Solanum lycopersicum (Tomato)-Derived Nanovesicles Accelerate Wound Healing by Eliciting the Migration of Keratinocytes and Fibroblasts
Abstract
1. Introduction
2. Results
2.1. Physico-Chemical Characterization of TDNVs
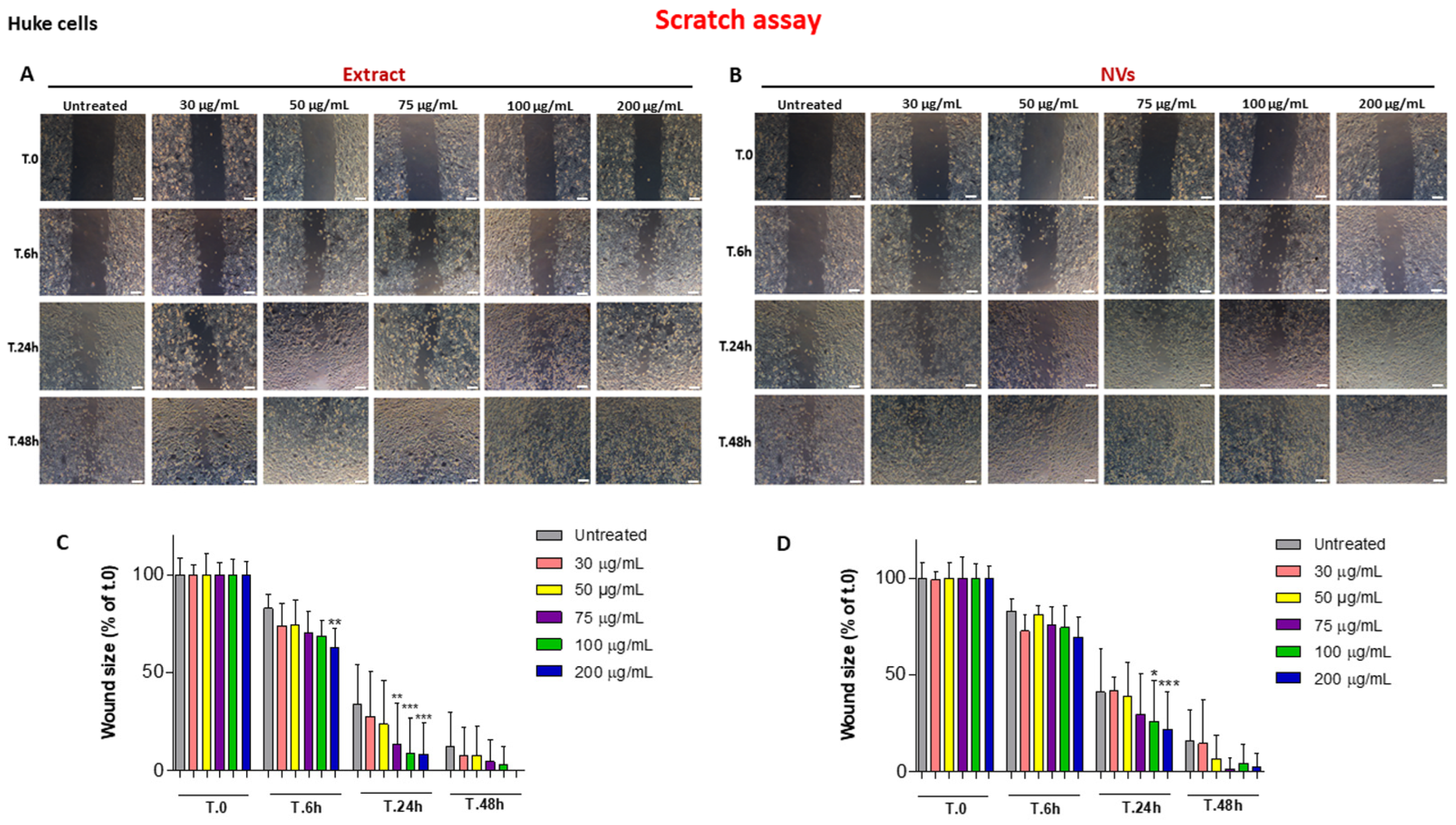
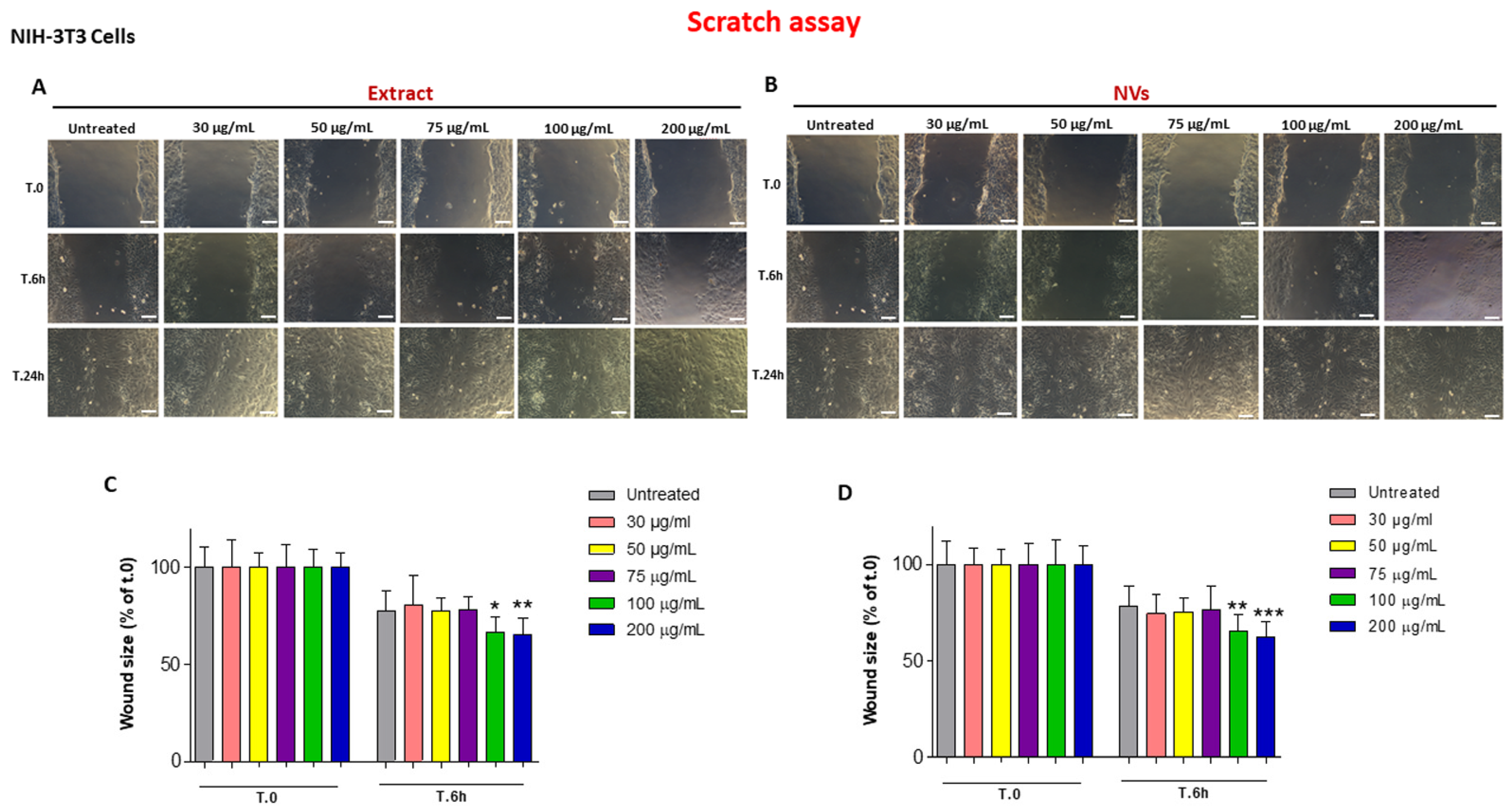
2.2. The Effect of Tomato NVs and Extract on the Wound Healing Process
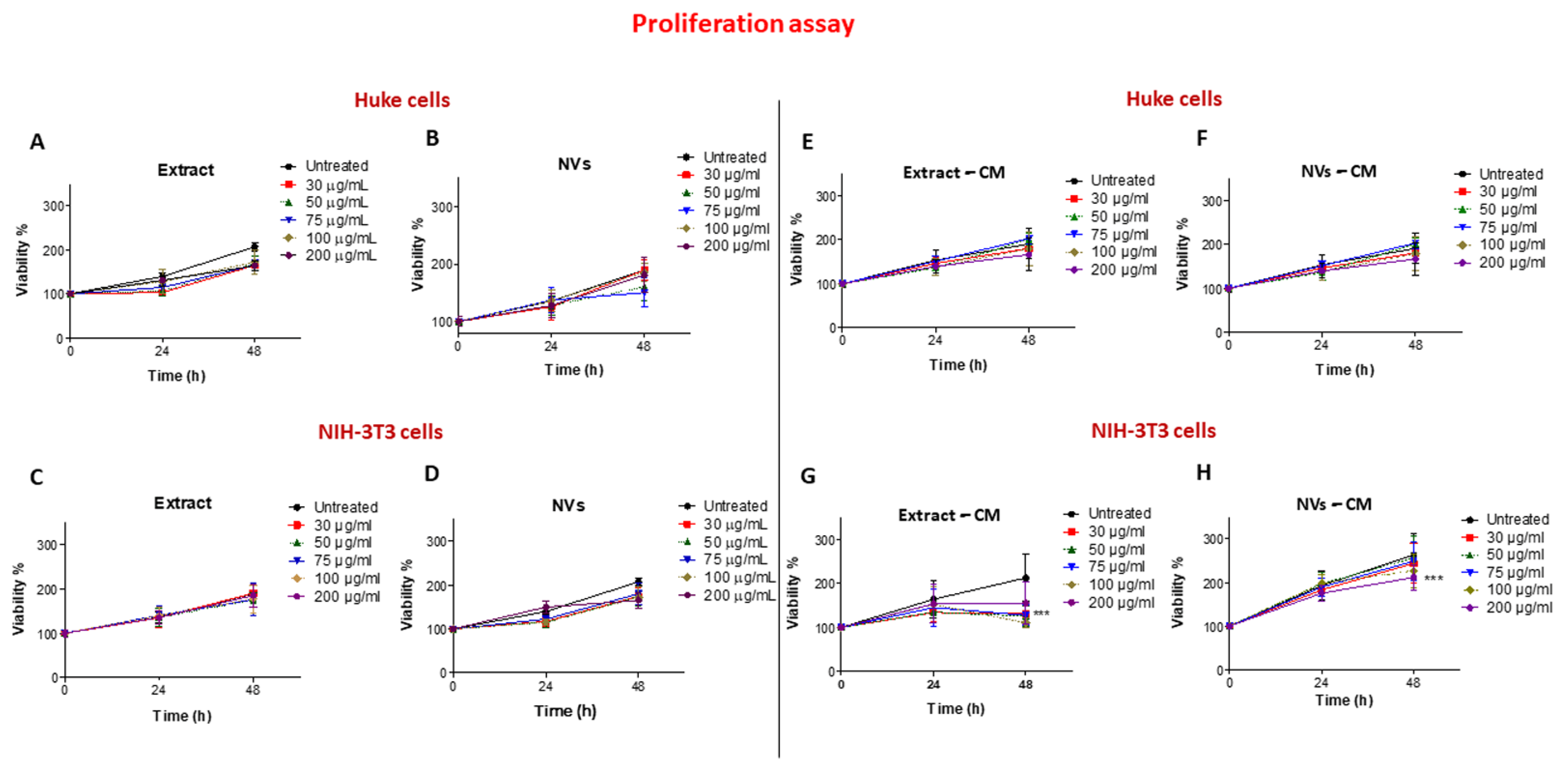
2.3. Effects of Tomato NVs and Extract on Cell Proliferation
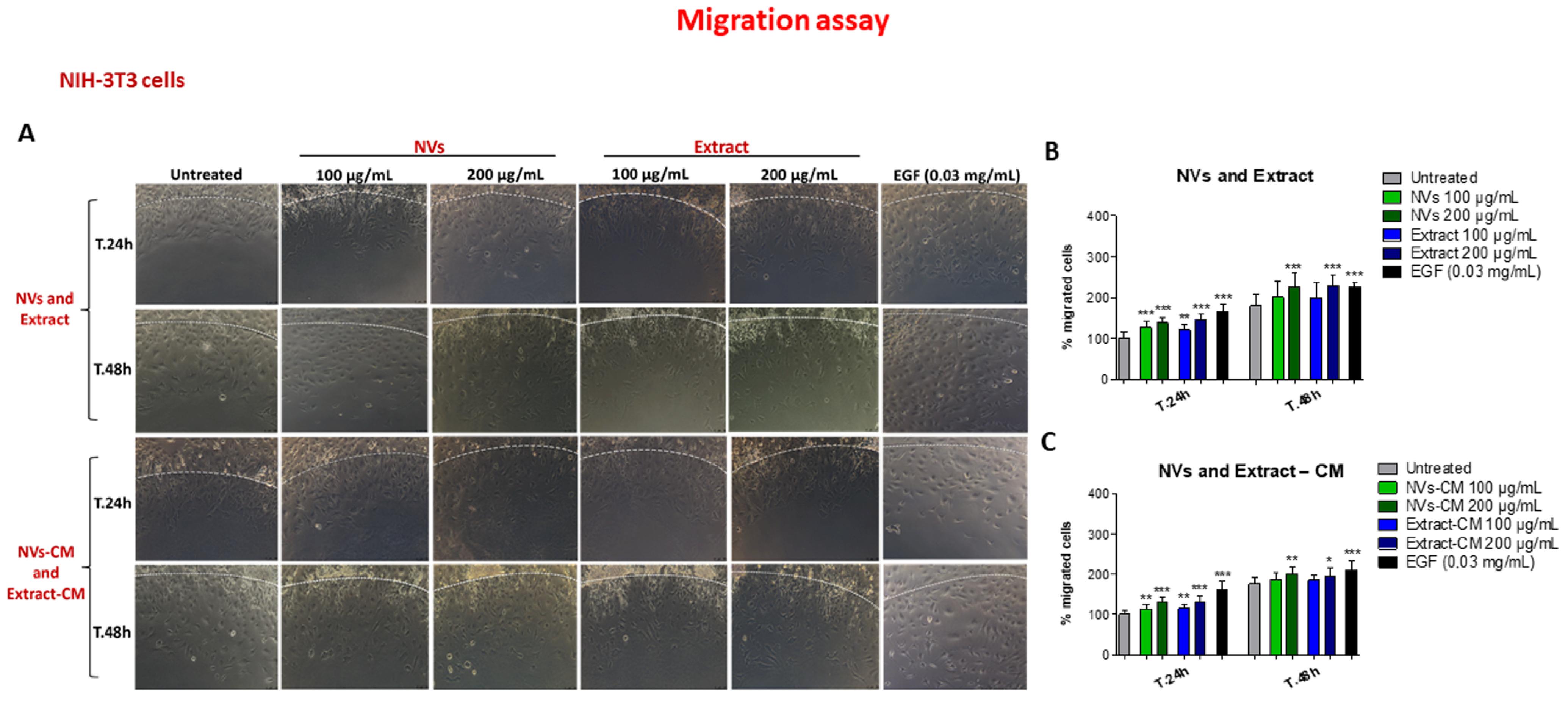
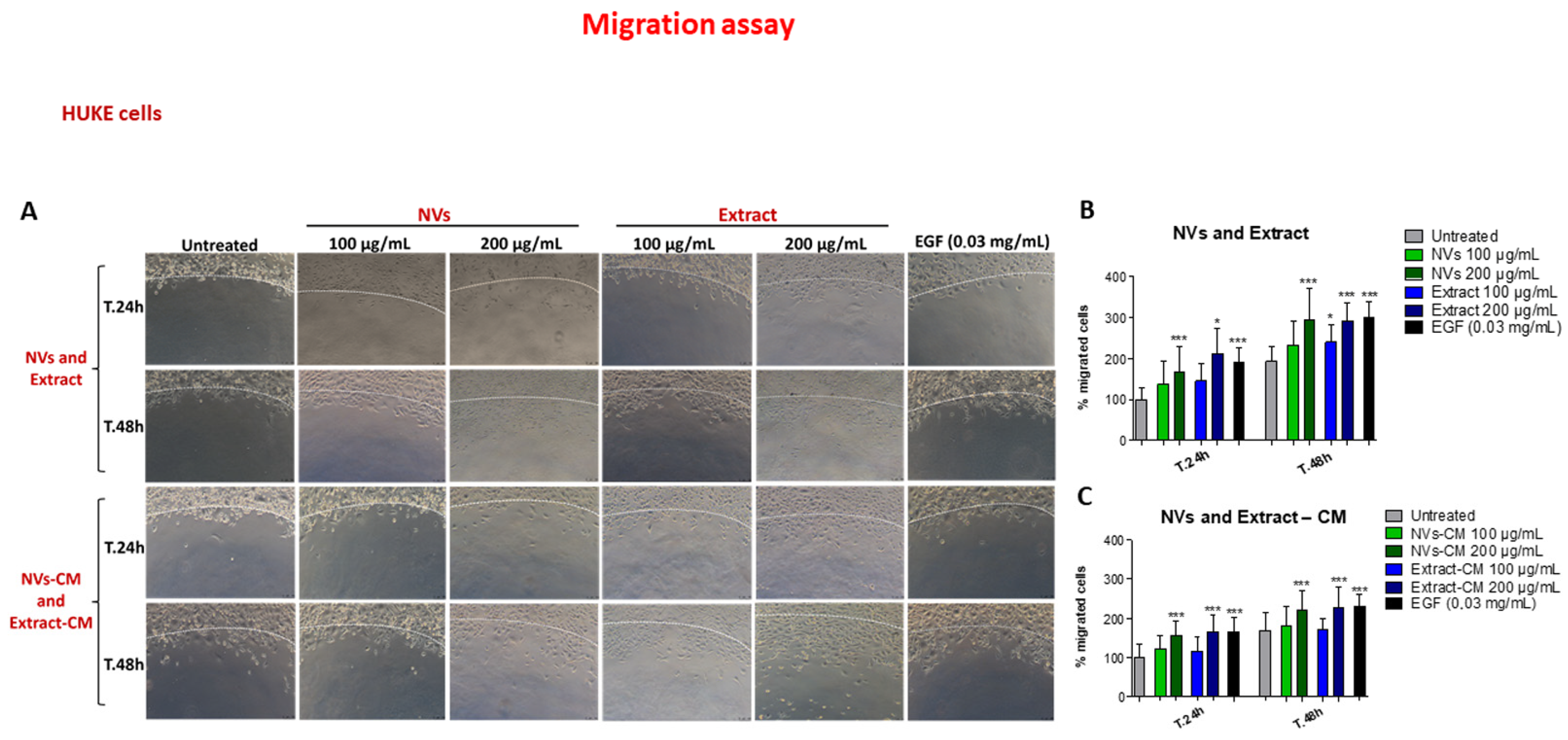
2.4. Effect of NVs and Extract on Cell Migration
3. Discussion
4. Materials and Methods
4.1. Isolation of Solanum lycopersicum (Tomato)-Derived Nanovesicles (TDNVs)
4.2. Physicochemical Characterization of Tomato NVs and Extract
4.3. Determination of Protein Concentration
4.4. Cell Cultures
4.5. Scratch Assay
4.6. Cell Proliferation Assay
4.7. Agarose Spot Assay
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Yates, C.C.; Hebda, P.; Wells, A. Skin wound healing and scarring: Fetal wounds and regenerative restitution. Birth Defects Res. C Embryo Today 2012, 96, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Reinke, J.M.; Sorg, H. Wound repair and regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Falanga, V. Wound healing and its impairment in the diabetic foot. Lancet 2005, 366, 1736–1743. [Google Scholar] [CrossRef] [PubMed]
- Adib, Y.; Bensussan, A.; Michel, L. Cutaneous Wound Healing: A Review about Innate Immune Response and Current Therapeutic Applications. Mediat. Inflamm. 2022, 2022, 5344085. [Google Scholar] [CrossRef] [PubMed]
- Sorg, H.; Tilkorn, D.J.; Hager, S.; Hauser, J.; Mirastschijski, U. Skin Wound Healing: An Update on the Current Knowledge and Concepts. Eur. Surg. Res. 2017, 58, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Gushiken, L.F.S.; Beserra, F.P.; Bastos, J.K.; Jackson, C.J.; Pellizzon, C.H. Cutaneous Wound Healing: An Update from Physiopathology to Current Therapies. Life 2021, 11, 665. [Google Scholar] [CrossRef] [PubMed]
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef]
- Choi, S.; Yoon, M.; Choi, K.Y. Approaches for Regenerative Healing of Cutaneous Wound with an Emphasis on Strategies Activating the Wnt/beta-Catenin Pathway. Adv. Wound Care 2022, 11, 70–86. [Google Scholar] [CrossRef]
- Hajialyani, M.; Tewari, D.; Sobarzo-Sanchez, E.; Nabavi, S.M.; Farzaei, M.H.; Abdollahi, M. Natural product-based nanomedicines for wound healing purposes: Therapeutic targets and drug delivery systems. Int. J. Nanomed. 2018, 13, 5023–5043. [Google Scholar] [CrossRef]
- Narauskaite, D.; Vydmantaite, G.; Rusteikaite, J.; Sampath, R.; Rudaityte, A.; Stasyte, G.; Aparicio Calvente, M.I.; Jekabsone, A. Extracellular Vesicles in Skin Wound Healing. Pharmaceuticals 2021, 14, 811. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, M.; Merlin, D. Advances in Plant-derived Edible Nanoparticle-based lipid Nano-drug Delivery Systems as Therapeutic Nanomedicines. J. Mater. Chem. B 2018, 6, 1312–1321. [Google Scholar] [CrossRef] [PubMed]
- Nemati, M.; Singh, B.; Mir, R.A.; Nemati, M.; Babaei, A.; Ahmadi, M.; Rasmi, Y.; Golezani, A.G.; Rezaie, J. Plant-derived extracellular vesicles: A novel nanomedicine approach with advantages and challenges. Cell Commun. Signal. 2022, 20, 69. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Mu, J.; Teng, Y.; Zhang, X.; Sundaram, K.; Sriwastva, M.K.; Kumar, A.; Lei, C.; Zhang, L.; Liu, Q.M.; et al. Restoring Oat Nanoparticles Mediated Brain Memory Function of Mice Fed Alcohol by Sorting Inflammatory Dectin-1 Complex into Microglial Exosomes. Small 2022, 18, e2105385. [Google Scholar] [CrossRef]
- Teng, Y.; Ren, Y.; Sayed, M.; Hu, X.; Lei, C.; Kumar, A.; Hutchins, E.; Mu, J.; Deng, Z.; Luo, C.; et al. Plant-Derived Exosomal MicroRNAs Shape the Gut Microbiota. Cell Host Microbe 2018, 24, 637–652.e8. [Google Scholar] [CrossRef]
- Dad, H.A.; Gu, T.W.; Zhu, A.Q.; Huang, L.Q.; Peng, L.H. Plant Exosome-like Nanovesicles: Emerging Therapeutics and Drug Delivery Nanoplatforms. Mol. Ther. 2021, 29, 13–31. [Google Scholar] [CrossRef]
- Zhang, M.; Collins, J.F.; Merlin, D. Do ginger-derived nanoparticles represent an attractive treatment strategy for inflammatory bowel diseases? Nanomedicine 2016, 11, 3035–3037. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Merlin, D. Nanoparticle-Based Oral Drug Delivery Systems Targeting the Colon for Treatment of Ulcerative Colitis. Inflamm. Bowel Dis. 2018, 24, 1401–1415. [Google Scholar] [CrossRef] [PubMed]
- Sahin, F.; Kocak, P.; Gunes, M.Y.; Ozkan, I.; Yildirim, E.; Kala, E.Y. In Vitro Wound Healing Activity of Wheat-Derived Nanovesicles. Appl. Biochem. Biotechnol. 2019, 188, 381–394. [Google Scholar] [CrossRef]
- Savci, Y.; Kirbas, O.K.; Bozkurt, B.T.; Abdik, E.A.; Tasli, P.N.; Sahin, F.; Abdik, H. Grapefruit-derived extracellular vesicles as a promising cell-free therapeutic tool for wound healing. Food Funct. 2021, 12, 5144–5156. [Google Scholar] [CrossRef]
- Sanchez-Lopez, C.M.; Manzaneque-Lopez, M.C.; Perez-Bermudez, P.; Soler, C.; Marcilla, A. Characterization and bioactivity of extracellular vesicles isolated from pomegranate. Food Funct. 2022, 13, 12870–12882. [Google Scholar] [CrossRef]
- Kim, M.; Park, J.H. Isolation of Aloe saponaria-Derived Extracellular Vesicles and Investigation of Their Potential for Chronic Wound Healing. Pharmaceutics 2022, 14, 1905. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Lu, S.; Ren, L.; Bian, S.; Zhao, D.; Liu, M.; Wang, J. Ginseng-derived nanoparticles induce skin cell proliferation and promote wound healing. J. Ginseng Res. 2023, 47, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Hazewindus, M.; Haenen, G.R.M.M.; Weseler, A.R.; Bast, A. The anti-inflammatory effect of lycopene complements the antioxidant action of ascorbic acid and α-tocopherol. Food Chem. 2012, 132, 954–958. [Google Scholar] [CrossRef]
- Choi, S.H.; Lee, S.H.; Kim, H.J.; Lee, I.S.; Kozukue, N.; Levin, C.E.; Friedman, M. Changes in free amino acid, phenolic, chlorophyll, carotenoid, and glycoalkaloid contents in tomatoes during 11 stages of growth and inhibition of cervical and lung human cancer cells by green tomato extracts. J. Agric. Food Chem. 2010, 58, 7547–7556. [Google Scholar] [CrossRef] [PubMed]
- Raiola, A.; Rigano, M.M.; Calafiore, R.; Frusciante, L.; Barone, A. Enhancing the health-promoting effects of tomato fruit for biofortified food. Mediat. Inflamm. 2014, 2014, 139873. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Deng, Z.; Liu, R.; Loewen, S.; Tsao, R. Bioaccessibility, in vitro antioxidant activities and in vivo anti-inflammatory activities of a purple tomato (Solanum lycopersicum L.). Food Chem. 2014, 159, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.Y.; Sina, A.A.; Khandker, S.S.; Neesa, L.; Tanvir, E.M.; Kabir, A.; Khalil, M.I.; Gan, S.H. Nutritional Composition and Bioactive Compounds in Tomatoes and Their Impact on Human Health and Disease: A Review. Foods 2020, 10, 45. [Google Scholar] [CrossRef]
- García-Valverde, V.; Navarro-González, I.; García-Alonso, J.; Periago, M.J. Antioxidant Bioactive Compounds in Selected Industrial Processing and Fresh Consumption Tomato Cultivars. Food Bioprocess. Technol. 2013, 6, 391–402. [Google Scholar] [CrossRef]
- Li, H.; Deng, Z.; Liu, R.; Loewen, S.; Tsao, R. Carotenoid compositions of coloured tomato cultivars and contribution to antioxidant activities and protection against H2O2-induced cell death in H9c2. Food Chem. 2013, 136, 878–888. [Google Scholar] [CrossRef]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef]
- Metibemu, D.S.; Ogungbe, I.V. Carotenoids in Drug Discovery and Medicine: Pathways and Molecular Targets Implicated in Human Diseases. Molecules 2022, 27, 6005. [Google Scholar] [CrossRef]
- Abete, I.; Perez-Cornago, A.; Navas-Carretero, S.; Bondia-Pons, I.; Zulet, M.A.; Martinez, J.A. A regular lycopene enriched tomato sauce consumption influences antioxidant status of healthy young-subjects: A crossover study. J. Funct. Foods 2013, 5, 28–35. [Google Scholar] [CrossRef]
- Mammadova, R.; Maggio, S.; Fiume, I.; Bokka, R.; Moubarak, M.; Gellen, G.; Schlosser, G.; Adamo, G.; Bongiovanni, A.; Trepiccione, F.; et al. Protein Biocargo and Anti-Inflammatory Effect of Tomato Fruit-Derived Nanovesicles Separated by Density Gradient Ultracentrifugation and Loaded with Curcumin. Pharmaceutics 2023, 15, 333. [Google Scholar] [CrossRef]
- Bokka, R.; Ramos, A.P.; Fiume, I.; Manno, M.; Raccosta, S.; Turiak, L.; Sugar, S.; Adamo, G.; Csizmadia, T.; Pocsfalvi, G. Biomanufacturing of Tomato-Derived Nanovesicles. Foods 2020, 9, 1852. [Google Scholar] [CrossRef] [PubMed]
- Tkachenko, V.; Vidal, L.; Josien, L.; Schmutz, M.; Poly, J.; Chemtob, A. Characterizing the Core-Shell Architecture of Block Copolymer Nanoparticles with Electron Microscopy: A Multi-Technique Approach. Polymers 2020, 12, 1656. [Google Scholar] [CrossRef] [PubMed]
- Veres-Szekely, A.; Pap, D.; Szebeni, B.; Orfi, L.; Szasz, C.; Pajtok, C.; Levai, E.; Szabo, A.J.; Vannay, A. Transient Agarose Spot (TAS) Assay: A New Method to Investigate Cell Migration. Int. J. Mol. Sci. 2022, 23, 2119. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Basheer, H.A.; Ayuso, J.M.; Ahmet, D.; Mazzini, M.; Patel, R.; Shnyder, S.D.; Vinader, V.; Afarinkia, K. Agarose Spot as a Comparative Method for in situ Analysis of Simultaneous Chemotactic Responses to Multiple Chemokines. Sci. Rep. 2017, 7, 1075. [Google Scholar] [CrossRef] [PubMed]
- Wiggins, H.; Rappoport, J. An agarose spot assay for chemotactic invasion. BioTechniques 2010, 48, 121–124. [Google Scholar] [CrossRef]
- Liu, H.; Luo, G.-F.; Shang, Z. Plant-derived nanovesicles as an emerging platform for cancer therapy. Acta Pharm. Sin. B 2024, 14, 133–154. [Google Scholar] [CrossRef]
- Cui, Y.; Gao, J.; He, Y.; Jiang, L. Plant extracellular vesicles. Protoplasma 2020, 257, 3–12. [Google Scholar] [CrossRef]
- Di Giulio, S.; Carata, E.; Mariano, S.; Panzarini, E. Plant Extracellular Vesicles: Investigating Their Utilization as Beneficial Nutrients in Diet. Appl. Sci. 2023, 13, 6656. [Google Scholar] [CrossRef]
- Di Gioia, S.; Hossain, M.N.; Conese, M. Biological properties and therapeutic effects of plant-derived nanovesicles. Open Med. 2020, 15, 1096–1122. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Wu, S.C.; Chien, H.Y.; Shen, T.L.; Hsu, W.H. Tomato-fruit-derived extracellular vesicles inhibit Fusobacterium nucleatum via lipid-mediated mechanism. Food Funct. 2023, 14, 8942–8950. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, M.; Unsal, N.; Kabatas, B.; Eren, O.; Sahin, F. Effect of Solanum lycopersicum and Citrus limon-Derived Exosome-like Vesicles on Chondrogenic Differentiation of Adipose-Derived Stem Cells. Appl. Biochem. Biotechnol. 2024, 196, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Kilasoniya, A.; Garaeva, L.; Shtam, T.; Spitsyna, A.; Putevich, E.; Moreno-Chamba, B.; Salazar-Bermeo, J.; Komarova, E.; Malek, A.; Valero, M.; et al. Potential of Plant Exosome Vesicles from Grapefruit (Citrus × paradisi) and Tomato (Solanum lycopersicum) Juices as Functional Ingredients and Targeted Drug Delivery Vehicles. Antioxidants 2023, 12, 943. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Diao, N.; Cai, X.; Chen, X.; Xiao, Y.; Guo, C.; Chen, D.; Zhang, X. Plant exosome nanovesicles (PENs): Green delivery platforms. Mater. Horiz. 2023, 10, 3879–3894. [Google Scholar] [CrossRef] [PubMed]
- Rousselle, P.; Braye, F.; Dayan, G. Re-epithelialization of adult skin wounds: Cellular mechanisms and therapeutic strategies. Adv. Drug Deliv. Rev. 2019, 146, 344–365. [Google Scholar] [CrossRef]
- Rousselle, P.; Montmasson, M.; Garnier, C. Extracellular matrix contribution to skin wound re-epithelialization. Matrix Biol. 2019, 75–76, 12–26. [Google Scholar] [CrossRef]
- Chen, X.; Xing, X.; Lin, S.; Huang, L.; He, L.; Zou, Y.; Zhang, X.; Su, B.; Lu, Y.; Zheng, D. Plant-derived nanovesicles: Harnessing nature’s power for tissue protection and repair. J. Nanobiotechnol. 2023, 21, 445. [Google Scholar] [CrossRef]
- Rodriguez, P.G.; Felix, F.N.; Woodley, D.T.; Shim, E.K. The role of oxygen in wound healing: A review of the literature. Dermatol. Surg. 2008, 34, 1159–1169. [Google Scholar] [CrossRef]
- Innocent, E.E.-E.; Wokpeogu, P.C.; Sudor, W.B.; Nwolim, P.J. The effect of ethanolic seed extract of Solanum lycopersicum on wound healing. Eur. J. Pharm. Med. Res. 2017, 5, 356–361. [Google Scholar]
- Fang, Y.; Li, G.; Huang, C.; Huang, K.; Zhao, Y.; Nie, T.; Wu, J. Tomato based gelatin methacryloyl hydrogel as an effective natural and low-cost scaffold for accelerative wound healing. Int. J. Biol. Macromol. 2023, 229, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, M.; Wondimu, B.; Bakhiet, M.; Modeer, T. Induction of interferon gamma in human gingival fibroblasts challenged with phytohaemagglutinin. Cytokine 2000, 12, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.; Prakash, J.; de Ruijter, M.; Beljaars, L.; Poelstra, K. Peptide-modified albumin carrier explored as a novel strategy for a cell-specific delivery of interferon gamma to treat liver fibrosis. Mol. Pharm. 2011, 8, 1899–1909. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.; Tomar, T.; Ostman, A.; Poelstra, K.; Prakash, J. Selective targeting of interferon gamma to stromal fibroblasts and pericytes as a novel therapeutic approach to inhibit angiogenesis and tumor growth. Mol. Cancer Ther. 2012, 11, 2419–2428. [Google Scholar] [CrossRef]
- Pakyari, M.; Farrokhi, A.; Maharlooei, M.K.; Ghahary, A. Critical Role of Transforming Growth Factor Beta in Different Phases of Wound Healing. Adv. Wound Care 2013, 2, 215–224. [Google Scholar] [CrossRef]
- Jiang, C.K.; Tomic-Canic, M.; Lucas, D.J.; Simon, M.; Blumenberg, M. TGF beta promotes the basal phenotype of epidermal keratinocytes: Transcriptional induction of K#5 and K#14 keratin genes. Growth Factors 1995, 12, 87–97. [Google Scholar]
- Safferling, K.; Sutterlin, T.; Westphal, K.; Ernst, C.; Breuhahn, K.; James, M.; Jager, D.; Halama, N.; Grabe, N. Wound healing revised: A novel reepithelialization mechanism revealed by in vitro and in silico models. J. Cell Biol. 2013, 203, 691–709. [Google Scholar] [CrossRef]
- Singer, A.J. Healing Mechanisms in Cutaneous Wounds: Tipping the Balance. Tissue Eng. Part B Rev. 2022, 28, 1151–1167. [Google Scholar] [CrossRef]
- Vig, K.; Chaudhari, A.; Tripathi, S.; Dixit, S.; Sahu, R.; Pillai, S.; Dennis, V.A.; Singh, S.R. Advances in Skin Regeneration Using Tissue Engineering. Int. J. Mol. Sci. 2017, 18, 789. [Google Scholar] [CrossRef]
- Conese, M.; Annacontini, L.; Carbone, A.; Beccia, E.; Cecchino, L.R.; Parisi, D.; Di Gioia, S.; Lembo, F.; Angiolillo, A.; Mastrangelo, F.; et al. The Role of Adipose-Derived Stem Cells, Dermal Regenerative Templates, and Platelet-Rich Plasma in Tissue Engineering-Based Treatments of Chronic Skin Wounds. Stem Cell Int. 2020, 2020, 7056261. [Google Scholar] [CrossRef] [PubMed]
- Md Fadilah, N.I.; Mohd Abdul Kader Jailani, M.S.; Badrul Hisham, M.A.I.; Sunthar Raj, N.; Shamsuddin, S.A.; Ng, M.H.; Fauzi, M.B.; Maarof, M. Cell secretomes for wound healing and tissue regeneration: Next generation acellular based tissue engineered products. J. Tissue Eng. 2022, 13, 20417314221114273. [Google Scholar] [CrossRef]
- Hossain, M.N.; De Leo, V.; Tamborra, R.; Laselva, O.; Ingrosso, C.; Daniello, V.; Catucci, L.; Losito, I.; Sollitto, F.; Loizzi, D.; et al. Characterization of anti-proliferative and anti-oxidant effects of nano-sized vesicles from Brassica oleracea L. (Broccoli). Sci. Rep. 2022, 12, 14362. [Google Scholar] [CrossRef]
- Beccia, E.; Daniello, V.; Laselva, O.; Leccese, G.; Mangiacotti, M.; Di Gioia, S.; La Bella, G.; Guerra, L.; Matteo, M.; Angiolillo, A.; et al. Human Amniotic Mesenchymal Stem Cells and Fibroblasts Accelerate Wound Repair of Cystic Fibrosis Epithelium. Life 2022, 12, 756. [Google Scholar] [CrossRef] [PubMed]
- Pusnik, M.; Imeri, M.; Deppierraz, G.; Bruinink, A.; Zinn, M. The agar diffusion scratch assay—A novel method to assess the bioactive and cytotoxic potential of new materials and compounds. Sci. Rep. 2016, 6, 20854. [Google Scholar] [CrossRef] [PubMed]
- Conese, M.; Portincasa, A. Mesenchymal stem cells, secretome and biomaterials in in-vivo animal models: Regenerative medicine application in cutaneous wound healing. Biocell 2022, 46, 1815–1826. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daniello, V.; De Leo, V.; Lasalvia, M.; Hossain, M.N.; Carbone, A.; Catucci, L.; Zefferino, R.; Ingrosso, C.; Conese, M.; Di Gioia, S. Solanum lycopersicum (Tomato)-Derived Nanovesicles Accelerate Wound Healing by Eliciting the Migration of Keratinocytes and Fibroblasts. Int. J. Mol. Sci. 2024, 25, 2452. https://doi.org/10.3390/ijms25052452
Daniello V, De Leo V, Lasalvia M, Hossain MN, Carbone A, Catucci L, Zefferino R, Ingrosso C, Conese M, Di Gioia S. Solanum lycopersicum (Tomato)-Derived Nanovesicles Accelerate Wound Healing by Eliciting the Migration of Keratinocytes and Fibroblasts. International Journal of Molecular Sciences. 2024; 25(5):2452. https://doi.org/10.3390/ijms25052452
Chicago/Turabian StyleDaniello, Valeria, Vincenzo De Leo, Maria Lasalvia, Md Niamat Hossain, Annalucia Carbone, Lucia Catucci, Roberto Zefferino, Chiara Ingrosso, Massimo Conese, and Sante Di Gioia. 2024. "Solanum lycopersicum (Tomato)-Derived Nanovesicles Accelerate Wound Healing by Eliciting the Migration of Keratinocytes and Fibroblasts" International Journal of Molecular Sciences 25, no. 5: 2452. https://doi.org/10.3390/ijms25052452
APA StyleDaniello, V., De Leo, V., Lasalvia, M., Hossain, M. N., Carbone, A., Catucci, L., Zefferino, R., Ingrosso, C., Conese, M., & Di Gioia, S. (2024). Solanum lycopersicum (Tomato)-Derived Nanovesicles Accelerate Wound Healing by Eliciting the Migration of Keratinocytes and Fibroblasts. International Journal of Molecular Sciences, 25(5), 2452. https://doi.org/10.3390/ijms25052452











