Assessing the Potential of HPV16 E6 Seroprevalence as a Biomarker for Anal Dysplasia and Cancer Screening—A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Literature Selection
2.2. Data Extraction
2.3. Statistical Analysis
3. Results and Discussion
3.1. Literature Selection and Study Characteristics
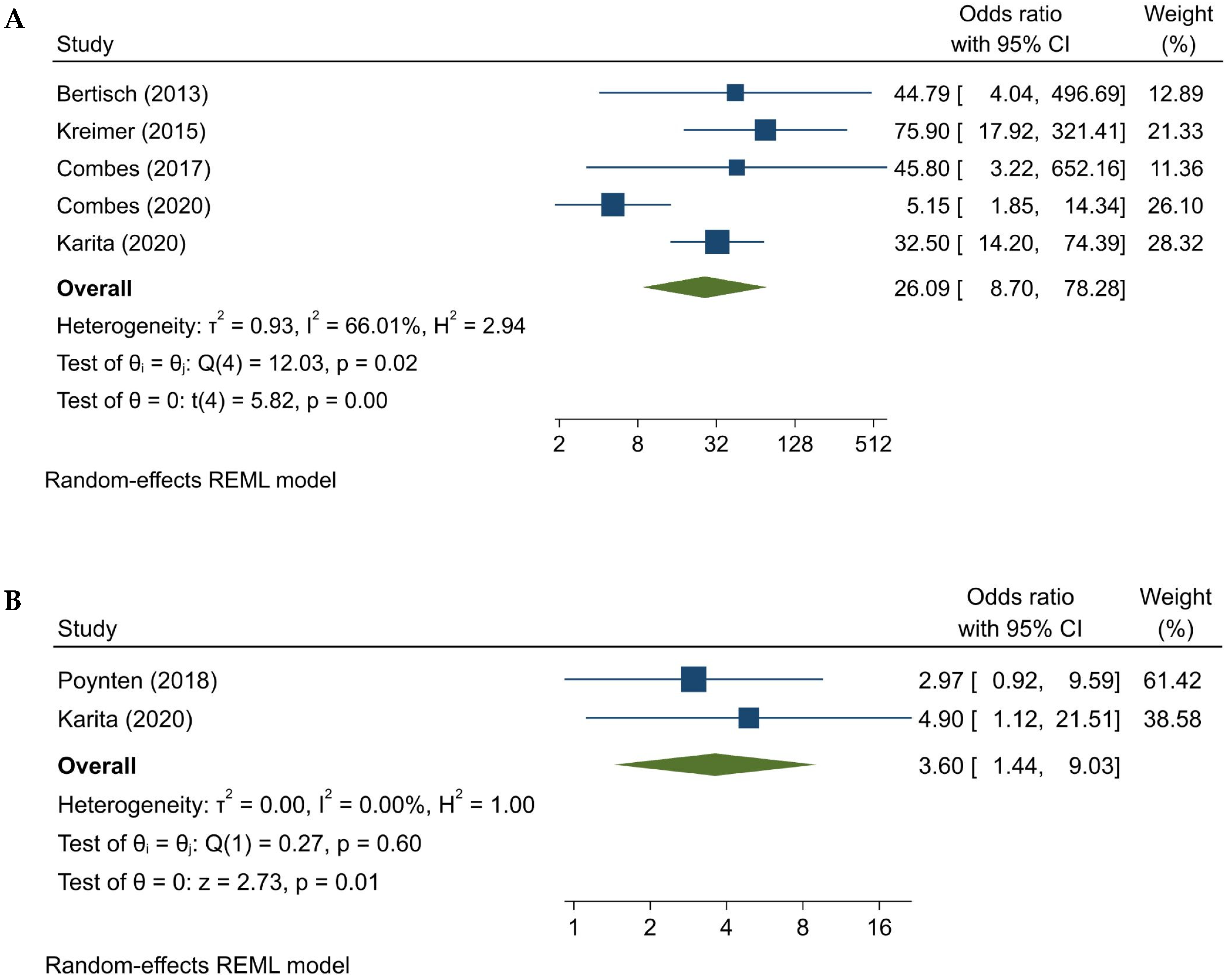
3.2. Meta-Analysis of HPV16 E6 Seropositivity in HSIL+ Patients and Publication Bias
3.3. Variability in HPV16 E6 Seroprevalence Studies among Patients with HSIL+
3.4. Diagnostic Performance of HPV16 E6 Seroprevalence
3.5. Limitations and Future Potentials of HPV16 E6 Seropositivity as a Biomarker for Anal Cancer Risk Stratification and Screening
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Clifford, G.M.; Georges, D.; Shiels, M.S.; Engels, E.A.; Albuquerque, A.; Poynten, I.M.; de Pokomandy, A.; Easson, A.M.; Stier, E.A. A Meta-Analysis of Anal Cancer Incidence by Risk Group: Toward a Unified Anal Cancer Risk Scale. Int. J. Cancer 2021, 148, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Loos, A.H.; McCarron, P.; Weiderpass, E.; Arbyn, M.; Møller, H.; Hakama, M.; Parkin, D.M. Trends in Cervical Squamous Cell Carcinoma Incidence in 13 European Countries: Changing Risk and the Effects of Screening. Cancer Epidemiol. Biomark. Prev. 2005, 14, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Franco, E.L.; Duarte-Franco, E.; Ferenczy, A. Cervical Cancer: Epidemiology, Prevention and the Role of Human Papillomavirus Infection. CMAJ Can. Med. Assoc. J. 2001, 164, 1017–1025. [Google Scholar]
- de Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide Burden of Cancer Attributable to HPV by Site, Country and HPV Type. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef] [PubMed]
- de Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global Burden of Cancer Attributable to Infections in 2018: A Worldwide Incidence Analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef] [PubMed]
- Stier, E.A.; Clarke, M.A.; Deshmukh, A.A.; Wentzensen, N.; Liu, Y.; Poynten, I.M.; Cavallari, E.N.; Fink, V.; Barroso, L.F.; Clifford, G.M.; et al. International Anal Neoplasia Society’s Consensus Guidelines for Anal Cancer Screening. Int. J. Cancer 2024, 154, 1694–1702. [Google Scholar] [CrossRef] [PubMed]
- Sendagorta, E.; Romero, M.P.; Bernardino, J.I.; Beato, M.J.; Alvarez-Gallego, M.; Herranz, P. Human Papillomavirus mRNA Testing for the Detection of Anal High-Grade Squamous Intraepithelial Lesions in Men Who Have Sex with Men Infected with HIV. J. Med. Virol. 2015, 87, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Berry, J.M.; Palefsky, J.M.; Jay, N.; Cheng, S.C.; Darragh, T.M.; Chin-Hong, P.V. Performance Characteristics of Anal Cytology and Human Papillomavirus Testing in Patients with High-Resolution Anoscopy-Guided Biopsy of High-Grade Anal Intraepithelial Neoplasia. Dis. Colon Rectum 2009, 52, 239–247. [Google Scholar] [CrossRef]
- Schofield, A.M.; Sadler, L.; Nelson, L.; Gittins, M.; Desai, M.; Sargent, A.; McMahon, R.F.T.; Hill, J.; Crosbie, E.J.; Patnick, J.; et al. A Prospective Study of Anal Cancer Screening in HIV-Positive and Negative MSM. Aids 2016, 30, 1375–1383. [Google Scholar] [CrossRef]
- Nathan, M.; Singh, N.; Garrett, N.; Hickey, N.; Prevost, T.; Sheaff, M. Performance of Anal Cytology in a Clinical Setting When Measured against Histology and High-Resolution Anoscopy Findings. Aids 2010, 24, 373–379. [Google Scholar] [CrossRef]
- Lorincz, A.T.; Nathan, M.; Reuter, C.; Warman, R.; Thaha, M.A.; Sheaff, M.; Vasiljevic, N.; Ahmad, A.; Cuzick, J.; Sasieni, P. Methylation of HPV and a Tumor Suppressor Gene Reveals Anal Cancer and Precursor Lesions. Oncotarget 2017, 8, 50510–50520. [Google Scholar] [CrossRef]
- Hillman, R.J.; Gunathilake, M.P.W.; Jin, F.; Tong, W.; Field, A.; Carr, A. Ability to Detect High-Grade Squamous Anal Intraepithelial Lesions at High Resolution Anoscopy Improves over Time. Sex. Health 2016, 13, 177–181. [Google Scholar] [CrossRef]
- Hillman, R.J.; Cuming, T.; Darragh, T.; Nathan, M.; Berry-Lawthorn, M.; Goldstone, S.; Law, C.; Palefsky, J.; Barroso, L.F.; Stier, E.A.; et al. 2016 IANS International Guidelines for Practice Standards in the Detection of Anal Cancer Precursors. J. Low. Genit. Tract Dis. 2016, 20, 283–291. [Google Scholar] [CrossRef]
- Wentzensen, N.; Massad, L.S.; Mayeaux, E.J.; Khan, M.J.; Waxman, A.G.; Einstein, M.H.; Conageski, C.; Schiffman, M.H.; Gold, M.A.; Apgar, B.S.; et al. Evidence-Based Consensus Recommendations for Colposcopy Practice for Cervical Cancer Prevention in the United States. J. Low. Genit. Tract Dis. 2017, 21, 216–222. [Google Scholar] [CrossRef]
- Clarke, M.A.; Deshmukh, A.A.; Suk, R.; Roberts, J.; Gilson, R.; Jay, N.; Stier, E.A.; Wentzensen, N. A Systematic Review and Meta-Analysis of Cytology and HPV-Related Biomarkers for Anal Cancer Screening among Different Risk Groups. Int. J. Cancer 2022, 151, 1889–1901. [Google Scholar] [CrossRef]
- Machalek, D.A.; Poynten, M.; Jin, F.; Fairley, C.K.; Farnsworth, A.; Garland, S.M.; Hillman, R.J.; Petoumenos, K.; Roberts, J.; Tabrizi, S.N.; et al. Anal Human Papillomavirus Infection and Associated Neoplastic Lesions in Men Who Have Sex with Men: A Systematic Review and Meta-Analysis. Lancet Oncol. 2012, 13, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Faber, M.T.; Frederiksen, K.; Palefsky, J.M.; Kjaer, S.K. Risk of Anal Cancer Following Benign Anal Disease and Anal Cancer Precursor Lesions: A Danish Nationwide Cohort Study. Cancer Epidemiol. Biomarkers Prev. 2020, 29, 185–192. [Google Scholar] [CrossRef]
- Hamid, N.A.; Brown, C.; Gaston, K. The Regulation of Cell Proliferation by the Papillomavirus Early Proteins. Cell. Mol. Life Sci. 2009, 66, 1700–1717. [Google Scholar] [CrossRef]
- Doorbar, J.; Egawa, N.; Griffin, H.; Kranjec, C.; Murakami, I. Human Papillomavirus Molecular Biology and Disease Association. Rev. Med. Virol. 2015, 25, 2–23. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Kundu, R. Human Papillomavirus E6 and E7: The Cervical Cancer Hallmarks and Targets for Therapy. Front. Microbiol. 2019, 10, 3116. [Google Scholar] [CrossRef]
- Rosendo-Chalma, P.; Antonio-Véjar, V.; Ortiz Tejedor, J.G.; Ortiz Segarra, J.; Vega Crespo, B.; Bigoni-Ordóñez, G.D. The Hallmarks of Cervical Cancer: Molecular Mechanisms Induced by Human Papillomavirus. Biology 2024, 13, 77. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Waterboer, T.; Sehr, P.; Michael, K.M.; Franceschi, S.; Nieland, J.D.; Joos, T.O.; Templin, M.F.; Pawlita, M. Multiplex Human Papillomavirus Serology Based on in Situ-Purified Glutathione S-Transferase Fusion Proteins. Clin. Chem. 2005, 51, 1845–1853. [Google Scholar] [CrossRef] [PubMed]
- Balachandra, S.; Kusin, S.B.; Lee, R.; Blackwell, J.-M.; Tiro, J.A.; Cowell, L.G.; Chiang, C.-M.; Wu, S.-Y.; Varma, S.; Rivera, E.L.; et al. Blood-Based Biomarkers of Human Papillomavirus-Associated Cancers: A Systematic Review and Meta-Analysis. Cancer 2021, 127, 850–864. [Google Scholar] [CrossRef]
- Clifford, G.M.; Shin, H.R.; Oh, J.K.; Waterboer, T.; Ju, Y.H.; Vaccarella, S.; Quint, W.; Pawlita, M.; Franceschi, S. Serologic Response to Oncogenic Human Papillomavirus Types in Male and Female University Students in Busan, South Korea. Cancer Epidemiol. Biomarkers Prev. 2007, 16, 1874–1879. [Google Scholar] [CrossRef]
- Combes, J.D.; Clifford, G.M.; Egger, M.; Cavassini, M.; Hirsch, H.H.; Hauser, C.; Calmy, A.; Schmid, P.; Bernasconi, E.; Günthard, H.F.; et al. Human Papillomavirus Antibody Response Following HAART Initiation among MSM. Aids 2017, 31, 561–569. [Google Scholar] [CrossRef]
- Bertisch, B.; Franceschi, S.; Lise, M.; Vernazza, P.; Keiser, O.; Schöni-Affolter, F.; Bouchardy, C.; Dehler, S.; Levi, F.; Jundt, G.; et al. Risk Factors for Anal Cancer in Persons Infected with HIV: A Nested Case-Control Study in the Swiss HIV Cohort Study. Am. J. Epidemiol. 2013, 178, 877–884. [Google Scholar] [CrossRef]
- Sweeting, M.J.; Sutton, A.J.; Lambert, P.C. What to Add to Nothing? Use and Avoidance of Continuity Corrections in Meta-Analysis of Sparse Data. Stat. Med. 2004, 23, 1351–1375. [Google Scholar] [CrossRef]
- Raudenbush, S.W. Analyzing Effect Sizes: Random Effects Models. In The Handbook of Research Synthesis and Meta-Analysis, 2nd ed.; Cooper, H., Hedges, L.V., Valentine, J.C., Eds.; Russell Sage Foundation: New York, NY, USA, 2009; pp. 295–315. [Google Scholar]
- Kreimer, A.R.; Brennan, P.; Lang Kuhs, K.A.; Waterboer, T.; Clifford, G.; Franceschi, S.; Michel, A.; Willhauck-Fleckenstein, M.; Riboli, E.; Castellsagué, X.; et al. Human Papillomavirus Antibodies and Future Risk of Anogenital Cancer: A Nested Case-Control Study in the European Prospective Investigation into Cancer and Nutrition Study. J. Clin. Oncol. 2015, 33, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Stankiewicz Karita, H.C.; Waterboer, T.; Magaret, A.; Doody, D.R.; Pawlita, M.; Brenner, N.; Galloway, D.A.; Wald, A.; Madeleine, M.M. Humoral Response to HPV16 Proteins in Persons with Anal High-Grade Squamous Intraepithelial Lesion or Anal Cancer. Cancer Epidemiol. Biomark. Prev. 2020, 29, 2255–2260. [Google Scholar] [CrossRef]
- Combes, J.D.; Clifford, G.M.; Günthard, H.F.; Hauser, C.; Darling, K.E.A.; Valladares, P.; Battegay, M.; Waldeck, F.; Bernasconi, E.; Bertisch, B.; et al. Antibodies against HPV16E6 Oncoprotein in the Swiss HIV Cohort Study: Kinetics and Anal Cancer Risk Prediction. Int. J. Cancer 2020, 147, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Poynten, I.M.; Waterboer, T.; Jin, F.; Templeton, D.J.; Hillman, R.J.; Law, C.; Cornall, A.; Tabrizi, S.; Roberts, J.M.; Garland, S.M.; et al. Human Papillomavirus Seroprevalence and Association with Anal HPV Infection and Squamous Intraepithelial Lesions in Australian Gay and Bisexual Men. Cancer Epidemiol. Biomark. Prev. 2018, 27, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Alberts, C.J.; Albuquerque, A.; Clifford, G.M. Impact of Human Papillomavirus Vaccine against Anal Human Papillomavirus Infection, Anal Intraepithelial Neoplasia, and Recurrence of Anal Intraepithelial Neoplasia: A Systematic Review and Meta-Analysis. J. Infect. Dis. 2023, 228, 1496–1504. [Google Scholar] [CrossRef]


| Authors (Year) | Cohort | Age Range (y.o.) | Location | Gender | HIV Status | Sample Size a (Cases/Controls) | Endpoint | Serologic Assay | Time of Serum Collection | HPV16 E6 Seroprevalence (Cases/Controls) | Effect Measured | OR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bertisch (2013) [27] | Swiss HIV Cohort Study | ≥25 | Switzerland | Men (predominant) and women | HIV- positive | 155 (41/114) | AC | Multiplex serology assay | At AC diagnosis | 21.95/0 | Adjusted OR (95%) b | Inf. e |
| Kreimer (2015) [30] | EPIC | ≤40–>70 | Europe | Men and women (predominant) | n.s. | 743 (24/719) | AC | Multiplex serology assay | Before AC diagnosis | 29.16/ 0.28 | Adjusted OR (95%) c | 75.90 (17.92, 321.41) |
| Combes (2017) [26] | Swiss HIV Cohort Study | 19.6–73.40 (mean age range) | Switzerland | Men (MSM) | HIV-positive | 281 (3/278) | AC | Multiplex serology assay | Before AC diagnosis | 33.33/ 1.08 | Unadjusted OR (95%) | 45.83 (3.22, 652.16) |
| Combes (2020) [32] | Swiss HIV Cohort Study | >25 | Switzerland | MSM (predominant), non-MSM and women | HIV-positive | 10,384 (76/10,308) | AC | Multiplex serology assay | Before AC diagnosis | 5.26/ 1.07 | Unadjusted OR (95%) | 5.15 (1.85, 14.34) |
| Karita (2020) [31] | Cancer Surveillance System | 18–78 | Washington (USA) | Men and women (predominant) | n.s. | 946 (116/830) | AC | Multiplex serology assay | After AC diagnosis | 29.31/ 0.73 | Adjusted OR (95%) d | 32.50 (14.20, 74.39) |
| Poynten (2018) [33] | SPANC | 35–79 | Sydney (Australia) | Men (GBM) | HIV-positive and HIV-negative | 568 (263/305) | HSIL | Multiplex serology assay | n.s. | 3.80/ 1.31 | Unadjusted OR (95%) | 2.97 (0.92, 9.59) |
| Karita (2020) [31] | Cancer Surveillance System | 18–78 | Washington (USA) | Men (predominant) and women | n.s. | 897 (67/830) | HSIL | Multiplex serology assay | After HSIL diagnosis | 4.48/ 0.73 | Adjusted OR (95%) d | 4.90 (1.12, 21.51) |
| Authors (Year) | Endpoint | Raw Sens | Raw Sp | Raw PPV | Raw NPV | Calculated Sens | Calculated Sp | Calculated PPV | Calculated NPV |
|---|---|---|---|---|---|---|---|---|---|
| Bertisch (2013) [27] | AC | 0.22 | 1.00 | 1.00 | 0.78 | 0.22 | 1.00 | 1.00 | 0.78 |
| Kreimer (2015) [30] | AC | 0.29 | 0.99 | 0.64 | 0.98 | 0.29 | 0.99 | 0.34 | 0.98 |
| Combes (2017) [26] | AC | 0.33 | 0.99 | 0.25 | 0.99 | 0.33 | 0.99 | 0.25 | 0.99 |
| Combes (2020) [32] | AC | 0.05 | 0.99 | 0.03 | 0.99 | 0.05 | 0.99 | 0.035 | 0.99 |
| Karita (2020) [31] | AC | 0.20 | 0.99 | 0.75 | 0.85 | 0.29 | 0.994 | 0.74 | 0.91 |
| Poynten (2018) [33] | HSIL | 0.04 | 0.99 | 0.71 | 0.54 | 0.04 | 0.99 | 0.71 | 0.54 |
| Karita (2020) [31] | HSIL | 0.20 | 0.99 | 0.75 | 0.85 | 0.04 | 0.994 | 0.20 | 0.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tous, S.; Guillamet, M.; Waterboer, T.; Alemany, L.; Paytubi, S. Assessing the Potential of HPV16 E6 Seroprevalence as a Biomarker for Anal Dysplasia and Cancer Screening—A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2024, 25, 3437. https://doi.org/10.3390/ijms25063437
Tous S, Guillamet M, Waterboer T, Alemany L, Paytubi S. Assessing the Potential of HPV16 E6 Seroprevalence as a Biomarker for Anal Dysplasia and Cancer Screening—A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences. 2024; 25(6):3437. https://doi.org/10.3390/ijms25063437
Chicago/Turabian StyleTous, Sara, Mariona Guillamet, Tim Waterboer, Laia Alemany, and Sonia Paytubi. 2024. "Assessing the Potential of HPV16 E6 Seroprevalence as a Biomarker for Anal Dysplasia and Cancer Screening—A Systematic Review and Meta-Analysis" International Journal of Molecular Sciences 25, no. 6: 3437. https://doi.org/10.3390/ijms25063437
APA StyleTous, S., Guillamet, M., Waterboer, T., Alemany, L., & Paytubi, S. (2024). Assessing the Potential of HPV16 E6 Seroprevalence as a Biomarker for Anal Dysplasia and Cancer Screening—A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences, 25(6), 3437. https://doi.org/10.3390/ijms25063437






