Silymarin Synergizes with Antiviral Therapy in Hepatitis B Virus-Related Liver Cirrhosis: A Propensity Score Matching Multi-Institutional Study
Abstract
1. Introduction
2. Results
2.1. Flowchart
2.2. Clinical Characteristics in the Two Propensity Score-Matched Cohorts
2.3. Comparison of the Cumulative Duration of Study Medication, Follow-Up Time, and Primary and Secondary Outcomes of the Two Cohorts
2.4. Comparing Laboratory Parameters and Clinical Indices of the Two Cohorts
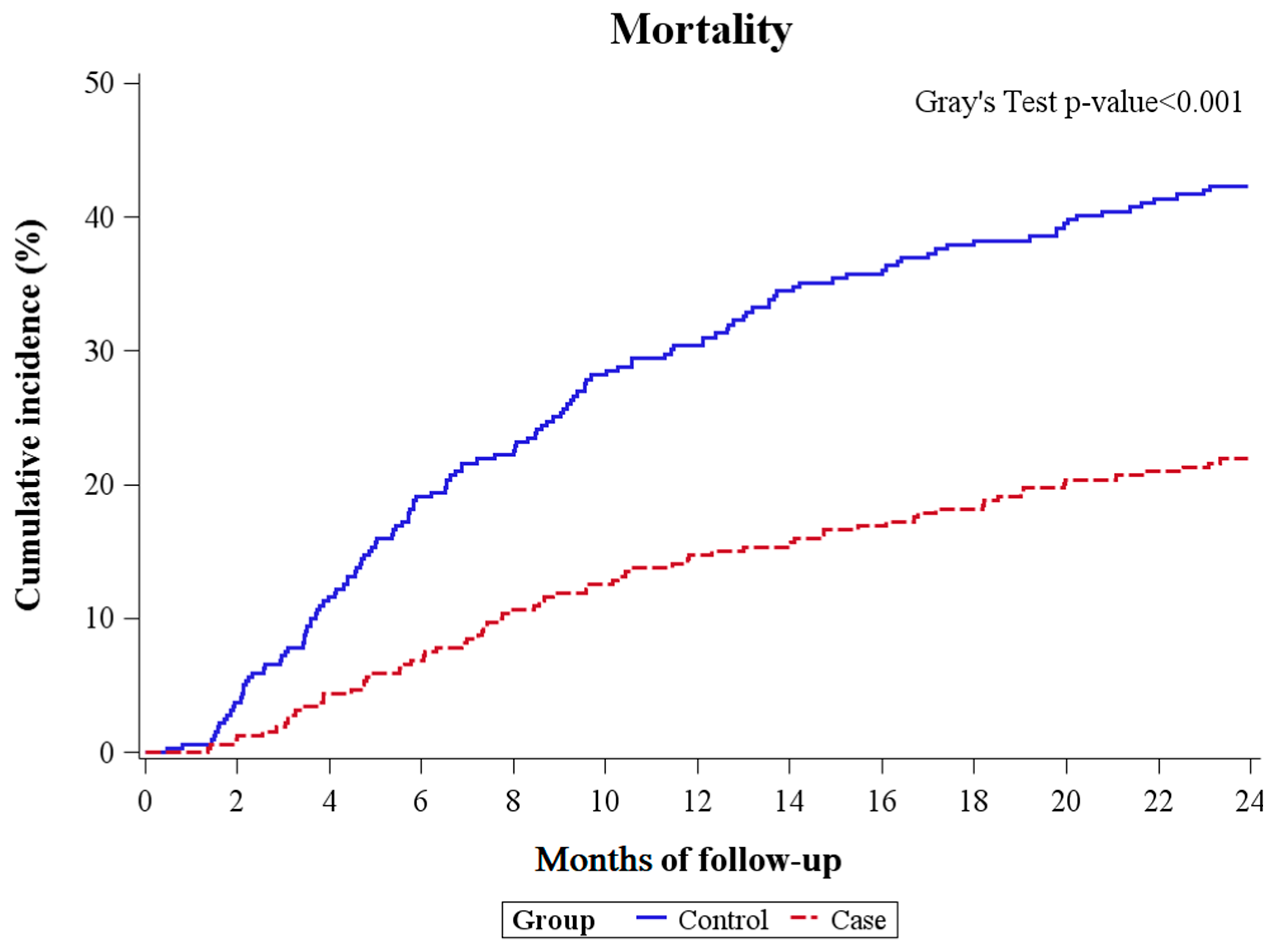
2.5. Competing Risk Analysis for the Primary Outcome
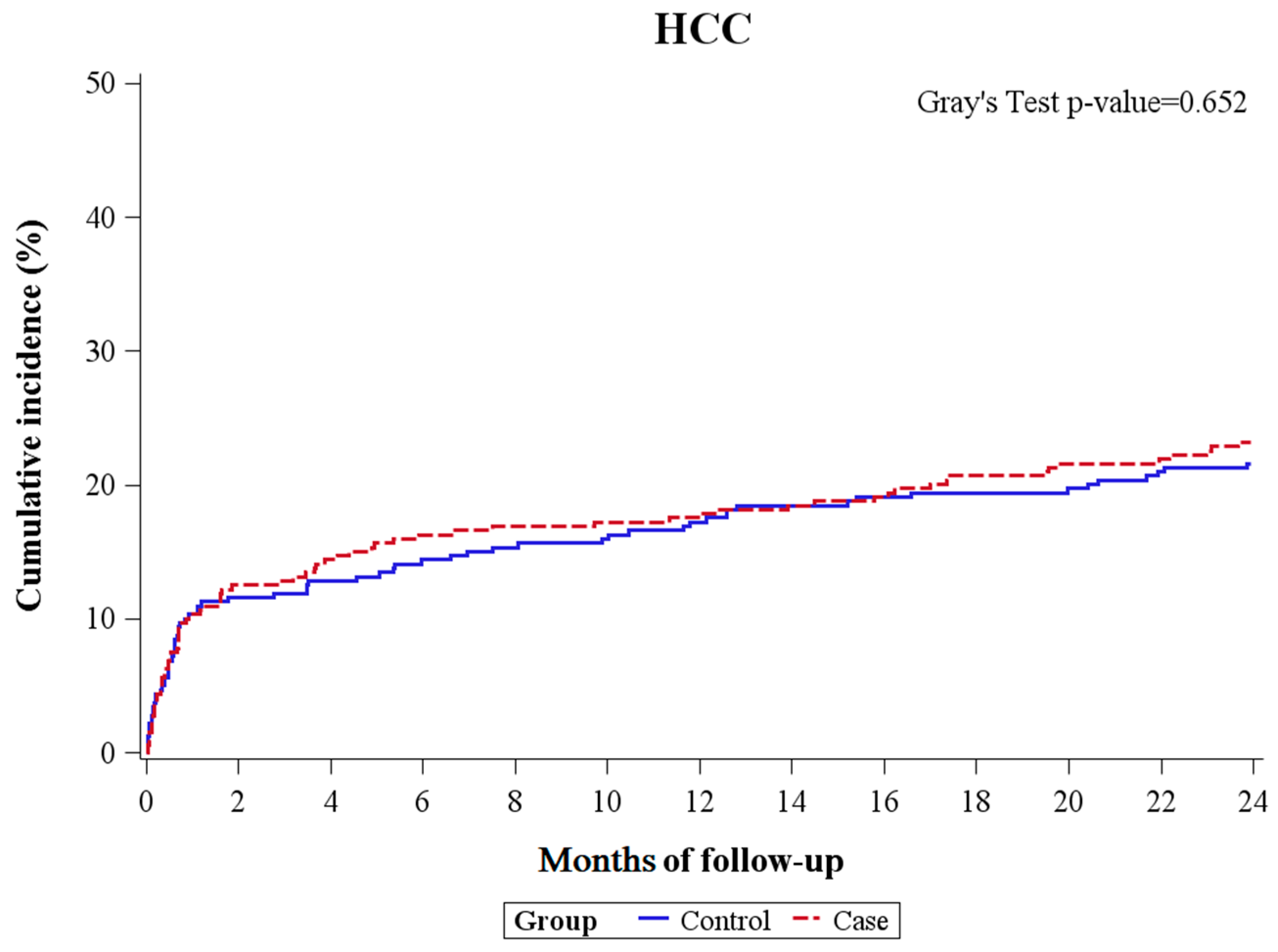
2.6. Competing Risk Analysis for the Secondary Outcome
3. Discussion
4. Materials and Methods
4.1. Study Design and Data Source
4.2. Study Population
4.3. Diagnostic Criteria for HBV-Related Liver Cirrhosis with AVT
4.4. Index Date and Follow-Up Time
4.5. Calculation of Medication Duration
4.6. Propensity Score Matching
4.7. Primary and Secondary Outcomes
4.8. Asessing the Magnitude of Change in Laboratory Parameters, Clinical Index, and Charlson Comorbidity Index
4.9. Statistical Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liaw, Y.F.; Chu, C.M. Hepatitis B virus infection. Lancet 2009, 373, 582–592. [Google Scholar] [CrossRef]
- Kaur, S.P.; Talat, A.; Karimi-Sari, H.; Grees, A.; Chen, H.W.; Lau, D.T.Y.; Catana, A.M. Hepatocellular Carcinoma in Hepatitis B Virus-Infected Patients and the Role of Hepatitis B Surface Antigen (HBsAg). J. Clin. Med. 2022, 11, 1126. [Google Scholar] [CrossRef] [PubMed]
- Razavi-Shearer, D.; Gamkrelidze, I.; Nguyen, M.H.; Chen, D.S.; Van Damme, P.; Abbas, Z.; Abdulla, M.; Abou Rached, A.; Adda, D.; Aho, I.; et al. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: A modelling study. Lancet Gastroenterol. Hepatol. 2018, 3, 383–403. [Google Scholar] [CrossRef] [PubMed]
- Chien, R.N.; Liaw, Y.F. Current Trend in Antiviral Therapy for Chronic Hepatitis B. Viruses 2022, 14, 434. [Google Scholar] [CrossRef]
- Liu, C.J.; Chen, P.J. Elimination of Hepatitis B in Highly Endemic Settings: Lessons Learned in Taiwan and Challenges Ahead. Viruses 2020, 12, 815. [Google Scholar] [CrossRef]
- Schuppan, D.; Afdhal, N.H. Liver cirrhosis. Lancet 2008, 371, 838–851. [Google Scholar] [CrossRef]
- Liaw, Y.F.; Sung, J.J.; Chow, W.C.; Farrell, G.; Lee, C.Z.; Yuen, H.; Tanwandee, T.; Tao, Q.M.; Shue, K.; Keene, O.N.; et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N. Engl. J. Med. 2004, 351, 1521–1531. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.L.; Chan, H.L.; Mak, C.W.; Lee, S.K.; Ip, Z.M.; Lam, A.T.; Iu, H.W.; Leung, J.M.; Lai, J.W.; Lo, A.O.; et al. Entecavir treatment reduces hepatic events and deaths in chronic hepatitis B patients with liver cirrhosis. Hepatology 2013, 58, 1537–1547. [Google Scholar] [CrossRef]
- Peng, C.Y.; Chien, R.N.; Liaw, Y.F. Hepatitis B virus-related decompensated liver cirrhosis: Benefits of antiviral therapy. J. Hepatol. 2012, 57, 442–450. [Google Scholar] [CrossRef]
- Jeng, W.J.; Papatheodoridis, G.V.; Lok, A.S.F. Hepatitis B. Lancet 2023, 401, 1039–1052. [Google Scholar] [CrossRef]
- Umetsu, T.; Inoue, J.; Kogure, T.; Kakazu, E.; Ninomiya, M.; Iwata, T.; Takai, S.; Nakamura, T.; Sano, A.; Shimosegawa, T. Inhibitory effect of silibinin on hepatitis B virus entry. Biochem. Biophys. Rep. 2018, 14, 20–25. [Google Scholar] [CrossRef]
- Li, G.; Zhu, Y.; Shao, D.; Chang, H.; Zhang, X.; Zhou, D.; Gao, Y.; Lan, K.; Deng, Q. Recombinant covalently closed circular DNA of hepatitis B virus induces long-term viral persistence with chronic hepatitis in a mouse model. Hepatology 2018, 67, 56–70. [Google Scholar] [CrossRef]
- Nassal, M. HBV cccDNA: Viral persistence reservoir and key obstacle for a cure of chronic hepatitis B. Gut. 2015, 64, 1972–1984. [Google Scholar] [CrossRef]
- Lee, H.W.; Yip, T.C.; Tse, Y.K.; Wong, G.L.; Kim, B.K.; Kim, S.U.; Park, J.Y.; Kim, D.Y.; Chan, H.L.; Ahn, S.H.; et al. Hepatic Decompensation in Cirrhotic Patients Receiving Antiviral Therapy for Chronic Hepatitis B. Clin. Gastroenterol. Hepatol. 2021, 19, 1950–1958.e7. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Tsai, M.H.; Lin, C.Y. Identifying the pivotal role of non-acute decompensation as a precursor to acute decompensation in cirrhosis. J. Hepatol. 2024. [Google Scholar] [CrossRef]
- Tonon, M.; D’Ambrosio, R.; Calvino, V.; Tosetti, G.; Barone, A.; Incicco, S.; Gambino, C.; Gagliardi, R.; Borghi, M.; Zeni, N.; et al. A new clinical and prognostic characterization of the patterns of decompensation of cirrhosis. J. Hepatol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.W.; Choi, J.Y.; Kim, Y.S.; Woo, H.Y.; Choi, S.K.; Lee, C.H.; Kim, T.Y.; Sohn, J.H.; Tak, W.Y.; Han, K.H. Long-term effect of antiviral therapy on disease course after decompensation in patients with hepatitis B virus-related cirrhosis. Hepatology 2015, 61, 1809–1820. [Google Scholar] [CrossRef] [PubMed]
- Fontana, R.J.; Hann, H.W.; Perrillo, R.P.; Vierling, J.M.; Wright, T.; Rakela, J.; Anschuetz, G.; Davis, R.; Gardner, S.D.; Brown, N.A. Determinants of early mortality in patients with decompensated chronic hepatitis B treated with antiviral therapy. Gastroenterology 2002, 123, 719–727. [Google Scholar] [CrossRef]
- Lee, H.A.; Lee, Y.S.; Jung, Y.K.; Kim, J.H.; Yim, H.J.; Yeon, J.E.; Seo, Y.S.; Lee, J.S.; Lee, H.W.; Kim, B.K.; et al. The clinical effect of antiviral therapy in patients with hepatitis B virus-related decompensated cirrhosis and undetectable DNA. J. Gastroenterol. Hepatol. 2023, 38, 716–723. [Google Scholar] [CrossRef]
- Mayer, K.E.; Myers, R.P.; Lee, S.S. Silymarin treatment of viral hepatitis: A systematic review. J. Viral Hepat. 2005, 12, 559–567. [Google Scholar] [CrossRef]
- Mastron, J.K.; Siveen, K.S.; Sethi, G.; Bishayee, A. Silymarin and hepatocellular carcinoma: A systematic, comprehensive, and critical review. Anticancer Drugs 2015, 26, 475–486. [Google Scholar] [CrossRef]
- Kisseleva, T.; Brenner, D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 151–166. [Google Scholar] [CrossRef]
- Surai, P.F. Silymarin as a Natural Antioxidant: An Overview of the Current Evidence and Perspectives. Antioxidants 2015, 4, 204–247. [Google Scholar] [CrossRef]
- Kim, M.; Yang, S.G.; Kim, J.M.; Lee, J.W.; Kim, Y.S.; Lee, J.I. Silymarin suppresses hepatic stellate cell activation in a dietary rat model of non-alcoholic steatohepatitis: Analysis of isolated hepatic stellate cells. Int. J. Mol. Med. 2012, 30, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Mendoza, N.; Madrigal-Santillan, E.; Morales-Gonzalez, A.; Esquivel-Soto, J.; Esquivel-Chirino, C.; Garcia-Luna, Y.G.-R.M.; Gayosso-de-Lucio, J.A.; Morales-Gonzalez, J.A. Hepatoprotective effect of silymarin. World J. Hepatol. 2014, 6, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.L.; Liaw, Y.F. Hepatitis B flares in chronic hepatitis B: Pathogenesis, natural course, and management. J. Hepatol. 2014, 61, 1407–1417. [Google Scholar] [CrossRef] [PubMed]
- Tapper, E.B.; Parikh, N.D. Mortality due to cirrhosis and liver cancer in the United States, 1999–2016: Observational study. BMJ 2018, 362, k2817. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Liu, S.K.; Liu, X.Y.; Li, Z.J.; Li, B.; Zhou, Y.L.; Zhang, H.Y.; Li, Y.W. Meta-analysis: Silymarin and its combination therapy for the treatment of chronic hepatitis B. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Liaw, Y.F.; Leung, N.; Guan, R.; Lau, G.K.; Merican, I.; McCaughan, G.; Gane, E.; Kao, J.H.; Omata, M.; Asian-Pacific Consensus Update Working Party on Chronic Hepatitis B. Asian-Pacific consensus statement on the management of chronic hepatitis B: A 2005 update. Liver Int. 2005, 25, 472–489. [Google Scholar] [CrossRef] [PubMed]
- Liaw, Y.F.; Leung, N.; Kao, J.H.; Piratvisuth, T.; Gane, E.; Han, K.H.; Guan, R.; Lau, G.K.; Locarnini, S.; Chronic Hepatitis, B.; et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: A 2008 update. Hepatol. Int. 2008, 2, 263–283. [Google Scholar] [CrossRef]
- Liaw, Y.F.; Kao, J.H.; Piratvisuth, T.; Chan, H.L.; Chien, R.N.; Liu, C.J.; Gane, E.; Locarnini, S.; Lim, S.G.; Han, K.H.; et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: A 2012 update. Hepatol. Int. 2012, 6, 531–561. [Google Scholar] [CrossRef] [PubMed]
- Sarin, S.K.; Kumar, M.; Lau, G.K.; Abbas, Z.; Chan, H.L.; Chen, C.J.; Chen, D.S.; Chen, H.L.; Chen, P.J.; Chien, R.N.; et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: A 2015 update. Hepatol. Int. 2016, 10, 1–98. [Google Scholar] [CrossRef] [PubMed]
- Jeng, W.J.; Liaw, Y.F. Erratum to: Finite Antiviral Therapy in Chronic Hepatitis B Patients with Cirrhosis. Semin. Liver Dis. 2021, 41, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Chien, R.N.; Kao, J.H.; Peng, C.Y.; Chen, C.H.; Liu, C.J.; Huang, Y.H.; Hu, T.H.; Yang, H.I.; Lu, S.N.; Ni, Y.H.; et al. Taiwan consensus statement on the management of chronic hepatitis B. J. Formos. Med. Assoc. 2019, 118, 7–38. [Google Scholar] [CrossRef]
- Ahmed, S.; Ullah, N.; Parveen, S.; Javed, I.; Jalil, N.A.C.; Murtey, M.D.; Sheikh, I.S.; Khan, S.; Ojha, S.C.; Chen, K. Effect of Silymarin as an Adjunct Therapy in Combination with Sofosbuvir and Ribavirin in Hepatitis C Patients: A Miniature Clinical Trial. Oxid. Med. Cell. Longev. 2022, 2022, 9199190. [Google Scholar] [CrossRef]
- Jacobs, B.P.; Dennehy, C.; Ramirez, G.; Sapp, J.; Lawrence, V.A. Milk thistle for the treatment of liver disease: A systematic review and meta-analysis. Am. J. Med. 2002, 113, 506–515. [Google Scholar] [CrossRef]
- Gillessen, A.; Schmidt, H.H. Silymarin as Supportive Treatment in Liver Diseases: A Narrative Review. Adv. Ther. 2020, 37, 1279–1301. [Google Scholar] [CrossRef]
- Polyak, S.J.; Morishima, C.; Shuhart, M.C.; Wang, C.C.; Liu, Y.; Lee, D.Y. Inhibition of T-cell inflammatory cytokines, hepatocyte NF-kappaB signaling, and HCV infection by standardized Silymarin. Gastroenterology 2007, 132, 1925–1936. [Google Scholar] [CrossRef]
- Polyak, S.J.; Morishima, C.; Lohmann, V.; Pal, S.; Lee, D.Y.; Liu, Y.; Graf, T.N.; Oberlies, N.H. Identification of hepatoprotective flavonolignans from silymarin. Proc. Natl. Acad. Sci. USA 2010, 107, 5995–5999. [Google Scholar] [CrossRef]
- Agarwal, R.; Agarwal, C.; Ichikawa, H.; Singh, R.P.; Aggarwal, B.B. Anticancer potential of silymarin: From bench to bed side. Anticancer Res. 2006, 26, 4457–4498. [Google Scholar]
- Polyak, S.J.; Ferenci, P.; Pawlotsky, J.M. Hepatoprotective and antiviral functions of silymarin components in hepatitis C virus infection. Hepatology 2013, 57, 1262–1271. [Google Scholar] [CrossRef] [PubMed]
- Gazak, R.; Walterova, D.; Kren, V. Silybin and silymarin--new and emerging applications in medicine. Curr. Med. Chem. 2007, 14, 315–338. [Google Scholar] [CrossRef]
- Morishima, C.; Shuhart, M.C.; Wang, C.C.; Paschal, D.M.; Apodaca, M.C.; Liu, Y.; Sloan, D.D.; Graf, T.N.; Oberlies, N.H.; Lee, D.Y.; et al. Silymarin inhibits in vitro T-cell proliferation and cytokine production in hepatitis C virus infection. Gastroenterology 2010, 138, 671–681.e2. [Google Scholar] [CrossRef] [PubMed]
- Wagoner, J.; Negash, A.; Kane, O.J.; Martinez, L.E.; Nahmias, Y.; Bourne, N.; Owen, D.M.; Grove, J.; Brimacombe, C.; McKeating, J.A.; et al. Multiple effects of silymarin on the hepatitis C virus lifecycle. Hepatology 2010, 51, 1912–1921. [Google Scholar] [CrossRef] [PubMed]
- Ahmed-Belkacem, A.; Ahnou, N.; Barbotte, L.; Wychowski, C.; Pallier, C.; Brillet, R.; Pohl, R.T.; Pawlotsky, J.M. Silibinin and related compounds are direct inhibitors of hepatitis C virus RNA-dependent RNA polymerase. Gastroenterology 2010, 138, 1112–1122. [Google Scholar] [CrossRef]
- Payer, B.A.; Reiberger, T.; Rutter, K.; Beinhardt, S.; Staettermayer, A.F.; Peck-Radosavljevic, M.; Ferenci, P. Successful HCV eradication and inhibition of HIV replication by intravenous silibinin in an HIV-HCV coinfected patient. J. Clin. Virol. 2010, 49, 131–133. [Google Scholar] [CrossRef]
- Federico, A.; Dallio, M.; Loguercio, C. Silymarin/Silybin and Chronic Liver Disease: A Marriage of Many Years. Molecules 2017, 22, 191. [Google Scholar] [CrossRef]
- Nam, J.Y.; Lee, J.H.; Kim, H.Y.; Kim, J.E.; Lee, D.H.; Chang, Y.; Cho, H.; Yoo, J.J.; Lee, M.; Cho, Y.Y.; et al. Oral Medications Enhance Adherence to Surveillance for Hepatocellular Carcinoma and Survival in Chronic Hepatitis B Patients. PLoS ONE 2017, 12, e0166188. [Google Scholar] [CrossRef]
- Ferenci, P.; Dragosics, B.; Dittrich, H.; Frank, H.; Benda, L.; Lochs, H.; Meryn, S.; Base, W.; Schneider, B. Randomized controlled trial of silymarin treatment in patients with cirrhosis of the liver. J. Hepatol. 1989, 9, 105–113. [Google Scholar] [CrossRef]
- Su, T.H.; Hu, T.H.; Chen, C.Y.; Huang, Y.H.; Chuang, W.L.; Lin, C.C.; Wang, C.C.; Su, W.W.; Chen, M.Y.; Peng, C.Y.; et al. Four-year entecavir therapy reduces hepatocellular carcinoma, cirrhotic events and mortality in chronic hepatitis B patients. Liver Int. 2016, 36, 1755–1764. [Google Scholar] [CrossRef]
- Garg, H.; Sarin, S.K.; Kumar, M.; Garg, V.; Sharma, B.C.; Kumar, A. Tenofovir improves the outcome in patients with spontaneous reactivation of hepatitis B presenting as acute-on-chronic liver failure. Hepatology 2011, 53, 774–780. [Google Scholar] [CrossRef]
- Chen, C.J.; Iloeje, U.H.; Yang, H.I. Long-term outcomes in hepatitis B: The REVEAL-HBV study. Clin. Liver Dis. 2007, 11, 797–816. [Google Scholar] [CrossRef]
- Chen, C.J.; Yang, H.I.; Su, J.; Jen, C.L.; You, S.L.; Lu, S.N.; Huang, G.T.; Iloeje, U.H.; Group, R.-H.S. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 2006, 295, 65–73. [Google Scholar] [CrossRef]
- National Toxicology, P. Toxicology and carcinogenesis studies of milk thistle extract (CAS No. 84604-20-6) in F344/N rats and B6C3F1 mice (Feed Studies). Natl. Toxicol. Program Tech. Rep. Ser. 2011, 565, 1–177. [Google Scholar]
- Tarao, K.; Nozaki, A.; Ikeda, T.; Sato, A.; Komatsu, H.; Komatsu, T.; Taguri, M.; Tanaka, K. Real impact of liver cirrhosis on the development of hepatocellular carcinoma in various liver diseases-meta-analytic assessment. Cancer Med. 2019, 8, 1054–1065. [Google Scholar] [CrossRef] [PubMed]
- Cortesi, P.A.; Fornari, C.; Conti, S.; Antonazzo, I.C.; Ferrara, P.; Ahmed, A.; Andrei, C.L.; Andrei, T.; Artamonov, A.A.; Banach, M.; et al. Hepatitis B and C in Europe: An update from the Global Burden of Disease Study 2019. Lancet. Public. Health. 2023, 8, e701–e716. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yuan, A.J.; Wu, Y.J.; Wu, L.M.; Zhang, L. Silymarin in cancer therapy: Mechanisms of action, protective roles in chemotherapy-induced toxicity, and nanoformulations. J. Funct. Foods 2023, 100, 105384. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Fuertes-Agudo, M.; Luque-Tevar, M.; Cucarella, C.; Martin-Sanz, P.; Casado, M. Advances in Understanding the Role of NRF2 in Liver Pathophysiology and Its Relationship with Hepatic-Specific Cyclooxygenase-2 Expression. Antioxidants 2023, 12, 1491. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Yuan, W.G.; He, P.; Lei, J.H.; Wang, C.X. Liver fibrosis and hepatic stellate cells: Etiology, pathological hallmarks and therapeutic targets. World J. Gastroenterol. 2016, 22, 10512–10522. [Google Scholar] [CrossRef] [PubMed]
- Freedman, N.D.; Curto, T.M.; Morishima, C.; Seeff, L.B.; Goodman, Z.D.; Wright, E.C.; Sinha, R.; Everhart, J.E.; Group, H.-C.T. Silymarin use and liver disease progression in the Hepatitis C Antiviral Long-Term Treatment against Cirrhosis trial. Aliment. Pharmacol. Ther. 2011, 33, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.S.; Lin, M.H.; Lee, C.P.; Yang, Y.H.; Chen, W.C.; Chang, G.H.; Tsai, Y.T.; Chen, P.C.; Tsai, Y.H. Chang Gung Research Database: A multi-institutional database consisting of original medical records. Biomed. J. 2017, 40, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.L.; Huang, C.H.; Wu, V.C.; Wu, C.L.; Huang, Y.T.; Chang, S.H. Prognostic Effects of Liver Fibrosis and Steatosis Determined Using Transient Elastography in Patients with Chronic Hepatitis B or C. Dig. Dis. Sci. 2023, 68, 2747–2756. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.C.; Chan, Y.Y.; Kao Yang, Y.H.; Lin, S.J.; Hung, M.J.; Chien, R.N.; Lai, C.C.; Lai, E.C. The Chang Gung Research Database-A multi-institutional electronic medical records database for real-world epidemiological studies in Taiwan. Pharmacoepidemiol. Drug Saf. 2019, 28, 593–600. [Google Scholar] [CrossRef]
- Kohutek, F.; Bystricky, B. Optimal dose of silymarin for the management of drug-induced liver injury in oncology. Mol. Clin. Oncol. 2023, 18, 35. [Google Scholar] [CrossRef]
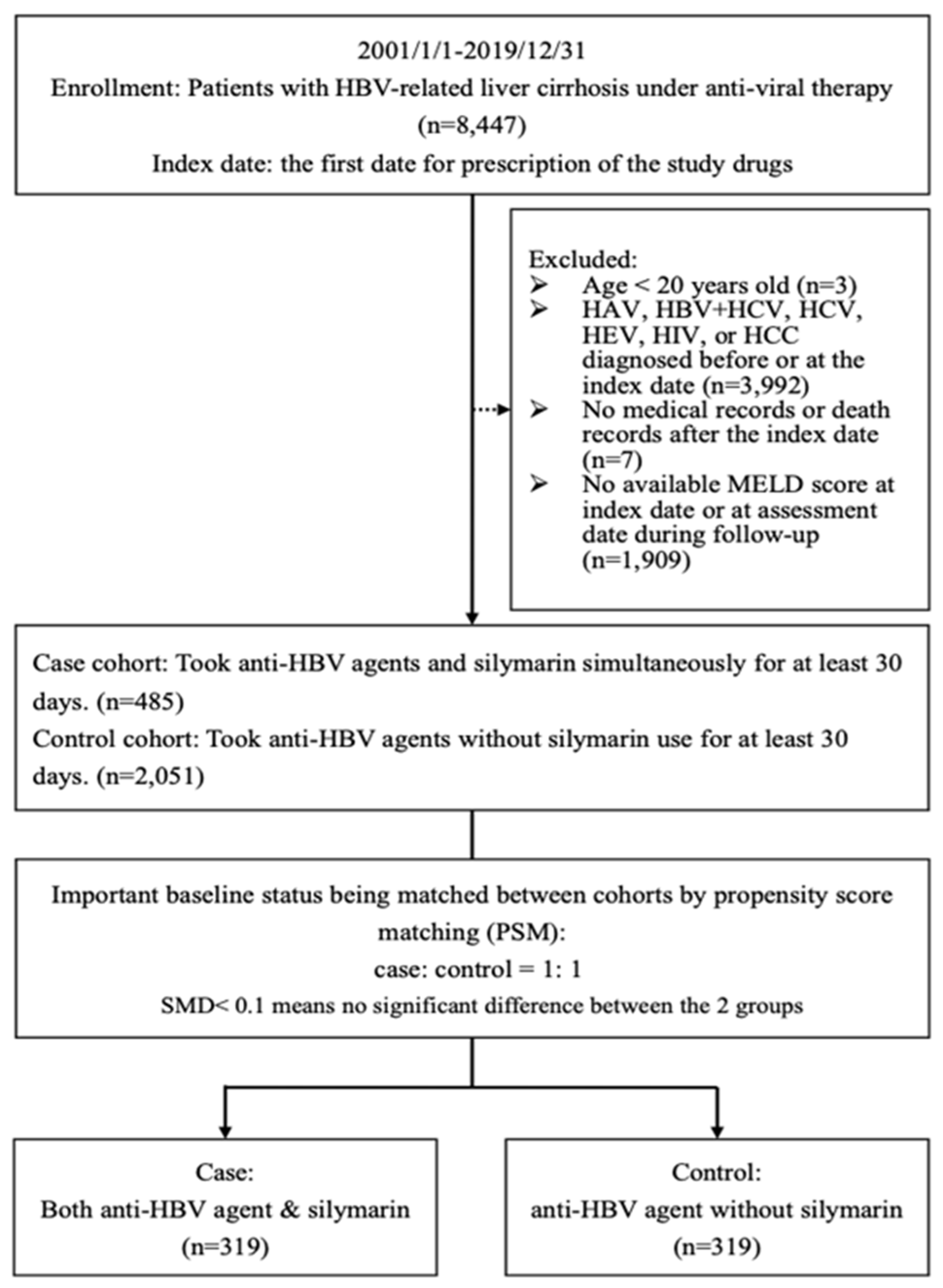
| Baseline Clinical Characteristics @ | Case (Anti-HBV + Silymarin) (n = 319) | Control (Anti-HBV Agent) (n = 319) | ASMD # |
|---|---|---|---|
| Age (years), mean ± SD | 55.94 ± 11.47 | 55.93 ± 13.03 | 0.001 |
| Sex, n (%) | 0.022 | ||
| Male | 241 (75.55) | 244 (76.49) | |
| Female | 78 (24.45) | 75 (23.51) | |
| Baseline biochemistry | |||
| Creatinine (mg/dL) | 1.16 ± 1.31 | 1.09 ± 1.39 | 0.050 |
| Na (mEq/L) | 137.48 ± 4.11 | 137.43 ± 4.31 | 0.014 |
| AST (U/L) | 122.69 ± 205.76 | 123.27 ± 246.9 | 0.003 |
| ALT (U/L) | 114.7 ± 231.02 | 106.41 ± 214.87 | 0.037 |
| Albumin (g/dL) | 3.27 ± 0.75 | 3.22 ± 0.73 | 0.079 |
| HBV-DNA (log10 IU/mL) | 7.37 ± 8.01 | 7.47 ± 8.1 | 0.053 |
| Hemogram | |||
| INR | 1.27 ± 0.33 | 1.26 ± 0.43 | 0.012 |
| Baseline decompensation status, n (%) | |||
| EV or GV bleeding | 75 (23.51) | 81 (25.39) | 0.044 |
| Ascites | 127 (39.81) | 115 (36.05) | 0.078 |
| Hepatic encephalopathy | 27 (8.46) | 35 (10.97) | 0.085 |
| Hepatorenal syndrome | 1 (0.31) | 1 (0.31) | 0.000 |
| Clinical index | |||
| MELD score | 12.89 ± 5.47 | 12.91 ± 5.7 | 0.003 |
| ALBI score | −1.77 ± 0.77 | −1.71 ± 0.76 | 0.081 |
| CCI (Charlson comorbidity index), mean ± SD | 1.93 ± 2.43 | 2.11 ± 2.63 | 0.072 |
| Case (Anti-HBV + Silymarin) (n = 319) | Control (Anti-HBV Agent) (n = 319) | ASMD # | |
|---|---|---|---|
| Cumulative duration of medication, mean ± SD (months) | 9.11 ± 13.33 | 9.62 ± 12.95 | 0.039 |
| Non-selective β-blocker | 31 (9.72) | 24 (7.52) | 0.078 |
| Follow-up time, mean ± SD (months) | 61.97 ± 47.77 | 42.24 ± 49.24 | 0.407 |
| Primary outcome, n (%) | 0.349 | ||
| Mortality | 154 (48.28) | 191 (59.87) | |
| LT $ | 6 (1.88) | 18 (5.64) | |
| Secondary outcome, n (%) | |||
| HCC | 107 (33.54) | 91 (28.53) | 0.109 |
| Cirrhotic complications, n (%) | |||
| EV or GV bleeding | 62 (19.44) | 86 (26.96) | 0.179 |
| Ascites | 23 (7.21) | 33 (10.34) | 0.111 |
| Hepatic encephalopathy | 6 (1.88) | 14 (4.39) | 0.144 |
| Hepatorenal syndrome | 107 (33.54) | 91 (28.53) | 0.109 |
| Case (Anti-HBV + Silymarin) (n = 319) | Control (Anti-HBV Agent) (n = 319) | p-Value | |
|---|---|---|---|
| Biochemistry, Δ mean ± SD | |||
| ΔCr (mg/dL) | 0.08 ± 0.62 | 0.19 ± 0.71 | 0.083 |
| ΔNa (mEq/L) | 0.70 ± 4.18 | −0.05 ± 6.18 | 0.227 |
| ΔAST (U/L) | −75.66 ± 244.16 | −72.95 ± 284.61 | 0.921 |
| ΔALT (U/L) | −87.2 ± 257.64 | −79.81 ± 320.27 | 0.804 |
| ΔAlbumin (g/dL) | 0.48 ± 0.77 | 0.37 ± 0.87 | 0.224 |
| ΔHBV-DNA (log10 IU/mL) | −7.31 ± 8.02 | −7.44 ± 8.10 | 0.434 |
| Hemogram | |||
| ΔINR | −0.07 ± 0.30 | 0.03 ± 0.47 | 0.038 |
| Clinical index | |||
| ΔMELD score | −1.65 ± 5.22 | −0.08 ± 6.75 | 0.025 |
| ΔALBI score | −0.51 ± 0.79 | −0.39 ± 0.92 | 0.198 |
| ΔCCI (Charlson comorbidity index) | 0.77 ± 3.00 | −0.30 ± 3.39 | <0.0001 |
| Competing Risk Analysis | ||||
|---|---|---|---|---|
| Follow-Up Duration | One Year | Two Years | ||
| Variable | HR (95% CI) | p-Value | HR (95% CI) | p-Value |
| Cohort | ||||
| Case b | 0.43 (0.311–0.61) | <0.001 | 0.44 (0.33–0.59) | <0.001 |
| Control c | 1 (reference) | 1 (reference) | ||
| Competing Risk Analysis | ||||
|---|---|---|---|---|
| Follow-Up Duration | One Year | Two Years | ||
| Variable | HR (95% CI) | p-Value | HR (95% CI) | p-Value |
| Cohort | ||||
| Case b | 1.02 (0.71–1.48) | 0.907 | 1.08 (0.78–1.50) | 0.651 |
| Control c | 1 (reference) | 1 (reference) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-H.; Wu, V.C.-C.; Wang, C.-L.; Wu, C.-L.; Huang, Y.-T.; Chang, S.-H. Silymarin Synergizes with Antiviral Therapy in Hepatitis B Virus-Related Liver Cirrhosis: A Propensity Score Matching Multi-Institutional Study. Int. J. Mol. Sci. 2024, 25, 3088. https://doi.org/10.3390/ijms25063088
Huang C-H, Wu VC-C, Wang C-L, Wu C-L, Huang Y-T, Chang S-H. Silymarin Synergizes with Antiviral Therapy in Hepatitis B Virus-Related Liver Cirrhosis: A Propensity Score Matching Multi-Institutional Study. International Journal of Molecular Sciences. 2024; 25(6):3088. https://doi.org/10.3390/ijms25063088
Chicago/Turabian StyleHuang, Chien-Hao, Victor Chien-Chia Wu, Chun-Li Wang, Chia-Ling Wu, Yu-Tung Huang, and Shang-Hung Chang. 2024. "Silymarin Synergizes with Antiviral Therapy in Hepatitis B Virus-Related Liver Cirrhosis: A Propensity Score Matching Multi-Institutional Study" International Journal of Molecular Sciences 25, no. 6: 3088. https://doi.org/10.3390/ijms25063088
APA StyleHuang, C.-H., Wu, V. C.-C., Wang, C.-L., Wu, C.-L., Huang, Y.-T., & Chang, S.-H. (2024). Silymarin Synergizes with Antiviral Therapy in Hepatitis B Virus-Related Liver Cirrhosis: A Propensity Score Matching Multi-Institutional Study. International Journal of Molecular Sciences, 25(6), 3088. https://doi.org/10.3390/ijms25063088






