Snail Transcriptionally Represses Brachyury to Promote the Mesenchymal-Epithelial Transition in Ascidian Notochord Cells
Abstract
1. Introduction
2. Results
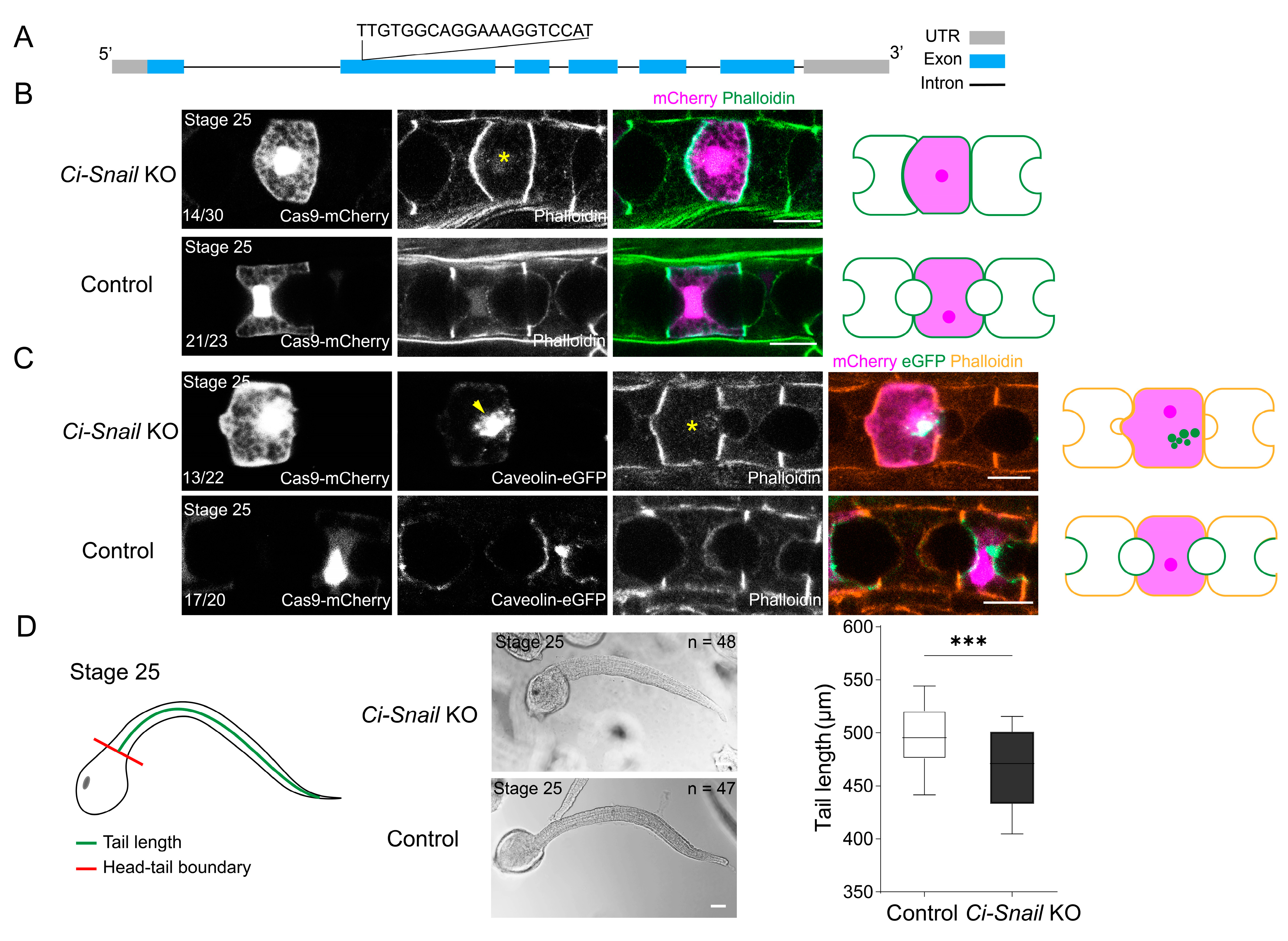
2.1. Snail Plays a Critical Role in Ciona Notochord MET
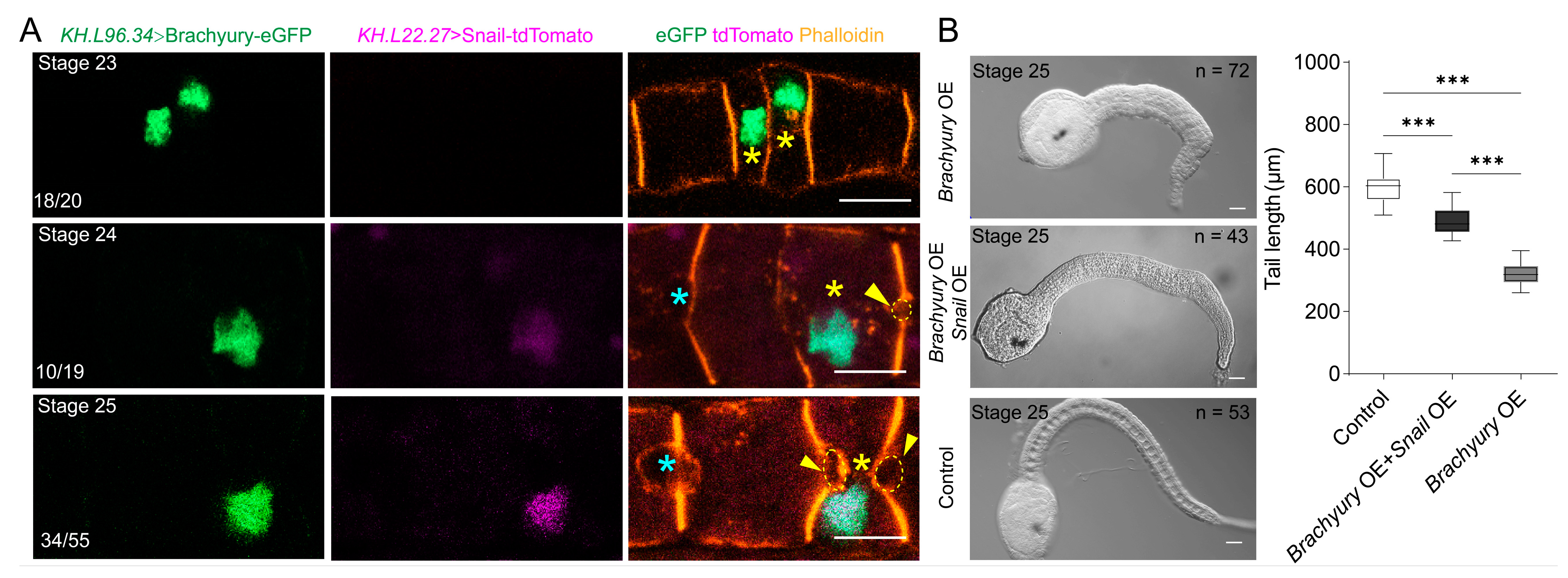
2.2. Brachyury Overexpression Affects Ciona Notochord Cell MET
2.3. Ci-Snail OE Partially Rescue the Delayed MET Caused by Brachyury OE
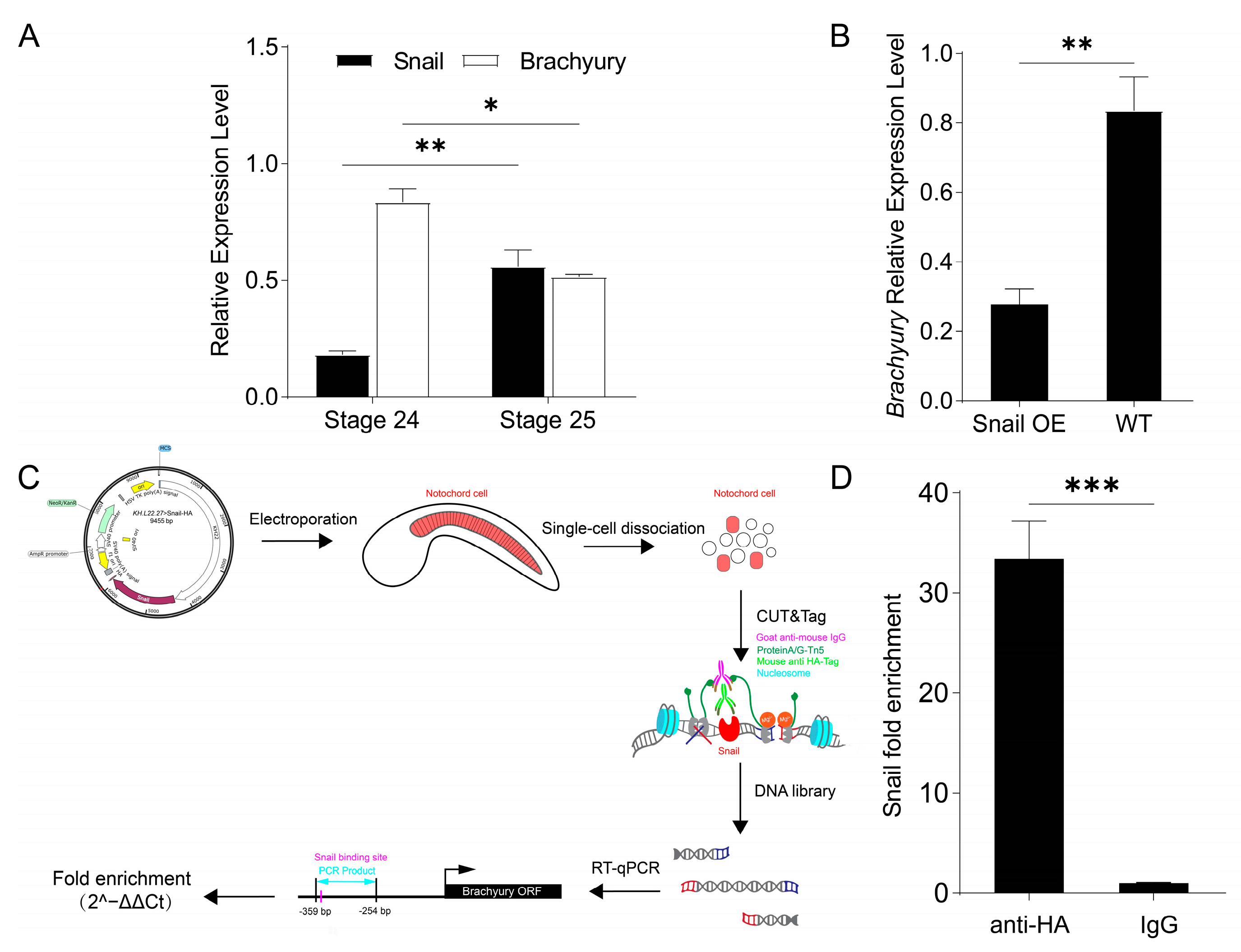
2.4. Ci-Snail Transcriptionally Represses Brachyury Expression to Regulate Notochord MET
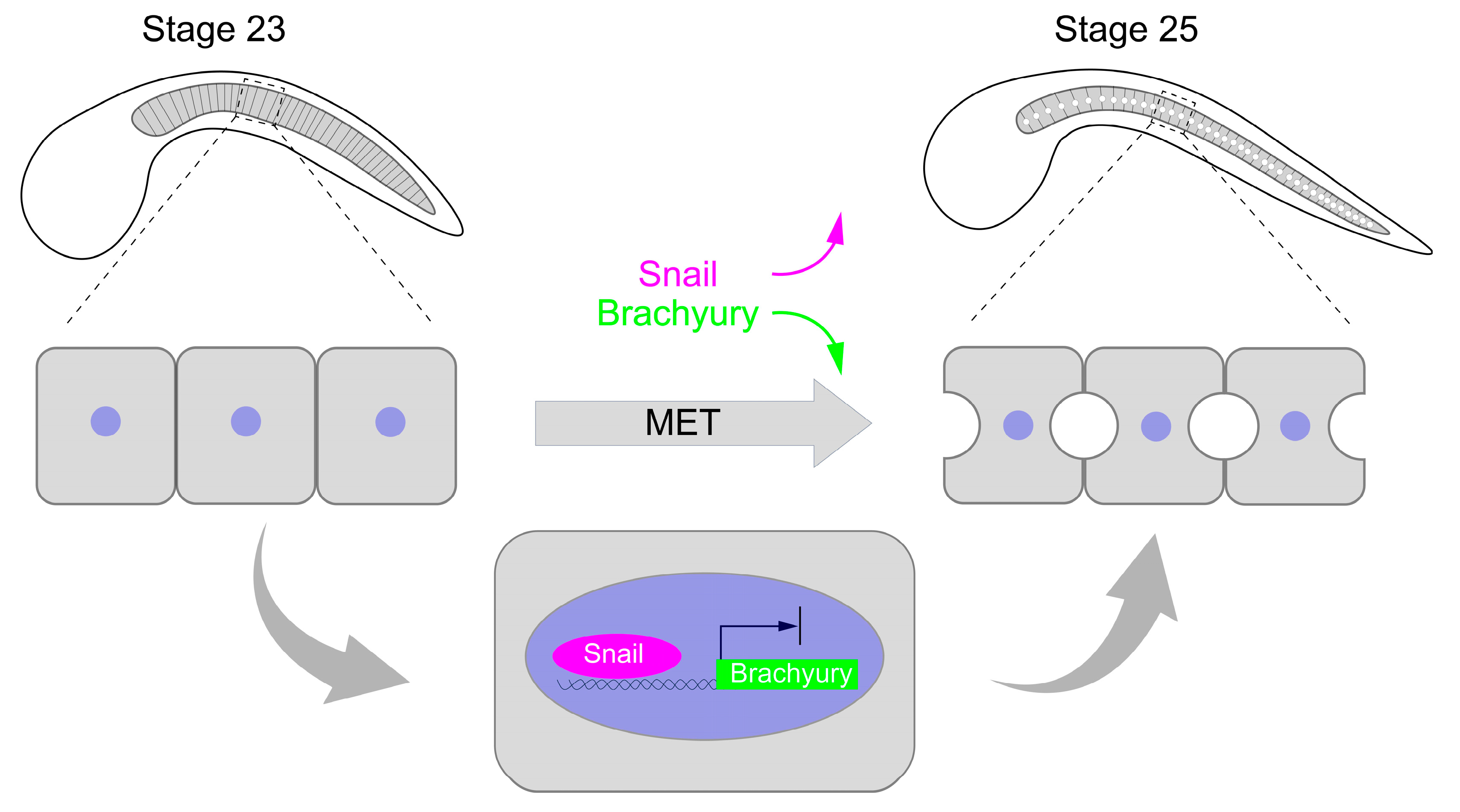
3. Discussion
4. Materials and Methods
4.1. Animal Breeding and Electroporation Experiments
4.2. Phylogenetic Analysis and Protein Domain Analysis
4.3. Immunofluorescence
4.4. Plasmid Construction
4.5. Gene Knockout by CRISPR/Cas9
4.6. RNA Extraction and RT-qPCR
4.7. CUT&Tag-qPCR
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pei, D.; Shu, X.; Gassama-Diagne, A.; Thiery, J.P. Mesenchymal–epithelial transition in development and reprogramming. Nat. Cell Biol. 2019, 21, 44–53. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Overeem, A.W.; Bryant, D.M.; van Ijzendoorn, S.C.D. Mechanisms of apical–basal axis orientation and epithelial lumen positioning. Trends Cell Biol. 2015, 25, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ocampo, A.; Belmonte, J.C.I. Cellular Metabolism and Induced Pluripotency. Cell 2016, 166, 1371–1385. [Google Scholar] [CrossRef]
- Grau, Y.; Carteret, C.; Simpson, P. Mutations and Chromosomal Rearrangements Affecting the Expression of Snail, a Gene Involved in Embryonic Patterning in Drosophila Melanogaster. Genetics 1984, 108, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Kosman, D.; Ip, Y.T.; Levine, M.; Arora, K. Establishment of the mesoderm-neuroectoderm boundary in the Drosophila embryo. Science 1991, 254, 118–122. [Google Scholar] [CrossRef]
- Nieto, M.A. The Snail superfamily of zinc-finger transcription factors. Nat. Rev. Mol. Cell Biol. 2002, 3, 155–166. [Google Scholar] [CrossRef]
- Corbo, C.J.; Albert, E.; Di, G.A. Dorsoventral patterning of the vertebrate neural tube is conserved in a protochordate. Development 1997, 124, 2335–2344. [Google Scholar] [CrossRef] [PubMed]
- Peinado, H.; Ballestar, E.; Esteller, M.; Cano, A. Snail Mediates E-Cadherin Repression by the Recruitment of the Sin3A/Histone Deacetylase 1 (HDAC1)/HDAC2 Complex. Mol. Cell. Biol. 2004, 24, 306–319. [Google Scholar] [CrossRef]
- Fernando, R.I.; Litzinger, M.; Trono, P.; Hamilton, D.H.; Schlom, J.; Palena, C. The T-box transcription factor Brachyury promotes epithelial-mesenchymal transition in human tumor cells. J. Clin. Investig. 2010, 120, 533–544. [Google Scholar] [CrossRef]
- Showell, C.; Binder, O.; Conlon, F.L. T-box Genes in Early Embryogenesis. Dev. Dyn. 2004, 229, 201–218. [Google Scholar] [CrossRef]
- Fujiwara, S.; Corbo, J.C.; Levine, M. The Snail repressor establishes a muscle/notochord boundary in the Ciona embryo. Development 1998, 125, 2511–2520. [Google Scholar] [CrossRef]
- Lu, Q.; Bhattachan, P.; Dong, B. Ascidian notochord elongation. Dev. Biol. 2019, 448, 147–153. [Google Scholar] [CrossRef]
- Peng, H.; Qiao, R.; Dong, B. Polarity Establishment and Maintenance in Ascidian Notochord. Front. Cell Dev. Biol. 2020, 8, 597446. [Google Scholar] [CrossRef]
- Denker, E.; Jiang, D. Ciona intestinalis notochord as a new model to investigate the cellular and molecular mechanisms of tubulogenesis. Semin. Cell Dev. Biol. 2012, 23, 308–319. [Google Scholar] [CrossRef]
- Dong, B.; Horie, T.; Denker, E.; Kusakabe, T.; Tsuda, M.; Smith, W.C.; Jiang, D. Tube formation by complex cellular processes in Ciona intestinalis notochord. Dev. Biol. 2009, 330, 237–249. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Li, Y.; Li, Y.; Dong, B.; Yu, H. Dynamics of Chromatin Opening across Larval Development in the Urochordate Ascidian Ciona savignyi. Int. J. Mol. Sci. 2024, 25, 2793. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.; Ayyanathan, K. Snail/Gfi-1 (SNAG) family zinc finger proteins in transcription regulation, chromatin dynamics, cell signaling, development, and disease. Cytokine Growth Factor Rev. 2013, 24, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Bhattachan, P.; Rae, J.; Yu, H.; Jung, W.; Wei, J.; Parton, R.G.; Dong, B. Ascidian caveolin induces membrane curvature and protects tissue integrity and morphology during embryogenesis. FASEB J. 2020, 34, 1345–1361. [Google Scholar] [CrossRef] [PubMed]
- Chiba, S.; Jiang, D.; Satoh, N.; Smith, W.C. Brachyury null mutant-induced defects in juvenile ascidian endodermal organs. Development 2009, 136, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Hotta, K.; Mitsuhara, K.; Takahashi, H.; Inaba, K.; Oka, K.; Gojobori, T.; Ikeo, K. A web-based interactive developmental table for the ascidian Ciona intestinalis, including 3D real-image embryo reconstructions: I. From fertilized egg to hatching larva. Dev. Dyn. 2007, 236, 1790–1805. [Google Scholar] [CrossRef]
- Cao, C.; Lemaire, L.A.; Wang, W.; Yoon, P.H.; Choi, Y.A.; Parsons, L.R.; Matese, J.C.; Wang, W.; Levine, M.; Chen, K. Comprehensive single-cell transcriptome lineages of a proto-vertebrate. Nature 2019, 571, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Dai, C.; Peng, S. Mechanism of the Mesenchymal–Epithelial Transition and Its Relationship with Metastatic Tumor Formation. Mol. Cancer Res. 2011, 9, 1608–1620. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, Z.; Li, Y.; Lu, T.; Hu, G. Silencing Snail Reverses Epithelial-Mesenchymal Transition and Increases Radiosensitivity in Hypopharyngeal Carcinoma. OncoTargets Ther. 2020, 13, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhou, B.P. Snail: More than EMT. Cell Adh. Migr. 2010, 4, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Gingold, J.A.; Fidalgo, M.; Guallar, D.; Lau, Z.; Sun, Z.; Zhou, H.; Faiola, F.; Huang, X.; Lee, D.F.; Waghray, A.; et al. A Genome-wide RNAi Screen Identifies Opposing Functions of Snai1 and Snai2 on the Nanog Dependency in Reprogramming. Mol. Cell 2014, 56, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Kudo-Saito, C.; Shirako, H.; Takeuchi, T.; Kawakami, Y. Cancer Metastasis Is Accelerated through Immunosuppression during Snail-Induced EMT of Cancer Cells. Cancer Cell 2009, 15, 195–206. [Google Scholar] [CrossRef]
- Zhu, J.; Kwan, K.M.; Mackem, S. Putative oncogene Brachyury (T) is essential to specify cell fate but dispensable for notochord progenitor proliferation and EMT. Proc. Natl. Acad. Sci. USA 2016, 113, 3820–3825. [Google Scholar] [CrossRef]
- Hemavathy, K.; Guru, S.C.; Harris, J.; Chen, J.D.; Ip, Y.T. Human Slug Is a Repressor That Localizes to Sites of Active Transcription. Mol. Cell. Biol. 2000, 55, 5087–5095. [Google Scholar] [CrossRef]
- Mezzasalma, M.; Andreone, F.; Glaw, F.; Guarino, F.M.; Odierna, G.; Petraccioli, A.; Picariello, O. Changes in heterochromatin content and ancient chromosome fusion. Salamandra 2019, 55, 140–144. [Google Scholar]
- Andrés, P.-P.; Mayra, F.-M. Heterochromatin as an Important Driver of Genome Organization. Front. Cell Dev. Biol. 2020, 8, 579137. [Google Scholar]
- Nibu, Y.; Zhang, H.; Bajor, E.; Barolo, S.; Small, S.; Levine, M. dCtBP mediates transcriptional repression by Knirps, Krüppel and Snail in the Drosophila embryo. EMBO J. 1998, 17, 7009–7020. [Google Scholar] [CrossRef] [PubMed]
- Lechner, M.S.; Dressler, G.R. The molecular basis of embryonic kidney development. Mech. Dev. 1997, 62, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Corbo, J.C.; Levine, M.; Zeller, R.W. Characterization of a notochord-specific enhancer from the Brachyury promoter region of the ascidian, Ciona intestinalis. Development 1997, 124, 589–602. [Google Scholar] [CrossRef]
- Stolfi, A.; Gandhi, S.; Salek, F.; Christiaen, L. Tissue-specific genome editing in Ciona embryos by CRISPR/Cas9. Development 2014, 141, 4115–4120. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, B.; Ouyang, X.; Yang, X.; Dong, B. Snail Transcriptionally Represses Brachyury to Promote the Mesenchymal-Epithelial Transition in Ascidian Notochord Cells. Int. J. Mol. Sci. 2024, 25, 3413. https://doi.org/10.3390/ijms25063413
Wu B, Ouyang X, Yang X, Dong B. Snail Transcriptionally Represses Brachyury to Promote the Mesenchymal-Epithelial Transition in Ascidian Notochord Cells. International Journal of Molecular Sciences. 2024; 25(6):3413. https://doi.org/10.3390/ijms25063413
Chicago/Turabian StyleWu, Bingtong, Xiuke Ouyang, Xiuxia Yang, and Bo Dong. 2024. "Snail Transcriptionally Represses Brachyury to Promote the Mesenchymal-Epithelial Transition in Ascidian Notochord Cells" International Journal of Molecular Sciences 25, no. 6: 3413. https://doi.org/10.3390/ijms25063413
APA StyleWu, B., Ouyang, X., Yang, X., & Dong, B. (2024). Snail Transcriptionally Represses Brachyury to Promote the Mesenchymal-Epithelial Transition in Ascidian Notochord Cells. International Journal of Molecular Sciences, 25(6), 3413. https://doi.org/10.3390/ijms25063413






