Constitutional BRCA1 and MGMT Methylation Are Significant Risk Factors for Triple-Negative Breast Cancer and High-Grade Serous Ovarian Cancer in Saudi Women
Abstract
1. Introduction
2. Results
2.1. Constitutional BRCA1 Promoter Methylation and MGMT Promoter Methylation Are Associated with BC in Saudi Breast Cancer Patients
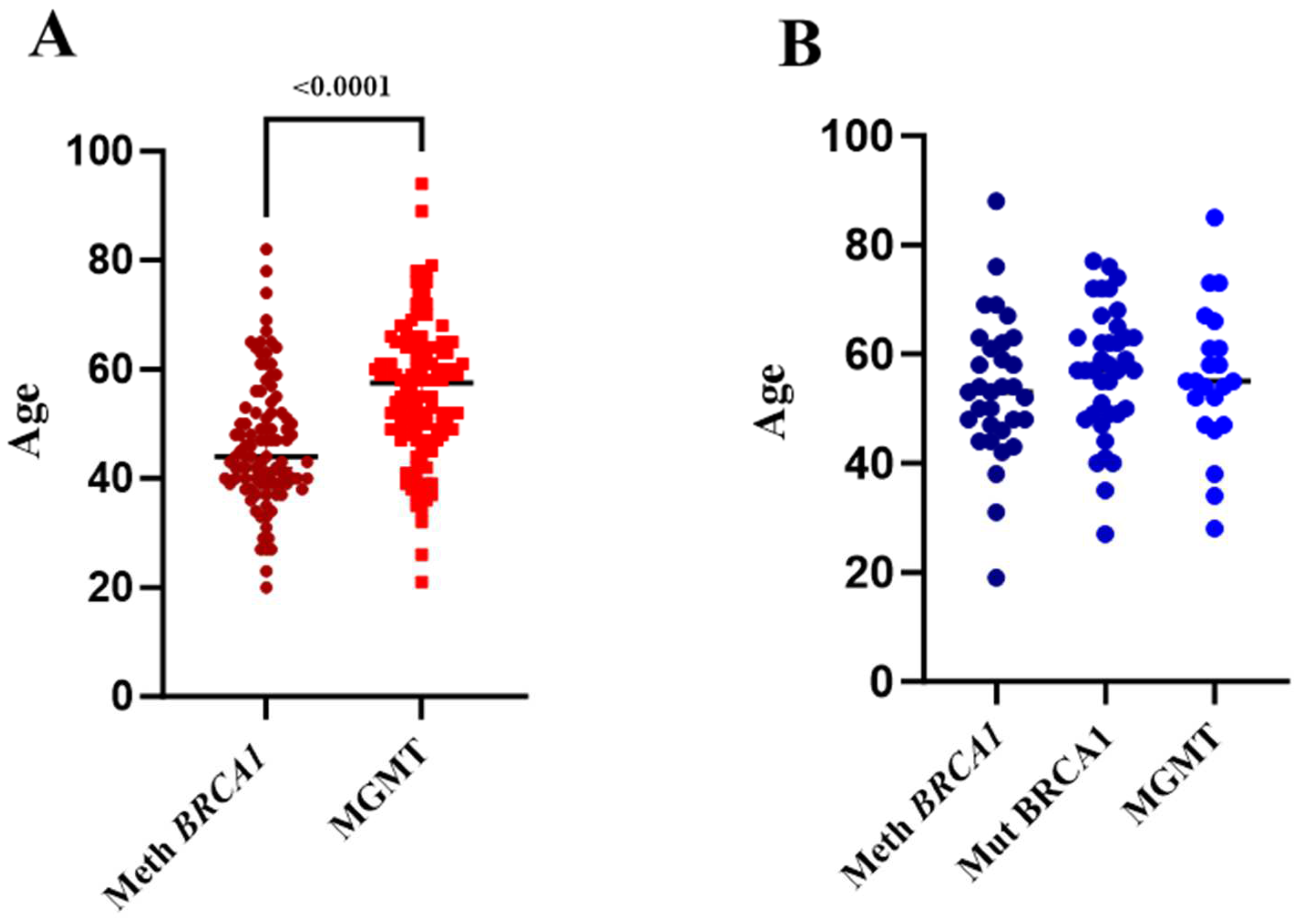
2.2. BRCA1 Methylation Is Associated with Early-Onset BC, While MGMT Methylation Is Associated with Late-Onset BC
2.3. Constitutional BRCA1 Promoter Methylation and MGMT Promoter Methylation Account for about One-Third of TNBC Instances in Saudi Breast Cancer Patients
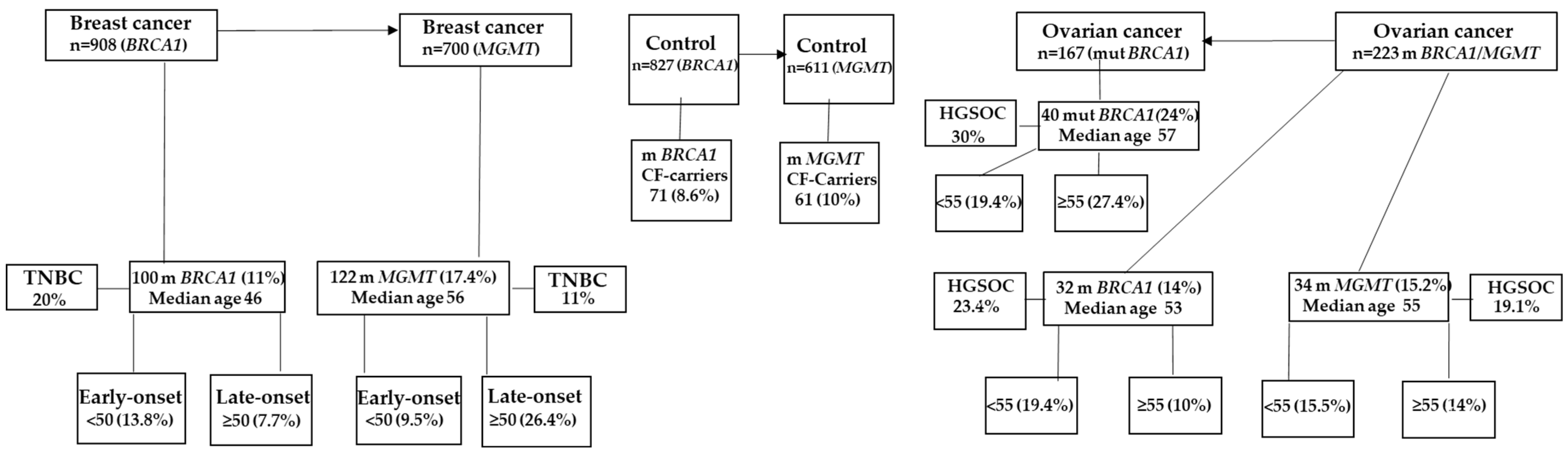
2.4. Constitutional BRCA1 Promoter Methylation and MGMT Promoter Methylation Contribute to a Greater Proportion of OC in Saudi Women Than Mutant BRCA1
2.5. Constitutional BRCA1 Promoter Methylation and MGMT Promoter Methylation Account for a Higher Proportion of HGSOC in Saudi Women with Ovarian Cancer Than Mutant BRCA1
2.6. Patients with BRCA1- and MGMT-Methylated Cancers Have a Family History of Cancer
2.7. BRCA1- and MGMT-Methylated Breast Cancer Patients, as Well as Cancer-Free Methylation Carriers, Express High Levels of BRCA1 and MGMT mRNA
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. DNA and RNA Isolation from WBCs
4.3. Methylation-Specific Polymerase Chain Reaction
4.4. Reverse Transcription Quantitative PCR (RT qPCR)
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, P.A.; Baylin, S.B. The fundamental role of epigenetic events in cancer. Nat. Rev. Genet. 2002, 3, 415–428. [Google Scholar] [CrossRef]
- Lonning, P.E.; Eikesdal, H.P.; Loes, I.M.; Knappskog, S. Constitutional Mosaic Epimutations—A hidden cause of cancer? Cell Stress 2019, 3, 118–135. [Google Scholar] [CrossRef]
- Lonning, P.E.; Nikolaienko, O.; Pan, K.; Kurian, A.W.; Eikesdal, H.P.; Pettinger, M.; Anderson, G.L.; Prentice, R.L.; Chlebowski, R.T.; Knappskog, S. Constitutional BRCA1 Methylation and Risk of Incident Triple-Negative Breast Cancer and High-grade Serous Ovarian Cancer. JAMA Oncol. 2022, 8, 1579–1587. [Google Scholar] [CrossRef]
- Al-Moghrabi, N.; Al-Showimi, M.; Al-Yousef, N.; Al-Shahrani, B.; Karakas, B.; Alghofaili, L.; Almubarak, H.; Madkhali, S.; Al Humaidan, H. Methylation of BRCA1 and MGMT genes in white blood cells are transmitted from mothers to daughters. Clin. Epigenetics 2018, 10, 99. [Google Scholar] [CrossRef]
- Al-Moghrabi, N.; Al-Showimi, M.; Al-Yousef, N.; AlOtai, L. MicroRNA-155-5p, Reduced by Curcumin-Re-Expressed Hypermethylated BRCA1, Is a Molecular Biomarker for Cancer Risk in BRCA1-methylation Carriers. Int. J. Mol. Sci. 2023, 24, 9021. [Google Scholar] [CrossRef] [PubMed]
- Al-Moghrabi, N.; Al-Qasem, A.J.; Aboussekhra, A. Methylation-related mutations in the BRCA1 promoter in peripheral blood cells from cancer-free women. Int. J. Oncol. 2011, 39, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Jacinto, F.V.; Esteller, M. Mutator pathways unleashed by epigenetic silencing in human cancer. Mutagenesis 2007, 22, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Welcsh, P.L.; King, M.C. BRCA1 and BRCA2 and the genetics of breast and ovarian cancer. Hum. Mol. Genet. 2001, 10, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Al-Showimi, M.; Al-Yousef, N.; Alharbi, W.; Alkhezayem, S.; Almalik, O.; Alhusaini, H.; Alghamdi, A.; Al-Moghrabi, N. MicroRNA-126 expression in the peripheral white blood cells of patients with breast and ovarian cancer is a potential biomarker for the early prediction of cancer risk in the carriers of methylated BRCA1. Oncol. Lett. 2022, 24, 276. [Google Scholar] [CrossRef]
- Lonning, P.E.; Berge, E.O.; Bjornslett, M.; Minsaas, L.; Chrisanthar, R.; Hoberg-Vetti, H.; Dulary, C.; Busato, F.; Bjorneklett, S.; Eriksen, C.; et al. White Blood Cell BRCA1 Promoter Methylation Status and Ovarian Cancer Risk. Ann. Intern. Med. 2018, 168, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, T.; Yamamoto, N.; Taguchi, T.; Tamaki, Y.; Noguchi, S. BRCA1 promoter methylation in peripheral blood cells is associated with increased risk of breast cancer with BRCA1 promoter methylation. Breast Cancer Res. Treat. 2011, 129, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Vos, S.; van Diest, P.J.; Moelans, C.B. A systematic review on the frequency of BRCA promoter methylation in breast and ovarian carcinomas of BRCA germline mutation carriers. Mutually exclusive, or not? Crit. Rev. Oncol. Hematol. 2018, 127, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Daniels, D.S.; Woo, T.T.; Luu, K.X.; Noll, D.M.; Clarke, N.D.; Pegg, A.E.; Tainer, J.A. DNA binding and nucleotide flipping by the human DNA repair protein AGT. Nat. Struct. Mol. Biol. 2004, 11, 714–720. [Google Scholar] [CrossRef]
- Kim, J.I.; Suh, J.T.; Choi, K.U.; Kang, H.J.; Shin, D.H.; Lee, I.S.; Moon, T.Y.; Kim, W.T. Inactivation of O6-methylguanine-DNA methyltransferase in soft tissue sarcomas. association with K-ras mutations. Hum. Pathol. 2009, 40, 934–941. [Google Scholar] [CrossRef]
- Gerson, S.L. MGMT: Its role in cancer aetiology and cancer therapeutics. Nat. Rev. Cancer 2004, 4, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.H.; Yazici, H.; Wu, H.C.; Terry, M.B.; Gonzalez, K.; Qu, M.; Dalay, N.; Santella, R.M. Aberrant promoter hypermethylation and genomic hypomethylation in tumor, adjacent normal tissues and blood from breast cancer patients. Anticancer Res. 2010, 30, 2489–2496. [Google Scholar]
- Cho, Y.H.; McCullough, L.E.; Gammon, M.D.; Wu, H.C.; Zhang, Y.J.; Wang, Q.; Xu, X.; Teitelbaum, S.L.; Neugut, A.I.; Chen, J.; et al. Promoter Hypermethylation in White Blood Cell DNA and Breast Cancer Risk. J. Cancer 2015, 6, 819–824. [Google Scholar] [CrossRef]
- Chen, R.; Zheng, Y.; Zhuo, L.; Wang, S. Association between MGMT Promoter Methylation and Risk of Breast and Gynecologic Cancers: A Systematic Review and Meta-Analysis. Sci. Rep. 2017, 7, 12783. [Google Scholar] [CrossRef]
- Fumagalli, C.; Pruneri, G.; Possanzini, P.; Manzotti, M.; Barile, M.; Feroce, I.; Colleoni, M.; Bonanni, B.; Maisonneuve, P.; Radice, P.; et al. Methylation of O6-methylguanine-DNA methyltransferase (MGMT) promoter gene in triple-negative breast cancer patients. Breast Cancer Res. Treat. 2012, 134, 131–137. [Google Scholar] [CrossRef]
- Roh, H.J.; Suh, D.S.; Choi, K.U.; Yoo, H.J.; Joo, W.D.; Yoon, M.S. Inactivation of O(6)-methyguanine-DNA methyltransferase by promoter hypermethylation: Association of epithelial ovarian carcinogenesis in specific histological types. J. Obstet. Gynaecol. Res. 2011, 37, 851–860. [Google Scholar] [CrossRef]
- Al-Othman, S.; Haoudi, A.; Alhomoud, S.; Alkhenizan, A.; Khoja, T.; Al-Zahrani, A. Tackling cancer control in the Gulf Cooperation Council Countries. Lancet Oncol. 2015, 16, e246–e257. [Google Scholar] [CrossRef]
- Basudan, A.M. Breast Cancer Incidence Patterns in the Saudi Female Population: A 17-Year Retrospective Analysis. Medicina 2022, 58, 1617. [Google Scholar] [CrossRef] [PubMed]
- Nakai, K.; Hung, M.C.; Yamaguchi, H. A perspective on anti-EGFR therapies targeting triple-negative breast cancer. Am. J. Cancer Res. 2016, 6, 1609–1623. [Google Scholar] [PubMed]
- Clarke-Pearson, D.L. Clinical practice. Screening for ovarian cancer. N. Engl. J. Med. 2009, 361, 170–177. [Google Scholar] [CrossRef]
- Al-Moghrabi, N.; Nofel, A.; Al-Yousef, N.; Madkhali, S.; Bin Amer, S.M.; Alaiya, A.; Shinwari, Z.; Al-Tweigeri, T.; Karakas, B.; Tulbah, A.; et al. The molecular significance of methylated BRCA1 promoter in white blood cells of cancer-free females. BMC Cancer 2014, 14, 830. [Google Scholar] [CrossRef] [PubMed]
- Alhuqail, A.J.; Alzahrani, A.; Almubarak, H.; Al-Qadheeb, S.; Alghofaili, L.; Almoghrabi, N.; Alhussaini, H.; Park, B.H.; Colak, D.; Karakas, B. High prevalence of deleterious BRCA1 and BRCA2 germline mutations in arab breast and ovarian cancer patients. Breast Cancer Res. Treat. 2018, 168, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Anders, C.K.; Johnson, R.; Litton, J.; Phillips, M.; Bleyer, A. Breast cancer before age 40 years. Semin. Oncol. 2009, 36, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Azim, H.A., Jr.; Partridge, A.H. Biology of breast cancer in young women. Breast Cancer Res. 2014, 16, 427. [Google Scholar] [CrossRef] [PubMed]
- Narod, S.A. Breast cancer in young women. Nat. Rev. Clin. Oncol. 2012, 9, 460–470. [Google Scholar] [CrossRef]
- Agha, N.; Alshamsan, B.; Al-Farsi, S.; Ateya, H.A.; Almugbel, F.A.; Alotaibi, H.A.; Omar, A.; Mohamed, A.S.; Alharthy, H.; Elhassan, T.; et al. Assessing frequency and clinical outcomes of BRCA mutated ovarian cancer in Saudi women. BMC Cancer 2022, 22, 18. [Google Scholar] [CrossRef]
- Prajzendanc, K.; Domagala, P.; Hybiak, J.; Rys, J.; Huzarski, T.; Szwiec, M.; Tomiczek-Szwiec, J.; Redelbach, W.; Sejda, A.; Gronwald, J.; et al. BRCA1 promoter methylation in peripheral blood is associated with the risk of triple-negative breast cancer. Int. J. Cancer 2020, 146, 1293–1298. [Google Scholar] [CrossRef]
- Muhammad, N.; Azeem, A.; Bakar, M.A.; Prajzendanc, K.; Loya, A.; Jakubowska, A.; Hamann, U.; Rashid, M.U. Contribution of constitutional BRCA1 promoter methylation to early-onset and familial breast cancer patients from Pakistan. Breast Cancer Res. Treat. 2023, 202, 377–387. [Google Scholar] [CrossRef]
- Nikolaienko, O.; Eikesdal, H.P.; Ognedal, E.; Gilje, B.; Lundgren, S.; Blix, E.S.; Espelid, H.; Geisler, J.; Geisler, S.; Janssen, E.A.M.; et al. Prenatal BRCA1 epimutations contribute significantly to triple-negative breast cancer development. Genome Med. 2023, 15, 104. [Google Scholar] [CrossRef]
- Wu, B.; Qi, L.; Chiang, H.C.; Pan, H.; Zhang, X.; Greenbaum, A.; Stark, E.; Wang, L.J.; Chen, Y.; Haddad, B.R.; et al. BRCA1 deficiency in mature CD8(+) T lymphocytes impairs antitumor immunity. J. Immunother. Cancer 2023, 11, e005852. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wei, W.; Jiang, Y.I.; Yang, H.; Liu, J. Promoter methylation and expression changes of BRCA1 in cancerous tissues of patients with sporadic breast cancer. Oncol. Lett. 2015, 9, 1807–1813. [Google Scholar] [CrossRef] [PubMed]
- Rice, J.C.; Ozcelik, H.; Maxeiner, P.; Andrulis, I.; Futscher, B.W. Methylation of the BRCA1 promoter is associated with decreased BRCA1 mRNA levels in clinical breast cancer specimens. Carcinogenesis 2000, 21, 1761–1765. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Fu, Y.; Xue, H.; Guo, K.; Song, Z.; Yu, Z.; Jia, T.; Yan, Y.; Zhao, L.; Mi, X.; et al. BRCA1 promoter hypermethylation in sporadic epithelial ovarian carcinoma. Association with low expression of BRCA1, improved survival and co-expression of DNA methyltransferases. Oncol. Lett. 2014, 7, 1088–1096. [Google Scholar] [CrossRef]
- Munot, K.; Bell, S.M.; Lane, S.; Horgan, K.; Hanby, A.M.; Speirs, V. Pattern of expression of genes linked to epigenetic silencing in human breast cancer. Hum. Pathol. 2006, 37, 989–999. [Google Scholar] [CrossRef]
- An, J.; Wei, Q.; Liu, Z.; Lu, K.H.; Cheng, X.; Mills, G.B.; Wang, L.E. Messenger RNA expression and methylation of candidate tumor-suppressor genes and risk of ovarian cancer-a case-control analysis. Int. J. Mol. Epidemiol. Genet. 2010, 1, 1–10. [Google Scholar]
- Al-Yousef, N.; Shinwari, Z.; Al-Shahrani, B.; Al-Showimi, M.; Al-Moghrabi, N. Curcumin induces re-expression of BRCA1 and suppression of gamma synuclein by modulating DNA promoter methylation in breast cancer cell lines. Oncol. Rep. 2020, 43, 827–838. [Google Scholar] [PubMed]
- Bhattacharyya, S.; Mandal, D.; Saha, B.; Sen, G.S.; Das, T.; Sa, G. Curcumin prevents tumor-induced T cell apoptosis through Stat-5a-mediated Bcl-2 induction. J. Biol. Chem. 2007, 282, 15954–15964. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]

| Meth (n%) | Unmeth (n%) | p Value, OR (95% CI) | |
|---|---|---|---|
| (A) | |||
| Control (n = 827) | 71 (8.6) | 756 (91,4) | |
| BC (n = 908) | 100 (11) | 808 (89) | 0.104, 1.32 (0.96, 1.18) |
| Age | |||
| <50 n = 492 | 68 (13.8) | 424 (86.2) | 0.003, 1.92 (1.24, 2.99) |
| ≥50 n = 416 | 32 (7.7) | 384 (92.3) | |
| TNBC n = 144 | 29 (20) | 115 (80) | 0.0001, 2.68 (1.64, 4.31) |
| (B) | |||
| Control (n = 611) | 61 (10) | 550 (90) | |
| BC (n = 700) | 122 (17.4) | 578 (82.6) | 0.0003, 1.9 (1.36, 2.64) |
| Age | |||
| <50 n = 37 | 35 (9.5) | 335 (90.5) | |
| ≥50 n = 330 | 87 (26.4) | 243 (73.6) | 0.0001, 3.42 (2.23, 5.24) |
| TNBC n = 110 | 12 (11) | 98 (89) | 0.153, 1.53 (0.85, 2.77) |
| Meth (n%) | Unmeth (n%) | p Value, OR (95% CI) | |
|---|---|---|---|
| (A) | |||
| Control (n = 827) | 71 (8.6) | 756 (91.4) | |
| OC (n = 223) | 32 (14) | 191 (86) | 0.011, 1.78 (1.14, 2.78) |
| Age | |||
| <55 n = 103 | 20 (19.4) | 83 (80.6) | 0.0007, 2.56 (1.48, 4.4) |
| ≥55 n = 120 | 12 (10) | 108 (90) | |
| HGSOC n = 47 | 11 (23.4) | 36 | 0.001, 3.25 (1.58, 6.68) |
| (B) | |||
| Control (n = 611) | 61 (10) | 550 (90) | |
| OC (n = 223) | 34 (15.2) | 189 (84.8) | 0.034, 1.62 (1.03, 2.54) |
| Age | |||
| <55 n = 103 | 16 (15.5) | 87 (84.5) | |
| ≥55 n = 120 | 17 (14) | 103 (86) | |
| HGSOC (n = 47) | 9 (19.1) | 38 | 0.054, 2.13 (0.98, 4.62) |
| (C) | |||
| Mut (n%) | WT (n%) | p Value, OR (95% CI) | |
| OC (n = 167) | 40 (24) | 127 (76) | |
| Age | |||
| <55 n = 72 | 14 (19.4) | 58 (80.5) | 0.236, 1.56 (0.74, 3.26) |
| ≥55 n = 95 | 26 (27.4) | 69 (27.6) | |
| HGSOC (n = 45) | 12 (26.7%) | 33 | |
| Sample # | Age | Affected FM | Type of Cancer |
|---|---|---|---|
| (A) | |||
| 162 | 42 | Grandmother | BC at age 70 |
| 165 | 50 | Sister | BC |
| 199 | 52 | Mother | BC |
| 235 | 31 | Cousin | BC |
| 237 | 48 | Cousin | BC |
| 315 | 64 | Sister | BC |
| 329 | 59 | Mother, Sister, Aunt | BC |
| 409 | 65 | Sister | BC |
| 547 | 59 | Sister | BC |
| 573 | 20 | ND | FH of BC |
| 617 | 39 | Sister | BC |
| 642 | 29 | Cousin | BC |
| 390 | 55 | Mother | OC |
| 172 | 46 | Mother | Jaw cancer |
| 181 | 34 | Mother | Thyroid cancer |
| 275 | 61 | ND | Bone and Lung cancer |
| 429 | 65 | Mother | Oropharyngeal cancer |
| 587 | 44 | Father | Urinary bladder cancer |
| 605 | 63 | Sister | ND |
| 650 | 74 | Daughter | Colon cancer |
| (B) | |||
| 60 | 61 | 2 cousins | Breast and Uterine cancer |
| 123 | 62 | Sister | BC |
| 104 | 50 | 2 cousins | BC |
| 30 | 46 | Sister | Cervical cancer |
| 31 | 53 | Aunt | ND |
| 122 | 63 | Son | Benign tumor in neck |
| 132 | 69 | Father | Brain cancer |
| Sample # | Age | Affected FM | Type of Cancer |
|---|---|---|---|
| (A) | |||
| 136 | 51 | FH in 2 of 2nd generation | BC |
| 212 | 69 | Cousin | BC |
| 341 | 60 | Sister | BC |
| 352 | 58 | Mother | BC at old age |
| 514 | 60 | Sister | BC |
| 638 | 66 | Daughter | BC |
| 566 | 39 | Grandma | BC |
| 646 | 64 | FH | BC |
| 559 | 76 | Cousin | BC |
| 625 | 41 | Mother | BC |
| 689 | 60 | Aunt | BC at old age |
| Uncle | Pancreatic cancer | ||
| 702 | 76 | Mother | BC |
| Cousin | Oropharyngeal cancer | ||
| 712 | 49 | Mother | BC at 70 years old |
| 733 | 78 | Cousin | BC |
| 353 | 52 | Sister | Uterine cancer |
| 711 | 65 | Sister | Endometrial cancer |
| Father | Neck and prostate cancer | ||
| 28 | 47 | Father | ND |
| 39 | 62 | FH | Liver cancer |
| Cousin | Colon cancer | ||
| 167 | 52 | Uncle | Thyroid cancer |
| 200 | 59 | Mother | Bowel cancer |
| 428 | 51 | Mother | Thyroid cancer |
| 464 | 68 | Brother | Prostate cancer |
| 465 | 53 | Brother | Bladder cancer |
| 511 | 59 | Sister | Bone marrow cancer |
| 522 | 67 | Daughter | Spinal cancer |
| Brother | Liver and prostate cancer | ||
| 556 | 58 | Uncle | Colon cancer |
| 562 | 63 | Cousin | Abdominal cancer |
| 569 | 35 | Father | Renal cancer |
| Uncle | Bladder cancer | ||
| 616 | 89 | Mother | Pancreatic cancer |
| 619 | 65 | Brother | Colon cancer |
| 659 | 50 | Mother | Oral cavity cancer |
| (B) | |||
| 53 | 73 | Mother | Uterine cancer |
| 55 | 55 | Son | Thyroid cancer |
| 59 | 52 | Sister | Uterine cancer |
| 79 | 64 | Sister (1) | Endometrial and thyroid cancer |
| Sister (2) | Colon Cancer | ||
| 163 | 61 | Two brothers | Colon cancer |
| Primer Name | Primer Sequence | Annealing Temp |
|---|---|---|
| RT BRCA1 | F5′-TGTAGGCTCCTTTTGGTTATATCATTC-3′ R5′-CATGCTGAAACTTCTCAACCAGAA-3′ | 59 °C |
| β-Actin | F5′-TCCCTGGAGAAGAGCTACGA-3′ R5′-TGAAGGTAGTTTCGTGGATGC-3′ | 59 °C |
| RT MGMT | F5′-GCGTTCGACGTTCGTAGGT-3′ R5′-CACTCTTCCGAAAACGAACG-3′ | 60 °C |
| F5′-AAACTGGAACGGTGAAGG TG-3′ | ||
| β-Actin | R5′-AGTGGGGTGGCTTTTAGGAT-3′ | 60 °C |
| M BRCA1 | F5′-GGTTAATTTAGAGTTTCGAGAGACG-3′ | |
| R5′-TCAACGAACTCACGCCGCGCAATCG-3′ | 65 °C | |
| UM BRCA1 | F5′-GGTTAATTTAGAGTTTTGAGAGATG-3′ | |
| R5′-TCAACAAACTCACACCACACAATCA-3′ | 65 °C | |
| M MGMT | F5′-TTTCGACGTTCGTAGGTTTTCGC-3′ | |
| R5′-GCACTCTTCCGAAAACGAAACG-3′ | 59 °C | |
| UM MGMT | F5′-TTTGTGTTTTGATGTTTGTAGGTTTTTGT-3′ | |
| R5′-AACTCCACACTCTTCCAAAAACAAAACA-3′ | 59 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Moghrabi, N.; Al-Showimi, M.; Alqahtani, A.; Almalik, O.; Alhusaini, H.; Almalki, G.; Saad, A.; Alsunayi, E. Constitutional BRCA1 and MGMT Methylation Are Significant Risk Factors for Triple-Negative Breast Cancer and High-Grade Serous Ovarian Cancer in Saudi Women. Int. J. Mol. Sci. 2024, 25, 3108. https://doi.org/10.3390/ijms25063108
Al-Moghrabi N, Al-Showimi M, Alqahtani A, Almalik O, Alhusaini H, Almalki G, Saad A, Alsunayi E. Constitutional BRCA1 and MGMT Methylation Are Significant Risk Factors for Triple-Negative Breast Cancer and High-Grade Serous Ovarian Cancer in Saudi Women. International Journal of Molecular Sciences. 2024; 25(6):3108. https://doi.org/10.3390/ijms25063108
Chicago/Turabian StyleAl-Moghrabi, Nisreen, Maram Al-Showimi, Amal Alqahtani, Osama Almalik, Hamed Alhusaini, Ghdah Almalki, Ajawhara Saad, and Elaf Alsunayi. 2024. "Constitutional BRCA1 and MGMT Methylation Are Significant Risk Factors for Triple-Negative Breast Cancer and High-Grade Serous Ovarian Cancer in Saudi Women" International Journal of Molecular Sciences 25, no. 6: 3108. https://doi.org/10.3390/ijms25063108
APA StyleAl-Moghrabi, N., Al-Showimi, M., Alqahtani, A., Almalik, O., Alhusaini, H., Almalki, G., Saad, A., & Alsunayi, E. (2024). Constitutional BRCA1 and MGMT Methylation Are Significant Risk Factors for Triple-Negative Breast Cancer and High-Grade Serous Ovarian Cancer in Saudi Women. International Journal of Molecular Sciences, 25(6), 3108. https://doi.org/10.3390/ijms25063108







