Overcoming the Low-Stability Bottleneck in the Clinical Translation of Liposomal Pressurized Metered-Dose Inhalers: A Shell Stabilization Strategy Inspired by Biomineralization
Abstract
1. Introduction
1.1. Liposomes Act as Superior Drug Delivery Systems
1.2. Realistic Needs and Advantages of Liposome Pulmonary Delivery
2. Pressurized Metered-Dose Inhalers Are Promising for Liposome Pulmonary Delivery
3. Poor Stability Hampers Liposomal pMDIs from Clinical Translation
4. Plausible Strategies for Improving the Stability of Liposomal pMDIs
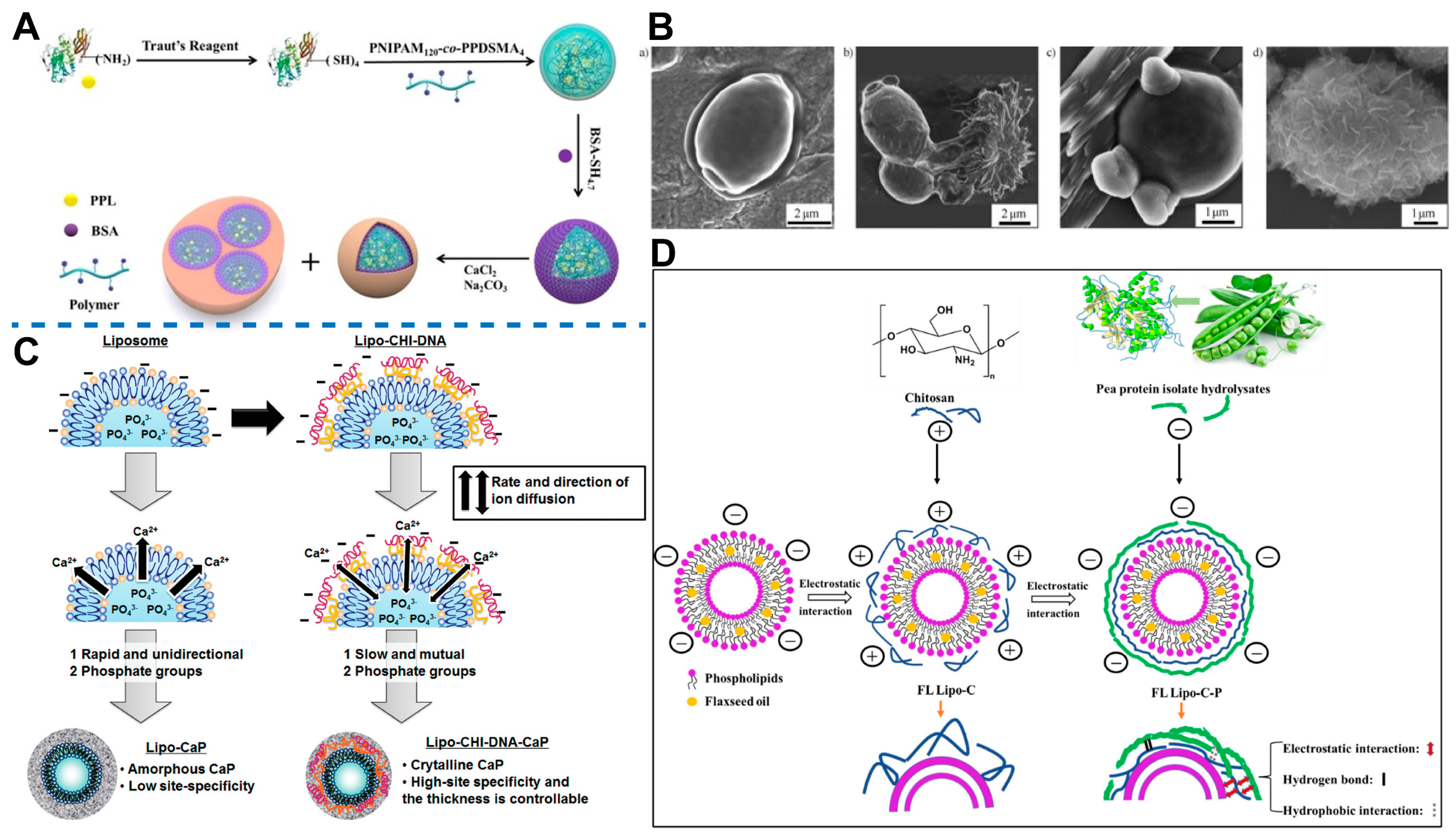
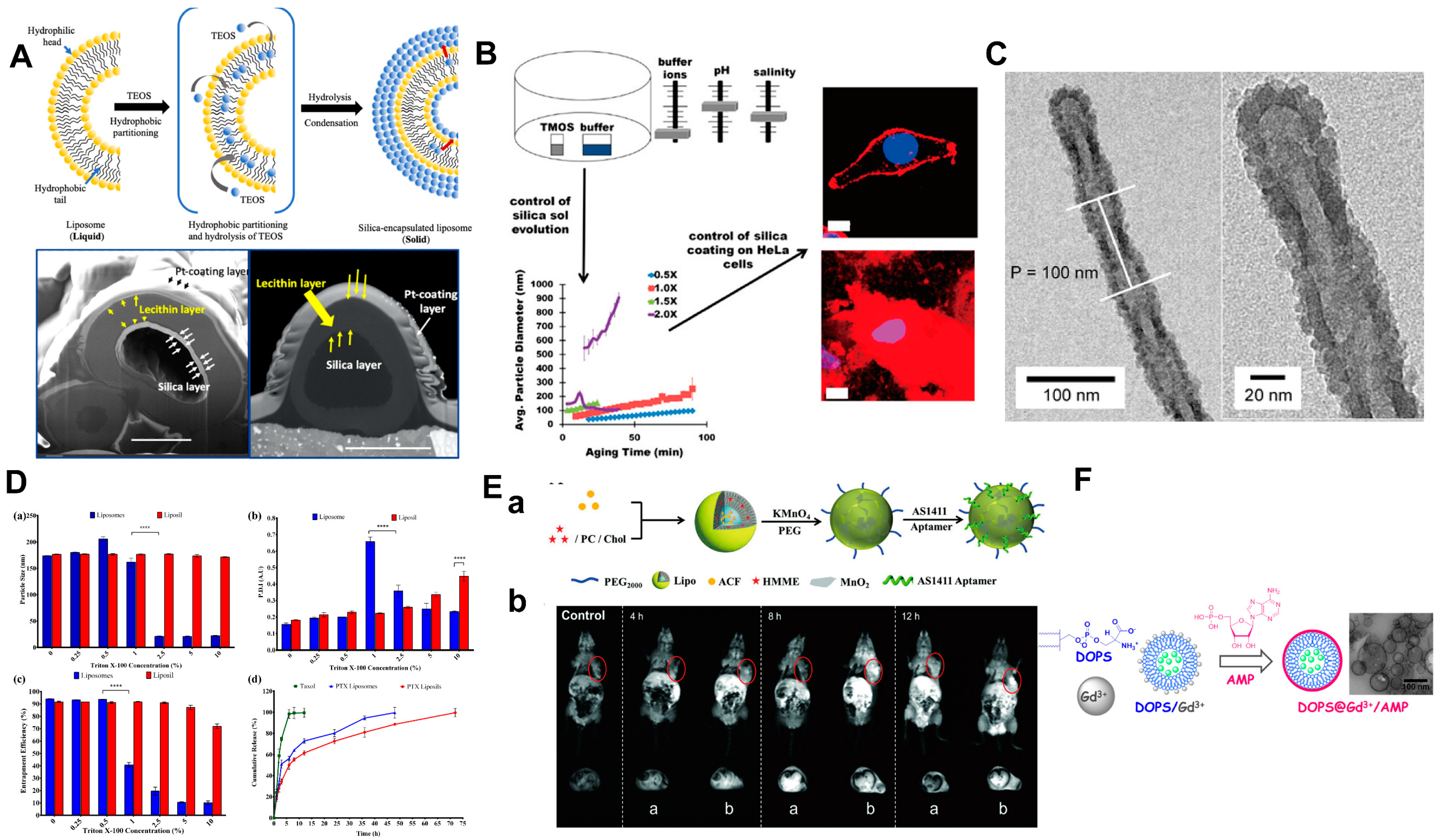
5. Shell Stabilization Strategy Inspired by Biomineralization
5.1. Biomineralization
5.1.1. Biomineralization Can Enhance the Structural Integrity and Durability of Biomaterials
5.1.2. Basic Construction Principles of Biomineralization
5.1.3. Outstanding Organic Template for Biomineralization: Liposome
5.1.4. Calcium-Based Shell
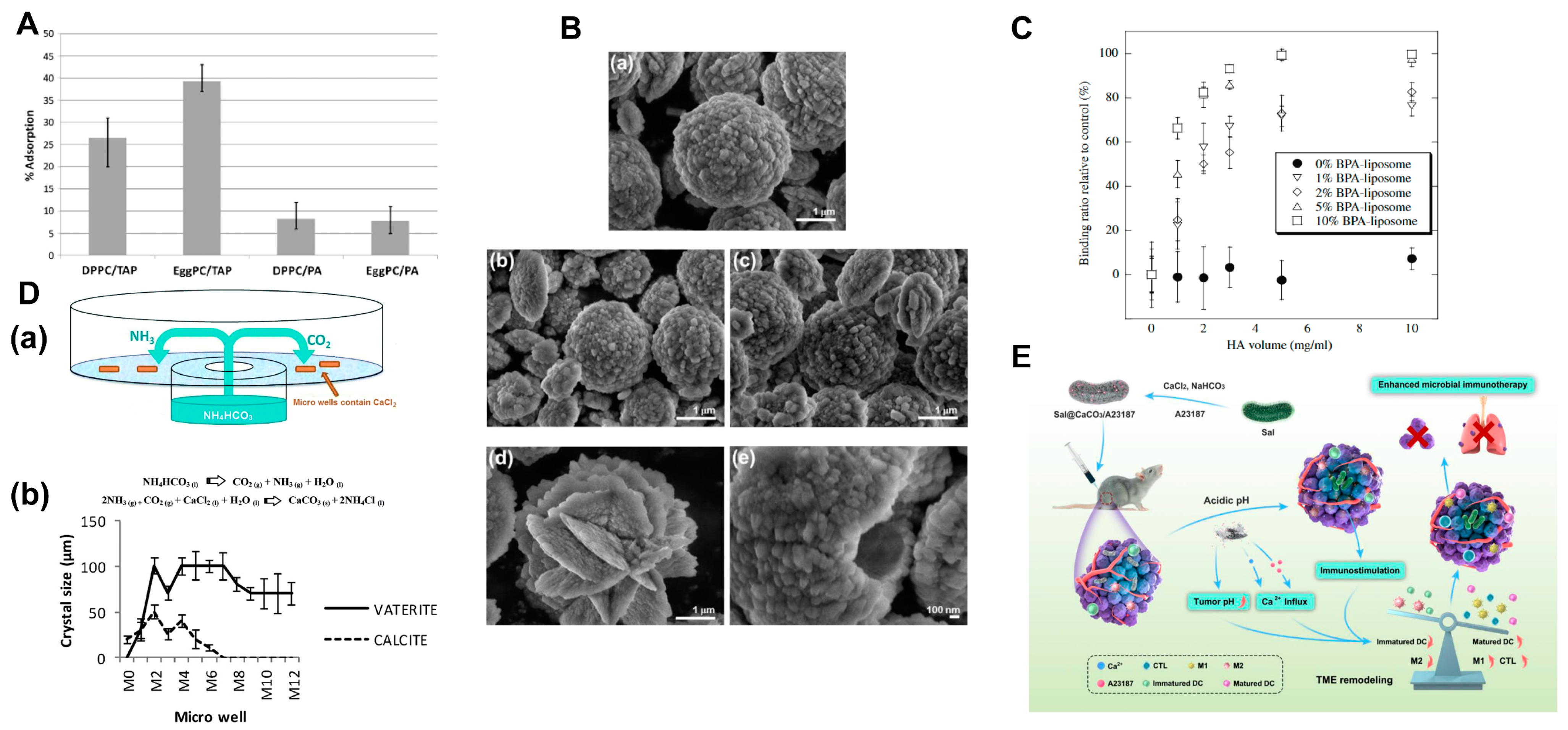
5.1.5. Shell Based on Other Material
5.1.6. Surface Modification
5.2. Biomineralization-like
5.2.1. Artificial Protein Corona
5.2.2. Potential Transformation of Protein Corona
5.3. LbL Assembly
6. Outlook
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Couvreur, P. Nanoparticles in drug delivery: Past, present and future. Adv. Drug Deliv. Rev. 2013, 65, 21–23. [Google Scholar] [CrossRef]
- Song, Y.; Peng, C.; Iqbal, Z.; Sirkar, K.K.; Peterson, G.W.; Mahle, J.J.; Buchanan, J.H. Graphene Oxide and Metal-Organic Framework-Based Breathable Barrier Membranes for Toxic Vapors. ACS Appl. Mater. Interfaces 2022, 14, 31321–31331. [Google Scholar] [CrossRef]
- Kinnear, C.; Moore, T.L.; Rodriguez-Lorenzo, L.; Rothen-Rutishauser, B.; Petri-Fink, A. Form Follows Function: Nanoparticle Shape and Its Implications for Nanomedicine. Chem. Rev. 2017, 117, 11476–11521. [Google Scholar] [CrossRef]
- Hussein, A.K. Applications of nanotechnology to improve the performance of solar collectors—Recent advances and overview. Renew. Sustain. Energy Rev. 2016, 62, 767–792. [Google Scholar] [CrossRef]
- Yan, M.; Chen, Q.; Liu, T.; Li, X.; Pei, P.; Zhou, L.; Zhou, S.; Zhang, R.; Liang, K.; Dong, J.; et al. Site-selective superassembly of biomimetic nanorobots enabling deep penetration into tumor with stiff stroma. Nat. Commun. 2023, 14, 4628. [Google Scholar] [CrossRef]
- Kiyomiya, K.; Matsuo, S.; Kurebe, M. Differences in intracellular sites of action of Adriamycin in neoplastic and normal differentiated cells. Cancer Chemother. Pharmacol. 2001, 47, 51–56. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Mittal, D.; Ali, A.; Md, S.; Baboota, S.; Sahni, J.K.; Ali, J. Insights into direct nose to brain delivery: Current status and future perspective. Drug Deliv. 2014, 21, 75–86. [Google Scholar] [CrossRef]
- Banerjee, P.; Qamar, I. Chapter 11—Novel anticancer drugs related to cardiotoxicity. In Cardiovascular Toxicity and Therapeutic Modalities Targeting Cardio-Oncology; Qamar, I., Maurya, P.K., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 195–213. [Google Scholar]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef]
- Urbán, P.; Valle-Delgado, J.J.; Moles, E.; Marques, J.; Díez, C.; Fernàndez-Busquets, X. Nanotools for the delivery of antimicrobial peptides. Curr. Drug Targets 2012, 13, 1158–1172. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, Y.S.; Pang, B.; Hyun, D.C.; Yang, M.; Xia, Y. Engineered Nanoparticles for Drug Delivery in Cancer Therapy. Nanomater. Neoplasms 2014, 53, 12320–12364. [Google Scholar] [CrossRef]
- Noda, M.; Shimanouchi, T.; Okuyama, M.; Kuboi, R. A bio-thermochemical microbolometer with immobilized intact liposome on sensor solid surface. Sens. Actuators B Chem. 2008, 135, 40–45. [Google Scholar] [CrossRef]
- Sabir, F.; Barani, M.; Randar, A.; Bilal, M.; Zafar, M.N.; Bungau, S.; Kyzas, G.Z. How to Face Skin Cancer with Nanomaterials: A Review. Biointerface Res. Appl. Chem. 2021, 11, 11931–11955. [Google Scholar] [CrossRef]
- Chen, T.; Gong, T.; Zhao, T.; Fu, Y.; Zhang, Z.; Gong, T. A comparison study between lycobetaine-loaded nanoemulsion and liposome using nRGD as therapeutic adjuvant for lung cancer therapy. Eur. J. Pharm. Sci. 2018, 111, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Marqués-Gallego, P.; de Kroon, A.I.P.M. Ligation Strategies for Targeting Liposomal Nanocarriers. BioMed Res. Int. 2014, 2014, 129458. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, W.; Xiao, Q.; Fan, L.; Huang, D.; Chen, W.; He, W. Liposomes and liposome-like nanoparticles: From anti-fungal infection to the COVID-19 pandemic treatment. Asian J. Pharm. Sci. 2022, 17, 817–837. [Google Scholar] [CrossRef]
- Dubey, A.K.; Chaudhry, S.K.; Singh, H.B.; Gupta, V.K.; Kaushik, A. Perspectives on nano-nutraceuticals to manage pre and post COVID-19 infections. Biotechnol. Rep. 2022, 33, e00712. [Google Scholar] [CrossRef]
- Yarborough, B.J.H.; Ahmedani, B.K.; Boggs, J.M.; Beck, A.; Coleman, K.J.; Sterling, S.; Schoenbaum, M.; Goldstein-Grumet, J.; Simon, G.E. Challenges of Population-based Measurement of Suicide Prevention Activities Across Multiple Health Systems. EGEMS 2019, 7, 13. [Google Scholar] [CrossRef]
- Lakshmanan, V.K.; Jindal, S.; Packirisamy, G.; Ojha, S.; Lian, S.; Kaushik, A.; Alzarooni, A.; Metwally, Y.A.F.; Thyagarajan, S.P.; Do Jung, Y.; et al. Nanomedicine-based cancer immunotherapy: Recent trends and future perspectives. Cancer Gene Ther. 2021, 28, 911–923. [Google Scholar] [CrossRef]
- Zar, H.J.; Ferkol, T.W. The global burden of respiratory disease-impact on child health. Pediatr. Pulmonol. 2014, 49, 430–434. [Google Scholar] [CrossRef]
- Sacks, D.; Baxter, B.; Campbell, B.C.V.; Carpenter, J.S.; Cognard, C.; Dippel, D.; Eesa, M.; Fischer, U.; Hausegger, K.; Hirsch, J.A.; et al. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. Int. J. Stroke Off. J. Int. Stroke Soc. 2018, 13, 612–632. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Nehra, M.; Khurana, S.; Dilbaghi, N.; Kumar, V.; Kaushik, A.; Kim, K.H. Aspects of Point-of-Care Diagnostics for Personalized Health Wellness. Int. J. Nanomed. 2021, 16, 383–402. [Google Scholar] [CrossRef] [PubMed]
- Lemjabbar-Alaoui, H.; Hassan, O.U.; Yang, Y.W.; Buchanan, P. Lung cancer: Biology and treatment options. Biochim. Biophys. Acta 2015, 1856, 189–210. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Skwarczynski, M.; Toth, I. Lipid Core Peptide System for Gene, Drug, and Vaccine Delivery. J Aust. J. Chem. 2009, 62, 956–967. [Google Scholar] [CrossRef]
- Peng, T.; Xu, W.; Li, Q.; Ding, Y.; Huang, Y. Pharmaceutical liposomal delivery-specific considerations of innovation and challenges. Biomater. Sci. 2022, 11, 62–75. [Google Scholar] [CrossRef]
- Ghaffar, K.A.; Giddam, A.K.; Zaman, M.; Skwarczynski, M.; Toth, I. Liposomes as nanovaccine delivery systems. Curr. Top. Med. Chem. 2014, 14, 1194–1208. [Google Scholar] [CrossRef]
- Kaur, G.; Narang, R.K.; Rath, G.; Goyal, A.K. Advances in pulmonary delivery of nanoparticles. Artif. Cells Blood Substit. Immobil. Biotechnol. 2012, 40, 75–96. [Google Scholar] [CrossRef]
- Patton, J.S.; Fishburn, C.S.; Weers, J.G. The lungs as a portal of entry for systemic drug delivery. Proc. Am. Thorac. Soc. 2004, 1, 338–344. [Google Scholar] [CrossRef]
- Tiwari, S.; Atluri, V.; Kaushik, A.; Yndart, A.; Nair, M. Alzheimer’s disease: Pathogenesis, diagnostics, and therapeutics. Int. J. Nanomed. 2019, 14, 5541–5554. [Google Scholar] [CrossRef]
- Han, Z.Y.; Chen, Q.W.; Zheng, D.W.; Chen, K.W.; Huang, Q.X.; Zhuang, Z.N.; Zhang, X.Z. Inhalable Capsular Polysaccharide-Camouflaged Gallium-Polyphenol Nanoparticles Enhance Lung Cancer Chemotherapy by Depleting Local Lung Microbiota. Adv. Mater. 2023, 35, e2302551. [Google Scholar] [CrossRef]
- Ponkshe, P.; Feng, S.; Tan, C. Inhalable liposomes for treating lung diseases: Clinical development and challenges. Biomed. Mater. 2021, 16, 054101. [Google Scholar] [CrossRef]
- Griffith, D.E.; Eagle, G.; Thomson, R.; Aksamit, T.R.; Hasegawa, N.; Morimoto, K.; Addrizzo-Harris, D.J.; O’Donnell, A.E.; Marras, T.K.; Flume, P.A.; et al. Amikacin Liposome Inhalation Suspension for Treatment-Refractory Lung Disease Caused by Mycobacterium avium Complex (CONVERT). A Prospective, Open-Label, Randomized Study. Am. J. Respir. Crit. Care Med. 2018, 198, 1559–1569. [Google Scholar] [CrossRef]
- Zhu, X.; Kong, Y.; Liu, Q.; Lu, Y.; Xing, H.; Lu, X.; Yang, Y.; Xu, J.; Li, N.; Zhao, D.; et al. Inhalable dry powder prepared from folic acid-conjugated docetaxel liposomes alters pharmacodynamic and pharmacokinetic properties relevant to lung cancer chemotherapy. Pulm. Pharmacol. Ther. 2019, 55, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.; Huang, K.; Li, Y.; Li, R.; Zhou, X.; Shi, J.; Tong, Z.; Sun, Z.; Yu, A. Optimization of formulation and atomization of lipid nanoparticles for the inhalation of mRNA. Int. J. Pharm. 2023, 640, 123050. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Mehta, P.; Shankar, K.R.; Rajora, M.A.K.; Mishra, Y.K.; Mostafavi, E.; Kaushik, A. Nanotechnology-Assisted Metered-Dose Inhalers (MDIs) for High-Performance Pulmonary Drug Delivery Applications. Pharm. Res. 2022, 39, 2831–2855. [Google Scholar] [CrossRef]
- Zeng, Q.X.; Xi, Q.Y.; Luo, Q.; Liu, Z.H.; Xiong, C.M. Etiological classification and manifestation of pulmonary artery dissection: A literature review and case analysis. Heliyon 2023, 9, e22570. [Google Scholar] [CrossRef]
- Martin, A.R.; Finlay, W.H. Nebulizers for drug delivery to the lungs. Expert. Opin. Drug Deliv. 2015, 12, 889–900. [Google Scholar] [CrossRef] [PubMed]
- Schuh, S.; Sweeney, J.; Rumantir, M.; Coates, A.L.; Willan, A.R.; Stephens, D.; Atenafu, E.G.; Finkelstein, Y.; Thompson, G.; Zemek, R.; et al. Effect of Nebulized Magnesium vs Placebo Added to Albuterol on Hospitalization Among Children With Refractory Acute Asthma Treated in the Emergency Department: A Randomized Clinical Trial. Jama 2020, 324, 2038–2047. [Google Scholar] [CrossRef]
- Scherließ, R.; Etschmann, C. DPI formulations for high dose applications—Challenges and opportunities. Int. J. Pharm. 2018, 548, 49–53. [Google Scholar] [CrossRef]
- Ibrahim, M.; Verma, R.; Garcia-Contreras, L. Inhalation drug delivery devices: Technology update. Med. Devices 2015, 8, 131–139. [Google Scholar] [CrossRef]
- Sanchis, J.; Corrigan, C.; Levy, M.L.; Viejo, J.L. Inhaler devices—From theory to practice. Respir. Med. 2013, 107, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Shu, L.; Huang, Y.; Wu, C.; Pan, X. Low Drug Loading Hampers the Clinical Translation of Peptide Drugs-Containing Metered-Dose Inhalers. Pharmaceuticals 2022, 15, 389. [Google Scholar] [CrossRef]
- Ivey, J.W.; Vehring, R.; Finlay, W.H. Understanding pressurized metered dose inhaler performance. Expert. Opin. Drug Deliv. 2015, 12, 901–916. [Google Scholar] [CrossRef] [PubMed]
- Vallorz, E.; Sheth, P.; Myrdal, P. Pressurized Metered Dose Inhaler Technology: Manufacturing. AAPS PharmSciTech 2019, 20, 177. [Google Scholar] [CrossRef]
- Mehta, P.P.; Kadam, S.S.; Pawar, A.P. Influence of modified induction port, modified DUSA assembly and device air-inlet geometry on the aerosolization pattern of a dry powder inhaler. J. Drug Deliv. Sci. Technol. 2020, 55, 101416. [Google Scholar] [CrossRef]
- Li, M.; Du, C.; Guo, N.; Teng, Y.; Meng, X.; Sun, H.; Li, S.; Yu, P.; Galons, H. Composition design and medical application of liposomes. Eur. J. Med. Chem. 2019, 164, 640–653. [Google Scholar] [CrossRef]
- Rau, J.L. The inhalation of drugs: Advantages and problems. Respir. Care 2005, 50, 367–382. [Google Scholar]
- Kuzmov, A.; Minko, T. Nanotechnology approaches for inhalation treatment of lung diseases. J. Control. Release 2015, 219, 500–518. [Google Scholar] [CrossRef] [PubMed]
- Janson, C.; Henderson, R.; Löfdahl, M.; Hedberg, M.; Sharma, R.; Wilkinson, A.J.K. Carbon footprint impact of the choice of inhalers for asthma and COPD. Thorax 2020, 75, 82–84. [Google Scholar] [CrossRef]
- Myrdal, P.B.; Sheth, P.; Stein, S.W. Advances in Metered Dose Inhaler Technology: Formulation Development. AAPS PharmSciTech 2014, 15, 434–455. [Google Scholar] [CrossRef]
- Soni, M.; Handa, M.; Singh, K.K.; Shukla, R. Recent nanoengineered diagnostic and therapeutic advancements in management of Sepsis. J. Control. Release Off. J. Control. Release Soc. 2022, 352, 931–945. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A. Methods of Liposomes Preparation: Formation and Control Factors of Versatile Nanocarriers for Biomedical and Nanomedicine Application. Pharmaceutics 2022, 14, 543. [Google Scholar] [CrossRef] [PubMed]
- Baldrick, P. Pharmaceutical excipient development: The need for preclinical guidance. Regul. Toxicol. Pharmacol. RTP 2000, 32, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Mossadeq, S.; Shah, R.; Shah, V.; Bagul, M. Formulation, Device, and Clinical Factors Influencing the Targeted Delivery of COVID-19 Vaccines to the Lungs. AAPS PharmSciTech 2022, 24, 2. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Liang, Q.; Gao, F.; Liu, T.; Wu, Y.; Zheng, Z.; Kang, J.; Xu, R.; Cao, Y.; Xiang, M. Design of high-performance biomimetic reverse osmosis membranes by introducing loose liposome as an artificial water channel. Chem. Eng. J. 2022, 431, 133878. [Google Scholar] [CrossRef]
- Labiris, N.R.; Dolovich, M.B. Pulmonary drug delivery. Part I: Physiological factors affecting therapeutic effectiveness of aerosolized medications. Br. J. Clin. Pharmacol. 2003, 56, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Hardy, C.L.; LeMasurier, J.S.; Mohamud, R.; Yao, J.; Xiang, S.D.; Rolland, J.M.; O’Hehir, R.E.; Plebanski, M. Differential Uptake of Nanoparticles and Microparticles by Pulmonary APC Subsets Induces Discrete Immunological Imprints. J. Immunol. 2013, 191, 5278–5290. [Google Scholar] [CrossRef] [PubMed]
- Stein, S.W.; Sheth, P.; Hodson, P.D.; Myrdal, P.B. Advances in metered dose inhaler technology: Hardware development. AAPS PharmSciTech 2014, 15, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.T.; Lee, H.; Kim, J.; Jung, Y.; Choi, E.; Jeong, J.H.; Jeong, J.-H.; Lee, J.H.; Youn, Y.S. Alveolar macrophage phagocytosis-evading inhaled microgels incorporating nintedanib-PLGA nanoparticles and pirfenidone-liposomes for improved treatment of pulmonary fibrosis. Bioact. Mater. 2024, 33, 262–278. [Google Scholar] [CrossRef]
- Gavtash, B.; Versteeg, H.K.; Hargrave, G.; Myatt, B.; Lewis, D.; Church, T.; Brambilla, G. Transient aerodynamic atomization model to predict aerosol droplet size of pressurized metered dose inhalers (pMDI). Aerosol Sci. Technol. 2017, 51, 998–1008. [Google Scholar] [CrossRef]
- Tang, S.; Davoudi, Z.; Wang, G.; Xu, Z.; Rehman, T.; Prominski, A.; Tian, B.; Bratlie, K.M.; Peng, H.; Wang, Q. Soft materials as biological and artificial membranes. Chem. Soc. Rev. 2021, 50, 12679–12701. [Google Scholar] [CrossRef]
- Wyrzykowska, E.; Mikolajczyk, A.; Lynch, I.; Jeliazkova, N.; Kochev, N.; Sarimveis, H.; Doganis, P.; Karatzas, P.; Afantitis, A.; Melagraki, G.; et al. Representing and describing nanomaterials in predictive nanoinformatics. Nat. Nanotechnol. 2022, 17, 924–932. [Google Scholar] [CrossRef]
- Alhowyan, A.A.; Altamimi, M.A.; Kalam, M.A.; Khan, A.A.; Badran, M.; Binkhathlan, Z.; Alkholief, M.; Alshamsan, A. Antifungal efficacy of Itraconazole loaded PLGA-nanoparticles stabilized by vitamin-E TPGS: In vitro and ex vivo studies. J. Microbiol. Methods 2019, 161, 87–95. [Google Scholar] [CrossRef]
- Sun, X.; Pan, C.; Ying, Z.; Yu, D.; Duan, X.; Huang, F.; Ling, J.; Ouyang, X.K. Stabilization of zein nanoparticles with k-carrageenan and tween 80 for encapsulation of curcumin. Int. J. Biol. Macromol. 2020, 146, 549–559. [Google Scholar] [CrossRef]
- Wang, Z.; Ji, L.; Ren, Y.; Liu, M.; Ai, X.; Yang, C. Preparation and Anti-tumor Study of Dextran 70,000-Selenium Nanoparticles and Poloxamer 188-Selenium Nanoparticles. AAPS PharmSciTech 2021, 23, 29. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Liu, Q.; Yan, J.; Pan, D.; Wang, L.; Xu, Y.; Wang, F.; Liu, Y.; Li, X.; et al. ROS-Responsive Boronate-Stabilized Polyphenol-Poloxamer 188 Assembled Dexamethasone Nanodrug for Macrophage Repolarization in Osteoarthritis Treatment. Adv. Healthc. Mater. 2021, 10, e2100883. [Google Scholar] [CrossRef] [PubMed]
- Piacentini, E.; Yan, M.; Giorno, L. Development of enzyme-loaded PVA microspheres by membrane emulsification. J. Membr. Sci. 2017, 524, 79–86. [Google Scholar] [CrossRef]
- Fraj, A.; Jaâfar, F.; Marti, M.; Coderch, L.; Ladhari, N. A comparative study of oregano (Origanum vulgare L.) essential oil-based polycaprolactone nanocapsules/ microspheres: Preparation, physicochemical characterization, and storage stability. Ind. Crops Prod. 2019, 140, 111669. [Google Scholar] [CrossRef]
- Lu, C.; Dong, P.; Pi, L.; Wang, Z.; Yuan, H.; Liang, H.; Ma, D.; Chai, K.Y. Hydroxyl-PEG-Phosphonic Acid-Stabilized Superparamagnetic Manganese Oxide-Doped Iron Oxide Nanoparticles with Synergistic Effects for Dual-Mode MR Imaging. Langmuir 2019, 35, 9474–9482. [Google Scholar] [CrossRef]
- Jayeoye, T.J.; Olatunde, O.O.; Benjakul, S.; Rujiralai, T. Synthesis and characterization of novel poly(3-aminophenyl boronic acid-co-vinyl alcohol) nanocomposite polymer stabilized silver nanoparticles with antibacterial and antioxidant applications. Colloids Surf. B Biointerfaces 2020, 193, 111112. [Google Scholar] [CrossRef]
- Oh, H.; Lee, J.S.; Lee, H.S.; Sung, D.; Choi, W.I. A Novel Polyvinylpyrrolidone-Stabilized Illite Microparticle with Enhanced Antioxidant and Antibacterial Effect. Polymers 2021, 13, 4275. [Google Scholar] [CrossRef]
- Bae, Y.; Ha, M.Y.; Bang, K.T.; Yang, S.; Kang, S.Y.; Kim, J.; Sung, J.; Kang, S.; Kang, D.; Lee, W.B.; et al. Conformation Dynamics of Single Polymer Strands in Solution. Adv. Mater. 2022, 34, e2202353. [Google Scholar] [CrossRef]
- Murdock, D.J.E. The ‘biomineralization toolkit’ and the origin of animal skeletons. Biol. Rev. Camb. Philos. Soc. 2020, 95, 1372–1392. [Google Scholar] [CrossRef]
- Wang, Y.N.; Jiang, S.Q.; Pan, H.H.; Tang, R.K. Less is more: Silicate in the crystallization of hydroxyapatite in simulated body fluids. CrystEngComm 2016, 18, 379–383. [Google Scholar] [CrossRef]
- DeVol, R.T.; Sun, C.-Y.; Marcus, M.A.; Coppersmith, S.N.; Myneni, S.C.B.; Gilbert, P.U.P.A. Nanoscale Transforming Mineral Phases in Fresh Nacre. J. Am. Chem. Soc. 2015, 137, 13325–13333. [Google Scholar] [CrossRef]
- Yang, G.; Liu, Y.; Jin, S.; Zhao, C.-X. Development of Core-Shell Nanoparticle Drug Delivery Systems Based on Biomimetic Mineralization. ChemBioChem 2020, 21, 2871–2879. [Google Scholar] [CrossRef]
- Kong, T.; Wang, L.; Wyss, H.M.; Shum, H.C. Capillary micromechanics for core-shell particles. Soft Matter 2014, 10, 3271–3276. [Google Scholar] [CrossRef]
- Mandal, B.; Bhattacharjee, H.; Mittal, N.; Sah, H.; Balabathula, P.; Thoma, L.A.; Wood, G.C. Core–shell-type lipid–polymer hybrid nanoparticles as a drug delivery platform. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 474–491. [Google Scholar] [CrossRef]
- Han, R.L.; Tang, K.Q.; Hou, Y.F.; Yu, J.C.; Wang, C.L.; Wang, Y. Fabrication of core/shell/shell structure nanoparticle with anticancer drug and dual-photosensitizer co-loading for synergistic chemotherapy and photodynamic therapy. Microporous Mesoporous Mater. 2020, 297, 110049. [Google Scholar] [CrossRef]
- Lancet, D.; Pecht, I. Spectroscopic and immunochemical studies with nitrobenzoxadiazolealanine, a fluorescent dinitrophenyl analog. Biochemistry 1977, 16, 5150–5157. [Google Scholar] [CrossRef]
- Estroff, L.A. Introduction: Biomineralization. Chem. Rev. 2008, 108, 4329–4331. [Google Scholar] [CrossRef]
- Kawasaki, K.; Buchanan, A.V.; Weiss, K.M. Biomineralization in humans: Making the hard choices in life. Annu. Rev. Genet. 2009, 43, 119–142. [Google Scholar] [CrossRef]
- Aizenberg, J.; Weaver, J.C.; Thanawala, M.S.; Sundar, V.C.; Morse, D.E.; Fratzl, P. Skeleton of Euplectella sp.: Structural hierarchy from the nanoscale to the macroscale. Science 2005, 309, 275–278. [Google Scholar] [CrossRef]
- Hamm, C.E.; Merkel, R.; Springer, O.; Jurkojc, P.; Maier, C.; Prechtel, K.; Smetacek, V. Architecture and material properties of diatom shells provide effective mechanical protection. Nature 2003, 421, 841–843. [Google Scholar] [CrossRef]
- Retzepi, M.; Donos, N. Guided Bone Regeneration: Biological principle and therapeutic applications. Clin. Oral. Implant. Res. 2010, 21, 567–576. [Google Scholar] [CrossRef]
- Chen, W.; Wang, G.C.; Tang, R.K. Nanomodification of living organisms by biomimetic mineralization. Nano Res. 2014, 7, 1404–1428. [Google Scholar] [CrossRef]
- Cano, M.; Giner-Casares, J.J. Biomineralization at fluid interfaces. Adv. Colloid. Interface Sci. 2020, 286, 102313. [Google Scholar] [CrossRef]
- Tang, S.; Dong, Z.; Ke, X.; Luo, J.; Li, J. Advances in biomineralization-inspired materials for hard tissue repair. Int. J. Oral. Sci. 2021, 13, 42. [Google Scholar] [CrossRef]
- Li, B.; Cui, Y.; Wang, X.; Tang, R. Novel nanomaterial-organism hybrids with biomedical potential. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1706. [Google Scholar] [CrossRef]
- Ying, G.; Zhang, G.; Yang, J.; Hao, Z.; Xing, W.; Lu, D.; Zhang, S.; Yan, L. Biomineralization and biotechnological applications of bacterial magnetosomes. Colloids Surf. B Biointerfaces 2022, 216, 112556. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, X.; Zheng, X.; Jin, H.J.; Li, X. Biomineralization: An Opportunity and Challenge of Nanoparticle Drug Delivery Systems for Cancer Therapy. Adv. Healthc. Mater. 2020, 9, e2001117. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Wang, W.; Wu, L.; Wang, W.; Huang, Z.; Huang, Y.; Wu, C.; Pan, X. Inhalable Biomineralized Liposomes for Cyclic Ca(2+)-Burst-Centered Endoplasmic Reticulum Stress Enhanced Lung Cancer Ferroptosis Therapy. ACS Nano 2023, 17, 5486–5502. [Google Scholar] [CrossRef] [PubMed]
- Veis, A. Materials science. A window on biomineralization. Science 2005, 307, 1419–1420. [Google Scholar] [CrossRef] [PubMed]
- Faivre, D.; Godec, T.U. From bacteria to mollusks: The principles underlying the biomineralization of iron oxide materials. Angew. Chem. (Int. Ed. Engl.) 2015, 54, 4728–4747. [Google Scholar] [CrossRef]
- Sharma, V.; Srinivasan, A.; Nikolajeff, F.; Kumar, S. Biomineralization process in hard tissues: The interaction complexity within protein and inorganic counterparts. Acta Biomater. 2021, 120, 20–37. [Google Scholar] [CrossRef]
- Zuo, L.; Yang, Y.; Zhang, H.; Ma, Z.; Xin, Q.; Ding, C.; Li, J. Bioinspired Multiscale Mineralization: From Fundamentals to Potential Applications. Macromol. Biosci. 2023, 24, e2300348. [Google Scholar] [CrossRef]
- Tang, R.; Suzuki, M. Special Issue on Biomineralization: From Principles to Practices. ACS Biomater. Sci. Eng. 2023, 9, 1730–1732. [Google Scholar] [CrossRef]
- Ben-Tabou de-Leon, S. The Evolution of Biomineralization through the Co-Option of Organic Scaffold Forming Networks. Cells 2022, 11, 595. [Google Scholar] [CrossRef]
- Dey, A.; de With, G.; Sommerdijk, N.A. In situ techniques in biomimetic mineralization studies of calcium carbonate. Chem. Soc. Rev. 2010, 39, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, H.; Yang, L.; Lv, W.; Zhang, L.; Jiang, X.; Sun, D. In Situ Strategy for Biomimetic Construction of Calcium Phosphate Mineral Shells on Microbial Cells. ACS Sustain. Chem. Eng. 2021, 9, 9854–9860. [Google Scholar] [CrossRef]
- Peacock, M. Phosphate Metabolism in Health and Disease. Calcif. Tissue Int. 2021, 108, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Yang, J.; Yang, D.; Wang, X.; Yang, Z.; Jin, Q.; Wang, M.; Lai, J.; Wang, X. Phospholipid Composition and Fat Globule Structure I: Comparison of Human Milk Fat from Different Gestational Ages, Lactation Stages, and Infant Formulas. J. Agric. Food Chem. 2019, 67, 13922–13928. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Zhang, J.; Yang, P. Hyperlipidemia induced by high-fat diet enhances dentin formation and delays dentin mineralization in mouse incisor. J. Mol. Histol. 2016, 47, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Du, T.-M.; Yang, H.-S.; Niu, X.-F. Phosphorus-containing compounds regulate mineralization. Mater. Today Chem. 2021, 22, 100579. [Google Scholar] [CrossRef]
- Collier, J.H.; Messersmith, P.B. Phospholipid Strategies in Biomineralization and Biomaterials Research. Annu. Rev. Mater. Res. 2001, 31, 237–263. [Google Scholar] [CrossRef]
- Wang, X.; Sun, H.; Xia, Y.; Chen, C.; Xu, H.; Shan, H.; Lu, J.R. Lysozyme mediated calcium carbonate mineralization. J. Colloid. Interface Sci. 2009, 332, 96–103. [Google Scholar] [CrossRef]
- Vist, M.R.; Davis, J.H. Phase equilibria of cholesterol/dipalmitoylphosphatidylcholine mixtures: Deuterium nuclear magnetic resonance and differential scanning calorimetry. Biochemistry 1990, 29, 451–464. [Google Scholar] [CrossRef]
- Luo, J.J.; Ning, T.Y.; Cao, Y.; Zhu, X.P.; Xu, X.H.; Tang, X.Y.; Chu, C.H.; Li, Q.L. Biomimic Enamel Remineralization by Hybridization Calcium- and Phosphate-Loaded Liposomes with Amelogenin-Inspired Peptide. Key Eng. Mater. 2012, 512, 1727–1730. [Google Scholar] [CrossRef]
- Newcomb, C.J.; Bitton, R.; Velichko, Y.S.; Snead, M.L.; Stupp, S.I. The Role of Nanoscale Architecture in Supramolecular Templating of Biomimetic Hydroxyapatite Mineralization. Small 2012, 8, 2195–2202. [Google Scholar] [CrossRef]
- Tester, C.C.; Joester, D. Chapter Twelve—Precipitation in Liposomes as a Model for Intracellular Biomineralization. In Methods in Enzymology; De Yoreo, J.J., Ed.; Academic Press: Cambridge, MA, USA, 2013; Volume 532, pp. 257–276. [Google Scholar]
- Ouyang, J.-M.; Duan, L.; Tieke, B. Effects of Carboxylic Acids on the Crystal Growth of Calcium Oxalate Nanoparticles in Lecithin−Water Liposome Systems. Langmuir 2003, 19, 8980–8985. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, D.; Wang, T. Hierarchical Structures of Bone and Bioinspired Bone Tissue Engineering. Small 2016, 12, 4611–4632. [Google Scholar] [CrossRef]
- Kahil, K.; Weiner, S.; Addadi, L.; Gal, A. Ion Pathways in Biomineralization: Perspectives on Uptake, Transport, and Deposition of Calcium, Carbonate, and Phosphate. J. Am. Chem. Soc. 2021, 143, 21100–21112. [Google Scholar] [CrossRef]
- Jamshidi, A. Analysis of Pavement Structures. By Animesh Das, CRC Press: Boca Raton, FL, USA, 2014; 194p, ISBN 978-1466558557. 2021, 13, 6098.
- Sikirić, M.D.; Füredi-Milhofer, H. The influence of surface active molecules on the crystallization of biominerals in solution. Adv. Colloid. Interface Sci. 2006, 128–130, 135–158. [Google Scholar] [CrossRef]
- Seifan, M.; Berenjian, A. Microbially induced calcium carbonate precipitation: A widespread phenomenon in the biological world. Appl. Microbiol. Biotechnol. 2019, 103, 4693–4708. [Google Scholar] [CrossRef]
- Ajili, W.; Tovani, C.B.; Fouassier, J.; de Frutos, M.; Laurent, G.P.; Bertani, P.; Djediat, C.; Marin, F.; Auzoux-Bordenave, S.; Azaïs, T.; et al. Inorganic phosphate in growing calcium carbonate abalone shell suggests a shared mineral ancestral precursor. Nat. Commun. 2022, 13, 1496. [Google Scholar] [CrossRef]
- Balantič, K.; Weiss, V.U.; Allmaier, G.; Kramar, P. Calcium ion effect on phospholipid bilayers as cell membrane analogues. Bioelectrochemistry 2022, 143, 107988. [Google Scholar] [CrossRef] [PubMed]
- Pabst, G.; Hodzic, A.; Štrancar, J.; Danner, S.; Rappolt, M.; Laggner, P. Rigidification of Neutral Lipid Bilayers in the Presence of Salts. Biophys. J. 2007, 93, 2688–2696. [Google Scholar] [CrossRef] [PubMed]
- Szcześ, A. Effects of DPPC/Cholesterol liposomes on the properties of freshly precipitated calcium carbonate. Colloids Surf. B Biointerfaces 2013, 101, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.; Yamaguchi, T.; Tagaya, M. Fabrication of Phospholipid Vesicle-Interacted Calcium Phosphate Films with Sterilization Stability. Cryst. Growth Des. 2017, 17, 4977–4983. [Google Scholar] [CrossRef]
- Yeo, C.-H.; Zein, S.H.S.; Ahmad, A.L.; McPhail, D.S. Comparison of DOPA and DPPA liposome templates for the synthesis of calcium phosphate nanoshells. Ceram. Int. 2012, 38, 561–570. [Google Scholar] [CrossRef]
- Fukui, Y.; Fujimoto, K. Control in Mineralization by the Polysaccharide-Coated Liposome via the Counter-Diffusion of Ions. Chem. Mater. 2011, 23, 4701–4708. [Google Scholar] [CrossRef]
- Nguyen, S.; Solheim, L.; Bye, R.; Rykke, M.; Hiorth, M.; Smistad, G. The influence of liposomal formulation factors on the interactions between liposomes and hydroxyapatite. Colloids Surf. B Biointerfaces 2010, 76, 354–361. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Erceg, I.; Kontrec, J.; Strasser, V.; Selmani, A.; Domazet Jurašin, D.; Ćurlin, M.; Džakula, B.N.; Matijaković Mlinarić, N.; Šegota, S.; Lyons, D.M.; et al. Precipitation of Calcium Phosphates and Calcium Carbonates in the Presence of Differently Charged Liposomes. Minerals 2022, 12, 208. [Google Scholar] [CrossRef]
- Anada, T.; Takeda, Y.; Honda, Y.; Sakurai, K.; Suzuki, O. Synthesis of calcium phosphate-binding liposome for drug delivery. Bioorganic Med. Chem. Lett. 2009, 19, 4148–4150. [Google Scholar] [CrossRef] [PubMed]
- Beato, C.; Fernández, M.S.; Fermani, S.; Reggi, M.; Neira-Carrillo, A.; Rao, A.; Falini, G.; Arias, J.L. Calcium carbonate crystallization in tailored constrained environments. CrystEngComm 2015, 17, 5953–5961. [Google Scholar] [CrossRef]
- Tester, C.C.; Brock, R.E.; Wu, C.-H.; Krejci, M.R.; Weigand, S.; Joester, D. In vitro synthesis and stabilization of amorphous calcium carbonate (ACC) nanoparticles within liposomes. CrystEngComm 2011, 13, 3975–3978. [Google Scholar] [CrossRef]
- Dalai, P.; Sahai, N. Mineral-Lipid Interactions in the Origins of Life. Trends Biochem. Sci. 2019, 44, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.T.; Ostafin, A.E. Liposome Directed Growth of Calcium Phosphate Nanoshells. Adv. Mater. 2002, 14, 532–535. [Google Scholar] [CrossRef]
- Tagaya, M.; Yamaguchi, T.; Shiba, K. Preparation of Phospholipid Vesicle-Templated Calcium Phosphate Nanostructures and Their Cytocompatibility. Cryst. Growth Des. 2016, 16, 2843–2849. [Google Scholar] [CrossRef]
- Eanes, E.D.; Hailer, A.W. Calcium phosphate precipitation in aqueous suspensions of phosphatidylserine-containing anionic liposomes. Calcif. Tissue Int. 1987, 40, 43–48. [Google Scholar] [CrossRef]
- Guo, L.; Ding, J.; Zhou, W. Converting bacteria into autologous tumor vaccine via surface biomineralization of calcium carbonate for enhanced immunotherapy. Acta Pharm. Sin. B 2023, 13, 5074–5090. [Google Scholar] [CrossRef]
- Bewernitz, M.A.; Lovett, A.C.; Gower, L.B. Liquid–Solid Core-Shell Microcapsules of Calcium Carbonate Coated Emulsions and Liposomes. Appl. Sci. 2020, 10, 8551. [Google Scholar] [CrossRef]
- Gopal, K.; Lu, Z.; de Villiers, M.M.; Lvov, Y. Composite Phospholipid−Calcium Carbonate Microparticles: Influence of Anionic Phospholipids on the Crystallization of Calcium Carbonate. J. Phys. Chem. B 2006, 110, 2471–2474. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Huang, Y.W.; Zhou, X.D.; Ma, Y. In vitro toxicity of silica nanoparticles in human lung cancer cells. Toxicol. Appl. Pharmacol. 2006, 217, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.E.; Jung, Y.J.; Choi, Y.J.; Choi, J.S.; Cho, Y.W. Artificial skin models for animal-free testing. J. Pharm. Investig. 2018, 48, 215–223. [Google Scholar] [CrossRef]
- Liu, H.; Geng, W.; Jin, C.-J.; Wu, S.-M.; Lu, Y.; Hu, J.; Yu, H.-Z.; Chang, G.-G.; Zhao, T.; Wan, Y.; et al. Silica coating with well-defined micro-nano hierarchy for universal and stable surface superhydrophobicity. Chem. Phys. Lett. 2019, 730, 594–599. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Y.; Su, T.; Feng, L.; Long, Y.; Chen, Z. Silica-coated flexible liposomes as a nanohybrid delivery system for enhanced oral bioavailability of curcumin. Int. J. Nanomed. 2012, 7, 5995–6002. [Google Scholar] [CrossRef]
- Pyo, C.E.; Song, M.; Chang, J.H. Preparation and In Vitro Cytotoxicity Assessments of Spherical Silica-Encapsulated Liposome Particles for Highly Efficient Drug Carriers. ACS Appl. Bio Mater. 2021, 4, 1350–1359. [Google Scholar] [CrossRef]
- Grant, A.; O’Dwyer, C. Thickness-Based Dispersion in Opal Photonic Crystals. ECS Meet. Abstr. 2022, MA2022-02, 1304. [Google Scholar] [CrossRef]
- Beloglazova, N.V.; Goryacheva, I.Y.; Shmelin, P.S.; Kurbangaleev, V.; De Saeger, S. Preparation and characterization of stable phospholipid-silica nanostructures loaded with quantum dots. J. Mater. Chem. B 2015, 3, 180–183. [Google Scholar] [CrossRef]
- Johnston, R.K.; Harper, J.C.; Tartis, M.S. Control over Silica Particle Growth and Particle-Biomolecule Interactions Facilitates Silica Encapsulation of Mammalian Cells with Thickness Control. ACS Biomater. Sci. Eng. 2017, 3, 2098–2109. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Xu, P.; He, J.; Lawson, L.; McPherson, G.L.; John, V.T. Highly aspherical silica nanoshells by templating tubular liposomes. Soft Matter 2009, 5, 3006–3009. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ingle, S.G.; Pai, R.V.; Monpara, J.D.; Vavia, P.R. Liposils: An effective strategy for stabilizing Paclitaxel loaded liposomes by surface coating with silica. Eur. J. Pharm. Sci. 2018, 122, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Niu, M.; Zheng, C.; Zhao, H.; Niu, X.; Li, L.; Hu, Y.; Zhang, Y.; Shi, J.; Zhang, Z. A Core-Shell Nanoplatform for Synergistic Enhanced Sonodynamic Therapy of Hypoxic Tumor via Cascaded Strategy. Adv. Healthc. Mater. 2018, 7, e1800819. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, J. Growing a Nucleotide/Lanthanide Coordination Polymer Shell on Liposomes. Langmuir 2019, 35, 11217–11224. [Google Scholar] [CrossRef] [PubMed]
- Szcześ, A.; Sternik, D. Properties of calcium carbonate precipitated in the presence of DPPC liposomes modified with the phospholipase A2. J. Therm. Anal. Calorim. 2016, 123, 2357–2365. [Google Scholar] [CrossRef]
- Ferreira, C.R.; Cruz, M.A.E.; Bolean, M.; Andrilli, L.H.d.S.; Millan, J.L.; Ramos, A.P.; Bottini, M.; Ciancaglini, P. Annexin A5 stabilizes matrix vesicle-biomimetic lipid membranes: Unravelling a new role of annexins in calcification. Eur. Biophys. J. 2023, 52, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.X.; Shen, M.J.; Wang, C.Y.; Wei, J.H.; Wan, Q.Q.; Zhu, Y.F.; Ye, T.; Luo, M.L.; Qin, W.P.; Li, Y.T.; et al. Regulation of biomineralization by proteoglycans: From mechanisms to application. Carbohydr. Polym. 2022, 294, 119773. [Google Scholar] [CrossRef]
- Wen, Y.; Dong, H.; Li, Y.; Shen, A.; Li, Y. Nano-assembly of bovine serum albumin driven by rare-earth-ion (Gd) biomineralization for highly efficient photodynamic therapy and tumor imaging. J. Mater. Chem. B 2016, 4, 743–751. [Google Scholar] [CrossRef]
- Wang, X.; Shen, X.; Li, J.; Ge, X.; Ouyang, J.; Na, N. Biomineralization of DNA Nanoframeworks for Intracellular Delivery, On-Demand Diagnosis, and Synergistic Cancer Treatments. Anal. Chem. 2022, 94, 16803–16812. [Google Scholar] [CrossRef]
- Zeinabad, H.A.; Kachooei, E.; Saboury, A.A.; Kostova, I.; Attar, F.; Vaezzadeh, M.; Falahati, M. Thermodynamic and conformational changes of protein toward interaction with nanoparticles: A spectroscopic overview. RSC Adv. 2016, 6, 105903–105919. [Google Scholar] [CrossRef]
- Arsalan, N.; Hassan Kashi, E.; Hasan, A.; Edalat Doost, M.; Rasti, B.; Ahamad Paray, B.; Zahed Nakhjiri, M.; Sari, S.; Sharifi, M.; Shahpasand, K.; et al. Exploring the Interaction of Cobalt Oxide Nanoparticles with Albumin, Leukemia Cancer Cells and Pathogenic Bacteria by Multispectroscopic, Docking, Cellular and Antibacterial Approaches. Int. J. Nanomed. 2020, 15, 4607–4623. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Zoulikha, M.; Qiu, M.; Teng, C.; Lin, C.; Li, X.; Sallam, M.A.; Xu, Q.; He, W. The effects of protein corona on in vivo fate of nanocarriers. Adv. Drug Deliv. Rev. 2022, 186, 114356. [Google Scholar] [CrossRef] [PubMed]
- Bashiri, G.; Padilla, M.S.; Swingle, K.L.; Shepherd, S.J.; Mitchell, M.J.; Wang, K. Nanoparticle protein corona: From structure and function to therapeutic targeting. Lab. A Chip 2023, 23, 1432–1466. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, Y.; Tang, Q.; Yin, D.; Tang, C.; He, E.; Zou, L.; Peng, Q. The protein corona and its effects on nanoparticle-based drug delivery systems. Acta Biomater. 2021, 129, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Doudna, J.A. CRISPR-Cas9 Structures and Mechanisms. Annu. Rev. Biophys. 2017, 46, 505–529. [Google Scholar] [CrossRef]
- Nakagawa, H.; Kataoka, M. Rigidity of protein structure revealed by incoherent neutron scattering. Biochim. Biophys. Acta. Gen. Subj. 2020, 1864, 129536. [Google Scholar] [CrossRef]
- Dominguez, R.; Holmes, K.C. Actin structure and function. Annu. Rev. Biophys. 2011, 40, 169–186. [Google Scholar] [CrossRef] [PubMed]
- Piella, J.; Bastús, N.G.; Puntes, V. Size-Dependent Protein–Nanoparticle Interactions in Citrate-Stabilized Gold Nanoparticles: The Emergence of the Protein Corona. Bioconjugate Chem. 2017, 28, 88–97. [Google Scholar] [CrossRef]
- Bilardo, R.; Traldi, F.; Vdovchenko, A.; Resmini, M. Influence of surface chemistry and morphology of nanoparticles on protein corona formation. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1788. [Google Scholar] [CrossRef] [PubMed]
- Kopac, T. Protein corona, understanding the nanoparticle-protein interactions and future perspectives: A critical review. Int. J. Biol. Macromol. 2021, 169, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Piloni, A.; Wong, C.K.; Chen, F.; Lord, M.; Walther, A.; Stenzel, M.H. Surface roughness influences the protein corona formation of glycosylated nanoparticles and alter their cellular uptake. Nanoscale 2019, 11, 23259–23267. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wu, E.; Tang, W.; Qian, J.; Zhan, C. Interplay between nanomedicine and protein corona. J. Mater. Chem. B 2021, 9, 6713–6727. [Google Scholar] [CrossRef]
- Khan, S.; Sharifi, M.; Gleghorn, J.P.; Babadaei, M.M.N.; Bloukh, S.H.; Edis, Z.; Amin, M.; Bai, Q.; ten Hagen, T.L.M.; Falahati, M.; et al. Artificial engineering of the protein corona at bio-nano interfaces for improved cancer-targeted nanotherapy. J. Control. Release 2022, 348, 127–147. [Google Scholar] [CrossRef]
- Arezki, Y.; Delalande, F.; Schaeffer-Reiss, C.; Cianférani, S.; Rapp, M.; Lebeau, L.; Pons, F.; Ronzani, C. Surface charge influences protein corona, cell uptake and biological effects of carbon dots. Nanoscale 2022, 14, 14695–14710. [Google Scholar] [CrossRef]
- Giulimondi, F.; Digiacomo, L.; Pozzi, D.; Palchetti, S.; Vulpis, E.; Capriotti, A.L.; Chiozzi, R.Z.; Laganà, A.; Amenitsch, H.; Masuelli, L.; et al. Interplay of protein corona and immune cells controls blood residency of liposomes. Nat. Commun. 2019, 10, 3686. [Google Scholar] [CrossRef]
- Dahanayake, J.N.; Shahryari, E.; Roberts, K.M.; Heikes, M.E.; Kasireddy, C.; Mitchell-Koch, K.R. Protein Solvent Shell Structure Provides Rapid Analysis of Hydration Dynamics. J. Chem. Inf. Model. 2019, 59, 2407–2422. [Google Scholar] [CrossRef]
- MacCarthy, E.; Perry, D.; Kc, D.B. Advances in Protein Super-Secondary Structure Prediction and Application to Protein Structure Prediction. In Protein Supersecondary Structures: Methods and Protocols; Kister, A.E., Ed.; Springer: New York, NY, USA, 2019; pp. 15–45. [Google Scholar]
- Ebert, S.; Koo, C.K.W.; Weiss, J.; McClements, D.J. Continuous production of core-shell protein nanoparticles by antisolvent precipitation using dual-channel microfluidization: Caseinate-coated zein nanoparticles. Food Res. Int. 2017, 92, 48–55. [Google Scholar] [CrossRef]
- Mady, M.M.; Darwish, M.M.; Khalil, S.; Khalil, W.M. Biophysical studies on chitosan-coated liposomes. Eur. Biophys. J. EBJ 2009, 38, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.K.; Kong, M.; Wei, Y.N.; Liu, Y.; Cheng, X.J.; Li, J.; Park, H.J.; Chen, X.G. Folate-modified–chitosan-coated liposomes for tumor-targeted drug delivery. J. Mater. Sci. 2013, 48, 1717–1728. [Google Scholar] [CrossRef]
- Hadinoto, K.; Sundaresan, A.; Cheow, W.S. Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: A review. Eur. J. Pharm. Biopharm. Off. J. Arbeitsgemeinschaft Fur Pharm. Verfahrenstechnik E.V. 2013, 85, 427–443. [Google Scholar] [CrossRef]
- Nie, Z.; Zhang, Y.; Tang, R.; Wang, X. Biomimetic mineralization: An emerging organism engineering strategy for biomedical applications. J. Inorg. Biochem. 2022, 232, 111815. [Google Scholar] [CrossRef]
- Saurer, E.M.; Jewell, C.M.; Roenneburg, D.A.; Bechler, S.L.; Torrealba, J.R.; Hacker, T.A.; Lynn, D.M. Polyelectrolyte multilayers promote stent-mediated delivery of DNA to vascular tissue. Biomacromolecules 2013, 14, 1696–1704. [Google Scholar] [CrossRef]
- Ren, K.; Hu, M.; Zhang, H.; Li, B.; Lei, W.; Chen, J.; Chang, H.; Wang, L.; Ji, J. Layer-by-layer assembly as a robust method to construct extracellular matrix mimic surfaces to modulate cell behavior. Prog. Polym. Sci. 2019, 92, 1–34. [Google Scholar] [CrossRef]
- Alkekhia, D.; Hammond, P.T.; Shukla, A. Layer-by-Layer Biomaterials for Drug Delivery. Annu. Rev. Biomed. Eng. 2020, 22, 1–24. [Google Scholar] [CrossRef]
- Wang, J.-Z.; Ding, Z.-Q.; Zhang, F.; Ye, W.-B. Recent development in cell encapsulations and their therapeutic applications. Mater. Sci. Eng. C 2017, 77, 1247–1260. [Google Scholar] [CrossRef]
- Yu, Q.; Ma, X.; Liu, Y.; Zhao, H. Biomimetic Mineralization of Protein Nanogels for Enzyme Protection. Chemistry 2019, 25, 16712–16717. [Google Scholar] [CrossRef] [PubMed]
- Fakhrullin, R.F.; Lvov, Y.M. “Face-Lifting” and “Make-Up” for Microorganisms: Layer-by-Layer Polyelectrolyte Nanocoating. ACS Nano 2012, 6, 4557–4564. [Google Scholar] [CrossRef] [PubMed]
- Meka, V.S.; Sing, M.K.G.; Pichika, M.R.; Nali, S.R.; Kolapalli, V.R.M.; Kesharwani, P. A comprehensive review on polyelectrolyte complexes. Drug Discov. Today 2017, 22, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- Borges, J.; Mano, J.F. Molecular Interactions Driving the Layer-by-Layer Assembly of Multilayers. Chem. Rev. 2014, 114, 8883–8942. [Google Scholar] [CrossRef]
- Piccinini, E.; Fenoy, G.E.; Knoll, W.; Marmisollé, W.A.; Azzaroni, O. Polyelectrolyte-Enzyme Assemblies Integrated into Graphene Field-Effect Transistors for Biosensing Applications. In Graphene Field-Effect Transistors; John Wiley & Sons: Hoboken, NJ, USA, 2023; pp. 285–299. [Google Scholar]
- De Villiers, M.M.; Otto, D.P.; Strydom, S.J.; Lvov, Y.M. Introduction to nanocoatings produced by layer-by-layer (LbL) self-assembly. Adv. Drug Deliv. Rev. 2011, 63, 701–715. [Google Scholar] [CrossRef]
- Salomäki, M.; Vinokurov, I.A.; Kankare, J. Effect of Temperature on the Buildup of Polyelectrolyte Multilayers. Langmuir 2005, 21, 11232–11240. [Google Scholar] [CrossRef]
- Dubas, S.T.; Schlenoff, J.B. Factors Controlling the Growth of Polyelectrolyte Multilayers. Macromolecules 1999, 32, 8153–8160. [Google Scholar] [CrossRef]
- Elizarova, I.S.; Luckham, P.F. Layer-by-layer adsorption: Factors affecting the choice of substrates and polymers. Adv. Colloid. Interface Sci. 2018, 262, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Grohmann, S.; Rothe, H.; Frant, M.; Liefeith, K. Colloidal Force Spectroscopy and Cell Biological Investigations on Biomimetic Polyelectrolyte Multilayer Coatings Composed of Chondroitin Sulfate and Heparin. Biomacromolecules 2011, 12, 1987–1997. [Google Scholar] [CrossRef] [PubMed]
- Gribova, V.; Auzely-Velty, R.; Picart, C. Polyelectrolyte Multilayer Assemblies on Materials Surfaces: From Cell Adhesion to Tissue Engineering. Chem. Mater. 2012, 24, 854–869. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, P.; Jiang, W.; Pan, H.; Xu, X.; Tang, R. Yeast cells with an artificial mineral shell: Protection and modification of living cells by biomimetic mineralization. Angew. Chem. 2008, 47, 3560–3564. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Chen, J.; Zhang, Z.; Tian, S. Preparation, characterization, and evaluation of flaxseed oil liposomes coated with chitosan and pea protein isolate hydrolysates. Food Chem. 2023, 404, 134547. [Google Scholar] [CrossRef] [PubMed]
- Potaś, J.; Winnicka, K. The Potential of Polyelectrolyte Multilayer Films as Drug Delivery Materials. Int. J. Mol. Sci. 2022, 23, 3496. [Google Scholar] [CrossRef]
- Rogosnitzky, M.; Branch, S. Gadolinium-based contrast agent toxicity: A review of known and proposed mechanisms. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2016, 29, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Wittenberg, N.J.; Haynes, C.L. Using nanoparticles to push the limits of detection. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 237–254. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, S.; Abidi, S.M.S.; Dar, A.I.; Acharya, A. The curious cases of nanoparticle induced amyloidosis during protein corona formation and anti-amyloidogenic nanomaterials: Paradox or prejudice? Int. J. Biol. Macromol. 2021, 193, 1009–1020. [Google Scholar] [CrossRef] [PubMed]
- Alipour, E.; Halverson, D.; McWhirter, S.; Walker, G.C. Phospholipid Bilayers: Stability and Encapsulation of Nanoparticles. Annu. Rev. Phys. Chem. 2017, 68, 261–283. [Google Scholar] [CrossRef]
- Waghule, T.; Saha, R.N.; Alexander, A.; Singhvi, G. Tailoring the multi-functional properties of phospholipids for simple to complex self-assemblies. J. Control. Release 2022, 349, 460–474. [Google Scholar] [CrossRef]
- Mura, S.; Manconi, M.; Sinico, C.; Valenti, D.; Fadda, A.M. Penetration enhancer-containing vesicles (PEVs) as carriers for cutaneous delivery of minoxidil. Int. J. Pharm. 2009, 380, 72–79. [Google Scholar] [CrossRef]
- Nyambura, B.K.; Kellaway, I.W.; Taylor, K.M. The processing of nanoparticles containing protein for suspension in hydrofluoroalkane propellants. Int. J. Pharm. 2009, 372, 140–146. [Google Scholar] [CrossRef]
- Alouache, A.I. Novel Phospholipid-Based Pressurised Metered Dose Inhaler Formulations. Ph.D. Thesis, University of London, University College London, London, UK, 2008. [Google Scholar]



| Drug | Company | Application | Date of First Approved | Types |
|---|---|---|---|---|
| Injectafer | American Regent, Shirley, NY, USA | Iron-deficient anemia | 2013 | Inorganic |
| Feraheme | AMAG, Zurich, Switzerland | Iron deficiency in chronic kidney disease | 2009 | |
| Venofer | American Regent | Iron deficiency in chronic kidney disease | 2000 | |
| Ferrlecit | Sanofi, Paris, France | Iron deficiency in chronic kidney disease | 1999 | |
| DexFerrum | American Regent | Iron-deficient anemia | 1996 | |
| INFeD | Allergan, Dublin, Ireland | Iron-deficient anemia | 1992 | |
| ADYNOVATE | Takeda, Tokyo, Japan | Hemophilia | 2015 | Polymer-based |
| Plegridy | Biogen, Cambridge, MA, USA | Multiple sclerosis | 2014 | |
| Cimiza | UCB, Brussels, Belgium | Crohn’s disease, rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis | 2008 | |
| Abraxane | Celgene, Summit, NJ, USA | Lung cancer, metastatic breast cancer, metastatic pancreatic cancer | 2005 | |
| Neulasta | Amgen, Thousand Oaks, CA, USA | Neutropenia, chemotherapy induced | 2002 | |
| Eligard | Tolmar, Fort Collins, CO, USA | Prostate cancer | 2002 | |
| PegIntron | Merck, Rahway, NJ, USA | Hepatitis C infection | 2001 | |
| Copaxone | Teva, Tel Aviv, Israel | Multiple sclerosis | 1996 | |
| Oncaspar | Servier Pharmaceuticals, Boston, MA, USA | Acute lymphoblastic leukemia | 1994 | |
| Onpattro | Alnylam Pharmaceuticals, Cambridge, MA, USA | Transthyretin-mediated amyloidosis | 2018 | Lipid-based |
| Vyxeos | Jazz Pharmaceuticals, Dublin, Ireland | Acute myeloid leukemia | 2017 | |
| Onivyde | Ipsen, Paris, France | Metastatic pancreatic cancer | 2015 | |
| Visudyne | Bausch and Lomb, Laval, Canada | Wet age-related macular degeneration, myopia, ocular histoplasmosis | 2000 | |
| AmBisome | Gilead Sciences, Foster City, CA, USA | Fungal/protozoal infections | 1997 | |
| DaunoXome | Galen, Craigavon, Northern Ireland | Kaposi’s sarcoma | 1996 | |
| Doxil | Janssen, Beerse, Belgium | Kaposi’s sarcoma, ovarian cancer, multiple myeloma | 1995 |
| Product Name | Approval Year | Company | Active Ingredient | Administration Route | Formulation | Indication |
|---|---|---|---|---|---|---|
| Ambisome® | 1990 | Astellas (Chuo-Ku, Tokyo, Japan) | Amphotericin B | intravenous | HSPC, DSPG, and Cholesterol | Fungal infection |
| Epaxal® | 1993 | Crucell Italy S.r.l. (Baranzate, Italy) | Inactivated hepatitis A virus (strain RGSB) | intramuscular | DOPC and DOPE | Hepatitis A |
| Abelcet® | 1995 | Leadiant Bioscience Inc. (Gaithersburg, MD, USA) | Amphotericin B | intravenous | DMPC and DMPG | Invasive severe fungal infections |
| Doxil®/Caelyx® | 1995/1996 | Baxter Healthcare Corp. (Deerfield, IL, USA)/Baxter Holding B.V.(Utrecht, The Netherlands) | Doxorubicin | intravenous | HSPC, Cholesterol, and PEG 2000-DSPE | Ovarian cancer and Kaposi’s sarcoma |
| Amphotec® | 1996 | Alkopharma USA (Sacramento, CA, USA) | Amphotericin B | intravenous | Cholesteryl sulphate | Severe fungal infections |
| DaunoXome® | 1996 | Galen (Northern Ireland, UK) | Daunorubicin | intravenous | DSPC and Cholesterol | Kaposi’s sarcoma infected with human immunodeficiency virus |
| Inflexal® V | 1997 | Crucell Berna Biotech (Bern, Switzerland) | Inactivated hemagglutinin of Influenza virus strains A and B | intramuscular | DOPC and DOPE | Influenza |
| Depocyt® | 1999 | Pacira Pharmaceuticals Inc (San Diego, CA, USA) | Cytarabine | spinal | DOPC, DPPG, Cholesterol, and Triolein | Neoplastic meningitis |
| Visudyne® | 2000 | V Valeant Luxem Bourg (Quebec, Canada) | Verteporfin | intravenous | DMPC and EPG | Choroidal neovascularisation |
| Myocet® | 2001 | Teva B.V. (City of Arkansas, Palestine) | Doxorubicin | intravenous | Lecithin and Cholesterol | Combination therapy with cyclophosphamide in metastatic breast cancer |
| Lipusu® | 2003 | Luye Pharma Group (Yantai, Shandong, China) | Paclitaxel | intravenously guttae | Lecithin, Cholesterol, Threonine, and Glucose | Ovarian cancer |
| DepoDurTM Epidural | 2004 | Pacira Pharmaceuticals Inc (San Diego, CA, USA) | Morphine | sulfate | DOPC, DPPG, Cholesterol, and Triolein | Pain management |
| Mepact® | 2009 | Takeda France SAS (Paris, France) | Mifamurtide | intravenous | DOPS and POPC | Non-metastatic osteosarcoma |
| Exparel® | 2011 | Pacira Pharmaceuticals Inc (San Diego, CA, USA) | Bupivacaine | intravenous | DEPC, DPPG, Cholesterol, and Tricaprylin | Pain management |
| Marqibo® | 2012 | Acrotech Biopharma Inc (East Windsor, NJ, USA) | Vincristine | intravenous | Sphingomyelin and Cholesterol | Acute lymphoblastic leukemia |
| OnivydeTM | 2015 | Ipsen S.A (Boulogne-Billancourt, France) | Irinotecan | intravenous | DSPC, mPEG-2000, and DSPE | Metastatic pancreatic cancer |
| Vyxeos® | 2017 | Celator Pharms (Princeton, NJ, USA) | Daunorubicin and Cytarabine | intravenous | DSPC, DSPG, and Cholesterol | Acute myeloid leukemia with myelodysplasia-related changes and therapy-related acute myeloid leukemia |
| Shingrix® | 2017 | GlaxoSmithkline Biologicals SA (London, UK) | Recombinant VZV glycoprotein E | intramuscular | DOPC and Chol | Against shingles and post-herpetic neuralgia |
| OnpattroTM | 2018 | Alnylam Pharmas.Inc (Cambridge, MA, USA) | siRNA | intravenous | DLin-MC3-DMA, DSPC, Cholesterol, and PEG2000-C-DMG | Polyneuropathy caused by hereditary transthyretin familial amyloidosis |
| Arikayce® Kit | 2018 | Insmed Inc (Bridgewater, NJ, USA) | Amikacin | inhalation administration | DPPC and CHO-HP | Non-tuberculous mycobacteria lung disease caused by mycobacterium avium complex |
| Comirnaty® | 2021 | BioNTech Manufacturing GmbH (Mainz, Germany) | BNT162b2 | intramuscular | ALC-0315, ALC-0159, DSPC, and Cholesterol | COVID-19 |
| Moderna SM-102 | 2021 | Moderna (Cambridge, MA, USA) | mRNA-1273 | intramuscular | PEG2000-DMG, Cholesterol, and DSPC | COVID-19 |
| Micro–Nano System | Polymer Excipient | Modification Method | Stable Period (Day) | Dispersion Medium | References |
|---|---|---|---|---|---|
| Polylactic-acetic acid copolymer nanoparticles | Vitamin E PEG Succinate | Non-covalent binding | 90 | Water | [64] |
| Zein nanoparticles | Polysorbate 80 | Non-covalent binding | 30 | Water | [65] |
| Selenate nanoparticles | Dextran T-70 | Non-covalent binding | 30 | Water | [66] |
| Tannic acid derivative nanoparticles | Poloxamer 188 | Non-covalent binding | 2 | Water | [67] |
| Glutaraldehyde crosslinked polyvinyl alcohol microspheres | Span 80 | Non-covalent binding | 15 | Water | [68] |
| Glutaraldehyde crosslinked polyvinyl alcohol microspheres | Polyvinyl alcohol | Non-covalent binding | 14 | Water | [69] |
| Iron oxide–manganese oxide nanoparticles | PEG3000 | Covalent binding | 90 | Water | [70] |
| Silver nanoparticles | Aminophenylboric acid-polyvinyl alcohol copolymer | Covalent binding | 70 | Water | [71] |
| Illite nanoparticles | Polyvinylpyrrolidone (PVP) K10 | Covalent binding | 14 | Water | [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Chang, Z.; Gao, Y.; Ren, C.; Lin, Y.; Zhang, X.; Wu, C.; Pan, X.; Huang, Z. Overcoming the Low-Stability Bottleneck in the Clinical Translation of Liposomal Pressurized Metered-Dose Inhalers: A Shell Stabilization Strategy Inspired by Biomineralization. Int. J. Mol. Sci. 2024, 25, 3261. https://doi.org/10.3390/ijms25063261
Huang Y, Chang Z, Gao Y, Ren C, Lin Y, Zhang X, Wu C, Pan X, Huang Z. Overcoming the Low-Stability Bottleneck in the Clinical Translation of Liposomal Pressurized Metered-Dose Inhalers: A Shell Stabilization Strategy Inspired by Biomineralization. International Journal of Molecular Sciences. 2024; 25(6):3261. https://doi.org/10.3390/ijms25063261
Chicago/Turabian StyleHuang, Yeqi, Ziyao Chang, Yue Gao, Chuanyu Ren, Yuxin Lin, Xuejuan Zhang, Chuanbin Wu, Xin Pan, and Zhengwei Huang. 2024. "Overcoming the Low-Stability Bottleneck in the Clinical Translation of Liposomal Pressurized Metered-Dose Inhalers: A Shell Stabilization Strategy Inspired by Biomineralization" International Journal of Molecular Sciences 25, no. 6: 3261. https://doi.org/10.3390/ijms25063261
APA StyleHuang, Y., Chang, Z., Gao, Y., Ren, C., Lin, Y., Zhang, X., Wu, C., Pan, X., & Huang, Z. (2024). Overcoming the Low-Stability Bottleneck in the Clinical Translation of Liposomal Pressurized Metered-Dose Inhalers: A Shell Stabilization Strategy Inspired by Biomineralization. International Journal of Molecular Sciences, 25(6), 3261. https://doi.org/10.3390/ijms25063261








