The MoLfa1 Protein Regulates Fungal Development and Septin Ring Formation in Magnaporthe oryzae
Abstract
1. Introduction
2. Results
2.1. Identification of MoLfa1 in M. oryzae
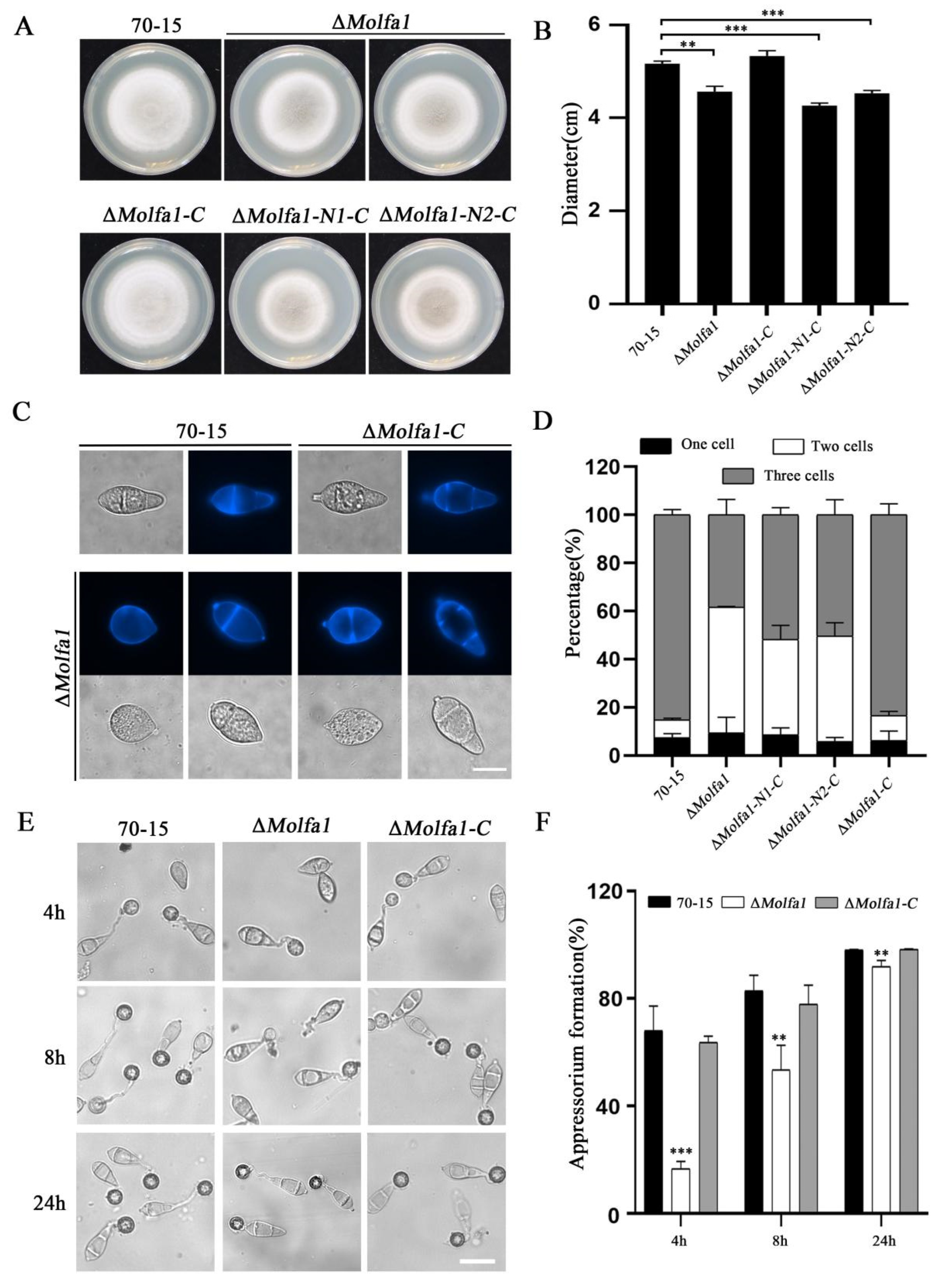
2.2. MoLfa1 Is Involved in the Growth, Conidia Development, and Appressorium Formation of M. oryzae
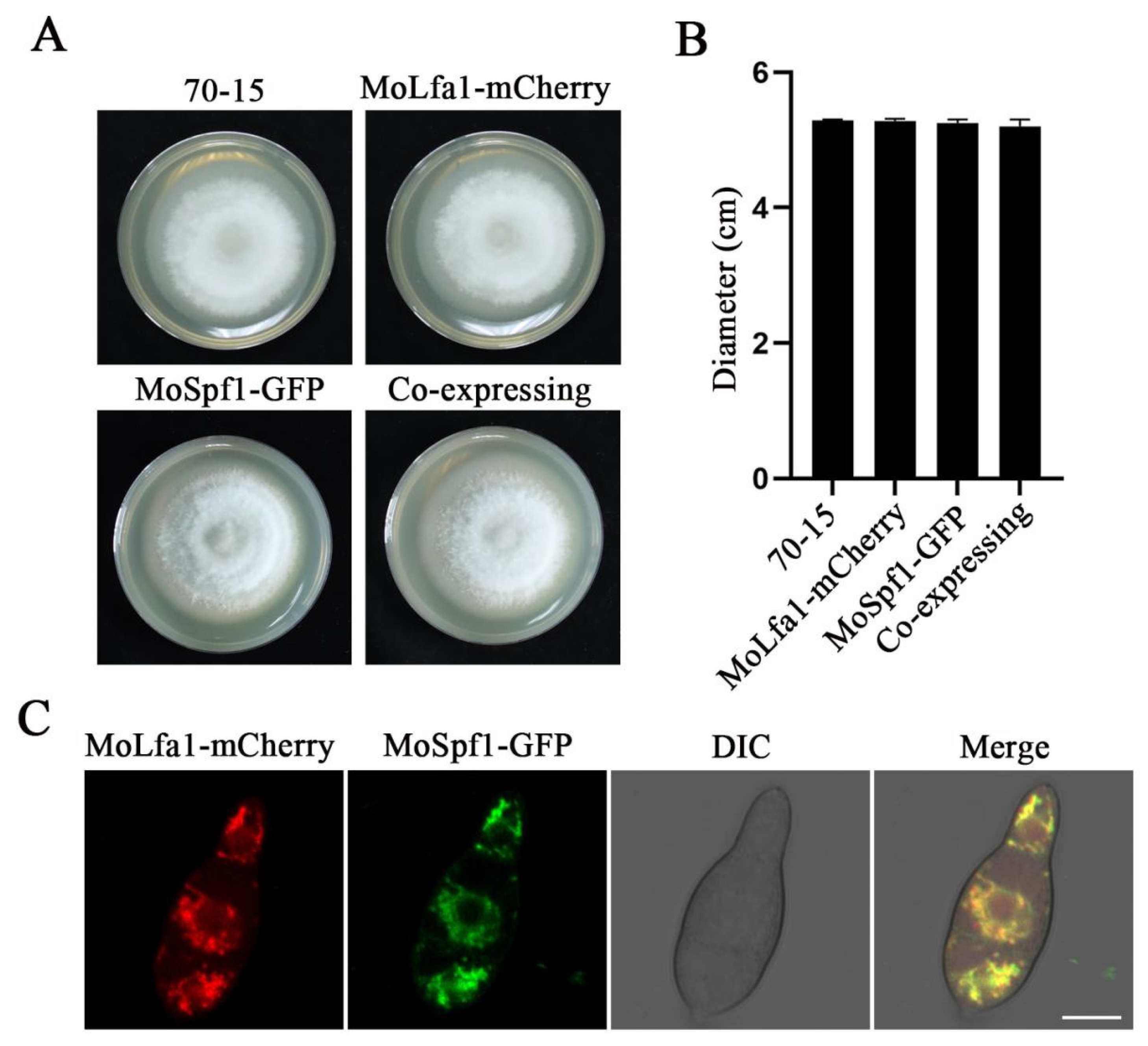
2.3. Subcellular Localization of MoLfa1
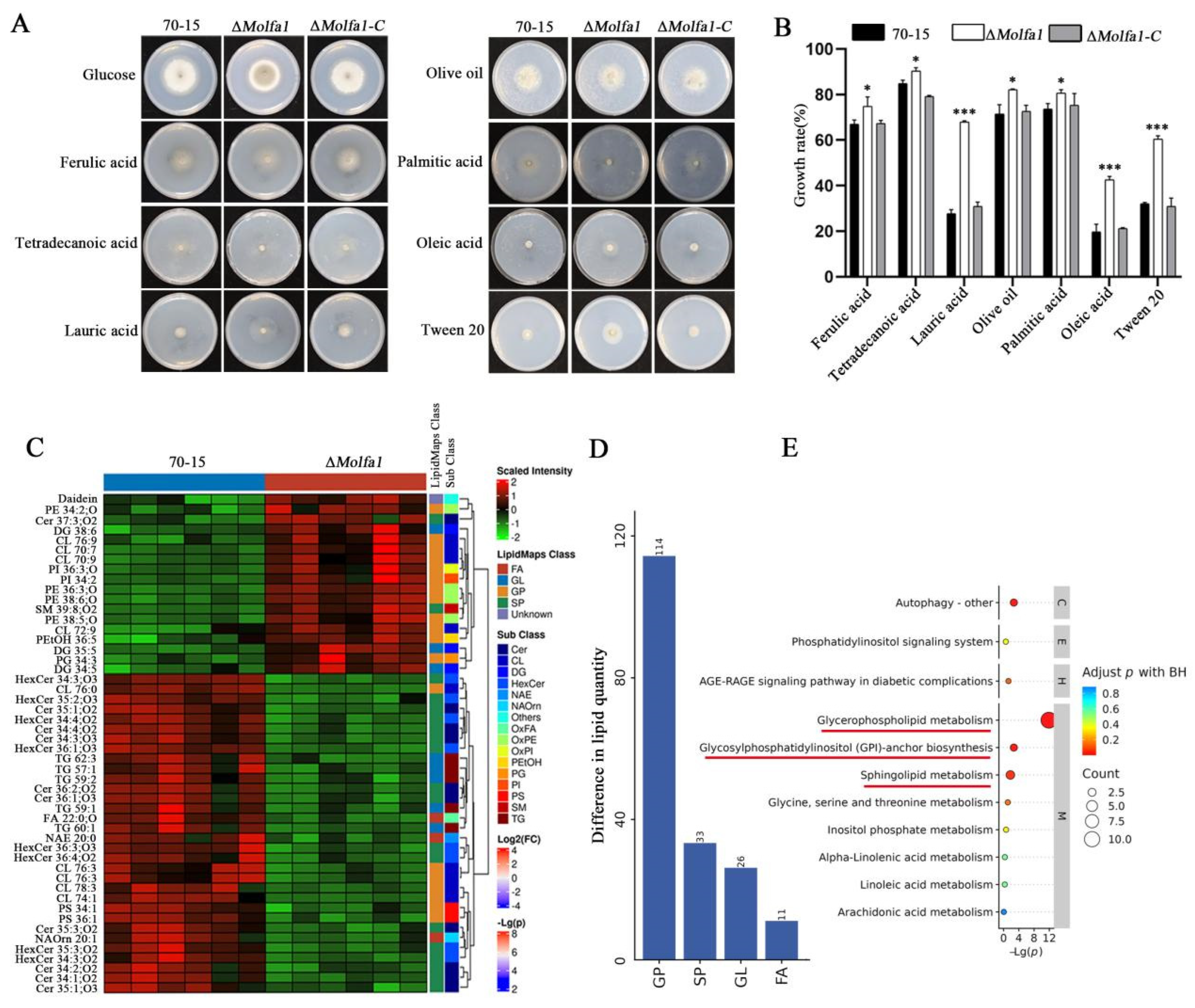
2.4. MoLfa1 Is Involved in Lipid Metabolism in M. oryzae
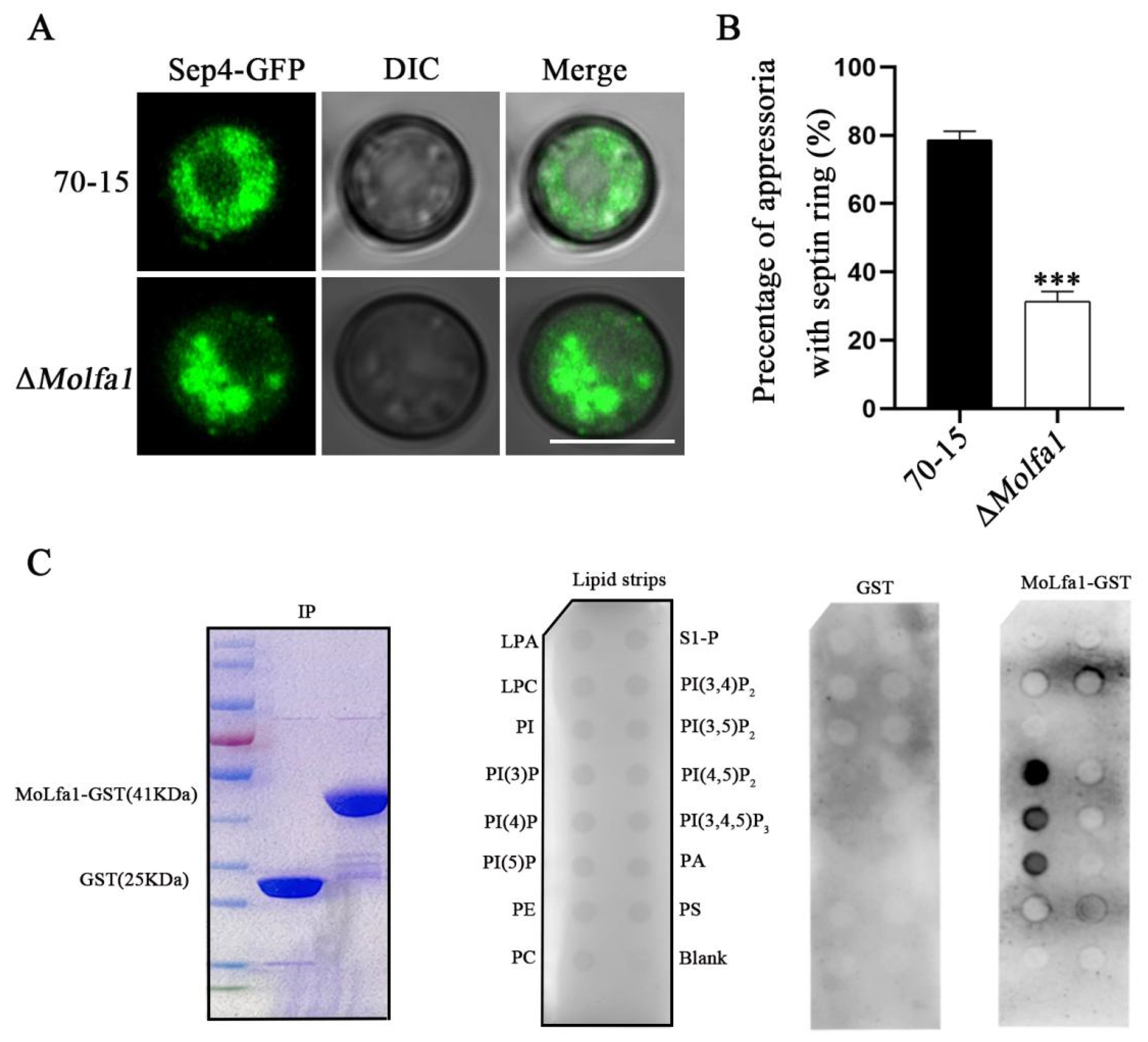
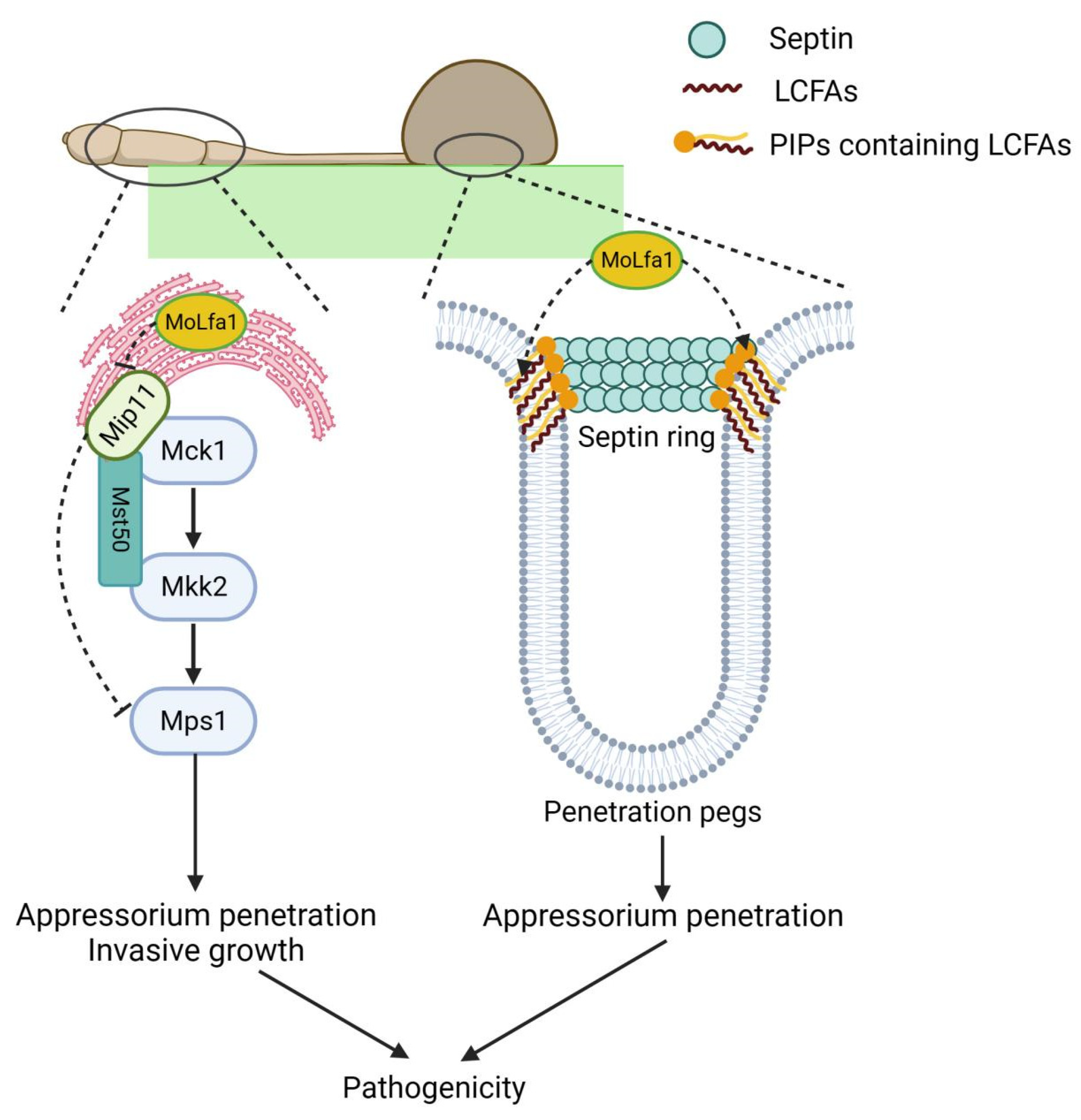
2.5. MoLfa1 Can Associate with PIPs to Regulate the Formation of Septin Ring
2.6. MoLfa1 Is Essential for Pathogenicity
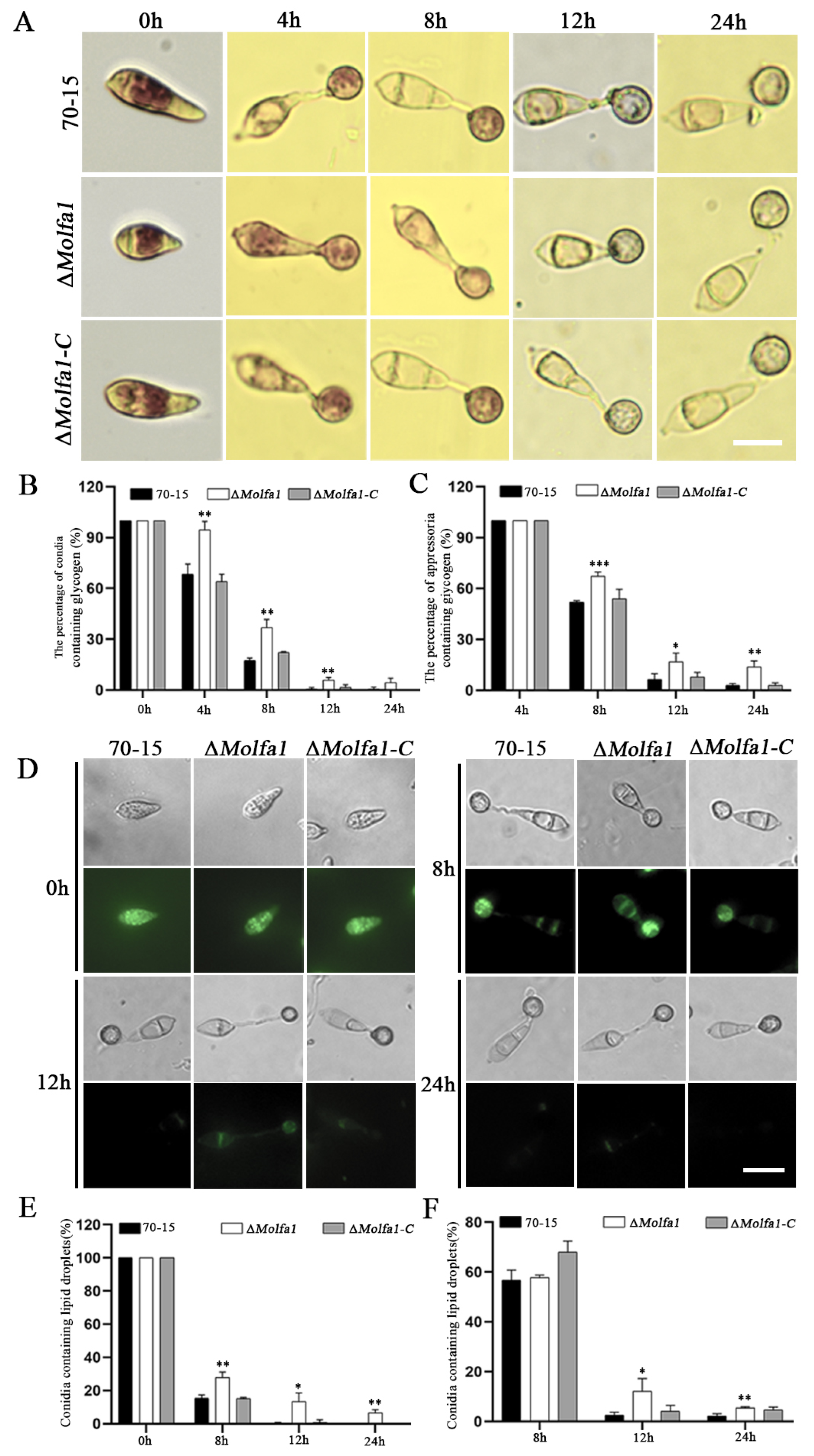
2.7. Disruption of MoLFA1 Delays the Mobilization and Degradation of Glycogen and Lipid Droplets
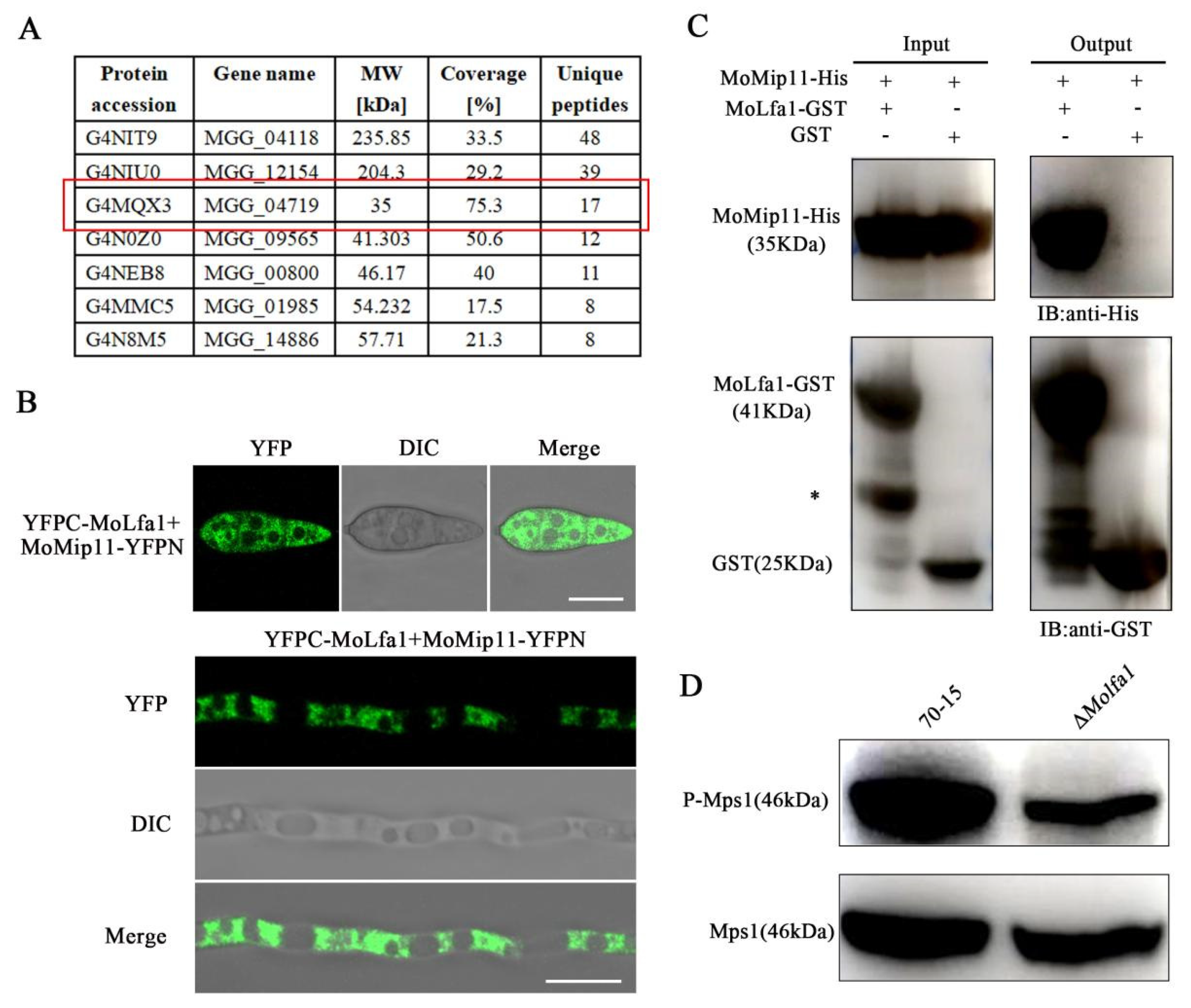
2.8. MoLfa1 Interacts with the RACK Protein MoMip11 and Is Involved in Mps1-MAPK Signaling Pathways in M. oryzae
3. Discussion
4. Materials and Methods
4.1. Strains and Cultural Conditions
4.2. Gene Deletion and Complement Strategy
4.3. Detection of Growth, Abnormal Conidia Rate, and Germination of M. oryzae
4.4. Pathogenicity Analysis Experiment
4.5. Degradation of Glycogen and Lipid Droplets
4.6. Subcellular Localization of MoLfa1
4.7. Detection of M. oryzae Growth Rate in Different LCFAs Media
4.8. Non-Targeted Lipidomics Assay
4.9. Mass Spectrometry Experiment
4.10. Septin Ring Observation and In Vitro Lipid Binding Experiment
4.11. In Vitro Protein Binding Experiments (Pull-Down)
4.12. Bimolecular Fluorescence Complementation Assay (BiFC) and Fluorescence Monitoring
4.13. Western Blot Analysis of Mps1 Phosphorylation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dagdas, Y.F.; Yoshino, K.; Dagdas, G.; Ryder, L.S.; Bielska, E.; Steinberg, G.; Talbot, N.J. Septin-mediated plant cell invasion by the rice blast fungus, Magnaporthe oryzae. Science 2012, 336, 1590–1595. [Google Scholar] [CrossRef] [PubMed]
- Mostowy, S.; Cossart, P. Septins: The fourth component of the cytoskeleton. Nat. Rev. Mol. Cell Biol. 2012, 13, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Hartwell, L.H. Genetic control of the cell division cycle in yeast. II. Genes controlling DNA replication and its initiation. J. Mol. Biol. 1971, 59, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Saunders, D.G.; Dagdas, Y.F.; Talbot, N.J. Spatial uncoupling of mitosis and cytokinesis during appressorium-mediated plant infection by the rice blast fungus Magnaporthe oryzae. Plant Cell 2010, 22, 2417–2428. [Google Scholar] [CrossRef]
- Bertin, A.; McMurray, M.A.; Thai, L.; Garcia, G., 3rd; Votin, V.; Grob, P.; Allyn, T.; Thorner, J.; Nogales, E. Phosphatidylinositol-4,5-bisphosphate promotes budding yeast septin filament assembly and organization. J. Mol. Biol. 2010, 404, 711–731. [Google Scholar] [CrossRef]
- Farkasovsky, M. Septin architecture and function in budding yeast. Biol. Chem. 2020, 401, 903–919. [Google Scholar] [CrossRef]
- McMurray, M.A.; Thorner, J. Reuse, replace, recycle. Specificity in subunit inheritance and assembly of higher-order septin structures during mitotic and meiotic division in budding yeast. Cell Cycle 2009, 8, 195–203. [Google Scholar] [CrossRef]
- Spiliotis, E.T.; Gladfelter, A.S. Spatial guidance of cell asymmetry: Septin GTPases show the way. Traffic 2012, 13, 195–203. [Google Scholar] [CrossRef]
- Woods, B.L.; Gladfelter, A.S. The state of the septin cytoskeleton from assembly to function. Curr. Opin. Cell Biol. 2021, 68, 105–112. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Su, J.; Xu, Y.; Chen, J.; Chern, M.; Lei, M.; Qi, T.; Wang, Z.; Ryder, L.S.; Tang, B.; et al. Discovery of broad-spectrum fungicides that block septin-dependent infection processes of pathogenic fungi. Nat. Microbiol. 2020, 5, 1565–1575. [Google Scholar] [CrossRef] [PubMed]
- Oshiro, J.; Han, G.S.; Carman, G.M. Diacylglycerol pyrophosphate phosphatase in Saccharomyces cerevisiae. Biochim. Biophys. Acta 2003, 1635, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Strahl, T.; Thorner, J. Synthesis and function of membrane phosphoinositides in budding yeast, Saccharomyces cerevisiae. Biochim. Biophys. Acta 2007, 1771, 353–404. [Google Scholar] [CrossRef]
- Roth, A.F.; Wan, J.; Bailey, A.O.; Sun, B.; Kuchar, J.A.; Green, W.N.; Phinney, B.S.; Yates, J.R., 3rd; Davis, N.G. Global analysis of protein palmitoylation in yeast. Cell 2006, 125, 1003–1013. [Google Scholar] [CrossRef]
- Henry, S.A.; Kohlwein, S.D.; Carman, G.M. Metabolism and regulation of glycerolipids in the yeast Saccharomyces cerevisiae. Genetics 2012, 190, 317–349. [Google Scholar] [CrossRef]
- Kim, Y.J.; Guzman-Hernandez, M.L.; Balla, T. A highly dynamic ER-derived phosphatidylinositol-synthesizing organelle supplies phosphoinositides to cellular membranes. Dev. Cell 2011, 21, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Jigami, Y. Lipid remodeling of GPI-anchored proteins and its function. Biochim. Biophys. Acta 2008, 1780, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Michell, R.H. Inositol phospholipids and cell surface receptor function. Biochim. Biophys. Acta 1975, 415, 81–147. [Google Scholar] [CrossRef]
- Chen, J.; Li, Z.; Sheng, X.; Huang, J.; Sun, Q.; Huang, Y.; Wang, R.; Wu, Y.; Long, M.; Bao, J.; et al. Heterologous Expressed NbSWP12 from Microsporidia Nosema bombycis Can Bind with Phosphatidylinositol 3-Phosphate and Affect Vesicle Genesis. J. Fungi 2022, 8, 764. [Google Scholar] [CrossRef]
- Balla, T.; Varnai, P. Visualization of cellular phosphoinositide pools with GFP-fused protein-domains. Curr. Protoc. Cell Biol. 2009, 42, 24.4.1–24.4.27. [Google Scholar] [CrossRef]
- Noack, L.C.; Jaillais, Y. Precision targeting by phosphoinositides: How PIs direct endomembrane trafficking in plants. Curr. Opin. Plant Biol. 2017, 40, 22–33. [Google Scholar] [CrossRef]
- Qin, L.; Zhou, Z.; Li, Q.; Zhai, C.; Liu, L.; Quilichini, T.D.; Gao, P.; Kessler, S.A.; Jaillais, Y.; Datla, R.; et al. Specific Recruitment of Phosphoinositide Species to the Plant-Pathogen Interfacial Membrane Underlies Arabidopsis Susceptibility to Fungal Infection. Plant Cell 2020, 32, 1665–1688. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Y.N.; Li, X.; Gu, W.; Moeketsi, E.K.; Zhou, R.; Zheng, X.; Zhang, Z.; Zhang, H. The Peroxisomal-CoA Synthetase MoPcs60 Is Important for Fatty Acid Metabolism and Infectious Growth of the Rice Blast Fungus. Front. Plant Sci. 2021, 12, 811041. [Google Scholar] [CrossRef] [PubMed]
- Listenberger, L.L.; Han, X.; Lewis, S.E.; Cases, S.; Farese, R.V., Jr.; Ory, D.S.; Schaffer, J.E. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc. Natl. Acad. Sci. USA 2003, 100, 3077–3082. [Google Scholar] [CrossRef] [PubMed]
- Wysham, W.Z.; Roque, D.R.; Han, J.; Zhang, L.; Guo, H.; Gehrig, P.A.; Zhou, C.; Bae-Jump, V.L. Effects of Fatty Acid Synthase Inhibition by Orlistat on Proliferation of Endometrial Cancer Cell Lines. Target. Oncol. 2016, 11, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, T.; Seki, N.; Kihara, A. Phytosphingosine degradation pathway includes fatty acid alpha-oxidation reactions in the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA 2017, 114, E2616–E2623. [Google Scholar] [CrossRef] [PubMed]
- Schneiter, R.; Brugger, B.; Amann, C.M.; Prestwich, G.D.; Epand, R.F.; Zellnig, G.; Wieland, F.T.; Epand, R.M. Identification and biophysical characterization of a very-long-chain-fatty-acid-substituted phosphatidylinositol in yeast subcellular membranes. Biochem. J. 2004, 381, 941–949. [Google Scholar] [CrossRef]
- Rezanka, T.; Kolouchova, I.; Gharwalova, L.; Palyzova, A.; Sigler, K. Identification and Characterization of Phospholipids with Very Long Chain Fatty Acids in Brewer’s Yeast. Lipids 2017, 52, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Tucker, S.L.; Talbot, N.J. Surface attachment and pre-penetration stage development by plant pathogenic fungi. Annu. Rev. Phytopathol. 2001, 39, 385–417. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, W.; Li, Y.; Li, G.; Xu, J.R. Expression of HopAI interferes with MAP kinase signalling in Magnaporthe oryzae. Environ. Microbiol. 2017, 19, 4190–4204. [Google Scholar] [CrossRef]
- Leng, Y.; Zhong, S. The Role of Mitogen-Activated Protein (MAP) Kinase Signaling Components in the Fungal Development, Stress Response and Virulence of the Fungal Cereal Pathogen Bipolaris sorokiniana. PLoS ONE 2015, 10, e0128291. [Google Scholar] [CrossRef]
- Jeon, J.; Goh, J.; Yoo, S.; Chi, M.H.; Choi, J.; Rho, H.S.; Park, J.; Han, S.S.; Kim, B.R.; Park, S.Y.; et al. A putative MAP kinase kinase kinase, MCK1, is required for cell wall integrity and pathogenicity of the rice blast fungus, Magnaporthe oryzae. Mol. Plant Microbe Interact. 2008, 21, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Jiang, C.; Zhang, Q.; Qi, L.; Li, C.; Xu, J.R. Thioredoxins are involved in the activation of the PMK1 MAP kinase pathway during appressorium penetration and invasive growth in Magnaporthe oryzae. Environ. Microbiol. 2016, 18, 3768–3784. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, X.; Tian, H.; Choi, Y.E.; Tao, W.A.; Xu, J.R. MST50 is involved in multiple MAP kinase signaling pathways in Magnaporthe oryzae. Environ. Microbiol. 2017, 19, 1959–1974. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Zhang, X.; Liu, H.; Xu, J.R. Mitogen-activated protein kinase signaling in plant pathogenic fungi. PLoS Pathog. 2018, 14, e1006875. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Wang, Q.; Dou, X.; Wang, W.; Zhao, Q.; Lv, R.; Zhang, H.; Zheng, X.; Wang, P.; Zhang, Z. MoSwi6, an APSES family transcription factor, interacts with MoMps1 and is required for hyphal and conidial morphogenesis, appressorial function and pathogenicity of Magnaporthe oryzae. Mol. Plant Pathol. 2012, 13, 677–689. [Google Scholar] [CrossRef]
- Yin, Z.; Zhang, X.; Wang, J.; Yang, L.; Feng, W.; Chen, C.; Gao, C.; Zhang, H.; Zheng, X.; Wang, P.; et al. MoMip11, a MoRgs7-interacting protein, functions as a scaffolding protein to regulate cAMP signaling and pathogenicity in the rice blast fungus Magnaporthe oryzae. Environ. Microbiol. 2018, 20, 3168–3185. [Google Scholar] [CrossRef]
- Lu, J.; Cao, H.; Zhang, L.; Huang, P.; Lin, F. Systematic analysis of Zn2Cys6 transcription factors required for development and pathogenicity by high-throughput gene knockout in the rice blast fungus. PLoS Pathog. 2014, 10, e1004432. [Google Scholar] [CrossRef]
- Tang, H.; Wang, X.; Xu, L.; Ran, X.; Li, X.; Chen, L.; Zhao, X.; Deng, H.; Liu, X. Establishment of local searching methods for orbitrap-based high throughput metabolomics analysis. Talanta 2016, 156–157, 163–171. [Google Scholar] [CrossRef]
- Cai, Y.Y.; Wang, J.Y.; Wu, X.Y.; Liang, S.; Zhu, X.M.; Li, L.; Lu, J.P.; Liu, X.H.; Lin, F.C. MoOpy2 is essential for fungal development, pathogenicity, and autophagy in Magnaporthe oryzae. Environ. Microbiol. 2022, 24, 1653–1671. [Google Scholar] [CrossRef]
- Xie, Y.; Vessey, J.P.; Konecna, A.; Dahm, R.; Macchi, P.; Kiebler, M.A. The GTP-binding protein Septin 7 is critical for dendrite branching and dendritic-spine morphology. Curr. Biol. 2007, 17, 1746–1751. [Google Scholar] [CrossRef] [PubMed]
- Saunders, D.G.; Aves, S.J.; Talbot, N.J. Cell cycle-mediated regulation of plant infection by the rice blast fungus. Plant Cell 2010, 22, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, S.; Docampo, M.D.; MacRae, J.I.; Ralton, J.E.; Rupasinghe, T.; McConville, M.J.; Striepen, B. The intracellular parasite Toxoplasma gondii depends on the synthesis of long-chain and very long-chain unsaturated fatty acids not supplied by the host cell. Mol. Microbiol. 2015, 97, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Casamayor, A.; Snyder, M. Molecular dissection of a yeast septin: Distinct domains are required for septin interaction, localization, and function. Mol. Cell Biol. 2003, 23, 2762–2777. [Google Scholar] [CrossRef]
- Talbot, N.J.; Ebbole, D.J.; Hamer, J.E. Identification and characterization of MPG1, a gene involved in pathogenicity from the rice blast fungus Magnaporthe grisea. Plant Cell 1993, 5, 1575–1590. [Google Scholar] [PubMed]
- He, M.; Xu, Y.; Chen, J.; Luo, Y.; Lv, Y.; Su, J.; Kershaw, M.J.; Li, W.; Wang, J.; Yin, J.; et al. MoSnt2-dependent deacetylation of histone H3 mediates MoTor-dependent autophagy and plant infection by the rice blast fungus Magnaporthe oryzae. Autophagy 2018, 14, 1543–1561. [Google Scholar] [CrossRef]
- Liu, X.H.; Chen, S.M.; Gao, H.M.; Ning, G.A.; Shi, H.B.; Wang, Y.; Dong, B.; Qi, Y.Y.; Zhang, D.M.; Lu, G.D.; et al. The small GTPase MoYpt7 is required for membrane fusion in autophagy and pathogenicity of Magnaporthe oryzae. Environ. Microbiol. 2015, 17, 4495–4510. [Google Scholar] [CrossRef]
- Song, H.; Xu, T.; Feng, X.; Lai, Y.; Yang, Y.; Zheng, H.; He, X.; Wei, G.; Liao, W.; Liao, Y.; et al. Itaconate prevents abdominal aortic aneurysm formation through inhibiting inflammation via activation of Nrf2. EBioMedicine 2020, 57, 102832. [Google Scholar] [CrossRef]
- Zhu, X.M.; Li, L.; Cai, Y.Y.; Wu, X.Y.; Shi, H.B.; Liang, S.; Qu, Y.M.; Naqvi, N.I.; Del Poeta, M.; Dong, B.; et al. A VASt-domain protein regulates autophagy, membrane tension, and sterol homeostasis in rice blast fungus. Autophagy 2021, 17, 2939–2961. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.-Q.; Zhu, X.-M.; Bao, J.-D.; Wang, J.-Y.; Yu, X.-P.; Lin, F.-C.; Li, L. The MoLfa1 Protein Regulates Fungal Development and Septin Ring Formation in Magnaporthe oryzae. Int. J. Mol. Sci. 2024, 25, 3434. https://doi.org/10.3390/ijms25063434
Wu J-Q, Zhu X-M, Bao J-D, Wang J-Y, Yu X-P, Lin F-C, Li L. The MoLfa1 Protein Regulates Fungal Development and Septin Ring Formation in Magnaporthe oryzae. International Journal of Molecular Sciences. 2024; 25(6):3434. https://doi.org/10.3390/ijms25063434
Chicago/Turabian StyleWu, Jia-Qi, Xue-Ming Zhu, Jian-Dong Bao, Jiao-Yu Wang, Xiao-Ping Yu, Fu-Cheng Lin, and Lin Li. 2024. "The MoLfa1 Protein Regulates Fungal Development and Septin Ring Formation in Magnaporthe oryzae" International Journal of Molecular Sciences 25, no. 6: 3434. https://doi.org/10.3390/ijms25063434
APA StyleWu, J.-Q., Zhu, X.-M., Bao, J.-D., Wang, J.-Y., Yu, X.-P., Lin, F.-C., & Li, L. (2024). The MoLfa1 Protein Regulates Fungal Development and Septin Ring Formation in Magnaporthe oryzae. International Journal of Molecular Sciences, 25(6), 3434. https://doi.org/10.3390/ijms25063434






