Characterization of Hyaluronic Acid-Coated PLGA Nanoparticles by Surface-Enhanced Raman Spectroscopy
Abstract
1. Introduction
2. Results and Discussion
2.1. DLS Measurements
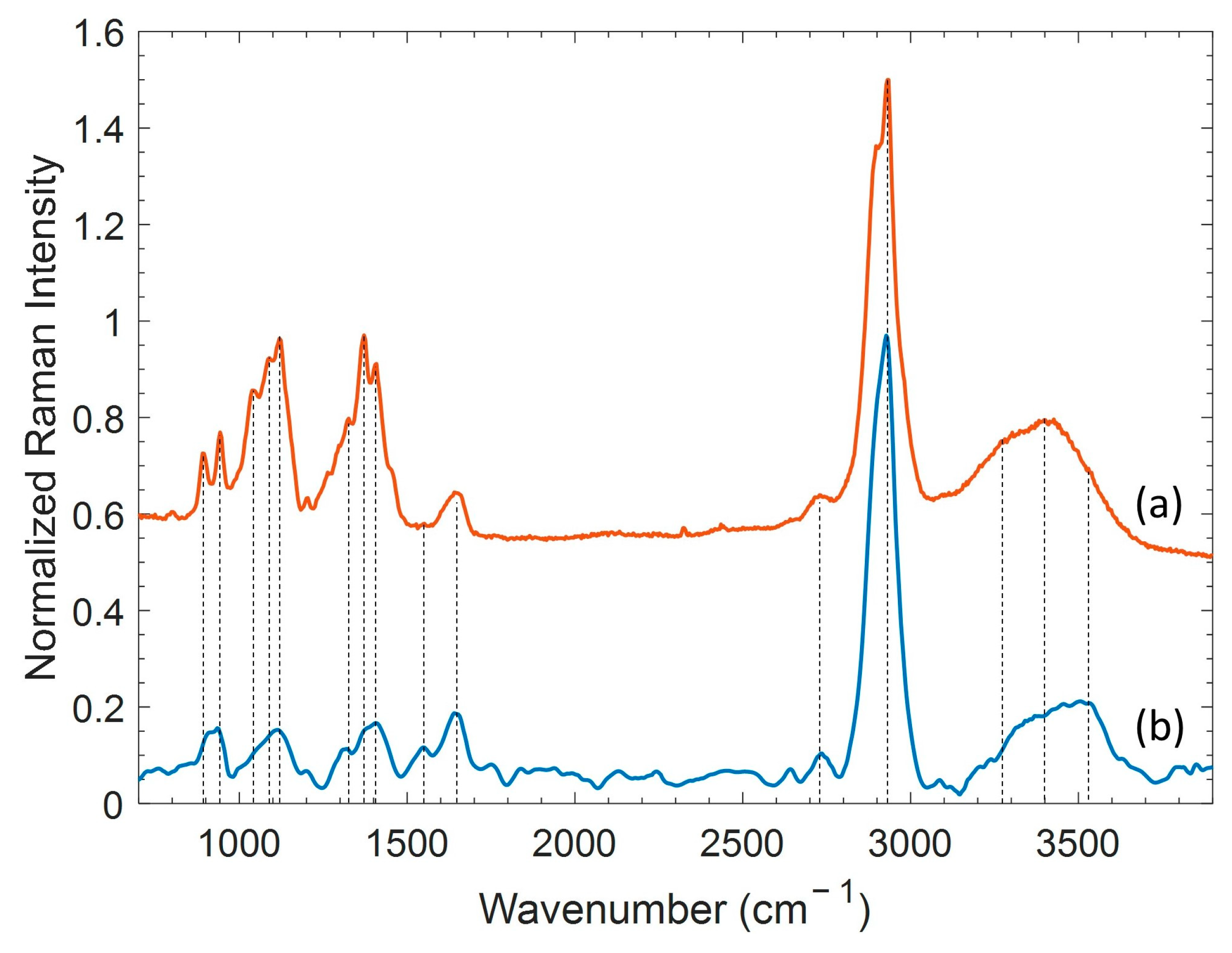
2.2. Spontaneous Raman Spectroscopy of Bulk Materials
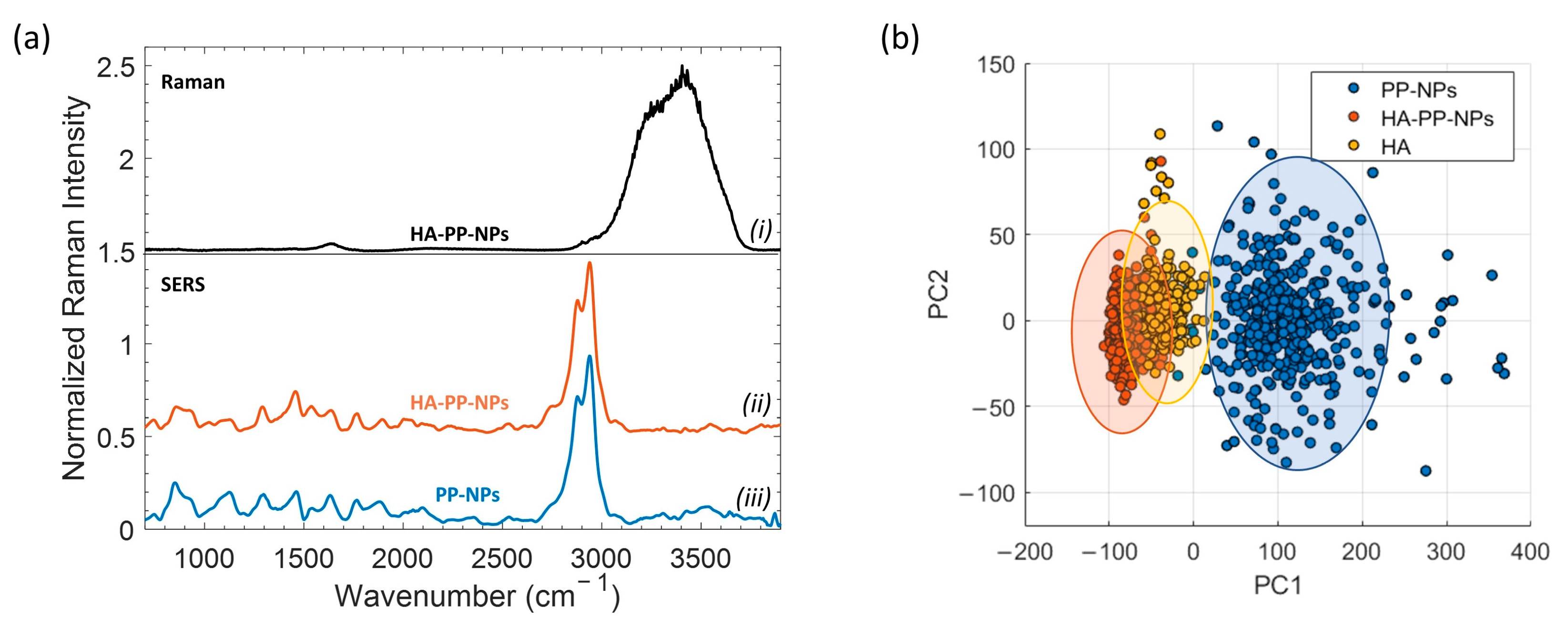
2.3. SERS and Raman Analysis of HA
2.4. SERS of PP-NPs and HA-PP-NPs
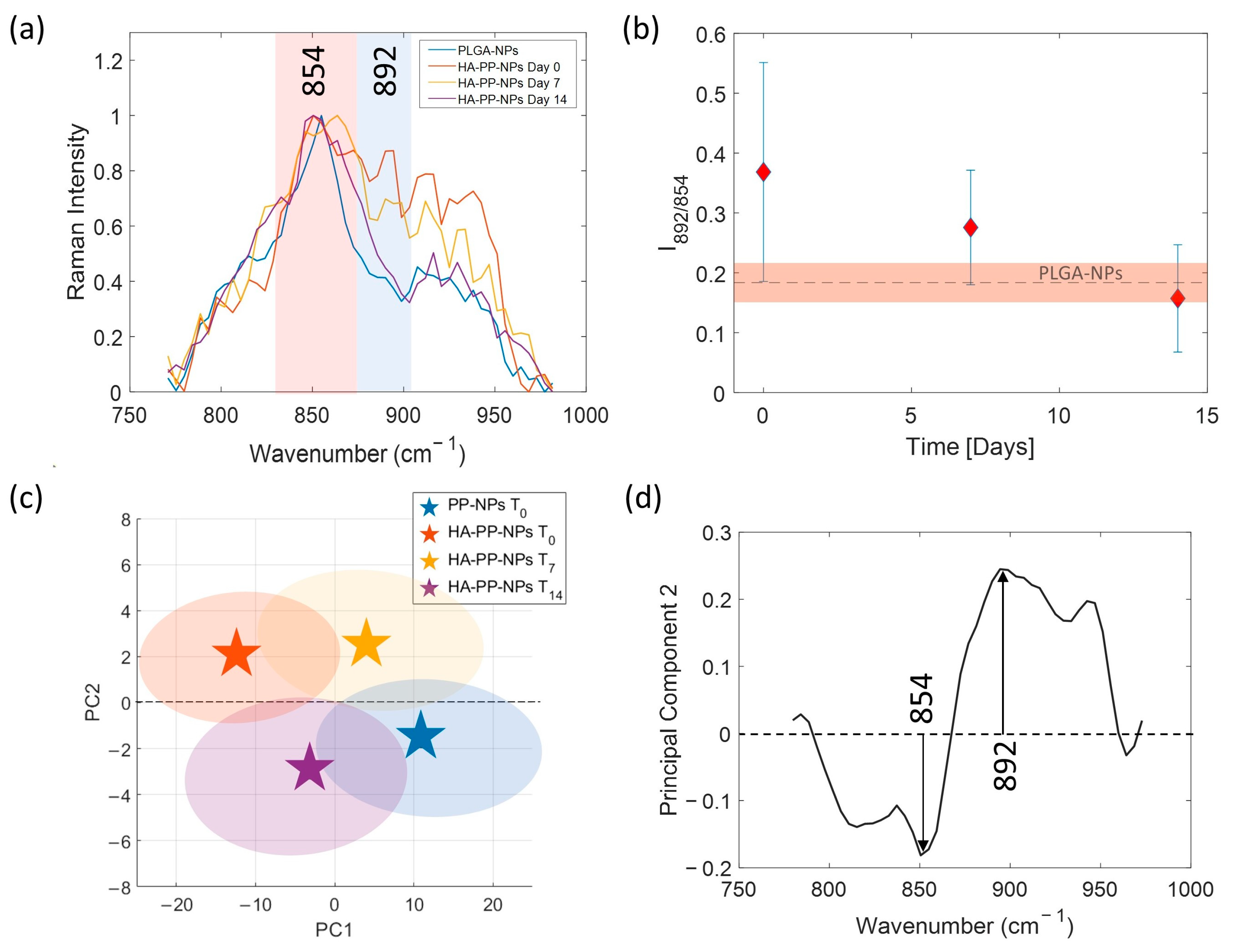
2.5. HA-PP-NP Aging SERS Analysis
3. Materials and Methods
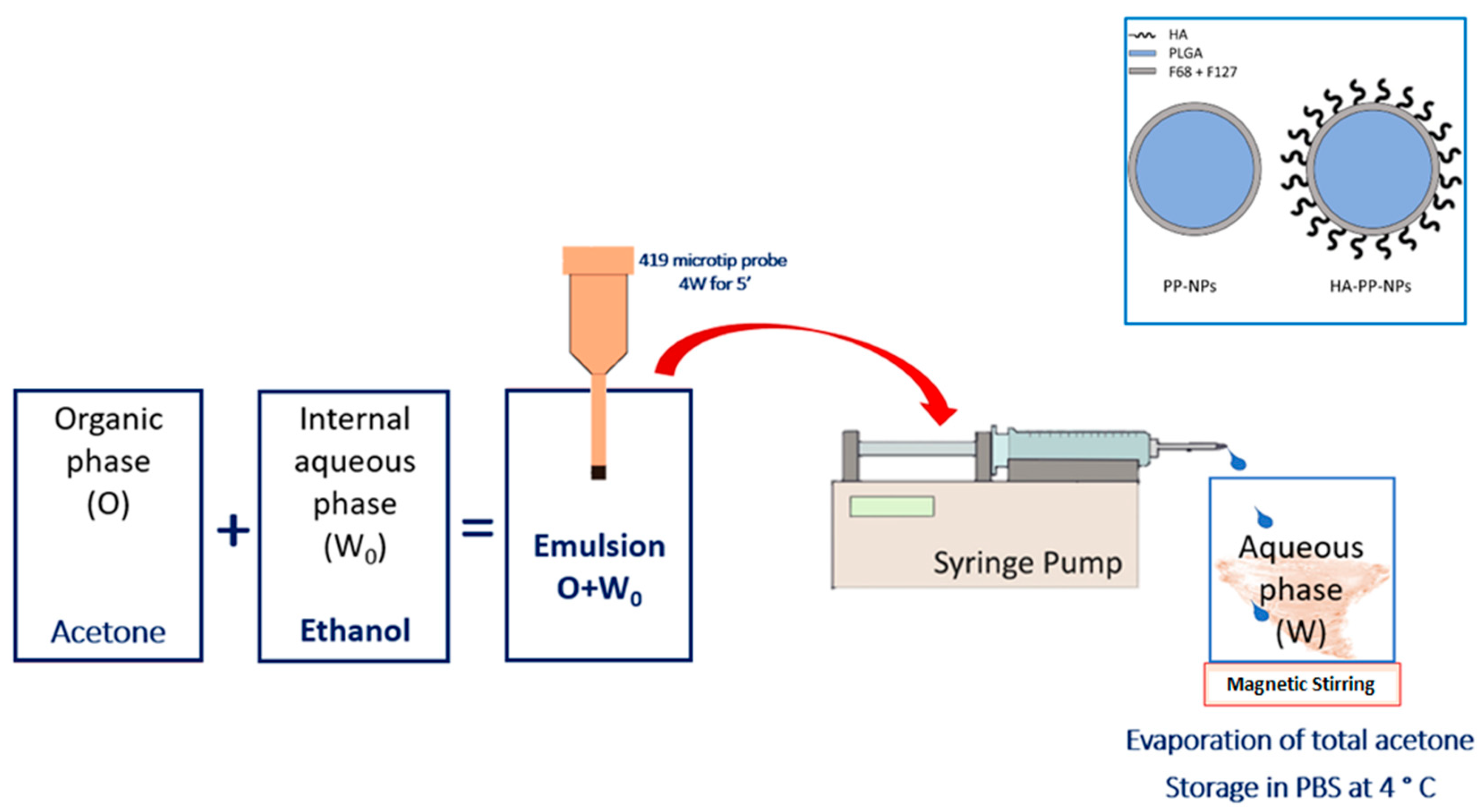
3.1. NP Formulation
3.2. Dynamic Light Scattering
3.3. Raman Analysis
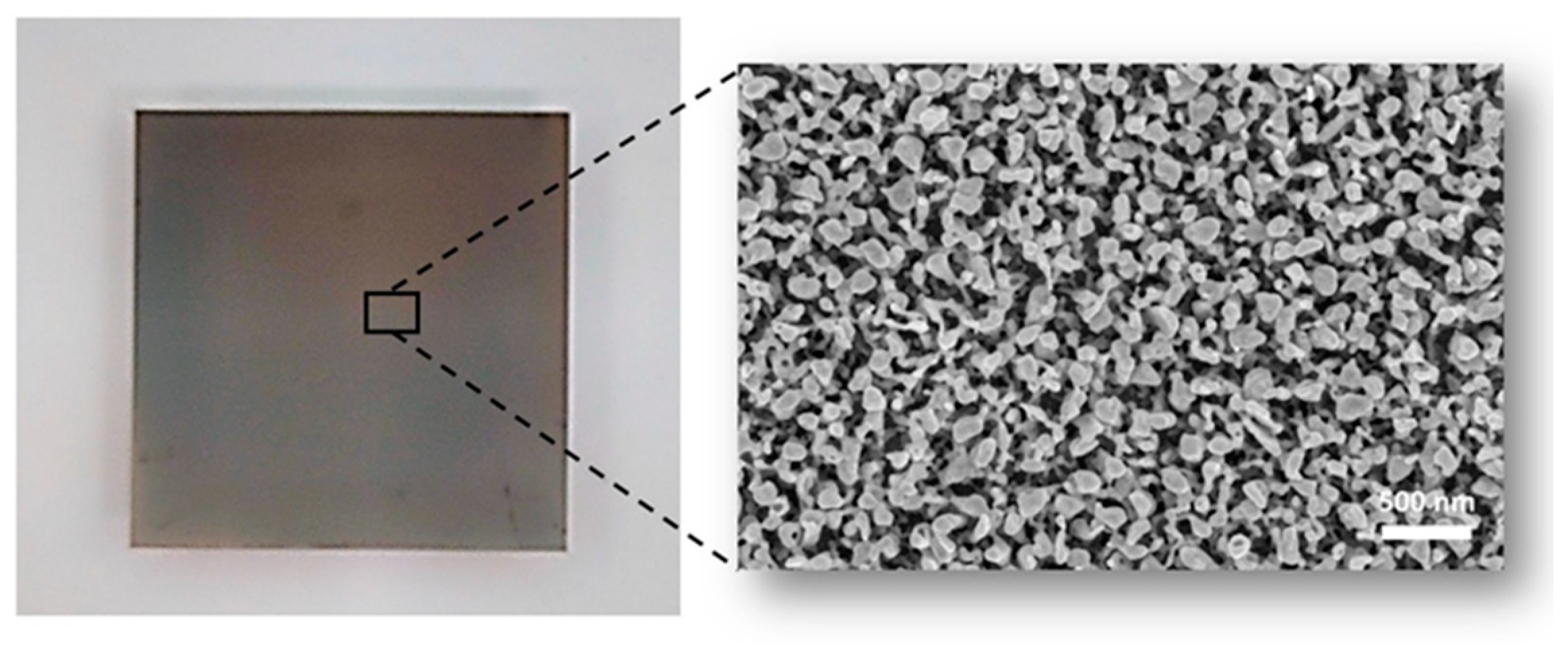
3.4. SERS Substrates
3.5. Statistical Analysis of DLS
3.6. Statistical Analysis of SERS Spectra
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zraik, I.M.; Heß-Busch, Y. Management of chemotherapy side effects and their long-term sequelae. Urologe A 2021, 60, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Baguley, B.C. Multidrug resistance in cancer. Methods Mol. Biol. 2010, 596, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Avitabile, E.; Bedognetti, D.; Ciofani, G.; Bianco, A.; Delogu, L.G. How can nanotechnology help the fight against breast cancer? Nanoscale 2018, 10, 11719–11731. [Google Scholar] [CrossRef]
- Strambeanu, N.; Demetrovici, L.; Dragos, D.; Lungu, M. Nanoparticles: Definition, Classification and General Physical Properties. In Nanoparticles’ Promises and Risks; Lungu, M., Neculae, A., Bunoiu, M., Biris, C., Eds.; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Su, X.Y.; Liu, P.D.; Wu, H.; Gu, N. Enhancement of radiosensitization by metal-based nanoparticles in cancer radiation therapy. Cancer Biol. Med. 2014, 11, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Panzetta, V.; La Verde, G.; Pugliese, M.; Artiola, V.; Arrichiello, C.; Muto, P.; La Commara, M.; Netti, P.A.; Fusco, S. Adhesion and migration response to radiation therapy of mammary epithelial and adenocarcinoma cells interacting with different stiffness substrates. Cancers 2020, 12, 1170. [Google Scholar] [CrossRef]
- La Verde, G.; Artiola, V.; Panzetta, V.; Pugliese, M.; Netti, P.A.; Fusco, S. Cytoskeleton Response to Ionizing Radiation: A Brief Review on Adhesion and Migration Effects. Biomedicines 2021, 9, 1102. [Google Scholar] [CrossRef]
- Davies, C.d.L.; Lundstrøm, L.M.; Frengen, J.; Eikenes, L.; Bruland, Ø.S.; Kaalhus, O.; Hjelstuen, M.H.; Brekken, C. Radiation improves the distribution and uptake of liposomal doxorubicin (caelyx) in human osteosarcoma xenografts. Cancer Res. 2004, 64, 547–553. [Google Scholar] [CrossRef]
- Panzetta, V.; Pugliese, M.; di Gennaro, G.; Federico, C.; Arrichiello, C.; Muto, P.; Netti, P.A.; Fusco, S. X-rays affect cytoskeleton assembly and nanoparticle uptake: Preliminary results. Il Nuovo Cim. 2018, 41, 204. [Google Scholar] [CrossRef]
- Owens, D.E., 3rd; Peppas, N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic nanoparticles and their targeted delivery applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, D.; Muscetti, O.; Falanga, A.; Fusco, S.; Belli, V.; Perillo, E.; Battista, E.; Panzetta, V.; Galdiero, S.; Netti, P.A. Sur-face decoration with gH625-membranotropic peptides as a method to escape the endo-lysosomal compartment and reduce nanoparticle toxicity. Nanotechnology 2015, 26, 415101. [Google Scholar] [CrossRef] [PubMed]
- Ponta, H.; Sherman, L.; Herrlich, P.A. CD44: From adhesion molecules to signalling regulators. Nat. Rev. Mol. Cell Biol. 2003, 4, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Naor, D.; Sionov, R.V.; Ish-Shalom, D. CD44: Structure, function, and association with the malignant process. Adv. Cancer Res. 1997, 71, 241–319. [Google Scholar] [CrossRef]
- Yan, Y.; Zuo, X.; Wei, D. Concise Review: Emerging Role of CD44 in Cancer Stem Cells: A Promising Biomarker and Therapeutic Target. Stem Cells Transl. Med. 2015, 4, 1033–1043. [Google Scholar] [CrossRef]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic Acid in the Third Millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef]
- Hou, X.; Zhong, D.; Chen, H.; Gu, Z.; Gong, Q.; Ma, X.; Zhang, H.; Zhu, H.; Luo, K. Recent advances in hyaluronic acid-based nanomedicines: Preparation and application in cancer therapy. Carbohydr. Polym. 2022, 292, 119662. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, J.; Watanabe, W. Physical and chemical stability of drug nanoparticles. Adv. Drug Deliv. Rev. 2011, 63, 456–469. [Google Scholar] [CrossRef]
- Menon, J.U.; Kona, S.; Wadajkar, A.S.; Desai, F.; Vadla, A.; Nguyen, K.T. Effects of surfactants on the properties of PLGA nanoparticles. J. Biomed. Mater. Res. Part A 2012, 100, 1998–2005. [Google Scholar] [CrossRef]
- Della Sala, F.; Silvestri, T.; Borzacchiello, A.; Mayol, L.; Ambrosio, L.; Biondi, M. Hyaluronan-coated nanoparticles for active tumor targeting: Influence of polysaccharide molecular weight on cell uptake. Colloids Surf. B Biointerfaces 2022, 210, 112240. [Google Scholar] [CrossRef]
- Cannavà, C.; De Gaetano, F.; Stancanelli, R.; Venuti, V.; Paladini, G.; Caridi, F.; Ghica, C.; Crupi, V.; Majolino, D.; Ferlazzo, G.; et al. Chitosan-Hyaluronan Nanoparticles for Vinblastine Sulfate Delivery: Characterization and Internalization Studies on K-562 Cells. Pharmaceutics 2022, 14, 942. [Google Scholar] [CrossRef] [PubMed]
- Almalik, A.; Karimi, S.; Ouasti, S.; Donno, R.; Wandrey, C.; Day, P.J.; Tirelli, N. Hyaluronic acid (HA) presentation as a tool to modulate and control the receptor-mediated uptake of HA-coated nanoparticles. Biomaterials 2013, 34, 5369–5380. [Google Scholar] [CrossRef] [PubMed]
- Gennari, A.; Rios de la Rosa, J.M.; Hohn, E.; Pelliccia, M.; Lallana, E.; Donno, R.; Tirella, A.; Tirelli, N. The different ways to chitosan/hyaluronic acid nanoparticles: Templated vs direct complexation. Influence of particle preparation on morphology, cell uptake and silencing efficiency. Beilstein J. Nanotechnol. 2019, 10, 2594–2608. [Google Scholar] [CrossRef]
- Shipp, D.W.; Sinjab, F.; Notingher, I. Raman spectroscopy: Techniques and applications in the life sciences. Adv. Opt. Photonics 2017, 9, 315–428. [Google Scholar] [CrossRef]
- Langer, J.; Jimenez de Aberasturi, D.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.; Boisen, A.; Brolo, A.G.; et al. Present and Future of Surface-Enhanced Raman Scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef]
- Lane, L.A.; Qian, X.; Nie, S. SERS Nanoparticles in Medicine: From Label-Free Detection to Spectroscopic Tagging. Chem. Rev. 2015, 115, 10489–10529. [Google Scholar] [CrossRef]
- Zhou Rui, X.X.; Liu, R.; Hao, L.T.; Liu, J.F. Identification of polystyrene nanoplastics using surface enhanced Raman spectroscopy. Talanta 2021, 221, 121552. [Google Scholar] [CrossRef]
- Xia, L.; Li, W.; Sun, M.; Wang, H. Application of SERS in the Detection of Fungi, Bacteria and Viruses. Nanomaterials 2022, 12, 3572. [Google Scholar] [CrossRef]
- Isticato, R.; Sirec, T.; Giglio, R.; Baccigalupi, L.; Rusciano, G.; Pesce, G.; Zito, G.; Sasso, A.; De Felice, M.; Ricca, E. Flexibility of the programme of spore coat formation in Bacillus sub-tilis:bypass of CotE requirement by over-production of CotH. PLoS ONE 2013, 8, e74949. [Google Scholar] [CrossRef]
- Rusciano, G.; Zito, G.; Isticato, R.; Sirec, T.; Ricca, E.; Bailo, E.; Sasso, A. Nanoscale chemical imaging of bacillus subtilis spores by combining tip-enhanced raman scattering and advanced statistical tools. ACS Nano 2014, 8, 12300–12309. [Google Scholar] [CrossRef]
- Giarra, S.; Serri, C.; Russo, L.; Zeppetelli, S.; De Rosa, G.; Borzacchiello, A.; Biondi, M.; Ambrosio, L.; Mayol, L. Spontaneous arrangement of a tumor targeting hyaluronic acid shell on irinotecan loaded PLGA nanoparticles. Carbohydr. Polym. 2016, 140, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Essendoubi, M.; Gobinet, C.; Reynaud, R.; Angiboust, J.F.; Manfait, M.; Piot, O. Human skin penetration of hyaluronic acid of different molecular weights as probed by Raman spectroscopy. Skin Res. Technol. 2016, 22, 55–62. [Google Scholar] [CrossRef]
- Kotzianova, A.; Rebicek, J.; Zidek, O.; Pokorny, M.; Hrbac, J.; Velebny, V. Raman spectroscopy based method for the evaluation of compositional consistency of nanofibrous layers. Anal. Methods 2015, 7, 9900–9905. [Google Scholar] [CrossRef]
- Barrett, T.W.; Peticolas, W.L. Laser Raman inelastic light scattering investigations of hyaluronic acid primary and secondary structure. J. Raman Spectrosc. 1979, 8, 35–38. [Google Scholar] [CrossRef]
- Van Apeldoorn, A.A.; Van Manen, H.J.; Bezemer, J.M.; de Bruijn, J.D.; van Blitterswijk, C.A.; Otto, C. Raman imaging of PLGA microsphere degradation inside macrophages. J. Am. Chem. Soc. 2004, 126, 13226–13227. [Google Scholar] [CrossRef] [PubMed]
- Geze, A.; Chourpa, I.; Boury, F.; Benoit, J.P.; Dubois, P. Direct qualitative and quantitative characterization of a radiosensitizer, 5-iodo-2′-deoxyuridine within biodegradable polymeric microspheres by FT-Raman spectroscopy. Analyst 1999, 124, 37–42. [Google Scholar] [CrossRef]
- Guo, C.; Liu, H.; Wang, J.; Chen, J. Conformational Structure of Triblock Copolymers by FT-Raman and FTIR Spectroscopy. J. Colloid Interface Sci. 1999, 209, 368–373. [Google Scholar] [CrossRef]
- Shaarani, S.; Hamid, S.S.; Mohd Kaus, N.H. The Influence of Pluronic F68 and F127 Nanocarrier on Physicochemical Properties, In vitro Release, and Antiproliferative Activity of Thymoquinone. Drug Pharmacogn. Res. 2017, 9, 12–20. [Google Scholar] [CrossRef]
- Al-Milaji, K.N.; Zhao, H. New Perspective of mitigating the coffee-ring effect: Interfacial assembly. J. Phys. Chem. C 2019, 123, 12029–12041. [Google Scholar] [CrossRef]
- Le Ru, E.C.; Meyer, S.A.; Artur, C.; Etchegoin, P.G.; Grand, J.; Lang, P.; Maurel, F. Experimental demonstration of surface selection rules for SERS on flat metallic surfaces. ChemComm 2011, 47, 3903–3905. [Google Scholar] [CrossRef]
- Serri, C.; Argirò, M.; Piras, L.; Mita, D.G.; Saija, A.; Mita, L.; Forte, M.; Giarra, S.; Biondi, M.; Crispi, S.; et al. Nano-precipitated curcumin loaded particles: Effect of carrier size and drug complexation with (2-hydroxypropyl)-β-cyclodextrin on their biological performances. Int. J. Pharm. 2017, 520, 21–28. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Honary, S.; Zahir, F. Effect of Zeta Potential on the Properties of Nano-Drug Delivery Systems—A Review (Part 1). Trop. J. Pharm. Res. 2013, 12, 255. [Google Scholar] [CrossRef]
- Honary, S.; Zahir, F. Effect of Zeta Potential on the Properties of Nano-Drug Delivery Systems—A Review (Part 2). Trop. J. Pharm. Res. 2013, 12, 265. [Google Scholar] [CrossRef]
- Capaccio, A.; Sasso, A.; Tarallo, O.; Rusciano, G. Coral-like plasmonic probes for tip-enhanced Raman spectroscopy. Nanoscale 2020, 12, 24376. [Google Scholar] [CrossRef]
- Capaccio, A.; Sasso, A.; Rusciano, G. A simple and reliable approach for the fabrication of nanoporous silver patterns for surface-enhanced Raman spectroscopy applications. Sci. Rep. 2021, 11, 22295. [Google Scholar] [CrossRef]

| Formulation | Mean Diameter (nm) | PDI | ζ (mV) | Time |
|---|---|---|---|---|
| PP-NPs | 109 ± 11 | 0.11 ± 0.04 | −26 ± 6 | T0 |
| HA-PP-NPs | 145 ± 16 | 0.14 ± 0.076 | −34 ± 8 | |
| PP-NPs | 118 ± 15 | 0.13 ± 0.05 | −26 ± 5 | T7 |
| HA-PP-NPs | 135 ± 23 | 0.15 ± 0.05 | −24 ± 4 | |
| PP-NPs | 117 ± 10 | 0.16 ± 0.05 | −23 ± 2 | T14 |
| HA-PP-NPs | 142 ± 17 | 0.13 ± 0.06 | −29 ± 4 |
| F68 | PLGA | HA | Tentative Assignment (cm−1) |
|---|---|---|---|
| 840 | 845 | ρCH2 | |
| 877 | νC-COO | ||
| 894 | ρCH2, νC-C | ||
| 892 | β-linkages | ||
| 925 | ρCH2 | ||
| 933 | ρCH2, νC-C | ||
| 943 | νC-C, νC-O-C | ||
| 1042 | νC-C, νC-O | ||
| 1060 | ρCH2, νC-O | ||
| 1087 | 1089 | δC-OH | |
| 1120 | δC-OH, δCH | ||
| 1130 | ρCH3 asym | ||
| 1136 | νCH2, νC-C | ||
| 1230 | τCH2 | ||
| 1275 | 1288 | δCH | |
| 1326 | Amide III | ||
| 1386 | 1371 | δCH3 sym | |
| 1408 | δCH, δCOO- | ||
| 1421 | δCH2 | ||
| 1449 | CH2 scissor | ||
| 1441 | 1454 | δCH2, δCH3 asym | |
| 1474 | CH2 scissor | ||
| 1654 | νC=C, Amide I C=O | ||
| 1760 | νC=O | ||
| 2840 | νCH2 sym | ||
| 2880 | 2879 | νCH2 asym | |
| 2897 | νCH | ||
| 2933 | νNH | ||
| 2931 | νCH3 sym | ||
| 2947 | νCH2 sym | ||
| 2964 | 2998 | νCH3 asym | |
| 3200/3500 | νOH |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Verde, G.; Sasso, A.; Rusciano, G.; Capaccio, A.; Fusco, S.; Mayol, L.; Biondi, M.; Silvestri, T.; Netti, P.A.; La Commara, M.; et al. Characterization of Hyaluronic Acid-Coated PLGA Nanoparticles by Surface-Enhanced Raman Spectroscopy. Int. J. Mol. Sci. 2023, 24, 601. https://doi.org/10.3390/ijms24010601
La Verde G, Sasso A, Rusciano G, Capaccio A, Fusco S, Mayol L, Biondi M, Silvestri T, Netti PA, La Commara M, et al. Characterization of Hyaluronic Acid-Coated PLGA Nanoparticles by Surface-Enhanced Raman Spectroscopy. International Journal of Molecular Sciences. 2023; 24(1):601. https://doi.org/10.3390/ijms24010601
Chicago/Turabian StyleLa Verde, Giuseppe, Antonio Sasso, Giulia Rusciano, Angela Capaccio, Sabato Fusco, Laura Mayol, Marco Biondi, Teresa Silvestri, Paolo A. Netti, Marco La Commara, and et al. 2023. "Characterization of Hyaluronic Acid-Coated PLGA Nanoparticles by Surface-Enhanced Raman Spectroscopy" International Journal of Molecular Sciences 24, no. 1: 601. https://doi.org/10.3390/ijms24010601
APA StyleLa Verde, G., Sasso, A., Rusciano, G., Capaccio, A., Fusco, S., Mayol, L., Biondi, M., Silvestri, T., Netti, P. A., La Commara, M., Panzetta, V., & Pugliese, M. (2023). Characterization of Hyaluronic Acid-Coated PLGA Nanoparticles by Surface-Enhanced Raman Spectroscopy. International Journal of Molecular Sciences, 24(1), 601. https://doi.org/10.3390/ijms24010601











