Natural Compounds and Biomimetic Engineering to Influence Fibroblast Behavior in Wound Healing
Abstract
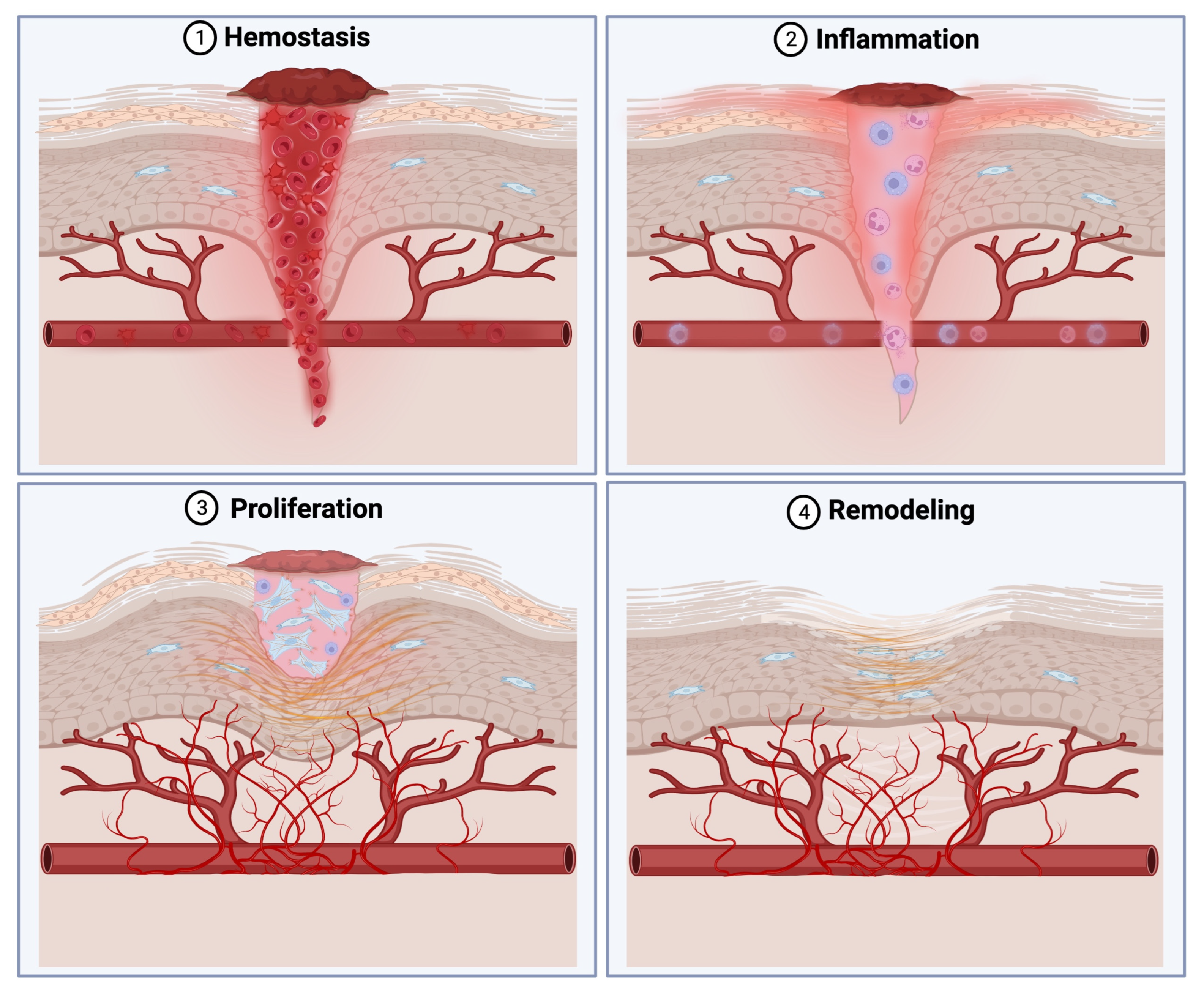
1. Introduction
2. Natural Compounds
2.1. Botanicals and Plant-Derived Products
2.2. Bacterial-Derived Products: Botulinum Toxin A
2.3. Animal-Derived Products
2.4. Exogenous Growth Factors and Growth Factor-Rich Products
3. Biomimetic Engineering of Biomaterials
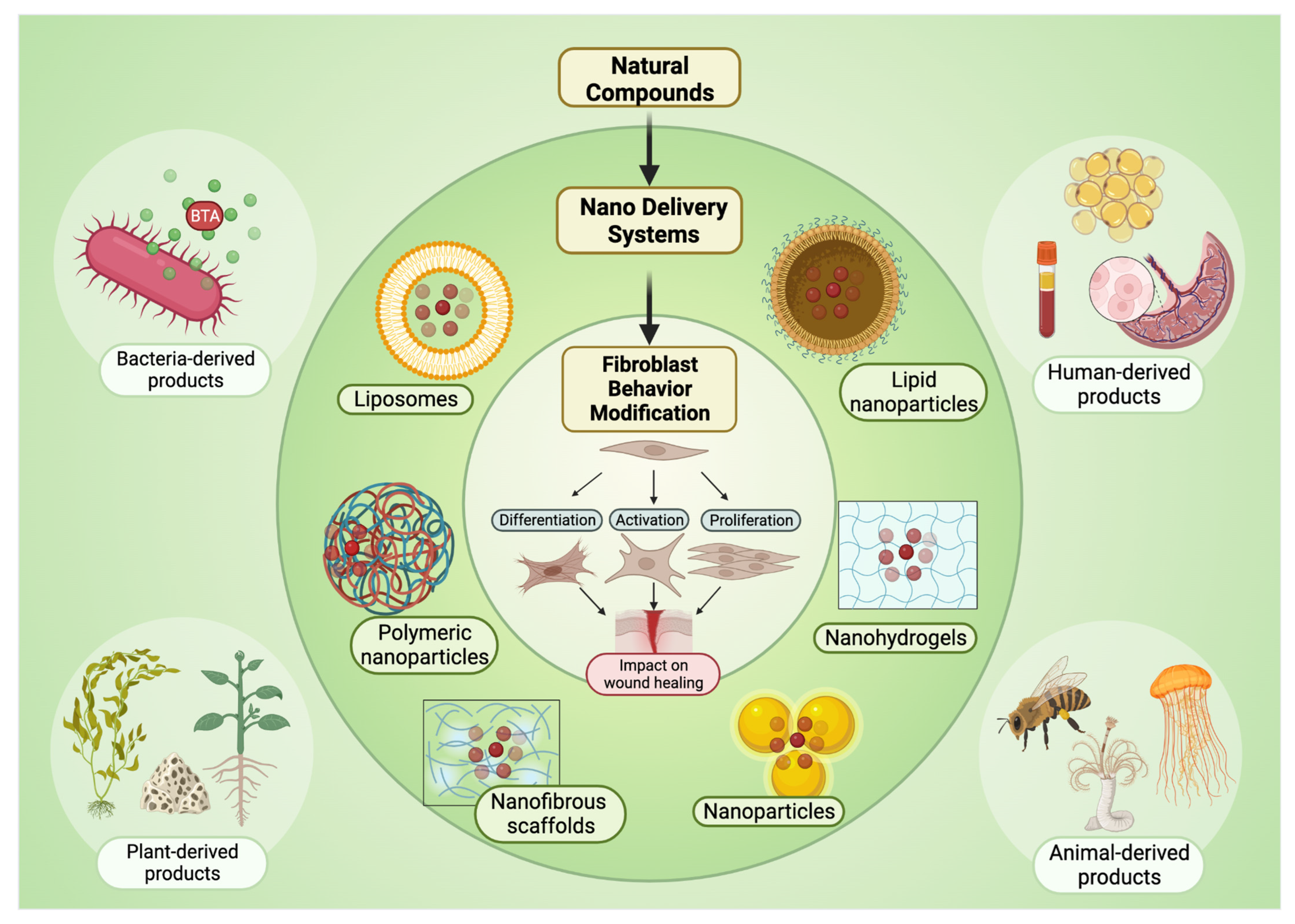
4. Natural Compound Delivery Systems
4.1. Micro Delivery Systems
4.2. Nano-Drug Delivery Systems
4.3. Liposomes and Transfersomes
4.4. Lipid Nanoparticles
4.5. Polymeric Nanoparticles
4.6. Inorganic Nanoparticles
4.7. Nanofibrous Structures (Nanofibers/Nanoscaffold)
4.8. Nanohydrogel and Hydrogels Loaded with Nanoparticles
5. Future Directions for the Field
5.1. Micro-Environment-Responsive Biomaterials
5.2. Smart Dressings
5.3. Stem Cells
6. Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AgNPs | Silver nanoparticles |
| bFGF | Basic fibroblast growth factor |
| BTA | Botulinum toxin type A |
| CTGF | Connective tissue growth factor |
| CXCL8 | C-X-C motif chemokine ligand 8 |
| DDHAM | Decellularized and dehydrated human amniotic membrane |
| ECM | Extracellular matrix |
| EGF | Epidermal growth factor |
| ES | Excretions/secretions |
| FAK | Focal adhesion kinase |
| FGF | Fibroblast growth factor |
| GF | Growth factors |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| HCM | Hydrocolloid membrane |
| IGF | Insulin-like growth factor |
| IL | Interleukin |
| KGF | Keratinocyte growth factor |
| MMPs | Matrix metalloproteinases |
| MSC | Mesenchymal stem cells |
| NPs | Nanoparticles |
| NRG1 | Neuregulin 1 |
| PCL | Polycaprolactone |
| PDGF | Platelet-derived growth factor |
| PHBV | Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) |
| PLGA | Poly(lactic-co-glycolic acid) |
| PRFM | Platelet-rich fibrin matrix |
| PRP | Platelet-rich plasma |
| PU | Polyurethane |
| ROS | Reactive oxygen species |
| SF | Silk fibroin |
| Smad | Suppressor of mothers against decapentaplegic |
| TGF-β | Transforming growth factor beta |
| TIMPs | Tissue inhibitors of metalloproteinases |
| TNF-α | Tumor necrosis factor alpha |
| YAP | Yes-associated protein |
| α-SMA | α-smooth muscle actin |
References
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Aarabi, S.; Bhatt, K.A.; Shi, Y.; Paterno, J.; Chang, E.I.; Loh, S.A.; Holmes, J.W.; Longaker, M.T.; Yee, H.; Gurtner, G.C. Mechanical load initiates hypertrophic scar formation through decreased cellular apoptosis. FASEB J. 2007, 21, 3250–3261. [Google Scholar] [CrossRef] [PubMed]
- Talbott, H.E.; Mascharak, S.; Griffin, M.; Wan, D.C.; Longaker, M.T. Wound healing, fibroblast heterogeneity, and fibrosis. Cell Stem Cell 2022, 29, 1161–1180. [Google Scholar] [CrossRef]
- Cialdai, F.; Risaliti, C.; Monici, M. Role of fibroblasts in wound healing and tissue remodeling on Earth and in space. Front. Bioeng. Biotechnol. 2022, 10, 958381. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.E.; Shabannezhad, A.; Kahrizi, A.; Akbar, A.; Safdari, S.M.; Hoseinnezhad, T.; Zahedi, M.; Sadeghi, S.; Mojarrad, M.G.; Safa, M. Tissue factor (coagulation factor III): A potential double-edge molecule to be targeted and re-targeted toward cancer. Biomark. Res. 2023, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Ellis, S.; Lin, E.J.; Tartar, D. Immunology of Wound Healing. Curr. Dermatol. Rep. 2018, 7, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Langevin, H.M.; Nedergaard, M.; Howe, A.K. Cellular control of connective tissue matrix tension. J. Cell. Biochem. 2013, 114, 1714–1719. [Google Scholar] [CrossRef] [PubMed]
- Mirastschijski, U.; Schnabel, R.; Claes, J.; Schneider, W.; Ågren, M.S.; Haaksma, C.; Tomasek, J.J. Matrix metalloproteinase inhibition delays wound healing and blocks the latent transforming growth factor-β1-promoted myofibroblast formation and function. Wound Repair Regen. 2010, 18, 223–234. [Google Scholar] [CrossRef]
- Thulabandu, V.; Chen, D.; Atit, R.P. Dermal fibroblast in cutaneous development and healing. Wiley Interdiscip. Rev. Dev. Biol. 2018, 7, e307. [Google Scholar] [CrossRef]
- Berry, C.E.; Downer, M.; Morgan, A.G.; Griffin, M.; Liang, N.E.; Kameni, L.; Parker, J.B.L.; Guo, J.; Longaker, M.T.; Wan, D.C. The effects of mechanical force on fibroblast behavior in cutaneous injury. Front. Surg. 2023, 10, 1167067. [Google Scholar] [CrossRef]
- Carmichael, S.W. The tangled web of Langer’s lines. Clin. Anat. 2014, 27, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Webb, K.; Hitchcock, R.W.; Smeal, R.M.; Li, W.; Gray, S.D.; Tresco, P.A. Cyclic strain increases fibroblast proliferation, matrix accumulation, and elastic modulus of fibroblast-seeded polyurethane constructs. J. Biomech. 2006, 39, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Barnes, L.A.; Marshall, C.D.; Leavitt, T.; Hu, M.S.; Moore, A.L.; Gonzalez, J.G.; Longaker, M.T.; Gurtner, G.C. Mechanical Forces in Cutaneous Wound Healing: Emerging Therapies to Minimize Scar Formation. Adv. Wound Care 2018, 7, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Bao, G.; Suresh, S. Cell and molecular mechanics of biological materials. Nat. Mater. 2003, 2, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Miranti, C.K.; Brugge, J.S. Sensing the environment: A historical perspective on integrin signal transduction. Nature 2002, 4, E83–E90. [Google Scholar] [CrossRef] [PubMed]
- Mascharak, S.; Desjardins-Park, H.E.; Davitt, M.F.; Griffin, M.; Borrelli, M.R.; Moore, A.L.; Chen, K.; Duoto, B.; Chinta, M.; Foster, D.S.; et al. Preventing Engrailed-1 activation in fibroblasts yields wound regeneration without scarring. Science 2021, 372, eaba2374. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, Y.-Y.; Sun, J.-E.; Lin, W.-H.; Zhou, R.-X. ILK–PI3K/AKT pathway participates in cutaneous wound contraction by regulating fibroblast migration and differentiation to myofibroblast. Lab Investig. 2016, 96, 741–751. [Google Scholar] [CrossRef]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef]
- Kuehlmann, B.; Bonham, C.A.; Zucal, I.; Prantl, L.; Gurtner, G.C. Mechanotransduction in Wound Healing and Fibrosis. J. Clin. Med. 2020, 9, 1423. [Google Scholar] [CrossRef]
- Modelling and Targeting Mechanical Forces in Organ Fibrosis|Nature Reviews Bioengineering. Available online: https://www.nature.com/articles/s44222-023-00144-3 (accessed on 29 January 2024).
- Rognoni, E.; Gomez, C.; Pisco, A.O.; Rawlins, E.L.; Simons, B.D.; Watt, F.M.; Driskell, R.R. Inhibition of β-catenin signalling in dermal fibroblasts enhances hair follicle regeneration during wound healing. Development 2016, 143, 2522–2535. [Google Scholar] [CrossRef]
- Mastrogiannaki, M.; Lichtenberger, B.M.; Reimer, A.; Collins, C.A.; Driskell, R.R.; Watt, F.M. β-Catenin Stabilization in Skin Fibroblasts Causes Fibrotic Lesions by Preventing Adipocyte Differentiation of the Reticular Dermis. J. Investig. Dermatol. 2016, 136, 1130–1142. [Google Scholar] [CrossRef]
- Hamburg-Shields, E.; DiNuoscio, G.J.; Mullin, N.K.; Lafyatis, R.; Atit, R.P. Sustained β-catenin activity in dermal fibroblasts promotes fibrosis by up-regulating expression of extracellular matrix protein-coding genes. J. Pathol. 2014, 235, 686–697. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Lin, K.; Choo, H.; He, J.; Wang, X.; Wu, Y.; Chen, X. β-Catenin Signaling Evokes Hair Follicle Senescence by Accelerating the Differentiation of Hair Follicle Mesenchymal Progenitors. Front. Cell Dev. Biol. 2022, 10, 839519. [Google Scholar] [CrossRef]
- Rahmani, W.; Sinha, S.; Biernaskie, J. Immune modulation of hair follicle regeneration. NPJ Regen. Med. 2020, 5, 9. [Google Scholar] [CrossRef]
- Chen, H.; Wang, X.; Han, J.; Fan, Z.; Sadia, S.; Zhang, R.; Guo, Y.; Jiang, Y.; Wu, Y. AKT and its related molecular feature in aged mice skin. PLoS ONE 2017, 12, e0178969. [Google Scholar] [CrossRef]
- Chen, H.; Wang, X.; Chen, Y.; Han, J.; Kong, D.; Zhu, M.; Fu, X.; Wu, Y. Pten loss in Lgr5+ hair follicle stem cells promotes SCC development. Theranostics 2019, 9, 8321–8331. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.; Tian, R.; Zhang, Y.; Drutskaya, M.S.; Wang, C.; Ge, J.; Fan, Z.; Kong, D.; Wang, X.; et al. Macrophages induce AKT/β-catenin-dependent Lgr5+ stem cell activation and hair follicle regeneration through TNF. Nat. Commun. 2017, 8, 14091. [Google Scholar] [CrossRef] [PubMed]
- Scepankova, H.; Combarros-Fuertes, P.; Fresno, J.M.; Tornadijo, M.E.; Dias, M.S.; Pinto, C.A.; Saraiva, J.A.; Estevinho, L.M. Role of Honey in Advanced Wound Care. Molecules 2021, 26, 4784. [Google Scholar] [CrossRef]
- Falbo, F.; Spizzirri, U.G.; Restuccia, D.; Aiello, F. Natural Compounds and Biopolymers-Based Hydrogels Join Forces to Promote Wound Healing. Pharmaceutics 2023, 15, 271. [Google Scholar] [CrossRef]
- Al-Qattan, M.M.; Al-Shanawani, B.N.; Alshomer, F. Botulinum toxin type A: Implications in wound healing, facial cutaneous scarring, and cleft lip repair. Ann. Saudi Med. 2013, 33, 482–488. [Google Scholar] [CrossRef]
- Geahchan, S.; Baharlouei, P.; Rahman, A. Marine Collagen: A Promising Biomaterial for Wound Healing, Skin Anti-Aging, and Bone Regeneration. Mar. Drugs 2022, 20, 61. [Google Scholar] [CrossRef]
- Ryall, C.; Duarah, S.; Chen, S.; Yu, H.; Wen, J. Advancements in Skin Delivery of Natural Bioactive Products for Wound Management: A Brief Review of Two Decades. Pharmaceutics 2022, 14, 1072. [Google Scholar] [CrossRef]
- Vitale, S.; Colanero, S.; Placidi, M.; Di Emidio, G.; Tatone, C.; Amicarelli, F.; D’alessandro, A.M. Phytochemistry and Biological Activity of Medicinal Plants in Wound Healing: An Overview of Current Research. Molecules 2022, 27, 3566. [Google Scholar] [CrossRef]
- Ataide, J.A.; Cefali, L.C.; Croisfelt, F.M.; Shimojo, A.A.M.; Oliveira-Nascimento, L.; Mazzola, P.G. Natural actives for wound healing: A review. Phytother. Res. 2018, 32, 1664–1674. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Ji, P.; Xia, P.; Song, H.; Guo, Z.; Hu, X.; Guo, Y.; Yuan, X.; Song, Y.; Shen, R.; et al. Identification and targeting of cancer-associated fibroblast signature genes for prognosis and therapy in Cutaneous melanoma. Comput. Biol. Med. 2023, 167, 107597. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.H.; Boettiger, A.N.; Moffitt, J.R.; Wang, S.; Zhuang, X. Spatially resolved, highly multiplexed RNA profiling in single cells. Science 2015, 348, aaa6090. [Google Scholar] [CrossRef] [PubMed]
- Woodley, J.P.; Lambert, D.W.; Asencio, I.O. Understanding Fibroblast Behavior in 3D Biomaterials. Tissue Eng. Part B Rev. 2022, 28, 569–578. [Google Scholar] [CrossRef]
- Combarros-Fuertes, P.; Fresno, J.M.; Estevinho, M.M.; Sousa-Pimenta, M.; Tornadijo, M.E.; Estevinho, L.M. Honey: Another Alternative in the Fight against Antibiotic-Resistant Bacteria? Antibiotics 2020, 9, 774. [Google Scholar] [CrossRef]
- Paudel, N.; Rai, M.; Adhikari, S.; Thapa, A.; Bharati, S.; Maharjan, B.; Shrestha, R.L.S.; Rav, K.; Singh, A.V. Green Extraction, Phytochemical Profiling, and Biological Evaluation of Dysphania ambrosioides: An In Silico and In Vitro Medicinal Investigation. J. Herbs Spices Med. Plants 2023, 30, 97–114. [Google Scholar] [CrossRef]
- Ponrasu, T.; Veerasubramanian, P.K.; Kannan, R.; Gopika, S.; Suguna, L.; Muthuvijayan, V. Morin incorporated polysaccharide–protein (psyllium–keratin) hydrogel scaffolds accelerate diabetic wound healing in Wistar rats. RSC Adv. 2018, 8, 2305–2314. [Google Scholar] [CrossRef]
- Hernandez-Hernandez, A.; Alarcon-Aguilar, F.; Almanza-Perez, J.; Nieto-Yañez, O.; Olivares-Sanchez, J.; Duran-Diaz, A.; Rodriguez-Monroy, M.; Canales-Martinez, M. Antimicrobial and anti-inflammatory activities, wound-healing effectiveness and chemical characterization of the latex of Jatropha neopauciflora Pax. J. Ethnopharmacol. 2017, 204, 1–7. [Google Scholar] [CrossRef]
- Tan, W.S.; Arulselvan, P.; Ng, S.-F.; Taib, C.N.M.; Sarian, M.N.; Fakurazi, S. Improvement of diabetic wound healing by topical application of Vicenin-2 hydrocolloid film on Sprague Dawley rats. BMC Complement. Altern. Med. 2019, 19, 20. [Google Scholar] [CrossRef]
- Mssillou, I.; Bakour, M.; Slighoua, M.; Laaroussi, H.; Saghrouchni, H.; Amrati, F.E.-Z.; Lyoussi, B.; Derwich, E. Investigation on wound healing effect of Mediterranean medicinal plants and some related phenolic compounds: A review. J. Ethnopharmacol. 2022, 298, 115663. [Google Scholar] [CrossRef]
- Trinh, X.-T.; Long, N.-V.; Van Anh, L.T.; Nga, P.T.; Giang, N.N.; Chien, P.N.; Nam, S.-Y.; Heo, C.-Y. A Comprehensive Review of Natural Compounds for Wound Healing: Targeting Bioactivity Perspective. Int. J. Mol. Sci. 2022, 23, 9573. [Google Scholar] [CrossRef]
- Natural Products and/or Isolated Compounds on Wound Healing. Available online: https://pubmed.ncbi.nlm.nih.gov/31186659/ (accessed on 22 February 2024).
- Wound Healing Properties of Selected Natural Products. Available online: https://pubmed.ncbi.nlm.nih.gov/30366427/ (accessed on 29 January 2024).
- Premarathna, A.D.; Ranahewa, T.; Wijesekera, S.; Harishchandra, D.; Karunathilake, K.; Waduge, R.N.; Wijesundara, R.; Jayasooriya, A.P.; Wijewardana, V.; Rajapakse, R. Preliminary screening of the aqueous extracts of twenty-three different seaweed species in Sri Lanka with in-vitro and in-vivo assays. Heliyon 2020, 6, e03918. [Google Scholar] [CrossRef]
- Hormozi, M.; Baharvand, P. Achillea biebersteinni Afan may inhibit scar formation: In vitro study. Mol. Genet. Genom. Med. 2019, 7, e640. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Fong, C.C.; Yu, W.K.; Chen, Y.; Wei, F.; Koon, C.M.; Lau, K.M.; Leung, P.C.; Lau, C.B.S.; Fung, K.P.; et al. Herbal formula Astragali Radix and Rehmanniae Radix exerted wound healing effect on human skin fibroblast cell line Hs27 via the activation of transformation growth factor (TGF-β) pathway and promoting extracellular matrix (ECM) deposition. Phytomedicine 2012, 20, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Ratcliffe, N.; Azambuja, P.; Mello, C.B. Recent advances in developing insect natural products as potential modern day medicines. Evid. Based Complement. Altern. Med. 2014, 2014, 904958. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Bian, D.; Xia, Y.; Gong, Z.; Tan, Q.; Chen, J.; Dai, Y. Identification of Major Active Ingredients Responsible for Burn Wound Healing of Centella asiatica Herbs. Evid. Based Complement. Altern. Med. 2012, 2012, 848093. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbeni, S.A.; Negm, W.A. The Wound Healing Effect of Botanicals and Pure Natural Substances Used in in Vivo Models. Inflammopharmacology 2023, 31, 755–772. [Google Scholar] [CrossRef] [PubMed]
- Singh, W.R.; Devi, H.S.; Kumawat, S.; Sadam, A.; Appukuttan, A.V.; Patel, M.R.; Lingaraju, M.C.; Singh, T.U.; Kumar, D. Angiogenic and MMPs Modulatory Effects of Icariin Improved Cutaneous Wound Healing in Rats. Eur. J. Pharmacol. 2019, 858, 172466. [Google Scholar] [CrossRef]
- Oryan, A.; Tabatabaei Naeini, A.; Moshiri, A.; Mohammadalipour, A.; Tabandeh, M.R. Modulation of Cutaneous Wound Healing by Silymarin in Rats. J. Wound Care 2012, 21, 457–464. [Google Scholar] [CrossRef]
- Li, W.; Kandhare, A.D.; Mukherjee, A.A.; Bodhankar, S.L. Hesperidin, a Plant Flavonoid Accelerated the Cutaneous Wound Healing in Streptozotocin-Induced Diabetic Rats: Role of TGF-ß/Smads and Ang-1/Tie-2 Signaling Pathways. EXCLI J. 2018, 17, 399–419. [Google Scholar] [CrossRef]
- Li, K.; Diao, Y.; Zhang, H.; Wang, S.; Zhang, Z.; Yu, B.; Huang, S.; Yang, H. Tannin Extracts from Immature Fruits of Terminalia Chebula Fructus Retz. Promote Cutaneous Wound Healing in Rats. BMC Complement. Altern. Med. 2011, 11, 86. [Google Scholar] [CrossRef]
- Porras-Reyes, B.H.; Lewis, W.H.; Roman, J.; Simchowitz, L.; Mustoe, T.A. Enhancement of wound healing by the alkaloid taspine defining mechanism of action. Exp. Biol. Med. 1993, 203, 18–25. [Google Scholar] [CrossRef]
- Salas-Oropeza, J.; Jimenez-Estrada, M.; Perez-Torres, A.; Castell-Rodriguez, A.E.; Becerril-Millan, R.; Rodriguez-Monroy, M.A.; Canales-Martinez, M.M. Wound Healing Activity of the Essential Oil of Bursera Morelensis, in Mice. Molecules 2020, 25, 1795. [Google Scholar] [CrossRef]
- Salas-Oropeza, J.; Jimenez-Estrada, M.; Perez-Torres, A.; Castell-Rodriguez, A.E.; Becerril-Millan, R.; Rodriguez-Monroy, M.A.; Jarquin-Yañez, K.; Canales-Martinez, M.M. Wound Healing Activity of α-Pinene and α-Phellandrene. Molecules 2021, 26, 2488. [Google Scholar] [CrossRef]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M.; Nabavi, S.M. Antibacterial and Antifungal Activities of Thymol: A Brief Review of the Literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Riella, K.R.; Marinho, R.R.; Santos, J.S.; Pereira-Filho, R.N.; Cardoso, J.C.; Albuquerque-Junior, R.L.C.; Thomazzi, S.M. Anti-Inflammatory and Cicatrizing Activities of Thymol, a Monoterpene of the Essential Oil from Lippia Gracilis, in Rodents. J. Ethnopharmacol. 2012, 143, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gao, Y.; Sun, X. Effect of Taspine hydrochloride on the repair of rat skin wounds by regulating keratinocyte growth factor signal. Bioengineered 2022, 13, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Vaisberg, A.J.; Milla, M.; Planas, M.d.C.; Cordova, J.L.; de Agusti, E.R.; Ferreyra, R.; Mustiga, M.d.C.; Carlin, L.; Hammond, G.B. Taspine is the cicatrizant principle in Sangre de Grado Extracted from Croton lechleri*. Plant. Med. 1989, 55, 140–143. [Google Scholar] [CrossRef]
- Wu, S.; Tian, C.; Tu, Z.; Guo, J.; Xu, F.; Qin, W.; Chang, H.; Wang, Z.; Hu, T.; Sun, X.; et al. Protective effect of total flavonoids of Engelhardia roxburghiana Wall. leaves against radiation-induced intestinal injury in mice and its mechanism. J. Ethnopharmacol. 2023, 311, 116428. [Google Scholar] [CrossRef]
- Algahtani, M.S.; Ahmad, M.Z.; Shaikh, I.A.; Abdel-Wahab, B.A.; Nourein, I.H.; Ahmad, J. Thymoquinone Loaded Topical Nanoemulgel for Wound Healing: Formulation Design and In-Vivo Evaluation. Molecules 2021, 26, 3863. [Google Scholar] [CrossRef]
- Bonnard, M.; Martin, E.; Parrot, I. Wound Healing Potential of an Oleoresin Essential Oil Chemotype from Canarium schweinfurthii Engl. Molecules 2022, 27, 7966. [Google Scholar] [CrossRef]
- Yang, D.; Chen, H.; Wei, H.; Liu, A.; Wei, D.-X.; Chen, J. Hydrogel Wound Dressings Containing Bioactive Compounds Originated from Traditional Chinese Herbs: A Review. Smart Mater. Med. 2024, 5, 153–165. [Google Scholar] [CrossRef]
- Xu, N.; Wang, L.; Guan, J.; Tang, C.; He, N.; Zhang, W.; Fu, S. Wound Healing Effects of a Curcuma Zedoaria Polysaccharide with Platelet-Rich Plasma Exosomes Assembled on Chitosan/Silk Hydrogel Sponge in a Diabetic Rat Model. Int. J. Biol. Macromol. 2018, 117, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Zhang, X.; Han, W.; Cheng, J.; Qin, Y. Wound healing effect of an Astragalus membranaceus polysaccharide and its mechanism. Mol. Med. Rep. 2017, 15, 4077–4083. [Google Scholar] [CrossRef] [PubMed]
- Prete, P.E. Growth effects of Phaenicia sericata larval extracts on fibroblasts: Mechanism for wound healing by maggot therapy. Life Sci. 1997, 60, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Baptista-Silva, S.; Borges, S.; Costa-Pinto, A.R.; Costa, R.; Amorim, M.; Dias, J.R.; Ramos, Ó.; Alves, P.; Granja, P.L.; Soares, R.; et al. In Situ Forming Silk Sericin-Based Hydrogel: A Novel Wound Healing Biomaterial. ACS Biomater. Sci. Eng. 2021, 7, 1573–1586. [Google Scholar] [CrossRef] [PubMed]
- Zancanela, D.C.; Funari, C.S.; Herculano, R.D.; Mello, V.M.; Rodrigues, C.M.; Borges, F.A.; de Barros, N.R.; Marcos, C.M.; Almeida, A.M.F.; Guastaldi, A.C. Natural rubber latex membranes incorporated with three different types of propolis: Physical-chemistry and antimicrobial behaviours. Mater. Sci. Eng. C 2019, 97, 576–582. [Google Scholar] [CrossRef]
- El-Ashram, S.; El-Samad, L.M.; Basha, A.A.; El Wakil, A. Naturally-derived targeted therapy for wound healing: Beyond classical strategies. Pharmacol. Res. 2021, 170, 105749. [Google Scholar] [CrossRef]
- Pierce, G.F.; Mustoe, T.A.; Senior, R.M.; Reed, J.; Griffin, G.L.; Thomason, A.; Deuel, T.F. In vivo incisional wound healing augmented by platelet-derived growth factor and recombinant c-sis gene homodimeric proteins. J. Exp. Med. 1988, 167, 974–987. [Google Scholar] [CrossRef]
- Dinh, T.; Braunagel, S.; Rosenblum, B.I. Growth factors in wound healing. Clin. Podiatr. Med. Surg. 2015, 32, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Larouche, J.; Sheoran, S.; Maruyama, K.; Martino, M.M. Immune Regulation of Skin Wound Healing: Mechanisms and Novel Therapeutic Targets. Adv. Wound Care 2018, 7, 209–231. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, E.S.; Sheikh, E.S.; Fetterolf, D.E. Use of dehydrated human amniotic membrane allografts to promote healing in patients with refractory non healing wounds. Int. Wound J. 2014, 11, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Todorović, V.; Peško, P.; Micev, M.; Bjelović, M.; Budeč, M.; Mićić, M.; Brašanac, D.; Ilić-Stojanović, O. Insulin-like growth factor-I in wound healing of rat skin. Regul. Pept. 2008, 150, 7–13. [Google Scholar] [CrossRef]
- Fotouhi, A.; Maleki, A.; Dolati, S.; Aghebati-Maleki, A.; Aghebati-Maleki, L. Platelet rich plasma, stromal vascular fraction and autologous conditioned serum in treatment of knee osteoarthritis. Biomed. Pharmacother. 2018, 104, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.; Yoon, D.; Cha, H.-J.; Lee, J.-S.; Chun, W. Enhancement of wound healing efficiency mediated by artificial dermis functionalized with EGF or NRG1. Biomed. Mater. 2018, 13, 045007. [Google Scholar] [CrossRef]
- Smiell, J.M.; Treadwell, T.; Hahn, H.D.; Hermans, M.H. Real-world Experience with a Decellularized Dehydrated Human Amniotic Membrane Allograft. Wounds 2015, 27, 158–169. [Google Scholar]
- Li, M.; Huang, X.; Tang, T.-Y.D.; Mann, S. Synthetic cellularity based on non-lipid micro-compartments and protocell models. Curr. Opin. Chem. Biol. 2014, 22, 1–11. [Google Scholar] [CrossRef]
- Lin, J.; Sclafani, A.P. Platelet-Rich Plasma for Skin Rejuvenation and Tissue Fill. Facial Plast. Surg. Clin. N. Am. 2018, 26, 439–446. [Google Scholar] [CrossRef]
- Hwang, J.; Jeong, Y.; Park, J.M.; Lee, K.H.; Hong, J.W.; Choi, J. Biomimetics: Forecasting the future of science, engineering, and medicine. Int. J. Nanomed. 2015, 10, 5701–5713. [Google Scholar] [CrossRef]
- Rodrigues, F.B.; Duarte, G.S.; Marques, R.E.; Castelão, M.; Ferreira, J.; Sampaio, C.; Moore, A.P.; Costa, J. Botulinum toxin type A therapy for cervical dystonia. Emergencias 2020, 11, CD003633. [Google Scholar] [CrossRef]
- Carrero, L.M.K.; Ma, W.; Liu, H.; Yin, X.; Zhou, B. Botulinum toxin type A for the treatment and prevention of hypertrophic scars and keloids: Updated review. J. Cosmet. Dermatol. 2018, 18, 10–15. [Google Scholar] [CrossRef]
- A Systematic Review and Meta-Analysis: Botulinum Toxin A Effect on Postoperative Facial Scar Prevention. Available online: https://pubmed.ncbi.nlm.nih.gov/34609526/ (accessed on 29 January 2024).
- Gassner, H.G.; Brissett, A.E.; Otley, C.C.; Boahene, D.K.; Boggust, A.J.; Weaver, A.L.; Sherris, D.A. Botulinum toxin to improve facial wound healing: A prospective, blinded, placebo-controlled study. Mayo Clin. Proc. 2006, 81, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- An, M.K.; Cho, E.B.; Park, E.J.; Kim, L.S.; Kim, K.J. Appropriate Timing of Early Postoperative Botulinum Toxin Type A Injection for Thyroidectomy Scar Management: A Split-Scar Study. Plast. Reconstr. Surg. 2019, 144, 659e–668e. [Google Scholar] [CrossRef]
- Liu, D.-Q.; Li, X.-J.; Weng, X.-J. Effect of BTXA on Inhibiting Hypertrophic Scar Formation in a Rabbit Ear Model. Aesthetic Plast. Surg. 2017, 41, 721–728. [Google Scholar] [CrossRef]
- Jeong, H.S.; Lee, B.H.; Sung, H.M.; Park, S.Y.; Ahn, D.K.; Jung, M.S.; Suh, I.S. Effect of Botulinum Toxin Type A on Differentiation of Fibroblasts Derived from Scar Tissue. Plast. Reconstr. Surg. 2015, 136, 171e–178e. [Google Scholar] [CrossRef]
- Austin, E.; Koo, E.; Jagdeo, J. The Cellular Response of Keloids and Hypertrophic Scars to Botulinum Toxin A: A Comprehensive Literature Review. Dermatol. Surg. 2018, 44, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Li, D.; Luo, Y.; Li, J.; Wang, Y. Effects of Botulinum Toxin Type A on Microvessels in Hypertrophic Scar Models on Rabbit Ears. BioMed Res. Int. 2020, 2020, 2170750. [Google Scholar] [CrossRef] [PubMed]
- Chandika, P.; Ko, S.-C.; Jung, W.-K. Marine-derived biological macromolecule-based biomaterials for wound healing and skin tissue regeneration. Int. J. Biol. Macromol. 2015, 77, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Dolati, S.; Yousefi, M.; Pishgahi, A.; Nourbakhsh, S.; Pourabbas, B.; Shakouri, S.K. Prospects for the application of growth factors in wound healing. Growth Factors 2020, 38, 25–34. [Google Scholar] [CrossRef]
- Walraven, M.; Akershoek, J.J.; Beelen, R.H.J.; Ulrich, M.M.W. In vitro cultured fetal fibroblasts have myofibroblast-associated characteristics and produce a fibrotic-like environment upon stimulation with TGF-β1: Is there a thin line between fetal scarless healing and fibrosis? Arch. Dermatol. Res. 2017, 309, 111–121. [Google Scholar] [CrossRef]
- Borena, B.M.; Martens, A.; Broeckx, S.Y.; Meyer, E.; Chiers, K.; Duchateau, L.; Spaas, J.H. Regenerative Skin Wound Healing in Mammals: State-of-the-Art on Growth Factor and Stem Cell Based Treatments. Cell. Physiol. Biochem. 2015, 36, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Nabipour, M.; Mellati, A.; Abasi, M.; Barough, S.E.; Karimizade, A.; Banikarimi, P.; Hasanzadeh, E. Preparation of bilayer tissue-engineered polyurethane/poly-L-lactic acid nerve conduits and their in vitro characterization for use in peripheral nerve regeneration. J. Biol. Eng. 2024, 18, 16. [Google Scholar] [CrossRef]
- Tolmacheva, N.; Bhattacharyya, A.; Noh, I. Calcium Phosphate Biomaterials for 3D Bioprinting in Bone Tissue Engineering. Biomimetics 2024, 9, 95. [Google Scholar] [CrossRef]
- Alfarano, M.; Pastore, D.; Fogliano, V.; Schalkwijk, C.G.; Oliviero, T. The Effect of Sulforaphane on Glyoxalase I Expression and Activity in Peripheral Blood Mononuclear Cells. Nutrients 2018, 10, 1773. [Google Scholar] [CrossRef]
- Ma, J.; Qin, C.; Wu, J.; Zhuang, H.; Du, L.; Xu, J.; Wu, C. 3D multicellular micropatterning biomaterials for hair regeneration and vascularization. Mater. Horiz. 2023, 10, 3773–3784. [Google Scholar] [CrossRef]
- Singh, V.; Marimuthu, T.; Makatini, M.M.; Choonara, Y.E. Biopolymer-Based Wound Dressings with Biochemical Cues for Cell-Instructive Wound Repair. Polymers 2022, 14, 5371. [Google Scholar] [CrossRef]
- Hama, R.; Reinhardt, J.W.; Ulziibayar, A.; Watanabe, T.; Kelly, J.; Shinoka, T. Recent Tissue Engineering Approaches to Mimicking the Extracellular Matrix Structure for Skin Regeneration. Biomimetics 2023, 8, 130. [Google Scholar] [CrossRef]
- Salber, J.; Gräter, S.; Harwardt, M.; Hofmann, M.; Klee, D.; Dujic, J.; Jinghuan, H.; Ding, J.; Kippenberger, S.; Bernd, A.; et al. Influence of different ECM mimetic peptide sequences embedded in a nonfouling environment on the specific adhesion of human-skin keratinocytes and fibroblasts on deformable substrates. Small 2007, 3, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Aghmiuni, A.I.; Keshel, S.H.; Sefat, F.; Khiyavi, A.A. Quince seed mucilage-based scaffold as a smart biological substrate to mimic mechanobiological behavior of skin and promote fibroblasts proliferation and h-ASCs differentiation into keratinocytes. Int. J. Biol. Macromol. 2020, 142, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Oria, R.; Wiegand, T.; Escribano, J.; Elosegui-Artola, A.; Uriarte, J.J.; Moreno-Pulido, C.; Platzman, I.; Delcanale, P.; Albertazzi, L.; Navajas, D.; et al. Force loading explains spatial sensing of ligands by cells. Nature 2017, 552, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Mobasseri, S.A.; Zijl, S.; Salameti, V.; Walko, G.; Stannard, A.; Garcia-Manyes, S.; Watt, F.M. Patterning of human epidermal stem cells on undulating elastomer substrates reflects differences in cell stiffness. Acta Biomater. 2019, 87, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.; Chung, H.; Lee, G.; Kim, C.; Kim, D.; Oh, S.J.; Kim, S.-H.; Lee, K. Hydrogel/Nanofiber Composite Wound Dressing Optimized for Skin Layer Regeneration through the Mechanotransduction-Based Microcellular Environment. ACS Appl. Bio Mater. 2023, 6, 1774–1786. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xiao, L.; Hu, C.; Shen, Z.; Zhou, E.; Zhang, S.; Wang, Y. Aligned lovastatin-loaded electrospun nanofibers regulate collagen organization and reduce scar formation. Acta Biomater. 2023, 164, 240–252. [Google Scholar] [CrossRef]
- Tracy, L.E.; Minasian, R.A.; Caterson, E. Extracellular Matrix and Dermal Fibroblast Function in the Healing Wound. Adv. Wound Care 2016, 5, 119–136. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Sainio, A.; Järveläinen, H. Extracellular matrix-cell interactions: Focus on therapeutic applications. Cell. Signal. 2020, 66, 109487. [Google Scholar] [CrossRef]
- Majno, G.; Gabbiani, G.; Hirschel, B.J.; Ryan, G.B.; Statkov, P.R. Contraction of granulation tissue in vitro: Similarity to smooth muscle. Science 1971, 173, 548–550. [Google Scholar] [CrossRef]
- Zahedi, G.; Amraei, S.; Biglari, M. Simulation and optimization of ethanol amine production plant. Korean J. Chem. Eng. 2009, 26, 1504–1511. [Google Scholar] [CrossRef]
- Kim, H.S.; Chen, J.; Wu, L.-P.; Wu, J.; Xiang, H.; Leong, K.W.; Han, J. Prevention of excessive scar formation using nanofibrous meshes made of biodegradable elastomer poly(3-hydroxybutyrate-co-3-hydroxyvalerate). J. Tissue Eng. 2020, 11, 2041731420949332. [Google Scholar] [CrossRef]
- Gholipourmalekabadi, M.; Sapru, S.; Samadikuchaksaraei, A.; Reis, R.L.; Kaplan, D.L.; Kundu, S.C. Silk fibroin for skin injury repair: Where do things stand? Adv. Drug Deliv. Rev. 2020, 153, 28–53. [Google Scholar] [CrossRef]
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Morshed, M.; Nasr-Esfahani, M.-H.; Ramakrishna, S. Electrospun poly(ɛ-caprolactone)/gelatin nanofibrous scaffolds for nerve tissue engineering. Biomaterials 2008, 29, 4532–4539. [Google Scholar] [CrossRef]
- Yang, M.; Yu, S.; Zhao, P.; Xie, L.; Lyu, G.; Yu, J. Fabrication of homogeneously-aligned nano-fillers encapsulated silk fibroin electrospun nanofibers for improved fibroblast attachment, epithelialization, and collagen depositions: In vitro and in vivo wound healing evaluation. J. Biomater. Sci. Polym. Ed. 2022, 33, 878–899. [Google Scholar] [CrossRef] [PubMed]
- Bugg, D.; Bretherton, R.C.; Kim, P.; Olszewski, E.; Nagle, A.; Schumacher, A.E.; Chu, N.; Gunaje, J.; DeForest, C.A.; Stevens, K.; et al. Infarct Collagen Topography Regulates Fibroblast Fate via p38-Yes-Associated Protein Transcriptional Enhanced Associate Domain Signals. Circ. Res. 2020, 127, 1306–1322. [Google Scholar] [CrossRef] [PubMed]
- Walraven, M.; Hinz, B. Therapeutic approaches to control tissue repair and fibrosis: Extracellular matrix as a game changer. Matrix Biol. 2018, 71–72, 205–224. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Hou, T.; Wang, L.; Liu, Y.; Guo, J.; Zhang, L.; Yang, T.; Tang, W.; An, M.; Wen, M. Aligned electrospun fibers of different diameters for improving cell migration capacity. Colloids Surf. B Biointerfaces 2023, 234, 113674. [Google Scholar] [CrossRef] [PubMed]
- Rippon, M.; Ousey, K.; Cutting, K. Wound healing and hyper-hydration: A counterintuitive model. J. Wound Care 2016, 25, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Barua, S.; Mitragotri, S. Challenges associated with Penetration of Nanoparticles across Cell and Tissue Barriers: A Review of Current Status and Future Prospects. Nano Today 2014, 9, 223–243. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.X.; Nguyen, C.N. Microneedle-Mediated Transdermal Delivery of Biopharmaceuticals. Pharmaceutics 2023, 15, 277. [Google Scholar] [CrossRef]
- Peng, S.; Cheng, L.; Wu, Q.; Li, Y.; Ran, L.; Wang, W.; Huang, K.; Zhu, R.; Xue, S.; Zhou, C.; et al. A Modified Hyaluronic Acid–Based Dissolving Microneedle Loaded With Daphnetin Improved the Treatment of Psoriasis. Front. Bioeng. Biotechnol. 2022, 10, 900274. [Google Scholar] [CrossRef]
- Vasudevan, D.T.; Rajan, R. Effect of permeation enhancers on the penetration mechanism of transfersomal gel of ketoconazole. J. Adv. Pharm. Technol. Res. 2012, 3, 112–116. [Google Scholar] [CrossRef]
- Zhong, S.P.; Zhang, Y.Z.; Lim, C.T. Tissue scaffolds for skin wound healing and dermal reconstruction. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 510–525. [Google Scholar] [CrossRef]
- Negut, I.; Dorcioman, G.; Grumezescu, V. Scaffolds for Wound Healing Applications. Polymers 2020, 12, 2010. [Google Scholar] [CrossRef]
- Kianvash, N.; Bahador, A.; Pourhajibagher, M.; Ghafari, H.; Nikoui, V.; Rezayat, S.M.; Dehpour, A.R.; Partoazar, A. Evaluation of Propylene Glycol Nanoliposomes Containing Curcumin on Burn Wound Model in Rat: Biocompatibility, Wound Healing, and Anti-Bacterial Effects. Drug Deliv. Transl. Res. 2017, 7, 654–663. [Google Scholar] [CrossRef]
- Li, Z.; Liu, M.; Wang, H.; Du, S. Increased Cutaneous Wound Healing Effect of Biodegradable Liposomes Containing Madecassoside: Preparation Optimization, in Vitro Dermal Permeation, and in Vivo Bioevaluation. Int. J. Nanomed. 2016, 11, 2995–3007. [Google Scholar] [CrossRef]
- Gainza, G.; Pastor, M.; Aguirre, J.J.; Villullas, S.; Pedraz, J.L.; Hernandez, R.M.; Igartua, M. A novel strategy for the treatment of chronic wounds based on the topical administration of rhEGF-loaded lipid nanoparticles: In vitro bioactivity and in vivo effectiveness in healing-impaired db/db mice. J. Control. Release 2014, 185, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Chen, P.; ZhuGe, D.; Zhu, Q.; Jin, B.; Shen, B.; Xiao, J.; Zhao, Y. Liposomes with Silk Fibroin Hydrogel Core to Stabilize bFGF and Promote the Wound Healing of Mice with Deep Second-Degree Scald. Adv. Health Mater. 2017, 6, 1700344. [Google Scholar] [CrossRef] [PubMed]
- Nunes, P.S.; Rabelo, A.S.; de Souza, J.C.C.; Santana, B.V.; da Silva, T.M.M.; Serafini, M.R.; Menezes, P.d.P.; Lima, B.d.S.; Cardoso, J.C.; Alves, J.C.S.; et al. Gelatin-based membrane containing usnic acid-loaded liposome improves dermal burn healing in a porcine model. Int. J. Pharm. 2016, 513, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Cho, S.-W.; Son, S.M.; Bogatyrev, S.R.; Singh, D.; Green, J.J.; Mei, Y.; Park, S.; Bhang, S.H.; Kim, B.-S.; et al. Genetic engineering of human stem cells for enhanced angiogenesis using biodegradable polymeric nanoparticles. Proc. Natl. Acad. Sci. USA 2010, 107, 3317–3322. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.U.; Lee, S.W.; Pangeni, R.; Byun, Y.; Yoon, I.-S.; Park, J.W. Preparation and in Vivo Evaluation of Cationic Elastic Liposomes Comprising Highly Skin-Permeable Growth Factors Combined with Hyaluronic Acid for Enhanced Diabetic Wound-Healing Therapy. Acta Biomater. 2017, 57, 197–215. [Google Scholar] [CrossRef]
- Chereddy, K.K.; Her, C.-H.; Comune, M.; Moia, C.; Lopes, A.; Porporato, P.E.; Vanacker, J.; Lam, M.C.; Steinstraesser, L.; Sonveaux, P.; et al. PLGA nanoparticles loaded with host defense peptide LL37 promote wound healing. J. Control. Release 2014, 194, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Ziv-Polat, O.; Topaz, M.; Brosh, T.; Margel, S. Enhancement of incisional wound healing by thrombin conjugated iron oxide nanoparticles. Biomaterials 2010, 31, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, A.; Paolino, D.; Costa, N.; Fresta, M.; Cosco, D. Zein- vs PLGA-based nanoparticles containing rutin: A comparative investigation. Mater. Sci. Eng. C 2020, 118, 111538. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Zhang, H.; Yang, S.; Xi, Z.; Tang, T.; Yin, R.; Zhang, W.; Pignatello, R.; Cenni, E.; Micieli, D.; et al. Electrospun PLGA membrane incorporated with andrographolide-loaded mesoporous silica nanoparticles for sustained antibacterial wound dressing. Nanomedicine 2018, 13, 2881–2899. [Google Scholar] [CrossRef]
- Ali, S.S.; Morsy, R.; El-Zawawy, N.A.; Fareed, M.F.; Bedaiwy, M.Y. Synthesized Zinc Peroxide Nanoparticles (ZnO2-NPs): A Novel Antimicrobial, Anti-Elastase, Anti-Keratinase, and Anti-Inflammatory Approach toward Polymicrobial Burn Wounds. Int. J. Nanomed. 2017, 12, 6059–6073. [Google Scholar] [CrossRef]
- Shan, Y.-H.; Peng, L.-H.; Liu, X.; Chen, X.; Xiong, J.; Gao, J.-Q. Silk fibroin/gelatin electrospun nanofibrous dressing functionalized with astragaloside IV induces healing and anti-scar effects on burn wound. Int. J. Pharm. 2015, 479, 291–301. [Google Scholar] [CrossRef]
- Sanchez, D.A.; Schairer, D.; Tuckman-Vernon, C.; Chouake, J.; Kutner, A.; Makdisi, J.; Friedman, J.M.; Nosanchuk, J.D.; Friedman, A.J. Amphotericin B Releasing Nanoparticle Topical Treatment of Candida Spp. in the Setting of a Burn Wound. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 269–277. [Google Scholar] [CrossRef]
- Aly, U.F.; Aboutaleb, H.A.; Abdellatif, A.A.; Tolba, N.S. Formulation and evaluation of simvastatin polymeric nanoparticles loaded in hydrogel for optimum wound healing purpose. Drug Des. Dev. Ther. 2019, 13, 1567–1580. [Google Scholar] [CrossRef]
- Nunes, D.; Andrade, S.; Ramalho, M.J.; Loureiro, J.A.; Pereira, M.C. Polymeric Nanoparticles-Loaded Hydrogels for Biomedical Applications: A Systematic Review on In Vivo Findings. Polymers 2022, 14, 1010. [Google Scholar] [CrossRef]
- Cardoso, A.M.; de Oliveira, E.G.; Coradini, K.; Bruinsmann, F.A.; Aguirre, T.; Lorenzoni, R.; Barcelos, R.C.S.; Roversi, K.; Rossato, D.R.; Pohlmann, A.R.; et al. Chitosan hydrogels containing nanoencapsulated phenytoin for cutaneous use: Skin permeation/penetration and efficacy in wound healing. Mater. Sci. Eng. C 2019, 96, 205–217. [Google Scholar] [CrossRef]
- Xie, Z.; Paras, C.B.; Weng, H.; Punnakitikashem, P.; Su, L.-C.; Vu, K.; Tang, L.; Yang, J.; Nguyen, K.T. Dual Growth Factor Releasing Multi-Functional Nanofibers for Wound Healing. Acta Biomater. 2013, 9, 9351–9359. [Google Scholar] [CrossRef] [PubMed]
- Abadehie, F.S.; Dehkordi, A.H.; Zafari, M.; Bagheri, M.; Yousefiasl, S.; Pourmotabed, S.; Mahmoodnia, L.; Validi, M.; Ashrafizadeh, M.; Zare, E.N.; et al. Lawsone-Encapsulated Chitosan/Polyethylene Oxide Nanofibrous Mat as a Potential Antibacterial Biobased Wound Dressing. Eng. Regen. 2021, 2, 219–226. [Google Scholar] [CrossRef]
- Liu, J.; Jia, B.; Li, Z.; Li, W. Reactive oxygen species-responsive polymer drug delivery systems. Front. Bioeng. Biotechnol. 2023, 11, 1115603. [Google Scholar] [CrossRef]
- Lokhande, G.; Carrow, J.K.; Thakur, T.; Xavier, J.R.; Parani, M.; Bayless, K.J.; Gaharwar, A.K. Nanoengineered Injectable Hydrogels for Wound Healing Application. Acta Biomater. 2018, 70, 35–47. [Google Scholar] [CrossRef]
- Xi Loh, E.Y.; Fauzi, M.B.; Ng, M.H.; Ng, P.Y.; Ng, S.F.; Ariffin, H.; Mohd Amin, M.C.I. Cellular and Molecular Interaction of Human Dermal Fibroblasts with Bacterial Nanocellulose Composite Hydrogel for Tissue Regeneration. ACS Appl. Mater. Interfaces 2018, 10, 39532–39543. [Google Scholar] [CrossRef] [PubMed]
- Dehkordi, N.K.; Minaiyan, M.; Talebi, A.; Akbari, V.; Taheri, A. Nanocrystalline cellulose–hyaluronic acid composite enriched with GM-CSF loaded chitosan nanoparticles for enhanced wound healing. Biomed. Mater. 2019, 14, 035003. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, B.; Lv, D.; Li, W.; Lu, Y.; Luo, G. Biomaterials releasing drug responsively to promote wound healing via regulation of pathological microenvironment. Adv. Drug Deliv. Rev. 2023, 196, 114778. [Google Scholar] [CrossRef]
- Karavana, S.Y.; Gökçe, E.H.; Rençber, S.; Özbal, S.; Pekçetin, Ç.; Güneri, P.; Ertan, G. A New Approach to the Treatment of Recurrent Aphthous Stomatitis with Bioadhesive Gels Containing Cyclosporine A Solid Lipid Nanoparticles: In Vivo/in Vitro Examinations. Int. J. Nanomed. 2012, 7, 5693–5704. [Google Scholar] [CrossRef]
- Thanusha, A.V.; Dinda, A.K.; Koul, V. Evaluation of nano hydrogel composite based on gelatin/HA/CS suffused with Asiatic acid/ZnO and CuO nanoparticles for second degree burns. Mater. Sci. Eng. C 2018, 89, 378–386. [Google Scholar] [CrossRef]
- Manukumar, H.M.; Chandrasekhar, B.; Rakesh, K.P.; Ananda, A.P.; Nandhini, M.; Lalitha, P.; Sumathi, S.; Qin, H.-L.; Umesha, S. Novel T-C@AgNPs mediated biocidal mechanism against biofilm associated methicillin-resistant Staphylococcus aureus (Bap-MRSA) 090, cytotoxicity and its molecular docking studies. MedChemComm 2017, 8, 2181–2194. [Google Scholar] [CrossRef]
- Maheen, S.; Younis, H.; Khan, H.U.; Ali, S.; Rehman, A.U.; Ilyas, S.; Zafar, M.N.; Shafqat, S.R.; Kalam, A.; Al-Ghamdi, A.A. Enhanced Antifungal and Wound Healing Efficacy of Statistically Optimized, Physicochemically Evaluated Econazole-Triamcinolone Loaded Silica Nanoparticles. Front. Chem. 2022, 10, 836678. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Sun, X.; Lee, J.-H.; Kim, H.-W.; Fu, X.; Leong, K.W. Advanced drug delivery systems and artificial skin grafts for skin wound healing. Adv. Drug Deliv. Rev. 2019, 146, 209–239. [Google Scholar] [CrossRef]
- Alexander, A.; Ajazuddin; Patel, R.J.; Saraf, S. Recent expansion of pharmaceutical nanotechnologies and targeting strategies in the field of phytopharmaceuticals for the delivery of herbal extracts and bioactives. J. Control. Release 2016, 241, 110–124. [Google Scholar] [CrossRef]
- Adhirajan, N.; Shanmugasundaram, N.; Shanmuganathan, S.; Babu, M. Functionally modified gelatin microspheres impregnated collagen scaffold as novel wound dressing to attenuate the proteases and bacterial growth. Eur. J. Pharm. Sci. 2009, 36, 235–245. [Google Scholar] [CrossRef]
- Ribeiro, M.P.; Morgado, P.I.; Miguel, S.A.P.; Coutinho, P.; Correia, I.J. Dextran-based hydrogel containing chitosan microparticles loaded with growth factors to be used in wound healing. Mater. Sci. Eng. C 2013, 33, 2958–2966. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Baker, A.B. Biomaterials and Nanotherapeutics for Enhancing Skin Wound Healing. Front. Bioeng. Biotechnol. 2016, 4, 82. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Orue, I.; Gainza, G.; Garcia-Garcia, P.; Gutierrez, F.B.; Aguirre, J.J.; Hernandez, R.M.; Delgado, A.; Igartua, M. Composite nanofibrous membranes of PLGA/Aloe vera containing lipid nanoparticles for wound dressing applications. Int. J. Pharm. 2019, 556, 320–329. [Google Scholar] [CrossRef]
- Wang, W.; Lu, K.-J.; Yu, C.-H.; Huang, Q.-L.; Du, Y.-Z. Nano-drug delivery systems in wound treatment and skin regeneration. J. Nanobiotechnol. 2019, 17, 82. [Google Scholar] [CrossRef] [PubMed]
- Losi, P.; Briganti, E.; Magera, A.; Spiller, D.; Ristori, C.; Battolla, B.; Balderi, M.; Kull, S.; Balbarini, A.; Di Stefano, R.; et al. Tissue response to poly(ether)urethane-polydimethylsiloxane-fibrin composite scaffolds for controlled delivery of pro-angiogenic growth factors. Biomaterials 2010, 31, 5336–5344. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Chattopadhyay, P.; Islam, J.; Ray, S.; Raju, P.S.; Mazumder, B. Aspects of Nanomaterials in Wound Healing. Curr. Drug Deliv. 2019, 16, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Sandri, G.; Miele, D.; Faccendini, A.; Bonferoni, M.C.; Rossi, S.; Grisoli, P.; Taglietti, A.; Ruggeri, M.; Bruni, G.; Vigani, B.; et al. Chitosan/Glycosaminoglycan Scaffolds: The Role of Silver Nanoparticles to Control Microbial Infections in Wound Healing. Polymers 2019, 11, 1207. [Google Scholar] [CrossRef]
- Mitragotri, S.; Burke, P.A.; Langer, R. Overcoming the challenges in administering biopharmaceuticals: Formulation and delivery strategies. Nat. Rev. Drug Discov. 2014, 13, 655–672. [Google Scholar] [CrossRef] [PubMed]
- Dawoud, M.H.S.; Yassin, G.E.; Ghorab, D.M.; Morsi, N.M. Insulin Mucoadhesive Liposomal Gel for Wound Healing: A Formulation with Sustained Release and Extended Stability Using Quality by Design Approach. AAPS PharmSciTech 2019, 20, 158. [Google Scholar] [CrossRef]
- Manconi, M.; Manca, M.L.; Caddeo, C.; Valenti, D.; Cencetti, C.; Diez-Sales, O.; Nacher, A.; Mir-Palomo, S.; Terencio, M.C.; Demurtas, D.; et al. Nanodesign of new self-assembling core-shell gellan-transfersomes loading baicalin and in vivo evaluation of repair response in skin. Nanomedicine 2018, 14, 569–579. [Google Scholar] [CrossRef]
- Shalaby, M.; Hamouda, D.; Khedr, S.M.; Mostafa, H.M.; Saeed, H.; Ghareeb, A.Z. Nanoparticles fabricated from the bioactive tilapia scale collagen for wound healing: Experimental approach. PLoS ONE 2023, 18, e0282557. [Google Scholar] [CrossRef]
- Chigurupati, S.; Mughal, M.R.; Okun, E.; Das, S.; Kumar, A.; McCaffery, M.; Seal, S.; Mattson, M.P. Effects of cerium oxide nanoparticles on the growth of keratinocytes, fibroblasts and vascular endothelial cells in cutaneous wound healing. Biomaterials 2013, 34, 2194–2201. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Lee, J.; Jang, M.; Kwak, M.H.; Go, J.; Kho, E.K.; Song, S.H.; Sung, J.E.; Lee, J.; Hwang, D.Y. Accelerated healing of cutaneous wounds using phytochemically stabilized gold nanoparticle deposited hydrocolloid membranes. Biomater. Sci. 2015, 3, 509–519. [Google Scholar] [CrossRef]
- Mahmoud, N.N.; Hikmat, S.; Abu Ghith, D.; Hajeer, M.; Hamadneh, L.; Qattan, D.; Khalil, E.A. Gold nanoparticles loaded into polymeric hydrogel for wound healing in rats: Effect of nanoparticles’ shape and surface modification. Int. J. Pharm. 2019, 565, 174–186. [Google Scholar] [CrossRef]
- Wallace, L.A.; Gwynne, L.; Jenkins, T. Challenges and opportunities of pH in chronic wounds. Ther. Deliv. 2019, 10, 719–735. [Google Scholar] [CrossRef]
- Miranda-Calderon, L.; Yus, C.; Landa, G.; Mendoza, G.; Arruebo, M.; Irusta, S. Pharmacokinetic control on the release of antimicrobial drugs from pH-responsive electrospun wound dressings. Int. J. Pharm. 2022, 624, 122003. [Google Scholar] [CrossRef]
- Pan, F.; Giovannini, G.; Zhang, S.; Altenried, S.; Zuber, F.; Chen, Q.; Boesel, L.F.; Ren, Q. pH-responsive silica nanoparticles for the treatment of skin wound infections. Acta Biomater. 2022, 145, 172–184. [Google Scholar] [CrossRef]
- Sobczak, M. Enzyme-Responsive Hydrogels as Potential Drug Delivery Systems—State of Knowledge and Future Prospects. Int. J. Mol. Sci. 2022, 23, 4421. [Google Scholar] [CrossRef]
- Mohanty, A.R.; Ravikumar, A.; Peppas, N.A. Recent advances in glucose-responsive insulin delivery systems: Novel hydrogels and future applications. Regen. Biomater. 2022, 9, rbac056. [Google Scholar] [CrossRef]
- Li, Z.; Huang, J.; Jiang, Y.; Liu, Y.; Qu, G.; Chen, K.; Zhao, Y.; Wang, P.; Wu, X.; Ren, J. Novel Temperature-Sensitive Hydrogel Promotes Wound Healing through YAP and MEK-Mediated Mechanosensitivity. Adv. Health Mater. 2022, 11, e2201878. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Fei, X.; Xu, L.; Wang, Y.; Tian, J.; Li, Y. Pressure-sensitive antibacterial hydrogel dressing for wound monitoring in bed ridden patients. J. Colloid Interface Sci. 2022, 627, 942–955. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Zhang, W.; Ren, M.; Li, Y.; Wang, Y.; Zhou, Y.; Wu, Y.; Zhang, Z.; Di, J. Moisture-Adaptive Contractile Biopolymer-Derived Fibers for Wound Healing Promotion. Small 2023, 19, e2300589. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, T.; Zhao, C.; Li, J.; Huang, R.; Zhang, Q.; Li, Y.; Li, X. An Integrated Smart Sensor Dressing for Real-Time Wound Microenvironment Monitoring and Promoting Angiogenesis and Wound Healing. Front. Cell Dev. Biol. 2021, 9, 701525. [Google Scholar] [CrossRef]
- Liang, Y.; Li, M.; Yang, Y.; Qiao, L.; Xu, H.; Guo, B. pH/Glucose Dual Responsive Metformin Release Hydrogel Dressings with Adhesion and Self-Healing via Dual-Dynamic Bonding for Athletic Diabetic Foot Wound Healing. ACS Nano 2022, 16, 3194–3207. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, L.; Scott, P.G.; Tredget, E.E. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 2007, 25, 2648–2659. [Google Scholar] [CrossRef]
- Kim, W.-S.; Park, B.-S.; Sung, J.-H.; Yang, J.-M.; Park, S.-B.; Kwak, S.-J.; Park, J.-S. Wound healing effect of adipose-derived stem cells: A critical role of secretory factors on human dermal fibroblasts. J. Dermatol. Sci. 2007, 48, 15–24. [Google Scholar] [CrossRef]
- François, S.; Mouiseddine, M.; Mathieu, N.; Semont, A.; Monti, P.; Dudoignon, N.; Saché, A.; Boutarfa, A.; Thierry, D.; Gourmelon, P.; et al. Human mesenchymal stem cells favour healing of the cutaneous radiation syndrome in a xenogenic transplant model. Ann. Hematol. 2007, 86, 1–8. [Google Scholar] [CrossRef]
- Vojtaššák, J.; Danišovič, Ľ.; Kubeš, M.; Bakoš, D.; Jarábek, L.; Uličná, M.; Blaško, M. Autologous biograft and mesenchymal stem cells in treatment of the diabetic foot. Neuro Endocrinol. Lett. 2006, 27 (Suppl. S2), 134–137. [Google Scholar]
- Yates, C.C.; Rodrigues, M.; Nuschke, A.; Johnson, Z.I.; Whaley, D.; Stolz, D.; Newsome, J.; Wells, A. Multipotent stromal cells/mesenchymal stem cells and fibroblasts combine to minimize skin hypertrophic scarring. Stem Cell Res. Ther. 2017, 8, 193. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Jiang, D.; Sindrilaru, A.; Stegemann, A.; Schatz, S.; Treiber, N.; Rojewski, M.; Schrezenmeier, H.; Vander Beken, S.; Wlaschek, M.; et al. TSG-6 released from intradermally injected mesenchymal stem cells accelerates wound healing and reduces tissue fibrosis in murine full-thickness skin wounds. J. Investig. Dermatol. 2014, 134, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.F.; Bártolo, P.J. Traditional Therapies for Skin Wound Healing. Adv. Wound Care 2016, 5, 208–229. [Google Scholar] [CrossRef] [PubMed]
- Dorai, A.A. Wound care with traditional, complementary and alternative medicine. Indian J. Plast. Surg. 2012, 45, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Piraino, F.; Selimović, Š. A Current View of Functional Biomaterials for Wound Care, Molecular and Cellular Therapies. BioMed Res. Int. 2015, 2015, 403801. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.; Garlick, J.A.; Egles, C. Self-assembling peptide nanofiber scaffolds accelerate wound healing. PLoS ONE 2008, 3, e1410. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zhao, L.; He, F.; Tan, H.; Li, Y.; Tang, Y.; Duan, X.; Li, Y. Palmatine-loaded electrospun poly(ε-caprolactone)/gelatin nanofibrous scaffolds accelerate wound healing and inhibit hypertrophic scar formation in a rabbit ear model. J. Biomater. Appl. 2021, 35, 869–886. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cheng, L.; Wang, Z.; Zhang, J.; Mao, X.; Liu, Z.; Zhang, Y.; Cui, W.; Sun, X. Conditioned medium-electrospun fiber biomaterials for skin regeneration. Bioact. Mater. 2021, 6, 361–374. [Google Scholar] [CrossRef]
- Fadilah, N.I.M.; Maarof, M.; Motta, A.; Tabata, Y.; Fauzi, M.B. The Discovery and Development of Natural-Based Biomaterials with Demonstrated Wound Healing Properties: A Reliable Approach in Clinical Trials. Biomedicines 2022, 10, 2226. [Google Scholar] [CrossRef]
- Al-Gharibi, K.A.; Sharstha, S.; Al-Faras, M.A. Cost-Effectiveness of Wound Care: A concept analysis. Sultan Qaboos Univ. Med. J. 2018, 18, e433–e439. [Google Scholar] [CrossRef] [PubMed]

| Compound Category | Compound Name | Improvement of Wound Healing | Modification of Fibroblast Behavior | Sources |
|---|---|---|---|---|
| Plant-derived products | Sarıçiçek (Achillea biebersteinni Afan) | Anti-microbial, anti-oxidant, and anti-inflammatory properties | Downregulates TGF-β1 and upregulates bFGF expression at the gene and protein level in murine embryonic fibroblasts | Hormozi, 2019 [50] Mssillou, 2022 [45] |
| Astragalus propinquus | Promotes re-epithelialization, revascularization, and immune function | Promotes cytokine secretion of TGF-β1, bFGF and EGF; promotes re-epithelialization; promotes proliferation, migration, and cell cycle progression of human skin fibroblasts | El-Ashram, 2021 [51] | |
| Astragali Radix and Rehmanniae Radix | Pro-angiogenic and anti-inflammatory properties | Activate genes in TGF-β1 pathway, regulate gene transcription for ECM synthesis via Smad pathway and cell motility via Ras/MAPK (non-Smad) pathway, and enhance skin fibroblast migration | Zhang, 2012 [52] | |
| Quercetin | Anti-bacterial, pro-angiogenic, and anti-oxidant properties | Modulates fibroblast activity, upregulate TGF-β1 | El-Sherbeni, 2023 [53] Mssillou, 2022 [45] Falbo, 2023 [31] | |
| Curcumin | Anti-bacterial, anti-oxidant, anti-inflammatory, and pro-angiogenic properties. | Induces fibroblast proliferation and collagen deposition | El-Ashram, 2021 [51] Mssillou, 2022 [45] Falbo, 2023 [31] | |
| Luteolin | Anti-bacterial, anti-oxidant, anti-inflammatory, and pro-angiogenic properties | Modulates IGF, PDGF, and FGF | Mssillou, 2022 [45] Falbo, 2023 [31] El-Sherbeni, 2023 [53] | |
| Kaempferol | Anti-neoplastic, anti-inflammatory, anti-bacterial, and anti-oxidant properties | Increases hydroxyproline and collagen in wound tissue | Mssillou, 2022 [45] El-Sherbeni, 2023 [53] | |
| Icariin | Anti-oxidant, anti-inflammatory, and anti-apoptotic properties | Accelerates collagen deposition | Singh, 2019 [54] Mssillou, 2022 [45] | |
| Morin | Anti-oxidant, anti-inflammatory, and anti-apoptotic properties | Accelerates collagen synthesis | Ponrasu, 2018 [42] Mssillou, 2022 [45] | |
| Naringin | Anti-oxidant, anti-inflammatory, anti-microbial, and astringent properties | Accelerates collagen synthesis | Mssillou, 2022 [45] | |
| Catechin and Epigallocatechin-3-Gallate (EGCG) | Anti-bacterial, anti-oxidant, anti-inflammatory, and pro-angiogenic properties | Enhance wound contraction and modulate growth factors | Hernandez-Hernandez, 2017 [43] Mssillou, 2022 [45] Falbo, 2023 [31] | |
| Silymarin | Anti-oxidant and anti-inflammatory properties | Increases number of fibrocytes, improves alignment of healing tissues, and enhances collagen fibers and fibroblasts | Oryan, 2012 [55] Mssillou, 2022 [45] | |
| Hesperidin | Anti-inflammatory, anti-microbial, anti-fungal, anti-oxidant, anti-neoplastic, anti-hypertensive, pro-angiogenic, and anti-atherogenic properties | Upregulates TGF-β and Smad 2/3 mRNA expression | Li, 2018 [56] Mssillou, 2022 [45] | |
| Vicenin-2 | Anti-oxidant, anti-inflammatory, and pro-angiogenic properties | Induces TGF-β to enhance fibroblast proliferation, migration and wound contraction | Tan, 2019 [43] | |
| Tannins | Anti-oxidant, pro-angiogenic, and antibacterial properties | Improves fibroblast proliferation and promote wound contraction | Li, 2011 [57] Falbo, 2023 [31] | |
| Terpinolene and α-phellandrene/α-pinene (PIN) and α-phellandrene | Anti-oxidant, anti-bacterial, anti-fungal, and anti-inflammatory properties | Improve migration and proliferation of fibroblasts | Bonnard, 2022 [58] Salas-Oropeza, 2020 [59] Salas-Oropeza, 2021 [60] Falbo, 2023 [31] | |
| Thymol | Anti-oxidant, anti-inflammatory, cicazitrant, anti-septic, anti-bacterial, and anti-fungal properties | Induces denser, thick, and parallel-arranged collagen fibers | Marchese, 2016 [61] Riella, 2012 [62] Falbo, 2023 [31] | |
| Taspine | Anti-bacterial, anti-inflammatory, anti-viral, and anti-neoplastic properties | Stimulates fibroblast chemotaxis, and induces hydroxyproline and KGF | Porras-Reyes, 1993 [63] Wang, 2022 [64] Vaisberg 1989 [65] Falbo, 2023 [31] | |
| Thymoquinone | Anti-microbial, anti-inflammatory, anti-oxidant, and anti-neoplastic properties | Enhances fibroblast formation and augments wound contraction | Algahtani, 2021 [66] El-Sherbeni, 2023 [53] | |
| APS2-1 from Atragalus | Anti-inflammatory, anti-oxidative, and immune-regulatory properties | Promotes fibroblast propagation and accelerate cell cycle progression, and promotes expression of TGF-β1, bFGF, and EGF | Zhao, 2017 [67] Yang, 2024 [68] | |
| ZWP from Curcuma zedoaria | Pro-angiogenic properties | Enhance collagen synthesis and deposition | Xu, 2018 [69] El-Sherbeni, 2023 [53] | |
| Extract of Sargasum Ilicifolium seaweed species | Increased speed of wound closure | Increases myofibroblast activity, promote TGF-β1 expression | Premarathna, 2020 [49] | |
| Madecassoside and Asiaticoside from C. asiatica | Improved speed and quality of wound healing | Activate the TGF-β/Smad pathway, enhancing type I and III collagen expression | Wu, 2012 [70] | |
| Animal-derived products | Honey | Anti-bacterial, anti-oxidant, anti-inflammatory, and pro-angiogenic properties | Induces fibroblast proliferation and migration, and induces collagen matrix development | Ratcliffe, 2014 [71] Ibrahim, 2018 [72] El-Ashram, 2021 [51] |
| Sericin | Anti-oxidant, pro-angiogenic, and anti-inflammatory properties | Regulates TGF-β1 and TGF-β3 expression, and activates collagen production | El-Ashram, 2021 [51] | |
| Maggot excretions/secretions of Phaenicia sericata | Anti-inflammatory, pro-angiogenic, anti-viral, and anti-neoplastic properties | Activate and enhance the growth rate of fibroblasts | Prete, 1997 [73] Ratcliffe, 2014 [71] | |
| Marine collagen | Pro-angiogenic, and anti-aging properties | Promote fibroblast migration | Geahchan, 2022 [33] Chandika, 2015 [74] | |
| Sea cucumbers | Anti-bacterial, anti-inflammatory, anti-oxidant, and immune regulatory properties | Stimulate fibroblast chemotaxis and proliferation, and breakdown of ECM proteins | El-Ashram, 2021 [51] Ibrahim, 2018 [72] | |
| Exogenous growth factors | TGF-β | Pro-angiogenic, and immune regulatory properties | Prompts differentiation of fibroblasts into myofibroblasts and ECM formation/ deposition via TGF-β1 and TGF-β2 chemotaxis of fibroblasts via TGF-β3 | Walraven, 2017 [75] Barrientos, 2008 [76] Dolati, 2020 [77] |
| EGF and NRG1 | Anti-inflammatory and pro-angiogenic properties, and enhanced kerotinocyte recruitment and cell motility | Stimulate recruitment of fibroblasts | Yoon, 2018 [78] Dolati, 2020 [77] | |
| FGF-2 (bFGF) | Anti-inflammatory and pro-angiogenic, properties and enhanced kerotinocyte recruitment and wound contraction | Modulates ECM formation and inhibits TGF-β1/Smad-dependent pathways | Borena, 2015 [79] Dolati, 2020 [77] | |
| IGF-1 | Anti-inflammatory, anti-apoptotic, and pro-angiogenic properties, and stimulator of keratinocyte proliferation | Stimulates proliferation of fibroblasts and collagen | Todorović, 2008 [80] Dolati, 2020 [77] | |
| hPDGF and rPDGF-B | Pro-angiogenic properties; stimulates chemotaxis of polymorphonuclear leukocytes and monocytes; induce MMPs and tissue inhibitors of metalloproteinases (TIMPs) | Stimulate fibroblast mitogenesis and chemotaxis; promote procollagen type I synthesis | Pierce, 1988 [81] Dolati, 2020 [77] | |
| Growth Factor-rich products | Platelet-rich plasma (PRP) | Anti-microbial, anti-inflammatory, and pro-angiogenic properties | Consists of high levels of PDGF and TGF-β1 | Fotouhi, 2018 [82] Dolati, 2020 [77] |
| Platelet-rich fibrin matrix (PRFM) | Anti-inflammatory and pro-angiogenic properties | Stimulates release of PDGF, TGF-β1, EGF, FGF-2, and IGF | Lin, 2018 [83] Dolati, 2020 [77] | |
| Decellularized and dehydrated human amniotic membrane (DDHAM) | Anti-bacterial, anti-inflammatory, and pro-angiogenic properties | Stimulate release of PDGF, TGF-β1, EGF, FGF-2, TGF-a, placental GF, G-CSF, Interleukin (IL)-4, IL-10, and various TIMPs | Sheikh, 2014 [84] Smiell, 2015 [85] Dolati, 2020 [77] |
| Nano DDS | Natural Compound | Modification of Fibroblast Behavior | Sources |
|---|---|---|---|
| Liposomes Ability to improve bioavailability, cause sustained transdermal delivery of different medicinal compounds, and overcome possible drug overdose and toxicity. | Curcumin | Shortens inflammatory process, inhibits bacterial growth, promotes fibrosis, angiogenesis, re-epithelialization, and wound contraction | Kianvash, 2017 [131] |
| Madecassoside | Significant burn wound healing effect | Li, 2016 [132] | |
| Usnic acid | Inhibits the secretion of pro-inflammatory cytokines, TNF-α, IL-6, IL-1B Induces nitric oxide and cyclooxygenase-2 (COX-2) Increases IL-10 and HO-1 in a dose-dependent relation Anti-bacterial activity Enhances maturation of granulation tissue and better collagen deposition | Nunes, 2016 [133] | |
| bFGF | Promotes fibroblast proliferation, migration, differentiation Expedites regeneration of vascular vessels and the synthesis of procollagen and collagen matrix | Xu, 2017 [134] | |
| Insulin/chitosan | Increases re-epithelialization collagen content, granulation tissue, wound tensile strength, and local production of insulin-like growth factors by fibroblasts. Increases proliferation and migration of human keratinocytes, which stimulates cell growth and enhances wound healing | Dawoud, 2019 [135] | |
| Transfersomes Deformable liposomes with an edge activator. | Gellan cholesterol nanohydrogels/baicalin | Inhibits TNF-αInhibits IL-1β Visually improves wound healing | Manconi, 2018 [136] |
| Polymeric nanoparticles Protect the degradation of drugs and release them in a controlled manner. | PDGF-A, IGF-1, EGF | Advanced granulation tissue formation, significantly enhances healing of chronic wounds | Choi, 2017 [137] |
| PLGA/LL-37 | Increases collagen deposition, and organization, enhancement of epithelialization, and neovascularization | Chereddy, 2014 [138] | |
| hVEGF gene/stem cells | Enhances angiogenesis, and reduces tissue degeneration and fibrosis in ischemic limbs | Yang, 2010 [136] | |
| Thymol/chitosan/AgNPs | Excellent anti-bacterial properties | Manukumar, 2017 [139] | |
| Inorganic nanoparticles Deprived from inorganic materials, including metallic nanoparticles, carbon-based nanoparticles, and ceramic nanoparticles. Benefiting from the intrinsic nature of materials, inorganic nanoparticles exhibit both similar merits in wound healing treatment and a strong anti-bacterial effect. | Iron oxide/thrombin | Increases tensile strength of wounds, decreases inflammation | Ziv-Polat, 2010 [140] |
| Cerium oxide | Enhances fibroblast proliferation, myofibroblast differentiation Accelerates migration and tube-forming ability of vascular endothelial cells | Chigurupati, 2013 [141] | |
| Zn02 | Shows anti-bacterial activity and enhances wound healing | Ali, 2017 [142] | |
| Gold | Enhances of wound healing, increases collagen expression, decreases MMP-1 expression and TGF-B1 Enhances VEGF, angiopoietin 1, and 2 | Kim, 2015 [143] | |
| Silane/amphotericin B | Shows efficacy in controlling Candida infection | Sanchez, 2014 [144] | |
| Lipid nanoparticles Introduced to overcome the limitation of liposomes. Controlled release of drugs due to their nontoxic colloidal dimensions. | rhEGF | Enhances proliferation and migration of fibroblasts, wound contraction, and epidermal regeneration | Gainza, 2013 [145] |
| Nanofibrous structures Mimic the ECM, provide favorable conditions for cell attachment and contact with drugs. Enhance variety of therapeutics agents due to their high area-to-volume ratio. | Andrographolide/silica | Accelerates wound healing, increases collagen deposition in the wound site, decreases inflammation | Jia, 2018 [146] |
| Astragaloside IV | Accelerates wound healing and inhibits scar formation, increasing angiogenesis, regulating newly formed types of collagen, and improving collagen organization | Shan, 2015 [147] | |
| PDGF-BB/VEGF | Accelerates wound healing, promotes fibroblast growth and inhibits bacteria in vitro | Xie, 2013 [148] | |
| Lawsone | Significantly increases TGF-β1 and collagen gene expression in vitro and promotes re-epithelialization of the wound in vivo | Abadehie, 2021 [149] | |
| Nanohydrogel High flexibility, high hydrophilicity, high mechanical strength, tunable structure, and the ability to absorb wound exudates as well as permeate oxygen and prevent wound dehydration. | Cellulose nanocrystal and hyaluronic acid/chitosan NPs/GM-CSF | Enhances proliferation and differentiation of fibroblasts, lowers inflammation, and increases collagen deposition | Dehkordi, 2019 [150] |
| Carrageenan/nano silicates | Enhances cell adhesion and spreading, reduces blood clotting time, facilitates in vitro tissue regeneration and wound healing | Lokhande, 2018 [151] | |
| Nanocellulose/acrylic acid hydrogels | Maintains the activity and morphology of human dermal fibroblasts, promotes rapid cell proliferation, and affects 9 genes’ expressions related to wound healing | Loh, 2018 [152] | |
| Hydrogels loaded with nanoparticles Synergistic effect between hydrogels and nanoparticles encapsulated. | Chitosan hydrogels/phenytoin | Increases the content of collagen fibers and fibroblasts in the wound tissue | Cardoso, 2019 [153] |
| Thermosensitive hydrogel/gold NPs | Enhances skin re-epithelialization, granulation tissue, vascularization, and collagen deposition Modulates gene expression of inflammatory and anti-inflammatory mediators | Mahmoud, 2019 [154] | |
| Hydrogels/cyclosporine A solid lipid NPs | Significantly increases rate of mucosal repair | Karavana, 2012 [155] | |
| Hyaluronic acid and chondroitin sulfate/asiatic acid/ZnO NPs/CuO NPs | Raises DNA, total protein, hexosamine and hydroxyproline content, and leads to superior re-epithelization, collagen fiber arrangement and angiogenesis | Thanusha 2018 [156] | |
| Hydrogel/Simvastatin polymeric NPs | Enhances of epithelialization and wound healing Decreases inflammatory cell infiltration | Aly, 2019 [157] | |
| PRP/collagen NPs | Enhances epithelialization and wound closure | Shalaby, 2023 [158] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berry, C.E.; Brenac, C.; Gonzalez, C.E.; Kendig, C.B.; Le, T.; An, N.; Griffin, M.F. Natural Compounds and Biomimetic Engineering to Influence Fibroblast Behavior in Wound Healing. Int. J. Mol. Sci. 2024, 25, 3274. https://doi.org/10.3390/ijms25063274
Berry CE, Brenac C, Gonzalez CE, Kendig CB, Le T, An N, Griffin MF. Natural Compounds and Biomimetic Engineering to Influence Fibroblast Behavior in Wound Healing. International Journal of Molecular Sciences. 2024; 25(6):3274. https://doi.org/10.3390/ijms25063274
Chicago/Turabian StyleBerry, Charlotte E., Camille Brenac, Caroline E. Gonzalez, Carter B. Kendig, Thalia Le, Nicholas An, and Michelle F. Griffin. 2024. "Natural Compounds and Biomimetic Engineering to Influence Fibroblast Behavior in Wound Healing" International Journal of Molecular Sciences 25, no. 6: 3274. https://doi.org/10.3390/ijms25063274
APA StyleBerry, C. E., Brenac, C., Gonzalez, C. E., Kendig, C. B., Le, T., An, N., & Griffin, M. F. (2024). Natural Compounds and Biomimetic Engineering to Influence Fibroblast Behavior in Wound Healing. International Journal of Molecular Sciences, 25(6), 3274. https://doi.org/10.3390/ijms25063274





