Antibacterial Properties of Rose Bengal Conjugated to Hyaluronic Acid
Abstract
1. Introduction
2. Results and Discussion
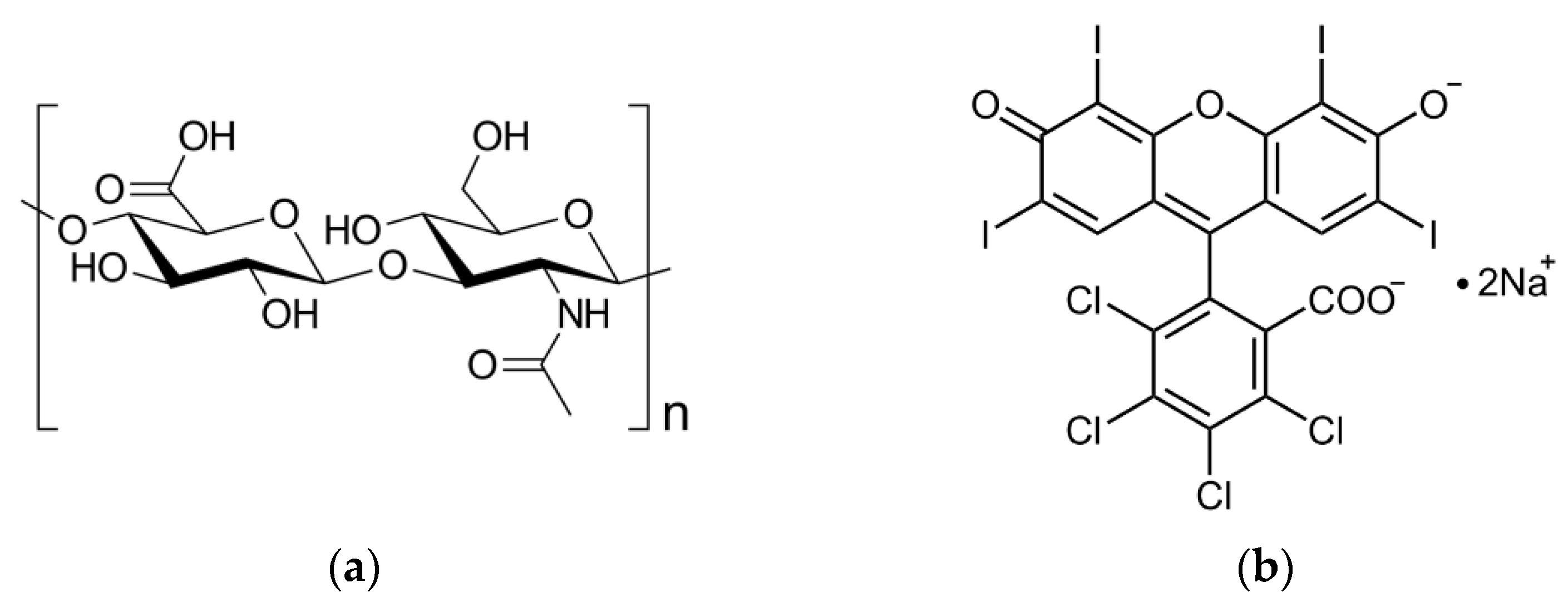
2.1. Characterization of RB-HA Conjugate
2.1.1. Composition of RB-HA Conjugate
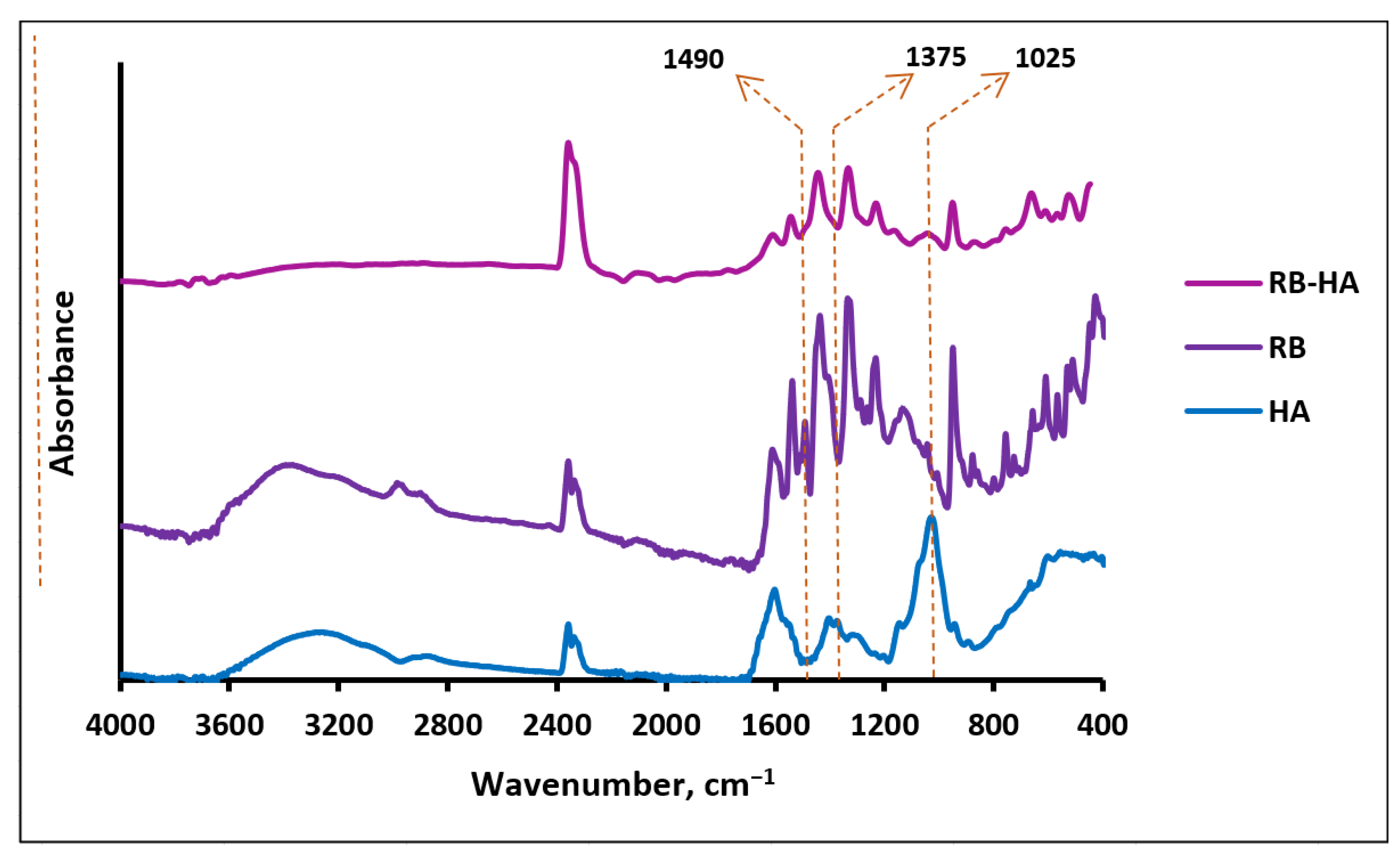
2.1.2. FTIR Assay of RB-HA
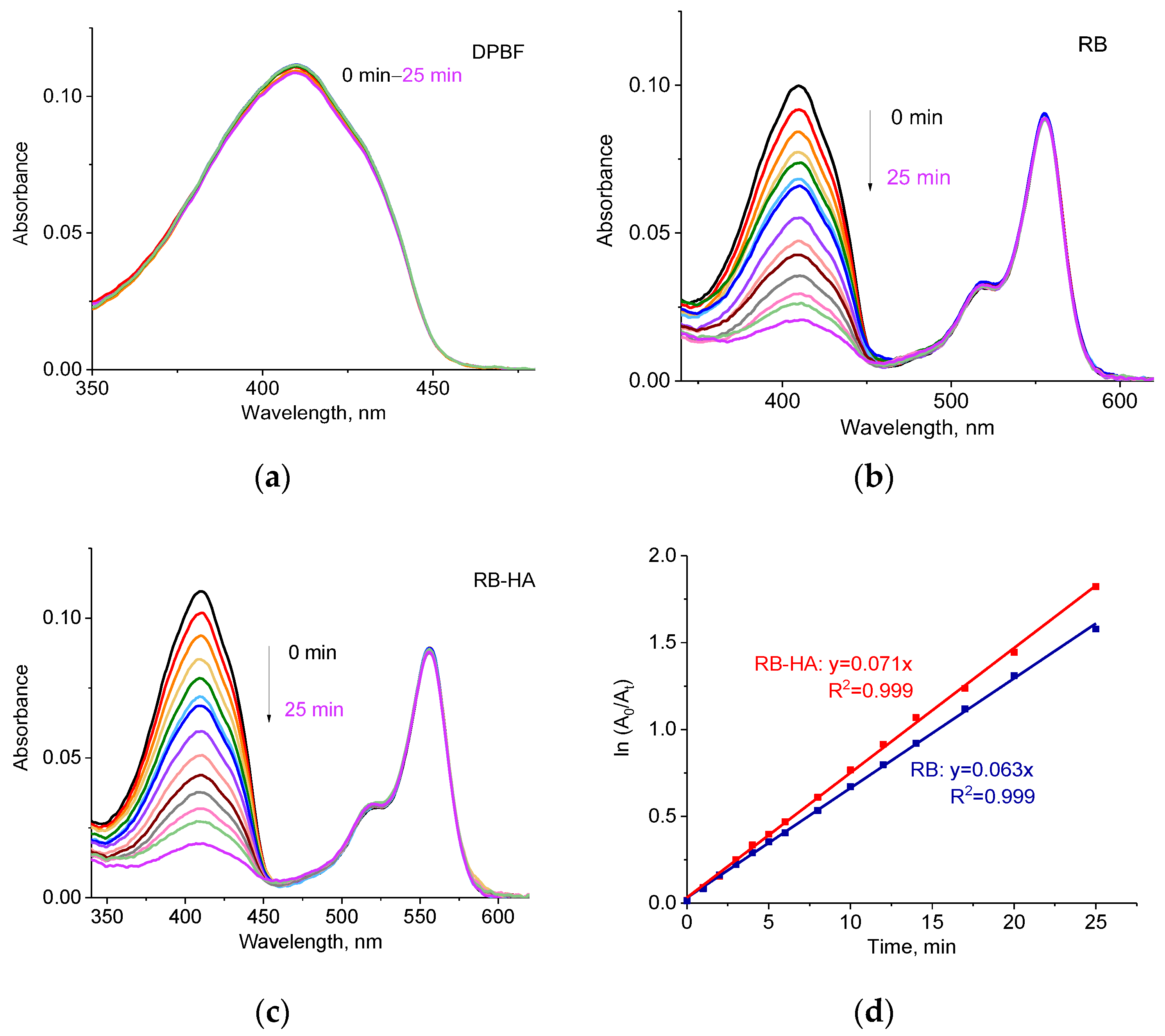
2.2. Generation ROS by RB-HA
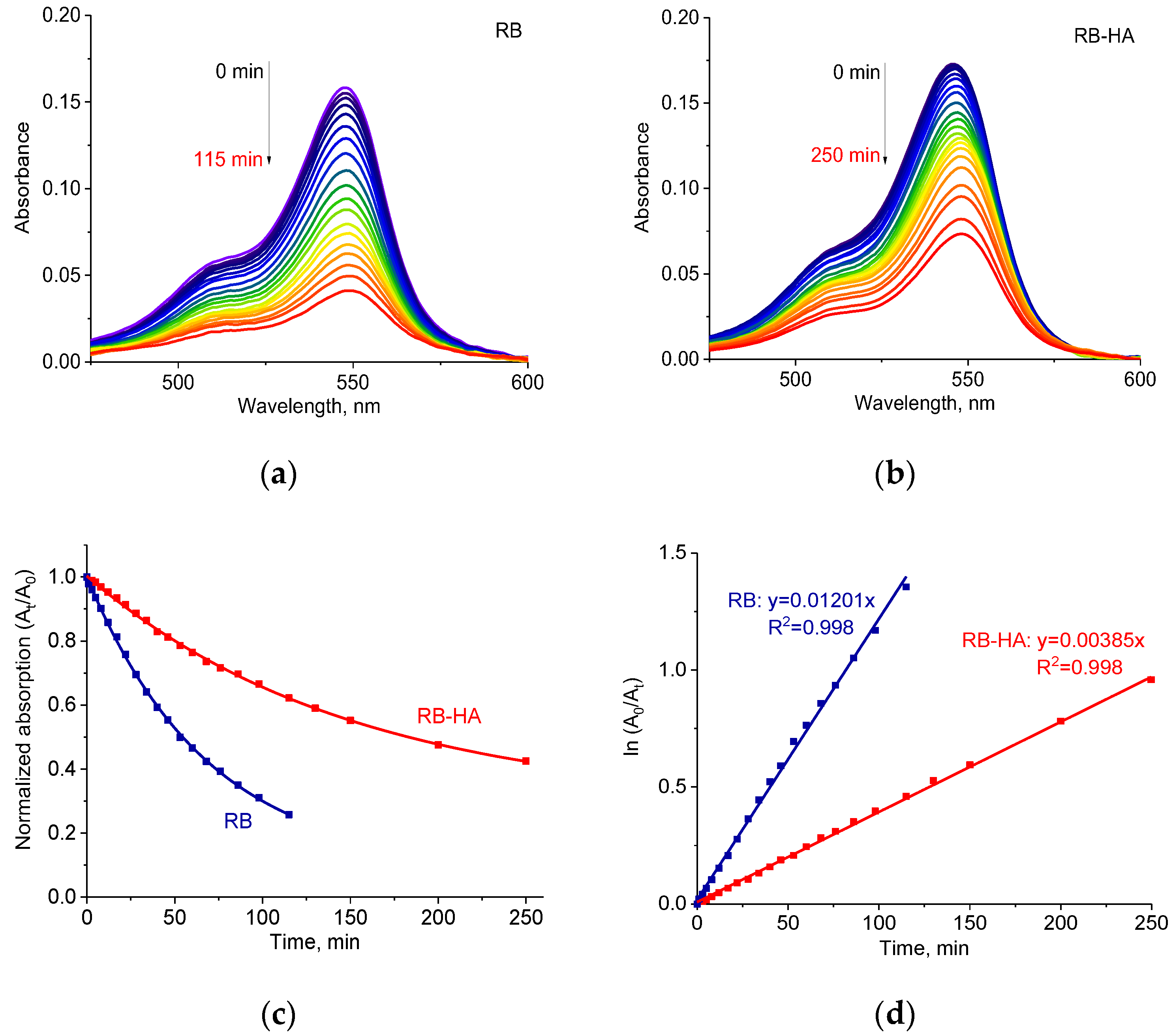
2.3. Photostability of RB-HA Conjugate
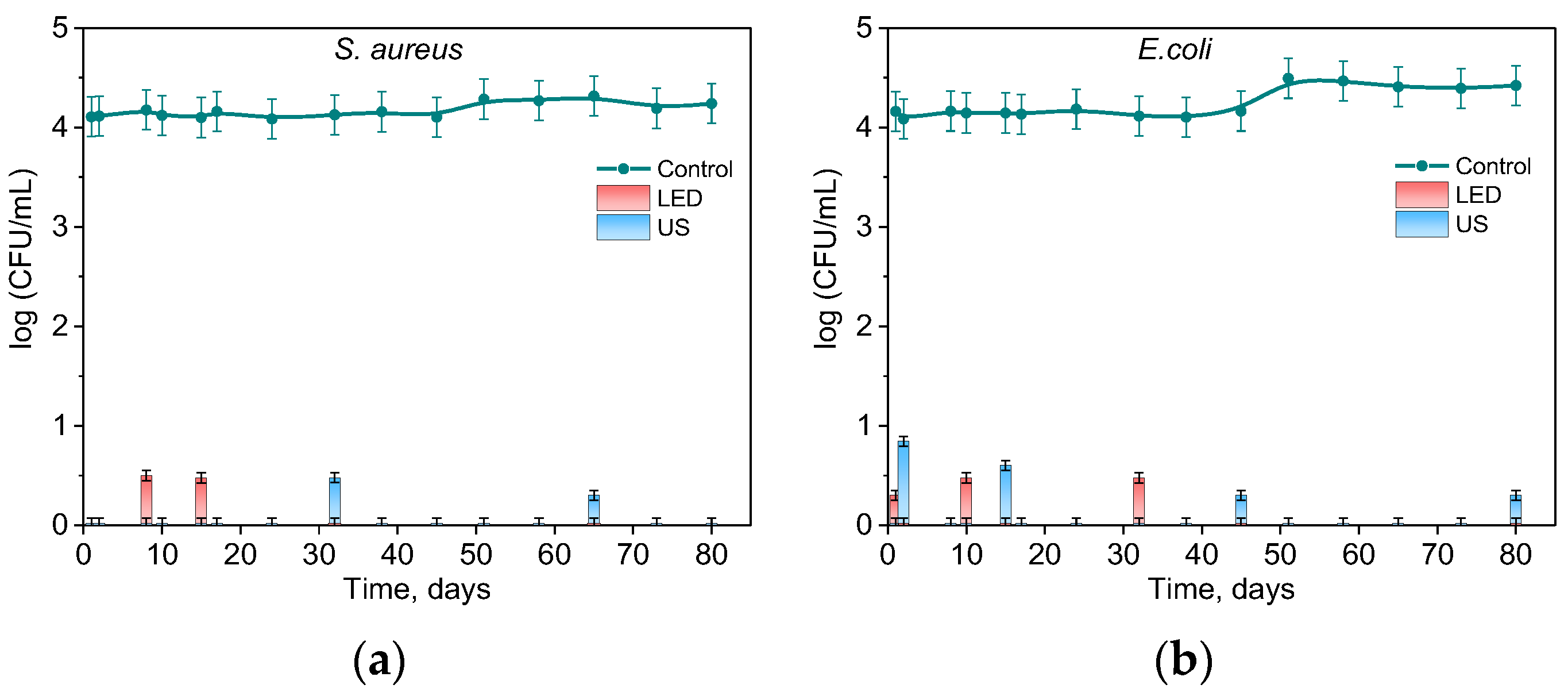
2.4. Photo- and Sonodynamic Activity of RB-HA
3. Methods and Materials
3.1. Synthesis and Characterization of RB-HA Conjugate
3.2. Checking the RB Load in the Conjugate
3.3. FTIR Analysis
3.4. Spectral Studies and Photostability Tests
3.5. Generation of ROS
3.6. Bacterial Growth
3.7. Antimicrobial Activity Test
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baelum, V. Dentistry and Population Approaches for Preventing Dental Diseases. J. Dent. 2011, 39, S9–S19. [Google Scholar] [CrossRef]
- Peres, M.A.; Macpherson, L.M.D.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreño, C.C.; Kearns, C.; et al. Oral Diseases: A Global Public Health Challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef]
- Kane, S.F. The Effects of Oral Health on Systemic Health. Gen. Dent. 2017, 65, 30–34. [Google Scholar]
- Laudenbach, J.M.; Kumar, S.S. Common Dental and Periodontal Diseases. Dermatol. Clin. 2020, 38, 413–420. [Google Scholar] [CrossRef]
- El Kholy, K.; Genco, R.J.; Van Dyke, T.E. Oral Infections and Cardiovascular Disease. Trends Endocrinol. Metab. 2015, 26, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, E.M.; Reis, C.; Manzanares-Céspedes, M.C. Chronic Periodontitis, Inflammatory Cytokines, and Interrelationship with Other Chronic Diseases. Postgrad. Med. 2018, 130, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; He, J.; Wang, L.; Zhou, S.; Peng, X.; Huang, S.; Zheng, L.; Cheng, L.; Hao, Y.; Li, J.; et al. Ecological Effect of Arginine on Oral Microbiota. Sci. Rep. 2017, 7, 7206. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, H.; Ebrahimi, A.; Ahmadi, F. Antibiotic Therapy in Dentistry. Int. J. Dent. 2021, 2021, 6667624. [Google Scholar] [CrossRef]
- Plotniece, A.; Sobolev, A.; Supuran, C.T.; Carta, F.; Björkling, F.; Franzyk, H.; Yli-Kauhaluoma, J.; Augustyns, K.; Cos, P.; De Vooght, L.; et al. Selected Strategies to Fight Pathogenic Bacteria. J. Enzyme Inhib. Med. Chem. 2023, 38, 2155816. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, A.; Das, P.; Maruthapandi, M.; Aryal, S.; Michaeli, S.; Mastai, Y.; Luong, J.H.T.; Gedanken, A. Heteroatom Co-Doping (N, NS, NB) on Carbon Dots and Their Antibacterial and Antioxidant Properties. Surf. Interfaces 2024, 46, 103857. [Google Scholar] [CrossRef]
- Mahmoudi, H.; Bahador, A.; Pourhajibagher, M.; Alikhani, M.Y. Antimicrobial Photodynamic Therapy: An Effective Alternative Approach to Control Bacterial Infections. J. Lasers Med. Sci. 2018, 9, 154–160. [Google Scholar] [CrossRef]
- Anas, A.; Sobhanan, J.; Sulfiya, K.M.; Jasmin, C.; Sreelakshmi, P.K.; Biju, V. Advances in Photodynamic Antimicrobial Chemotherapy. J. Photochem. Photobiol. C Photochem. Rev. 2021, 49, 100452. [Google Scholar] [CrossRef]
- Marasini, S.; Leanse, L.G.; Dai, T. Can Microorganisms Develop Resistance against Light Based Anti-Infective Agents? Adv. Drug Deliv. Rev. 2021, 175, 113822. [Google Scholar] [CrossRef]
- Takasaki, A.A.; Aoki, A.; Mizutani, K.; Schwarz, F.; Sculean, A.; Wang, C.Y.; Koshy, G.; Romanos, G.; Ishikawa, I.; Izumi, Y. Application of antimicrobial photodynamic therapy in periodontal and peri-implant diseases. Periodontol. 2000 2009, 51, 109–140. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.; Lunardi, A.J.L.; Barros, A.C.S.M.; Mandetta, A.R.H.; Grudzien, E.; San-Martín, M.; Horliana, A.C.R.T.; Bussadori, S.K.; Motta, L.J. Application of Photodynamic Therapy in Pediatric Dentistry: Literature Review. Pharmaceutics 2023, 15, 2335. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, A. Photodynamic therapy in dentistry: A literature review. J. Dent. Panacea 2023, 5, 17–20. [Google Scholar] [CrossRef]
- Roy, J.; Pandey, V.; Gupta, I.; Shekhar, H. Antibacterial Sonodynamic Therapy: Current Status and Future Perspectives. ACS Biomater. Sci. Eng. 2021, 7, 5326–5338. [Google Scholar] [CrossRef] [PubMed]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef]
- Dharshana, S.; Krishnan, V.; Divya, V. Therapeutic Applications of Ultrasound in Dentistry. J. Pharm. Sci. Res. 2020, 12, 1394–1399. [Google Scholar]
- Zhang, Y.; Jiang, R.; Lei, L.; Yang, Y.; Hu, T. Drug delivery systems for oral disease applications. J. Appl. Oral. Sci. 2022, 30, e20210349. [Google Scholar] [CrossRef]
- Hosseinpour-Moghadam, R.; Mehryab, F.; Torshabi, M.; Haeri, A. Applications of Novel and Nanostructured Drug Delivery Systems for the Treatment of Oral Cavity Diseases. Clin. Ther. 2021, 43, e377–e402. [Google Scholar] [CrossRef]
- Sabbahi, S.; Ben Ayed, L.; Jemli, M. Staphylococcus Aureus Photodynamic Inactivation Mechanisms by Rose Bengal: Use of Antioxidants and Spectroscopic Study. Appl. Water Sci. 2018, 8, 56. [Google Scholar] [CrossRef]
- Niazi, F.H.; Qamar, Z.; Noushad, M.; Muhareb, A.K. Bin Use of Rose Bengal, Methylene Blue and Curcumin Photosensitizers Activated Using Light Emitting Diode on Post Space Disinfection Bonded to Fiber Post: An Assessment of Extrusion Bond Strength. Pakistan J. Med. Sci. 2022, 38, 34–39. [Google Scholar] [CrossRef]
- Ghoniem, D.F.; Abdelkawi, S.A.; Fadel, M.; Abdel Fadeel, D.; Fouly, M.; El-Kholy, A.I.; Hassan, A.A. Novel Photodynamic/Photothermal Treatment of Fungal Keratitis Using Rose Bengal-Loaded Polypyrrole-Gold Nanoparticles in Wistar Albino Rats. J. Ocul. Pharmacol. Ther. 2023, 39, 379–388. [Google Scholar] [CrossRef]
- Kurosu, M.; Mitachi, K.; Yang, J.; Pershing, E.V.; Horowitz, B.D.; Wachter, E.A.; Lacey, J.W.; Ji, Y.; Rodrigues, D.J. Antibacterial Activity of Pharmaceutical-Grade Rose Bengal: An Application of a Synthetic Dye in Antibacterial Therapies. Molecules 2022, 27, 322. [Google Scholar] [CrossRef]
- Nisnevitch, M.; Nakonechny, F.; Nitzan, Y. Photodynamic Antimicrobial Chemotherapy by Liposome-Encapsulated Water-Soluble Photosensitizers. Russ. J. Bioorg. Chem. 2010, 36, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.F.; Borges, A.; Freitas, C.F.; Hioka, N.; Mikcha, J.M.G.; Simões, M. Antimicrobial Photodynamic Inactivation Mediated by Rose Bengal and Erythrosine Is Effective in the Control of Food-Related Bacteria in Planktonic and Biofilm States. Molecules 2018, 23, 2288. [Google Scholar] [CrossRef] [PubMed]
- Valkov, A.; Nakonechny, F.; Nisnevitch, M. Antibacterial Properties of Rose Bengal Immobilized in Polymer Supports. Appl. Mech. Mater. 2015, 719–720, 21–24. [Google Scholar] [CrossRef]
- Nakonechny, F.; Nisnevitch, M.; Nitzan, Y.; Nisnevitch, M. Sonodynamic Excitation of Rose Bengal for Eradication of Gram-Positive and Gram-Negative Bacteria. Biomed Res. Int. 2013, 2013, 684930. [Google Scholar] [CrossRef] [PubMed]
- Valkov, A.; Nakonechny, F.; Nisnevitch, M. Polymer-Immobilized Photosensitizers for Continuous Eradication of Bacteria. Int. J. Mol. Sci. 2014, 15, 14984–14996. [Google Scholar] [CrossRef] [PubMed]
- Gurianov, Y.; Meistelman, M.; Albo, Y.; Nisnevitch, M.; Nakonechny, F. Antibacterial Activity of Rose Bengal Entrapped in Organically Modified Silica Matrices. Int. J. Mol. Sci. 2022, 23, 3716. [Google Scholar] [CrossRef] [PubMed]
- Alarcon, E.I.; Poblete, H.; Roh, H.G.; Couture, J.F.; Comer, J.; Kochevar, I.E. Rose Bengal Binding to Collagen and Tissue Photobonding. ACS Omega 2017, 2, 6646–6657. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Hamblin, M.R.; Kishen, A. Characterization of a Conjugate between Rose Bengal and Chitosan for Targeted Antibiofilm and Tissue Stabilization Effects as a Potential Treatment of Infected Dentin. Antimicrob. Agents Chemother. 2012, 56, 4876–4884. [Google Scholar] [CrossRef] [PubMed]
- Son, T.I.; Sakuragi, M.; Takahashi, S.; Obuse, S.; Kang, J.; Fujishiro, M.; Matsushita, H.; Gong, J.; Shimizu, S.; Tajima, Y.; et al. Visible Light-Induced Crosslinkable Gelatin. Acta Biomater. 2010, 6, 4005–4010. [Google Scholar] [CrossRef] [PubMed]
- Schiraldi, C.; La, A.; De, M. Biotechnological Production and Application of Hyaluronan. Biopolymers 2010, 20, 10271. [Google Scholar] [CrossRef]
- Milipore Sigma IR Spectrum Table & Charts. Available online: https://www.sigmaaldrich.com/PH/en/technical-documents/technical-article/analytical-chemistry/photometry-and-reflectometry/ir-spectrum-table (accessed on 2 January 2024).
- Liao, L.F.; Lien, C.F.; Shieh, D.L.; Chen, F.C.; Lin, J.L. FTIR Study of Adsorption and Photochemistry of Amide on Powdered TiO2: Comparison of Benzamide with Acetamide. Phys. Chem. Chem. Phys. 2002, 4, 4584–4589. [Google Scholar] [CrossRef]
- Mordechai, S.; Mordehai, J.; Ramesh, J.; Levi, C.; Huleihal, M.; Erukhimovitch, V.; Moser, A.; Kapelushnik, J. Application of FTIR Microspectroscopy for the Follow-up of Childhood Leukemia Chemotherapy. Subsurf. Surf. Sens. Technol. Appl. III 2001, 4491, 243–250. [Google Scholar] [CrossRef]
- Gokulakumar, B.; Narayanaswamy, R. Fourier Transform—Infrared Spectra (FT-IR) Analysis of Root Rot Disease in Sesame (Sesamum Indicum). Rom. J. Biophys. 2008, 18, 217–223. [Google Scholar]
- Osmond, G.; Boon, J.J.; Puskar, L.; Drennan, J. Metal Stearate Distributions in Modern Artists’ Oil Paints: Surface and Cross-Sectional Investigation of Reference Paint Films Using Conventional and Synchrotron Infrared Microspectroscopy. Appl. Spectrosc. 2012, 66, 1136–1144. [Google Scholar] [CrossRef]
- Ryu, S.R.; Noda, I.; Jung, Y.M. What Is the Origin of Positional Fluctuation of Spectral Features: True Frequency Shift or Relative Intensity Changes of Two Overlapped Bands? Appl. Spectrosc. 2010, 64, 1017–1021. [Google Scholar] [CrossRef]
- Ryu, S.R.; Noda, I.; Jung, Y.M. Positional Fluctuation of IR Absorption Peaks: Frequency Shift of a Single Band or Relative Intensity Changes of Overlapped Bands? Am. Lab. 2011, 43, 40–43. [Google Scholar]
- Adre, E.; Durkee, H.; Arboleda, A.; Alawa, K.; Maestre, J.; Mintz, K.J.; Leblanc, R.M.; Amescua, G.; Parel, J.M.; Miller, D. Rose Bengal and Riboflavin Mediated Photodynamic Antimicrobial Therapy Against Selected South Florida Nocardia Keratitis Isolates. Transl. Vis. Sci. Technol. 2022, 11, 6–12. [Google Scholar] [CrossRef]
- Fujii, M.; Usui, M.; Hayashi, S.; Gross, E.; Kovalev, D.; Künzner, N.; Diener, J.; Timoshenko, V.Y. Chemical Reaction Mediated by Excited States of Si Nanocrystals—Singlet Oxygen Formation in Solution. J. Appl. Phys. 2004, 95, 3689–3693. [Google Scholar] [CrossRef]
- Żamojć, K.; Zdrowowicz, M.; Rudnicki-Velasquez, P.B.; Krzymiński, K.; Zaborowski, B.; Niedziałkowski, P.; Jacewicz, D.; Chmurzyński, L. The Development of 1,3-Diphenylisobenzofuran as a Highly Selective Probe for the Detection and Quantitative Determination of Hydrogen Peroxide. Free Radic. Res. 2017, 51, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Carloni, P.; Damiani, E.; Greci, L.; Stipa, P.; Tanfani, F.; Tartaglini, E.; Wozniak, M. On the Use of 1,3-Diphenylisobenzofuran (DPBF). Reactions with Carbon and Oxygen Centered Radicals in Model and Natural Systems. Res. Chem. Intermed. 1993, 19, 395–405. [Google Scholar] [CrossRef]
- Nardello, V.; Brault, D.; Chavalle, P.; Aubry, J.M. Measurement of Photogenerated Singlet Oxygen (1O2 (1Δ(g))) in Aqueous Solution by Specific Chemical Trapping with Sodium 1,3-Cyclohexadiene-1,4-Diethanoate. J. Photochem. Photobiol. B Biol. 1997, 39, 146–155. [Google Scholar] [CrossRef]
- Harada, N.; Kataoka, M.; Nakanosho, M.; Uyama, H. Penetration of Singlet Oxygen into Films with Oxygen Permeability Coefficient Close to That of Skin. Photochem. Photobiol. 2021, 97, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Liu, B.M.; Bennett, T.F.; Monsour, C.G.; Selke, M.; Liu, Y. Determination of Singlet Oxygen Quantum Yield of a Porphyrinic Metal-Organic Framework. J. Phys. Chem. C 2021, 125, 7392–7400. [Google Scholar] [CrossRef]
- Cantelli, A.; Piro, F.; Pecchini, P.; Di Giosia, M.; Danielli, A.; Calvaresi, M. Concanavalin A-Rose Bengal Bioconjugate for Targeted Gram-Negative Antimicrobial Photodynamic Therapy. J. Photochem. Photobiol. B Biol. 2020, 206, 111852. [Google Scholar] [CrossRef]
- Wilkinson, F.; Helman, W.P.; Ross, A.B. Quantum Yields for the Photosensitized Formation of the Lowest Electronically Excited Singlet State of Molecular Oxygen in Solution. J. Phys. Chem. Ref. Data 1993, 22, 113–262. [Google Scholar] [CrossRef]
- Fadel, M.; Kassab, K. Evaluation of the Photostability and Photodynamic Efficacy of Rose Bengal Loaded in Multivesicular Liposomes. Trop. J. Pharm. Res. 2011, 10, 289–297. [Google Scholar] [CrossRef]
- Zhang, Y.; Neal, S.L. Reaction Monitoring of Rose Bengal Photodegradation in Alcohols Using Multivariate Frequency-Domain Dynamic Fluorescence. J. Photochem. Photobiol. A Chem. 2023, 436, 114348. [Google Scholar] [CrossRef]
- Rytwo, G.; Zelkind, A.L. Evaluation of Kinetic Pseudo-Order in the Photocatalytic Degradation of Ofloxacin. Catalysts 2022, 12, 24. [Google Scholar] [CrossRef]
- Pierański, M.K.; Kosiński, J.G.; Szymczak, K.; Sadowski, P.; Grinholc, M. Antimicrobial Photodynamic Inactivation: An Alternative for Group B Streptococcus Vaginal Colonization in a Murine Experimental Model. Antioxidants 2023, 12, 847. [Google Scholar] [CrossRef]
- Nakonechny, F.; Barel, M.; David, A.; Koretz, S.; Litvak, B.; Ragozin, E.; Etinger, A.; Livne, O.; Pinhasi, Y.; Gellerman, G.; et al. Dark Antibacterial Activity of Rose Bengal. Int. J. Mol. Sci. 2019, 20, 3196. [Google Scholar] [CrossRef]
- Sugita, N.; Iwase, Y.; Yumita, N.; Ikeda, T.; Umemura, S.I. Sonodynamically Induced Cell Damage Using Rose Bengal Derivative. Anticancer Res. 2010, 30, 3361–3366. [Google Scholar]
- Štacková, L.; Muchová, E.; Russo, M.; Slavíček, P.; Štacko, P.; Klán, P. Deciphering the structure-property relations in substituted heptamethine cyanines. J Org Chem. 2020, 85, 9776–9790. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atrash, M.; Hovor, I.; Gurianov, Y.; Barel, M.; Semenova, O.; Brider, T.; Nisnevitch, M.; Nakonechny, F. Antibacterial Properties of Rose Bengal Conjugated to Hyaluronic Acid. Int. J. Mol. Sci. 2024, 25, 3330. https://doi.org/10.3390/ijms25063330
Atrash M, Hovor I, Gurianov Y, Barel M, Semenova O, Brider T, Nisnevitch M, Nakonechny F. Antibacterial Properties of Rose Bengal Conjugated to Hyaluronic Acid. International Journal of Molecular Sciences. 2024; 25(6):3330. https://doi.org/10.3390/ijms25063330
Chicago/Turabian StyleAtrash, Melad, Iryna Hovor, Yanna Gurianov, Margarita Barel, Olga Semenova, Tamara Brider, Marina Nisnevitch, and Faina Nakonechny. 2024. "Antibacterial Properties of Rose Bengal Conjugated to Hyaluronic Acid" International Journal of Molecular Sciences 25, no. 6: 3330. https://doi.org/10.3390/ijms25063330
APA StyleAtrash, M., Hovor, I., Gurianov, Y., Barel, M., Semenova, O., Brider, T., Nisnevitch, M., & Nakonechny, F. (2024). Antibacterial Properties of Rose Bengal Conjugated to Hyaluronic Acid. International Journal of Molecular Sciences, 25(6), 3330. https://doi.org/10.3390/ijms25063330







