Chicoric Acid Ameliorated Beta-Amyloid Pathology and Enhanced Expression of Synaptic-Function-Related Markers via L1CAM in Alzheimer’s Disease Models
Abstract
1. Introduction
2. Results
2.1. CA Reduced Aβ1–42, APP and BACE1 Expression Levels in APPswe/Ind Transgenic SH-SY5Y Cells
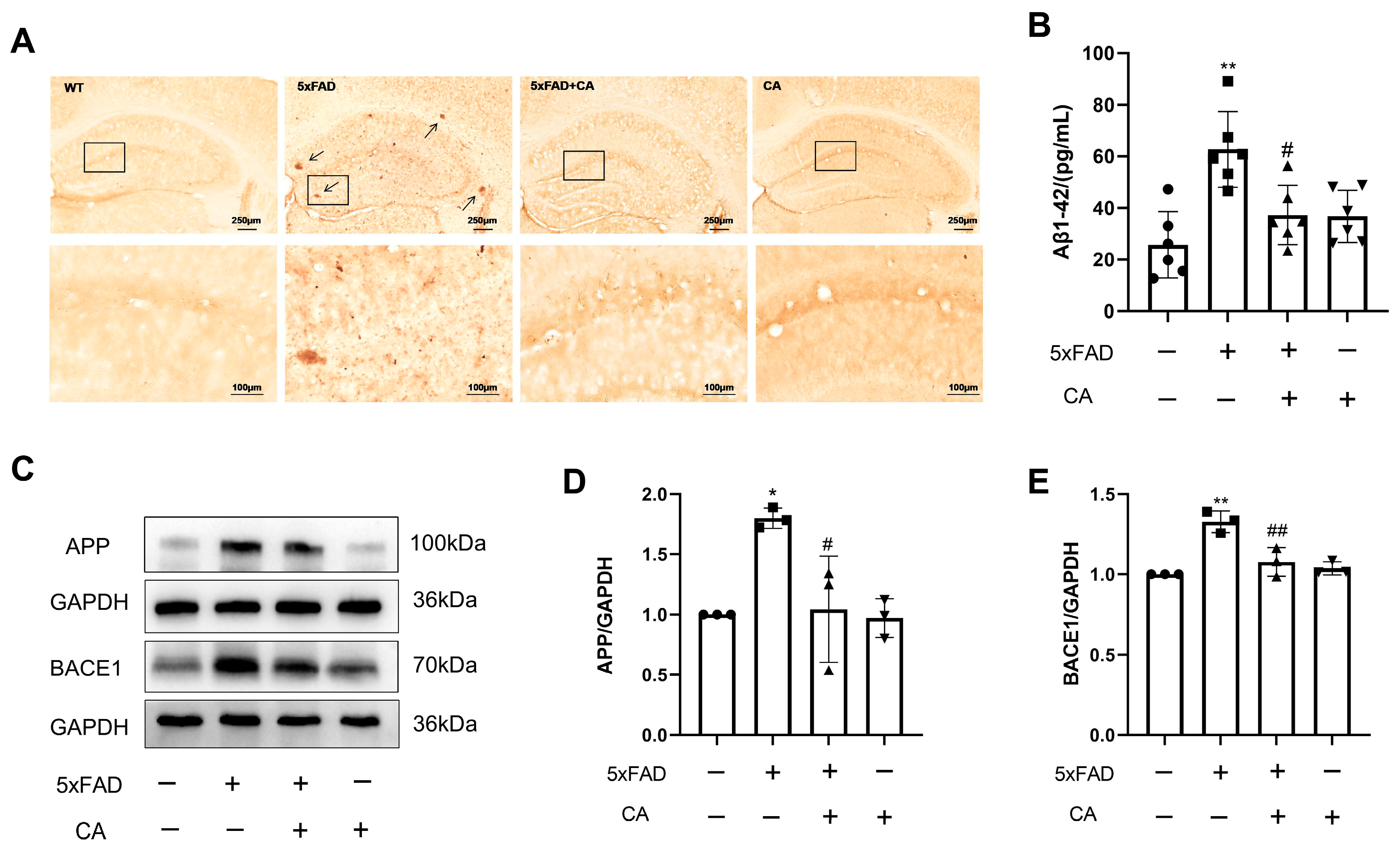
2.2. CA Decreased the Amyloid-β Plaque Load and Levels of APP and BACE1 in 5xFAD Mice
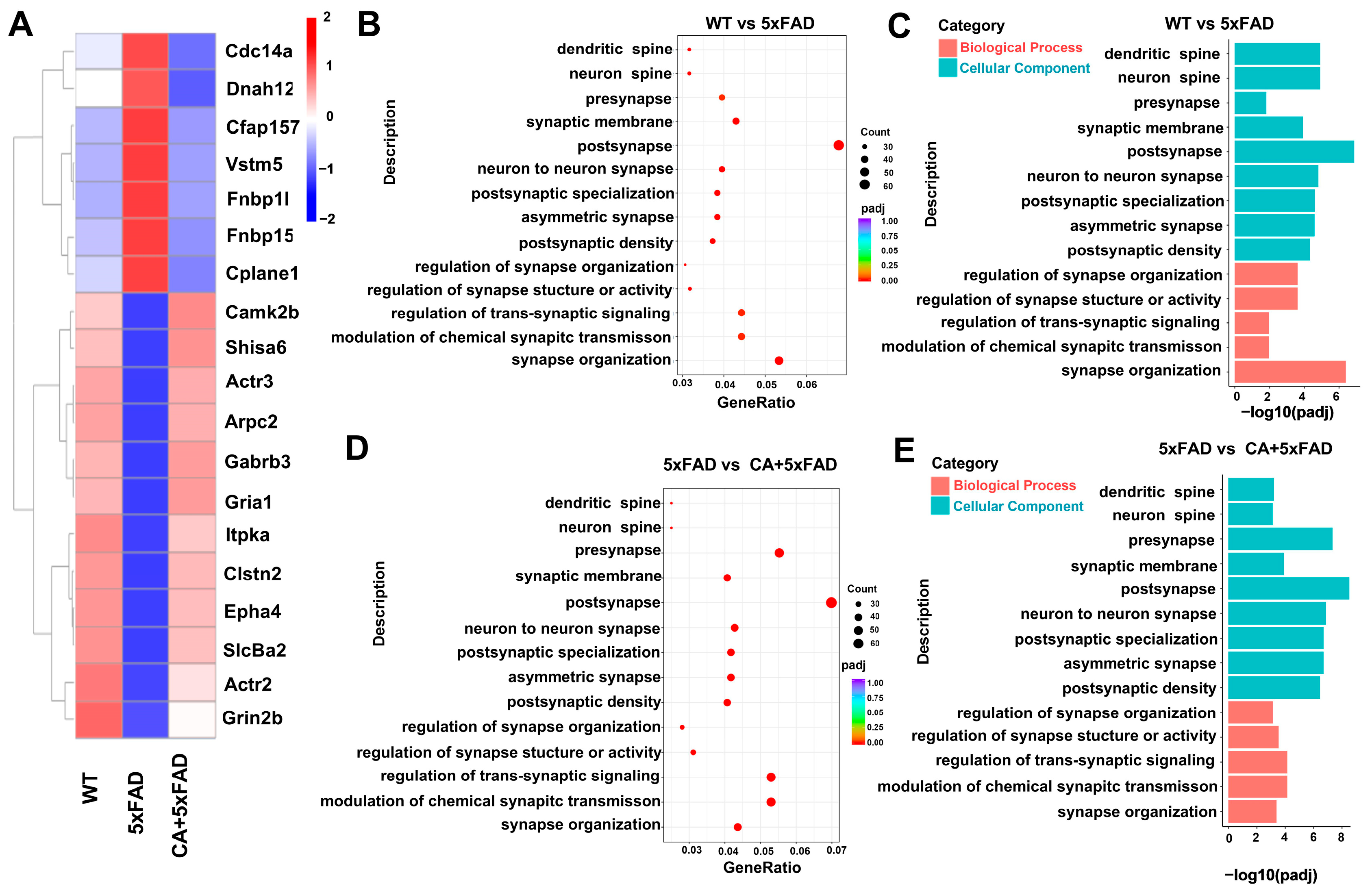
2.3. Transcriptomic and Bioinformatic Analyses of the Hippocampi in 5xFAD Mice
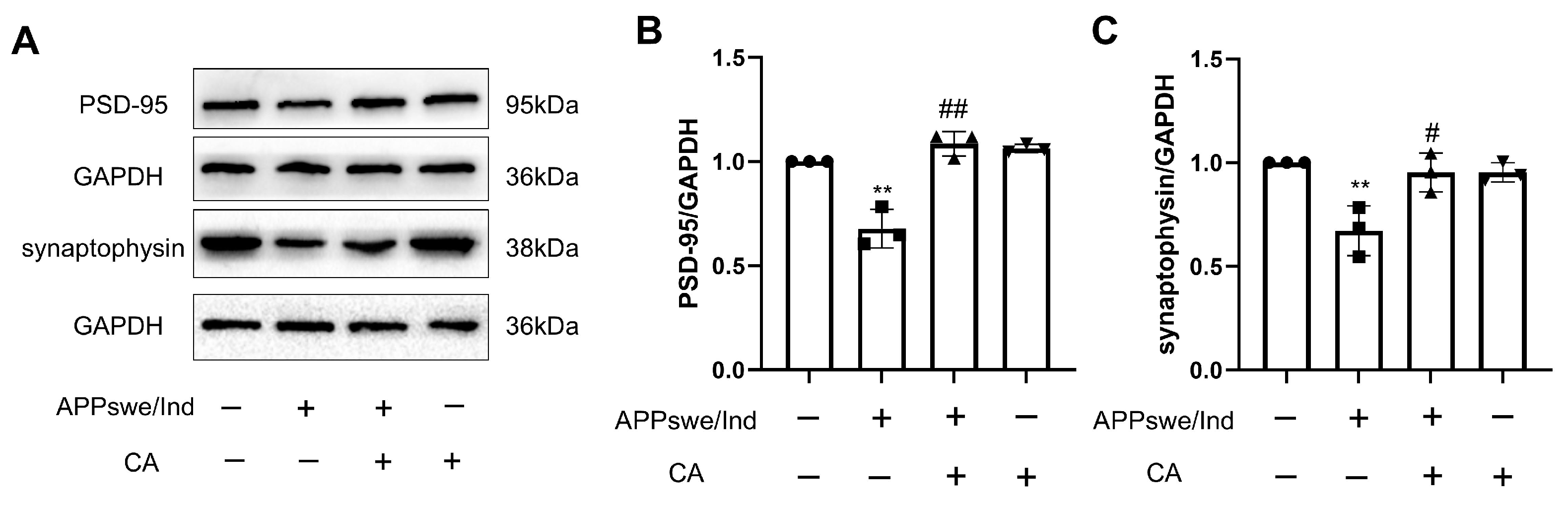
2.4. CA Increased Synaptic Density in APPswe/Ind Transgenic SH-SY5Y Cells
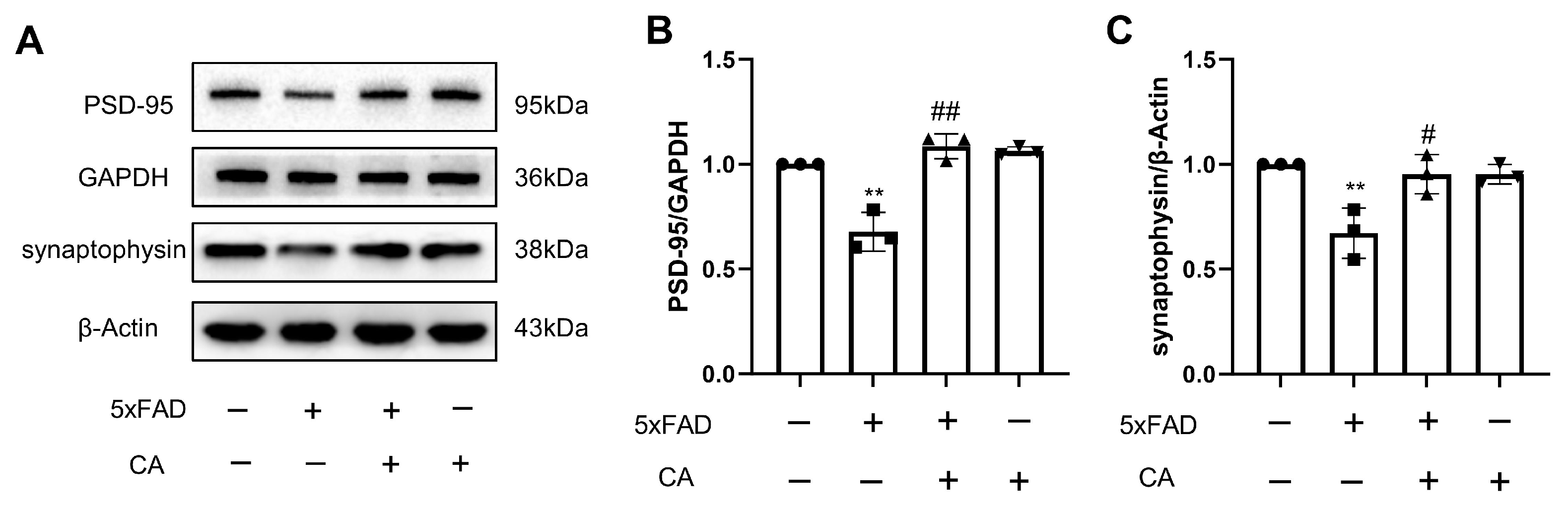
2.5. CA Enhanced Hippocampal Synaptic Density in 5xFAD Mice
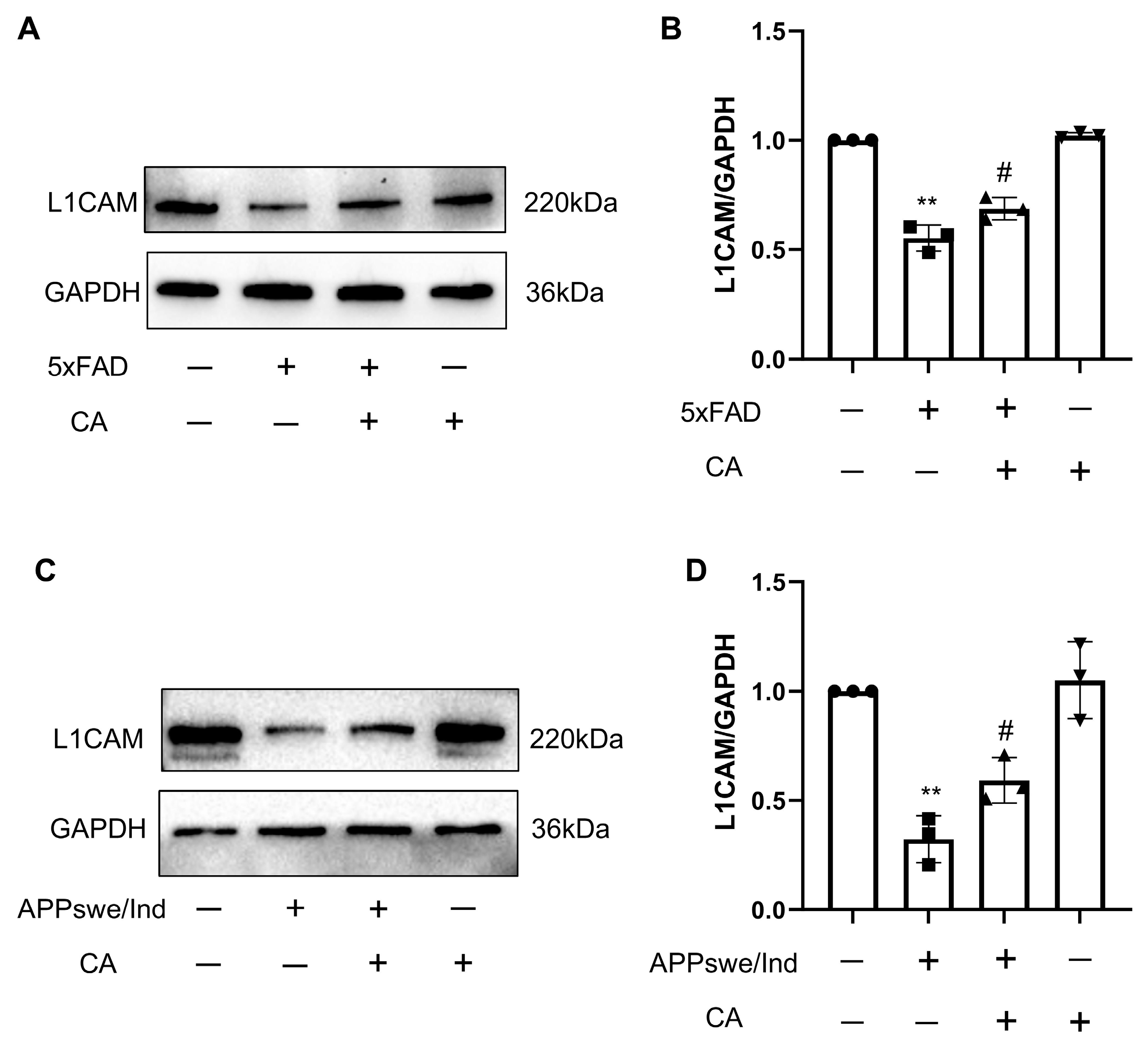
2.6. CA Treatment Increased L1 Levels in APPswe/Ind Transgenic SH-SY5Y Cells and 5xFAD Mice
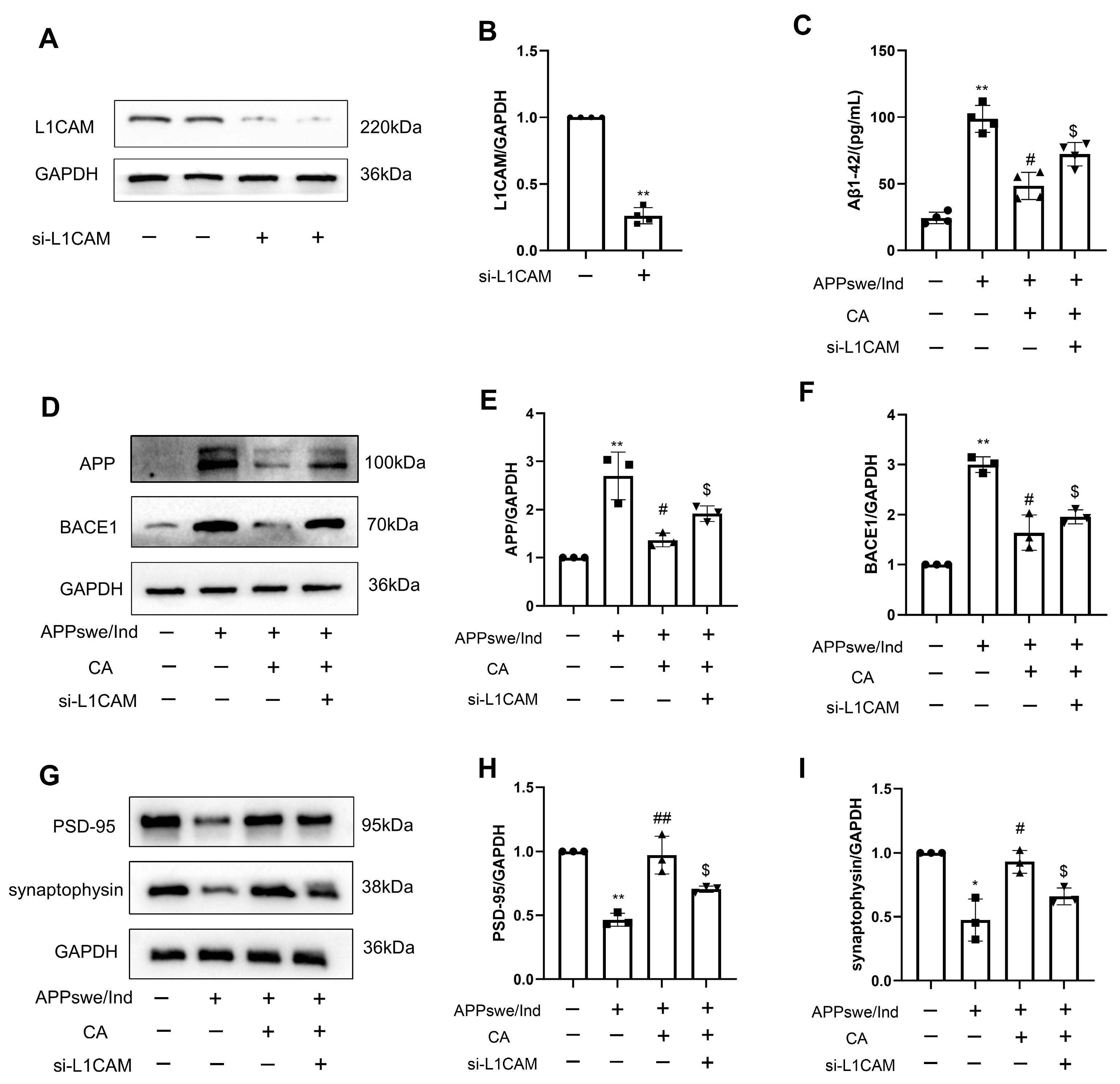
2.7. siL1-RNA Affects CA-Regulated Levels of AD- and Synapse-Related Proteins in APPswe/Ind Transgenic SH-SY5Y Cells and 5xFAD Mice
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture and Treatment
4.3. Mice and Treatments
4.4. Western Blot Analysis
4.5. Quantitative Real-Time Polymerase Chain RDXeaction (qRT-PCR)
4.6. Immunohistochemistry
4.7. Enzyme-Linked Immunosorbent Assay (ELISA) Analysis
4.8. Bioinformatics Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| Aβ | Amyloid-beta |
| CA | Chicoric acid |
| WHO | World Health Organization |
| APP | Amyloid precursor protein |
| BACE1 | β-secretase |
| MPTP | N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| PD | Parkinson’s disease |
| LPS | Lipopolysaccharides |
| Swe/Ind | Swedish/Indiana |
| PSD-95 | Postsynaptic density protein 95 |
| L1CAM | L1 cell adhesion molecule |
| WT | Wild-type |
| PBS | Phosphate-buffered saline |
| RIPA | Radio immunoprecipitation assay lysis buffer |
| SDS-PAGE | Sodium dodecyl sulfate–polyacrylamide gel electrophoresis |
| PVDF | Polyvinylidene fluoride |
| HRP | Horseradish peroxidase |
| ECL | Enhanced chemiluminescence |
| qRT-PCR | Quantitative real-time polymerase chain reaction |
| ELISA | Enzyme-linked immunosorbent assay |
References
- Weller, J.; Budson, A. Current understanding of Alzheimer’s disease diagnosis and treatment. F1000Research 2018, 7, 1161. [Google Scholar] [CrossRef]
- Brody, H. Alzheimer’s disease. Nature 2011, 475, S1. [Google Scholar] [CrossRef]
- Ogbodo, J.O.; Agbo, C.P.; Njoku, U.O.; Ogugofor, M.O.; Egba, S.I.; Ihim, S.A.; Echezona, A.C.; Brendan, K.C.; Upaganlawar, A.B.; Upasani, C.D. Alzheimer’s Disease: Pathogenesis and Therapeutic Interventions. Curr. Aging Sci. 2022, 15, 2–25. [Google Scholar] [CrossRef] [PubMed]
- Roselli, S.; Satir, T.M.; Camacho, R.; Fruhwürth, S.; Bergström, P.; Zetterberg, H.; Agholme, L. APP-BACE1 Interaction and Intracellular Localization Regulate Aβ Production in iPSC-Derived Cortical Neurons. Cell. Mol. Neurobiol. 2023, 43, 3653–3668. [Google Scholar] [CrossRef]
- Karran, E.; De Strooper, B. The amyloid hypothesis in Alzheimer disease: New insights from new therapeutics. Nat. Rev. Drug Discov. 2022, 21, 306–318. [Google Scholar] [CrossRef]
- Deng, C.; Chen, H.; Meng, Z.; Meng, S. Roles of traditional chinese medicine regulating neuroendocrinology on AD treatment. Front. Endocrinol. 2022, 13, 955618. [Google Scholar] [CrossRef]
- Peng, Y.; Sun, Q.C.; Park, Y. The Bioactive Effects of Chicoric Acid As a Functional Food Ingredient. J. Med. Food 2019, 22, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Hu, Y.; Cao, Y.; Song, G.; Liu, Z.; Liu, X. Chicoric Acid Ameliorates Lipopolysaccharide-Induced Oxidative Stress via Promoting the Keap1/Nrf2 Transcriptional Signaling Pathway in BV-2 Microglial Cells and Mouse Brain. J. Agric. Food Chem. 2017, 65, 338–347. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, Y.; Shen, C.; Xiao, Y.; Wang, Y.; Liu, Z.; Liu, X. Chicoric acid supplementation prevents systemic inflammation- induced memory impairment and amyloidogenesis via inhibition of NF-κB. FASEB J. 2017, 31, 1494–1507. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Sun, Q.; Gao, R.; Park, Y. AAK-2 and SKN-1 Are Involved in Chicoric-Acid-Induced Lifespan Extension in Caenorhabditis elegans. J. Agric. Food Chem. 2019, 67, 9178–9186. [Google Scholar] [CrossRef]
- Wang, N.; Li, R.; Feng, B.; Cheng, Y.; Guo, Y.; Qian, H. Chicoric Acid Prevents Neuroinflammation and Neurodegeneration in a Mouse Parkinson’s Disease Model: Immune Response and Transcriptome Profile of the Spleen and Colon. Int. J. Mol. Sci. 2022, 23, 2031. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Feng, B.-N.; Hu, B.; Cheng, Y.-L.; Guo, Y.-H.; Qian, H. Neuroprotection of chicoric acid in a mouse model of Parkinson’s disease involves gut microbiota and TLR4 signaling pathway. Food Funct. 2022, 13, 2019–2032. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhao, M.; Pu, Z.; Yin, Q.; Shui, Y. Chicoric Acid Presented NLRP3-Mediated Pyroptosis through Mitochondrial Damage by PDPK1 Ubiquitination in an Acute Lung Injury Model. Am. J. Chin. Med. 2023, 51, 1431–1457. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Bestard-Lorigados, I.; Song, W.H. The synapse as a treatment avenue for Alzheimer’s Disease. Mol. Psychiatry 2022, 27, 2940–2949. [Google Scholar] [CrossRef] [PubMed]
- Colombo, F.; Meldolesi, J. L1-CAM and N-CAM: From Adhesion Proteins to Pharmacological Targets. Trends Pharmacol. Sci. 2015, 36, 769–781. [Google Scholar] [CrossRef]
- Djogo, N.; Jakovcevski, I.; Müller, C.; Lee, H.J.; Xu, J.-C.; Jakovcevski, M.; Kügler, S.; Loers, G.; Schachner, M. Adhesion molecule L1 binds to amyloid beta and reduces Alzheimer’s disease pathology in mice. Neurobiol. Dis. 2013, 56, 104–115. [Google Scholar] [CrossRef]
- Tan, W.; Qi, L.; Hu, X.; Tan, Z. Research progress in traditional Chinese medicine in the treatment of Alzheimer’s disease and related dementias. Front. Pharmacol. 2022, 13, 921794. [Google Scholar] [CrossRef]
- Yan, H.; Feng, L.; Li, M. The Role of Traditional Chinese Medicine Natural Products in β-Amyloid Deposition and Tau Protein Hyperphosphorylation in Alzheimer’s Disease. Drug Des. Dev. Ther. 2023, 17, 3295–3323. [Google Scholar] [CrossRef]
- Pei, H.; Ma, L.; Cao, Y.; Wang, F.; Li, Z.; Liu, N.; Liu, M.; Wei, Y.; Li, H. Traditional Chinese Medicine for Alzheimer’s Disease and Other Cognitive Impairment: A Review. Am. J. Chin. Med. 2020, 48, 487–511. [Google Scholar] [CrossRef]
- Budd Haeberlein, S.; Aisen, P.; Barkhof, F.; Chalkias, S.; Chen, T.; Cohen, S.; Dent, G.; Hansson, O.; Harrison, K.; von Hehn, C.; et al. Two Randomized Phase 3 Studies of Aducanumab in Early Alzheimer’s Disease. J. Prev. Alzheimer’s Dis. 2022, 9, 197–210. [Google Scholar] [CrossRef]
- Huang, R.; Im, S.H. Dynamics of wrinkle growth and coarsening in stressed thin films. Phys. Rev. E Stat. Nonlin. Soft Matter. Phys. 2006, 74 Pt 2, 026214. [Google Scholar] [CrossRef] [PubMed]
- Hauptmann, S.; Scherping, I.; Dröse, S.; Brandt, U.; Schulz, K.L.; Jendrach, M.; Leuner, K.; Eckert, A.; Müller, W.E. Mitochondrial dysfunction: An early event in Alzheimer pathology accumulates with age in AD transgenic mice. Neurobiol. Aging 2009, 30, 1574–1586. [Google Scholar] [CrossRef]
- Yin, F.; Sancheti, H.; Patil, I.; Cadenas, E. Energy metabolism and inflammation in brain aging and Alzheimer’s disease. Free Radic. Biol. Med. 2016, 100, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Swain, B.C.; Subadini, S.; Rout, J.; Sakshi; Mishra, P.P.; Sahoo, H.; Tripathy, U. Biophysical study on complex formation between β-Lactoglobulin and vitamin B12. Food Chem. 2020, 312, 126064. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Q.; Jiang, X.F.; Ma, L.N.; Wei, W.; Li, Z.H.; Chang, S.R.; Wen, J.Y.; Sun, J.H.; Li, I. Role of Aβ in Alzheimer’s-related synaptic dysfunction. Front. Cell Dev. Biol. 2022, 10, 964075. [Google Scholar] [CrossRef] [PubMed]
- Dore, K.; Carrico, Z.; Alfonso, S.; Marino, M.; Koymans, K.; Kessels, H.W.; Malinow, R. PSD-95 protects synapses from β-amyloid. Cell Rep. 2021, 35, 109194. [Google Scholar] [CrossRef] [PubMed]
- Gylys, K.H.; Fein, J.A.; Yang, F.; Wiley, D.J.; Miller, C.A.; Cole, G.M. Synaptic changes in Alzheimer’s disease-beta: Increased amyloid-β and gliosis in surviving terminals is accompanied by decreased PSD-95 fluorescence. Am. J. Pathol. 2004, 165, 1809–1817. [Google Scholar] [CrossRef] [PubMed]
- Zhan, S.S.; Beyreuther, K.; Schmitt, H.P. Quantitative assessment of the synaptophysin immuno-reactivity of the cortical neuropil in various neurodegenerative disorders with dementia. Dementia 1993, 4, 66–74. [Google Scholar]
- Hu, J.; Lin, S.L.; Schachner, M. A fragment of cell adhesion molecule L1 reduces amyloid-beta plaques in a mouse model of Alzheimer’s disease. Cell Death Dis. 2022, 13, 48. [Google Scholar] [CrossRef]
- Yamanaka, H.; Okubo, M.; Kobayashi, K.; Noguchi, K. Aberrant Axo-Axonic Synaptic Reorganization in the Phosphorylated L1-CAM/Calcium Channel Subunit α2δ-1-Containing Central Terminals of Injured c-Fibers in the Spinal Cord of a Neuropathic Pain Model. Eneuro 2021, 8, 21. [Google Scholar] [CrossRef]
- Liu, Q.; Fang, J.; Chen, P.; Die, Y.; Wang, J.; Liu, Z.; Liu, X. Chicoric acid improves neuron survival against inflammation by promoting mitochondrial function and energy metabolism. Food Funct. 2019, 10, 6157–6169. [Google Scholar] [CrossRef] [PubMed]
- Birsa, M.L.; Sarbu, L.G. Health Benefits of Key Constituents in Cichorium intybus L. Nutrients 2023, 15, 1322. [Google Scholar] [CrossRef] [PubMed]
- Darrag, H.M.; Almuhanna, H.T.; Hakami, E.H. Secondary Metabolites in Basil, Bio-Insecticide, Inhibition Effect, and In Silico Molecular Docking against Proteolytic Enzymes of the Red PalmWeevil (Rhynchophorus ferrugineus). Plants 2022, 11, 1087. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.S.; Rai, S.N.; Birla, H.; Zahra, W.; Rathore, A.S.; Dilnashin, H.; Singh, R.; Singh, S.P. Neuroprotective Effect of Chlorogenic Acid on Mitochondrial Dysfunction-Mediated Apoptotic Death of DA Neurons in a Parkinsonian Mouse Model. Oxidative Med. Cell. Longev. 2020, 2020, 6571484. [Google Scholar] [CrossRef]
- Rai, S.N.; Yadav, S.K.; Singh, D.; Singh, S.P. Ursolic acid attenuates oxidative stress in nigrostriatal tissue and improves neurobehavioral activity in MPTP-induced Parkinsonian mouse model. J. Chem. Neuroanat. 2016, 71, 41–49. [Google Scholar] [CrossRef]

| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| APP | 5′-TGGAGGTACCCACTGATGGT-3′ | 5′-ACTGCATGTCTCTTTGGCGA-3′ |
| BACE1 | 5′-CAGGCTTGTTCTTCACAGGG-3′ | 5′-ACCACAAAGCCTGGCAATCTC-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Kang, S.; Zhao, Z.; Jin, L.; Cui, X.; Chen, L.; Schachner, M.; Li, S.; Guo, Y.; Zhao, J. Chicoric Acid Ameliorated Beta-Amyloid Pathology and Enhanced Expression of Synaptic-Function-Related Markers via L1CAM in Alzheimer’s Disease Models. Int. J. Mol. Sci. 2024, 25, 3408. https://doi.org/10.3390/ijms25063408
Wang R, Kang S, Zhao Z, Jin L, Cui X, Chen L, Schachner M, Li S, Guo Y, Zhao J. Chicoric Acid Ameliorated Beta-Amyloid Pathology and Enhanced Expression of Synaptic-Function-Related Markers via L1CAM in Alzheimer’s Disease Models. International Journal of Molecular Sciences. 2024; 25(6):3408. https://doi.org/10.3390/ijms25063408
Chicago/Turabian StyleWang, Ruonan, Shijia Kang, Zirui Zhao, Lingling Jin, Xiaolin Cui, Lili Chen, Melitta Schachner, Sheng Li, Yanjie Guo, and Jie Zhao. 2024. "Chicoric Acid Ameliorated Beta-Amyloid Pathology and Enhanced Expression of Synaptic-Function-Related Markers via L1CAM in Alzheimer’s Disease Models" International Journal of Molecular Sciences 25, no. 6: 3408. https://doi.org/10.3390/ijms25063408
APA StyleWang, R., Kang, S., Zhao, Z., Jin, L., Cui, X., Chen, L., Schachner, M., Li, S., Guo, Y., & Zhao, J. (2024). Chicoric Acid Ameliorated Beta-Amyloid Pathology and Enhanced Expression of Synaptic-Function-Related Markers via L1CAM in Alzheimer’s Disease Models. International Journal of Molecular Sciences, 25(6), 3408. https://doi.org/10.3390/ijms25063408







