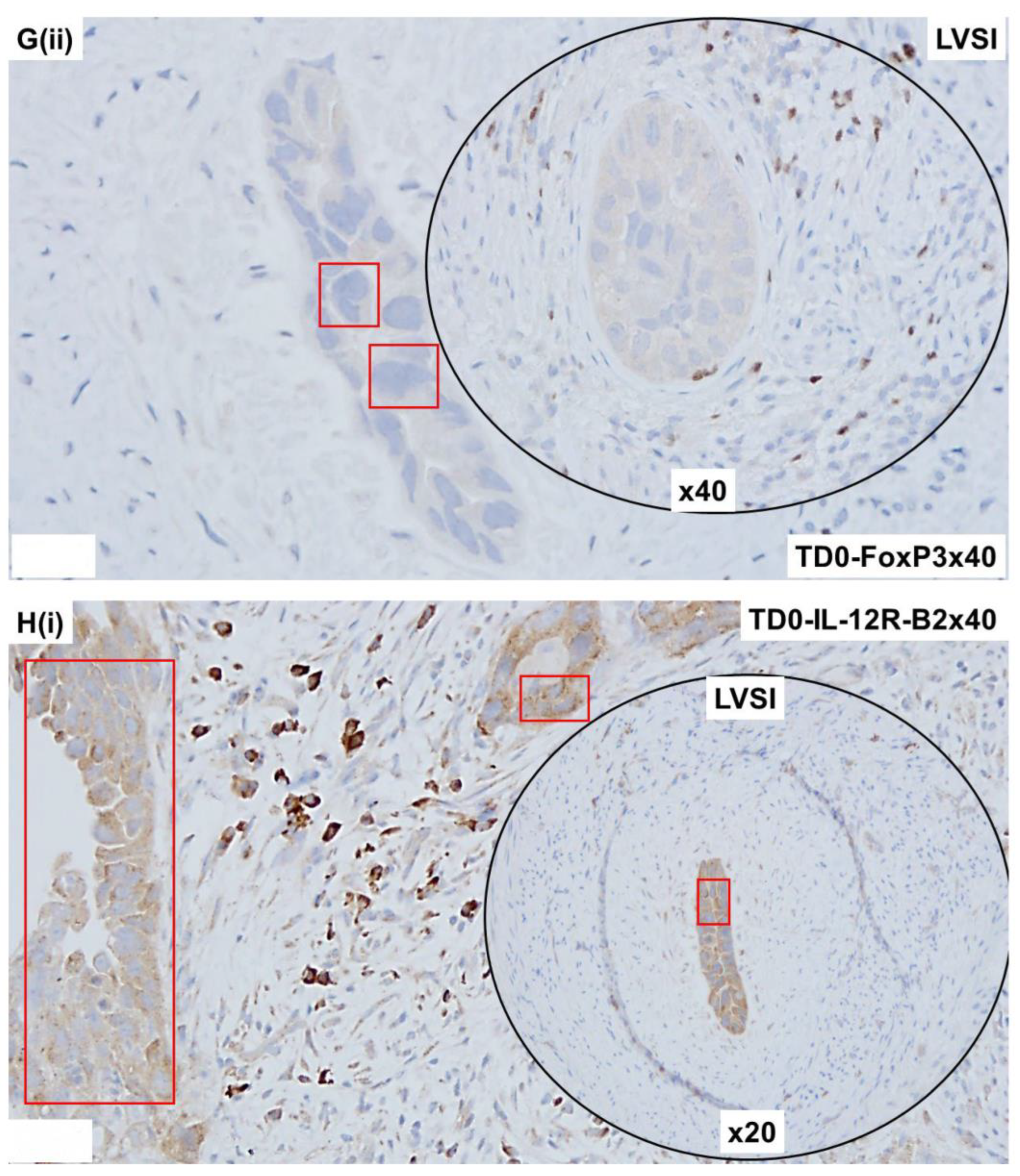
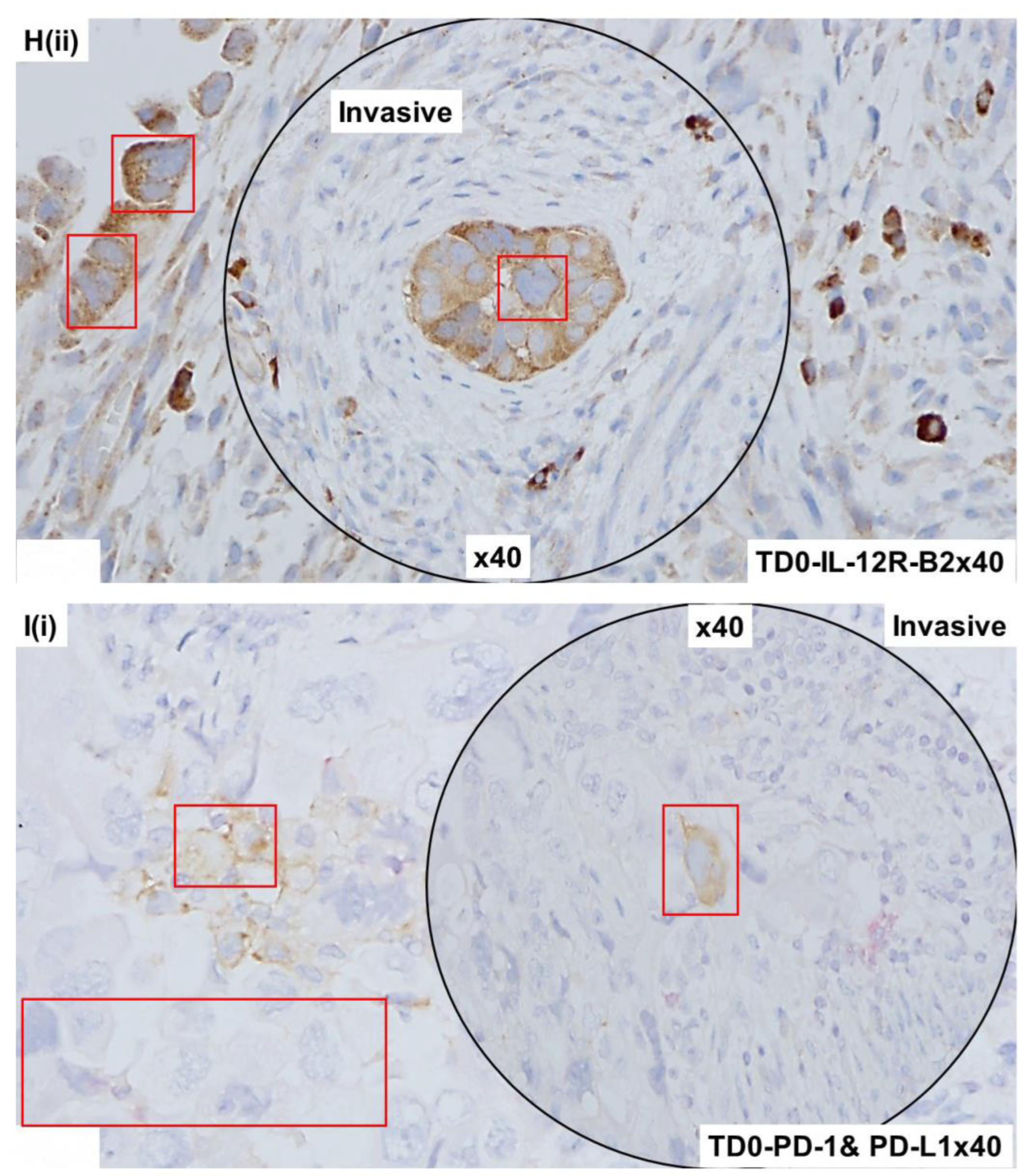
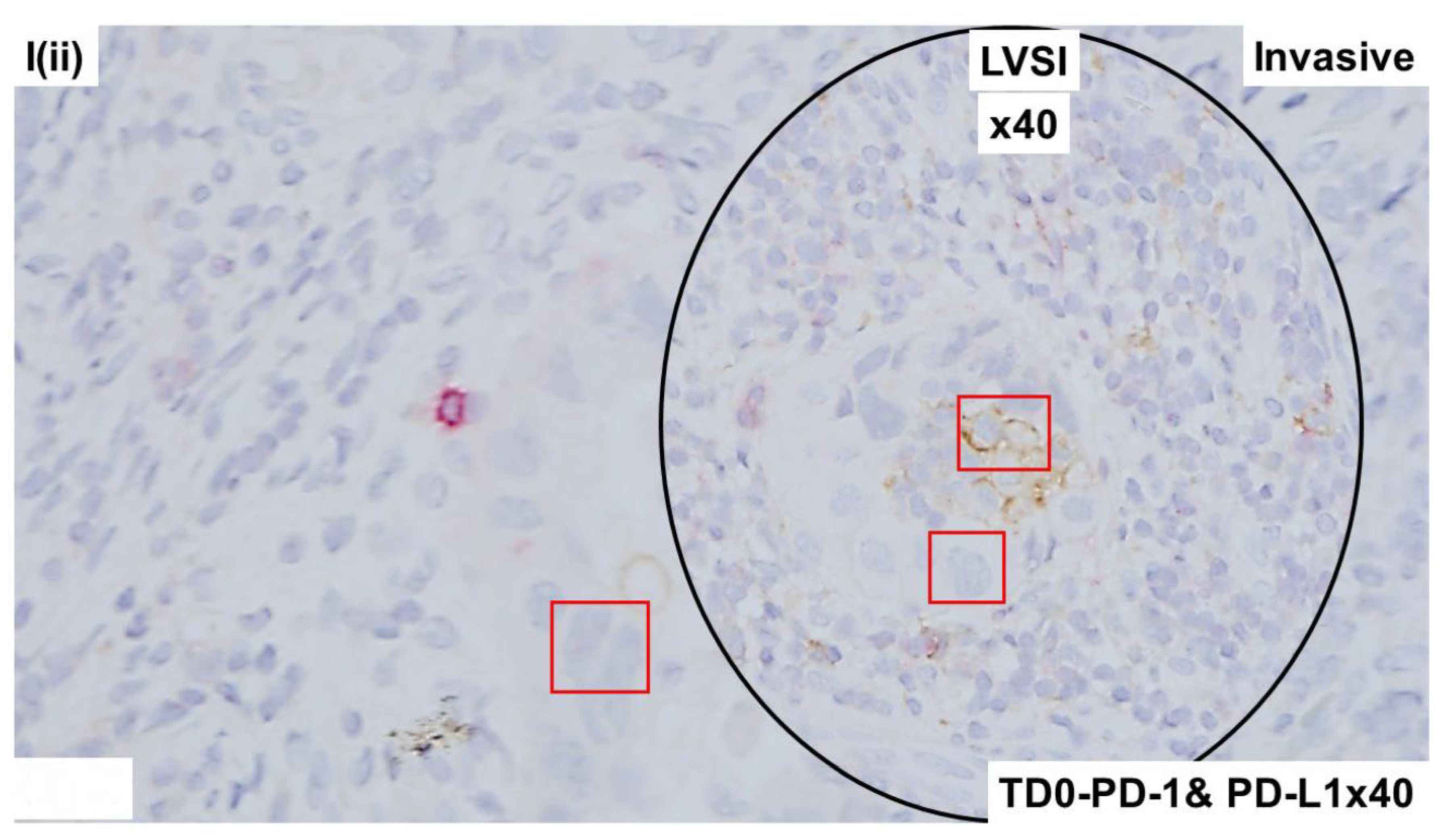
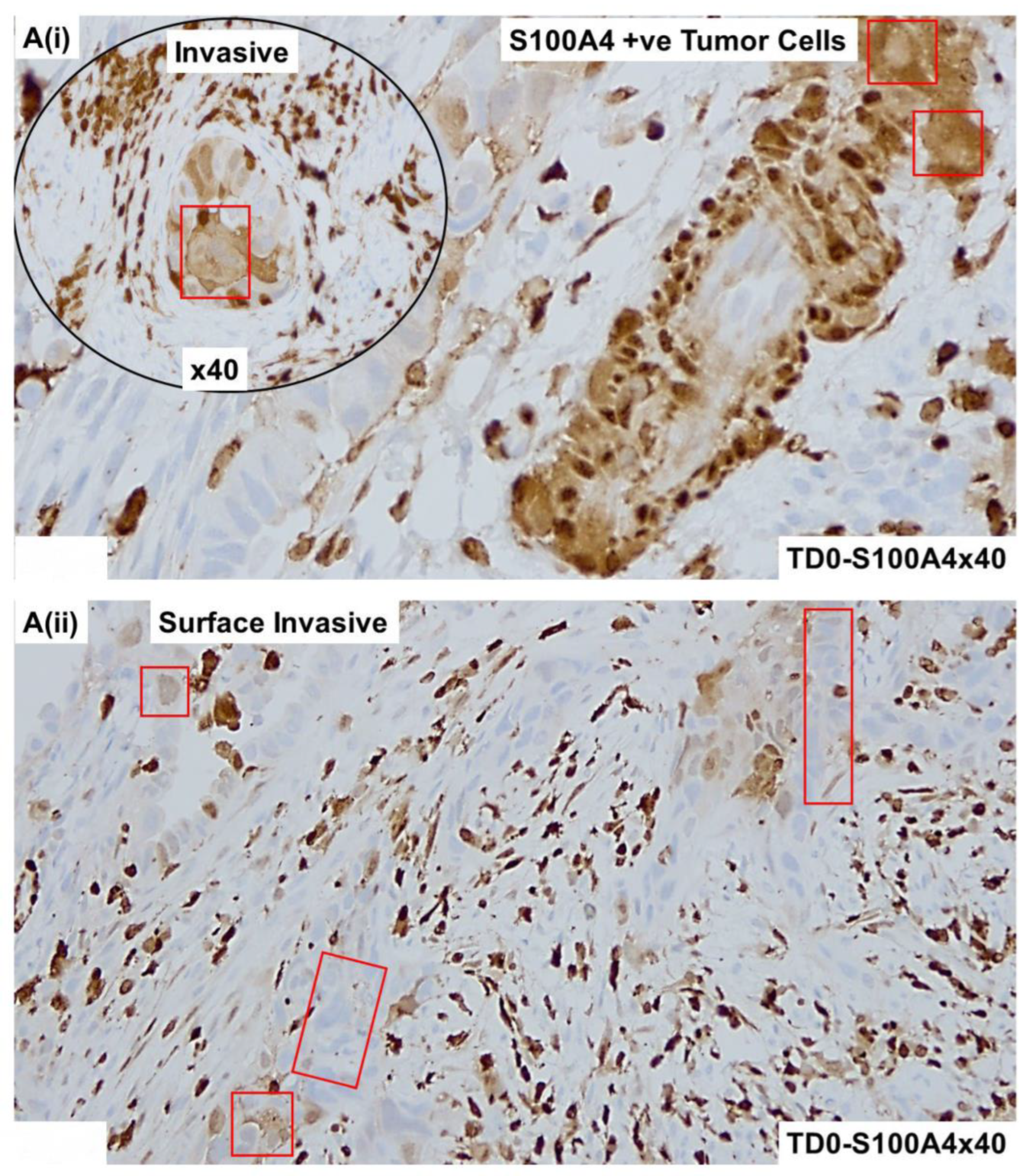
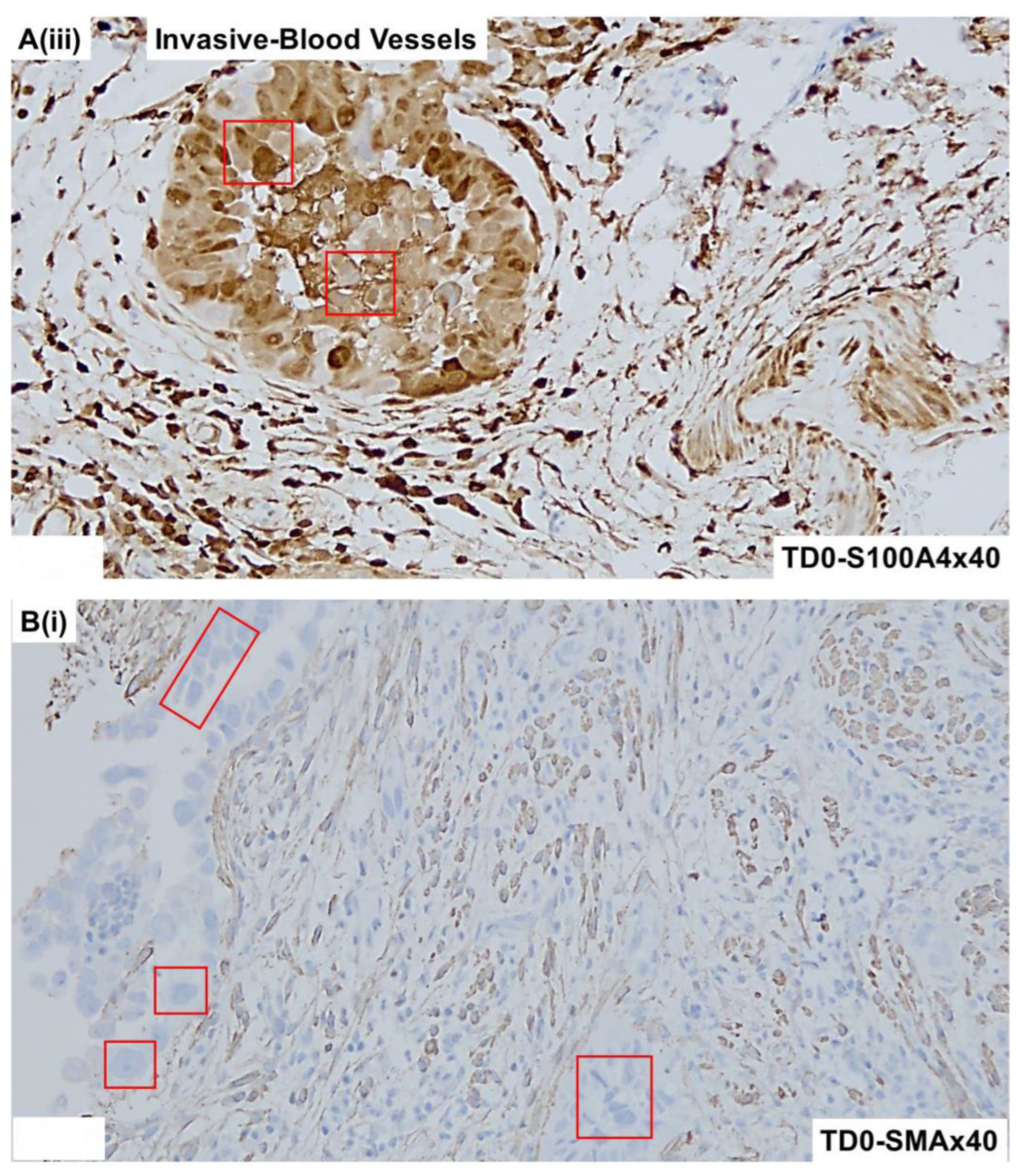
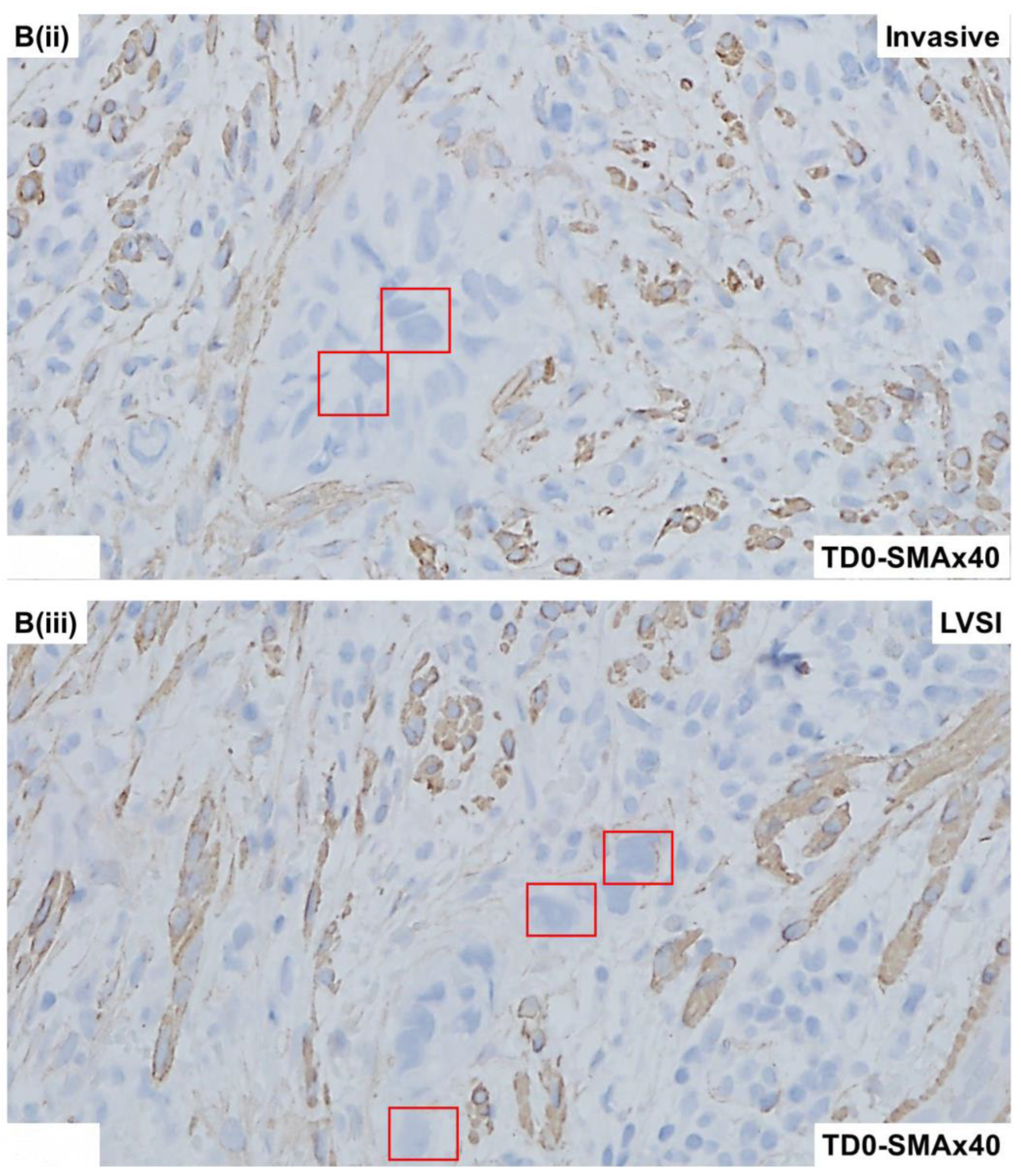
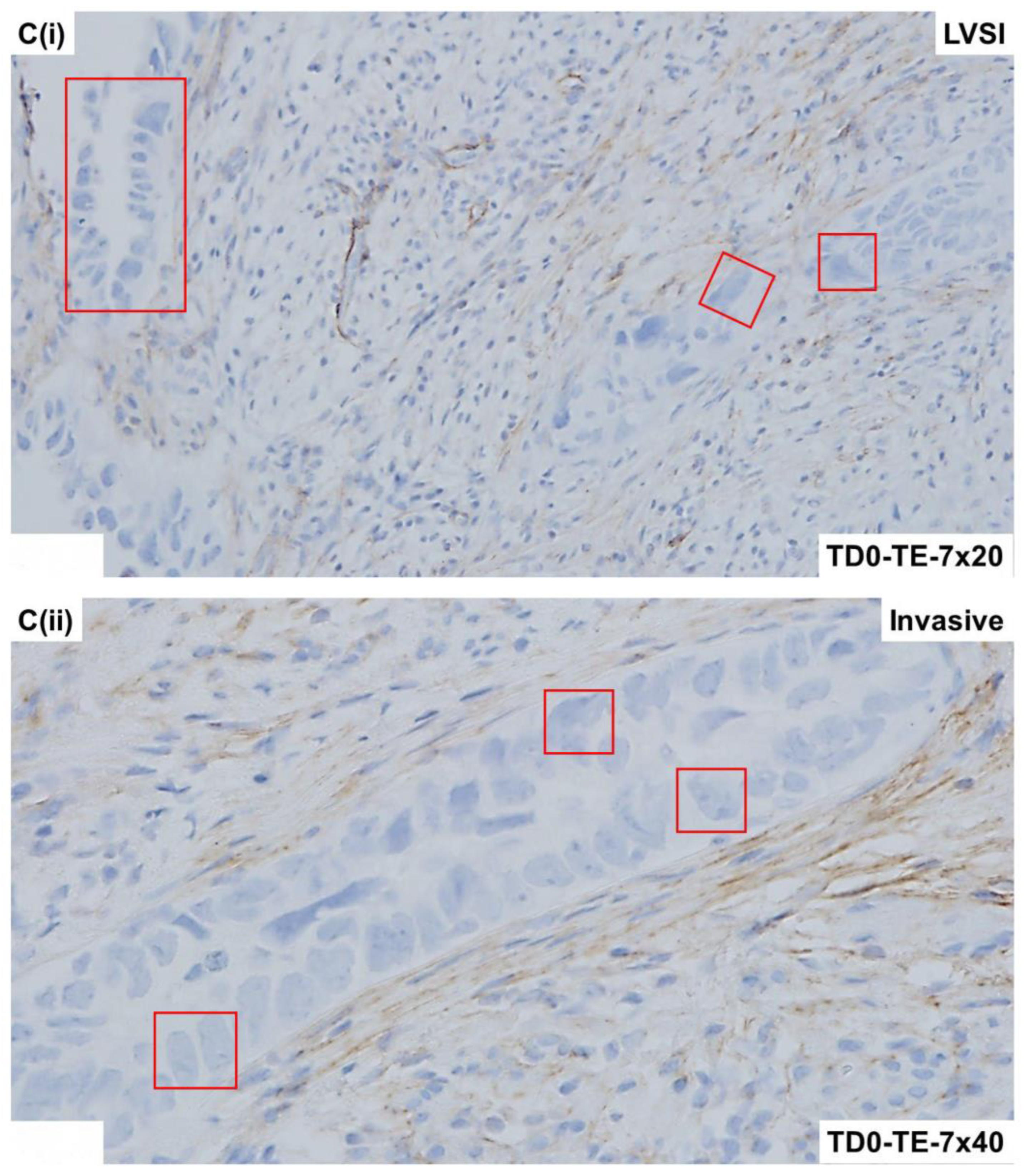
2.4. Immunohistological Marker-Based Characterization of Tumor and TME Compartments
2.5. Expression of Immuno-Histological Markers in Tumor Cells and Cells of TME Compartments
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Tortorella, L.; Restaino, S.; Zannoni, G.F.; Vizzielli, G.; Chiantera, V.; Cappuccio, S.; Gioe, A.; La Fera, E.; Dinoi, G.; Angelico, G.; et al. Substantial lymph-vascular space invasion (LVSI) as predictor of distant relapse and poor prognosis in low-risk early-stage endometrial cancer. J. Gynecol. Oncol. 2021, 32, e11. [Google Scholar] [CrossRef] [PubMed]
- Bogani, G.; Ray-Coquard, I.; Concin, N.; Ngoi, N.Y.L.; Morice, P.; Enomoto, T.; Takehara, K.; Denys, H.; Nout, R.A.; Lorusso, D.; et al. Uterine serous carcinoma. Gynecol. Oncol. 2021, 162, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Dewdney, S.B.; Kizer, N.T.; Andaya, A.A.; Babb, S.A.; Luo, J.; Mutch, D.G.; Schmidt, A.P.; Brinton, L.A.; Broaddus, R.R.; Ramirez, N.C.; et al. Uterine serous carcinoma: Increased familial risk for lynch-associated malignancies. Cancer Prev. Res. 2012, 5, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.N.; Fader, A.N. Uterine papillary serous carcinoma. Clin. Obstet. Gynecol. 2011, 54, 278–291. [Google Scholar] [CrossRef]
- Winer, I.; Ahmed, Q.F.; Mert, I.; Bandyopadhyay, S.; Cote, M.; Munkarah, A.R.; Hussein, Y.; Al-Wahab, Z.; Elshaikh, M.A.; Alosh, B.; et al. Significance of lymphovascular space invasion in uterine serous carcinoma: What matters more; extent or presence? Int. J. Gynecol. Pathol. 2015, 34, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Pollom, E.L.; Nwachukwu, C.; Seiger, K.; von Eyben, R.; Folkins, A.K.; Kidd, E.A. Extent of lymphovascular space invasion may predict lymph node metastasis in uterine serous carcinoma. Gynecol. Oncol. 2017, 147, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Lavie, O.; Ben-Arie, A.; Segev, Y.; Faro, J.; Barak, F.; Haya, N.; Auslender, R.; Gemer, O. BRCA germline mutations in women with uterine serous carcinoma—Still a debate. Int. J. Gynecol. Cancer 2010, 20, 1531–1534. [Google Scholar]
- Sulaiman, R.; De, P.; Aske, J.C.; Lin, X.; Dale, A.; Koirala, N.; Gaster, K.; Espaillat, L.R.; Starks, D.; Dey, N. Tumor-TME Bipartite Landscape of PD-1/PD-L1 in Endometrial Cancers. Int. J. Mol. Sci. 2023, 24, 11079. [Google Scholar] [CrossRef]
- Guntupalli, S.R.; Zighelboim, I.; Kizer, N.T.; Zhang, Q.; Powell, M.A.; Thaker, P.H.; Goodfellow, P.J.; Mutch, D.G. Lymphovascular space invasion is an independent risk factor for nodal disease and poor outcomes in endometrioid endometrial cancer. Gynecol. Oncol. 2012, 124, 31–35. [Google Scholar] [CrossRef]
- Raffone, A.; Travaglino, A.; Raimondo, D.; Neola, D.; Maletta, M.; Santoro, A.; Insabato, L.; Casadio, P.; Fanfani, F.; Zannoni, G.F.; et al. Lymphovascular space invasion in endometrial carcinoma: A prognostic factor independent from molecular signature. Gynecol. Oncol. 2022, 165, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, P.; Yang, X.; Yu, Q.; Xu, X.; Zou, G.; Zhang, X. Association of Myometrial Invasion With Lymphovascular Space Invasion, Lymph Node Metastasis, Recurrence, and Overall Survival in Endometrial Cancer: A Meta-Analysis of 79 Studies With 68,870 Patients. Front. Oncol. 2021, 11, 762329. [Google Scholar] [CrossRef] [PubMed]
- Peters, E.E.M.; Bartosch, C.; McCluggage, W.G.; Genestie, C.; Lax, S.F.; Nout, R.; Oosting, J.; Singh, N.; Smit, H.; Smit, V.; et al. Reproducibility of lymphovascular space invasion (LVSI) assessment in endometrial cancer. Histopathology 2019, 75, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, A.; Mohammadizadeh, F. Association of Lymphovascular Space Invasion (LVSI) with Histological Tumor Grade and Myometrial Invasion in Endometrial Carcinoma: A Review Study. Adv. Biomed. Res. 2023, 12, 159. [Google Scholar] [CrossRef]
- Sulaiman, R.; De, P.; Aske, J.C.; Lin, X.; Dale, A.; Vaselaar, E.; Ageton, C.; Gaster, K.; Espaillat, L.R.; Starks, D.; et al. Identification and Morphological Characterization of Features of Circulating Cancer-Associated Macrophage-like Cells (CAMLs) in Endometrial Cancers. Cancers 2022, 14, 4577. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, R.; De, P.; Aske, J.C.; Lin, X.; Dale, A.; Vaselaar, E.; Koirala, N.; Ageton, C.; Gaster, K.; Plorde, J.; et al. A Laboratory-Friendly CTC Identification: Comparable Double-Immunocytochemistry with Triple-Immunofluorescence. Cancers 2022, 14, 2871. [Google Scholar] [CrossRef] [PubMed]
- Hua, T.; Wang, X.; Chi, S.; Liu, Y.; Feng, D.; Zhao, Y.; Wang, H. Estrogen-related receptor gamma promotes the migration and metastasis of endometrial cancer cells by targeting S100A4. Oncol. Rep. 2018, 40, 823–832. [Google Scholar] [CrossRef]
- Hua, T.; Liu, S.; Xin, X.; Cai, L.; Shi, R.; Chi, S.; Feng, D.; Wang, H. S100A4 promotes endometrial cancer progress through epithelial-mesenchymal transition regulation. Oncol. Rep. 2016, 35, 3419–3426. [Google Scholar] [CrossRef]
- Tochimoto, M.; Oguri, Y.; Hashimura, M.; Konno, R.; Matsumoto, T.; Yokoi, A.; Kodera, Y.; Saegusa, M. S100A4/non-muscle myosin II signaling regulates epithelial-mesenchymal transition and stemness in uterine carcinosarcoma. Lab. Investig. 2020, 100, 682–695. [Google Scholar] [CrossRef]
- De, P.; Aske, J.C.; Dale, A.; Rojas Espaillat, L.; Starks, D.; Dey, N. Addressing activation of WNT beta-catenin pathway in diverse landscape of endometrial carcinogenesis. Am. J. Transl. Res. 2021, 13, 12168–12180. [Google Scholar] [PubMed]
- Dunn, G.P.; Bruce, A.T.; Ikeda, H.; Old, L.J.; Schreiber, R.D. Cancer immunoediting: From immunosurveillance to tumor escape. Nat. Immunol. 2002, 3, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Mittal, D.; Gubin, M.M.; Schreiber, R.D.; Smyth, M.J. New insights into cancer immunoediting and its three component phases--elimination, equilibrium and escape. Curr. Opin. Immunol. 2014, 27, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef] [PubMed]
- Quezada, S.A.; Peggs, K.S.; Simpson, T.R.; Allison, J.P. Shifting the equilibrium in cancer immunoediting: From tumor tolerance to eradication. Immunol. Rev. 2011, 241, 104–118. [Google Scholar] [CrossRef]
- Tao, Y.; Tao, T.; Gross, N.; Peng, X.; Li, Y.; Huang, Z.; Liu, L.; Li, G.; Chen, X.; Yang, J. Combined Effect of IL-12Rbeta2 and IL-23R Expression on Prognosis of Patients with Laryngeal Cancer. Cell Physiol. Biochem. 2018, 50, 1041–1054. [Google Scholar] [CrossRef]





| Compartments | IHC Stains * | |||
|---|---|---|---|---|
| Tumor Compartment | TME Compartment | |||
| Epithelial Cell Markers | CK 8, 18 | |||
| EpCAM | ||||
| LCA Marker | CD45 | |||
| Proliferation Marker | Ki67 | |||
| Apoptosis Markers | Cleaved-Caspase3 | |||
| Cleaved-PARP | ||||
| Immune-Cell Markers | CD3 | |||
| CD4 | ||||
| CD8 | ||||
| CD65 (NCAM1) | ||||
| FoxP2 | ||||
| PD-1 | ||||
| IL-12R-B2 | ||||
| Immune Marker | PD-L1 | |||
| Macrophage Markers | CD68 | |||
| CD163 | ||||
| Fibroblast Markers | S100A4 | |||
| SMA | ||||
| TE-7 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sulaiman, R.; Dale, A.; Lin, X.; Aske, J.C.; Gaster, K.; Starks, D.; Espaillat, L.R.; De, P.; Dey, N. Atlas of Tumor and Tumor Microenvironment Cells of Lymphovascular Space Invasion (LVSI) in High-Grade Serous Endometrial Adenocarcinoma: A Case Study. Int. J. Mol. Sci. 2024, 25, 3441. https://doi.org/10.3390/ijms25063441
Sulaiman R, Dale A, Lin X, Aske JC, Gaster K, Starks D, Espaillat LR, De P, Dey N. Atlas of Tumor and Tumor Microenvironment Cells of Lymphovascular Space Invasion (LVSI) in High-Grade Serous Endometrial Adenocarcinoma: A Case Study. International Journal of Molecular Sciences. 2024; 25(6):3441. https://doi.org/10.3390/ijms25063441
Chicago/Turabian StyleSulaiman, Raed, Adam Dale, Xiaoqian Lin, Jennifer C. Aske, Kris Gaster, David Starks, Luis Rojas Espaillat, Pradip De, and Nandini Dey. 2024. "Atlas of Tumor and Tumor Microenvironment Cells of Lymphovascular Space Invasion (LVSI) in High-Grade Serous Endometrial Adenocarcinoma: A Case Study" International Journal of Molecular Sciences 25, no. 6: 3441. https://doi.org/10.3390/ijms25063441
APA StyleSulaiman, R., Dale, A., Lin, X., Aske, J. C., Gaster, K., Starks, D., Espaillat, L. R., De, P., & Dey, N. (2024). Atlas of Tumor and Tumor Microenvironment Cells of Lymphovascular Space Invasion (LVSI) in High-Grade Serous Endometrial Adenocarcinoma: A Case Study. International Journal of Molecular Sciences, 25(6), 3441. https://doi.org/10.3390/ijms25063441





