Biomarkers in Contrast-Induced Acute Kidney Injury: Towards A New Perspective
Abstract
1. Introduction
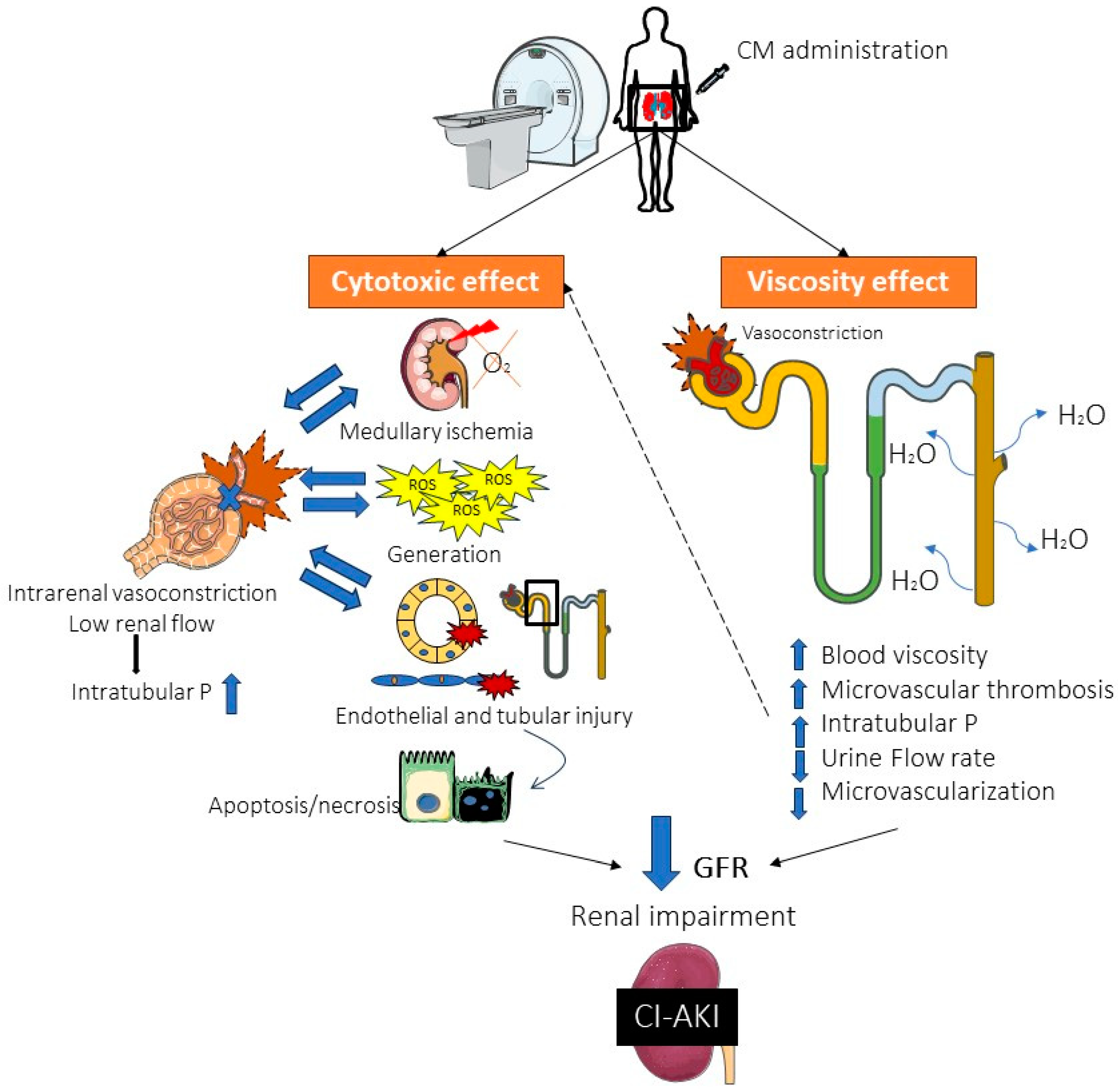
2. Pathophysiology of AKI and CI-AKI
3. Traditional “Gold Standard” Markers
3.1. Serum Creatinine (SCr)
3.2. Glomerular Filtration Rate (GFR)
3.3. Urinary Output (UO)
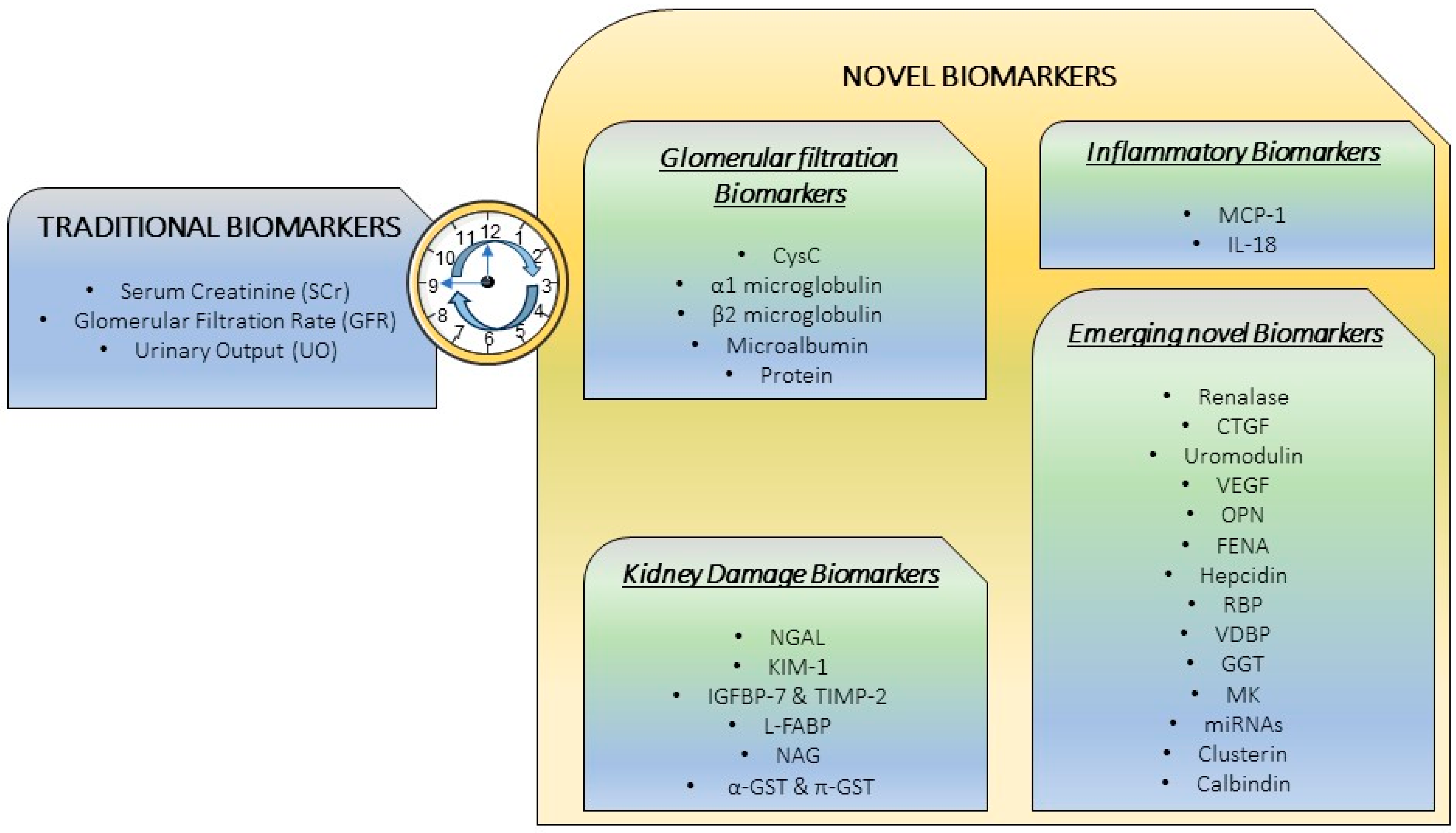
4. Novel Biomarkers: The Necessity for a Precise CI-AKI Diagnosis
4.1. Glomerular Filtration Biomarkers
4.1.1. Cystatin C (CysC)
4.1.2. α1-Microglobulin (α1-m)
4.1.3. β2-Microglobulin (β2-m)
4.1.4. Microalbuminuria
4.1.5. Proteinuria
4.2. Inflammatory Biomarkers
4.2.1. Monocyte Chemoattractant Protein 1 (MCP-1)
4.2.2. Interleukin 18 (IL-18)
4.3. Kidney Damage Biomarkers
4.3.1. Neutrophil Gelatinase-Associated Lipocalin (NGAL)
4.3.2. Kidney Injury Molecule 1 (KIM-1)
4.3.3. Insulin-Like Growth Factor-Binding Protein 7 (IGFBP-7) and Tissue Inhibitor of Metalloproteinases-2 (TIMP-2)
4.3.4. Liver Fatty Acid-Binding Protein (L-FABP)
4.3.5. N-acetyl-β-d-glucosaminidase (NAG)
4.3.6. α,π glutathione S-Transferase (α-GST, π-GST)
4.4. Emerging Novel Biomarkers
4.4.1. Renalase
4.4.2. Connective Tissue Growth Factor (CTGF)
4.4.3. Uromodulin
4.4.4. Vascular Endothelial Growth Factor (VEGF)
4.4.5. Osteopontin (OPN)
4.4.6. Fractional Excretion of Sodium (FENa)
4.4.7. Hepcidin
4.4.8. Retinal Binding Protein (RBP)
4.4.9. Vitamin D Binding Protein (VDBP)
4.4.10. Gamma Glutamyl Transferase (GGT)
4.4.11. Midkine (MK)
4.4.12. MicroRNAs (miRNAs)
4.4.13. Clusterin
4.4.14. Calbindin
5. Combination of Biomarkers: A Future Approach
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kurzhagen, J.T.; Dellepiane, S.; Cantaluppi, V.; Rabb, H. AKI: An increasingly recognized risk factor for CKD development and progression. J. Nephrol. 2020, 33, 1171–1187. [Google Scholar] [CrossRef] [PubMed]
- Batte, A.; Shahrin, L.; Claure-Del Granado, R.; Luyckx, V.A.; Conroy, A.L. Infections and Acute Kidney Injury: A Global Perspective. Semin. Nephrol. 2023, 28, 151466. [Google Scholar] [CrossRef] [PubMed]
- Ulasi, I.I.; Awobusuyi, O.; Nayak, S.; Ramachandran, R.; Musso, C.G.; Depine, S.A.; Aroca-Martinez, G.; Solarin, A.U.; Onuigbo, M.; Luyckx, V.A.; et al. Chronic Kidney Disease Burden in Low-Resource Settings: Regional Perspectives. Semin. Nephrol. 2022, 42, 151336. [Google Scholar] [CrossRef] [PubMed]
- Pomara, C.; Pascale, N.; Maglietta, F.; Neri, M.; Riezzo, I.; Turillazzi, E. Use of contrast media in diagnostic imaging: Medico-legal considerations. Radiol. Med. 2015, 120, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.H.; Miller, J.E.; Forman, H.P. The Critical Shortage of Iodinated Contrast Material—Will Value Prevail? N. Engl. J. Med. 2022, 387, 491–493. [Google Scholar] [CrossRef] [PubMed]
- Amukotuwa, S.A.; Bammer, R.; Jackson, D.M.; Sutherland, T. Iodinated contrast media shortage: Insights and guidance from two major public hospitals. J. Med. Imaging Radiat. Oncol. 2022, 66, 946–956. [Google Scholar] [CrossRef]
- Andreucci, M.; Solomon, R.; Tasanarong, A. Side effects of radiographic contrast media: Pathogenesis, risk factors, and prevention. BioMed. Res. Int. 2014, 2014, 741018. [Google Scholar] [CrossRef]
- Mehran, R.; Nikolsky, E. Contrast-induced nephropathy: Definition, epidemiology, and patients at risk. Kidney Int. Suppl. 2006, 100, S11–S15. [Google Scholar] [CrossRef]
- Weisbord, S.D.; Palevsky, P.M. Contrast-associated Acute Kidney Injury. Crit. Care Clin. 2015, 31, 725–735. [Google Scholar] [CrossRef]
- Wu, M.J.; Tsai, S.F. Patients with Different Stages of Chronic Kidney Disease Undergoing Intravenous Contrast-Enhanced Computed Tomography-The Incidence of Contrast-Associated Acute Kidney Injury. Diagnostics 2022, 12, 864. [Google Scholar] [CrossRef]
- Thomsen, H.S. Guidelines for contrast media from the European Society of Urogenital Radiology. AJR Am. J. Roentgenol. 2003, 181, 1463–1471. [Google Scholar] [CrossRef]
- McCullough, P.A. Contrast-induced acute kidney injury. J. Am. Coll. Cardiol. 2008, 51, 1419–1428. [Google Scholar] [CrossRef]
- Andreucci, M.; Faga, T.; Pisani, A.; Sabbatini, M.; Russo, D.; Michael, A. Prevention of contrast-induced nephropathy through a knowledge of its pathogenesis and risk factors. Sci. World J. 2014, 2014, 823169. [Google Scholar] [CrossRef] [PubMed]
- Andreucci, M.; Faga, T.; Pisani, A.; Sabbatini, M.; Michael, A. Acute kidney injury by radiographic contrast media: Pathogenesis and prevention. Biomed. Res. Int. 2014, 2014, 362725. [Google Scholar] [CrossRef] [PubMed]
- Andreucci, M.; Faga, T.; Serra, R.; De Sarro, G.; Michael, A. Update on the renal toxicity of iodinated contrast drugs used in clinical medicine. Drug Healthc. Patient Saf. 2017, 9, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Mehran, R.; Dangas, G.D.; Weisbord, S.D. Contrast-Associated Acute Kidney Injury. N. Engl. J. Med. 2019, 380, 2146–2155. [Google Scholar] [CrossRef] [PubMed]
- McCullough, P.A.; Choi, J.P.; Feghali, G.A.; Schussler, J.M.; Stoler, R.M.; Vallabahn, R.C.; Mehta, A. Contrast-Induced Acute Kidney Injury. J. Am. Coll. Cardiol. 2016, 68, 1465–1473. [Google Scholar] [CrossRef]
- Kidney International Supplements. Off. J. Int. Soc. Nephrol. 2012, 2, 69–80. Available online: https://kdigo.org/wp-content/uploads/2016/10/KDIGO-2012-AKI-Guideline-English.pdf (accessed on 31 January 2024).
- Nusca, A.; Miglionico, M.; Proscia, C.; Ragni, L.; Carassiti, M.; Pepe, F.L.; Di Sciascio, G. Early prediction of contrast-induced acute kidney injury by a “bedside” assessment of Neutrophil Gelatinase-Associated Lipocalin during elective percutaneous coronary interventions. PLoS ONE 2018, 13, e0197833. [Google Scholar] [CrossRef]
- Khandy, A.H.; Shiekh, R.; Nabi, T.; Sheikh, M.T.; Sheikh, R.Y. Incidence, Determinants, and Outcome of Contrast-induced Acute Kidney Injury following Percutaneous Coronary Intervention at a Tertiary Care Hospital. Saudi J. Kidney Dis. Transpl. 2023, 34, 214–223. [Google Scholar] [CrossRef]
- Kellum, J.A.; Romagnani, P.; Ashuntantang, G.; Ronco, C.; Zarbock, A.; Anders, H.J. Acute kidney injury. Nat. Rev. Dis. Primers 2021, 7, 52. [Google Scholar] [CrossRef]
- Makris, K.; Spanou, L. Acute Kidney Injury: Definition, Pathophysiology and Clinical Phenotypes. Clin. Biochem. Rev. 2016, 37, 85–98. [Google Scholar] [PubMed]
- Wu, Z.H.; Chiu, C.H.; Chen, C.C.; Chyau, C.C.; Cheng, C.H. Amelioration of Cyclosporine A-Induced Acute Nephrotoxicity by Cordyceps cicadae Mycelia via Mg(+2) Reabsorption and the Inhibition of GRP78-IRE1-CHOP Pathway: In Vivo and In Vitro. Int. J. Mol. Sci. 2023, 24, 772. [Google Scholar] [CrossRef] [PubMed]
- Jado, J.C.; Humanes, B.; González-Nicolás, M.A.; Camaño, S.; Lara, J.M.; López, B.; Cercenado, E.; García-Bordas, J.; Tejedor, A.; Lázaro, A. Nephroprotective Effect of Cilastatin against Gentamicin-Induced Renal Injury In Vitro and In Vivo without Altering Its Bactericidal Efficiency. Antioxidants 2020, 9, 821. [Google Scholar] [CrossRef]
- Pabla, N.; Dong, Z. Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int. 2008, 73, 994–1007. [Google Scholar] [CrossRef]
- Humanes, B.; Camaño, S.; Lara, J.M.; Sabbisetti, V.; González-Nicolás, M.A.; Bonventre, J.V.; Tejedor, A.; Lázaro, A. Cisplatin-induced renal inflammation is ameliorated by cilastatin nephroprotection. Nephrol. Dial. Transplant. 2017, 32, 1645–1655. [Google Scholar] [CrossRef]
- Mazer, M.; Perrone, J. Acetaminophen-induced nephrotoxicity: Pathophysiology, clinical manifestations, and management. J. Med. Toxicol. 2008, 4, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Briguori, C.; Quintavalle, C.; Donnarumma, E.; Condorelli, G. Novel biomarkers for contrast-induced acute kidney injury. BioMed. Res. Int. 2014, 2014, 568738. [Google Scholar] [CrossRef] [PubMed]
- Andreucci, M.; Faga, T.; Riccio, E.; Sabbatini, M.; Pisani, A.; Michael, A. The potential use of biomarkers in predicting contrast-induced acute kidney injury. Int. J. Nephrol. Renovasc. Dis. 2016, 9, 205–221. [Google Scholar] [CrossRef]
- Mussap, M.; Noto, A.; Fanos, V.; Van Den Anker, J.N. Emerging biomarkers and metabolomics for assessing toxic nephropathy and acute kidney injury (AKI) in neonatology. BioMed. Res. Int. 2014, 2014, 602526. [Google Scholar] [CrossRef][Green Version]
- Charlton, J.R.; Portilla, D.; Okusa, M.D. A basic science view of acute kidney injury biomarkers. Nephrol. Dial. Transplant. 2014, 29, 1301–1311. [Google Scholar] [CrossRef]
- Kusirisin, P.; Apaijai, N.; Noppakun, K.; Kuanprasert, S.; Chattipakorn, S.C.; Chattipakorn, N. Circulating mitochondrial dysfunction as an early biomarker for contrast media-induced acute kidney injury in chronic kidney disease patients. J. Cell. Mol. Med. 2023, 27, 2059–2070. [Google Scholar] [CrossRef]
- Damman, K.; Voors, A.A.; Navis, G.; van Veldhuisen, D.J.; Hillege, H.L. Current and novel renal biomarkers in heart failure. Heart Fail. Rev. 2012, 17, 241–250. [Google Scholar] [CrossRef]
- Cockcroft, D.W.; Gault, M.H. Prediction of creatinine clearance from serum creatinine. Nephron 1976, 16, 31–41. [Google Scholar] [CrossRef]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann. Intern. Med. 1999, 130, 461–470. [Google Scholar] [CrossRef]
- Solomon, R.; Goldstein, S. Real-time measurement of glomerular filtration rate. Curr. Opin. Crit. Care 2017, 23, 470–474. [Google Scholar] [CrossRef]
- QPI-1002 Phase 3 for Prevention of Major Adverse Kidney Events (MAKE) in Subjects at High Risk for AKI Following Cardiac Surgery. 2021. Available online: https://clinicaltrials.gov/study/NCT03510897 (accessed on 31 January 2024).
- Randomized Double Blind Placebo-Controlled Phase II Study on the Effects of EA-230 on the Innate Immune Response Following On-Pump Cardiac Surgery. 2016. Available online: https://www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2015-005600-28 (accessed on 31 January 2024).
- Stevens, M.A.; McCullough, P.A.; Tobin, K.J.; Speck, J.P.; Westveer, D.C.; Guido-Allen, D.A.; Timmis, G.C.; O’Neill, W.W. A prospective randomized trial of prevention measures in patients at high risk for contrast nephropathy: Results of the P.R.I.N.C.E. Study. Prevention of Radiocontrast Induced Nephropathy Clinical Evaluation. J. Am. Coll. Cardiol. 1999, 33, 403–411. [Google Scholar] [CrossRef]
- Kellum, J.A.; Sileanu, F.E.; Murugan, R.; Lucko, N.; Shaw, A.D.; Clermont, G. Classifying AKI by Urine Output versus Serum Creatinine Level. J. Am. Soc. Nephrol. 2015, 26, 2231–2238. [Google Scholar] [CrossRef]
- Sudarsky, D.; Nikolsky, E. Contrast-induced nephropathy in interventional cardiology. Int. J. Nephrol. Renovasc. Dis. 2011, 4, 85–99. [Google Scholar] [CrossRef][Green Version]
- Slocum, J.L.; Heung, M.; Pennathur, S. Marking renal injury: Can we move beyond serum creatinine? Transl. Res. 2012, 159, 277–289. [Google Scholar] [CrossRef]
- Royakkers, A.A.; van Suijlen, J.D.; Hofstra, L.S.; Kuiper, M.A.; Bouman, C.S.C.; Spronk, P.E.; Schultz, M.J. Serum cystatin C-A useful endogenous marker of renal function in intensive care unit patients at risk for or with acute renal failure? Curr. Med. Chem. 2007, 14, 2314–2317. [Google Scholar] [CrossRef]
- Peng, L.; Wong, K.; Chio, S.; Tam, K.; Hun, W.; Tao, T.; Xiao, H. Diagnostic value of cystatin C in contrast-induced acute kidney injury after percutaneous coronary intervention. Zhonghua Nei Ke Za Zhi 2015, 54, 188–192. [Google Scholar]
- Briguori, C.; Visconti, G.; Rivera, N.V.; Focaccio, A.; Golia, B.; Giannone, R.; Castaldo, D.; De Micco, F.; Ricciardelli, B.; Colombo, A. Cystatin C and contrast-induced acute kidney injury. Circulation 2010, 121, 2117–2122. [Google Scholar] [CrossRef]
- Budano, C.; Andreis, A.; De Filippo, O.; Bissolino, A.; Lanfranco, G.; Usmiani, T.; Gai, M.; Levis, M.; Bergamasco, L.; Marra, S.; et al. A single cystatin C determination before coronary angiography can predict short and long-term adverse events. Int. J. Cardiol. 2020, 300, 73–79. [Google Scholar] [CrossRef]
- Bachorzewska-Gajewska, H.; Malyszko, J.; Sitniewska, E.; Malyszko, J.S.; Dobrzycki, S. Neutrophil gelatinase-associated lipocalin (NGAL) correlations with cystatin C, serum creatinine and eGFR in patients with normal serum creatinine undergoing coronary angiography. Nephrol. Dial. Transplant. 2007, 22, 295–296. [Google Scholar] [CrossRef][Green Version]
- Rickli, H.; Benou, K.; Ammann, P.; Fehr, T.; Brunner-La Rocca, H.P.; Petridis, H.; Riesen, W.; Wüthrich, R.P. Time course of serial cystatin C levels in comparison with serum creatinine after application of radiocontrast media. Clin. Nephrol. 2004, 61, 98–102. [Google Scholar] [CrossRef]
- Solomon, R.J.; Mehran, R.; Natarajan, M.K.; Doucet, S.; Katholi, R.E.; Staniloae, C.S.; Sharma, S.K.; Labinaz, M.; Gelormini, J.L.; Barrett, B.J. Contrast-induced nephropathy and long-term adverse events: Cause and effect? Clin. J. Am. Soc. Nephrol. 2009, 4, 1162–1169. [Google Scholar] [CrossRef]
- Thiele, H.; Hildebrand, L.; Schirdewahn, C.; Eitel, I.; Adams, V.; Fuernau, G.; Erbs, S.; Linke, A.; Diederich, K.-W.; Nowak, M.; et al. Impact of high-dose N-acetylcysteine versus placebo on contrast-induced nephropathy and myocardial reperfusion injury in unselected patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. The LIPSIA-N-ACC (Prospective, Single-Blind, Placebo-Controlled, Randomized Leipzig Immediate PercutaneouS Coronary Intervention Acute Myocardial Infarction N-ACC) Trial. J. Am. Coll. Cardiol. 2010, 55, 2201–2209. [Google Scholar] [CrossRef] [PubMed]
- Droppa, M.; Desch, S.; Blasé, P.; Eitel, I.; Fuernau, G.; Schuler, G.; Adams, V.; Thiele, H. Impact of N-acetylcysteine on contrast-induced nephropathy defined by cystatin C in patients with ST-elevation myocardial infarction undergoing primary angioplasty. Clin. Res. Cardiol. 2011, 100, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Guan, Y.; Xu, S.; Li, Q.; Sun, Y.; Han, R.; Jiang, C. Early Predictors of Acute Kidney Injury: A Narrative Review. Kidney Blood Press. Res. 2016, 41, 680–700. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.X.; Gao, M.; Yang, T.H.; Tian, C.; Jin, S. The preventive effects of different doses of atorvastatin on contrast-induced acute kidney injury after CT perfusion. J. Clin. Lab. Anal. 2022, 36, e24386. [Google Scholar] [CrossRef] [PubMed]
- Banda, J.; Duarte, R.; Dix-Peek, T.; Dickens, C.; Manga, P.; Naicker, S. Biomarkers for Diagnosis and Prediction of Outcomes in Contrast-Induced Nephropathy. Int. J. Nephrol. 2020, 2020, 8568139. [Google Scholar] [CrossRef]
- Terzi, I.; Papaioannou, V.; Papanas, N.; Dragoumanis, C.; Petala, A.; Theodorou, V.; Gioka, T.; Vargemezis, V.; Maltezos, E.; Pneumatikos, I. Alpha1-microglobulin as an early biomarker of sepsis-associated acute kidney injury: A prospective cohort study. Hippokratia 2014, 18, 262–268. [Google Scholar]
- Husain-Syed, F.; Wilhelm, J.; Kassoumeh, S.; Birk, H.W.; Herold, S.; Vadász, I.; Walmrath, H.D.; Kellum, J.A.; Ronco, C.; Seeger, W. Acute kidney injury and urinary biomarkers in hospitalized patients with coronavirus disease-2019. Nephrol. Dial. Transplant. 2020, 35, 1271–1274. [Google Scholar] [CrossRef]
- Afifi, W.M.; Ghorab, A.A.M.; Mahmoud, M.I.; Sherif, M.M.; Hassaneen, A.M. Serum Alpha1-Microglobulin (α1MG) as an Early Predictor of Contrast induced Nephropathy. Br. J. Sci. 2013, 8, 1–11. [Google Scholar]
- D’Amore, C.; Nuzzo, S.; Briguori, C. Biomarkers of Contrast-Induced Nephropathy: Which Ones are Clinically Important? Interv. Cardiol. Clin. 2020, 9, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Heise, D.; Rentsch, K.; Braeuer, A.; Friedrich, M.; Quintel, M. Comparison of urinary neutrophil glucosaminidase-associated lipocalin, cystatin C, and α1-microglobulin for early detection of acute renal injury after cardiac surgery. Eur. J. Cardiothorac. Surg. 2011, 39, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Chu, W.; Han, S.; Gao, B.; Wang, X. Urinary proteomics investigations into contrast-induced acute kidney injury. PLoS ONE 2021, 16, e0258736. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zheng, Z.; Tang, X.; Peng, L.; Luo, Y.; Dong, R.; Zhao, Y.; Liu, J. Preprocedure and Postprocedure Predictive Values of Serum β2-Microglobulin for Contrast-Induced Nephropathy in Patients Undergoing Coronary Computed Tomography Angiography: A Comparison With Creatinine-Based Parameters and Cystatin C. J. Comput. Assist. Tomogr. 2015, 39, 969–974. [Google Scholar] [CrossRef]
- Barton, K.T.; Kakajiwala, A.; Dietzen, D.J.; Goss, C.W.; Gu, H.; Dharnidharka, V.R. Using the newer Kidney Disease: Improving Global Outcomes criteria, beta-2-microglobulin levels associate with severity of acute kidney injury. Clin. Kidney J. 2018, 11, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Adiyanti, S.S.; Loho, T. Acute Kidney Injury (AKI) biomarker. Acta Med. Indones. 2012, 44, 246–255. [Google Scholar] [PubMed]
- Levin, A.; Pate, G.E.; Shalansky, S.; Al-Shamari, A.; Webb, J.G.; Buller, C.E.; Humphries, K.H. N-acetylcysteine reduces urinary albumin excretion following contrast administration: Evidence of biological effect. Nephrol. Dial. Transplant. 2007, 22, 2520–2524. [Google Scholar] [CrossRef]
- Hu, M.; Luo, E.; Yan, G.; Tang, C.; Wang, L.; Zhang, Q.; Gong, J. Microalbuminuria Complicated with Low Estimated Glomerular Filtration Rate: Early Risk Factors for Contrast-Induced Acute Kidney Injury After Coronary Intervention. Med. Sci. Monit. 2022, 28, e935455. [Google Scholar] [CrossRef] [PubMed]
- Parr, S.K.; Matheny, M.E.; Abdel-Kader, K.; Greevy, R.A., Jr.; Bian, A.; Fly, J.; Chen, G.; Speroff, T.; Hung, A.M.; Ikizler, T.A.; et al. Acute kidney injury is a risk factor for subsequent proteinuria. Kidney Int. 2018, 93, 460–469. [Google Scholar] [CrossRef]
- Piskinpasa, S.; Altun, B.; Akoglu, H.; Yildirim, T.; Agbaht, K.; Yilmaz, R.; Peynircioglu, B.; Cil, B.; Aytemir, K.; Turgan, C. An uninvestigated risk factor for contrast-induced nephropathy in chronic kidney disease: Proteinuria. Ren. Fail. 2013, 35, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Parikh, C.R.; Devarajan, P. New biomarkers of acute kidney injury. Crit. Care Med. 2008, 36 (Suppl. 4), S159–S165. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Dong, W.; Li, Z.; Chen, Y.; Liang, H.; Li, R.; Mo, L.; Xu, L.; Liu, S.; Shi, W.; et al. Proteinuria as an independent risk factor for contrast-induced acute kidney injury and mortality in patients with stroke undergoing cerebral angiography. J. Neurointerv. Surg. 2017, 9, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Munshi, R.; Johnson, A.; Siew, E.D.; Ikizler, T.A.; Ware, L.B.; Wurfel, M.M.; Himmelfarb, J.; Zager, R.A. MCP-1 gene activation marks acute kidney injury. J. Am. Soc. Nephrol. 2011, 22, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Lucarelli, G.; Mancini, V.; Galleggiante, V.; Rutigliano, M.; Vavallo, A.; Battaglia, M.; Ditonno, P. Emerging urinary markers of renal injury in obstructive nephropathy. Biomed. Res. Int. 2014, 2014, 303298. [Google Scholar] [CrossRef]
- Shinke, H.; Masuda, S.; Togashi, Y.; Ikemi, Y.; Ozawa, A.; Sato, T.; Kim, Y.H.; Mishima, M.; Ichimura, T.; Bonventre, J.V.; et al. Urinary kidney injury molecule-1 and monocyte chemotactic protein-1 are noninvasive biomarkers of cisplatin-induced nephrotoxicity in lung cancer patients. Cancer Chemother. Pharmacol. 2015, 76, 989–996. [Google Scholar] [CrossRef]
- Gong, X.; Duan, Y.; Zheng, J.; Ye, Z.; Hei, T.K. Tetramethylpyrazine Prevents Contrast-Induced Nephropathy via Modulating Tubular Cell Mitophagy and Suppressing Mitochondrial Fragmentation, CCL2/CCR2-Mediated Inflammation, and Intestinal Injury. Oxid. Med. Cell Longev. 2019, 2019, 7096912. [Google Scholar] [CrossRef]
- Ling, W.; Zhaohui, N.; Ben, H.; Leyi, G.; Jianping, L.; Huili, D.; Jiaqi, Q. Urinary IL-18 and NGAL as early predictive biomarkers in contrast-induced nephropathy after coronary angiography. Nephron Clin. Pract. 2008, 108, c176–c181. [Google Scholar] [CrossRef]
- He, H.; Li, W.; Qian, W.; Zhao, X.; Wang, L.; Yu, Y.; Liu, J.; Cheng, J. Urinary interleukin-18 as an early indicator to predict contrast-induced nephropathy in patients undergoing percutaneous coronary intervention. Exp. Ther. Med. 2014, 8, 1263–1266. [Google Scholar] [CrossRef]
- Duan, S.B.; Liu, G.L.; Yu, Z.Q.; Pan, P. Urinary KIM-1, IL-18 and Cys-c as early predictive biomarkers in gadolinium-based contrast-induced nephropathy in the elderly patients. Clin. Nephrol. 2013, 80, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Bulent Gul, C.; Gullulu, M.; Oral, B.; Aydinlar, A.; Oz, O.; Budak, F.; Yilmaz, Y.; Yurtkuran, M. Urinary IL-18: A marker of contrast-induced nephropathy following percutaneous coronary intervention? Clin. Biochem. 2008, 41, 544–547. [Google Scholar] [CrossRef]
- Connolly, M.; Kinnin, M.; McEneaney, D.; Menown, I.; Kurth, M.; Lamont, J.; Morgan, N.; Harbinson, M. Prediction of contrast induced acute kidney injury using novel biomarkers following contrast coronary angiography. QJM 2018, 111, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Zdziechowska, M.; Gluba-Brzózka, A.; Poliwczak, A.R.; Franczyk, B.; Kidawa, M.; Zielinska, M.; Rysz, J. Serum NGAL, KIM-1, IL-18, L-FABP: New biomarkers in the diagnostics of acute kidney injury (AKI) following invasive cardiology procedures. Int. Urol. Nephrol. 2020, 52, 2135–2143. [Google Scholar] [CrossRef]
- Mishra, J.; Ma, Q.; Prada, A.; Mitsnefes, M.; Zahedi, K.; Yang, J.; Barasch, J.; Devarajan, P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J. Am. Soc. Nephrol. 2003, 14, 2534–2543. [Google Scholar] [CrossRef]
- Mishra, J.; Mori, K.; Ma, Q.; Kelly, C.; Barasch, J.; Devarajan, P. Neutrophil gelatinase-associated lipocalin: A novel early urinary biomarker for cisplatin nephrotoxicity. Am. J. Nephrol. 2004, 24, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Alge, J.L.; Arthur, J.M. Biomarkers of AKI: A review of mechanistic relevance and potential therapeutic implications. Clin. J. Am. Soc. Nephrol. 2015, 10, 147–155. [Google Scholar] [CrossRef]
- Paragas, N.; Qiu, A.; Zhang, Q.; Samstein, B.; Deng, S.-X.; Schmidt-Ott, K.M.; Viltard, M.; Yu, W.; Forster, C.S.; Gong, G.; et al. The Ngal reporter mouse detects the response of the kidney to injury in real time. Nat. Med. 2011, 17, 216–222. [Google Scholar] [CrossRef]
- Filiopoulos, V.; Biblaki, D.; Lazarou, D.; Chrisis, D.; Fatourou, M.; Lafoyianni, S.; Vlassopoulos, D. Plasma neutrophil gelatinase-associated lipocalin (NGAL) as an early predictive marker of contrast-induced nephropathy in hospitalized patients undergoing computed tomography. Clin. Kidney J. 2013, 6, 578–583. [Google Scholar] [CrossRef]
- Filiopoulos, V.; Biblaki, D.; Vlassopoulos, D. Neutrophil gelatinase-associated lipocalin (NGAL): A promising biomarker of contrast-induced nephropathy after computed tomography. Ren. Fail. 2014, 36, 979–986. [Google Scholar] [CrossRef]
- Schilcher, G.; Ribitsch, W.; Otto, R.; Portugaller, R.H.; Quehenberger, F.; Truschnig-Wilders, M.; Zweiker, R.; Stiegler, P.; Brodmann, M.; Weinhandl, K.; et al. Early detection and intervention using neutrophil gelatinase-associated lipocalin (NGAL) may improve renal outcome of acute contrast media induced nephropathy: A randomized controlled trial in patients undergoing intra-arterial angiography (ANTI-CIN Study). BMC Nephrol. 2011, 12, 39. [Google Scholar] [CrossRef]
- Tasanarong, A.; Hutayanon, P.; Piyayotai, D. Urinary Neutrophil Gelatinase-Associated Lipocalin predicts the severity of contrast-induced acute kidney injury in chronic kidney disease patients undergoing elective coronary procedures. BMC Nephrol. 2013, 14, 270. [Google Scholar] [CrossRef]
- Quintavalle, C.; Anselmi, C.V.; De Micco, F.; Roscigno, G.; Visconti, G.; Golia, B.; Focaccio, A.; Ricciardelli, B.; Perna, E.; Papa, L.; et al. Neutrophil Gelatinase-Associated Lipocalin and Contrast-Induced Acute Kidney Injury. Circ. Cardiovasc. Interv. 2015, 8, e002673. [Google Scholar] [CrossRef] [PubMed]
- Kafkas, N.; Liakos, C.; Zoubouloglou, F.; Dagadaki, O.; Dragasis, S.; Makris, K. Neutrophil Gelatinase-Associated Lipocalin as an Early Marker of Contrast-Induced Nephropathy After Elective Invasive Cardiac Procedures. Clin. Cardiol. 2016, 39, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Reyes, L.F.; Severiche-Bueno, D.F.; Bustamante, C.A.; Murillo, S.; Soni, N.J.; Poveda, M.; Gomez, E.; Buitrago, R.; Rodriguez, A. Serum levels of neutrophil Gelatinase associated Lipocalin (NGAL) predicts hemodialysis after coronary angiography in high risk patients with acute coronary syndrome. BMC Nephrol. 2020, 21, 143. [Google Scholar] [CrossRef] [PubMed]
- Byeon, J.; Choi, I.J.; Lee, D.; Ahn, Y.; Kim, M.J.; Jeon, D.S. Predictive and Prognostic Value of Serum Neutrophil Gelatinase-Associated Lipocalin for Contrast-Induced Acute Kidney Injury and Long-Term Clinical Outcomes after Percutaneous Coronary Intervention. J. Clin. Med. 2022, 11, 5971. [Google Scholar] [CrossRef] [PubMed]
- Ribitsch, W.; Schilcher, G.; Quehenberger, F.; Pilz, S.; Portugaller, R.H.; Truschnig-Wilders, M.; Zweiker, R.; Brodmann, M.; Stiegler, P.; Rosenkranz, A.R.; et al. Neutrophil gelatinase-associated lipocalin (NGAL) fails as an early predictor of contrast induced nephropathy in chronic kidney disease (ANTI-CI-AKI study). Sci. Rep. 2017, 7, 41300. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, T.; Bonventre, J.V.; Bailly, V.; Wei, H.; Hession, C.A.; Cate, R.L.; Sanicolaet, M. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J. Biol. Chem. 1998, 273, 4135–4142. [Google Scholar] [CrossRef] [PubMed]
- Prozialeck, W.C.; Vaidya, V.S.; Liu, J.; Waalkes, M.P.; Edwards, J.R.; Lamar, P.C.; Bernard, A.M.; Dumont, X.; Bonventre, J.V. Kidney injury molecule-1 is an early biomarker of cadmium nephrotoxicity. Kidney Int. 2007, 72, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Han, W.K.; Bailly, V.; Abichandani, R.; Thadhani, R.; Bonventre, J.V. Kidney Injury Molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int. 2002, 62, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, T.; Hung, C.C.; Yang, S.A.; Stevens, J.L.; Bonventre, J.V. Kidney injury molecule-1: A tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am. J. Physiol. Ren. Physiol. 2004, 286, F552–F563. [Google Scholar] [CrossRef]
- Han, W.K.; Waikar, S.S.; Johnson, A.; Betensky, R.A.; Dent, C.L.; Devarajan, P.; Bonventre, J.V. Urinary biomarkers in the early diagnosis of acute kidney injury. Kidney Int. 2008, 73, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Parikh, C.R.; Thiessen-Philbrook, H.; Garg, A.X.; Kadiyala, D.; Shlipak, M.G.; Koyner, J.L.; Edelstein, C.L.; Devarajan, P.; Patel, U.D.; Zappitelli, M.; et al. Performance of kidney injury molecule-1 and liver fatty acid-binding protein and combined biomarkers of AKI after cardiac surgery. Clin. J. Am. Soc. Nephrol. 2013, 8, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Sabbisetti, V.; Waikar, S.S.; Antoine, D.J.; Smiles, A.; Wang, C.; Ravisankar, A.; Ito, K.; Sharma, S.; Ramadesikan, S.; Lee, M.; et al. Blood kidney injury molecule-1 is a biomarker of acute and chronic kidney injury and predicts progression to ESRD in type I diabetes. J. Am. Soc. Nephrol. 2014, 25, 2177–2186. [Google Scholar] [CrossRef] [PubMed]
- Rosner, M.H.; Ronco, C.; Okusa, M.D. The role of inflammation in the cardio-renal syndrome: A focus on cytokines and inflammatory mediators. Semin. Nephrol. 2012, 32, 70–78. [Google Scholar] [CrossRef]
- Mamoulakis, C.; Fragkiadoulaki, I.; Karkala, P.; Georgiadis, G.; Zisis, I.-E.; Stivaktakis, P.; Kalogeraki, A.; Tsiaoussis, I.; Burykina, T.; Lazopoulos, G.; et al. Contrast-induced nephropathy in an animal model: Evaluation of novel biomarkers in blood and tissue samples. Toxicol. Rep. 2019, 6, 395–400. [Google Scholar] [CrossRef]
- Rouse, R.L.; Stewart, S.R.; Thompson, K.L.; Zhang, J. Kidney injury biomarkers in hypertensive, diabetic, and nephropathy rat models treated with contrast media. Toxicol. Pathol. 2013, 41, 662–680. [Google Scholar] [CrossRef]
- Akdeniz, D.; Celik, H.T.; Kazanci, F.; Yilmaz, H.; Yalcin, S.; Bilgic, M.A.; Ruzgaresen, N.; Akcay, A.; Eryonucu, B. Is Kidney Injury Molecule 1 a Valuable Tool for the Early Diagnosis of Contrast-Induced Nephropathy? J. Investig. Med. 2015, 63, 930–934. [Google Scholar] [CrossRef]
- Torregrosa, I.; Montoliu, C.; Urios, A.; Andrés-Costa, M.J.; Giménez-Garzó, C.; Juan, I.; Puchades, M.J.; Blasco, M.L.; Carratalá, A.; Sanjuán, R.; et al. Urinary KIM-1, NGAL and L-FABP for the diagnosis of AKI in patients with acute coronary syndrome or heart failure undergoing coronary angiography. Heart Vessels 2015, 30, 703–711. [Google Scholar] [CrossRef]
- Fuhrman, D.Y.; Kellum, J.A. Biomarkers for Diagnosis, Prognosis and Intervention in Acute Kidney Injury. Contrib. Nephrol. 2016, 187, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C.; Ricci, Z. The concept of risk and the value of novel markers of acute kidney injury. Crit. Care 2013, 17, 117. [Google Scholar] [CrossRef]
- Kashani, K.; Al-Khafaji, A.; Ardiles, T.; Artigas, A.; Bagshaw, S.M.; Bell, M.; Bihorac, A.; Birkhahn, R.; Cely, C.M.; Chawla, L.S.; et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit. Care 2013, 17, R25. [Google Scholar] [CrossRef] [PubMed]
- Koyner, J.L.; Shaw, A.D.; Chawla, L.S.; Hoste, E.A.J.; Bihorac, A.; Kashani, K.; Haase, M.; Shi, J.; Kellum, J.A.; Sapphire Investigators. Tissue Inhibitor Metalloproteinase-2 (TIMP-2)⋅IGF-Binding Protein-7 (IGFBP7) Levels Are Associated with Adverse Long-Term Outcomes in Patients with AKI. J. Am. Soc. Nephrol. 2015, 26, 1747–1754. [Google Scholar] [CrossRef]
- Gocze, I.; Koch, M.; Renner, P.; Zeman, F.; Graf, B.M.; Dahlke, M.H.; Nerlich, M.; Schlitt, H.J.; Kellum, J.A.; Bein, T. Urinary biomarkers TIMP-2 and IGFBP7 early predict acute kidney injury after major surgery. PLoS ONE 2015, 10, e0120863. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Kang, Z.; Li, Z.; Xun, M. Urinary NGAL, IGFBP-7, and TIMP-2: Novel biomarkers to predict contrast medium-induced acute kidney injury in children. Ren. Fail. 2022, 44, 1201–1206. [Google Scholar] [CrossRef]
- Landrier, J.F.; Thomas, C.; Grober, J.; Duez, H.; Percevault, F.; Souidi, M.; Linard, C.; Staels, B.; Besnard, P. Statin induction of liver fatty acid-binding protein (L-FABP) gene expression is peroxisome proliferator-activated receptor-alpha-dependent. J. Biol. Chem. 2004, 279, 45512–45518. [Google Scholar] [CrossRef]
- Yamamoto, T.; Noiri, E.; Ono, Y.; Doi, K.; Negishi, K.; Kamijo, A.; Kimura, K.; Fujita, T.; Kinukawa, T.; Taniguchi, H.; et al. Renal L-type fatty acid-binding protein in acute ischemic injury. J. Am. Soc. Nephrol. 2007, 18, 2894–2902. [Google Scholar] [CrossRef]
- Kamijo-Ikemori, A.; Sugaya, T.; Matsui, K.; Yokoyama, T.; Kimura, K. Roles of human liver type fatty acid binding protein in kidney disease clarified using hL-FABP chromosomal transgenic mice. Nephrology 2011, 16, 539–544. [Google Scholar] [CrossRef]
- Portilla, D.; Dent, C.; Sugaya, T.; Nagothu, K.K.; Kundi, I.; Moore, P.; Noiri, E.; Devarajan, P. Liver fatty acid-binding protein as a biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2008, 73, 465–472. [Google Scholar] [CrossRef]
- Ho, J.; Tangri, N.; Komenda, P.; Kausha, A.; Sood, M.; Brar, R.; Gill, K.; Walker, S.; MacDonald, K.; Hiebert, B.M.; et al. Urinary, Plasma, and Serum Biomarkers’ Utility for Predicting Acute Kidney Injury Associated With Cardiac Surgery in Adults: A Meta-analysis. Am. J. Kidney Dis. 2015, 66, 993–1005. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Sugaya, T.; Node, K.; Ueda, Y.; Koide, H. Urinary excretion of liver-type fatty acid-binding protein in contrast medium-induced nephropathy. Am. J. Kidney Dis. 2006, 47, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Peabody, J.; Paculdo, D.; Valdenor, C.; McCullough, P.A.; Noiri, E.; Sugaya, T.; Dahlen, J.R. Clinical Utility of a Biomarker to Detect Contrast-Induced Acute Kidney Injury during Percutaneous cardiovascular Procedures. Cardiorenal Med. 2022, 12, 11–19. [Google Scholar] [CrossRef]
- Liangos, O.; Perianayagam, M.C.; Vaidya, V.S.; Han, W.K.; Wald, R.; Tighiouart, H.; W MacKinnon, R.W.; Li, L.; Balakrishnan, V.S.; Pereira, B.J.G.; et al. Urinary N-acetyl-beta-(D)-glucosaminidase activity and kidney injury molecule-1 level are associated with adverse outcomes in acute renal failure. J. Am. Soc. Nephrol. 2007, 18, 904–912. [Google Scholar] [CrossRef]
- Bazzi, C.; Petrini, C.; Rizza, V.; Arrigo, G.; Napodano, P.; Paparella, M.; D’Amico, G. Urinary N-acetyl-beta-glucosaminidase excretion is a marker of tubular cell dysfunction and a predictor of outcome in primary glomerulonephritis. Nephrol. Dial. Transplant. 2002, 17, 1890–1896. [Google Scholar] [CrossRef]
- Mukhopadhyay, B.; Chinchole, S.; Lobo, V.; Gang, S.; Rajapurkar, M. Enzymuria pattern in early post renal transplant period: Diagnostic usefulness in graft dysfunction. Indian J. Clin. Biochem. 2004, 19, 14–19. [Google Scholar] [CrossRef]
- Arakawa, Y.; Tamura, M.; Sakuyama, T.; Aiba, K.; Eto, S.; Yuda, M.; Tanaka, Y.; Matsumoto, A.; Nishikawa, K. Early measurement of urinary N-acetyl-β-glucosaminidase helps predict severe hyponatremia associated with cisplatin-containing chemotherapy. J. Infect. Chemother. 2015, 21, 502–506. [Google Scholar] [CrossRef]
- Vaidya, V.S.; Waikar, S.S.; Ferguson, M.A.; Collings, F.B.; Sunderland, K.; Gioules, C.; Bradwin, G.; Matsouaka, R.; Betensky, R.A.; Curhan, G.C.; et al. Urinary biomarkers for sensitive and specific detection of acute kidney injury in humans. Clin. Transl. Sci. 2008, 1, 200–208. [Google Scholar] [CrossRef]
- Ren, L.; Ji, J.; Fang, Y.; Jiang, S.H.; Lin, Y.M.; Bo, J.; Qian, J.Y.; Xu, X.H.; Ding, X.Q. Assessment of urinary N-acetyl-β-glucosaminidase as an early marker of contrast-induced nephropathy. J. Int. Med. Res. 2011, 39, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Benzer, M.; Alpay, H.; Baykan, Ö.; Erdem, A.; Demir, I.H. Serum NGAL, cystatin C and urinary NAG measurements for early diagnosis of contrast-induced nephropathy in children. Ren. Fail. 2016, 38, 27–34. [Google Scholar] [CrossRef][Green Version]
- Nishida, M.; Kubo, S.; Morishita, Y.; Nishikawa, K.; Ikeda, K.; Itoi, T.; Hosoi, H. Kidney injury biomarkers after cardiac angiography in children with congenital heart disease. Congenit. Heart Dis. 2019, 14, 1087–1093. [Google Scholar] [CrossRef]
- Seabra, V.F.; Perianayagam, M.C.; Tighiouart, H.; Liangos, O.; dos Santos, O.F.; Jaber, B.L. Urinary α-GST and π-GST for prediction of dialysis requirement or in-hospital death in established acute kidney injury. Biomarkers 2011, 16, 709–717. [Google Scholar] [CrossRef] [PubMed]
- McMahon, B.A.; Koyner, J.L.; Murray, P.T. Urinary glutathione S-transferases in the pathogenesis and diagnostic evaluation of acute kidney injury following cardiac surgery: A critical review. Curr. Opin. Crit. Care 2010, 16, 550–555. [Google Scholar] [CrossRef]
- Susantitaphong, P.; Perianayagam, M.C.; Tighiouart, H.; Kouznetsov, D.; Liangos, O.; Jaber, B.L. Urinary α- and π-glutathione s-transferases for early detection of acute kidney injury following cardiopulmonary bypass. Biomarkers 2013, 18, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Shu, K.H.; Wang, C.H.; Wu, C.H.; Huang, T.M.; Wu, P.C.; Lai, C.H.; Tseng, L.J.; Tsai, P.R.; Connolly, R.; Wu, V.C. Urinary π-glutathione S-transferase Predicts Advanced Acute Kidney Injury Following Cardiovascular Surgery. Sci. Rep. 2016, 6, 26335. [Google Scholar] [CrossRef]
- Xu, J.; Li, G.; Wang, P.; Velazquez, H.; Yao, X.; Li, Y.; Wu, Y.; Peixoto, A.; Crowley, S.; Desir, G.V. Renalase is a novel, soluble monoamine oxidase that regulates cardiac function and blood pressure. J. Clin. Investig. 2005, 115, 1275–1280. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.T.; Kim, J.Y.; Kim, M.; Wang, P.; Tang, L.; Baroni, S.; D’Agati, V.D.; Desir, G.V. Renalase protects against ischemic AKI. J. Am. Soc. Nephrol. 2013, 24, 445–455. [Google Scholar] [CrossRef]
- Wang, L.; Velazquez, H.; Moeckel, G.; Chang, J.; Ham, A.; Lee, H.T.; Safirstein, R.; Desir, G.V. Renalase prevents AKI independent of amine oxidase activity. J. Am. Soc. Nephrol. 2014, 25, 1226–1235. [Google Scholar] [CrossRef]
- Zhao, B.; Zhao, Q.; Li, J.; Xing, T.; Wang, F.; Wang, N. Renalase protects against contrast-induced nephropathy in Sprague-Dawley rats. PLoS ONE 2015, 10, e0116583. [Google Scholar] [CrossRef]
- Wybraniec, M.T.; Bożentowicz-Wikarek, M.; Chudek, J.; Mizia-Stec, K. Urinary renalase concentration in patients with preserved kidney function undergoing coronary angiography. Nephrology 2018, 23, 133–138. [Google Scholar] [CrossRef]
- Wybraniec, M.T.; Mizia-Stec, K. Renalase and Biomarkers of Contrast-Induced Acute Kidney Injury. Cardiorenal Med. 2015, 6, 25–36. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Rayego, S.; Rodrigues-Díez, R.; Rodriguez, J.S.; Rodrigues-Díez, R.; Rodríguez-Vita, J.; Carvajal, G.; Aroeira, L.S.; Selgas, R.; Mezzano, S.A.; et al. CTGF promotes inflammatory cell infiltration of the renal interstitium by activating NF-kappaB. J. Am. Soc. Nephrol. 2009, 20, 1513–1526. [Google Scholar] [CrossRef]
- Kilari, S.; Yang, B.; Sharma, A.; McCall, D.L.; Misra, S. Increased transforming growth factor beta (TGF-β) and pSMAD3 signaling in a Murine Model for Contrast Induced Kidney Injury. Sci. Rep. 2018, 8, 6630. [Google Scholar] [CrossRef]
- El-Achkar, T.M.; Wu, X.R. Uromodulin in kidney injury: An instigator, bystander, or protector? Am. J. Kidney Dis. 2012, 59, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Dawnay, A.B.; Thornley, C.; Nockler, I.; Webb, J.A.; Cattell, W.R. Tamm-Horsfall glycoprotein excretion and aggregation during intravenous urography. Relevance to acute renal failure. Investig. Radiol. 1985, 20, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Dawson, P.; Freedman, D.B.; Howell, M.J.; Hine, A.L. Contrast-medium-induced acute renal failure and Tamm-Horsfall proteinuria. Br. J. Radiol. 1984, 57, 577–579. [Google Scholar] [CrossRef]
- Bakris, G.L.; Gaber, A.O.; Jones, J.D. Oxygen free radical involvement in urinary Tamm-Horsfall protein excretion after intrarenal injection of contrast medium. Radiology 1990, 175, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Garimella, P.; Jaber, B.L.; Tighiouart, H.; Liangos, O.; Bennett, M.R.; Devarajan, P.; El-Achkar, T.M.; Sarnak, M.J. Association of Preoperative Urinary Uromodulin with AKI after Cardiac Surgery. Clin. J. Am. Soc. Nephrol. 2017, 12, 10–18. [Google Scholar] [CrossRef]
- Bennett, M.R.; Pyles, O.; Ma, Q.; Devarajan, P. Preoperative levels of urinary uromodulin predict acute kidney injury after pediatric cardiopulmonary bypass surgery. Pediatr. Nephrol. 2018, 33, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Schrijvers, B.F.; Flyvbjerg, A.; De Vriese, A.S. The role of vascular endothelial growth factor (VEGF) in renal pathophysiology. Kidney Int. 2004, 65, 2003–2017. [Google Scholar] [CrossRef]
- Ari, E.; Kedrah, A.E.; Alahdab, Y.; Bulut, G.; Eren, Z.; Baytekin, O.; Odabasi, D. Antioxidant and renoprotective effects of paricalcitol on experimental contrast-induced nephropathy model. Br. J. Radiol. 2012, 85, 1038–1043. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Wang, B.; Xie, Y.; Wang, Y.; Jiang, X.; Wang, R.; Ren, K. Evaluation of Renal Pathophysiological Processes Induced by an Iodinated Contrast Agent in a Diabetic Rabbit Model Using Intravoxel Incoherent Motion and Blood Oxygenation Level-Dependent Magnetic Resonance Imaging. Korean J. Radiol. 2019, 20, 830–843. [Google Scholar] [CrossRef]
- Kaleta, B. The role of osteopontin in kidney diseases. Inflamm. Res. 2019, 68, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.E.; McCarthy, C.P.; Shrestha, S.; Gaggin, H.K.; Mukai, R.; Magaret, C.A.; Rhyne, R.F.; Januzzi Jr, J.L. A clinical, proteomics, and artificial intelligence-driven model to predict acute kidney injury in patients undergoing coronary angiography. Clin. Cardiol. 2019, 42, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Beitland, S.; Nakstad, E.R.; Berg, J.P.; Trøseid, A.M.S.; Brusletto, B.S.; Brunborg, C.; Lundqvist, C.; Sunde, K. Urine β-2-Microglobulin, Osteopontin, and Trefoil Factor 3 May Early Predict Acute Kidney Injury and Outcome after Cardiac Arrest. Crit. Care Res. Pract. 2019, 2019, 4384796. [Google Scholar] [CrossRef]
- Mohebi, R.; van Kimmenade, R.; McCarthy, C.; Gaggin, H.; Mehran, R.; Dangas, G.; Januzzi Jr, J.L. A Biomarker-Enhanced Model for Prediction of Acute Kidney Injury and Cardiovascular Risk Following Angiographic Procedures: CASABLANCA AKI Prediction Substudy. J. Am. Heart Assoc. 2022, 11, e025729. [Google Scholar] [CrossRef]
- Espinel, C.H. The FENa test. Use in the differential diagnosis of acute renal failure. JAMA 1976, 236, 579–581. [Google Scholar] [CrossRef]
- Russo, D.; Minutolo, R.; Cianciaruso, B.; Memoli, B.; Conte, G.; De Nicola, L. Early effects of contrast media on renal hemodynamics and tubular function in chronic renal failure. J. Am. Soc. Nephrol. 1995, 6, 1451–1458. [Google Scholar] [CrossRef]
- Murakami, R.; Kumazaki, T.; Tajima, H.; Hayashi, H.; Kuwako, T.; Hakozaki, K.; Kiriyama, T. Urinary excretion of vasoactive factors following contrast media exposure in humans. Nephron Clin. Pract. 2005, 101, c150–c154. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.B.; Yang, S.K.; Zhou, Q.Y.; Pan, P.; Zhang, H.; Liu, F.; Xu, X.Q. Mitochondria-targeted peptides prevent on contrast-induced acute kidney injury in the rats with hypercholesterolemia. Ren. Fail. 2013, 35, 1124–1129. [Google Scholar] [CrossRef]
- Calzavacca, P.; Ishikawa, K.; Bailey, M.; May, C.N.; Bellomo, R. Systemic and renal hemodynamic effects of intra-arterial radiocontrast. Intensive Care Med. Exp. 2014, 2, 32. [Google Scholar] [CrossRef]
- Ostermann, M.; Philips, B.J.; Forni, L.G. Clinical review: Biomarkers of acute kidney injury: Where are we now? Crit. Care 2012, 16, 233. [Google Scholar] [CrossRef] [PubMed]
- Prowle, J.R.; Ostland, V.; Calzavacca, P.; Licari, E.; Ligabo, E.V.; Echeverri, J.E.; Bagshaw, S.M.; Haase-Fielitz, A.; Haase, M.; Westerman, M.; et al. Greater increase in urinary hepcidin predicts protection from acute kidney injury after cardiopulmonary bypass. Nephrol. Dial. Transplant. 2012, 27, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Prowle, J.R.; Westerman, M.; Bellomo, R. Urinary hepcidin: An inverse biomarker of acute kidney injury after cardiopulmonary bypass? Curr. Opin. Crit. Care 2010, 16, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Leaf, D.E.; Rajapurkar, M.; Lele, S.S.; Mukhopadhyay, B.; Boerger, E.A.S.; Mc Causland, F.R.; Eisenga, M.F.; Singh, K.; Babitt, J.L.; Kellum, J.A.; et al. Iron, Hepcidin, and Death in Human AKI. J. Am. Soc. Nephrol. 2019, 30, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Malyszko, J.; Bachorzewska-Gajewska, H.; Malyszko, J.S.; Koc-Zorawska, E.; Matuszkiewicz-Rowinska, J.; Dobrzycki, S. Hepcidin—Potential biomarker of contrast-induced acute kidney injury in patients undergoing percutaneous coronary interventions. Adv. Med. Sci. 2019, 64, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Ayatse, J.O. Human retinol-binding protein: Its relationship to renal function in renal diseases. West. Afr. J. Med. 1991, 10, 226–231. [Google Scholar]
- Sadat, U.; Walsh, S.R.; Norden, A.G.; Gillard, J.H.; Boyle, J.R. Does oral N-acetylcysteine reduce contrast-induced renal injury in patients with peripheral arterial disease undergoing peripheral angiography? A randomized-controlled study. Angiology 2011, 62, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wan, X.; Zhang, H.; Li, W.; Ma, M.; Pan, B.; Liang, X.; Cao, C. Retinoic acid attenuates contrast-induced acute kidney injury in a miniature pig model. Biochem. Biophys. Res. Commun. 2019, 512, 163–169. [Google Scholar] [CrossRef]
- Chaykovska, L.; Heunisch, F.; von Einem, G.; Alter, M.L.; Hocher, C.F.; Tsuprykov, O.; Dschietzig, T.; Kretschmer, A.; Hocher, B. Urinary Vitamin D Binding Protein and KIM-1 Are Potent New Biomarkers of Major Adverse Renal Events in Patients Undergoing Coronary Angiography. PLoS ONE 2016, 11, e0145723. [Google Scholar] [CrossRef]
- Donadio, C.; Tramonti, G.; Lucchesi, A.; Giordani, R.; Lucchetti, A.; Bianchi, C. Gamma-glutamyltransferase is a reliable marker for tubular effects of contrast media. Ren. Fail. 1998, 20, 319–324. [Google Scholar] [CrossRef]
- Oksuz, F.; Yarlioglues, M.; Cay, S.; Celik, I.E.; Mendi, M.A.; Kurtul, A.; Cankurt, T.; Kuyumcu, S.; Canpolat, U.; Turak, O. Predictive Value of Gamma-Glutamyl Transferase Levels for Contrast-Induced Nephropathy in Patients With ST-Segment Elevation Myocardial Infarction Who Underwent Primary Percutaneous Coronary Intervention. Am. J. Cardiol. 2015, 116, 711–716. [Google Scholar] [CrossRef]
- Malyszko, J.; Bachorzewska-Gajewska, H.; Koc-Zorawska, E.; Malyszko, J.S.; Kobus, G.; Dobrzycki, S. Midkine: A novel and early biomarker of contrast-induced acute kidney injury in patients undergoing percutaneous coronary interventions. Biomed. Res. Int. 2015, 2015, 879509. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Ibrahim, G.H.; Adel, M.; Ismail, A.; Almaghraby, A.; Abdelnabi, M. Midkine as an Early Biomarker of Contrast-induced Acute Kidney Injury in Chronic Kidney Disease Patients Undergoing Percutaneous Coronary Intervention for Acute Coronary Syndrome: A Single-center Prospective Study. Maced. J. Med. Sci. 2021, 9, 983–989. [Google Scholar] [CrossRef]
- Jones, T.F.; Bekele, S.; O’Dwyer, M.J.; Prowle, J.R. MicroRNAs in Acute Kidney Injury. Nephron 2018, 140, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, Y.; Zhu, X.; Jiang, C. MicroRNA as an early diagnostic biomarker for contrast-induced acute kidney injury. Drug Chem. Toxicol. 2022, 45, 1552–1557. [Google Scholar] [CrossRef]
- Li, Y.F.; Jing, Y.; Hao, J.; Frankfort, N.C.; Zhou, X.; Shen, B.; Liu, X.; Wang, L.; Li, R. MicroRNA-21 in the pathogenesis of acute kidney injury. Protein Cell 2013, 4, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Cao, X.; Zou, L.; Chen, Y.; Guo, J.; Chen, Z.; Hu, S.; Zheng, Z. MicroRNA-21 and risk of severe acute kidney injury and poor outcomes after adult cardiac surgery. PLoS ONE 2013, 8, e63390. [Google Scholar] [CrossRef]
- Sun, S.Q.; Zhang, T.; Ding, D.; Zhang, W.F.; Wang, X.L.; Sun, Z.; Hu, L.H.; Qin, S.Y.; Shen, L.H.; He, B. Circulating MicroRNA-188, -30a, and -30e as Early Biomarkers for Contrast-Induced Acute Kidney Injury. J. Am. Heart Assoc. 2016, 5, e004138. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Moon, A. Drug-induced nephrotoxicity and its biomarkers. Biomol. Ther. 2012, 20, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Dieterle, F.; Perentes, E.; Cordier, A.; Roth, D.R.; Verdes, P.; Grenet, O.; Pantano, S.; Moulin, P.; Wahl, D.; Mahl, A.; et al. Urinary clusterin, cystatin C, beta2-microglobulin and total protein as markers to detect drug-induced kidney injury. Nat. Biotechnol. 2010, 28, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Vinken, P.; Starckx, S.; Barale-Thomas, E.; Looszova, A.; Sonee, M.; Goeminne, N.; Versmissen, L.; Buyens, K.; Lampo, A. Tissue Kim-1 and urinary clusterin as early indicators of cisplatin-induced acute kidney injury in rats. Toxicol. Pathol. 2012, 40, 1049–1062. [Google Scholar] [CrossRef] [PubMed]
- Nguan, C.Y.C.; Guan, Q.; Gleave, M.E.; Du, C. Promotion of cell proliferation by clusterin in the renal tissue repair phase after ischemia-reperfusion injury. Am. J. Physiol. Ren. Physiol. 2014, 306, F724–F733. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Guan, Q.; Liu, X.; Wang, H.; Gleave, M.E.; Nguan, C.Y.C.; Du, C. Relationship of clusterin with renal inflammation and fibrosis after the recovery phase of ischemia-reperfusion injury. BMC Nephrol. 2016, 17, 133. [Google Scholar] [CrossRef]
- Deng, Y.H.; Wang, X.F.; Wu, X.; Yan, P.; Liu, Q.; Wu, T.; Duan, S.B. Differential renal proteomics analysis in a novel rat model of iodinated contrast-induced acute kidney injury. Ren. Fail. 2023, 45, 2178821. [Google Scholar] [CrossRef]
- Da, Y.; Akalya, K.; Murali, T.; Vathsala, A.; Tan, C.S.; Low, S.; Lim, H.N.; Teo, B.W.; Lau, T.; Ong, L.; et al. Serial Quantification of Urinary Protein Biomarkers to Predict Drug-induced Acute Kidney Injury. Curr. Drug Metab. 2019, 20, 656–664. [Google Scholar] [CrossRef]
- Hoffmann, D.; Fuchs, T.C.; Henzler, T.; Matheis, K.A.; Herget, T.; Dekant, W.; Hewitt, P.; Mally, A. Evaluation of a urinary kidney biomarker panel in rat models of acute and subchronic nephrotoxicity. Toxicology 2010, 277, 49–58. [Google Scholar] [CrossRef]
- Palviainen, M.; Raekallio, M.; Rajamäki, M.M.; Linden, J.; Vainio, O. Kidney-derived proteins in urine as biomarkers of induced acute kidney injury in sheep. Vet. J. 2012, 193, 287–289. [Google Scholar] [CrossRef] [PubMed]
- George, B.; Wen, X.; Mercke, N.; Gomez, M.; O’Bryant, C.; Bowles, D.W.; Hu, Y.; Hogan, S.L.; Joy, M.S.; Aleksunes, L.M. Profiling of Kidney Injury Biomarkers in Patients Receiving Cisplatin: Time-dependent Changes in the Absence of Clinical Nephrotoxicity. Clin. Pharmacol. Ther. 2017, 101, 510–518. [Google Scholar] [CrossRef] [PubMed]
- George, B.; Wen, X.; Mercke, N.; Gomez, M.; O’Bryant, C.; Bowles, D.W.; Hu, Y.; Hogan, S.L.; Joy, M.S.; Aleksunes, L.M. Time-dependent changes in kidney injury biomarkers in patients receiving multiple cycles of cisplatin chemotherapy. Toxicol. Rep. 2020, 7, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.F.; Li, J.M.; Tan, Y.; Wang, Z.F.; He, Y.; Chang, J.; Zhang, H.; Zhao, H.; Bai, X.; Xie, F.; et al. Performance of urinary NGAL and L-FABP in predicting acute kidney injury and subsequent renal recovery: A cohort study based on major surgeries. Clin. Chem. Lab. Med. 2014, 52, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Arthur, J.M.; Hill, E.G.; Alge, J.L.; Lewis, E.C.; Neely, B.A.; Janech, M.G.; Tumlin, J.A.; Chawla, L.S.; Shaw, A.D.; SAKInet Investigators. Evaluation of 32 urine biomarkers to predict the progression of acute kidney injury after cardiac surgery. Kidney Int. 2014, 85, 431–438. [Google Scholar] [CrossRef]
- Wybraniec, M.T.; Chudek, J.; Bożentowicz-Wikarek, M.; Mizia-Stec, K. Prediction of contrast-induced acute kidney injury by early post-procedural analysis of urinary biomarkers and intra-renal Doppler flow indices in patients undergoing coronary angiography. J. Interv. Cardiol. 2017, 30, 465–472. [Google Scholar] [CrossRef] [PubMed]
| Biomarker | Location | Sample | Advantages | Disadvantages | Cutoff for CI-AKI Prediction | Detection Methods |
|---|---|---|---|---|---|---|
| Creatinine | Glomerulus | Blood/urine | * Routine measurement * Cheap | * Depends on age, gender, muscle mass and nutrition * No correlation with renal function in acute situations * Decreases with intensive serum therapy | ≥0.3 mg/dL at 48 h or 50% above baseline over the next 7 days, or by urine volume reduction of 0.5 mL/kg/h for 6 h | * Enzymatic assay * Automated biochemical analyzer |
| CysC | Glomerulus Proximal tubule | Blood/urine | * Early increase and related to renal function * The best diagnostic marker for CI-AKI | Not routinely available in all laboratories | ≥10% increase at 24 h | * ELISA * Nephelometric and turbidimetric assays |
| α1-m | Glomerulus Proximal tubule | Urine | * Considered as a sensitive indicator of impaired renal function * Associated with increased risk of kidney disease progression and all-cause mortality | Not routinely available in all laboratories | NA | * Immuno-nephelometric assay * Automated biochemical analyzer |
| β2-m | Glomerulus Proximal tubule | Blood/urine | * Routine measurement * Difference if the damage is of glomerular or tubular origin | * It is not a diagnostic test for any specific disease* Should be measured in blood and urine along with other renal function tests | >1.26 mg/dL at baseline | * ELISA * Nephelometric assays |
| Proteinuria | Glomerulus | Blood/urine | * Routine measurement * Cheap | Not a good prognostic or mortality biomarker | NA | * Colorimetric methods * Automated biochemical analyzer |
| MCP-1 | Renal cells | Urine | Reports the presence of inflammation | Not routinely used | NA | * ELISA * Bio-Plex |
| IL-18 | Proximal tubule Distal tubule Distal collecting tubule Tubular cells | Urine | * Early increase in AKI before renal function deterioration * Strong predictor of AKI in the subsequent 48 h * Sensitive biomarker of AKI especially post-cardiopulmonary bypass | * Not routinely used * Its use in CI-AKi is inconclusive | ≥25% increase at 24 h | * ELISA * Bio-Plex |
| NGAL | Glomerulus Proximal tubule Distal tubule | Blood/urine | * Increases its urinary concentration before creatinine * Predicts diagnosis and prognosis of CI-AKI | Not routinely used | ≥20 ng/mL uNGAL; ≥179 sNGAL | * ELISA * Immunoblotting * Turbidimetric assays |
| KIM-1 | Proximal tubule | Blood/urine | * Very sensitive and specific marker of proximal tubular kidney injury * Distinguishes ischemic acute tubular necrosis from prerenal azotemia | Not routinely used | >0.425 ng/mL | * ELISA * Immunoblotting |
| IGFBP7xTIMP-2 | Renal epithelial cells | Urine | * Strong predictor of AKI * Its early detection could facilitate new therapeutic and protective strategies | * Not validated in CI-AK * Not routinely available in all laboratories | > 0.3 is a strong predictor of AKI and a value > 2.0 indicates higher kidney stress and probable AKI within 12–24 h | NephroCheck® |
| L-FABP | Proximal tubule | Urine | * Useful predictive biomarker to detect CI-AKI onset before CM exposure * Sensitivity for predicting the need for dialysis | Not routinely used | ≥24.5 µg/g | ELISA |
| NAG | Proximal tubule Lysosomal enzymes | Urine | * Correlates with histological evidence of renal proximal tubule damage * Sensitive marker of tubular injury in AKI and CI-AKI | * Increased levels have been reported in a variety of non-clinical AKI conditions * Limited use as an AKI marker | NA | * ELISA * Spectrophotometric assays |
| α-GST/π-GST | Proximal tubule Distal tubule | Urine | Promising biomarkers for early detection of AKI | * Not routinely used * Limited studies in CI-AKI | NA | * ELISA * Bio-Plex |
| Renalase | Proximal tubule | Blood/urine | In animal models, its increase is related to renal improvement | * Not routinely used * Limited studies in CI-AKI | NA | ELISA |
| CTGF | All tissues | Urine | Important profibrotic biomarker upregulated in AKI | * Not routinely used * Limited studies in CI-AKI | NA | ELISA |
| Uromodulin | Distal tubule Loop of Henle | Urine | Lower Uro/Crea ratio is associated with AKI after cardiac surgery | * Not routinely used * Limited studies in CI-AKI | NA | * ELISA * Bio-Plex |
| VEGF | Glomerulus Proximal tubule | Blood | Its increase is related to advanced kidney damage | * Not routinely used * Limited studies in CI-AKI | NA | * ELISA * Bio-Plex |
| OPN | Proximal tubule Loop of Henle Distal tubule | Blood | It is associated with the risk of AKI in patients undergoing CA | Not routinely used | NA | * ELISA * Bio-Plex |
| Hepcidin | Glomerulus | Blood/urine | Early changes could predict a later CI-AKI onset | Not routinely used | NA | * ELISA * Dot blot * SELDI-TOF MS |
| RBP | Glomerulus Proximal tubule | Blood/urine | * Rapidly elevated, useful for the diagnosis and monitoring of kidney disease progression * Biomarker of proximal tubular dysfunction. Used as a diagnostic tool in some proximal tubulopathies and renal interstitial diseases | Not routinely available in all laboratories | NA | ELISA |
| VDBP | Glomerulus Proximal tubule | Urine | Possible predictor of dialysis use and CM-associated death. | Not routinely used | NA | ELISA |
| GGT | Proximal tubule | Urine | Predictive value for CI-AKI progression | Not routinely used | NA | ELISA |
| Midkine | Proximal tubule Distal tubule | Blood | Early increase in CI-AKI | Not routinely used | NA | ELISA |
| miRNAs | Preinjury biomarkers | Blood/urine | Early potential biomarkers for CI-AKI | * Not routinely used * The role of circRNAs or lncRNAs in CI-AKI remains unknown * More studies are needed to verify the putative miRNA-mRNA pairs in CI-AKI, as well as the interaction mechanisms and downstream signaling pathways | NA | * RT-qPCR * RNA-seq technology |
| Clusterin | Proximal tubule Distal tubule | Urine | * Increases after treatment with CM * Early detection of nephrotoxicity and prediction of AKI | * Not routinely used * Few studies in CI-AKI | NA | Bio-Plex |
| Calbindin | Distal tubule Collecting duct tubule | Urine | Elevated levels of nephrotoxicity | * Not routinely used * No studies in CI-AKI | NA | * ELISA * Arrays * Bio-Plex |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Nicolás, M.Á.; González-Guerrero, C.; Goicoechea, M.; Boscá, L.; Valiño-Rivas, L.; Lázaro, A. Biomarkers in Contrast-Induced Acute Kidney Injury: Towards A New Perspective. Int. J. Mol. Sci. 2024, 25, 3438. https://doi.org/10.3390/ijms25063438
González-Nicolás MÁ, González-Guerrero C, Goicoechea M, Boscá L, Valiño-Rivas L, Lázaro A. Biomarkers in Contrast-Induced Acute Kidney Injury: Towards A New Perspective. International Journal of Molecular Sciences. 2024; 25(6):3438. https://doi.org/10.3390/ijms25063438
Chicago/Turabian StyleGonzález-Nicolás, María Ángeles, Cristian González-Guerrero, Marian Goicoechea, Lisardo Boscá, Lara Valiño-Rivas, and Alberto Lázaro. 2024. "Biomarkers in Contrast-Induced Acute Kidney Injury: Towards A New Perspective" International Journal of Molecular Sciences 25, no. 6: 3438. https://doi.org/10.3390/ijms25063438
APA StyleGonzález-Nicolás, M. Á., González-Guerrero, C., Goicoechea, M., Boscá, L., Valiño-Rivas, L., & Lázaro, A. (2024). Biomarkers in Contrast-Induced Acute Kidney Injury: Towards A New Perspective. International Journal of Molecular Sciences, 25(6), 3438. https://doi.org/10.3390/ijms25063438







