Targeting BTK in B Cell Malignancies: From Mode of Action to Resistance Mechanisms
Abstract
1. Introduction
2. Covalent BTK Inhibitors
2.1. Covalent BTK Inhibitors in CLL
2.2. Covalent BTK Inhibitors in MCL
2.3. Covalent BTK Inhibitors in LPL
2.4. Covalent BTK Inhibitors in DLBCL
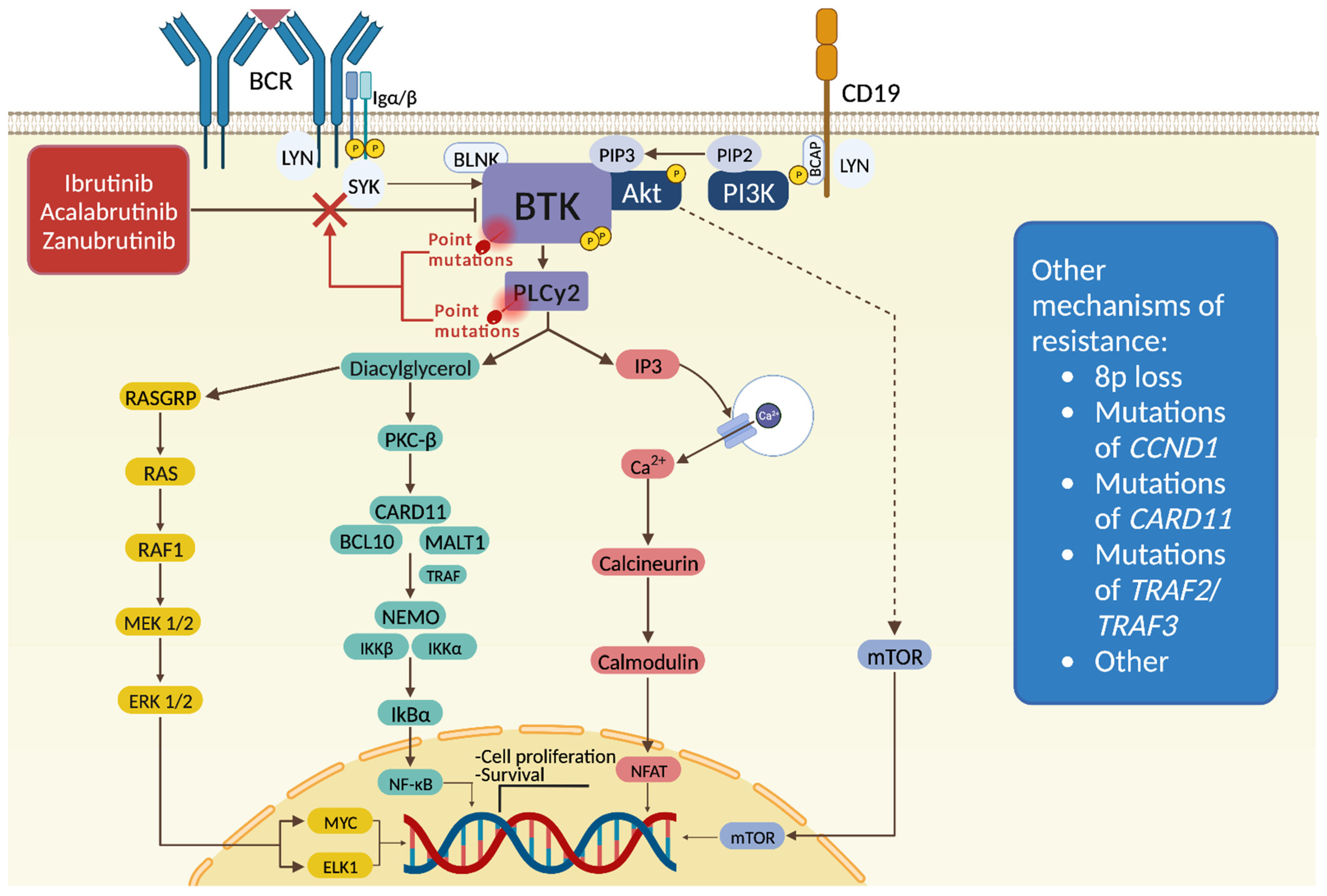
3. Mechanisms of Resistance to Covalent BTK Inhibitors
4. BTK Targeting Approaches to Overcome Covalent BTKi Resistance
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Swerdlow, S.H.; Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2017; Volume 2. [Google Scholar]
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.O.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef]
- Shirley, M. Bruton Tyrosine Kinase Inhibitors in B-Cell Malignancies: Their Use and Differential Features. Target Oncol. 2022, 17, 69–84. [Google Scholar] [CrossRef]
- Montoya, S.; Thompson, M.C. Non-Covalent Bruton’s Tyrosine Kinase Inhibitors in the Treatment of Chronic Lymphocytic Leukemia. Cancers 2023, 15, 3648. [Google Scholar] [CrossRef]
- Kim, H.O. Development of BTK inhibitors for the treatment of B-cell malignancies. Arch. Pharm. Res. 2019, 42, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Dal Porto, J.M.; Gauld, S.B.; Merrell, K.T.; Mills, D.; Pugh-Bernard, A.E.; Cambier, J. B cell antigen receptor signaling 101. Mol. Immunol. 2004, 41, 599–613. [Google Scholar] [CrossRef]
- Ahn, I.E.; Brown, J.R. Targeting Bruton’s Tyrosine Kinase in CLL. Front. Immunol. 2021, 12, 687458. [Google Scholar] [CrossRef]
- Pal Singh, S.; Dammeijer, F.; Hendriks, R.W. Role of Bruton’s tyrosine kinase in B cells and malignancies. Mol. Cancer 2018, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.A. Bruton Tyrosine Kinase Inhibitors: Present and Future. Cancer J. 2019, 25, 386–393. [Google Scholar] [CrossRef]
- Burger, J.A.; Chiorazzi, N. B cell receptor signaling in chronic lymphocytic leukemia. Trends Immunol. 2013, 34, 592–601. [Google Scholar] [CrossRef]
- Burger, J.A.; Wiestner, A. Targeting B cell receptor signalling in cancer: Preclinical and clinical advances. Nat. Rev. Cancer 2018, 18, 148–167. [Google Scholar] [CrossRef] [PubMed]
- Tkachenko, A.; Kupcova, K.; Havranek, O. B-Cell Receptor Signaling and Beyond: The Role of Igα (CD79a)/Igβ (CD79b) in Normal and Malignant B Cells. Int. J. Mol. Sci. 2023, 25, 10. [Google Scholar] [CrossRef]
- Maher, N.; Mouhssine, S.; Matti, B.F.; Alwan, A.F.; Gaidano, G. Treatment Refractoriness in Chronic Lymphocytic Leukemia: Old and New Molecular Biomarkers. Int. J. Mol. Sci. 2023, 24, 10374. [Google Scholar] [CrossRef]
- Nasnas, P.; Cerchione, C.; Musuraca, G.; Martinelli, G.; Ferrajoli, A. How I Manage Chronic Lymphocytic Leukemia. Hematol. Rep. 2023, 15, 454–464. [Google Scholar] [CrossRef]
- Phelan, J.D.; Scheich, S.; Choi, J.; Wright, G.; Häupl, B.; Young, R.M.; Rieke, S.; Pape, M.; Ji, Y.; Urlaub, H.; et al. Exceptional Response to BTK Inhibitors in Aggressive Lymphomas Linked to Chronic Selective Autophagy. Blood 2023, 142, 850. [Google Scholar] [CrossRef]
- Buske, C.; Jurczak, W.; Salem, J.E.; Dimopoulos, M.A. Managing Waldenström’s macroglobulinemia with BTK inhibitors. Leukemia 2023, 37, 35–46. [Google Scholar] [CrossRef]
- Shadman, M. Diagnosis and Treatment of Chronic Lymphocytic Leukemia: A Review. JAMA 2023, 329, 918–932. [Google Scholar] [CrossRef] [PubMed]
- Surveillance Epidemiology and End Results Program. Chronic Lymphocytic Leukemia—Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/clyl.html (accessed on 15 January 2024).
- Surveillance Epidemiology and End Results Program. Non-Hodgkin Lymphoma—Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/nhl.html (accessed on 15 January 2024).
- Arora, R.; Lefante, J.; Saba, N.S. Updated Trends in Incidence and Survival of Mantle Cell Lymphoma from 2000 to 2020. Blood 2023, 142, 5160. [Google Scholar] [CrossRef]
- Cortelazzo, S.; Ponzoni, M.; Ferreri, A.J.M.; Dreyling, M. Mantle cell lymphoma. Crit. Rev. Oncol./Hematol. 2012, 82, 78–101. [Google Scholar] [CrossRef] [PubMed]
- Draetta, G.F. Mammalian G1 cyclins. Curr. Opin. Cell Biol. 1994, 6, 842–846. [Google Scholar] [CrossRef]
- Meral, M.; Demirpençe, M.; Gönen, C.; Akarsu, M.; Kayahan, H.; Demirkan, F.; Kargi, A.; Akpinar, H. Diffuse gastrointestinal involvement of mantle cell lymphoma. Turk. J. Gastroenterol. 2008, 19, 117–120. [Google Scholar] [PubMed]
- Patel, D.; Kahl, B. SOHO State of the Art Updates and Next Questions: Tailoring Upfront Therapy in Mantle Cell Lymphoma. Clin. Lymphoma Myeloma Leuk. 2023, 23, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.D.; Ohana, Z.; Pham, H.M. Pirtobrutinib: A novel non-covalent BTK inhibitor for the treatment of adults with relapsed/refractory mantle cell lymphoma. J. Oncol. Pharm. Pract. 2023, 30, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Tivey, A.; Shotton, R.; Eyre, T.A.; Lewis, D.; Stanton, L.; Allchin, R.; Walter, H.S.; Miall, F.; Zhao, R.; Santarsieri, A.; et al. Ibrutinib as first line therapy for mantle cell lymphoma: A multicentre, real-world UK study. Blood Adv. 2024, 8, 1209–1219. [Google Scholar] [CrossRef] [PubMed]
- Le Gouill, S.; Długosz-Danecka, M.; Rule, S.; Zinzani, P.L.; Goy, A.; Smith, S.D.; Doorduijn, J.K.; Panizo, C.; Shah, B.D.; Davies, A.J.; et al. Final results and overall survival data from a phase II study of acalabrutinib monotherapy in patients with relapsed/refractory mantle cell lymphoma, including those with poor prognostic factors. Haematologica 2024, 109, 343–350. [Google Scholar] [CrossRef]
- Alsuhebany, N.; Pan, C.; Holovac, E.; Do, B.; McBride, A. Zanubrutinib in Mantle Cell Lymphoma Management: A Comprehensive Review. Blood Lymphat. Cancer 2023, 13, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Pratt, G.; El-Sharkawi, D.; Kothari, J.; D’Sa, S.; Auer, R.; McCarthy, H.; Krishna, R.; Miles, O.; Kyriakou, C.; Owen, R. Diagnosis and management of Waldenström macroglobulinaemia—A British Society for Haematology guideline. Br. J. Haematol. 2022, 197, 171–187. [Google Scholar] [CrossRef]
- Minderman, M.; Lantermans, H.; van der Zwaan, C.; Hoogendijk, A.J.; van den Biggelaar, M.; Kersten, M.J.; Spaargaren, M.; Pals, S.T. The oncogenic human B-cell lymphoma MYD88 L265P mutation genocopies activation by phosphorylation at the Toll/interleukin-1 receptor (TIR) domain. Blood Cancer J. 2023, 13, 125. [Google Scholar] [CrossRef]
- Alcoceba, M.; García-Álvarez, M.; Medina, A.; Maldonado, R.; González-Calle, V.; Chillón, M.C.; Sarasquete, M.E.; González, M.; García-Sanz, R.; Jiménez, C. MYD88 Mutations: Transforming the Landscape of IgM Monoclonal Gammopathies. Int. J. Mol. Sci. 2022, 23, 5570. [Google Scholar] [CrossRef]
- Gertz, M.A.; Fonseca, R.; Rajkumar, S.V. Waldenström’s macroglobulinemia. Oncologist 2000, 5, 63–67. [Google Scholar] [CrossRef]
- Hobbs, M.; Fonder, A.; Hwa, Y.L. Waldenström Macroglobulinemia: Clinical Presentation, Diagnosis, and Management. J. Adv. Pract. Oncol. 2020, 11, 381–389. [Google Scholar] [CrossRef]
- Durot, E.; Tomowiak, C. Advances in Treatment of Waldenström Macroglobulinemia. Curr. Oncol. Rep. 2023, 25, 1375–1386. [Google Scholar] [CrossRef] [PubMed]
- Varettoni, M.; Matous, J.V. BTK Inhibitors in the Frontline Management of Waldenström Macroglobulinemia. Hematol. Oncol. Clin. N. Am. 2023, 37, 707–717. [Google Scholar] [CrossRef]
- López-Guillermo, A.; Colomo, L.; Jiménez, M.; Bosch, F.; Villamor, N.; Arenillas, L.; Muntañola, A.; Montoto, S.; Giné, E.; Colomer, D.; et al. Diffuse large B-cell lymphoma: Clinical and biological characterization and outcome according to the nodal or extranodal primary origin. J. Clin. Oncol. 2005, 23, 2797–2804. [Google Scholar] [CrossRef] [PubMed]
- Coiffier, B.; Thieblemont, C.; Van Den Neste, E.; Lepeu, G.; Plantier, I.; Castaigne, S.; Lefort, S.; Marit, G.; Macro, M.; Sebban, C.; et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: A study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood 2010, 116, 2040–2045. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.A.; Eisen, M.B.; Davis, R.E.; Ma, C.; Lossos, I.S.; Rosenwald, A.; Boldrick, J.C.; Sabet, H.; Tran, T.; Yu, X.; et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 2000, 403, 503–511. [Google Scholar] [CrossRef]
- Lenz, G.; Wright, G.; Dave, S.S.; Xiao, W.; Powell, J.; Zhao, H.; Xu, W.; Tan, B.; Goldschmidt, N.; Iqbal, J.; et al. Stromal gene signatures in large-B-cell lymphomas. N. Engl. J. Med. 2008, 359, 2313–2323. [Google Scholar] [CrossRef]
- Davis, R.E.; Ngo, V.N.; Lenz, G.; Tolar, P.; Young, R.M.; Romesser, P.B.; Kohlhammer, H.; Lamy, L.; Zhao, H.; Yang, Y.; et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature 2010, 463, 88–92. [Google Scholar] [CrossRef]
- Schmitz, R.; Wright, G.W.; Huang, D.W.; Johnson, C.A.; Phelan, J.D.; Wang, J.Q.; Roulland, S.; Kasbekar, M.; Young, R.M.; Shaffer, A.L.; et al. Genetics and Pathogenesis of Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2018, 378, 1396–1407. [Google Scholar] [CrossRef]
- Wright, G.W.; Huang, D.W.; Phelan, J.D.; Coulibaly, Z.A.; Roulland, S.; Young, R.M.; Wang, J.Q.; Schmitz, R.; Morin, R.D.; Tang, J.; et al. A Probabilistic Classification Tool for Genetic Subtypes of Diffuse Large B Cell Lymphoma with Therapeutic Implications. Cancer Cell 2020, 37, 551–568.e14. [Google Scholar] [CrossRef] [PubMed]
- Chapuy, B.; Stewart, C.; Dunford, A.J.; Kim, J.; Kamburov, A.; Redd, R.A.; Lawrence, M.S.; Roemer, M.G.M.; Li, A.J.; Ziepert, M.; et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat. Med. 2018, 24, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Chirino, A.; Montoya, S.; Safronenka, A.; Taylor, J. Resisting the Resistance: Navigating BTK Mutations in Chronic Lymphocytic Leukemia (CLL). Genes 2023, 14, 2182. [Google Scholar] [CrossRef] [PubMed]
- Small, S. Ibrutinib approved for the treatment of mantle cell lymphoma. Clin. Adv. Hematol. Oncol. 2013, 11, 808. [Google Scholar] [PubMed]
- Pan, Z.; Scheerens, H.; Li, S.J.; Schultz, B.E.; Sprengeler, P.A.; Burrill, L.C.; Mendonca, R.V.; Sweeney, M.D.; Scott, K.C.; Grothaus, P.G.; et al. Discovery of selective irreversible inhibitors for Bruton’s tyrosine kinase. ChemMedChem 2007, 2, 58–61. [Google Scholar] [CrossRef]
- Guarini, A.; Chiaretti, S.; Tavolaro, S.; Maggio, R.; Peragine, N.; Citarella, F.; Ricciardi, M.R.; Santangelo, S.; Marinelli, M.; De Propris, M.S.; et al. BCR ligation induced by IgM stimulation results in gene expression and functional changes only in IgV H unmutated chronic lymphocytic leukemia (CLL) cells. Blood 2008, 112, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Ganatra, S.; Sharma, A.; Shah, S.; Chaudhry, G.M.; Martin, D.T.; Neilan, T.G.; Mahmood, S.S.; Barac, A.; Groarke, J.D.; Hayek, S.S.; et al. Ibrutinib-Associated Atrial Fibrillation. JACC Clin. Electrophysiol. 2018, 4, 1491–1500. [Google Scholar] [CrossRef] [PubMed]
- Kaur, V.; Swami, A. Ibrutinib in CLL: A focus on adverse events, resistance, and novel approaches beyond ibrutinib. Ann. Hematol. 2017, 96, 1175–1184. [Google Scholar] [CrossRef]
- Borge, M.; Belén Almejún, M.; Podaza, E.; Colado, A.; Fernández Grecco, H.; Cabrejo, M.; Bezares, R.F.; Giordano, M.; Gamberale, R. Ibrutinib impairs the phagocytosis of rituximab-coated leukemic cells from chronic lymphocytic leukemia patients by human macrophages. Haematologica 2015, 100, e140–e142. [Google Scholar] [CrossRef]
- Acalabrutinib Approved for MCL. Cancer Discov. 2018, 8, Of6. [CrossRef]
- Danilov, A.V.; Persky, D.O. Incorporating acalabrutinib, a selective next-generation Bruton tyrosine kinase inhibitor, into clinical practice for the treatment of haematological malignancies. Br. J. Haematol. 2021, 193, 15–25. [Google Scholar] [CrossRef]
- Muñoz, J.; Wang, Y.; Jain, P.; Wang, M. Zanubrutinib in lymphoproliferative disorders: A comprehensive review. Ther. Adv. Hematol. 2022, 13, 20406207221093980. [Google Scholar] [CrossRef]
- Byrd, J.C.; Hillmen, P.; Ghia, P.; Kater, A.P.; Chanan-Khan, A.; Furman, R.R.; O’Brien, S.; Yenerel, M.N.; Illés, A.; Kay, N.; et al. Acalabrutinib Versus Ibrutinib in Previously Treated Chronic Lymphocytic Leukemia: Results of the First Randomized Phase III Trial. J. Clin. Oncol. 2021, 39, 3441–3452. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.R.; Eichhorst, B.; Hillmen, P.; Jurczak, W.; Kaźmierczak, M.; Lamanna, N.; O’Brien, S.M.; Tam, C.S.; Qiu, L.; Zhou, K.; et al. Zanubrutinib or Ibrutinib in Relapsed or Refractory Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2023, 388, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Wolska-Washer, A.; Robak, T. Zanubrutinib for the treatment of lymphoid malignancies: Current status and future directions. Front. Oncol. 2023, 13, 1130595. [Google Scholar] [CrossRef] [PubMed]
- Proskuriakova, E.; Shrestha, D.B.; Jasaraj, R.; Reddy, V.K.; Shtembari, J.; Raut, A.; Gaire, S.; Khosla, P.; Kadariya, D. Cardiovascular Adverse Events Associated with Second-Generation Bruton Tyrosine Kinase Inhibitor Therapy: A Systematic Review and Meta-Analysis. Clin. Ther. 2023, 46, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Dangi-Garimella, S. FDA grants accelerated approval for ibrutinib for CLL. Am. J. Manag. Care 2014, 20, E10. [Google Scholar] [PubMed]
- Byrd, J.C.; Furman, R.R.; Coutre, S.E.; Flinn, I.W.; Burger, J.A.; Blum, K.; Sharman, J.P.; Wierda, W.; Zhao, W.; Heerema, N.A.; et al. Ibrutinib Treatment for First-Line and Relapsed/Refractory Chronic Lymphocytic Leukemia: Final Analysis of the Pivotal Phase Ib/II PCYC-1102 Study. Clin. Cancer Res. 2020, 26, 3918–3927. [Google Scholar] [CrossRef] [PubMed]
- Munir, T.; Brown, J.R.; O’Brien, S.; Barrientos, J.C.; Barr, P.M.; Reddy, N.M.; Coutre, S.; Tam, C.S.; Mulligan, S.P.; Jaeger, U.; et al. Final analysis from RESONATE: Up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am. J. Hematol. 2019, 94, 1353–1363. [Google Scholar] [CrossRef]
- Barr, P.M.; Owen, C.; Robak, T.; Tedeschi, A.; Bairey, O.; Burger, J.A.; Hillmen, P.; Coutre, S.E.; Dearden, C.; Grosicki, S.; et al. Up to 8-year follow-up from RESONATE-2: First-line ibrutinib treatment for patients with chronic lymphocytic leukemia. Blood Adv. 2022, 6, 3440–3450. [Google Scholar] [CrossRef]
- Woyach, J.A.; Ruppert, A.S.; Heerema, N.A.; Zhao, W.; Booth, A.M.; Ding, W.; Bartlett, N.L.; Brander, D.M.; Barr, P.M.; Rogers, K.; et al. Long-Term Results of Alliance A041202 Show Continued Advantage of Ibrutinib-Based Regimens Compared with Bendamustine Plus Rituximab (BR) Chemoimmunotherapy. Blood 2021, 138, 639. [Google Scholar] [CrossRef]
- Shanafelt, T.D.; Wang, X.V.; Hanson, C.A.; Paietta, E.M.; O’Brien, S.; Barrientos, J.; Jelinek, D.F.; Braggio, E.; Leis, J.F.; Zhang, C.C.; et al. Long-term outcomes for ibrutinib-rituximab and chemoimmunotherapy in CLL: Updated results of the E1912 trial. Blood 2022, 140, 112–120. [Google Scholar] [CrossRef]
- Sharman, J.P.; Egyed, M.; Jurczak, W.; Skarbnik, A.; Pagel, J.M.; Flinn, I.W.; Kamdar, M.; Munir, T.; Walewska, R.; Corbett, G.; et al. Efficacy and safety in a 4-year follow-up of the ELEVATE-TN study comparing acalabrutinib with or without obinutuzumab versus obinutuzumab plus chlorambucil in treatment-naïve chronic lymphocytic leukemia. Leukemia 2022, 36, 1171–1175. [Google Scholar] [CrossRef]
- Ghia, P.; Pluta, A.; Wach, M.; Lysak, D.; Šimkovič, M.; Kriachok, I.; Illés, Á.; de la Serna, J.; Dolan, S.; Campbell, P.; et al. Acalabrutinib Versus Investigator’s Choice in Relapsed/Refractory Chronic Lymphocytic Leukemia: Final ASCEND Trial Results. Hemasphere 2022, 6, e801. [Google Scholar] [CrossRef]
- Tam, C.S.; Brown, J.R.; Kahl, B.S.; Ghia, P.; Giannopoulos, K.; Jurczak, W.; Šimkovič, M.; Shadman, M.; Österborg, A.; Laurenti, L.; et al. Zanubrutinib versus bendamustine and rituximab in untreated chronic lymphocytic leukaemia and small lymphocytic lymphoma (SEQUOIA): A randomised, controlled, phase 3 trial. Lancet Oncol. 2022, 23, 1031–1043. [Google Scholar] [CrossRef] [PubMed]
- Gribbin, C.; Chen, J.; Martin, P.; Ruan, J. Novel treatment for mantle cell lymphoma—Impact of BTK inhibitors and beyond. Leuk. Lymphoma 2024, 65, 1–13. [Google Scholar] [CrossRef]
- Wang, M.L.; Jain, P.; Zhao, S.; Lee, H.J.; Nastoupil, L.; Fayad, L.; Ok, C.Y.; Kanagal-Shamanna, R.; Hill, H.A.; Yao, Y.; et al. Ibrutinib-rituximab followed by R-HCVAD as frontline treatment for young patients (≤65 years) with mantle cell lymphoma (WINDOW-1): A single-arm, phase 2 trial. Lancet Oncol. 2022, 23, 406–415. [Google Scholar] [CrossRef]
- Dreyling, M.; Doorduijn, J.K.; Gine, E.; Jerkeman, M.; Walewski, J.; Hutchings, M.; Mey, U.; Riise, J.; Trneny, M.; Vergote, V.K.J.; et al. Efficacy and Safety of Ibrutinib Combined with Standard First-Line Treatment or As Substitute for Autologous Stem Cell Transplantation in Younger Patients with Mantle Cell Lymphoma: Results from the Randomized Triangle Trial by the European MCL Network. Blood 2022, 140, 1–3. [Google Scholar] [CrossRef]
- Song, Y.; Zhou, K.; Zou, D.; Zhou, J.; Hu, J.; Yang, H.; Zhang, H.; Ji, J.; Xu, W.; Jin, J.; et al. Zanubrutinib in relapsed/refractory mantle cell lymphoma: Long-term efficacy and safety results from a phase 2 study. Blood 2022, 139, 3148–3158. [Google Scholar] [CrossRef] [PubMed]
- Dreyling, M.; Tam, C.S.; Wang, M.; Smith, S.D.; Ladetto, M.; Huang, H.; Novotny, W.; Co, M.; Romano, A.; Holmgren, E.; et al. A Phase III study of zanubrutinib plus rituximab versus bendamustine plus rituximab in transplant-ineligible, untreated mantle cell lymphoma. Future Oncol. 2020, 17, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Sarosiek, S.; Castillo, J.J. Waldenström Macroglobulinemia: Targeted Agents Taking Center Stage. Drugs 2023, 84, 17–25. [Google Scholar] [CrossRef]
- Treon, S.P.; Tripsas, C.K.; Meid, K.; Warren, D.; Varma, G.; Green, R.; Argyropoulos, K.V.; Yang, G.; Cao, Y.; Xu, L.; et al. Ibrutinib in previously treated Waldenström’s macroglobulinemia. N. Engl. J. Med. 2015, 372, 1430–1440. [Google Scholar] [CrossRef]
- Buske, C.; Tedeschi, A.; Trotman, J.; García-Sanz, R.; MacDonald, D.; Leblond, V.; Mahe, B.; Herbaux, C.; Matous, J.V.; Tam, C.S.; et al. Ibrutinib Plus Rituximab Versus Placebo Plus Rituximab for Waldenström’s Macroglobulinemia: Final Analysis from the Randomized Phase III iNNOVATE Study. J. Clin. Oncol. 2022, 40, 52–62. [Google Scholar] [CrossRef]
- Treon, S.P.; Meid, K.; Gustine, J.; Yang, G.; Xu, L.; Liu, X.; Patterson, C.J.; Hunter, Z.R.; Branagan, A.R.; Laubach, J.P.; et al. Long-Term Follow-Up of Ibrutinib Monotherapy in Symptomatic, Previously Treated Patients with Waldenström Macroglobulinemia. J. Clin. Oncol. 2021, 39, 565–575. [Google Scholar] [CrossRef]
- Castillo, J.J.; Meid, K.; Gustine, J.N.; Leventoff, C.; White, T.; Flynn, C.A.; Sarosiek, S.; Demos, M.G.; Guerrera, M.L.; Kofides, A.; et al. Long-term follow-up of ibrutinib monotherapy in treatment-naive patients with Waldenstrom macroglobulinemia. Leukemia 2022, 36, 532–539. [Google Scholar] [CrossRef]
- Castillo, J.J.; Buske, C.; Trotman, J.; Sarosiek, S.; Treon, S.P. Bruton tyrosine kinase inhibitors in the management of Waldenström macroglobulinemia. Am. J. Hematol. 2023, 98, 338–347. [Google Scholar] [CrossRef]
- Tam, C.S.; Opat, S.; D’Sa, S.; Jurczak, W.; Lee, H.P.; Cull, G.; Owen, R.G.; Marlton, P.; Wahlin, B.E.; Sanz, R.G.; et al. A randomized phase 3 trial of zanubrutinib vs. ibrutinib in symptomatic Waldenström macroglobulinemia: The ASPEN study. Blood 2020, 136, 2038–2050. [Google Scholar] [CrossRef]
- Owen, R.G.; McCarthy, H.; Rule, S.; D’Sa, S.; Thomas, S.K.; Tournilhac, O.; Forconi, F.; Kersten, M.J.; Zinzani, P.L.; Iyengar, S.; et al. Acalabrutinib monotherapy in patients with Waldenström macroglobulinemia: A single-arm, multicentre, phase 2 study. Lancet Haematol. 2020, 7, e112–e121. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.H.; Young, R.M.; Schmitz, R.; Yang, Y.; Pittaluga, S.; Wright, G.; Lih, C.J.; Williams, P.M.; Shaffer, A.L.; Gerecitano, J.; et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat. Med. 2015, 21, 922–926. [Google Scholar] [CrossRef] [PubMed]
- Younes, A.; Sehn, L.H.; Johnson, P.; Zinzani, P.L.; Hong, X.; Zhu, J.; Patti, C.; Belada, D.; Samoilova, O.; Suh, C.; et al. Randomized Phase III Trial of Ibrutinib and Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone in Non-Germinal Center B-Cell Diffuse Large B-Cell Lymphoma. J. Clin. Oncol. 2019, 37, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.H.; Wright, G.W.; Huang, D.W.; Hodkinson, B.; Balasubramanian, S.; Fan, Y.; Vermeulen, J.; Shreeve, M.; Staudt, L.M. Effect of ibrutinib with R-CHOP chemotherapy in genetic subtypes of DLBCL. Cancer Cell 2021, 39, 1643–1653.e3. [Google Scholar] [CrossRef] [PubMed]
- Phelan, J.D.; Scheich, S.; Choi, J.; Wright, G.W.; Häupl, B.; Young, R.M.; Rieke, S.A.; Pape, M.; Ji, Y.; Urlaub, H.; et al. Response to Bruton’s tyrosine kinase inhibitors in aggressive lymphomas linked to chronic selective autophagy. Cancer Cell 2024, 42, 238–252.e9. [Google Scholar] [CrossRef] [PubMed]
- Ahn, I.E.; Tian, X.; Ipe, D.; Cheng, M.; Albitar, M.; Tsao, L.C.; Zhang, L.; Ma, W.; Herman, S.E.M.; Gaglione, E.M.; et al. Prediction of Outcome in Patients With Chronic Lymphocytic Leukemia Treated with Ibrutinib: Development and Validation of a Four-Factor Prognostic Model. J. Clin. Oncol. 2021, 39, 576–585. [Google Scholar] [CrossRef]
- Woyach, J.A.; Furman, R.R.; Liu, T.M.; Ozer, H.G.; Zapatka, M.; Ruppert, A.S.; Xue, L.; Li, D.H.; Steggerda, S.M.; Versele, M.; et al. Resistance mechanisms for the Bruton’s tyrosine kinase inhibitor ibrutinib. N. Engl. J. Med. 2014, 370, 2286–2294. [Google Scholar] [CrossRef]
- Bonfiglio, S.; Sutton, L.-A.; Ljungström, V.; Capasso, A.; Pandzic, T.; Weström, S.; Foroughi-Asl, H.; Skaftason, A.; Gellerbring, A.; Lyander, A.; et al. BTK and PLCG2 remain unmutated in one-third of patients with CLL relapsing on ibrutinib. Blood Adv. 2023, 7, 2794–2806. [Google Scholar] [CrossRef] [PubMed]
- Chiron, D.; Di Liberto, M.; Martin, P.; Huang, X.; Sharman, J.; Blecua, P.; Mathew, S.; Vijay, P.; Eng, K.; Ali, S.; et al. Cell-cycle reprogramming for PI3K inhibition overrides a relapse-specific C481S BTK mutation revealed by longitudinal functional genomics in mantle cell lymphoma. Cancer Discov. 2014, 4, 1022–1035. [Google Scholar] [CrossRef] [PubMed]
- Hershkovitz-Rokah, O.; Pulver, D.; Lenz, G.; Shpilberg, O. Ibrutinib resistance in mantle cell lymphoma: Clinical, molecular and treatment aspects. Br. J. Haematol. 2018, 181, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, A.; Sandoval, N.; Das, M.; Pillai, R.; Chen, L.; Chen, R.W.; Amin, H.M.; Wang, M.; Marcucci, G.; Weisenburger, D.D.; et al. CCND1 mutations increase protein stability and promote ibrutinib resistance in mantle cell lymphoma. Oncotarget 2016, 7, 73558–73572. [Google Scholar] [CrossRef] [PubMed]
- Rahal, R.; Frick, M.; Romero, R.; Korn, J.M.; Kridel, R.; Chan, F.C.; Meissner, B.; Bhang, H.E.; Ruddy, D.; Kauffmann, A.; et al. Pharmacological and genomic profiling identifies NF-κB-targeted treatment strategies for mantle cell lymphoma. Nat. Med. 2014, 20, 87–92. [Google Scholar] [CrossRef]
- Sun, S.C. The non-canonical NF-κB pathway in immunity and inflammation. Nat. Rev. Immunol. 2017, 17, 545–558. [Google Scholar] [CrossRef]
- Wu, C.; de Miranda, N.F.; Chen, L.; Wasik, A.M.; Mansouri, L.; Jurczak, W.; Galazka, K.; Dlugosz-Danecka, M.; Machaczka, M.; Zhang, H.; et al. Genetic heterogeneity in primary and relapsed mantle cell lymphomas: Impact of recurrent CARD11 mutations. Oncotarget 2016, 7, 38180–38190. [Google Scholar] [CrossRef]
- Decombis, S.; Bellanger, C.; Le Bris, Y.; Madiot, C.; Jardine, J.; Santos, J.C.; Boulet, D.; Dousset, C.; Menard, A.; Kervoelen, C.; et al. CARD11 gain of function upregulates BCL2A1 expression and promotes resistance to targeted therapies combination in B-cell lymphoma. Blood 2023, 142, 1543–1555. [Google Scholar] [CrossRef]
- Piazza, F.; Di Paolo, V.; Scapinello, G.; Manni, S.; Trentin, L.; Quintieri, L. Determinants of Drug Resistance in B-Cell Non-Hodgkin Lymphomas: The Case of Lymphoplasmacytic Lymphoma/Waldenström Macroglobulinemia. Front. Oncol. 2021, 11, 801124. [Google Scholar] [CrossRef]
- Kaiser, L.M.; Hunter, Z.R.; Treon, S.P.; Buske, C. CXCR4 in Waldenström’s Macroglobulinema: Chances and challenges. Leukemia 2021, 35, 333–345. [Google Scholar] [CrossRef]
- Pozzobon, T.; Goldoni, G.; Viola, A.; Molon, B. CXCR4 signaling in health and disease. Immunol. Lett. 2016, 177, 6–15. [Google Scholar] [CrossRef]
- Balabanian, K.; Lagane, B.; Pablos, J.L.; Laurent, L.; Planchenault, T.; Verola, O.; Lebbe, C.; Kerob, D.; Dupuy, A.; Hermine, O.; et al. WHIM syndromes with different genetic anomalies are accounted for by impaired CXCR4 desensitization to CXCL12. Blood 2005, 105, 2449–2457. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Choi, U.; Whiting-Theobald, N.L.; Linton, G.F.; Brenner, S.; Sechler, J.M.; Murphy, P.M.; Malech, H.L. Enhanced function with decreased internalization of carboxy-terminus truncated CXCR4 responsible for WHIM syndrome. Exp. Hematol. 2005, 33, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.J.; Xu, L.; Gustine, J.N.; Keezer, A.; Meid, K.; Dubeau, T.E.; Liu, X.; Demos, M.G.; Kofides, A.; Tsakmaklis, N.; et al. CXCR4 mutation subtypes impact response and survival outcomes in patients with Waldenström macroglobulinaemia treated with ibrutinib. Br. J. Haematol. 2019, 187, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Compagno, M.; Lim, W.K.; Grunn, A.; Nandula, S.V.; Brahmachary, M.; Shen, Q.; Bertoni, F.; Ponzoni, M.; Scandurra, M.; Califano, A.; et al. Mutations of multiple genes cause deregulation of NF-kappaB in diffuse large B-cell lymphoma. Nature 2009, 459, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Nakhoda, S.; Vistarop, A.; Wang, Y.L. Resistance to Bruton tyrosine kinase inhibition in chronic lymphocytic leukaemia and non-Hodgkin lymphoma. Br. J. Haematol. 2023, 200, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Phelan, J.D.; Wright, G.W.; Häupl, B.; Huang, D.W.; Shaffer, A.L., 3rd; Young, R.M.; Wang, Z.; Zhao, H.; Yu, X.; et al. Regulation of B cell receptor-dependent NF-κB signaling by the tumor suppressor KLHL14. Proc. Natl. Acad. Sci. USA 2020, 117, 6092–6102. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, I.; Li, Y.; Sharma, A.; Zhu, H.; Bodo, J.; Xu, W.; Hsi, E.D.; Hill, B.T.; Almasan, A. Resistance to BTK inhibition by ibrutinib can be overcome by preventing FOXO3a nuclear export and PI3K/AKT activation in B-cell lymphoid malignancies. Cell Death Dis. 2019, 10, 924. [Google Scholar] [CrossRef]
- Shaffer, A.L., 3rd; Phelan, J.D.; Wang, J.Q.; Huang, D.; Wright, G.W.; Kasbekar, M.; Choi, J.; Young, R.M.; Webster, D.E.; Yang, Y.; et al. Overcoming Acquired Epigenetic Resistance to BTK Inhibitors. Blood Cancer Discov. 2021, 2, 630–647. [Google Scholar] [CrossRef] [PubMed]
- Bond, D.A.; Woyach, J.A. Targeting BTK in CLL: Beyond Ibrutinib. Curr. Hematol. Malign Rep. 2019, 14, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Puła, B.; Gołos, A.; Górniak, P.; Jamroziak, K. Overcoming Ibrutinib Resistance in Chronic Lymphocytic Leukemia. Cancers 2019, 11, 1384. [Google Scholar] [CrossRef] [PubMed]
- Frustaci, A.M.; Deodato, M.; Zamprogna, G.; Cairoli, R.; Montillo, M.; Tedeschi, A. Next Generation BTK Inhibitors in CLL: Evolving Challenges and New Opportunities. Cancers 2023, 15, 1504. [Google Scholar] [CrossRef]
- Keam, S.J. Pirtobrutinib: First Approval. Drugs 2023, 83, 547–553. [Google Scholar] [CrossRef]
- Cohen, J.B.; Shah, N.N.; Jurczak, W.; Zinzani, P.L.; Cheah, C.Y.; Eyre, T.A.; Ujjani, C.S.; Koh, Y.; Kim, W.S.; Nasta, S.D.; et al. Pirtobrutinib in Relapsed/Refractory (R/R) Mantle Cell Lymphoma (MCL) Patients with Prior cBTKi: Safety and Efficacy Including High-Risk Subgroup Analyses from the Phase 1/2 BRUIN Study. Blood 2023, 142, 981. [Google Scholar] [CrossRef]
- Mato, A.R.; Shah, N.N.; Jurczak, W.; Cheah, C.Y.; Pagel, J.M.; Woyach, J.A.; Fakhri, B.; Eyre, T.A.; Lamanna, N.; Patel, M.R.; et al. Pirtobrutinib in relapsed or refractory B-cell malignancies (BRUIN): A phase 1/2 study. Lancet 2021, 397, 892–901. [Google Scholar] [CrossRef]
- Scarfò, L.; Patel, M.R.; Eyre, T.A.; Jurczak, W.; Lewis, D.; Gastinne, T.; Ma, S.; Cohen, J.B.; Patel, K.; Brown, J.R.; et al. P1108: Efficacy of pirtobrutinib, a highly selective, non-covalent (reversible) btk inhibitor in relapsed/refractory waldenström macroglobulinemia: Results from the phase 1/2 bruin study. Hemasphere 2023, 7, e852670f. [Google Scholar] [CrossRef]
- Wang, E.; Mi, X.; Thompson, M.C.; Montoya, S.; Notti, R.Q.; Afaghani, J.; Durham, B.H.; Penson, A.; Witkowski, M.T.; Lu, S.X.; et al. Mechanisms of Resistance to Noncovalent Bruton’s Tyrosine Kinase Inhibitors. N. Engl. J. Med. 2022, 386, 735–743. [Google Scholar] [CrossRef]
- Blombery, P.; Thompson, E.R.; Lew, T.E.; Tiong, I.S.; Bennett, R.; Cheah, C.Y.; Lewis, K.L.; Handunnetti, S.M.; Tang, C.P.S.; Roberts, A.; et al. Enrichment of BTK Leu528Trp mutations in patients with CLL on zanubrutinib: Potential for pirtobrutinib cross-resistance. Blood Adv. 2022, 6, 5589–5592. [Google Scholar] [CrossRef]
- Brown, J.R.; Desikan, S.P.; Nguyen, B.; Won, H.; Tantawy, S.I.; McNeely, S.; Marella, N.; Ebata, K.; Woyach, J.A.; Patel, K.; et al. Genomic Evolution and Resistance during Pirtobrutinib Therapy in Covalent BTK-Inhibitor (cBTKi) Pre-Treated Chronic Lymphocytic Leukemia Patients: Updated Analysis from the BRUIN Study. Blood 2023, 142, 326. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, Z.; Zhang, Z.; Li, Z.; Guo, L.; Pan, H.; Luo, X.; Liu, D.; Rao, Y. Design, synthesis, and evaluation of BTK-targeting PROTACs with optimized bioavailability in vitro and in vivo. RSC Med. Chem. 2023, 14, 1562–1566. [Google Scholar] [CrossRef] [PubMed]
- Buhimschi, A.D.; Armstrong, H.A.; Toure, M.; Jaime-Figueroa, S.; Chen, T.L.; Lehman, A.M.; Woyach, J.A.; Johnson, A.J.; Byrd, J.C.; Crews, C.M. Targeting the C481S Ibrutinib-Resistance Mutation in Bruton’s Tyrosine Kinase Using PROTAC-Mediated Degradation. Biochemistry 2018, 57, 3564–3575. [Google Scholar] [CrossRef] [PubMed]
- Mato, A.R.; Wierda, W.G.; Ai, W.Z.; Flinn, I.W.; Tees, M.; Patel, M.R.; Patel, K.; O’Brien, S.; Bond, D.A.; Roeker, L.E.; et al. NX-2127-001, a First-in-Human Trial of NX-2127, a Bruton’s Tyrosine Kinase-Targeted Protein Degrader, in Patients with Relapsed or Refractory Chronic Lymphocytic Leukemia and B-Cell Malignancies. Blood 2022, 140, 2329–2332. [Google Scholar] [CrossRef]
- Robbins, D.W.; Kelly, A.; Tan, M.; McIntosh, J.; Wu, J.; Konst, Z.; Kato, D.; Peng, G.; Mihalic, J.; Weiss, D.; et al. Nx-2127, a Degrader of BTK and IMiD Neosubstrates, for the Treatment of B-Cell Malignancies. Blood 2020, 136, 34. [Google Scholar] [CrossRef]
- Chanan-Khan, A.A.; Swaika, A.; Paulus, A.; Kumar, S.K.; Mikhael, J.R.; Rajkumar, S.V.; Dispenzieri, A.; Lacy, M.Q. Pomalidomide: The new immunomodulatory agent for the treatment of multiple myeloma. Blood Cancer J. 2013, 3, e143. [Google Scholar] [CrossRef]
- Georgopoulos, K.; Moore, D.D.; Derfler, B. Ikaros, an early lymphoid-specific transcription factor and a putative mediator for T cell commitment. Science 1992, 258, 808–812. [Google Scholar] [CrossRef]
- Morgan, B.; Sun, L.; Avitahl, N.; Andrikopoulos, K.; Ikeda, T.; Gonzales, E.; Wu, P.; Neben, S.; Georgopoulos, K. Aiolos, a lymphoid restricted transcription factor that interacts with Ikaros to regulate lymphocyte differentiation. EMBO J. 1997, 16, 2004–2013. [Google Scholar] [CrossRef]
- Griggio, V.; Perutelli, F.; Salvetti, C.; Boccellato, E.; Boccadoro, M.; Vitale, C.; Coscia, M. Immune Dysfunctions and Immune-Based Therapeutic Interventions in Chronic Lymphocytic Leukemia. Front. Immunol. 2020, 11, 594556. [Google Scholar] [CrossRef]
| NCT Identifier | Phase | Condition | Intervention |
|---|---|---|---|
| NCT05734495 | II | LPL | Pirtobrutinib + venetoclax |
| NCT05317936 | II | CLL | Pirtobrutinib + venetoclax |
| NCT05254743 | III | CLL | Pirtobrutinib vs. ibrutinib |
| NCT05529069 | II | R/R MCL | Pirtobrutinib + venetoclax |
| NCT05006716 | I/II | R/R B cell malignancies, namely CLL, MM, MCL, LPL, MZL | Pirtobrutinib + LOXO-338 |
| NCT04965493 | III | R/R CLL | Pirtobrutinib + venetoclax + rituximab |
| NCT03740529 | I/II | R/R B cell malignancies, namely CLL, MCL, LPL, MZL | Pirtobrutinib ± venetoclax ± rituximab |
| NCT05990465 | I | R/R NHL, namely MCL, DLBCL, MZL, BL, FL | Pirtobrutinib + LV20.19 CAR T cells |
| NCT05833763 | II | BTKi-refractory MCL | Pirtobrutinib + glofitamab + obinutuzumab |
| NCT04849416 | II | R/R B cell malignancies, namely CLL, MCL, DLBCL, MZL | Pirtobrutinib |
| NCT04662255 | III | R/R BTKi naïve MCL | Pirtobrutinib vs. ibrutinib/acalabrutinib/zanubrutinib |
| NCT05536349 | II | Treatment-naïve CLL/RS | Pirtobrutinib + venetoclax+ obinutuzumab |
| NCT05677919 | II | Treatment-naïve CLL | Pirtobrutinib + venetoclax |
| NCT04666038 | III | R/R CLL | Pirtobrutinib vs. BR/idelalisib + rituximab |
| NCT03162536 | I/II | R/R B cell malignancies, namely CLL, MCL, LPL, DLBCL, MZL, FL, RS | Nemtabrutinib |
| NCT05683717 | I | R/R B cell malingnancies, including CLL, DLBCL, other NHL | TT-01488 |
| NCT05275504 | I | R/R B cell malignancies, including CLL, LPL, FL, MZL, DLBCL, other NHL | TT-01488 |
| NCT05023980 | III | Treatment-naïve CLL | Pirtobrutinib vs. BR |
| NCT Identifier | Phase | Condition | Intervention |
|---|---|---|---|
| NCT04830137 | I | CLL, MCL, LPL, MZL, FL, DLBCL, PCNSL | NX-2127 |
| NCT05294731 | I | CLL, MCL, LPL, MZL, FL, DLBCL, RS | BGB-16673 |
| NCT05131022 | I | CLL, MCL, LPL, MZL, FL, DLBCL, PCNSL | NX-5948 |
| NCT05753501 | I | CLL, MCL, LPL, MZL, FL, DLBCL | ABBV-101 |
| NCT05006716 | I/II | CLL, MCL, LPL, MZL, FL, DLBCL | BGB-16673 |
| NCT05780034 | I | R/R NHL, namely CLL, MCL, LPL, MZL, FL, DLBCL | AC676 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mouhssine, S.; Maher, N.; Matti, B.F.; Alwan, A.F.; Gaidano, G. Targeting BTK in B Cell Malignancies: From Mode of Action to Resistance Mechanisms. Int. J. Mol. Sci. 2024, 25, 3234. https://doi.org/10.3390/ijms25063234
Mouhssine S, Maher N, Matti BF, Alwan AF, Gaidano G. Targeting BTK in B Cell Malignancies: From Mode of Action to Resistance Mechanisms. International Journal of Molecular Sciences. 2024; 25(6):3234. https://doi.org/10.3390/ijms25063234
Chicago/Turabian StyleMouhssine, Samir, Nawar Maher, Bassam Francis Matti, Alaa Fadhil Alwan, and Gianluca Gaidano. 2024. "Targeting BTK in B Cell Malignancies: From Mode of Action to Resistance Mechanisms" International Journal of Molecular Sciences 25, no. 6: 3234. https://doi.org/10.3390/ijms25063234
APA StyleMouhssine, S., Maher, N., Matti, B. F., Alwan, A. F., & Gaidano, G. (2024). Targeting BTK in B Cell Malignancies: From Mode of Action to Resistance Mechanisms. International Journal of Molecular Sciences, 25(6), 3234. https://doi.org/10.3390/ijms25063234








