Assessing the Impact of Influenza Vaccination Timing on Experimental Arthritis: Effects on Disease Progression and Inflammatory Biomarkers
Abstract
1. Introduction
2. Results
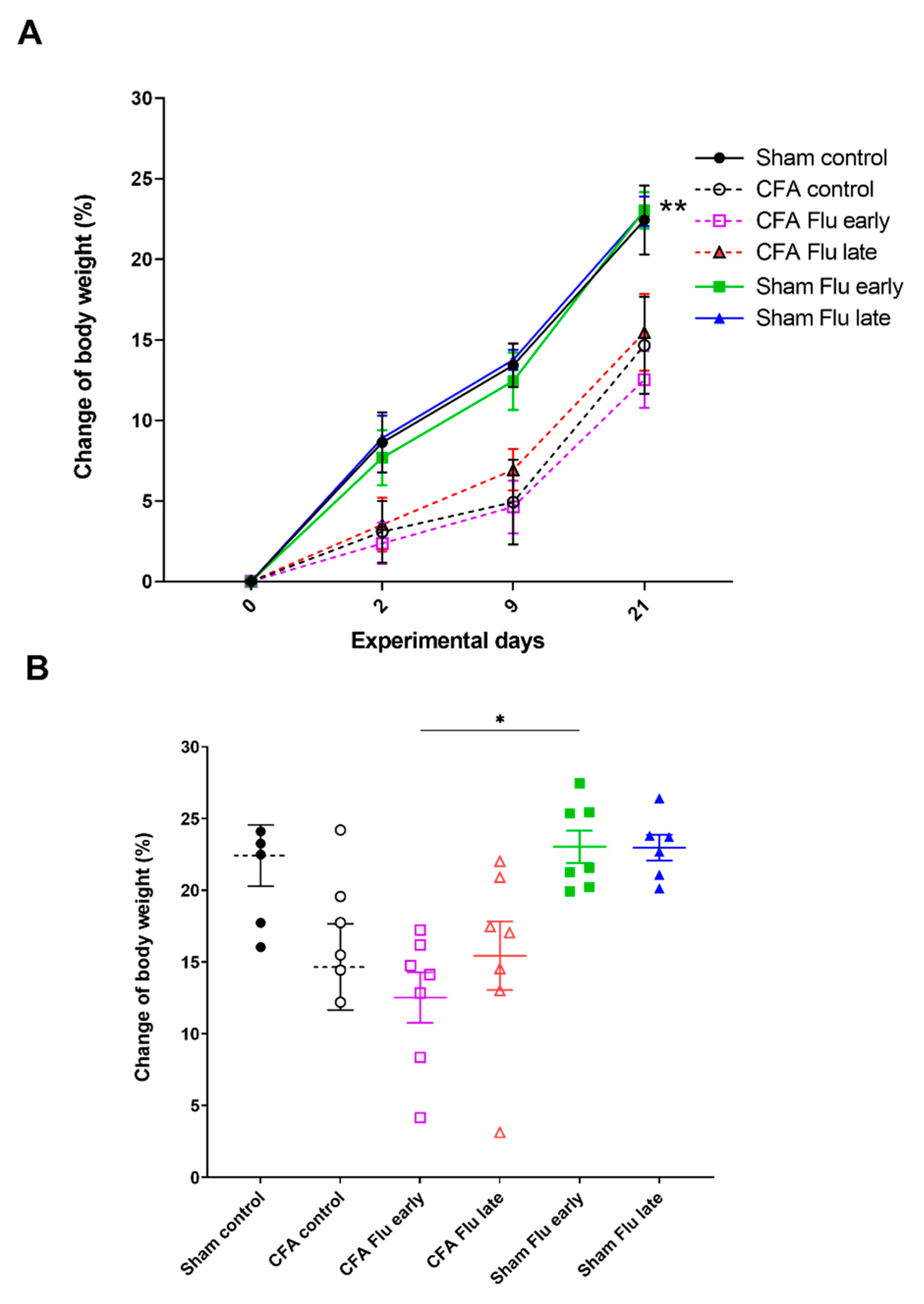
2.1. Influenza Vaccination Has No Effect on Body Weight Changes
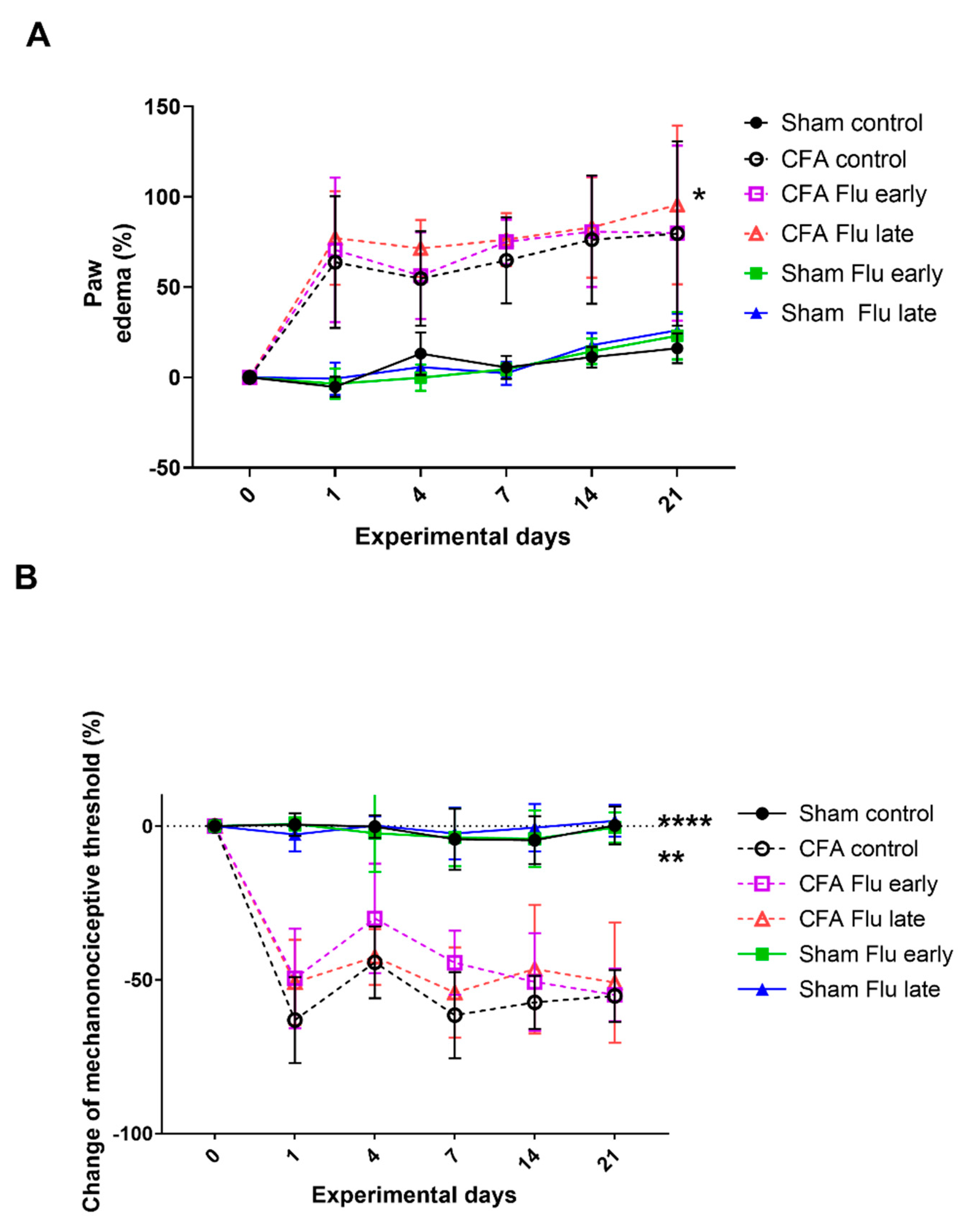
2.2. Influenza Vaccination Has No Effect on CFA-Induced Paw Edema and Mechanical Allodynia
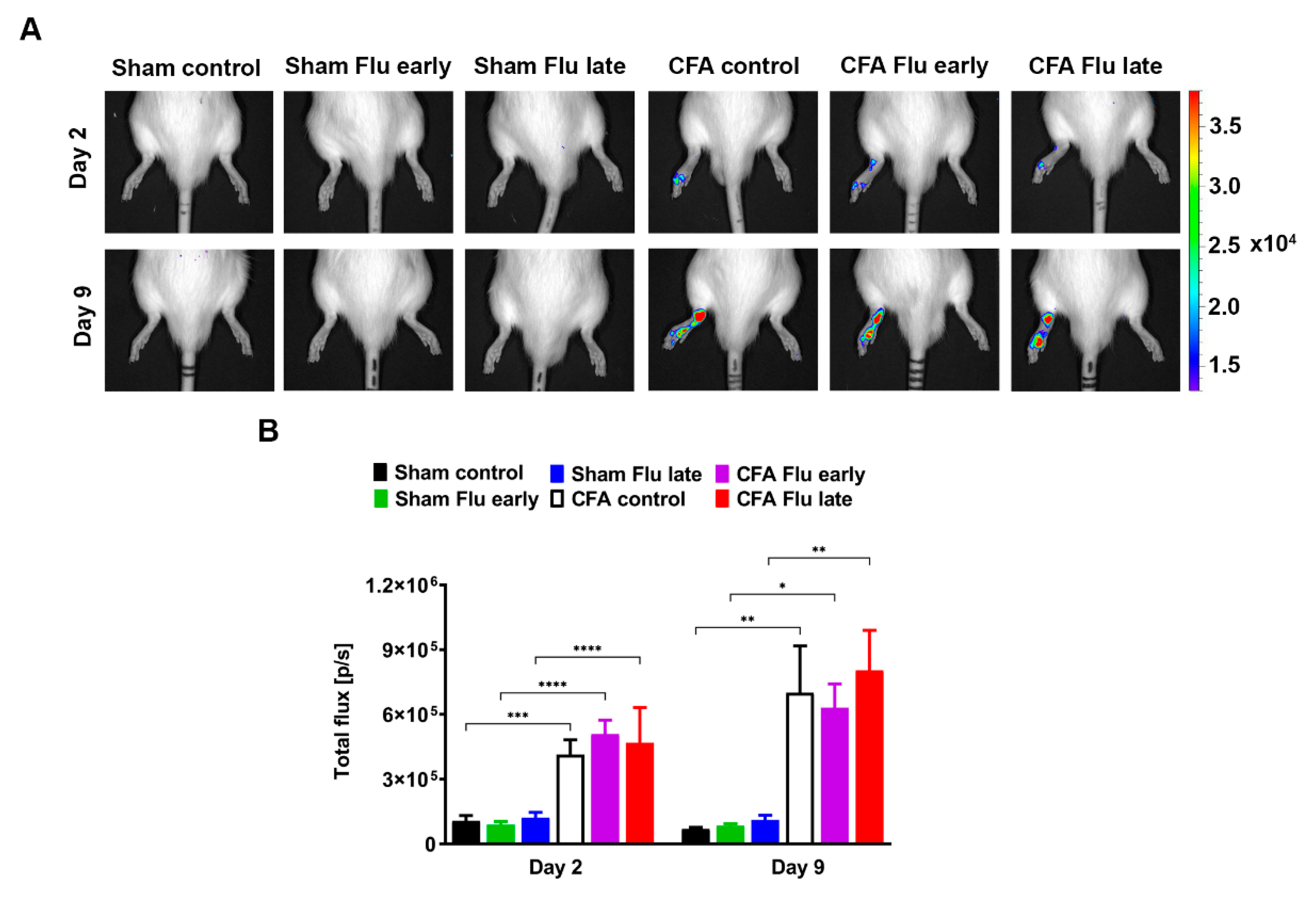
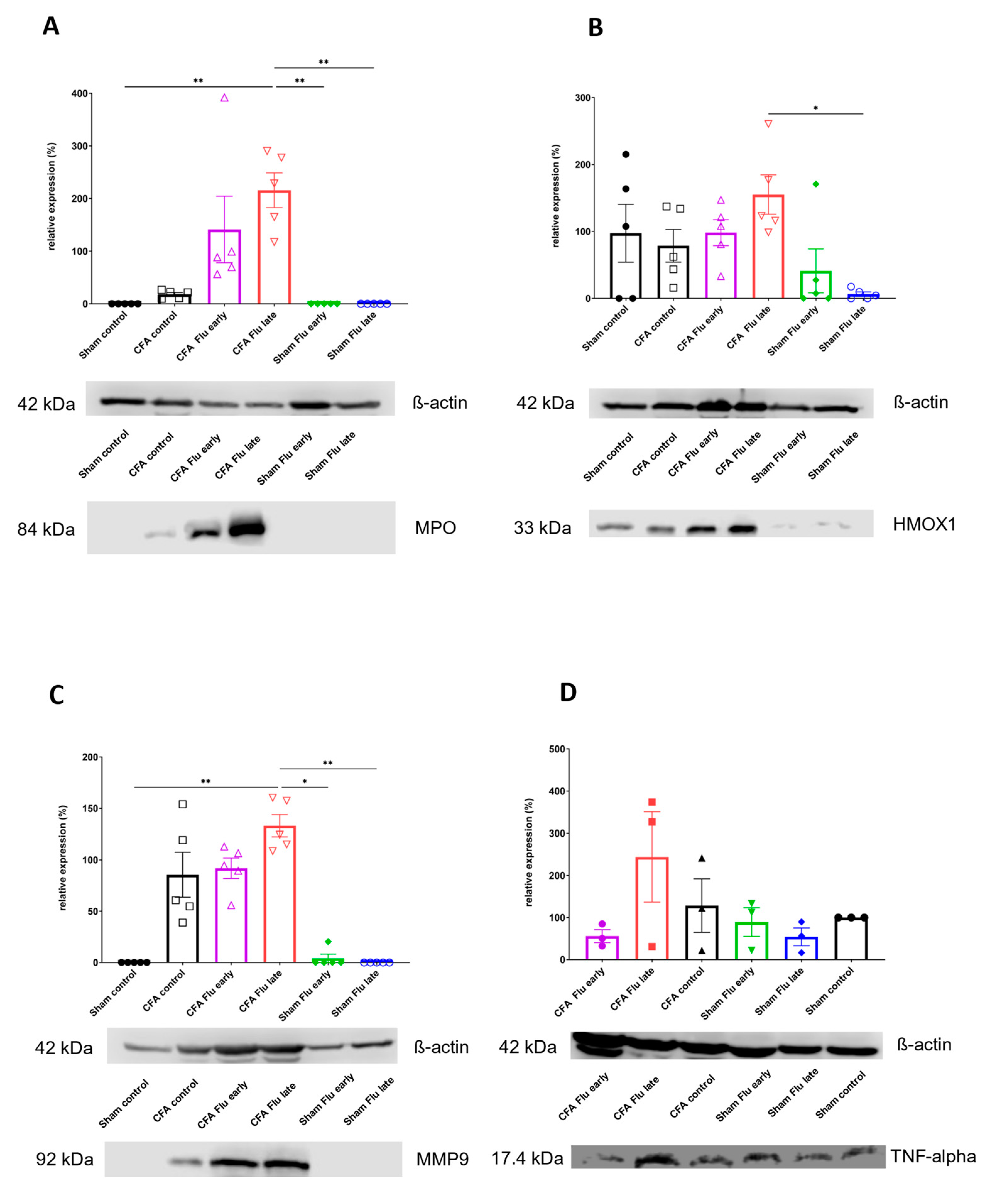
2.3. Influenza Vaccination Does Not Influence the Neutrophil Myeloperoxidase (MPO) Activity in the CFA-Injected Hind Paws
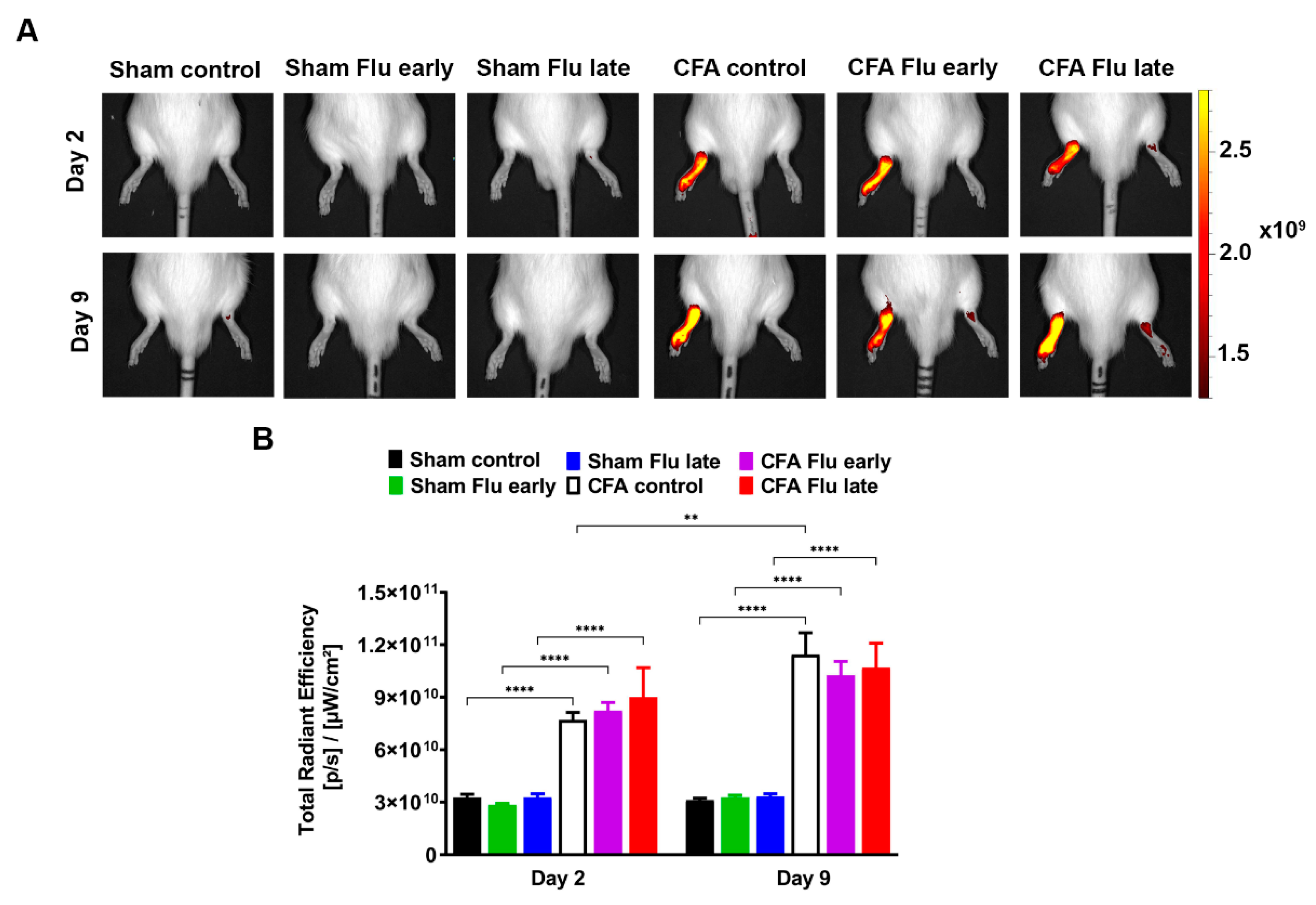
2.4. Influenza Vaccination Does Not Influence Plasma Extravasation in the CFA-Injected Tibiotarsal Joints
2.5. Influenza Vaccination Has No Effect on the CFA-Induced Inflammatory Biomarker Expression Increase
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Induction of the Experimental Disease
4.3. Treatment
4.4. Measurement of Body Weight
4.5. Measurement of Paw Edema
4.6. Measurement of Mechanonociceptive Threshold
4.7. In Vivo Bioluminescence Imaging of Neutrophil MPO Activity
4.8. In Vivo Fluorescence Imaging of Vascular Leakage
4.9. Western Blot
4.10. Microscopic Morphometry of Joint Inflammation
4.11. Ethics
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Collins, F.S.; Stoffels, P. Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV): An Unprecedented Partnership for Unprecedented Times. JAMA 2020, 323, 2455–2457. [Google Scholar] [CrossRef]
- Getts, D.R.; Chastain, E.M.; Terry, R.L.; Miller, S.D. Virus infection, antiviral immunity, and autoimmunity. Immunol. Rev. 2013, 255, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Segal, Y.; Shoenfeld, Y. Vaccine-induced autoimmunity: The role of molecular mimicry and immune crossreaction. Cell Mol. Immunol. 2018, 15, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Wraith, D.C.; Goldman, M.; Lambert, P.H. Vaccination and autoimmune disease: What is the evidence? Lancet 2003, 362, 1659–1666. [Google Scholar] [CrossRef] [PubMed]
- Vadalà, M.; Poddighe, D.; Laurino, C.; Palmieri, B. Vaccination and autoimmune diseases: Is prevention of adverse health effects on the horizon? EPMA J. 2017, 8, 295–311. [Google Scholar] [CrossRef] [PubMed]
- Nakken, B.; Papp, G.; Bosnes, V.; Zeher, M.; Nagy, G.; Szodoray, P. Biomarkers for rheumatoid arthritis: From molecular processes to diagnostic applications-current concepts and future perspectives. Immunol. Lett. 2017, 189, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., 3rd; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis. Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef]
- Wang, X.; Fan, D.; Cao, X.; Ye, Q.; Wang, Q.; Zhang, M.; Xiao, C. The Role of Reactive Oxygen Species in the Rheumatoid Arthritis-Associated Synovial Microenvironment. Antioxidants 2022, 11, 1153. [Google Scholar] [CrossRef]
- Joe, B.; Wilder, R.L. Animal models of rheumatoid arthritis. Mol. Med. Today 1999, 5, 367–369. [Google Scholar] [CrossRef]
- Billiau, A.; Matthys, P. Modes of action of Freund’s adjuvants in experimental models of autoimmune diseases. J. Leukoc. Biol. 2001, 70, 849–860. [Google Scholar] [CrossRef]
- Noh, A.S.M.; Chuan, T.D.; Khir, N.A.M.; Zin, A.A.M.; Ghazali, A.K.; Long, I.; Ab Aziz, C.B.; Ismail, C.A.N. Effects of different doses of complete Freund’s adjuvant on nociceptive behaviour and inflammatory parameters in polyarthritic rat model mimicking rheumatoid arthritis. PLoS ONE 2021, 16, e0260423. [Google Scholar] [CrossRef]
- da Fonseca, L.J.S.; Nunes-Souza, V.; Goulart, M.O.F.; Rabelo, L.A. Oxidative Stress in Rheumatoid Arthritis: What the Future Might Hold regarding Novel Biomarkers and Add-On Therapies. Oxid. Med. Cell Longev. 2019, 2019, 7536805. [Google Scholar] [CrossRef]
- Ryter, S.W. Heme Oxygenase-1: An Anti-Inflammatory Effector in Cardiovascular, Lung, and Related Metabolic Disorders. Antioxidants 2022, 11, 555. [Google Scholar] [CrossRef]
- Lal, R.; Dhaliwal, J.; Dhaliwal, N.; Dharavath, R.N.; Chopra, K. Activation of the Nrf2/HO-1 signaling pathway by dimethyl fumarate ameliorates complete Freund’s adjuvant-induced arthritis in rats. Eur. J. Pharmacol. 2021, 899, 174044. [Google Scholar] [CrossRef]
- Gabay, C.; Kushner, I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999, 340, 448–454. [Google Scholar] [CrossRef]
- Patel, S.S.; Shah, P.V. Evaluation of anti-inflammatory potential of the multidrug herbomineral formulation in male Wistar rats against rheumatoid arthritis. J. Ayurveda Integr. Med. 2013, 4, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.F.; Smidt, N.; Sterne, J.A.; Harbord, R.; Burton, A.; Burke, M.; Beynon, R.; Ben-Shlomo, Y.; Axford, J.; Dieppe, P. Systematic review: Accuracy of anti-citrullinated Peptide antibodies for diagnosing rheumatoid arthritis. Ann. Intern. Med. 2010, 152, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, A. Role of autoantibody testing. Best Pract. Res. Clin. Rheumatol. 2014, 28, 907–920. [Google Scholar] [CrossRef] [PubMed]
- Gad, S.S.; Fayez, A.M.; Abdelaziz, M.; Abou El-Ezz, D. Amelioration of autoimmunity and inflammation by zinc oxide nanoparticles in experimental rheumatoid arthritis. Naunyn. Schmiedebergs Arch. Pharmacol. 2021, 394, 1975–1981. [Google Scholar] [CrossRef] [PubMed]
- Nielen, M.M.; van Schaardenburg, D.; Reesink, H.W.; Twisk, J.W.; van de Stadt, R.J.; van der Horst-Bruinsma, I.E.; de Koning, M.H.; Habibuw, M.R.; Dijkmans, B.A. Simultaneous development of acute phase response and autoantibodies in preclinical rheumatoid arthritis. Ann. Rheum. Dis. 2006, 65, 535–537. [Google Scholar] [CrossRef] [PubMed]
- Pincus, T. Advantages and limitations of quantitative measures to assess rheumatoid arthritis: Joint counts, radiographs, laboratory tests, and patient questionnaires. Bull. NYU Hosp. Jt. Dis. 2006, 64, 32–39. [Google Scholar]
- Emery, P.; Gabay, C.; Kraan, M.; Gomez-Reino, J. Evidence-based review of biologic markers as indicators of disease progression and remission in rheumatoid arthritis. Rheumatol. Int. 2007, 27, 793–806. [Google Scholar] [CrossRef]
- Johnson, T.M.; Register, K.A.; Schmidt, C.M.; O’Dell, J.R.; Mikuls, T.R.; Michaud, K.; England, B.R. Correlation of the Multi-Biomarker Disease Activity Score With Rheumatoid Arthritis Disease Activity Measures: A Systematic Review and Meta-Analysis. Arthritis Care Res. 2019, 71, 1459–1472. [Google Scholar] [CrossRef]
- Davis, J.M., 3rd. Editorial: The Multi-Biomarker Disease Activity Test for Rheumatoid Arthritis: Is It a Valid Measure of Disease Activity? Arthritis Rheumatol. 2016, 68, 2061–2066. [Google Scholar] [CrossRef]
- Weinblatt, M.E.; Schiff, M.; Valente, R.; van der Heijde, D.; Citera, G.; Zhao, C.; Maldonado, M.; Fleischmann, R. Head-to-head comparison of subcutaneous abatacept versus adalimumab for rheumatoid arthritis: Findings of a phase IIIb, multinational, prospective, randomized study. Arthritis Rheum. 2013, 65, 28–38. [Google Scholar] [CrossRef]
- Rech, J.; Hueber, A.J.; Finzel, S.; Englbrecht, M.; Haschka, J.; Manger, B.; Kleyer, A.; Reiser, M.; Cobra, J.F.; Figueiredo, C.; et al. Prediction of disease relapses by multibiomarker disease activity and autoantibody status in patients with rheumatoid arthritis on tapering DMARD treatment. Ann. Rheum. Dis. 2016, 75, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Szekanecz, Z.; Halloran, M.M.; Volin, M.V.; Woods, J.M.; Strieter, R.M.; Kenneth Haines, G., 3rd; Kunkel, S.L.; Burdick, M.D.; Koch, A.E. Temporal expression of inflammatory cytokines and chemokines in rat adjuvant-induced arthritis. Arthritis Rheum. 2000, 43, 1266–1277. [Google Scholar] [CrossRef] [PubMed]
- Paquet, J.; Goebel, J.C.; Delaunay, C.; Pinzano, A.; Grossin, L.; Cournil-Henrionnet, C.; Gillet, P.; Netter, P.; Jouzeau, J.Y.; Moulin, D. Cytokines profiling by multiplex analysis in experimental arthritis: Which pathophysiological relevance for articular versus systemic mediators? Arthritis Res. Ther. 2012, 14, R60. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Jian, Z.; Guo, J.; Ning, X. Increased levels of serum myeloperoxidase in patients with active rheumatoid arthritis. Life Sci. 2014, 117, 19–23. [Google Scholar] [CrossRef]
- Pradhan, A.; Bagchi, A.; De, S.; Mitra, S.; Mukherjee, S.; Ghosh, P.; Ghosh, A.; Chatterjee, M. Role of redox imbalance and cytokines in mediating oxidative damage and disease progression of patients with rheumatoid arthritis. Free Radic. Res. 2019, 53, 768–779. [Google Scholar] [CrossRef]
- Odobasic, D.; Yang, Y.; Muljadi, R.C.; O’Sullivan, K.M.; Kao, W.; Smith, M.; Morand, E.F.; Holdsworth, S.R. Endogenous myeloperoxidase is a mediator of joint inflammation and damage in experimental arthritis. Arthritis Rheumatol. 2014, 66, 907–917. [Google Scholar] [CrossRef]
- Gelderman, M.P.; Stuart, R.; Vigerust, D.; Fuhrmann, S.; Lefkowitz, D.L.; Allen, R.C.; Lefkowitz, S.S.; Graham, S. Perpetuation of inflammation associated with experimental arthritis: The role of macrophage activation by neutrophilic myeloperoxidase. Mediators Inflamm. 1998, 7, 381–389. [Google Scholar] [CrossRef]
- Alcaraz, M.J.; Ferrándiz, M.L. Relevance of Nrf2 and heme oxygenase-1 in articular diseases. Free Radic. Biol. Med. 2020, 157, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Sanada, Y.; Tan, S.J.O.; Adachi, N.; Miyaki, S. Pharmacological Targeting of Heme Oxygenase-1 in Osteoarthritis. Antioxidants 2021, 10, 419. [Google Scholar] [CrossRef] [PubMed]
- Devesa, I.; Ferrándiz, M.L.; Guillén, I.; Cerdá, J.M.; Alcaraz, M.J. Potential role of heme oxygenase-1 in the progression of rat adjuvant arthritis. Lab. Investig. 2005, 85, 34–44. [Google Scholar] [CrossRef][Green Version]
- Toplak, N.; Avcin, T. Influenza and autoimmunity. Ann. N. Y. Acad. Sci. 2009, 1173, 619–626. [Google Scholar] [CrossRef]
- Furer, V.; Rondaan, C.; Heijstek, M.W.; Agmon-Levin, N.; van Assen, S.; Bijl, M.; Breedveld, F.C.; D’Amelio, R.; Dougados, M.; Kapetanovic, M.C.; et al. 2019 update of EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann. Rheum. Dis. 2020, 79, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Dirven, L.; Huizinga, T.W.; Allaart, C.F. Risk factors for reported influenza and influenza-like symptoms in patients with rheumatoid arthritis. Scand. J. Rheumatol. 2012, 41, 359–365. [Google Scholar] [CrossRef]
- Adler, S.; Krivine, A.; Weix, J.; Rozenberg, F.; Launay, O.; Huesler, J.; Guillevin, L.; Villiger, P.M. Protective effect of A/H1N1 vaccination in immune-mediated disease—A prospectively controlled vaccination study. Rheumatology 2012, 51, 695–700. [Google Scholar] [CrossRef]
- Chen, C.M.; Chen, H.J.; Chen, W.S.; Lin, C.C.; Hsu, C.C.; Hsu, Y.H. Clinical effectiveness of influenza vaccination in patients with rheumatoid arthritis. Int. J. Rheum. Dis. 2018, 21, 1246–1253. [Google Scholar] [CrossRef]
- Shoenfeld, Y.; Isenberg, D.A. The mosaic of autoimmunity. Immunol. Today 1989, 10, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Shoenfeld, Y.; Agmon-Levin, N. ‘ASIA’—Autoimmune/inflammatory syndrome induced by adjuvants. J. Autoimmun. 2011, 36, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Naik, S.R.; Wala, S.M. Arthritis, a complex connective and synovial joint destructive autoimmune disease: Animal models of arthritis with varied etiopathology and their significance. J. Postgrad. Med. 2014, 60, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Granado, M.; Martín, A.I.; Villanúa, M.A.; López-Calderón, A. Experimental arthritis inhibits the insulin-like growth factor-I axis and induces muscle wasting through cyclooxygenase-2 activation. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E1656–E1665. [Google Scholar] [CrossRef] [PubMed]
- Pinho-Ribeiro, F.A.; Verri, W.A., Jr.; Chiu, I.M. Nociceptor Sensory Neuron-Immune Interactions in Pain and Inflammation. Trends Immunol. 2017, 38, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Tseng, J.C.; Kung, A.L. In vivo imaging method to distinguish acute and chronic inflammation. J. Vis. Exp. 2013, 78, e50690. [Google Scholar]
- Van den Steen, P.E.; Proost, P.; Grillet, B.; Brand, D.D.; Kang, A.H.; Van Damme, J.; Opdenakker, G. Cleavage of denatured natural collagen type II by neutrophil gelatinase B reveals enzyme specificity, post-translational modifications in the substrate, and the formation of remnant epitopes in rheumatoid arthritis. FASEB J. 2002, 16, 379–389. [Google Scholar] [CrossRef]
- O’Neil, L.J.; Kaplan, M.J. Neutrophils in Rheumatoid Arthritis: Breaking Immune Tolerance and Fueling Disease. Trends Mol. Med. 2019, 25, 215–227. [Google Scholar] [CrossRef]
- Gruber, B.L.; Sorbi, D.; French, D.L.; Marchese, M.J.; Nuovo, G.J.; Kew, R.R.; Arbeit, L.A. Markedly elevated serum MMP-9 (gelatinase B) levels in rheumatoid arthritis: A potentially useful laboratory marker. Clin. Immunol. Immunopathol. 1996, 78, 161–171. [Google Scholar] [CrossRef]
- Helyes, Z.; Szabó, A.; Németh, J.; Jakab, B.; Pintér, E.; Bánvölgyi, A.; Kereskai, L.; Kéri, G.; Szolcsányi, J. Antiinflammatory and analgesic effects of somatostatin released from capsaicin-sensitive sensory nerve terminals in a Freund’s adjuvant-induced chronic arthritis model in the rat. Arthritis Rheum. 2004, 50, 1677–1685. [Google Scholar] [CrossRef]
- Sandell, L.J.; Aigner, T. Articular cartilage and changes in arthritis. An introduction: Cell biology of osteoarthritis. Arthritis Res. 2001, 3, 107–113. [Google Scholar] [CrossRef]
- Morel, M.; Ruscitto, A.; Pylawka, S.; Reeve, G.; Embree, M.C. Extracellular matrix turnover and inflammation in chemically-induced TMJ arthritis mouse models. PLoS ONE 2019, 14, e0223244. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B. Osteoarthritis and cartilage: The role of cytokines. Curr. Rheumatol. Rep. 2000, 2, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Kurucz, A.; Bombicz, M.; Kiss, R.; Priksz, D.; Varga, B.; Hortobágyi, T.; Trencsényi, G.; Szabó, R.; Pósa, A.; Gesztelyi, R.; et al. Heme Oxygenase-1 Activity as a Correlate to Exercise-Mediated Amelioration of Cognitive Decline and Neuropathological Alterations in an Aging Rat Model of Dementia. Biomed Res. Int. 2018, 2018, 7212861. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Takeno, M.; Saito, T.; Takeda, Y.; Kirino, Y.; Noyori, K.; Hayashi, T.; Ueda, A.; Ishigatsubo, Y. Regulatory role of heme oxygenase 1 in inflammation of rheumatoid arthritis. Arthritis Rheum. 2006, 54, 1132–1142. [Google Scholar] [CrossRef] [PubMed]
- Horváth, Á.; Tékus, V.; Boros, M.; Pozsgai, G.; Botz, B.; Borbély, É.; Szolcsányi, J.; Pintér, E.; Helyes, Z. Transient receptor potential ankyrin 1 (TRPA1) receptor is involved in chronic arthritis: In vivo study using TRPA1-deficient mice. Arthritis Res. Ther. 2016, 18, 6. [Google Scholar] [CrossRef] [PubMed]
- Nirogi, R.; Goura, V.; Shanmuganathan, D.; Jayarajan, P.; Abraham, R. Comparison of manual and automated filaments for evaluation of neuropathic pain behavior in rats. J. Pharmacol. Toxicol. Methods 2012, 66, 8–13. [Google Scholar] [CrossRef]
- Gross, S.; Gammon, S.T.; Moss, B.L.; Rauch, D.; Harding, J.; Heinecke, J.W.; Ratner, L.; Piwnica-Worms, D. Bioluminescence imaging of myeloperoxidase activity in vivo. Nat. Med. 2009, 15, 455–461. [Google Scholar] [CrossRef]
- Kenne, E.; Lindbom, L. Imaging inflammatory plasma leakage in vivo. Thromb. Haemost 2011, 105, 783–789. [Google Scholar]
- Schwab, W.; Bilgiçyildirim, A.; Funk, R.H. Microtopography of the autonomic nerves in the rat knee: A fluorescence microscopic study. Anat. Rec. 1997, 247, 109–118. [Google Scholar] [CrossRef]
| Potential Diagnostic Biomarkers | Diagnostic Biomarkers in Humans According to ACR/EULAR 2010 | Correlation with Disease Activity in Humans | Correlation with Disease Activity in Experimental Arthritis | References for Human Biomarkers | References for Experimental Biomarkers | |
|---|---|---|---|---|---|---|
| ESR | + | + | + | [15] | [16] | |
| ACPAs | + | − | + | [17,18] | [19,20] | |
| RF | + | − | + | [18,21] | [19,20] | |
| CRP | + | + | + | [22] | [16,20] | |
| MBDA | TNF-α VCAM-1 EGF VEGF-A IL-6 TNF-R1 MMP-1 MMP-3 YKL-40 leptin resistin SAA CRP | − | controversial data | controversial data | [23,24,25,26] | [27,28] |
| MPO | − | + | + | [29,30] | [31,32] | |
| HMOX-1 | − | not known | not known | [33,34] | [35] | |
| Sham Control | CFA Control | CFA Early | CFA Late | Sham Flu Early | Sham Flu Late | |
|---|---|---|---|---|---|---|
| CFA administration | − | + | + | + | − | − |
| 3Fluart | − | − | + day 1 | + day 7 | + day 1 | + day 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarjányi, V.; Ménes, Á.; Hamid, L.; Kurucz, A.; Priksz, D.; Varga, B.; Gesztelyi, R.; Kiss, R.; Horváth, Á.I.; Szentes, N.; et al. Assessing the Impact of Influenza Vaccination Timing on Experimental Arthritis: Effects on Disease Progression and Inflammatory Biomarkers. Int. J. Mol. Sci. 2024, 25, 3292. https://doi.org/10.3390/ijms25063292
Tarjányi V, Ménes Á, Hamid L, Kurucz A, Priksz D, Varga B, Gesztelyi R, Kiss R, Horváth ÁI, Szentes N, et al. Assessing the Impact of Influenza Vaccination Timing on Experimental Arthritis: Effects on Disease Progression and Inflammatory Biomarkers. International Journal of Molecular Sciences. 2024; 25(6):3292. https://doi.org/10.3390/ijms25063292
Chicago/Turabian StyleTarjányi, Vera, Ákos Ménes, Leila Hamid, Andrea Kurucz, Dániel Priksz, Balázs Varga, Rudolf Gesztelyi, Rita Kiss, Ádám István Horváth, Nikolett Szentes, and et al. 2024. "Assessing the Impact of Influenza Vaccination Timing on Experimental Arthritis: Effects on Disease Progression and Inflammatory Biomarkers" International Journal of Molecular Sciences 25, no. 6: 3292. https://doi.org/10.3390/ijms25063292
APA StyleTarjányi, V., Ménes, Á., Hamid, L., Kurucz, A., Priksz, D., Varga, B., Gesztelyi, R., Kiss, R., Horváth, Á. I., Szentes, N., Helyes, Z., Szilvássy, Z., & Bombicz, M. (2024). Assessing the Impact of Influenza Vaccination Timing on Experimental Arthritis: Effects on Disease Progression and Inflammatory Biomarkers. International Journal of Molecular Sciences, 25(6), 3292. https://doi.org/10.3390/ijms25063292









