Metabolic Dysregulation and Its Role in Postoperative Pain among Knee Osteoarthritis Patients
Abstract
1. Introduction
2. Results
2.1. Clinical Parameters of the Examined Patients with End-Stage KOA before Surgery
2.2. Whole-Blood Gene Expression
2.3. Protein Levels of the Examined Genes in Isolated PBMCs
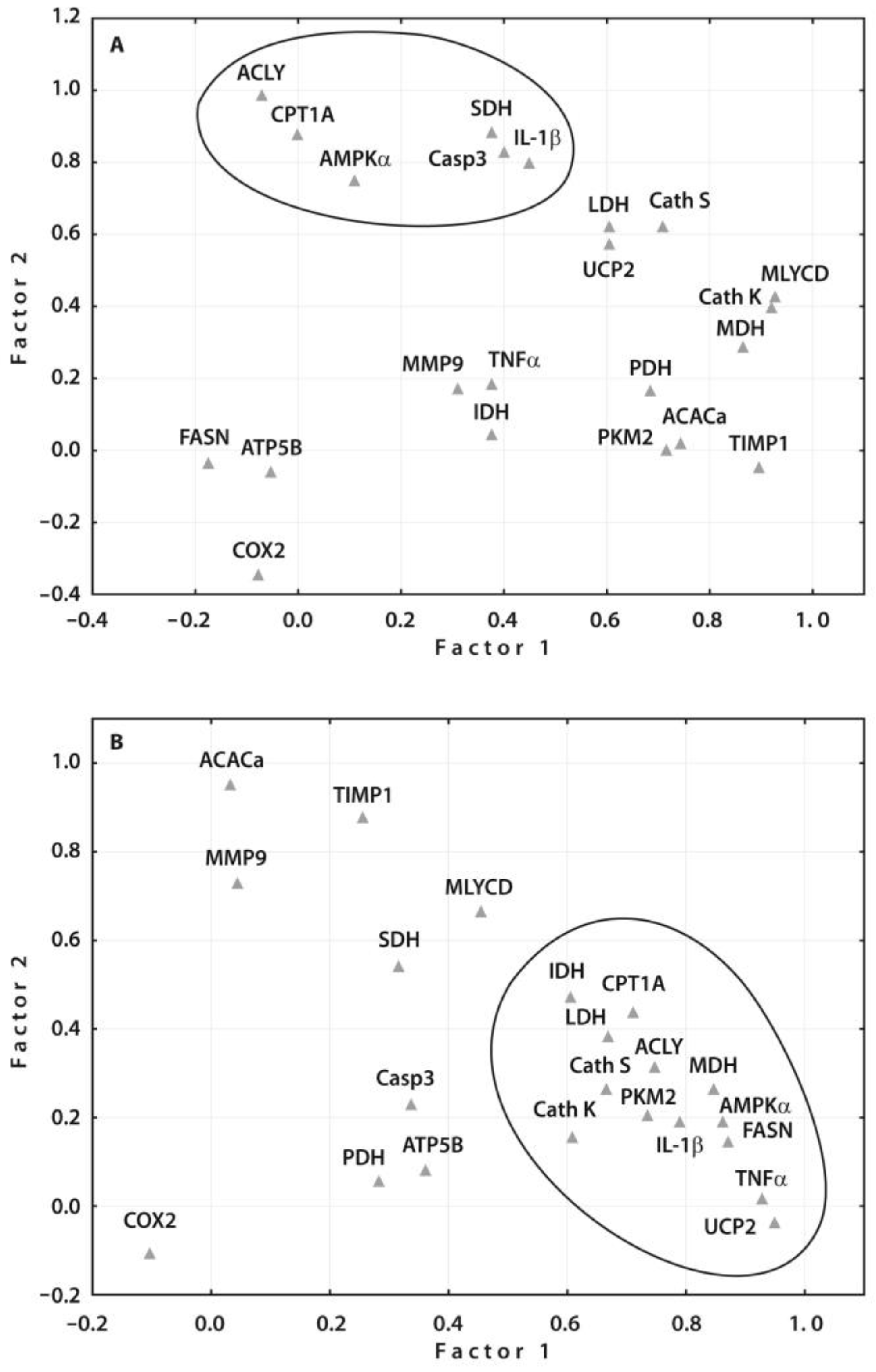
2.4. Correlation Analyses of the Gene Expressions with Clinical and Radiographic Parameters
2.5. Validation of Prognostic Values of the Identified Gene Expressions
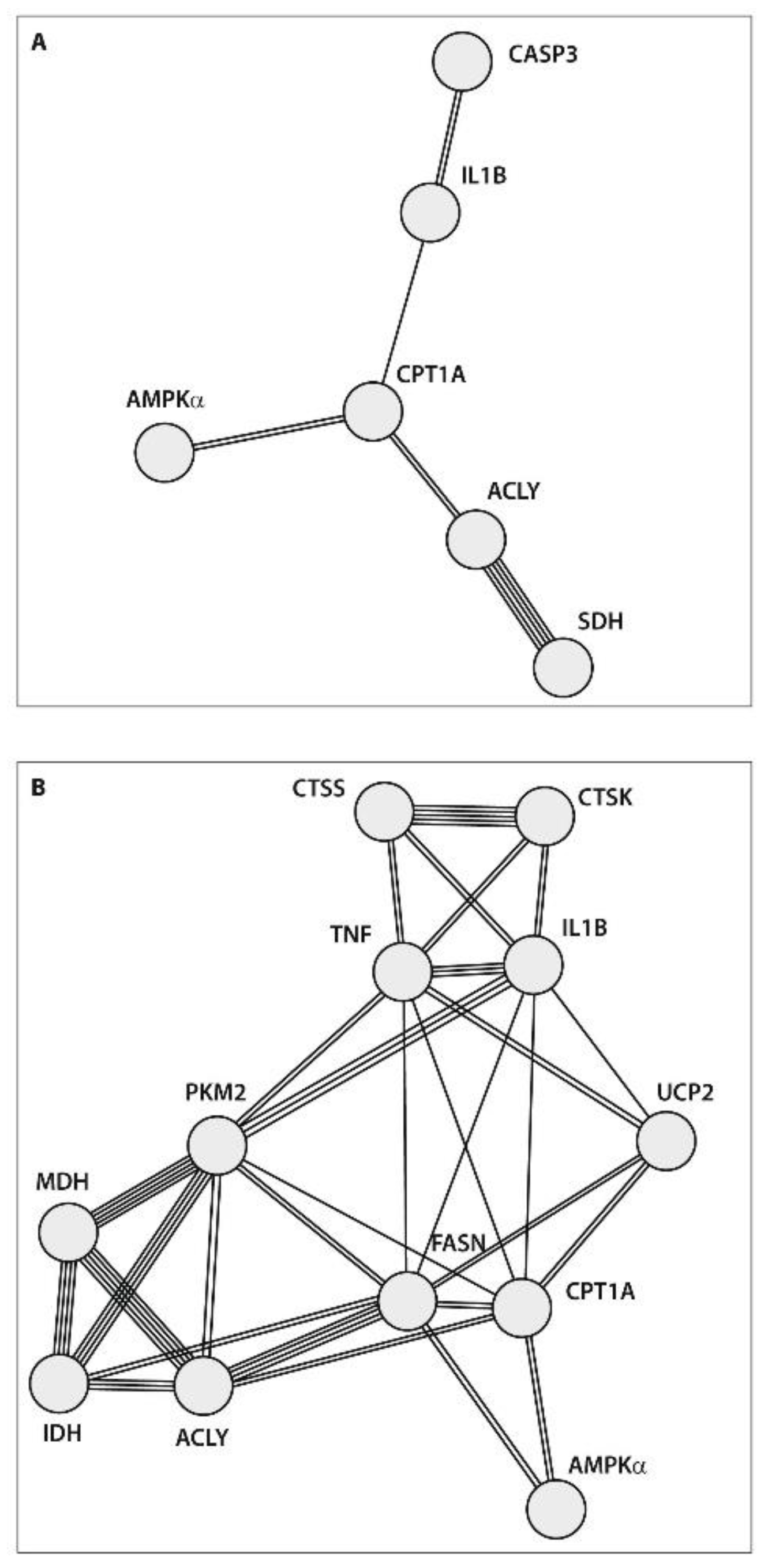
2.6. Protein–Protein Interaction (PPI) Network Construction
3. Discussion
4. Materials and Methods
4.1. Clinical Testing
4.2. Quantification of Protein Levels by the Enzyme-Linked Immunosorbent Assay (ELISA)
4.3. Total RNA Isolation and Reverse Transcriptase (RT) Reaction
4.4. Real-Time Quantitative PCR
4.5. Statistical Analysis
5. Conclusions
6. Patients
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hunter, D.J.; March, L.; Chew, M. Osteoarthritis in 2020 and beyond: A Lancet Commission. Lancet 2020, 396, 1711–1712. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L. Osteoarthritis of the Knee. N. Engl. J. Med. 2021, 384, 51–59. [Google Scholar] [CrossRef]
- Moseng, T.; Vlieland, T.P.M.V.; Battista, S.; Beckwée, D.; Boyadzhieva, V.; Conaghan, P.G.; Costa, D.; Doherty, M.; Finney, A.G.; Georgiev, T.; et al. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis: 2023 update. Ann. Rheum. Dis. 2024. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Lee, K.; Ju, J.H. Recent updates of diagnosis, pathophysiology, and treatment on osteoarthritis of the knee. Int. J. Mol. Sci. 2021, 22, 2619. [Google Scholar] [CrossRef]
- Katz, J.N.; Arant, K.R.; Loeser, R.F. Diagnosis and treatment of hip and knee osteoarthritis: A review. JAMA. 2021, 325, 568–578. [Google Scholar] [CrossRef]
- Feng, H.; Feng, M.-L.; Cheng, J.-B.; Zhang, X.; Tao, H.-C. Meta-analysis of factors influencing anterior knee pain after total knee arthroplasty. World J. Orthop. 2024, 15, 180–191. [Google Scholar] [CrossRef]
- Macrae, W.A.; Davies, H.T.O. Chronic postsurgical pain. In Epidemiology of Pain; Crombie, I.K., Ed.; IASP Press: Seattle, WA, USA, 1999; pp. 125–142. [Google Scholar]
- Macdonald, L.; Bruce, J.; Scott, N.W.; Smith, W.C.S.; Chambers, W.A. Long-term follow-up of breast cancer survivors with post-mastectomy pain syndrome. Br. J. Cancer 2005, 92, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Simpson, A.H.R.W.; Clement, N.D.; Simpson, S.A.; Pandit, H.; Smillie, S.; Leeds, A.R.; Conaghan, P.G.; Kingsbury, S.R.; Hamilton, D.; Craig, P.; et al. A preoperative package of care for osteoarthritis, consisting of weight loss, orthotics, rehabilitation, and topical and oral analgesia (OPPORTUNITY): A two-centre, open-label, randomised controlled feasibility trial. Lancet Rheumatol. 2024, 6, 237–246. [Google Scholar] [CrossRef]
- Umoh, I.O.; dos Reis, H.J.; de Oliveira, A.C.P. Molecular Mechanisms Linking Osteoarthritis and Alzheimer’s Disease: Shared Pathways, Mechanisms and Breakthrough Prospects. Int. J. Mol. Sci. 2024, 25, 3044. [Google Scholar] [CrossRef]
- Attur, M.; Belitskaya-Lévy, I.; Oh, C.; Krasnokutsky, S.; Greenberg, J.; Samuels, J.; Smiles, S.; Lee, S.; Patel, J.; Al-Mussawir, H.; et al. Increased interleukin-1β gene expression in peripheral blood leukocytes is associated with increased pain and predicts risk for progression of symptomatic knee osteoarthritis. Arthritis Rheum. 2011, 63, 1908–1917. [Google Scholar] [CrossRef]
- Tchetina, E.V.; Glemba, K.E.; Markova, G.A.; Naryshkin, E.A.; Taskina, E.A.; Makarov, M.A.; Lila, A.M. Development of Postoperative Pain in Patients with End-Stage Knee Osteoarthritis Is Associated with Upregulation of Genes Related to Extracellular Matrix Degradation, Inflammation, and Apoptosis Measured in the Peripheral Blood before Knee Surgery. Life 2020, 10, 224. [Google Scholar] [CrossRef] [PubMed]
- Sellam, J.; Berenbaum, F. Is osteoarthritis a metabolic disease? Jt. Bone Spine 2013, 80, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Leichsenring, A.; Bäcker, I.; Wendt, W.; Andriske, M.; Schmitz, B.; Stichel, C.C.; Lübbert, H. Differential expression of Cathepsin S and X in the spinal cord of a rat neuropathic pain model. BMC Neurosci. 2008, 9, 80. [Google Scholar] [CrossRef]
- Yang, X.; Chen, W.; Zhao, X.; Chen, L.; Lidong, W.; Ran, J.; Wu, L.; Wanli, L. Pyruvate Kinase M2 Modulates the Glycolysis of Chondrocyte and Extracellular Matrix in Osteoarthritis. DNA Cell Biol. 2018, 37, 271–277. [Google Scholar] [CrossRef]
- Johnson, K.; Jung, A.; Murphy, A.; Andreyev, A.; Dykens, J.; Terkeltaub, R. Mitochondrial oxidative phosphorylation is a downstream regulator of nitric oxide effects on chondrocyte matrix synthesis and mineralization. Arthritis Rheum. 2000, 43, 1560–1570. [Google Scholar] [CrossRef]
- O’Neill, L.A.J. Glycolytic reprogramming by TLRs in dendritic cells. Nat. Immunol. 2014, 15, 314–315. [Google Scholar] [CrossRef]
- Azzu, V.; Jastroch, M.; Divakaruni, A.S.; Brand, M.D. The regulation and turnover of mitochondrial uncoupling proteins. Biochim. Biophys. Acta (BBA) Bioenerg. 2010, 1797, 785–791. [Google Scholar] [CrossRef]
- Chen, L.; Zheng, J.J.Y.; Li, G.; Yuan, J.; Ebert, J.R.; Li, H.; Papadimitriou, J.; Wang, Q.; Wood, D.; Jones, C.W.; et al. Pathogenesis and clinical management of obesity-related knee osteoarthritis: Impact of mechanical loading. J. Orthop. Transl. 2020, 24, 66–75. [Google Scholar] [CrossRef]
- Raud, B.; Gay, C.; Guiguet-Auclair, C.; Bonnin, A.; Gerbaud, L.; Pereira, B.; Duclos, M.; Boirie, Y.; Coudeyre, E. Level of obesity is directly associated with the clinical and functional consequences of knee osteoarthritis. Sci. Rep. 2020, 10, 3601. [Google Scholar] [CrossRef]
- Zechner, R.; Strauss, J.G.; Haemmerle, G.; Lass, A.; Zimmermann, R. Lipolysis: Pathway under construction. Curr. Opin. Infect. Dis. 2005, 16, 333–340. [Google Scholar] [CrossRef]
- Stryer, L. Fatty acid metabolism. In Biochemistry, 4th ed.; W.H. Freeman and Company: New York, NY, USA, 1995; pp. 603–628. ISBN 0-7167-2009-4. [Google Scholar]
- Anderson, C.M.; Stahl, A. SLC27 fatty acid transport proteins. Mol. Asp. Med. 2013, 34, 516–528. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, N.; Swinnen, J.V.; Smans, K. ATP-citrate lyase: A key player in cancer metabolism. Cancer Res. 2012, 72, 3709–3714. [Google Scholar] [CrossRef] [PubMed]
- Ferré, P.; Foufelle, F. SREBP-1c Transcription Factor and Lipid Homeostasis: Clinical Perspective. Horm. Res. Paediatr. 2007, 68, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Yang, Z.; Mao, E.; Chen, E. Regulation of fatty acid synthesis in immune cells. Scand. J. Immunol. 2018, 88, e12713. [Google Scholar] [CrossRef]
- Sacksteder, K.A.; Morrell, J.C.; Wanders, R.J.A.; Matalon, R.; Gould, S.J. MCD encodes peroxisomal and cytoplasmic forms of malonyl-CoA decarboxylase and is mutated in malonyl-CoA decarboxylase deficiency. J. Biol. Chem. 1999, 274, 24461–24468. [Google Scholar] [CrossRef] [PubMed]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Wedan, R.J.; Longenecker, J.Z.; Nowinski, S.M. Mitochondrial fatty acid synthesis is an emergent central regulator of mammalian oxidative metabolism. Cell Metab. 2024, 36, 36–47. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A.J.; Hardie, D.G. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature 2013, 493, 346–355. [Google Scholar] [CrossRef]
- Liu, T.F.; Brown, C.M.; El Gazzar, M.; McPhail, L.; Millet, P.; Rao, A.; Vachharajani, V.T.; Yoza, B.K.; McCall, C.E. Fueling the flame: Bioenergy couples metabolism and inflammation. J. Leukoc. Biol. 2012, 92, 499–507. [Google Scholar] [CrossRef]
- Jaworska, M.; Szczudło, J.; Pietrzyk, A.; Shah, J.; Trojan, S.E.; Ostrowska, B.; Kocemba-Pilarczyk, K.A. The Warburg effect: A score for many instruments in the concert of cancer and cancer niche cells. Pharmacol. Rep. 2023, 75, 876–890. [Google Scholar] [CrossRef]
- Jia, L.; Zhang, L.; Liu, M.; Ji, H.; Wen, Z.; Wang, C. Mitochondrial control for healthy and autoimmune T cells. Cells 2023, 12, 1800. [Google Scholar] [CrossRef] [PubMed]
- Van Pevenage, P.M.; Birchmier, J.T.; June, R.K. Utilizing metabolomics to identify potential biomarkers and perturbed metabolic pathways in osteoarthritis: A systematic review. Semin. Arthritis Rheum. 2023, 59, 152163. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.-X.; Zhai, M.-N.; Zhu, M.; He, C.; Wang, H.; Wang, J.; Zhang, Z.-J. Inflammation in pathogenesis of chronic pain: Foe and friend. Mol. Pain 2023, 19, 17448069231178176. [Google Scholar] [CrossRef] [PubMed]
- Altman, F.P. A metabolic dysfunction in early murine osteoarthritis. Ann. Rheum. Dis. 1981, 40, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Nahir, A.M.; Vitis, N.; Silbermann, M. Cellular enzymatic activities in the articular cartilage of osteoarthritic and osteoporotic hip joints of humans: A quantitative cytochemical study. Aging Clin. Exp. Res. 1990, 2, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.L.; Kelly, B.; Logan, A.; Costa, A.S.H.; Varma, M.; Bryant, C.E.; Tourlomousis, P.; Däbritz, J.H.M.; Gottlieb, E.; Latorre, I.; et al. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell 2016, 167, 457–470.e13. [Google Scholar] [CrossRef] [PubMed]
- Williams, N.C.; O’neill, L.A.J. A Role for the Krebs Cycle Intermediate Citrate in Metabolic Reprogramming in Innate Immunity and Inflammation. Front. Immunol. 2018, 9, 141. [Google Scholar] [CrossRef] [PubMed]
- Sibille, K.T.; King, C.; Garrett, T.J.; Glover, T.L.; Zhang, H.; Chen, H.; Reddy, D.; Goodin, B.R.; Sotolongo, A.; Petrov, M.E.; et al. Omega-6:Omega-3 PUFA Ratio, Pain, Functioning, and Distress in Adults With Knee Pain. Clin. J. Pain 2018, 34, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Asou, H.K.; Matsugae, N.; Hirahara, K.; Shinoda, K.; Tumes, D.J.; Tokuyama, H.; Yokote, K.; Nakayama, T. Obesity drives Th17 cell differentiation by inducing the lipid metabolic kinase, ACC1. Cell Rep. 2015, 12, 1042–1055. [Google Scholar] [CrossRef]
- Miao, Y.; Wu, X.; Xue, X.; Ma, X.; Yang, L.; Zeng, X.; Hu, Y.; Dai, Y.; Wei, Z. Morin, the PPARγ agonist, inhibits Th17 differentiation by limiting fatty acid synthesis in collagen-induced arthritis. Cell Biol. Toxicol. 2022, 39, 1433–1452. [Google Scholar] [CrossRef]
- Paik, W.K.; Pearson, D.; Lee, H.W.; Kim, S. Nonenzymatic acetylation of histones with acetyl-CoA. Biochim. Biophys. Acta (BBA) Nucleic Acids Protein Synth. 1970, 213, 513–522. [Google Scholar] [CrossRef]
- Shi, L.; Tu, B.P. Protein acetylation as a means to regulate protein function in tune with metabolic state. Biochem. Soc. Trans. 2014, 42, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.R.; Hirschey, M.D. Nonenzymatic protein acylation as a carbon stress regulated by sirtuin deacylases. Mol. Cell 2014, 54, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Beaumont, G.; Castro-Dominguez, F.; Migliore, A.; Naredo, E.; Largo, R.; Reginster, J.-Y. Systemic osteoarthritis: The difficulty of categorically naming a continuous condition. Aging Clin. Exp. Res. 2024, 36, 45. [Google Scholar] [CrossRef] [PubMed]
- Lungu, E.; Vendittoli, P.-A.; Desmeules, F. Preoperative Determinants of Patient-reported Pain and Physical Function Levels Following Total Knee Arthroplasty: A Systematic Review. Open Orthop. J. 2016, 10, 213–231. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-X.; Xu, S.-Z.; Tian, T.; Wang, J.; Jiang, L.-Q.; He, T.; Meng, S.-Y.; Ni, J.; Pan, H.-F. Genetic Links Between Metabolic Syndrome and Osteoarthritis: Insights from Cross-Trait Analysis. J. Clin. Endocrinol. Metab. 2024, 14, dgae169. [Google Scholar] [CrossRef]
- Olsen, U.; Sellevold, V.B.; Gay, C.L.; Aamodt, A.; Lerdal, A.; Hagen, M.; Dihle, A.; Lindberg, M.F. Factors associated with pain and functional impairment five years after total knee arthroplasty: A prospective observational study. BMC Musculoskelet. Disord. 2024, 25, 22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, Y.; Li, Y.; Liu, R.; Rai, S.; Li, J.; Hong, P. Enhanced recovery after surgery in patients after hip and knee arthroplasty: A systematic review and meta-analysis. Postgrad. Med. J. 2023, 100, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Maccagnano, G.; Pesce, V.; Noia, G.; Coviello, M.; Vicenti, G.; Vitiello, R.; Ziranu, A.; Spinarelli, A.; Moretti, B. The effects of a new protocol on blood loss in total knee arthroplasty. Orthop. Rev. 2022, 14, 37625. [Google Scholar] [CrossRef]
- Kellgren, J.H.; Lawrence, J.S. Radiological Assessment of Osteo-Arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef]
- Bellamy, N. WOMAC Osteoarthritis Index: A User’s Guide; University of Western Ontario: London, ON, Canada, 1995. [Google Scholar]
- Freynhagen, R.; Baron, R.; Gockel, U.; Tölle, T.R. painDETECT: A new screening questionnaire to identify neuropathic components in patients with back pain. Curr. Med. Res. Opin. 2006, 22, 1911–1920. [Google Scholar] [CrossRef]
- Bouhassira, D.; Attal, N.; Alchaar, H.; Boureau, F.; Brochet, B.; Bruxelle, J.; Cunin, G.; Fermanian, J.; Ginies, P.; Grun-Overdyking, A.; et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain 2005, 114, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Cleeland, C.S. Measurement of pain by subjective report. In Issues in Pain Measurement. Advances in Pain Research and Therapy; Chapman, C.R., Loeser, J.D., Eds.; Raven Press: New York, NY, USA, 1989; Volume 12, pp. 391–403. [Google Scholar]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Son, B.K.; Roberts, R.L.; Ank, B.J.; Stiehm, E.R. Effects of anticoagulant, serum, and temperature on the natural killer activity of human peripheral blood mononuclear cells stored overnight. Clin. Diagn. Lab. Immunol. 1996, 3, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Tchetina, E.V.; Poole, A.R.; Zaitseva, E.M.; Sharapova, E.P.; Kashevarova, N.G.; Taskina, E.A.; Alekseeva, L.I.; Semyonova, L.A.; Glukhova, S.I.; Kuzin, A.N.; et al. Differences in mTOR (mammalian target of rapamycin) gene expression in the peripheral blood and articular cartilages of osteoarthritic patients and disease activity. Arthritis 2013, 2013, 461486. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J. Comparative Ct method. ABI Prism 7700 sequence detection system. In User Bulletin No. 2; PE Applied Biosystems: Foster City, CA, USA, 1997. [Google Scholar]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Altman, R.; Asch, E.; Bloch, D.; Bole, G.; Borenstein, D.; Brandt, K.; Christy, W.; Cooke, T.D.; Greenwald, R.; Hochberg, M.; et al. Development of criteria for the classification and reporting of osteoarthritis: Classification of osteoarthritis of the knee. Arthritis Rheum. 1986, 29, 1039–1049. [Google Scholar] [CrossRef]
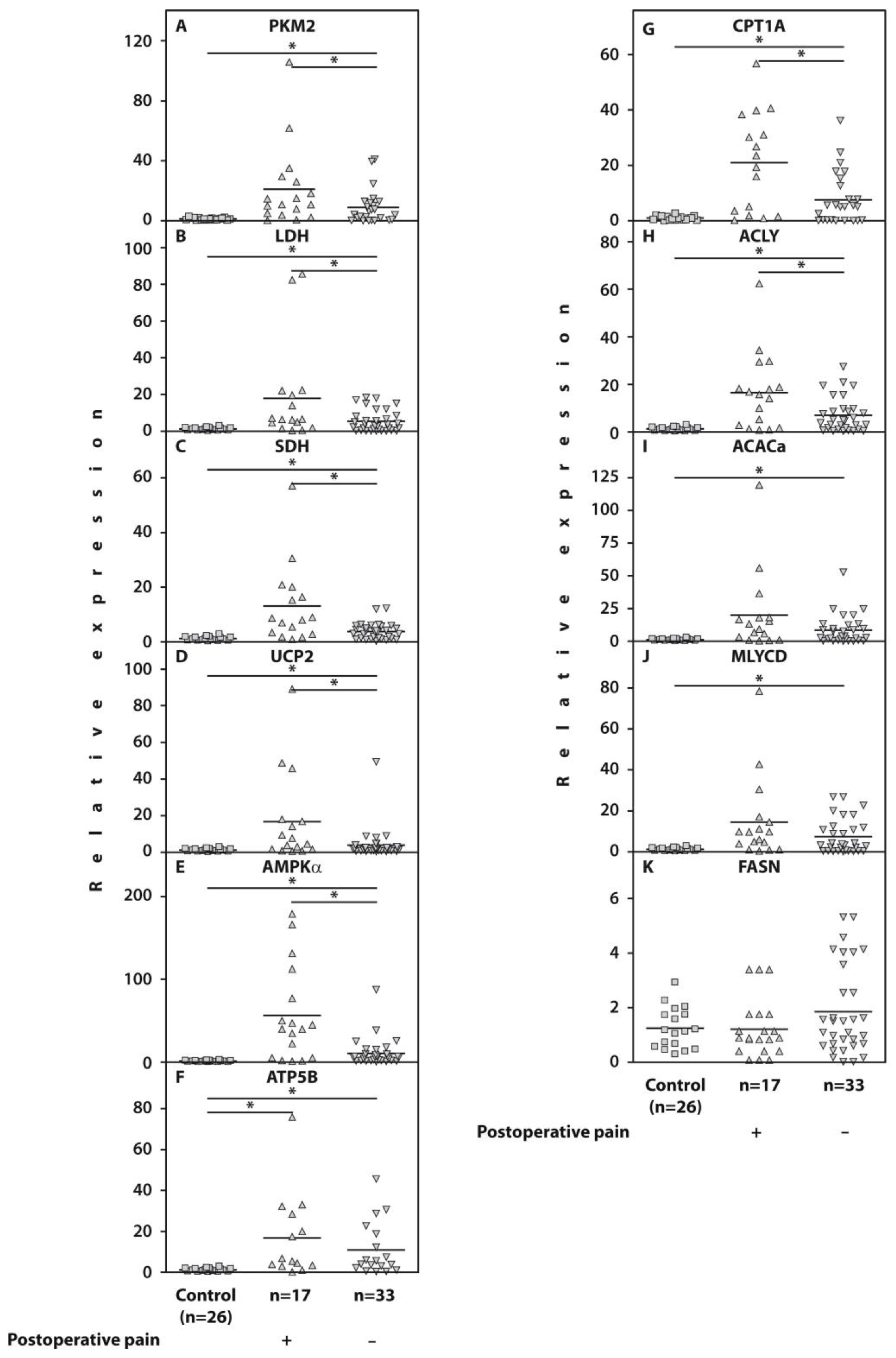


| Gene | Patients Who Developed POP n = 17 | Pain-Free Subjects n = 33 | p (Mann–Whitney U-Test) |
|---|---|---|---|
| PKM2 | 12.6 [5.7; 27.8] | 4.1 [0.3; 11.5] | 0.03 |
| LDH | 6.4 [3.2; 20.9] | 3.9 [0.9; 7.6] | 0.04 |
| SDH | 8.3 [2.3; 20.5] | 3.7 [1.0; 5.7] | 0.02 |
| MDH | 5.8 [1.4; 30.6] | 3.2 [0.6; 5.5] | ns |
| IDH | 4.8 [2.1; 18.3] | 4.8 [2.1; 8.5] | ns |
| UCP2 | 6.1 [1.6; 17.4] | 1.8 [0.8; 2.8] | 0.02 |
| AMPKα | 39.9 [3.6; 122.0] | 6.4 [1.6; 16.8] | 0.001 |
| ATP5B | 6.1 [3.2; 30.4] | 4.8 [1.7; 20.6] | ns |
| CPT1A | 21.7 [2.9; 35.0] | 6.0 [0.9; 17.0] | 0.01 |
| ACLY | 16.2 [3.9; 24.0] | 5.0 [1.7; 9.1] | 0.01 |
| ACACa | 13.2 [3.2; 18.2] | 4.0 [1.3; 12.9] | ns |
| MLYCD | 9.8 [3.8; 17.1] | 3.9 [0.7; 13.2] | ns |
| FASN | 1.1 [0.7; 3.0] | 1.5 [0.7; 2.8] | ns |
| AMPKα | PKM2 | UCP2 | ACLY | MLYCD | CPT1A | |
|---|---|---|---|---|---|---|
| DN4 | 0.423 p = 0.013 | 0.471 p = 0.004 | 0.33 p = 0.046 | 0.446 p = 0.006 | 0.368 p = 0.03 | 0.368 p = 0.027 |
| PainDETECT | 0.343 p = 0.063 | 0.342 p = 0.06 | 0.456 p = 0.011 | 0.459 p = 0.012 | 0.388 p = 0.037 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tchetina, E.V.; Glemba, K.E.; Markova, G.A.; Glukhova, S.I.; Makarov, M.A.; Lila, A.M. Metabolic Dysregulation and Its Role in Postoperative Pain among Knee Osteoarthritis Patients. Int. J. Mol. Sci. 2024, 25, 3857. https://doi.org/10.3390/ijms25073857
Tchetina EV, Glemba KE, Markova GA, Glukhova SI, Makarov MA, Lila AM. Metabolic Dysregulation and Its Role in Postoperative Pain among Knee Osteoarthritis Patients. International Journal of Molecular Sciences. 2024; 25(7):3857. https://doi.org/10.3390/ijms25073857
Chicago/Turabian StyleTchetina, Elena V., Kseniya E. Glemba, Galina A. Markova, Svetlana I. Glukhova, Maksim A. Makarov, and Aleksandr M. Lila. 2024. "Metabolic Dysregulation and Its Role in Postoperative Pain among Knee Osteoarthritis Patients" International Journal of Molecular Sciences 25, no. 7: 3857. https://doi.org/10.3390/ijms25073857
APA StyleTchetina, E. V., Glemba, K. E., Markova, G. A., Glukhova, S. I., Makarov, M. A., & Lila, A. M. (2024). Metabolic Dysregulation and Its Role in Postoperative Pain among Knee Osteoarthritis Patients. International Journal of Molecular Sciences, 25(7), 3857. https://doi.org/10.3390/ijms25073857






