Integration Analysis of Hair Follicle Transcriptome and Proteome Reveals the Mechanisms Regulating Wool Fiber Diameter in Angora Rabbits
Abstract
1. Introduction
2. Results
2.1. Identification of Proteins in Hair Follicles of Coarse and Fine Wool
2.2. Identification of Differential Proteins in Hair Follicles of Coarse and Fine Wool
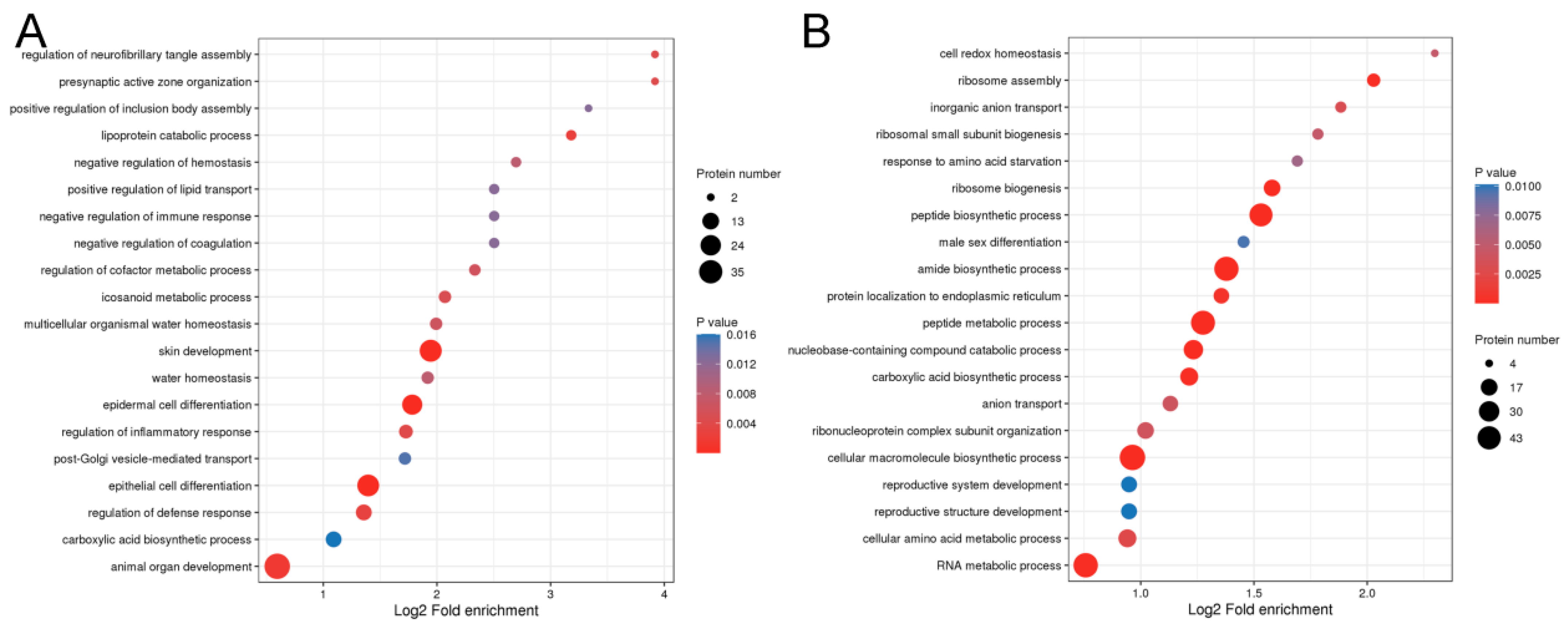
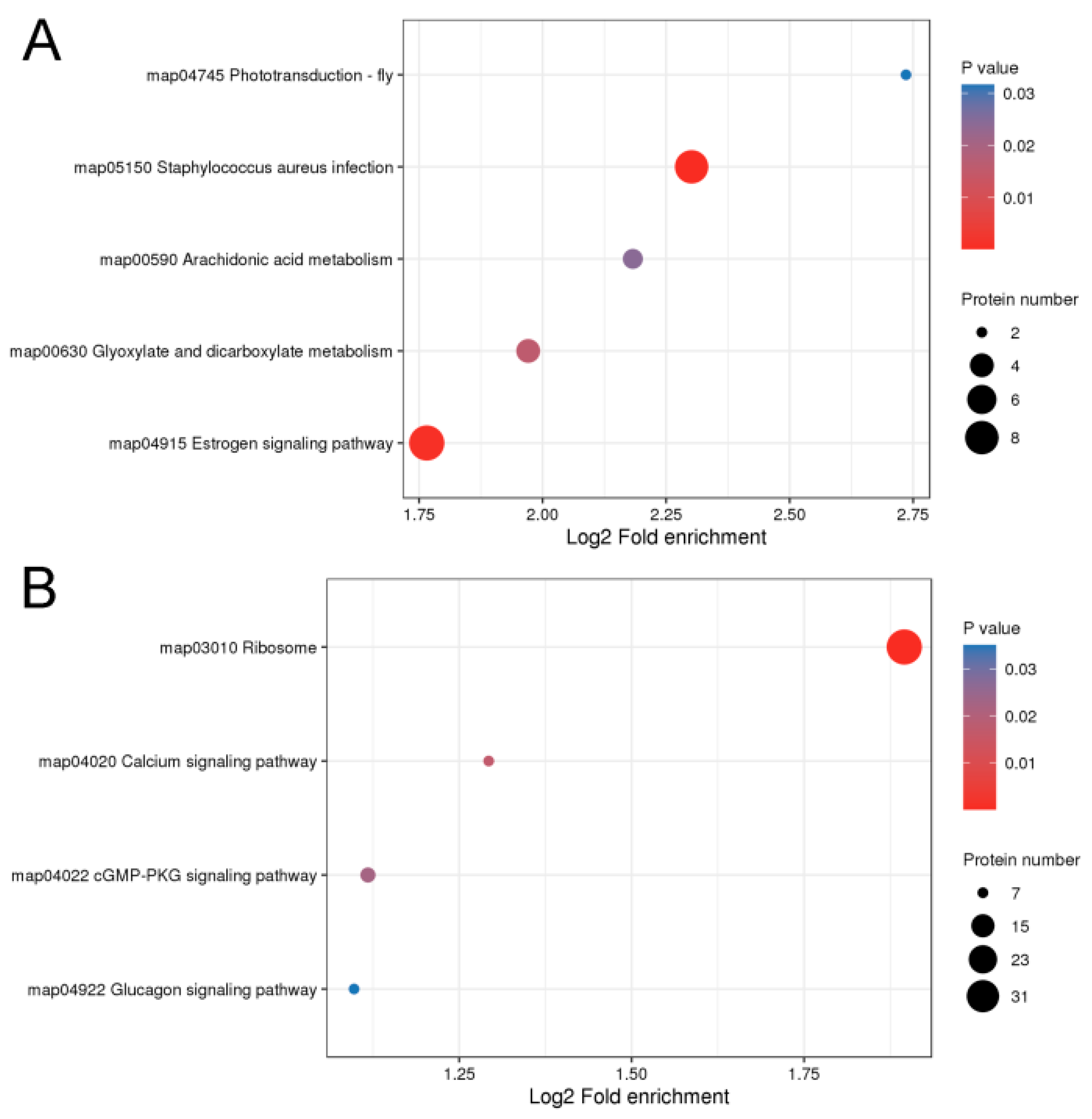
2.3. Functional Enrichment Analysis of DEPs from Hair Follicles of Coarse and Fine Wool
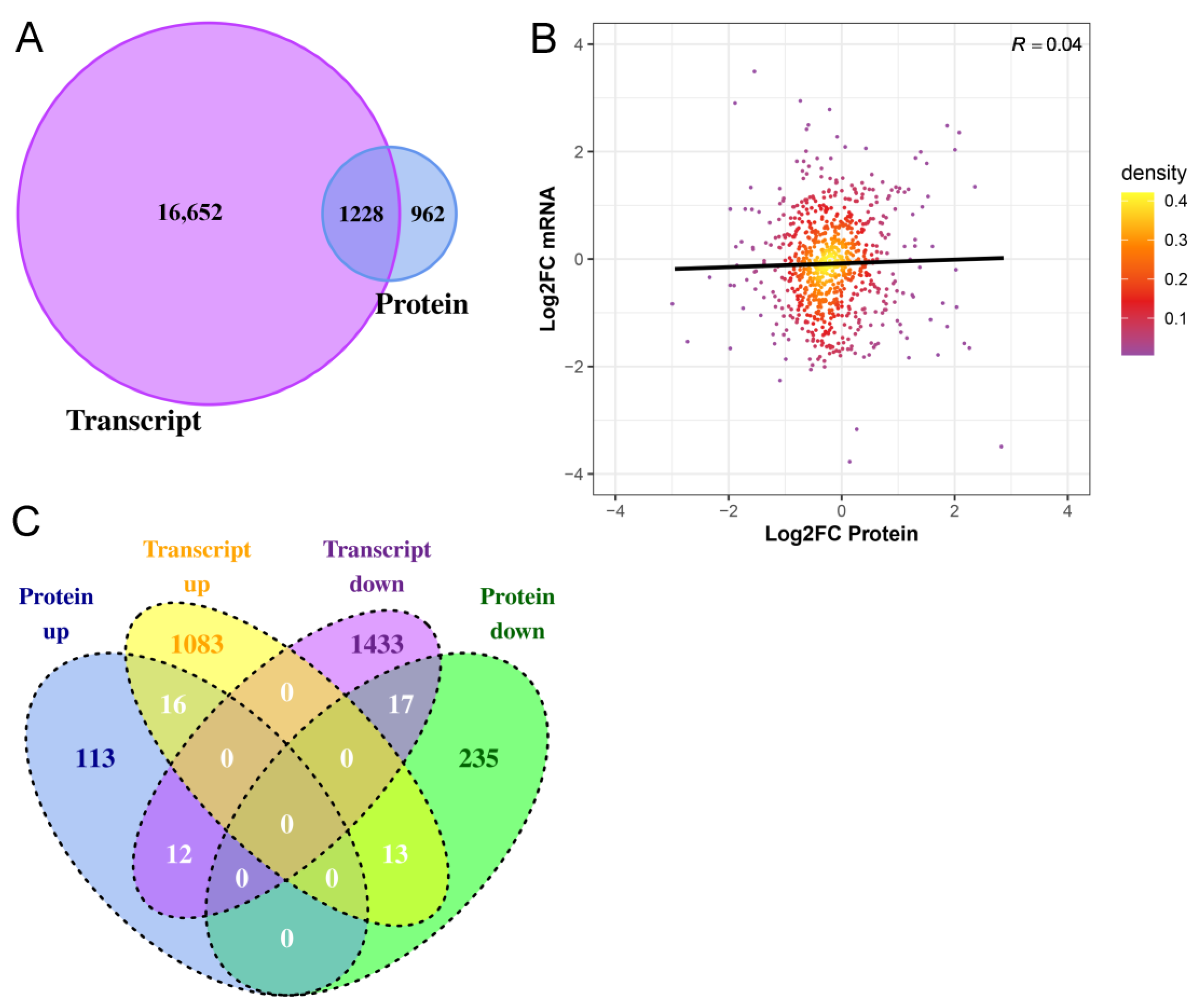
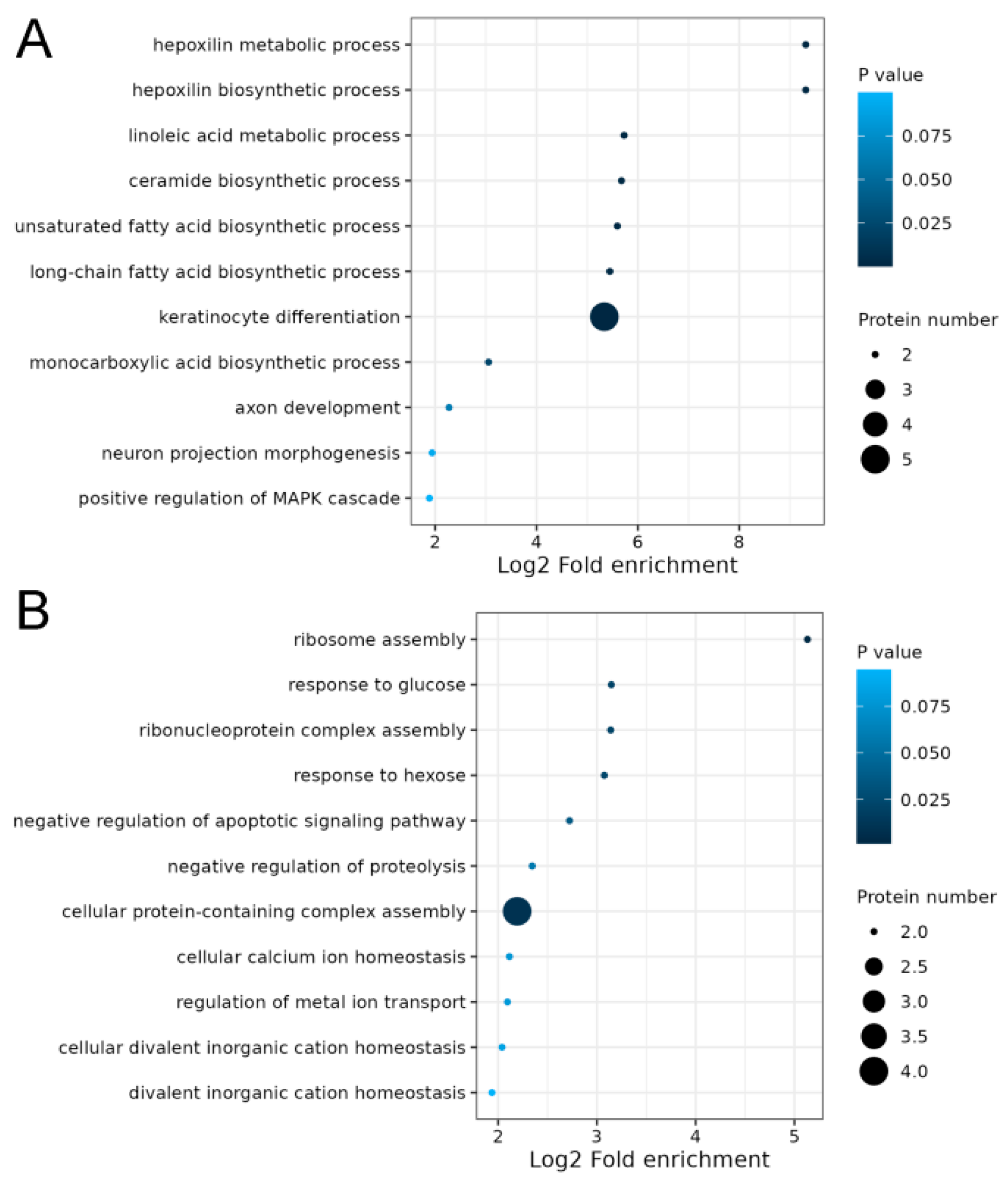
2.4. Integrated Analysis of Transcriptome and Proteome of Hair Follicles from Coarse and Fine Wool
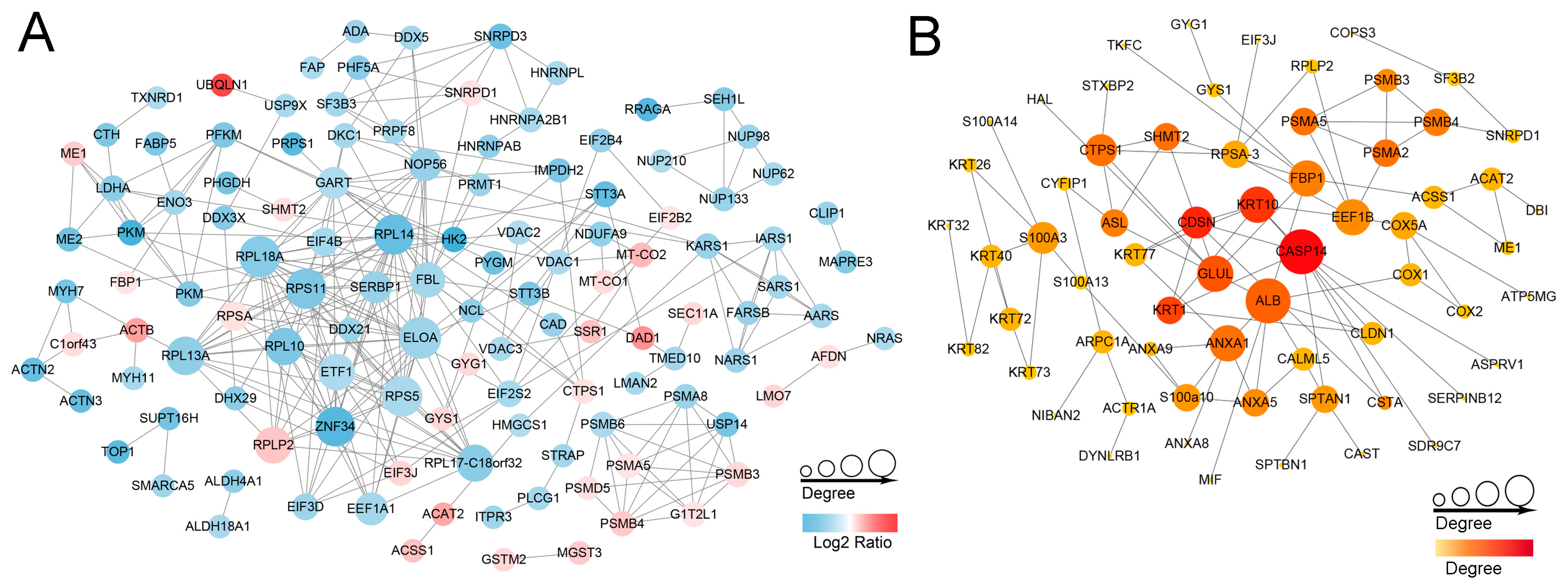
2.5. Protein-Protein Interaction (PPI) Networks
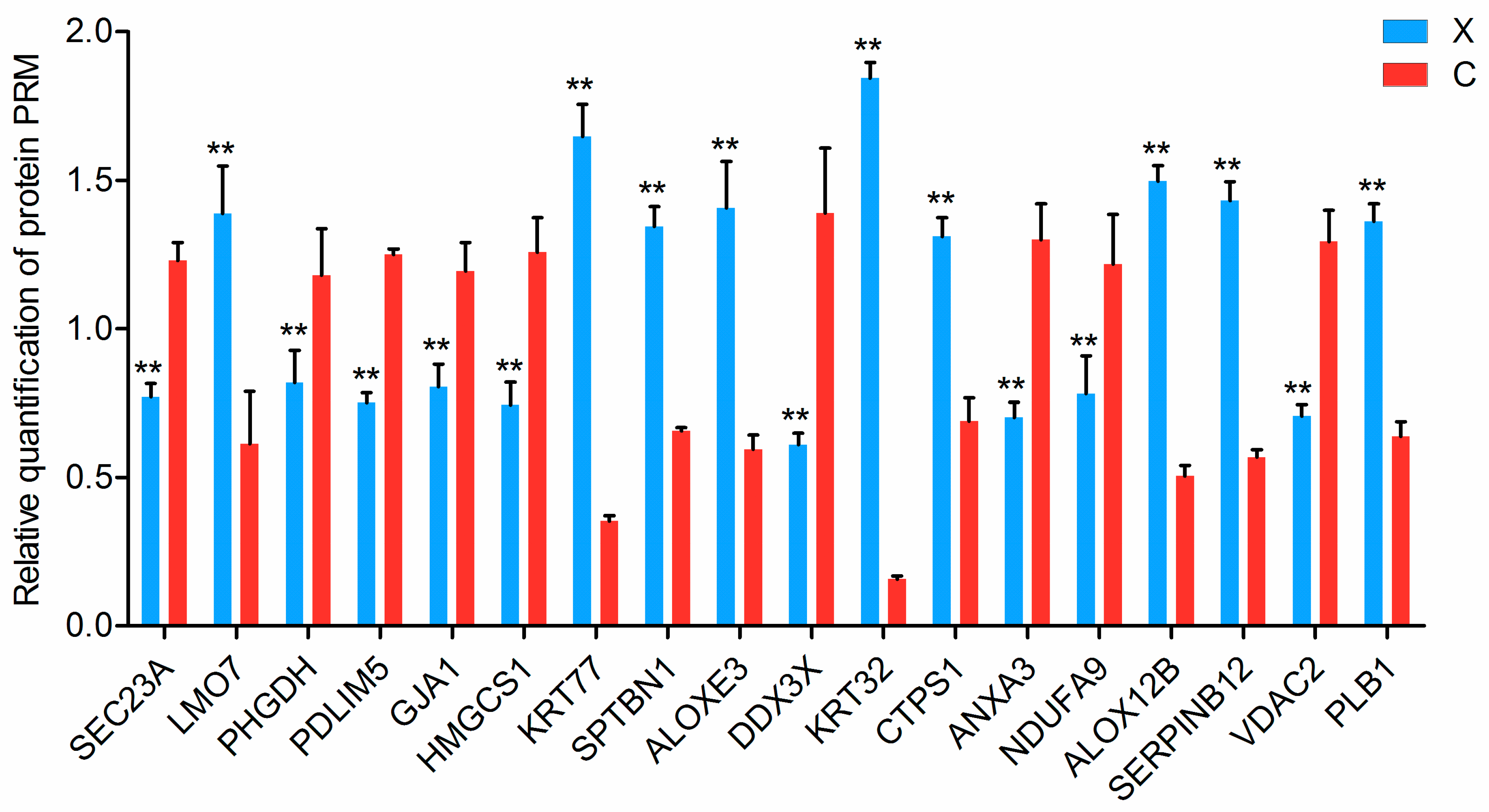
2.6. Validation of Proteins via Parallel Reaction Monitoring (PRM)
3. Discussion
4. Materials and Methods
4.1. Animals and Sample Collection
4.2. Hair Follicle Protein Extraction and Tryptic Digestion
4.3. Liquid Chromatography Tandem Mass Spectrometry Analysis
4.4. Data and Bioinformatics Analysis
4.5. Validation of Protein Expression Levels Using Parallel Reaction Monitoring
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, C.; Qin, Q.; Liu, Z.; Xu, X.; Lan, M.; Xie, Y.; Wang, Z.; Li, J.; Liu, Z. Identification of the key proteins associated with different hair types in sheep and goats. Front. Genet. 2022, 13, 993192. [Google Scholar] [CrossRef] [PubMed]
- Plowman, J.E.; Harland, D.P.; Campos, A.; Rocha, E.S.S.; Thomas, A.; Vernon, J.A.; van Koten, C.; Hefer, C.; Clerens, S.; de Almeida, A.M. The wool proteome and fibre characteristics of three distinct genetic ovine breeds from Portugal. J. Proteom. 2020, 225, 103853. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.Y.; Sheng, S.D.; Hui, T.Y.; Yue, C.; Sun, J.M.; Guo, D.; Guo, S.L.; Li, B.J.; Xue, H.L.; Wang, Z.Y.; et al. An Integrated Analysis of Cashmere Fineness lncRNAs in Cashmere Goats. Genes 2019, 10, 266. [Google Scholar] [CrossRef] [PubMed]
- Dorozynska, K.; Maj, D. Rabbits—Their domestication and molecular genetics of hair coat development and quality. Anim. Genet. 2021, 52, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Yang, Y.; Li, S.; Guo, Y.; Shui, W.; Cao, Q. Discrimination and quantification of homologous keratins from goat and sheep with dual protease digestion and PRM assays. J. Proteom. 2018, 186, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, H.; Luo, Y.; Zhao, M.; Gong, H.; Hao, Z.; Hu, J.; Hickford, J. Variation in the Caprine KAP24-1 Gene Affects Cashmere Fibre Diameter. Animals 2019, 9, 15. [Google Scholar] [CrossRef]
- Zhao, J.; Qin, H.; Xin, J.; Liu, N.; Han, R.; Perez-Campo, F.M.; Li, H. Discovery of genes and proteins possibly regulating mean wool fibre diameter using cDNA microarray and proteomic approaches. Sci. Rep. 2020, 10, 7726. [Google Scholar] [CrossRef]
- Safari, E.; Fogarty, N.M.; Gilmour, A.R. A review of genetic parameter estimates for wool, growth, meat and reproduction traits in sheep. Livest. Prod. Sci. 2005, 92, 271–289. [Google Scholar] [CrossRef]
- Galbraith, H. Fundamental hair follicle biology and fine fibre production in animals. Animal 2010, 4, 1490–1509. [Google Scholar] [CrossRef]
- Yue, Y.J.; Liu, J.B.; Yang, M.; Han, J.L.; Guo, T.T.; Guo, J.; Feng, R.L.; Yang, B.H. De novo assembly and characterization of skin transcriptome using RNAseq in sheep (Ovis aries). Genet. Mol. Res. 2015, 14, 1371–1384. [Google Scholar] [CrossRef]
- Ibraheem, M.; Galbraith, H.; Scaife, J.; Ewen, S. Growth of secondary hair follicles of the Cashmere goat in vitro and their response to prolactin and melatonin. J. Anat. 1994, 185, 135–142. [Google Scholar] [PubMed]
- Zhu, B.; Xu, T.; Yuan, J.; Guo, X.; Liu, D. Transcriptome sequencing reveals differences between primary and secondary hair follicle-derived dermal papilla cells of the Cashmere goat (Capra hircus). PLoS ONE 2013, 8, e76282. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ding, H.; Wang, Y.; Cheng, G.; Wang, X.; Leng, T.; Zhao, H. Hair Follicle Transcriptome Analysis Reveals Differentially Expressed Genes That Regulate Wool Fiber Diameter in Angora Rabbits. Biology 2023, 12, 445. [Google Scholar] [CrossRef]
- Tian, Y.Z.; Usman, T.; Tian, K.C.; Di, J.; Huang, X.X.; Xu, X.M.; Tulafu, H.; Wu, W.W.; Fu, X.F.; Bai, Y.; et al. Comparative study of 13 candidate genes applying multi-reference normalization to detect the expression of different fineness in skin tissues of wool sheep. Genet. Mol. Res. 2017, 16. [Google Scholar] [CrossRef]
- Li, C.; He, X.; Wu, Y.; Li, J.; Zhang, R.; An, X.; Yue, Y. Single-Cell Transcriptome Sequence Profiling on the Morphogenesis of Secondary Hair Follicles in Ordos Fine-Wool Sheep. Int. J. Mol. Sci. 2024, 25, 584. [Google Scholar] [CrossRef]
- He, X.; Chao, Y.; Zhou, G.; Chen, Y. Fibroblast growth factor 5-short (FGF5s) inhibits the activity of FGF5 in primary and secondary hair follicle dermal papilla cells of cashmere goats. Gene 2016, 575, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Liu, C.; Zhang, Y.; Sun, H.; Yang, L.; Bai, L.; Gao, S. Hair Follicle Development of Rex Rabbits Is Regulated Seasonally by Wnt10b/β-Catenin, TGFβ-BMP, IGF1, and EGF Signaling Pathways. Animals 2023, 13, 3742. [Google Scholar] [CrossRef]
- Rishikaysh, P.; Dev, K.; Diaz, D.; Qureshi, W.M.; Filip, S.; Mokry, J. Signaling involved in hair follicle morphogenesis and development. Int. J. Mol. Sci. 2014, 15, 1647–1670. [Google Scholar] [CrossRef]
- Fatima, N.; Jia, L.; Liu, B.; Li, L.; Bai, L.; Wang, W.; Zhao, S.; Wang, R.; Liu, E. A homozygous missense mutation in the fibroblast growth factor 5 gene is associated with the long-hair trait in Angora rabbits. BMC Genom. 2023, 24, 298. [Google Scholar] [CrossRef]
- Zhao, H.; Hu, R.; Li, F.; Yue, X. Five SNPs within the FGF5 Gene Significantly Affect Both Wool Traits and Growth Performance in Fine-Wool Sheep (Ovis aries). Front. Genet. 2021, 12, 732097. [Google Scholar] [CrossRef]
- Yue, L.; Lu, Z.; Guo, T.; Liu, J.; Yuan, C.; Yang, B. Association of SLIT3 and ZNF280B Gene Polymorphisms with Wool Fiber Diameter. Animals 2023, 13, 3552. [Google Scholar] [CrossRef] [PubMed]
- McLaren, R.J.; Rogers, G.R.; Davies, K.P.; Maddox, J.F.; Montgomery, G.W. Linkage mapping of wool keratin and keratin-associated protein genes in sheep. Mamm. Genome 1997, 8, 938–940. [Google Scholar] [CrossRef] [PubMed]
- Plowman, J.E.; Deb-Choudhury, S.; Clerens, S.; Thomas, A.; Cornellison, C.D.; Dyer, J.M. Unravelling the proteome of wool: Towards markers of wool quality traits. J. Proteom. 2012, 75, 4315–4324. [Google Scholar] [CrossRef] [PubMed]
- Powell, B.C.; Rogers, G.E. The role of keratin proteins and their genes in the growth, structure and properties of hair. Exs 1997, 78, 59–148. [Google Scholar] [PubMed]
- Koehn, H.; Clerens, S.; Deb-Choudhury, S.; Morton, J.D.; Dyer, J.M.; Plowman, J.E. The proteome of the wool cuticle. J. Proteome Res. 2010, 9, 2920–2928. [Google Scholar] [CrossRef] [PubMed]
- Bawden, C.S.; Powell, B.C.; Walker, S.K.; Rogers, G.E. Expression of a wool intermediate filament keratin transgene in sheep fibre alters structure. Transgenic Res. 1998, 7, 273–287. [Google Scholar] [CrossRef]
- Clerens, S.; Cornellison, C.D.; Deb-Choudhury, S.; Thomas, A.; Plowman, J.E.; Dyer, J.M. Developing the wool proteome. J. Proteom. 2010, 73, 1722–1731. [Google Scholar] [CrossRef]
- Plowman, J.E.; Harland, D.P.; Ganeshan, S.; Woods, J.L.; van Shaijik, B.; Deb-Choudhury, S.; Thomas, A.; Clerens, S.; Scobie, D.R. The proteomics of wool fibre morphogenesis. J. Struct. Biol. 2015, 191, 341–351. [Google Scholar] [CrossRef]
- Almeida, A.M.; Bassols, A.; Bendixen, E.; Bhide, M.; Ceciliani, F.; Cristobal, S.; Eckersall, P.D.; Hollung, K.; Lisacek, F.; Mazzucchelli, G.; et al. Animal board invited review: Advances in proteomics for animal and food sciences. Animal 2015, 9, 1–17. [Google Scholar] [CrossRef]
- Plowman, J.; Thomas, A.; Perloiro, T.; Clerens, S.; de Almeida, A.M. Characterisation of white and black merino wools: A proteomics study. Animal 2019, 13, 659–665. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, G.; Zhang, R.; Guo, J.; Li, C.; Martin, G.; Chen, Y.; Wang, X. Comparative proteomic analyses using iTRAQ-labeling provides insights into fiber diversity in sheep and goats. J. Proteom. 2018, 172, 82–88. [Google Scholar] [CrossRef]
- Guo, T.; Han, J.; Yuan, C.; Liu, J.; Niu, C.; Lu, Z.; Yue, Y.; Yang, B. Comparative proteomics reveals genetic mechanisms underlying secondary hair follicle development in fine wool sheep during the fetal stage. J. Proteom. 2020, 223, 103827. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, Y.; Tang, H.; Wang, X.; Wang, L.; Zhou, P. Proteomics-based screening of differentially expressed proteins in skin of Chinese merino fine sheep (JunKen type) of different gender. Sheng Wu Gong Cheng Xue Bao 2022, 38, 3925–3939. [Google Scholar] [PubMed]
- Liu, Y.; Ding, Y.; Liu, Z.; Chen, Q.; Li, X.; Xue, X.; Pu, Y.; Ma, Y.; Zhao, Q. Integration Analysis of Transcriptome and Proteome Reveal the Mechanisms of Goat Wool Bending. Front. Cell Dev. Biol. 2022, 10, 836913. [Google Scholar] [CrossRef]
- Zhao, B.; Wu, C.; Sammad, A.; Ma, Z.; Suo, L.; Wu, Y.; Fu, X. The fiber diameter traits of Tibetan cashmere goats are governed by the inherent differences in stress, hypoxic, and metabolic adaptations: An integrative study of proteome and transcriptome. BMC Genom. 2022, 23, 191. [Google Scholar] [CrossRef]
- Niranjan, S.K.; Sharma, S.R.; Gowane, G.R. Estimation of Genetic Parameters for Wool Traits in Angora Rabbit. Asian Austral. J. Anim. 2011, 24, 1335–1340. [Google Scholar] [CrossRef]
- Rafat, S.A.; de Rochambeau, H.; Brims, M.; Thebault, R.G.; Deretz, S.; Bonnet, M.; Allain, D. Characteristics of Angora rabbit fiber using optical fiber diameter analyzer. J. Anim. Sci. 2007, 85, 3116–3122. [Google Scholar] [CrossRef][Green Version]
- Chen, Y.; Bao, Z.; Liu, M.; Li, J.; Dai, Y.; Wang, F.; Zhang, X.; Zhai, P.; Zhao, B.; Wu, X. Promoter Methylation Changes in KRT17: A Novel Epigenetic Marker for Wool Production in Angora Rabbit. Int. J. Mol. Sci. 2022, 23, 6077. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xi, Q.; Zhao, F.; Wang, J.; He, Z.; Hu, J.; Liu, X.; Luo, Y. A highly polymorphic caprine keratin-associated protein gene identified and its effect on cashmere traits. J. Anim. Sci. 2021, 99, skab233. [Google Scholar] [CrossRef] [PubMed]
- Hearle, J.W. A critical review of the structural mechanics of wool and hair fibres. Int. J. Biol. Macromol. 2000, 27, 123–138. [Google Scholar] [CrossRef]
- Seki, Y.; Yokohama, M.; Wada, K.; Fujita, M.; Kotani, M.; Nagura, Y.; Kanno, M.; Nomura, K.; Amano, T.; Kikkawa, Y. Expression analysis of the type I keratin protein keratin 33A in goat coat hair. Anim. Sci. J. 2011, 82, 773–781. [Google Scholar] [CrossRef]
- Maytin, E.V.; Lin, J.C.; Krishnamurthy, R.; Batchvarova, N.; Ron, D.; Mitchell, P.J.; Habener, J.F. Keratin 10 gene expression during differentiation of mouse epidermis requires transcription factors C/EBP and AP-2. Dev. Biol. 1999, 216, 164–181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wu, D.; Ahmed, Z.; Liu, X.; Chen, J.; Ma, J.; Wang, M.; Liu, J.; Zhang, J.; Huang, B.; et al. The genetic secrets of adaptation: Decoding the significance of the 30-bp insertion in the KRT77 gene for Chinese cattle. Anim. Biotechnol. 2023, 34, 3847–3854. [Google Scholar] [CrossRef]
- Cai, P.; Zhang, W.; Jiang, S.; Xiong, Y.; Yuan, H.; Gao, Z.; Gao, X.; Ma, C.; Zhou, Y.; Gong, Y.; et al. Insulin-like Androgenic Gland Hormone Induced Sex Reversal and Molecular Pathways in Macrobrachium nipponense: Insights into Reproduction, Growth, and Sex Differentiation. Int. J. Mol. Sci. 2023, 24, 14306. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Effect of Different LED Light on Hair Follicle Structure and Fur Quality of Angora Rabbits and Its Mechanism. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2017. [Google Scholar]
- Sun, B.K.; Boxer, L.D.; Ransohoff, J.D.; Siprashvili, Z.; Qu, K.; Lopez-Pajares, V.; Hollmig, S.T.; Khavari, P.A. CALML5 is a ZNF750- and TINCR-induced protein that binds stratifin to regulate epidermal differentiation. Genes Dev. 2015, 29, 2225–2230. [Google Scholar] [CrossRef]
- Ohnemus, U.; Uenalan, M.; Inzunza, J.; Gustafsson, J.A.; Paus, R. The hair follicle as an estrogen target and source. Endocr. Rev. 2006, 27, 677–706. [Google Scholar] [CrossRef]
- Ohnemus, U.; Uenalan, M.; Conrad, F.; Handjiski, B.; Mecklenburg, L.; Nakamura, M.; Inzunza, J.; Gustafsson, J.A.; Paus, R. Hair cycle control by estrogens: Catagen induction via estrogen receptor (ER)-alpha is checked by ER beta signaling. Endocrinology 2005, 146, 1214–1225. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Guo, T.; Liu, J.; Guo, J.; Yuan, C.; Feng, R.; Niu, C.; Sun, X.; Yang, B. Exploring Differentially Expressed Genes and Natural Antisense Transcripts in Sheep (Ovis aries) Skin with Different Wool Fiber Diameters by Digital Gene Expression Profiling. PLoS ONE 2015, 10, e129249. [Google Scholar] [CrossRef]
- Kawa, D. Twist of Fate: Ribosomal Stress Reprograms Root Hair Patterning. Plant Cell 2020, 32, 2079–2080. [Google Scholar] [CrossRef]
- Wang, W.; Ryu, K.H.; Bruex, A.; Barron, C.; Schiefelbein, J. Molecular Basis for a Cell Fate Switch in Response to Impaired Ribosome Biogenesis in the Arabidopsis Root Epidermis. Plant Cell 2020, 32, 2402–2423. [Google Scholar] [CrossRef]
- Gao, Y.; Li, T.; Sun, Q.; Huo, B. Gradient fluid shear stress regulates migration of osteoclast precursors. Cell Adh. Migr. 2019, 13, 183–191. [Google Scholar] [CrossRef]
- Wen, X.; Yang, M.; Zhou, K.; Huang, J.; Fan, X.; Zhang, W.; Luo, J. Transcriptomic and proteomic analyses reveal the common and unique pathway(s) underlying different skin colors of leopard coral grouper (Plectropomus leopardus). J. Proteom. 2022, 266, 104671. [Google Scholar] [CrossRef] [PubMed]
- Ellis, D.Z.; Nathanson, J.A.; Sweadner, K.J. Carbachol inhibits Na(+)-K(+)-ATPase activity in choroid plexus via stimulation of the NO/cGMP pathway. Am. J. Physiol. Cell Physiol. 2000, 279, C1685–C1693. [Google Scholar] [CrossRef] [PubMed]
- Wolin, M.S.; Ahmad, M.; Gupte, S.A. Oxidant and redox signaling in vascular oxygen sensing mechanisms: Basic concepts, current controversies, and potential importance of cytosolic NADPH. Am. J. Physiol. Lung Cell Mol. Physiol. 2005, 289, L159–L173. [Google Scholar] [CrossRef]
- Buccitelli, C.; Selbach, M. mRNAs, proteins and the emerging principles of gene expression control. Nat. Rev. Genet. 2020, 21, 630–644. [Google Scholar] [CrossRef] [PubMed]
- Ghazalpour, A.; Bennett, B.; Petyuk, V.A.; Orozco, L.; Hagopian, R.; Mungrue, I.N.; Farber, C.R.; Sinsheimer, J.; Kang, H.M.; Furlotte, N.; et al. Comparative analysis of proteome and transcriptome variation in mouse. PLoS Genet. 2011, 7, e1001393. [Google Scholar] [CrossRef]
- Pascal, L.E.; True, L.D.; Campbell, D.S.; Deutsch, E.W.; Risk, M.; Coleman, I.M.; Eichner, L.J.; Nelson, P.S.; Liu, A.Y. Correlation of mRNA and protein levels: Cell type-specific gene expression of cluster designation antigens in the prostate. BMC Genom. 2008, 9, 246. [Google Scholar] [CrossRef]
- Ng, P.C.; Henikoff, S. SIFT: Predicting amino acid changes that affect protein function. Nucleic Acids Res. 2003, 31, 3812–3814. [Google Scholar] [CrossRef]
- Aebersold, R.; Agar, J.N.; Amster, I.J.; Baker, M.S.; Bertozzi, C.R.; Boja, E.S.; Costello, C.E.; Cravatt, B.F.; Fenselau, C.; Garcia, B.A.; et al. How many human proteoforms are there? Nat. Chem. Biol. 2018, 14, 206–214. [Google Scholar] [CrossRef]
- Salovska, B.; Zhu, H.; Gandhi, T.; Frank, M.; Li, W.; Rosenberger, G.; Wu, C.; Germain, P.L.; Zhou, H.; Hodny, Z.; et al. Isoform-resolved correlation analysis between mRNA abundance regulation and protein level degradation. Mol. Syst. Biol. 2020, 16, e9170. [Google Scholar] [CrossRef]
- Lundberg, E.; Fagerberg, L.; Klevebring, D.; Matic, I.; Geiger, T.; Cox, J.; Algenas, C.; Lundeberg, J.; Mann, M.; Uhlen, M. Defining the transcriptome and proteome in three functionally different human cell lines. Mol. Syst. Biol. 2010, 6, 450. [Google Scholar] [CrossRef]
- de Godoy, L.M.; Olsen, J.V.; Cox, J.; Nielsen, M.L.; Hubner, N.C.; Frohlich, F.; Walther, T.C.; Mann, M. Comprehensive mass-spectrometry-based proteome quantification of haploid versus diploid yeast. Nature 2008, 455, 1251–1254. [Google Scholar] [CrossRef]
- Feng, X.; Coulombe, P.A. A role for disulfide bonding in keratin intermediate filament organization and dynamics in skin keratinocytes. J. Cell Biol. 2015, 209, 59–72. [Google Scholar] [CrossRef]
- Trueb, R.M. Pharmacologic interventions in aging hair. Clin. Interv. Aging 2006, 1, 121–129. [Google Scholar] [CrossRef]
- Tan, T.H.; Li, S.W.; Chang, C.W.; Chen, Y.C.; Liu, Y.H.; Ma, J.T.; Chang, C.P.; Liao, P.C. Rat Hair Metabolomics Analysis Reveals Perturbations of Unsaturated Fatty Acid Biosynthesis, Phenylalanine, and Arachidonic Acid Metabolism Pathways Are Associated with Amyloid-beta-Induced Cognitive Deficits. Mol. Neurobiol. 2023, 60, 4373–4395. [Google Scholar] [CrossRef] [PubMed]
- Westerberg, R.; Tvrdik, P.; Unden, A.B.; Mansson, J.E.; Norlen, L.; Jakobsson, A.; Holleran, W.H.; Elias, P.M.; Asadi, A.; Flodby, P.; et al. Role for ELOVL3 and fatty acid chain length in development of hair and skin function. J. Biol. Chem. 2004, 279, 5621–5629. [Google Scholar] [CrossRef] [PubMed]
- Eckhart, L.; Ban, J.; Fischer, H.; Tschachler, E. Caspase-14: Analysis of gene structure and mRNA expression during keratinocyte differentiation. Biochem. Biophys. Res. Commun. 2000, 277, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Levy-Nissenbaum, E.; Betz, R.C.; Frydman, M.; Simon, M.; Lahat, H.; Bakhan, T.; Goldman, B.; Bygum, A.; Pierick, M.; Hillmer, A.M.; et al. Hypotrichosis simplex of the scalp is associated with nonsense mutations in CDSN encoding corneodesmosin. Nat. Genet. 2003, 34, 151–153. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]


| Total Spectra | Matched Spectra | Peptides | Unique Peptides | Identified Proteins | Quantifiable Proteins |
|---|---|---|---|---|---|
| 1,167,434 | 83,708 | 14,655 | 12,463 | 2275 | 1431 |
| Protein Accession | Gene Name | X Average | C Average | X/C Ratio | X/C p Value | Regulation Type |
|---|---|---|---|---|---|---|
| G1SDF4 | KRT82 | 1.6102455 | 0.3897545 | 4.131 | 1.10187 × 10−10 | Up |
| G1SHX4 | KRT84 | 0.646035 | 1.35396525 | 0.477 | 5.18384 × 10−10 | Down |
| G1SHZ4 | KRT7 | 0.5288665 | 1.47113325 | 0.359 | 8.82596 × 10−8 | Down |
| G1SKE3 | KRT77 | 1.579011 | 0.420989 | 3.751 | 1.05656 × 10−8 | Up |
| G1SUH1 | KRT72 | 1.4606215 | 0.5393785 | 2.708 | 4.71087 × 10−10 | Up |
| G1SWB4 | KRT26 | 1.2793635 | 0.7206365 | 1.775 | 7.79235 × 10−5 | Up |
| G1T1V0 | KRT10 | 1.57839425 | 0.42160575 | 3.744 | 7.48266 × 10−9 | Up |
| G1T4P0 | KRT32 | 1.49585425 | 0.50414575 | 2.967 | 5.87822 × 10−11 | Up |
| G1T4R6 | KRT16 | 0.74950175 | 1.25049825 | 0.599 | 1.71792 × 10−8 | Down |
| G1T6X1 | KRT15 | 1.44077525 | 0.559225 | 2.576 | 3.67408 × 10−6 | Up |
| G1T8T1 | KRT28 | 1.40851325 | 0.5914865 | 2.381 | 1.81837 × 10−9 | Up |
| G1T9T2 | KRT40 | 1.46848275 | 0.53151725 | 2.763 | 1.43752 × 10−8 | Up |
| G1TDN8 | KRT73 | 1.25889925 | 0.74110075 | 1.699 | 6.19365 × 10−8 | Up |
| G1U9I8 | KRT1 | 1.4189405 | 0.5810595 | 2.442 | 3.43761 × 10−11 | Up |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, D.; Ding, H.; Wang, Y.; Wang, X.; Zhao, H. Integration Analysis of Hair Follicle Transcriptome and Proteome Reveals the Mechanisms Regulating Wool Fiber Diameter in Angora Rabbits. Int. J. Mol. Sci. 2024, 25, 3260. https://doi.org/10.3390/ijms25063260
Huang D, Ding H, Wang Y, Wang X, Zhao H. Integration Analysis of Hair Follicle Transcriptome and Proteome Reveals the Mechanisms Regulating Wool Fiber Diameter in Angora Rabbits. International Journal of Molecular Sciences. 2024; 25(6):3260. https://doi.org/10.3390/ijms25063260
Chicago/Turabian StyleHuang, Dongwei, Haisheng Ding, Yuanlang Wang, Xiaofei Wang, and Huiling Zhao. 2024. "Integration Analysis of Hair Follicle Transcriptome and Proteome Reveals the Mechanisms Regulating Wool Fiber Diameter in Angora Rabbits" International Journal of Molecular Sciences 25, no. 6: 3260. https://doi.org/10.3390/ijms25063260
APA StyleHuang, D., Ding, H., Wang, Y., Wang, X., & Zhao, H. (2024). Integration Analysis of Hair Follicle Transcriptome and Proteome Reveals the Mechanisms Regulating Wool Fiber Diameter in Angora Rabbits. International Journal of Molecular Sciences, 25(6), 3260. https://doi.org/10.3390/ijms25063260





