Abstract
This study aimed to examine the changes in biomarker levels in responders and non-responders to tumor necrosis factor alpha inhibitor (TNFi) and interleukin-17A inhibitor (IL-17Ai) in psoriatic arthritis (PsA) patients over a 4-month period after treatment initiation. A total of 68 PsA patients initiating either TNFi, IL-17Ai, or methotrexate treatment were included. Blood plasma and clinical outcome measures were collected adjacent to treatment initiation and after four months. A commercially available multiplex immunoassay was included to evaluate 54 biomarkers. Mean changes were used to evaluate change over time. A statistically significant decrease in pro-inflammatory cytokines IL-6 (log-transformed mean change −0.97, 95%CI −4.30; 2.37, [p = 0.032]) and an increase in anti-inflammatory IL-10 (0.38, 95%CI 1.74; 2.50 [p = 0.010]) were seen in TNFi responders. Meanwhile, a statistically significant increase in the target cytokine IL-17A was seen in both IL-17Ai responders (2.49, 95%CI −1.84; 6.85 [p = 0.031]) and non-responders (2.48, 95%CI −1.46; 6.41 [p = 0.001]). This study demonstrated differing changes in cytokine levels when comparing treatment responders and non-responders, highlighting the need to improve the understanding of the different immune response mechanisms explaining different responses to medical treatment in PsA patients.
1. Introduction
Psoriatic arthritis (PsA) is an immune-mediated inflammatory disease characterized by heterogeneous clinical and patient-reported manifestations with a great impact on patients’ quality of life [1]. The introduction of biological disease-modifying anti-rheumatic drugs (bDMARDs) have improved the disease control of arthritis significantly [2]. Further, it has bettered PsA patients’ health-related quality of life [3]. Nonetheless, around 30% of PsA patients have no effect of treatment [4], and the unknown associations between clinical manifestations, immunopathogenesis, and treatment response mechanisms complicate treatment.
PsA has been considered an inflammatory disease of both auto-inflammatory and auto-immune origin [5], constituted by both innate and adaptive immune components [6]. The main theorized mechanisms include the upregulation of the interleukin (IL)-23/IL-17 inflammatory pathway [7], promoted by an abnormal innate response to physical stress [8,9]. The increased levels of pro-inflammatory cytokines are associated with the induction of T cells polarization towards the T helper cell type 17 (Th17) subtype, resulting in IL-17A secretion [10]. However, several additional cell subtypes, cell mediators, and cytokines have been suspected to play an important part in the immune response mechanisms inducing, amplifying, and sustaining the inflammatory mechanisms in PsA, which are the targets of available treatments [6].
Treatments include conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs), for which pharmacodynamics remain unknown [11], and bDMARDs that target (pro-)inflammatory cytokines known to PsA immunopathogenesis and include treatments such as tumor necrosis factor alpha inhibitor (TNFi), interleukin-17A inhibitor (IL-17Ai), interleukin-23 inhibitor, interleukin-12/23 inhibitor, etc. [12]. Nevertheless, the literature reveals the different effectiveness of individual types of treatment on the diverse manifestations of PsA [13]. The different effects of TNFi and IL-17Ai on PsA disease activity have been associated with different cytokine signatures at baseline, but the significance of the change in biomarkers over time and the association with a clinical outcome remains unclear [14]. The observations indicate the continuous need to improve the understanding of PsA immunopathogenesis and the effect of bDMARDs on inflammation-associated biomarkers in association with different PsA clinical phenotypes.
The primary aim of this study was to assess the effect of medical treatments, TNFi and IL-17Ai, on the levels of 54 cytokines, chemokines, and additional biomarkers in PsA patients stratified by the effect of treatment. The secondary aim was to investigate the associations between biomarkers at baseline and individual clinical- and patient-reported outcomes, as well as the difference in biomarker levels at baseline when comparing patients initiating bDMARDs and methotrexate (MTX), i.e., bDMARD-naïve patients.
2. Results
2.1. Baseline Characteristics
A total of 68 PsA patients were included in the study: 29 TNFi initiators, 19 IL-17Ai initiators, and 20 MTX initiators. No statistically significant differences were found between the patient treatment groups at baseline when comparing sex, age, or disease duration, or when comparing clinical outcome disease activity scores in PsA (DAPSA), the Psoriasis Area Severity Index (PASI), and VAS patient pain. Statistically significant differences between treatment groups were found when comparing the number of previous bDMARDs and VAS patient fatigue (Table 1). Evaluating the differences between individual groups, a post hoc Dunn’s test with Bonferroni correction revealed a statistically significant difference between IL-17Ai and MTX initiators. When comparing treatment groups, statistically significant differences in biomarker levels at baseline were found in IL-31, with higher levels in MTX initiators compared with IL-17Ai initiators; eotaxin, with higher levels in MTX initiators compared with TNFi initiators; and Tie-2, with higher levels in MTX initiators compared with both TNFi and IL-17Ai initiators. TNFα had higher levels in patients initiating IL-17Ai compared with both TNFi and MTX initiators, and IL-17A had higher levels in patients initiating IL-17Ai compared with MTX initiators. Additionally, IL-15 was statistically significantly lower in MTX initiators compared with both TNFi and IL-17Ai initiators (Table S1).

Table 1.
Baseline characteristics.
Twenty-two biomarkers were excluded due to missing data (>10%) caused by biomarkers being below the detection range or by the intra-assay coefficient of variation (CV) being deemed too large. Spearman rank correlations between the remaining 32 biomarkers and 9 PsA disease manifestations revealed no associations, including none of the correlation coefficients reaching ≥0.60, defining the threshold for moderate correlation (Figure 1) [15].
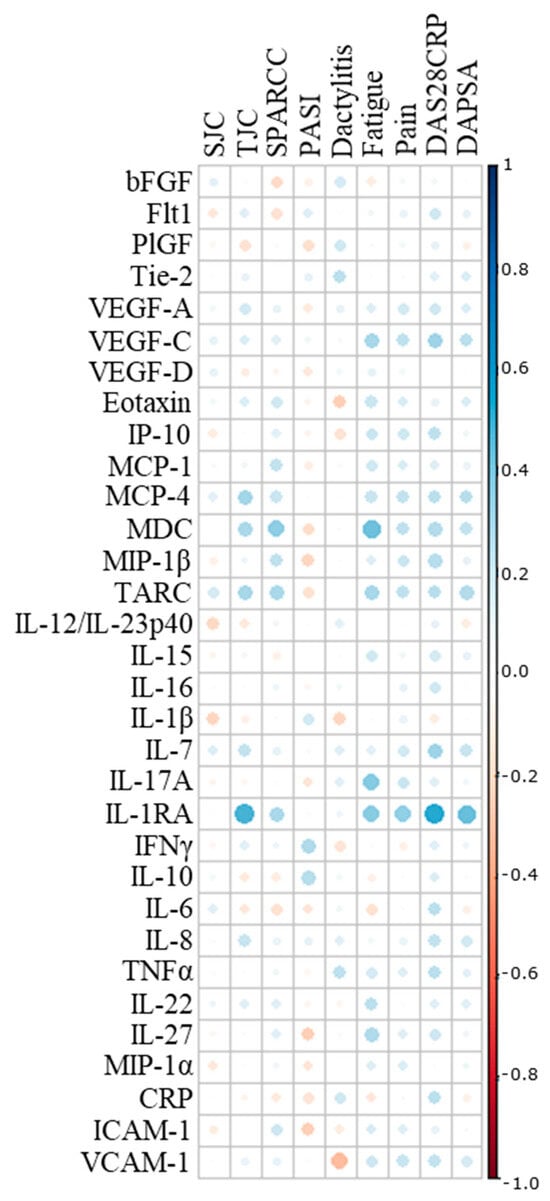
Figure 1.
Correlation analysis. Spearman’s correlation coefficient was implemented to assess associations between individual biomarkers and clinical and patient-reported outcomes. SJC, swollen joint count; TJC, tender joint count; SPARCC, Spondyloarthritis Research Consortium of Canada; PASI, Psoriasis Area Severity Index; DAS28CRP, Disease Activity Score calculated with 28 joints and c-reactive protein; DAPSA, disease activity in psoriatic arthritis; bFGF, basic fibroblast growth factor; Flt-1, Fms-related receptor tyrosine kinase-1; PlGF, placental growth factor; Tie-2, endothelial receptor tyrosine kinase; VEGF, vascular endothelial growth factor; IP-10, IFN-induced protein-10; MCP, monocyte chemoattractant protein; MDC, macrophage-derived chemokine; MIP, macrophage inflammatory protein; TARC, thymus and activation-regulated chemokine; IL, interleukin; IL-1RA, interleukin-1 receptor antagonist; IFN, interferon; TNF, tumor necrosis factor; CRP, c-reactive protein; ICAM, intercellular adhesion molecule; VCAM, vascular cell adhesion molecule.
2.2. Change in Clinical Outcome Measures in Responders and Non-Responders to Treatment
Follow-up data were obtained from 20 TNFi initiators and 19 IL-17Ai initiators. MTX initiators were only included for the analysis of baseline data due to a low number of patients with follow-up data, limiting additional stratification based on treatment response. Data on PsA patients were only included if they presented with complete clinical data to calculate composite clinical outcome measures. Response to treatment was defined either by a 50% improvement in DAPSA, i.e., DAPSA50, or PASI, i.e., PASI50. Of the PsA patients initiating TNFi, 11 and 9 patients were considered as DAPSA50 responders and non-responders, respectively, while 12 and 6 patients were considered as PASI50 responders and non-responders, respectively. Of the PsA patients initiating IL-17Ai, 7 and 12 were considered as DAPSA50 responders and non-responders, respectively, while 7 and 4 were considered as PASI50 responders and non-responders, respectively (Table 2). Stratifying by the DAPSA50 response demonstrated statistically significant decreases in DAPSA for all treatment responders; however, this was also the case for TNFi DAPSA50 non-responders (mean change −7.16 95%CI −49.90; 35.59 [p = 0.039]). Thus, the decrease in DAPSA was higher for TNFi DAPSA50 responders (−28.75 95%CI −81.81; 24.32 [p < 0.001]). Stratifying by the PASI50 response demonstrated statistically significant decreases in PASI for TNFi responders (−4.08 95%CI −15.61; 7.45 [p = 0.003]) and IL-17Ai responders (−4.97 95%CI −22.95; 13.00 [p = 0.016]) (Table 2).

Table 2.
Change in clinical outcomes of DAPSA50 and PASI50 responders and non-responders to TNFi and IL-17Ai.
2.3. Change in Biomarker Level from Baseline to Follow-Up
Log-transformed biomarker levels were evaluated to assess changes from baseline to follow-up in the TNFi and IL-17Ai initiators, stratifying by response to treatment. Results were visualized by bar charts for TNFi and IL-17Ai DAPSA50 responders versus non-responders (Figure 2 and Figure 3) and for TNFi and IL-17i PASI50 responders and non-responders (Figure 4 and Figure 5). Changes in biomarkers from baseline to the follow-up, presented with numeric values, are available in Table S2–S5.
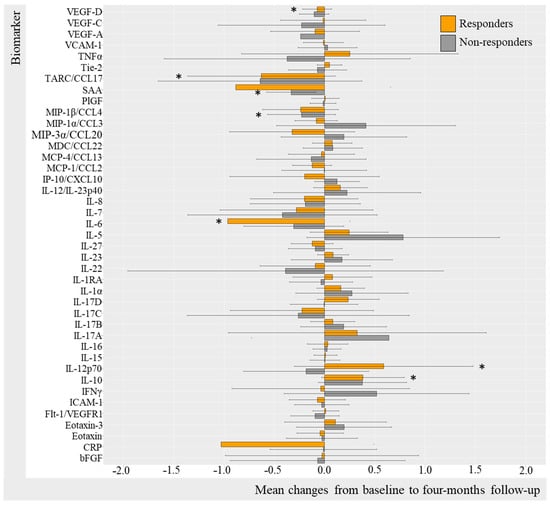
Figure 2.
Mean changes in biomarker levels in TNFi initiators stratified by DAPSA50. Log-transformed mean changes from baseline to four-month follow-up stratified by DAPSA50 corresponding to a 50% improvement in DAPSA, included as a measure of arthritic joint disease (Table S2). * (star) represents changes that are statistically significant. TNFi, tumor necrosis factor alpha inhibitor; DAPSA, disease activity in psoriatic arthritis; VEGF, vascular endothelial growth factor; VCAM, vascular cell adhesion molecule; TNF, tumor necrosis factor; Tie-2, endothelial receptor tyrosine kinase; TARC, thymus and activation regulated chemokine; CCL, CC chemokine ligand; SAA, serum amyloid A; PlGF, placental growth factor; MIP, macrophage inflammatory protein; MDC, macrophage-derived chemokine; MCP, monocyte chemoattractant protein; IP-10, IFN-induced protein-10; CXCL, CX chemokine ligand; IL, interleukin; IL-1RA, interleukin-1 receptor antagonist; IFN, interferon; ICAM, intercellular adhesion molecule; Flt-1, Fms-related receptor tyrosine kinase-1; CRP, c-reactive protein; bFGF, basic fibroblast growth factor.
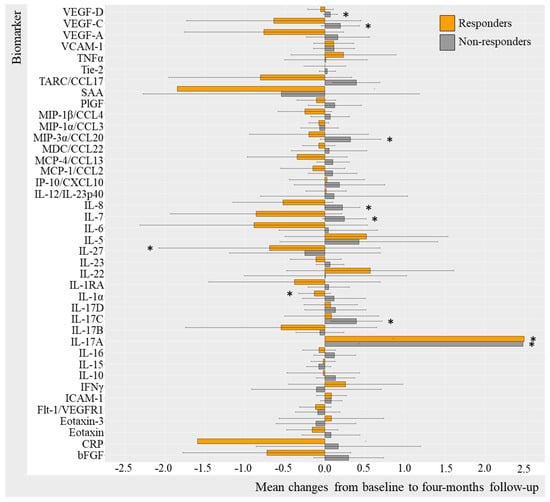
Figure 3.
Mean changes in biomarker level in IL-17Ai initiators stratified by DAPSA50. Log-transformed mean changes from baseline to four-month follow-up stratified by DAPSA50 corresponding to a 50% improvement in DAPSA, included as a measure of arthritic joint disease (Table S3). * (star) represents changes that are statistically significant. IL-17Ai, interleukin-17A inhibitor; DAPSA, disease activity in psoriatic arthritis; VEGF, vascular endothelial growth factor; VCAM, vascular cell adhesion molecule; TNF, tumor necrosis factor; Tie-2, endothelial receptor tyrosine kinase; TARC, thymus and activation regulated chemokine; CCL, CC chemokine ligand; SAA, serum amyloid A; PlGF, placental growth factor; MIP, macrophage inflammatory protein; MDC, macrophage-derived chemokine; MCP, monocyte chemoattractant protein; IP-10, IFN-induced protein-10; CXCL, CX chemokine ligand; IL, interleukin; IL-1RA, interleukin-1 receptor antagonist; IFN, interferon; ICAM, intercellular adhesion molecule; Flt-1, Fms-related receptor tyrosine kinase-1; CRP, c-reactive protein; bFGF, basic fibroblast growth factor.
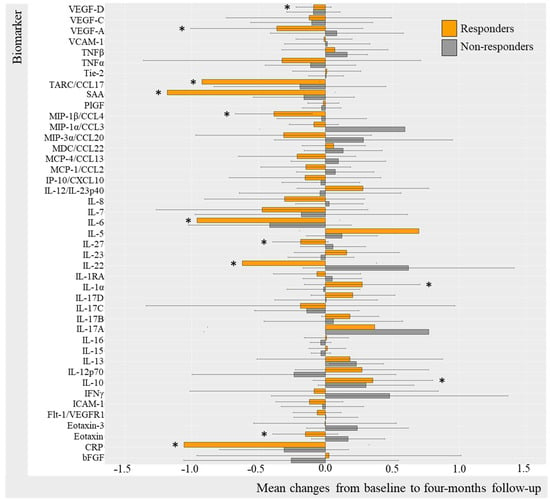
Figure 4.
Mean changes in biomarker levels in TNFi initiators stratified by PASI50. Log-transformed mean changes from baseline to four-month follow-up stratified by PASI50 corresponding to 50% improvement in PASI included as a measure of cutaneous psoriasis (Table S4). * (star) represents changes that are statistically significant. TNFi, Tumor Necrosis Factor alpha inhibitor; PASI, Psoriasis Area Severity Index; VEGF, Vascular Endothelial Growth Factor; VCAM, vascular cell adhesion molecule; TNF, Tumor Necrosis Factor; Tie-2, endothelial receptor tyrosine kinase; TARC, Thymus and activation regulated chemokine; CCL, CC chemokine ligand; SAA, serum amyloid A; PlGF, Placental Growth Factor; MIP, macrophage inflammatory protein; MDC, macrophage-derived chemokine; MCP, monocyte chemoattractant protein; IP-10, IFN-induced protein-10; CXCL, CX chemokine ligand; IL, interleukin; IL-1RA, interleukin-1 receptor antagonist; IFN, interferon; ICAM, intercellular adhesion molecule; Flt-1, Fms-related receptor tyrosine kinase-1; CRP, c-reactive protein; bFGF, basic fibroblast growth factor.
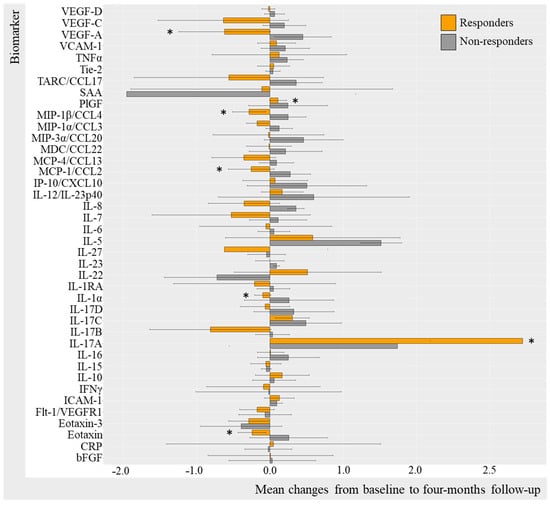
Figure 5.
Mean changes in biomarker level in IL-17Ai initiators stratified by PASI50. Log-transformed mean changes from baseline to four-month follow-up stratified by PASI50 corresponding to 50% improvement in PASI included as a measure of cutaneous psoriasis (Table S5). * (star) represents changes that are statistically significant. IL-17i, interleukin-17 inhibitor; PASI, Psoriasis Area Severity Index; VEGF, Vascular Endothelial Growth Factor; VCAM, vascular cell adhesion molecule; TNF, Tumor Necrosis Factor; Tie-2, endothelial receptor tyrosine kinase; TARC, Thymus and activation regulated chemokine; CCL, CC chemokine ligand; SAA, serum amyloid A; PlGF, Placental Growth Factor; MIP, macrophage inflammatory protein; MDC, macrophage-derived chemokine; MCP, monocyte chemoattractant protein; IP-10, IFN-induced protein-10; CXCL, CX chemokine ligand; IL, interleukin; IL-1RA, interleukin-1 receptor antagonist; IFN, interferon; ICAM, intercellular adhesion molecule; Flt-1, Fms-related receptor tyrosine kinase-1; CRP, c-reactive protein; bFGF, basic fibroblast growth factor.
2.3.1. Change in Biomarkers in TNFi Responders and Non-Responders (DAPSA50 and PASI50)
Changes in biomarker levels did differentiate over time depending on the DAPSA50 response to TNFi (Figure 2). For DAPSA50 responders, statistically significant log-transformed decreases were seen in CRP (−1.03 95%CI −4.50; 2.44 [p = 0.049]), IL-6 (−0.97, 95%CI −4.30; 2.37 [p = 0.032]), TARC (−0.629 95%CI −3.446; 2.188 [p = 0.042]), and VEGF-D (−0.07, 95%CI −0.98; 0.83 [p = 0.042]) and, while statistically significant, increases were demonstrated in IL-12p70 (0.59, 95%CI −2.30; 3.47 [p = 0.049]) and IL-10 (0.38, 95%CI −1.74; 2.50 [p = 0.009]). DAPSA50 non-responders demonstrated a statistically significant decrease in SAA (−0.33, 95%CI −3.15; 2.48, [p = 0.031]) (Table S2).
Additional results based on PASI50 response stratification (Figure 4) included PASI50 responders demonstrating statistically significant decreases in VEGF-D (−0.09, 95%CI −0.99; 0.82, [p = 0.021]), VEGF-A (−0.365 95%CI −2.747; 2.017 [p = 0.042]), eotaxin (−0.15 95%CI −1.58 1.27 [0.042]), TARC (−0.92, 95%CI −3.47; 1.62, [p = 0.012]), SAA (−1.18, 95%CI −4.40; 2.04, [p = 0.004]), MIP-1β (−0.39, 95%CI −2.37; 1.60, [p < 0.001]), IL-6 (−0.96, 95%CI −4.18; 2.26, [p = 0.012]), IL-27 (−0.187 95%CI −2.13; 1.75 [p = 0.016]), IL-22 (−0.62 95%CI −3.52; 2.28 [p = 0.042), and CRP (−1.06 95%CI −4.72; 2.61 [p = 0.027]) and a statistically significant increase in IL-10 (0.355 95%CI −2.238; 2.80 [p = 0.010]) and IL-1α (0.274 95%CI −1.64; 2.91 [p = 0.032]) (Table S4).
2.3.2. Change in Biomarkers in IL-17Ai Responders and Non-Responders (DAPSA50 and PASI50)
Differences in biomarker changes over the 4-month trial period were also evident when comparing IL-17i DAPSA50 responders and non-responders (Figure 4). There were statistically significant decreases in IL-1α (mc −0.13, 95%CI −2.59; 2.32 [p = 0.031]) and IL-27 (mc −0.69 95%CI −4.33; 2.94 [p = 0.031]), while an increase in IL-17A (2.49, 95%CI −1.86; 6.84, [p = 0.031]) was found in DAPSA50 responders. Non-responders revealed statistically significant increases in MIP-3α (0.32, 95%CI −1.02; 1.66, [p = 0.016]), IL-8 (0.22, 95%CI −1.76; 2.19, [p = 0.007]), IL-17C (0.39, 95%CI −1.09; 1.87, [p = 0.010]), IL-7 (0.24, 95%CI −1.71; 2.20, [p = 0.003]), IL-17A (2.48, 95%CI −1.46; 6.41, [p = 0.009]), VEGF-D (mc 0.66 95%CI −1.20; 1.34 [p = 0.027]), and VEGF-C (mc 0.19 (−1.28; 1.66) [p = 0.039]) (Table S4).
PASI50 IL-17Ai responders (Figure 5) demonstrated statistically significant decreases in IL-1α (mc −0.096 (−2.56; 2.37) [p = 0.031]), MIP-1β (mc −0.29 95%CI −2.58; 2.01 [p = 0.031]), MCP-1 (−0.25 95%CI −2.37; 1.82 [p = 0.047]), and eotaxin (mc −0.24 95%CI −1.75; 1.26 [p = 0.016]) VEGF-A (mc −0.62 95%CI −2.50; 1.26 [p = 0.047]),and increases in IL-17A (3.45 95%CI 0.10; 6.81 [p = 0.016]) and PlGF (p = 0.047) (Table S5). There were no statistically significant changes from baseline to follow-up in PASI50 non-responders, probably due to insufficient power (n = 4). For additional biomarkers, it applied to IL-17Ai DAPSA50 responders that most biomarker levels decreased; however, this was not the case for TNFα, IL-5, IL-22, and IFNγ levels which increased, whereas DAPSA50 non-responders increased in biomarker levels. Thus, these results were not statistically significant (Table S5).
3. Discussion
This study evaluated the effect of different medical treatments, TNFi and IL-17Ai, on inflammation-associated biomarkers in a group of PsA patients stratified by the DAPSA50 and PASI50 response to treatment. Biomarker levels in MTX initiators were quantified at baseline to establish biomarker levels in a PsA patient group comprising mainly bio-naïve patients (90%), i.e., medical treatments have had limited effect on the biomarker milieu. Statistically significant differences were only seen in 6 out of 54 biomarkers at baseline when comparing TNFi, IL-17A, and MTX initiators.
The evaluation of biomarkers at baseline revealed differences between patient groups in PsA-associated cytokines, TNFα, and IL-17A. TNFα levels were similar in TNFi and MTX initiators, which might be associated with the majority of patients being bio-naïve. In comparison, TNFα levels displaying a statistically significant increase were demonstrated in IL-17Ai initiators. Higher levels of IL-17A were demonstrated in IL-17Ai initiators compared with both TNFi and MTX initiators. Increased levels of treatment target cytokines TNFα and IL-17A have been associated with previous TNFi treatment, which in other studies has been shown to induce increases in TNFα and IL-17 levels [16,17]. Additional baseline differences between groups include inflammatory cytokines, IL-31 and IL-15, and angiogenesis-associated biomarker Tie-2, which have all been associated with PsA immunopathogenesis [18,19,20]. Nevertheless, it is important to acknowledge that none of the biomarkers measured have been compared with healthy controls, i.e., whether the biomarkers are significantly associated with PsA patients.
Differences in patient characteristics when comparing groups were found in the number of previous bDMARDs, VAS fatigue, and VAS pain, which were all increased in IL-17Ai initiators. This is possibly related to Danish treatment guidelines, including TNFi being the first bDMARD of choice in the PsA treatment. IL-17Ai was typically initiated after TNFi failure. The results implied that included IL-17Ai initiators suffer from higher disease severity or a degree of chronicity, explaining the statistically significant higher VAS pain and VAS fatigue [21], which was supported by the trend indicating longer disease duration for IL-17Ai. None of the clinical and patient-reported outcomes reached a strong correlation, with individual biomarkers reporting the complexity and difficulties of translational examinations finding the link between clinical presentation and PsA immunopathogenesis.
Changes in biomarker levels from baseline to four-month follow-up were evaluated to compare differences between treatment responders and non-responders. It was decided to examine the change in arthritis by DAPSA stratifying by the DAPSA50 response and change in cutaneous psoriasis by PASI stratifying the PASI50 response. Stratification by two clinical symptoms were performed to recognize the sometimes differential effect of treatments in joint arthritis and cutaneous psoriasis [22,23,24].
Previous studies have demonstrated increased cytokine levels, TNFα, IL-17, and IL-10, together with decreases in IL-6 and IL-8 in response to TNFi [16,25], as demonstrated in this study. However, studies exploring changes in response to IL-17Ai remain limited. DAPSA50 responders to TNFi demonstrated a statistically significant increase in IL-10, suggesting the contribution and upregulation of anti-inflammatory properties in responders to TNFi. The increase in IL-10 of responders to treatment is in line with previous data demonstrating an upregulation of IL-10 mRNA expression in response to TNFi [26]. DAPSA50 responders further showed a statistically significant decrease in the pro-inflammatory cytokines IL-6 and CRP and increasing IL-12p70, the last of which is somewhat surprising to previous studies, revealing decreases in pro-inflammatory cytokines that were not the direct target of specific cytokine inhibitors [16]. Decreasing IL-6 levels and the strong trend of decreasing IL-8, most pronounced in PASI50 responders, might imply that TNFi works through the inhibition of the inflammatory amplification process recently proposed [27], which was further supported by the overall decrease in inflammation implied by decreasing CRP. However, increasing IL-17A, IL-1, IL-17B, and IL-17C seems conflicting. Additional statistically significant decreases were seen in acute-phase reactant serum amyloid A [28] and (pro-)inflammatory cytokines IL-27 and IL-22 in responders to TNFi when stratifying by PASI50, suggesting a downregulation of cytokines involved in the recruitment and differentiation of T cells into inflammatory subtypes, such as T helper cell type 1 and 17 (Th1 and Th17) [29,30,31]. Moreover, IL-27 and IL-22 have been considered as important contributors to PsA immunopathogenesis [14]. Increasing TNFα in response to TNFα inhibitor, as demonstrated by previous studies [16,25], was also implied in the current study. However, the increase in TNFα did not reach statistical significance.
PsA patients initiating IL-17Ai revealed statistically significant increases in cytokines IL-8, IL-17C, IL-7, and IL-17A for DAPSA50 non-responders after four months of treatment. Trends of decreasing pro-inflammatory cytokines IL-6, IL-7, and IL-8 were implied in IL-17i DAPSA50 responders, suggesting that IL-17Ai inhibits the amplification process [27]. Interestingly, the same pattern with a statistically significant increase in the target cytokine IL-17A was demonstrated for DAPSA50 and PASI50 responders as well, indicating similar mechanisms with increasing levels in the target cytokine. The increase in target cytokines may be explained by a higher half-life of the cytokines promoted by binding to the TNFi and IL-17Ai, respectively, shown in human subjects treated with TNFi [26]; thus, the bound target cytokine is suggestively inactive [25]. This is supported by studies demonstrating the differential effect on post-treatment TNFα levels depending on generic drugs with different cytokine-binding properties [25]. Further, negative feedback mechanisms have been suggested by the upregulation of TNFα- and IFNγ-positive T cells in response to TNFi [17], which have been contradicted in IL-17Ai initiators by studies revealing the downregulation of mRNA encoding IL-12, IL-17A, IL-21, IL-22, etc. [32]. In this PsA patient cohort, the markedly increased IL-17A after IL-17Ai are also believed to exist as inactive cytokines due to the significant concomitant decrease in pro-inflammatory cytokines IL-8 and IL-6 otherwise induced by IL-17 [27]. However, additional studies are needed to confirm this theory.
The limitations of the study included PsA patients with overall low PASI scores, indicating the need for caution in concluding baseline associations between immune characteristics and cutaneous psoriasis. However, changes over time indicated larger changes, especially in patients experiencing total clearing or exacerbation of cutaneous psoriasis, which is considered interesting in relation to immune response mechanisms. Nonetheless, it is important to consider the possible bias introduced by factors associated with differences in cytokine levels such as BMI, time of day of plasma sample retrieval, etc. Furthermore, stratifying patients further resulted in smaller populations that might reduce the power of the statistical analysis. PsA patients were grouped based on the type of treatment planned for initiation due to the different target cytokines, which suggests different immune response mechanisms. However, 89% of PsA initiating IL-17Ai had been treated with other bDMARDs prior to baseline, which most likely have had an effect on the cytokine milieu. In line with Danish treatment guidelines during the inclusion period, the majority of PsA patients who have received previous bDMARDs received a TNFi. Additionally, several PsA patients were receiving concomitant csDMARDs at a stable dose, which might have an immune modulatory effect as well. Nevertheless, the results are still considered of interest as these patients had high disease activity and the design represents the real-life clinical complexity of PsA patients, and it is the change in cytokine levels in association with response to treatment that remains interesting. Furthermore, it is believed that no stratification will bias the understanding of important immune mechanisms explaining differences between PsA patients and their response to treatment. However, PsA patients were stratified as responders and non-responders based on DAPSA50, which corresponds to a minor improvement in disease activity [33]. PsA patients reaching DAPSA50 will still present an ongoing inflammation possibly reflecting the measured cytokines. It is believed that stratifying by DAPSA70 or DAPSA80 may result in a clearer understanding of treatment response mechanisms in PsA.
4. Materials and Methods
4.1. Study Participants
Samples to evaluate biomarkers were obtained from the Parker Institute’s clinical observational PsA patient cohort (PIPA), enrolling patients initiating new treatment at Departments of Rheumatology in Region Zealand and the Capital Region of Denmark in accordance with Danish treatment guideline for PsA. Inclusion and exclusion criteria have been published in the PIPA cohort article [34]. An additional inclusion criterion for the current study was added to ensure different treatments including plasma samples from 68 PsA patients initiating TNFi (n = 29), IL-17i (n = 19), or methotrexate (MTX) (n = 20), which were obtained with corresponding clinical and patient-reported data from a baseline visit adjacent to treatment start and after four months of treatment.
4.2. Study Visits
The baseline and four-month follow-up visits included clinical examination, blood sampling, and questionnaires collecting relevant patient-reported measures. The clinical examination consisted of a 66/68 swollen and tender joint count, the evaluation of psoriatic skin lesions quantified by the Psoriasis Area Severity Index (PASI), Spondyloarthritis Research Consortium of Canada (SPARCC) enthesitis score, and the examination of dactylitis reported with the number of digits involved. The patient-reported outcome retrieved included the Visual Analog Scale (VAS) patient global, pain, and fatigue. Additionally, the composite measures of Disease Activity Score (DAS28CRP) and Disease Activity in Psoriatic Arthritis (DAPSA) were retrieved to evaluate the response to treatment. Peripheral blood was collected in EDTA vacutainer tubes (Greiner bio-one, Kremsmünster, Germany) and centrifuged to collect plasma. EDTA plasma was transferred and stored at −80 °C until analysis. All procedures were conducted on the same day.
4.3. Quantifying Biomarker Levels
The MSD V-plex 54-plex kit (Mesoscale Discovery (MSD), Meso Scale Diagnostic, LCC, Rockville, MD, USA) was used. Patient samples were thawed, prepared in technical doublets, and diluted in-plate. Plate preparation and analysis were conducted in line with the manufacturer’s instructions. Analysis was conducted on the MSD QuickPlex SQ 120 instrument with the software Discovery Workbench version 4.0.12. Biomarker a bsolute values were reported as absolute concentrations in picograms per milliliter (pg/mL).
4.4. Statistical Analysis
Before the statistical analysis, biomarker measurements exceeding the allowed intra-assay coefficient of variation (CV) of 30 between doublet samples were excluded. Patient demographics and biomarker levels at baseline were presented as medians with interquartile ranges (IQRs) and numbers with percentages for continuous and categorical variables, respectively. Biomarker levels were presented in pg/mL. The Kruskal–Wallis test was applied at baseline to evaluate differences between treatment groups, i.e., TNFi initiators, IL-17Ai initiators, or MTX initiators. A post hoc Dunn’s test with Bonferroni correction was applied to evaluate statistically significant differences between the individual groups. A Spearman’s rank correlation coefficient (ρ) was included to examine the correlation between (1) individual biomarkers and (2) individual biomarkers and PsA outcomes. A spearman’s value of ρ ≤ 0.29 was considered poor, between 0.3 and 0.59 was considered fair, from 0.6 to 0.8 was considered moderately strong, and >0.8 was considered very strong [15]. To compare responders and non-responders to treatment, PsA patients were stratified based on the DAPSA50 and PASI50 response (yes/no), i.e., whether an improvement of at least 50% in DAPSA and PASI score was achieved from baseline to follow-up. The primary analysis was performed on DAPSA50 responders as not all included PsA patients presented with active psoriasis at baseline. QQ plots applied to evaluate for normal distribution. Biomarker levels were log-transformed, and the change over time was calculated as mean changes with standard deviations to be visualized in bar charts. Biomarkers with >10% missing were excluded from Spearman’s ρ and the mean change bar chart. A Wilcoxon signed-rank test was used to examine differences between paired measures: clinical, patient-reported, and biomarker levels. It applied to all analyses, and p < 0.05 was considered statistically significant. The statistical analysis was carried out using the statistical software R with additional relevant packages.
5. Conclusions
This study aimed to assess the effect of TNFi and IL-17Ai on various biomarkers, revealing interesting results regarding immune treatment response mechanisms. The results demonstrated the importance of studying immune response mechanisms in different well-defined subgroups of PsA to improve the understanding of PsA clinical heterogeneity and different responses to treatments.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms25053002/s1.
Author Contributions
Conceptualization, M.S. and L.E.K.; methodology, M.S. and S.B.D.; software, M.F.S.; validation, M.S. and S.B.D.; formal analysis, M.F.S.; investigation, M.S.; resources, L.E.K.; data curation, M.S.; writing—original draft preparation, M.S.; writing—review and editing, all authors.; visualization, M.S.; supervision, S.B.D. and L.E.K.; project administration, M.S.; funding acquisition, M.S. and L.E.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by an Investigator-Initiated Research Grant from Lilly (I1F-NS-O004), The Danish Rheumatism Association (R188-A6597)), Elisabeth and Karl Ejnar Nis-Hanssens Mindelegat and Minister Erna Hamiltons Legat for Videnskab og Kunst. The study was further supported by an unrestricted core grant to the Parker Institute from the Oak Foundation (OCAY-18-774-OFIL).
Institutional Review Board Statement
This study was conducted in accordance with the Helsinki Declaration with ethical approval obtained from the Danish Ethical Committee of the Capital Region of Denmark (J.no.: H-18024568), and the General Data Protection Regulation was approved by the Capital Region of Denmark (J.no.: BFH-2015-043). All patients provided written informed consent before inclusion. This study was registered at clinicaltrials.gov (NCT02572700). The pre-specified protocol is available from the Parker Institute’s website (www.parkerinst.dk).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The dataset supporting the conclusions of this article cannot be shared publicly due to the privacy of the individuals who participated in the study. Data may be shared as part of research collaborations between participating institutions in line with GDPR and if approved by the Parker Institute and Danish authorities.
Acknowledgments
We wish to acknowledge all patients participating in this study, Dorte Kongshøj Marcussen for valuable talks during the project, and Christian Cato Holm from the Parker Institute for the assistance during the collection and retrieval of data to conduct the study.
Conflicts of Interest
Marie Skougaard has received research funding from the Danish Rheumatism Association, the Danish National Psoriasis Foundation, Lilly and Pfizer, and a speaker fee from Janssen-Cilag. Sisse B. Ditlev has no conflicts of interest. Magnus Friis Søndergaard has no conflicts of interest. Lars Erik Kristensen has received fees for speaking and consultancy from AbbVie, Amgen, Biogen, Bristol Myers Squibb, Eli Lilly, Gilead, Janssen, MSD, Novartis, Pfizer, and UCB, as well as IIT grants from Biogen, Eli Lilly, Janssen, Novartis, Pfizer, and UCB. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Husted, J.A.; Gladman, D.D.; Farewell, V.T.; Cook, R.J. Health-related quality of life of patients with psoriatic arthritis: A comparison with patients with rheumatoid arthritis. Arthritis Care Res. 2001, 45, 151–158. [Google Scholar] [CrossRef]
- Scott, D.L. Biologics-based therapy for the treatment of rheumatoid arthritis. Clin. Pharmacol. Ther. 2012, 91, 30–43. [Google Scholar] [CrossRef]
- Skougaard, M.; Jørgensen, T.S.; Jensen, M.J.; Ballegaard, C.; Guldberg-Møller, J.; Egeberg, A.; Christensen, R.; Benzin, P.; Stisen, Z.R.; Merola, J.F.; et al. Change in psoriatic arthritis outcome measures impacts SF-36 physical and mental component score differently: An observational cohort study. Rheumatol. Adv. Pract. 2021, 5, rkab076. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, L.E.; Lie, E.; Jacobsson, L.T.; Christensen, R.; Mease, P.J.; Bliddal, H.; Geborek, P. Effectiveness and feasibility associated with switching to a second or third TNF inhibitor in patients with psoriatic arthritis: A cohort study from southern Sweden. J. Rheumatol. 2016, 43, 81–87. [Google Scholar] [CrossRef]
- McGonagle, D.; McDermott, M.F. A proposed classification of the immunological diseases. PLoS Med. 2006, 3, 1242–1248. [Google Scholar] [CrossRef] [PubMed]
- Veale, D.J.; Fearon, U. The pathogenesis of psoriatic arthritis. Lancet 2018, 391, 2273–2284. [Google Scholar] [CrossRef] [PubMed]
- Lubberts, E. The IL-23-IL-17 axis in inflammatory arthritis. Nat. Rev. Rheumatol. 2015, 11, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Tinazzi, I.; McGonagle, D.; Aydin, S.Z.; Chessa, D.; Marchetta, A.; Macchioni, P. ‘Deep Koebner’ phenomenon of the flexor tendon-associated accessory pulleys as a novel factor in tenosynovitis and dactylitis in psoriatic arthritis. Ann. Rheum. Dis. 2018, 77, 922–925. [Google Scholar] [CrossRef]
- McGonagle, D. Enthesitis: An autoinflammatory lesion linking nail and joint involvement in psoriatic disease. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 9–13. [Google Scholar] [CrossRef]
- Blauvelt, A.; Chiricozzi, A. The Immunologic Role of IL-17 in Psoriasis and Psoriatic Arthritis Pathogenesis. Clin. Rev. Allergy Immunol. 2018, 55, 379–390. [Google Scholar] [CrossRef]
- Grim, J.; Chládek, J.; Martínková, J. Pharmacokinetics and pharmacodynamics of methotrexate in non-neoplastic diseases. Clin. Pharmacokinet. 2003, 42, 139–151. [Google Scholar] [CrossRef]
- Chao, R.; Kavanaugh, A. Psoriatic Arthritis: Newer and Older Therapies. Curr. Rheumatol. Rep. 2019, 21, 75. [Google Scholar] [CrossRef]
- Bravo, A.; Kavanaugh, A. Bedside to bench: Defining the immunopathogenesis of psoriatic arthritis. Nat. Rev. Rheumatol. 2019, 15, 645–656. [Google Scholar] [CrossRef]
- Skougaard, M.; Ditlev, S.B.; Søndergaard, M.F.; Kristensen, L.E. Cytokine Signatures in Psoriatic Arthritis Patients Indicate Different Phenotypic Traits Comparing Responders and Non-Responders of IL-17A and TNFα Inhibitors. Int. J. Mol. Sci. 2023, 24, 6343. [Google Scholar] [CrossRef]
- Chan, Y.H. Biostatistics 104: Correlational Analysis. Singap. Med. J. 2003, 44, 614–619. [Google Scholar]
- Walters, H.M.; Pan, N.; Lehman, T.J.A.; Adams, A.; Kalliolias, G.D.; Zhu, Y.; Santiago, F.; Nguyen, J.; Sitaras, L.; Cunningham-Rundles, S.; et al. The impact of disease activity and tumour necrosis factor-α inhibitor therapy on cytokine levels in juvenile idiopathic arthritis. Clin. Exp. Immunol. 2016, 184, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Rudwaleit, M.; Brandt, J.; Thiel, A.; Braun, J.; Sieper, J. Up regulation of the production of tumour necrosis factor α and interferon γ by T cells in ankylosing spondylitis during treatment with etanercept. Ann. Rheum. Dis. 2003, 62, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Hueber, A.J.; McInnes, I.B. Immune regulation in psoriasis and psoriatic arthritis-Recent developments. Immunol. Lett. 2007, 114, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Herrera, L.A.; De la Cruz-Mosso, U.; Román-Fernández, I.V.; Parra-Rojas, I.; Soñanez-Organis, J.; Hernández-Bello, J.; Morales-Zambrano, R.; Villanueva-Quintero, G.; Muñoz-Valle, J. A potential inflammatory role of IL-31 in psoriatic arthritis: A correlation with Th17 cytokine profile. Int. J. Immunopathol. Pharmacol. 2020, 34, 2058738420907186. [Google Scholar] [CrossRef]
- Fromm, S.; Cunningham, C.C.; Dunne, M.R.; Veale, D.J.; Fearon, U.; Wade, S.M. Enhanced angiogenic function in response to fibroblasts from psoriatic arthritis synovium compared to rheumatoid arthritis. Arthritis Res. Ther. 2019, 21, 297. [Google Scholar] [CrossRef] [PubMed]
- Skougaard, M.; Jørgensen, T.S.; Rifbjerg-Madsen, S.; Coates, L.C.; Egeberg, A.; Amris, K.; Dreyer, L.; Højgaard, P.; Guldberg-Møller, J.; Merola, J.F.; et al. Relationship between Fatigue and Inflammation, Disease Duration, and Chronic Pain in Psoriatic Arthritis: An Observational DANBIO Registry Study. J. Rheumatol. 2020, 47, 548–552. [Google Scholar] [CrossRef] [PubMed]
- McInnes, I.B.; Mease, P.J.; Kirkham, B.; Kavanaugh, A.; Ritchlin, C.T.; Rahman, P.; van der Heijde, D.; Landewé, R.; Conaghan, P.G.; Gottlieb, A.B.; et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2015, 386, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Mease, P.J.; Van Der Heijde, D.; Ritchlin, C.T.; Okada, M.; Cuchacovich, R.S.; Shuler, C.L.; Lin, C.-Y.; Braun, D.K.; Lee, C.H.; Gladman, D.D. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: Results from the 24-week randomised, double-blind, placebocontrolled and active (adalimumab)-controlled period of th. Ann. Rheum. Dis. 2017, 76, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Furue, K.; Ito, T.; Furue, M. Differential efficacy of biologic treatments targeting the TNF-α/IL-23/IL-17 axis in psoriasis and psoriatic arthritis. Cytokine 2018, 111, 182–188. [Google Scholar] [CrossRef]
- Schulz, M.; Dotzlaw, H.; Neeck, G. Ankylosing spondylitis and rheumatoid arthritis: Serum levels of TNF- α and its soluble receptors during the course of therapy with etanercept and infliximab. Biomed. Res. Int. 2014, 2014, 675108. [Google Scholar] [CrossRef]
- Gane, J.M.; Stockley, R.A.; Sapey, E. TNF- α Autocrine Feedback Loops in Human Monocytes: The Pro- and Anti-Inflammatory Roles of the TNF- α Receptors Support the Concept of Selective TNFR1 Blockade in vivo. J. Immunol. Res. 2016, 2016, 1079851. [Google Scholar] [CrossRef]
- Miossec, P. Local and systemic effects of IL-17 in joint inflammation: A historical perspective from discovery to targeting. Cell. Mol. 2021, 18, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Mc Ardle, A.; Flatley, B.; Pennington, S.R.; FitzGerald, O. Early biomarkers of joint damage in rheumatoid and psoriatic arthritis. Arthritis Res. Ther. 2015, 17, 141. [Google Scholar] [CrossRef]
- Meka, R.R.; Venkatesha, S.H.; Dudics, S.; Acharya, B.; Moudgil, K.D. IL-27-induced modulation of autoimmunity and its therapeutic potential. Autoimmun. Rev. 2015, 14, 1131–1141. [Google Scholar] [CrossRef]
- van Roon, J.A.G.; Glaudemans, K.A.F.M.; Bijilsma, J.W.J.; Lafeber FP, J.G. Interleukin 7 stimulates tumour necrosis factor α and Th 1 cytokine production in joints of patients with rheumatoid arthritis. Ann. Rheum. Dis. 2003, 62, 113–119. [Google Scholar] [CrossRef]
- Ogata, A.; Kumanogoh, A.; Tanaka, T. Pathological Role of Interleukin-6 in Psoriatic Arthritis. Arthritis 2012, 2012, 1713618. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, B.W.; Kavanaugh, A.; Reich, K. Interleukin-17A: A unique pathway in immune-mediated diseases: Psoriasis, psoriatic arthritis and rheumatoid arthritis. Immunology 2014, 141, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Schoels, M.M.; Aletaha, D.; Alasti, F.; Smolen, J.S. Disease activity in psoriatic arthritis (PsA): Defining remission and treatment success using the DAPSA score. Ann. Rheum. Dis. 2016, 75, 811–818. [Google Scholar] [CrossRef]
- Højgaard, P.; Christensen, R.; Dreyer, L.; Mease, P.; de Wit, M.; Skov, L.; Glintborg, B.; Christensen, A.W.; Ballegaard, C.; Bliddal, H.; et al. Pain mechanisms and ultrasonic inflammatory activity as prognostic factors in patients with psoriatic arthritis: Protocol for a prospective, exploratory cohort study. BMJ Open 2016, 6, e010650. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).