Challenges in the Therapeutic Targeting of KCa Channels: From Basic Physiology to Clinical Applications
Abstract
1. Introduction
2. BK Channels
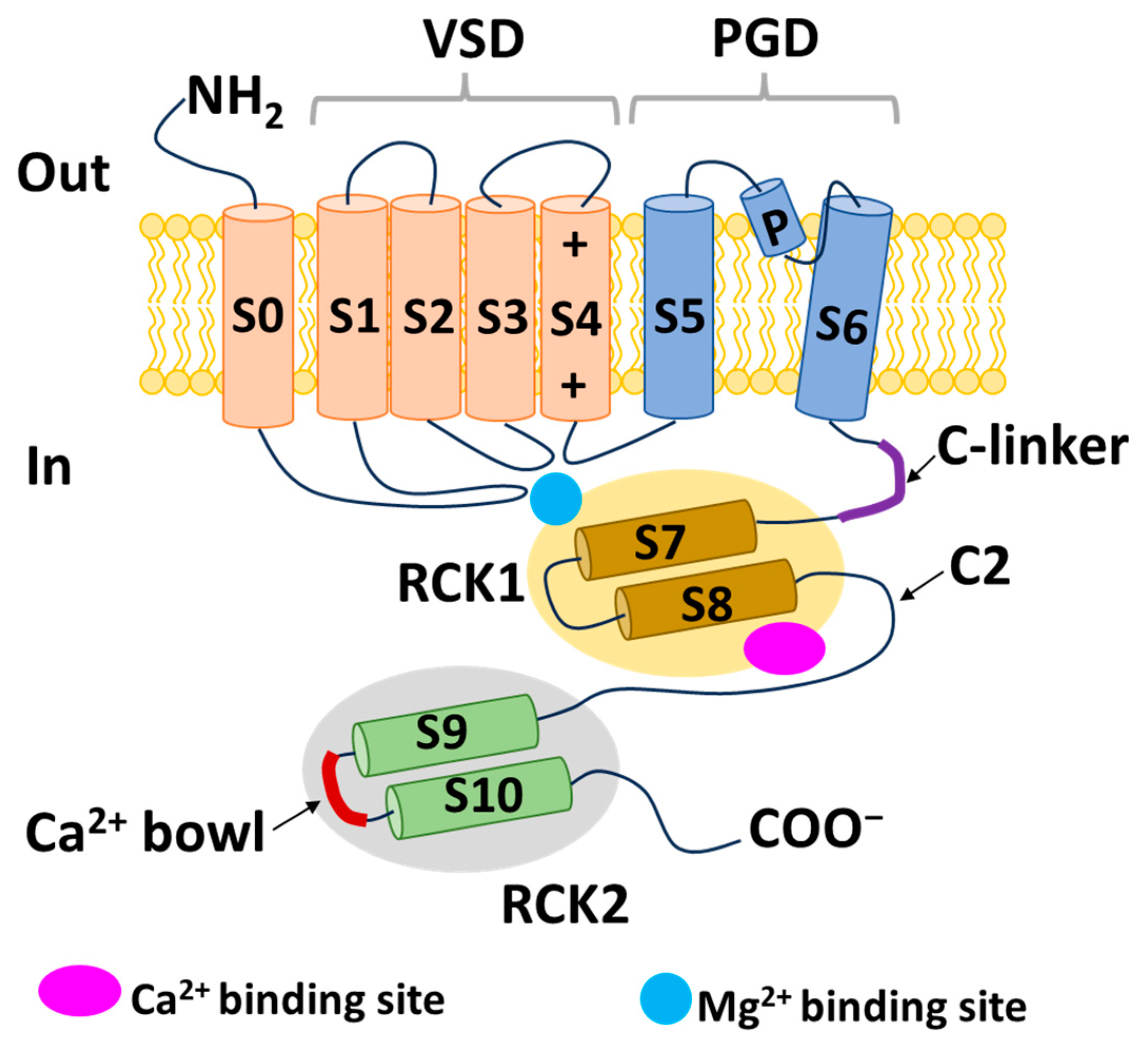
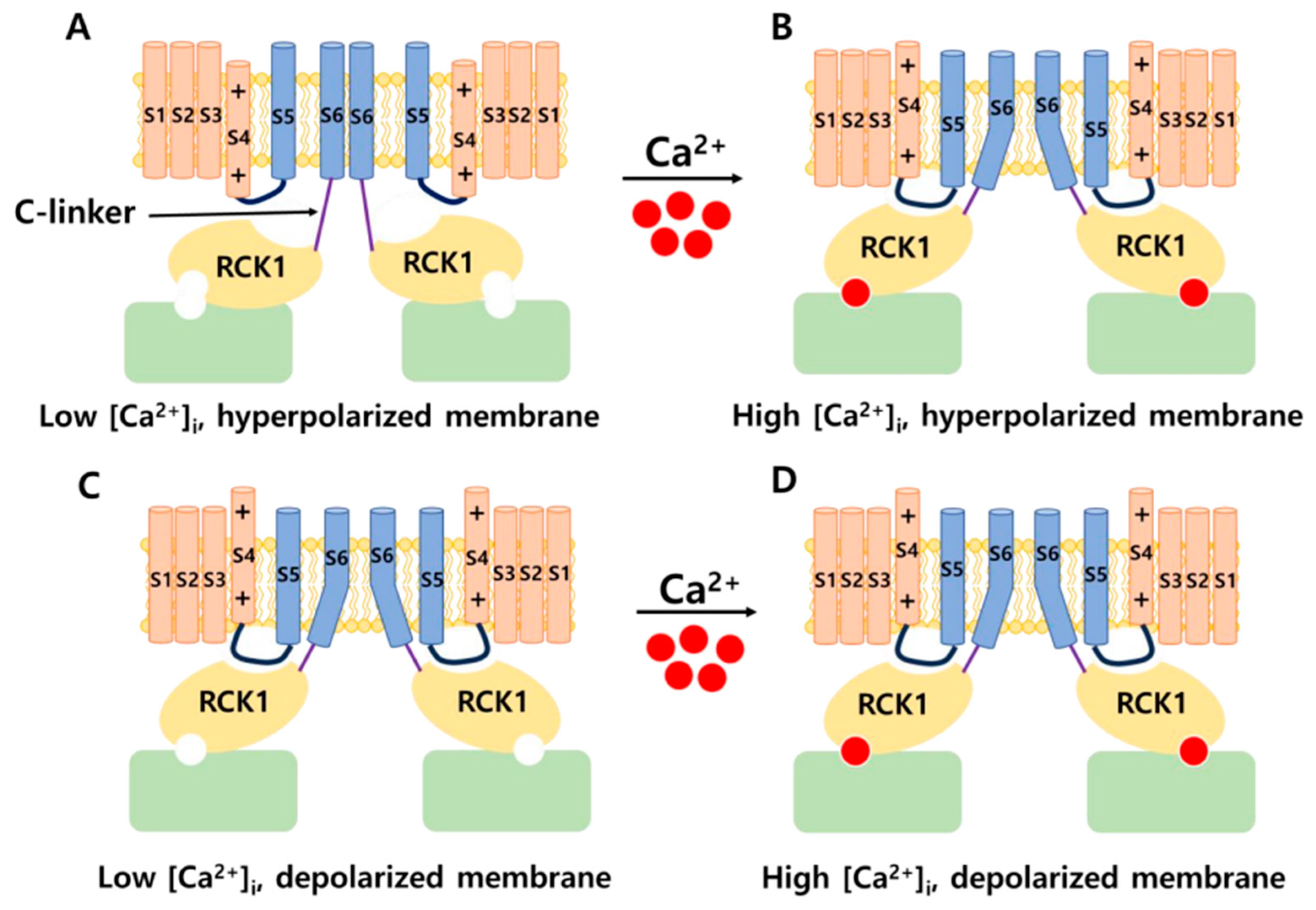
2.1. Structure of BK Channels
2.1.1. α Subunits of BK Channels
2.1.2. Auxiliary Subunits: BK-β and BK-γ
2.2. Distribution and Physiology of BK Channels
2.3. Modulators Targeting BK Channels
2.3.1. Neurological Diseases
2.3.2. Cardiovascular Disorders
2.3.3. Cancers
3. IK Channels
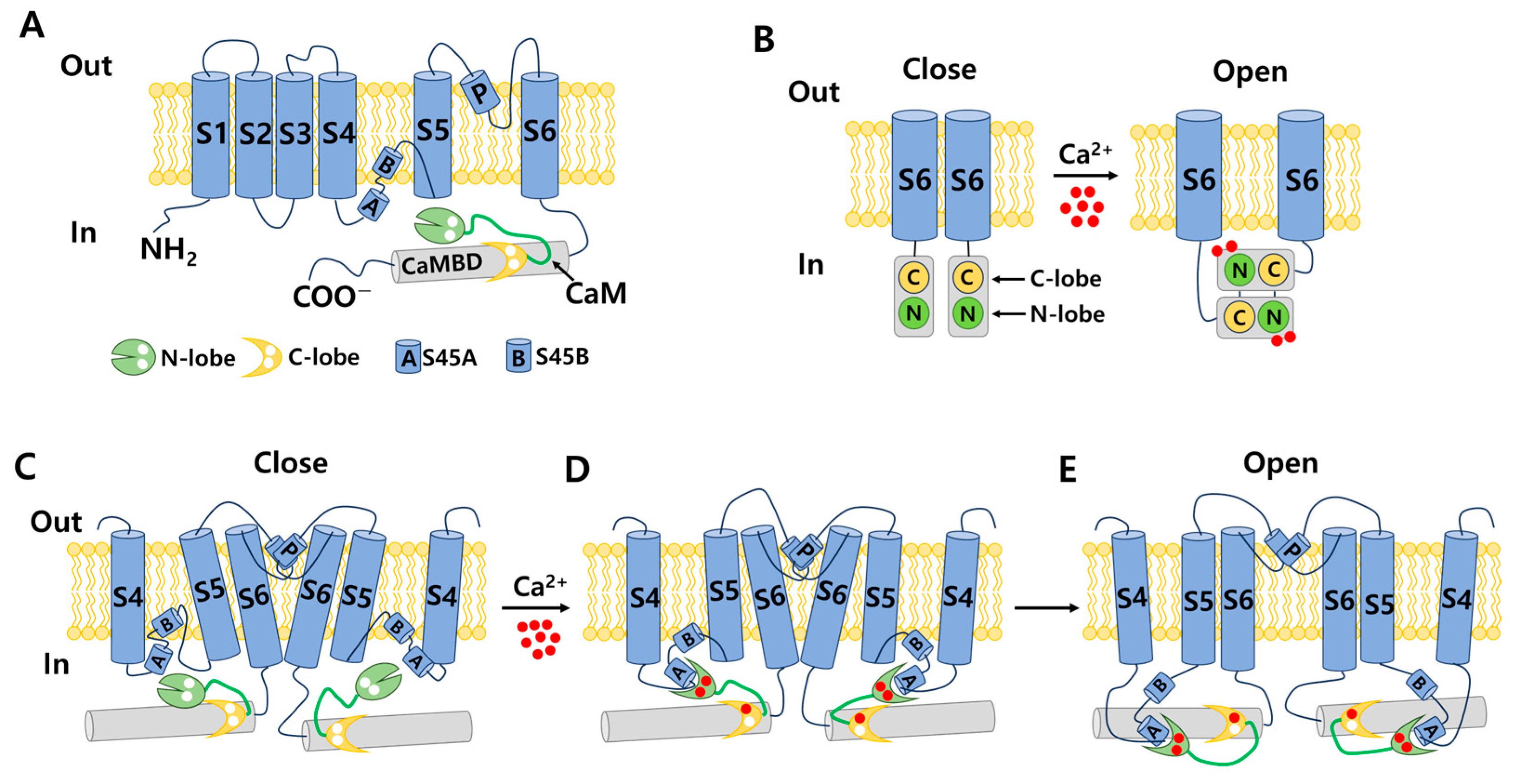
3.1. Structure of IK and SK Channels
3.2. Distribution and Physiology of IK Channels
3.3. Modulators Targeting IK Channels
3.3.1. Blood Cell Disorders
3.3.2. Cancer
3.3.3. Neurological Diseases
4. SK Channels
4.1. Distribution and Physiology of SK Channels
4.2. Modulators Targeting SK Channels
4.2.1. Neurological Diseases
4.2.2. Cardiovascular Diseases
5. Discussion
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gardos, G. The function of calcium in the potassium permeability of human erythrocytes. Biochim. Biophys. Acta 1958, 30, 653–654. [Google Scholar] [CrossRef]
- Meech, R.W. Intracellular calcium injection causes increased potassium conductance in Aplysia nerve cells. Comp. Biochem. Physiol. A Comp. Physiol. 1972, 42, 493–499. [Google Scholar] [CrossRef]
- Krnjević, K.; Lisiewicz, A. Injections of calcium ions into spinal motoneurones. J. Physiol. 1972, 225, 363–390. [Google Scholar] [CrossRef]
- Weaver, A.K.; Bomben, V.C.; Sontheimer, H. Expression and function of calcium-activated potassium channels in human glioma cells. Glia 2006, 54, 223–233. [Google Scholar] [CrossRef]
- Marty, A. Ca-dependent K channels with large unitary conductance in chromaffin cell membranes. Nature 1981, 291, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.M.; Silvia, C.; Hirschberg, B.; Bond, C.T.; Adelman, J.P.; Maylie, J. A human intermediate conductance calcium-activated potassium channel. Proc. Natl. Acad. Sci. USA 1997, 94, 11651–11656. [Google Scholar] [CrossRef] [PubMed]
- Maylie, J.; Bond, C.T.; Herson, P.S.; Lee, W.S.; Adelman, J.P. Small conductance Ca2+-activated K+ channels and calmodulin. J. Physiol. 2004, 554, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Ghatta, S.; Nimmagadda, D.; Xu, X.; O’Rourke, S.T. Large-conductance, calcium-activated potassium channels: Structural and functional implications. Pharmacol. Ther. 2006, 110, 103–116. [Google Scholar] [CrossRef]
- Adams, P.R.; Constanti, A.; Brown, D.A.; Clark, R.B. Intracellular Ca2+ activates a fast voltage-sensitive K+ current in vertebrate sympathetic neurones. Nature 1982, 296, 746–749. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.M.; Fakler, B.; Rivard, A.; Wayman, G.; Johnson-Pais, T.; Keen, J.E.; Ishii, T.; Hirschberg, B.; Bond, C.T.; Lutsenko, S.; et al. Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature 1998, 395, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Horrigan, F.T.; Aldrich, R.W. Coupling between voltage sensor activation, Ca2+ binding and channel opening in large conductance (BK) potassium channels. J. Gen. Physiol. 2002, 120, 267–305. [Google Scholar] [CrossRef]
- Sweet, T.B.; Cox, D.H. Measurements of the BKCa channel’s high-affinity Ca2+ binding constants: Effects of membrane voltage. J. Gen. Physiol. 2008, 132, 491–505. [Google Scholar] [CrossRef]
- Brown, B.M.; Shim, H.; Christophersen, P.; Wulff, H. Pharmacology of Small- and Intermediate-Conductance Calcium-Activated Potassium Channels. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 219–240. [Google Scholar] [CrossRef]
- Wei, A.; Solaro, C.; Lingle, C.; Salkoff, L. Calcium sensitivity of BK-type KCa channels determined by a separable domain. Neuron 1994, 13, 671–681. [Google Scholar] [CrossRef]
- Fanger, C.M.; Ghanshani, S.; Logsdon, N.J.; Rauer, H.; Kalman, K.; Zhou, J.; Beckingham, K.; Chandy, K.G.; Cahalan, M.D.; Aiyar, J. Calmodulin mediates calcium-dependent activation of the intermediate conductance KCa channel, IKCa1. J. Biol. Chem. 1999, 274, 5746–5754. [Google Scholar] [CrossRef]
- Adelman, J.P.; Shen, K.Z.; Kavanaugh, M.P.; Warren, R.A.; Wu, Y.N.; Lagrutta, A.; Bond, C.T.; North, R.A. Calcium-activated potassium channels expressed from cloned complementary DNAs. Neuron 1992, 9, 209–216. [Google Scholar] [CrossRef]
- Köhler, M.; Hirschberg, B.; Bond, C.T.; Kinzie, J.M.; Marrion, N.V.; Maylie, J.; Adelman, J.P. Small-conductance, calcium-activated potassium channels from mammalian brain. Science 1996, 273, 1709–1714. [Google Scholar] [CrossRef] [PubMed]
- Logsdon, N.J.; Kang, J.; Togo, J.A.; Christian, E.P.; Aiyar, J. A novel gene, hKCa4, encodes the calcium-activated potassium channel in human T lymphocytes. J. Biol. Chem. 1997, 272, 32723–32726. [Google Scholar] [CrossRef]
- Lee, C.H.; MacKinnon, R. Activation mechanism of a human SK-calmodulin channel complex elucidated by cryo-EM structures. Science 2018, 360, 508–513. [Google Scholar] [CrossRef]
- Li, Q.; Yan, J. Modulation of BK Channel Function by Auxiliary Beta and Gamma Subunits. Int. Rev. Neurobiol. 2016, 128, 51–90. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Lou, X.J.; Horrigan, F.T. Role of charged residues in the S1–S4 voltage sensor of BK channels. J. Gen. Physiol. 2006, 127, 309–328. [Google Scholar] [CrossRef]
- Koval, O.M.; Fan, Y.; Rothberg, B.S. A role for the S0 transmembrane segment in voltage-dependent gating of BK channels. J. Gen. Physiol. 2007, 129, 209–220. [Google Scholar] [CrossRef]
- Bao, L.; Kaldany, C.; Holmstrand, E.C.; Cox, D.H. Mapping the BKCa channel’s “Ca2+ bowl”: Side-chains essential for Ca2+ sensing. J. Gen. Physiol. 2004, 123, 475–489. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, Y.; Ye, S.; Jiang, Y. Structure of the gating ring from the human large-conductance Ca2+-gated K+ channel. Nature 2010, 466, 393–397. [Google Scholar] [CrossRef]
- Li, Q.; Li, Y.; Wei, H.; Pan, H.M.; Vouga, A.G.; Rothberg, B.S.; Wu, Y.; Yan, J. Molecular determinants of Ca2+ sensitivity at the intersubunit interface of the BK channel gating ring. Sci. Rep. 2018, 8, 509. [Google Scholar] [CrossRef] [PubMed]
- Budelli, G.; Geng, Y.; Butler, A.; Magleby, K.L.; Salkoff, L. Properties of Slo1 K+ channels with and without the gating ring. Proc. Natl. Acad. Sci. USA 2013, 110, 16657–16662. [Google Scholar] [CrossRef]
- Lee, U.S.; Cui, J. BK channel activation: Structural and functional insights. Trends Neurosci. 2010, 33, 415–423. [Google Scholar] [CrossRef]
- Niu, X.; Qian, X.; Magleby, K.L. Linker-gating ring complex as passive spring and Ca2+-dependent machine for a voltage- and Ca2+-activated potassium channel. Neuron 2004, 42, 745–756. [Google Scholar] [CrossRef]
- Tao, X.; Hite, R.K.; MacKinnon, R. Cryo-EM structure of the open high-conductance Ca2+-activated K+ channel. Nature 2017, 541, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Hite, R.K.; Tao, X.; MacKinnon, R. Structural basis for gating the high-conductance Ca2+-activated K+ channel. Nature 2017, 541, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Shi, J.; Zhang, G.; Yang, J.; Delaloye, K.; Cui, J. Activation of Slo1 BK channels by Mg2+ coordinated between the voltage sensor and RCK1 domains. Nat. Struct. Mol. Biol. 2008, 15, 1152–1159. [Google Scholar] [CrossRef]
- Yang, H.; Hu, L.; Shi, J.; Delaloye, K.; Horrigan, F.T.; Cui, J. Mg2+ mediates interaction between the voltage sensor and cytosolic domain to activate BK channels. Proc. Natl. Acad. Sci. USA 2007, 104, 18270–18275. [Google Scholar] [CrossRef]
- Chen, R.S.; Geng, Y.; Magleby, K.L. Mg2+ binding to open and closed states can activate BK channels provided that the voltage sensors are elevated. J. Gen. Physiol. 2011, 138, 593–607. [Google Scholar] [CrossRef] [PubMed]
- Horrigan, F.T.; Ma, Z. Mg2+ enhances voltage sensor/gate coupling in BK channels. J. Gen. Physiol. 2008, 131, 13–32. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Xu, R.; Heinemann, S.H.; Hoshi, T. Reciprocal regulation of the Ca2+ and H+ sensitivity in the SLO1 BK channel conferred by the RCK1 domain. Nat. Struct. Mol. Biol. 2008, 15, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Xi, L.; Wang, H.; Huang, X.; Ma, X.; Han, Z.; Wu, P.; Ma, X.; Lu, Y.; Wang, G.; et al. The potassium ion channel opener NS1619 inhibits proliferation and induces apoptosis in A2780 ovarian cancer cells. Biochem. Biophys. Res. Commun. 2008, 375, 205–209. [Google Scholar] [CrossRef]
- Schubert, R.; Krien, U.; Gagov, H. Protons inhibit the BKCa channel of rat small artery smooth muscle cells. J. Vasc. Res. 2001, 38, 30–38. [Google Scholar] [CrossRef]
- Avdonin, V.; Tang, X.D.; Hoshi, T. Stimulatory action of internal protons on Slo1 BK channels. Biophys. J. 2003, 84, 2969–2980. [Google Scholar] [CrossRef]
- Zhou, X.B.; Wulfsen, I.; Utku, E.; Sausbier, U.; Sausbier, M.; Wieland, T.; Ruth, P.; Korth, M. Dual role of protein kinase C on BK channel regulation. Proc. Natl. Acad. Sci. USA 2010, 107, 8005–8010. [Google Scholar] [CrossRef]
- Wallner, M.; Meera, P.; Toro, L. Molecular basis of fast inactivation in voltage and Ca2+-activated K+ channels: A transmembrane beta-subunit homolog. Proc. Natl. Acad. Sci. USA 1999, 96, 4137–4142. [Google Scholar] [CrossRef]
- Brenner, R.; Jegla, T.J.; Wickenden, A.; Liu, Y.; Aldrich, R.W. Cloning and functional characterization of novel large conductance calcium-activated potassium channel beta subunits, hKCNMB3 and hKCNMB4. J. Biol. Chem. 2000, 275, 6453–6461. [Google Scholar] [CrossRef]
- Liu, G.; Niu, X.; Wu, R.S.; Chudasama, N.; Yao, Y.; Jin, X.; Weinberg, R.; Zakharov, S.I.; Motoike, H.; Marx, S.O.; et al. Location of modulatory beta subunits in BK potassium channels. J. Gen. Physiol. 2010, 135, 449–459. [Google Scholar] [CrossRef]
- Orio, P.; Latorre, R. Differential effects of beta 1 and beta 2 subunits on BK channel activity. J. Gen. Physiol. 2005, 125, 395–411. [Google Scholar] [CrossRef]
- Nimigean, C.M.; Magleby, K.L. Functional coupling of the beta(1) subunit to the large conductance Ca2+-activated K+ channel in the absence of Ca2+. Increased Ca2+ sensitivity from a Ca2+-independent mechanism. J. Gen. Physiol. 2000, 115, 719–736. [Google Scholar] [CrossRef]
- Bentrop, D.; Beyermann, M.; Wissmann, R.; Fakler, B. NMR structure of the “ball-and-chain” domain of KCNMB2, the beta 2-subunit of large conductance Ca2+- and voltage-activated potassium channels. J. Biol. Chem. 2001, 276, 42116–42121. [Google Scholar] [CrossRef]
- Hu, S.; Labuda, M.Z.; Pandolfo, M.; Goss, G.G.; McDermid, H.E.; Ali, D.W. Variants of the KCNMB3 regulatory subunit of maxi BK channels affect channel inactivation. Physiol. Genom. 2003, 15, 191–198. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Uebele, V.N.; Lagrutta, A.; Wade, T.; Figueroa, D.J.; Liu, Y.; McKenna, E.; Austin, C.P.; Bennett, P.B.; Swanson, R. Cloning and functional expression of two families of beta-subunits of the large conductance calcium-activated K+ channel. J. Biol. Chem. 2000, 275, 23211–23218. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Hoa, N.T.; Wilson, Z.; Arismendi-Morillo, G.; Kong, X.T.; Tajhya, R.B.; Beeton, C.; Jadus, M.R. Big Potassium (BK) ion channels in biology, disease and possible targets for cancer immunotherapy. Int. Immunopharmacol. 2014, 22, 427–443. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Rothberg, B.S.; Brenner, R. Mechanism of beta4 subunit modulation of BK channels. J. Gen. Physiol. 2006, 127, 449–465. [Google Scholar] [CrossRef]
- Yan, J.; Aldrich, R.W. LRRC26 auxiliary protein allows BK channel activation at resting voltage without calcium. Nature 2010, 466, 513–516. [Google Scholar] [CrossRef]
- Yan, J.; Aldrich, R.W. BK potassium channel modulation by leucine-rich repeat-containing proteins. Proc. Natl. Acad. Sci. USA 2012, 109, 7917–7922. [Google Scholar] [CrossRef]
- Hanner, M.; Schmalhofer, W.A.; Munujos, P.; Knaus, H.G.; Kaczorowski, G.J.; Garcia, M.L. The beta subunit of the high-conductance calcium-activated potassium channel contributes to the high-affinity receptor for charybdotoxin. Proc. Natl. Acad. Sci. USA 1997, 94, 2853–2858. [Google Scholar] [CrossRef]
- Meera, P.; Wallner, M.; Toro, L. A neuronal beta subunit (KCNMB4) makes the large conductance, voltage- and Ca2+-activated K+ channel resistant to charybdotoxin and iberiotoxin. Proc. Natl. Acad. Sci. USA 2000, 97, 5562–5567. [Google Scholar] [CrossRef]
- Gan, G.; Yi, H.; Chen, M.; Sun, L.; Li, W.; Wu, Y.; Ding, J. Structural basis for toxin resistance of beta4-associated calcium-activated potassium (BK) channels. J. Biol. Chem. 2008, 283, 24177–24184. [Google Scholar] [CrossRef]
- Petkov, G.V.; Bonev, A.D.; Heppner, T.J.; Brenner, R.; Aldrich, R.W.; Nelson, M.T. Beta1-subunit of the Ca2+-activated K+ channel regulates contractile activity of mouse urinary bladder smooth muscle. J. Physiol. 2001, 537, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Grimm, P.R.; Foutz, R.M.; Brenner, R.; Sansom, S.C. Identification and localization of BK-beta subunits in the distal nephron of the mouse kidney. Am. J. Physiol. Ren. Physiol. 2007, 293, F350–F359. [Google Scholar] [CrossRef] [PubMed]
- Contet, C.; Goulding, S.P.; Kuljis, D.A.; Barth, A.L. BK Channels in the Central Nervous System. Int. Rev. Neurobiol. 2016, 128, 281–342. [Google Scholar] [CrossRef] [PubMed]
- Brenner, R.; Peréz, G.J.; Bonev, A.D.; Eckman, D.M.; Kosek, J.C.; Wiler, S.W.; Patterson, A.J.; Nelson, M.T.; Aldrich, R.W. Vasoregulation by the beta1 subunit of the calcium-activated potassium channel. Nature 2000, 407, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Behrens, R.; Nolting, A.; Reimann, F.; Schwarz, M.; Waldschütz, R.; Pongs, O. hKCNMB3 and hKCNMB4, cloning and characterization of two members of the large-conductance calcium-activated potassium channel beta subunit family. FEBS Lett. 2000, 474, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.S.; Marx, S.O. The BK potassium channel in the vascular smooth muscle and kidney: α- and β-subunits. Kidney Int. 2010, 78, 963–974. [Google Scholar] [CrossRef] [PubMed]
- Weiger, T.M.; Holmqvist, M.H.; Levitan, I.B.; Clark, F.T.; Sprague, S.; Huang, W.J.; Ge, P.; Wang, C.; Lawson, D.; Jurman, M.E.; et al. A novel nervous system beta subunit that downregulates human large conductance calcium-dependent potassium channels. J. Neurosci. 2000, 20, 3563–3570. [Google Scholar] [CrossRef]
- Imlach, W.L.; Finch, S.C.; Miller, J.H.; Meredith, A.L.; Dalziel, J.E. A role for BK channels in heart rate regulation in rodents. PLoS ONE 2010, 5, e8698. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Lu, R.; Bopassa, J.C.; Meredith, A.L.; Stefani, E.; Toro, L. MitoBKCa is encoded by the Kcnma1 gene, and a splicing sequence defines its mitochondrial location. Proc. Natl. Acad. Sci. USA 2013, 110, 10836–10841. [Google Scholar] [CrossRef] [PubMed]
- Szteyn, K.; Singh, H. BKCa Channels as Targets for Cardioprotection. Antioxidants 2020, 9, 760. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy-Natarajan, G.; Koide, M. BK Channels in the Vascular System. Int. Rev. Neurobiol. 2016, 128, 401–438. [Google Scholar] [CrossRef] [PubMed]
- Sachse, G.; Faulhaber, J.; Seniuk, A.; Ehmke, H.; Pongs, O. Smooth muscle BK channel activity influences blood pressure independent of vascular tone in mice. J. Physiol. 2014, 592, 2563–2574. [Google Scholar] [CrossRef]
- Taylor, J.L.; Walsh, K.R.; Mosneag, I.E.; Danby, T.G.E.; Luka, N.; Chanda, B.; Schiessl, I.; Dunne, R.A.; Hill-Eubanks, D.; Hennig, G.W.; et al. Uncoupling of Ca2+ sparks from BK channels in cerebral arteries underlies hypoperfusion in hypertension-induced vascular dementia. Proc. Natl. Acad. Sci. USA 2023, 120, e2307513120. [Google Scholar] [CrossRef]
- Petkov, G.V. Central role of the BK channel in urinary bladder smooth muscle physiology and pathophysiology. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R571–R584. [Google Scholar] [CrossRef]
- Semenov, I.; Wang, B.; Herlihy, J.T.; Brenner, R. BK channel beta1-subunit regulation of calcium handling and constriction in tracheal smooth muscle. Am. J. Physiol. Lung Cell Mol. Physiol. 2006, 291, L802–L810. [Google Scholar] [CrossRef]
- Zuccolini, P.; Gavazzo, P.; Pusch, M. BK Channel in the Physiology and in the Cancer of Pancreatic Duct: Impact and Reliability of BK Openers. Front. Pharmacol. 2022, 13, 906608. [Google Scholar] [CrossRef]
- Bromfield, E.B.; Cavazos, J.E.; Sirven, J.I. (Eds.) An Introduction to Epilepsy. West Hartford (CT): American Epilepsy Society 2006, Chapter 1, Basic Mechanisms Underlying Seizures and Epilepsy. Available online: https://www.ncbi.nlm.nih.gov/books/NBK2510/ (accessed on 6 January 2024).
- N’Gouemo, P. BKCa channel dysfunction in neurological diseases. Front. Physiol. 2014, 5, 373. [Google Scholar] [CrossRef]
- Du, W.; Bautista, J.F.; Yang, H.; Diez-Sampedro, A.; You, S.A.; Wang, L.; Kotagal, P.; Lüders, H.O.; Shi, J.; Cui, J.; et al. Calcium-sensitive potassium channelopathy in human epilepsy and paroxysmal movement disorder. Nat. Genet. 2005, 37, 733–738. [Google Scholar] [CrossRef]
- Brenner, R.; Chen, Q.H.; Vilaythong, A.; Toney, G.M.; Noebels, J.L.; Aldrich, R.W. BK channel beta4 subunit reduces dentate gyrus excitability and protects against temporal lobe seizures. Nat. Neurosci. 2005, 8, 1752–1759. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W.; Hoffmann, K.; Twele, F.; Potschka, H.; Töllner, K. The novel antiepileptic drug imepitoin compares favourably to other GABA-mimetic drugs in a seizure threshold model in mice and dogs. Pharmacol. Res. 2013, 77, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.W.; Huang, C.C.; Wu, S.N. Activation by zonisamide, a newer antiepileptic drug, of large-conductance calcium-activated potassium channel in differentiated hippocampal neuron-derived H19-7 cells. J. Pharmacol. Exp. Ther. 2007, 321, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Wilfong, A.A.; Willmore, L.J. Zonisamide—A review of experience and use in partial seizures. Neuropsychiatr. Dis. Treat. 2006, 2, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Biton, V. Clinical pharmacology and mechanism of action of zonisamide. Clin. Neuropharmacol. 2007, 30, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Pallàs, M.; Ortuño-Sahagún, D.; Andrés-Benito, P.; Ponce-Regalado, M.D.; Rojas-Mayorquín, A.E. Resveratrol in epilepsy: Preventive or treatment opportunities? Front. Biosci. 2014, 19, 1057–1064. [Google Scholar] [CrossRef]
- Wang, Y.J.; Chan, M.H.; Chen, L.; Wu, S.N.; Chen, H.H. Resveratrol attenuates cortical neuron activity: Roles of large conductance calcium-activated potassium channels and voltage-gated sodium channels. J. Biomed. Sci. 2016, 23, 47. [Google Scholar] [CrossRef]
- Zhou, Y.; Lingle, C.J. Paxilline inhibits BK channels by an almost exclusively closed-channel block mechanism. J. Gen. Physiol. 2014, 144, 415–440. [Google Scholar] [CrossRef]
- Sheehan, J.J.; Benedetti, B.L.; Barth, A.L. Anticonvulsant effects of the BK-channel antagonist paxilline. Epilepsia 2009, 50, 711–720. [Google Scholar] [CrossRef]
- Jin, W.; Sugaya, A.; Tsuda, T.; Ohguchi, H.; Sugaya, E. Relationship between large conductance calcium-activated potassium channel and bursting activity. Brain Res. 2000, 860, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Mehranfard, N.; Gholamipour-Badie, H.; Motamedi, F.; Janahmadi, M.; Naderi, N. Long-term increases in BK potassium channel underlie increased action potential firing in dentate granule neurons following pilocarpine-induced status epilepticus in rats. Neurosci. Lett. 2015, 585, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.L.; Pritchard, H.A.T.; Walsh, K.R.; Strangward, P.; White, C.; Hill-Eubanks, D.; Alakrawi, M.; Hennig, G.W.; Allan, S.M.; Nelson, M.T.; et al. Functionally linked potassium channel activity in cerebral endothelial and smooth muscle cells is compromised in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2022, 119, e2204581119. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Kang, H.; Li, Y.; Shui, Y.; Yamamoto, R.; Sugai, T.; Kato, N. Cognitive recovery by chronic activation of the large-conductance calcium-activated potassium channel in a mouse model of Alzheimer’s disease. Neuropharmacology 2015, 92, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Jafari, A.; Noursadeghi, E.; Khodagholi, F.; Saghiri, R.; Sauve, R.; Aliaghaei, A.; Eliassi, A. Brain mitochondrial ATP-insensitive large conductance Ca⁺²-activated K⁺ channel properties are altered in a rat model of amyloid-β neurotoxicity. Exp. Neurol. 2015, 269, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; LeVine, H., 3rd. Alzheimer’s disease and the amyloid-beta peptide. J. Alzheimers Dis. 2010, 19, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Chi, S.; Qi, Z. Regulatory effect of sulphatides on BKCa channels. Br. J. Pharmacol. 2006, 149, 1031–1038. [Google Scholar] [CrossRef]
- Irizarry, M.C. A turn of the sulfatide in Alzheimer’s disease. Ann. Neurol. 2003, 54, 7–8. [Google Scholar] [CrossRef]
- Hébert, B.; Pietropaolo, S.; Même, S.; Laudier, B.; Laugeray, A.; Doisne, N.; Quartier, A.; Lefeuvre, S.; Got, L.; Cahard, D.; et al. Rescue of fragile X syndrome phenotypes in Fmr1 KO mice by a BKCa channel opener molecule. Orphanet J. Rare Dis. 2014, 9, 124. [Google Scholar] [CrossRef]
- Carreno-Munoz, M.I.; Martins, F.; Medrano, M.C.; Aloisi, E.; Pietropaolo, S.; Dechaud, C.; Subashi, E.; Bony, G.; Ginger, M.; Moujahid, A.; et al. Potential Involvement of Impaired BKCa Channel Function in Sensory Defensiveness and Some Behavioral Disturbances Induced by Unfamiliar Environment in a Mouse Model of Fragile X Syndrome. Neuropsychopharmacology 2018, 43, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Zaman, T.; De Oliveira, C.; Smoka, M.; Narla, C.; Poulter, M.O.; Schmid, S. BK Channels Mediate Synaptic Plasticity Underlying Habituation in Rats. J. Neurosci. 2017, 37, 4540–4551. [Google Scholar] [CrossRef] [PubMed]
- Hewawasam, P.; Gribkoff, V.K.; Pendri, Y.; Dworetzky, S.I.; Meanwell, N.A.; Martinez, E.; Boissard, C.G.; Post-Munson, D.J.; Trojnacki, J.T.; Yeleswaram, K.; et al. The synthesis and characterization of BMS-204352 (MaxiPost) and related 3-fluorooxindoles as openers of maxi-K potassium channels. Bioorg. Med. Chem. Lett. 2002, 12, 1023–1026. [Google Scholar] [CrossRef] [PubMed]
- Jensen, B.S. BMS-204352: A potassium channel opener developed for the treatment of stroke. CNS Drug Rev. 2002, 8, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Al-Karagholi, M.A.; Ghanizada, H.; Nielsen, C.A.W.; Skandarioon, C.; Snellman, J.; Lopez Lopez, C.; Hansen, J.M.; Ashina, M. Opening of BKCa channels alters cerebral hemodynamic and causes headache in healthy volunteers. Cephalalgia 2020, 40, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.H.; Wu, Y.; Gao, Z.; Anderson, M.E.; Dalziel, J.E.; Meredith, A.L. BK channels regulate sinoatrial node firing rate and cardiac pacing in vivo. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H1327–H1338. [Google Scholar] [CrossRef]
- Patel, N.H.; Johannesen, J.; Shah, K.; Goswami, S.K.; Patel, N.J.; Ponnalagu, D.; Kohut, A.R.; Singh, H. Inhibition of BKCa negatively alters cardiovascular function. Physiol. Rep. 2018, 6, e13748. [Google Scholar] [CrossRef]
- Chen, L.; Tian, L.; MacDonald, S.H.; McClafferty, H.; Hammond, M.S.; Huibant, J.M.; Ruth, P.; Knaus, H.G.; Shipston, M.J. Functionally diverse complement of large conductance calcium- and voltage-activated potassium channel (BK) alpha-subunits generated from a single site of splicing. J. Biol. Chem. 2005, 280, 33599–33609. [Google Scholar] [CrossRef]
- Ryckwaert, F.; Virsolvy, A.; Fort, A.; Murat, B.; Richard, S.; Guillon, G.; Colson, P.H. Terlipressin, a provasopressin drug exhibits direct vasoconstrictor properties: Consequences on heart perfusion and performance. Crit. Care Med. 2009, 37, 876–881. [Google Scholar] [CrossRef]
- Zou, J.G.; Wang, Z.R.; Huang, Y.Z.; Cao, K.J.; Wu, J.M. Effect of red wine and wine polyphenol resveratrol on endothelial function in hypercholesterolemic rabbits. Int. J. Mol. Med. 2003, 11, 317–320. [Google Scholar] [CrossRef]
- Calderone, V.; Martelli, A.; Testai, L.; Martinotti, E.; Breschi, M.C. Functional contribution of the endothelial component to the vasorelaxing effect of resveratrol and NS 1619, activators of the large-conductance calcium-activated potassium channels. Naunyn Schmiedebergs Arch. Pharmacol. 2007, 375, 73–80. [Google Scholar] [CrossRef]
- Aon, M.A.; Cortassa, S.; Wei, A.C.; Grunnet, M.; O’Rourke, B. Energetic performance is improved by specific activation of K+ fluxes through KCa channels in heart mitochondria. Biochim. Biophys. Acta 2010, 1797, 71–80. [Google Scholar] [CrossRef]
- Goswami, S.K.; Ponnalagu, D.; Hussain, A.T.; Shah, K.; Karekar, P.; Gururaja Rao, S.; Meredith, A.L.; Khan, M.; Singh, H. Expression and Activation of BKCa Channels in Mice Protects Against Ischemia-Reperfusion Injury of Isolated Hearts by Modulating Mitochondrial Function. Front. Cardiovasc. Med. 2018, 5, 194. [Google Scholar] [CrossRef]
- Wang, X.; Yin, C.; Xi, L.; Kukreja, R.C. Opening of Ca2+-activated K+ channels triggers early and delayed preconditioning against I/R injury independent of NOS in mice. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H2070–H2077. [Google Scholar] [CrossRef]
- Shi, Y.; Jiang, M.T.; Su, J.; Hutchins, W.; Konorev, E.; Baker, J.E. Mitochondrial big conductance KCa channel and cardioprotection in infant rabbit heart. J. Cardiovasc. Pharmacol. 2007, 50, 497–502. [Google Scholar] [CrossRef]
- Shintani, Y.; Node, K.; Asanuma, H.; Sanada, S.; Takashima, S.; Asano, Y.; Liao, Y.; Fujita, M.; Hirata, A.; Shinozaki, Y.; et al. Opening of Ca2+-activated K+ channels is involved in ischemic preconditioning in canine hearts. J. Mol. Cell Cardiol. 2004, 37, 1213–1218. [Google Scholar] [CrossRef]
- Park, W.S.; Kang, S.H.; Son, Y.K.; Kim, N.; Ko, J.H.; Kim, H.K.; Ko, E.A.; Kim, C.D.; Han, J. The mitochondrial Ca2+-activated K+ channel activator, NS 1619 inhibits L-type Ca2+ channels in rat ventricular myocytes. Biochem. Biophys. Res. Commun. 2007, 362, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Edwards, G.; Niederste-Hollenberg, A.; Schneider, J.; Noack, T.; Weston, A.H. Ion channel modulation by NS 1619, the putative BKCa channel opener, in vascular smooth muscle. Br. J. Pharmacol. 1994, 113, 1538–1547. [Google Scholar] [CrossRef]
- Wrzosek, A. The potassium channel opener NS1619 modulates calcium homeostasis in muscle cells by inhibiting SERCA. Cell Calcium 2014, 56, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Bentzen, B.H.; Nardi, A.; Calloe, K.; Madsen, L.S.; Olesen, S.P.; Grunnet, M. The small molecule NS11021 is a potent and specific activator of Ca2+-activated big-conductance K+ channels. Mol. Pharmacol. 2007, 72, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Maqoud, F.; Curci, A.; Scala, R.; Pannunzio, A.; Campanella, F.; Coluccia, M.; Passantino, G.; Zizzo, N.; Tricarico, D. Cell Cycle Regulation by Ca2+-Activated K⁺ (BK) Channels Modulators in SH-SY5Y Neuroblastoma Cells. Int. J. Mol. Sci. 2018, 19, 2442. [Google Scholar] [CrossRef]
- Rosa, P.; Sforna, L.; Carlomagno, S.; Mangino, G.; Miscusi, M.; Pessia, M.; Franciolini, F.; Calogero, A.; Catacuzzeno, L. Overexpression of Large-Conductance Calcium-Activated Potassium Channels in Human Glioblastoma Stem-Like Cells and Their Role in Cell Migration. J. Cell Physiol. 2017, 232, 2478–2488. [Google Scholar] [CrossRef]
- Goda, A.A.; Siddique, A.B.; Mohyeldin, M.; Ayoub, N.M.; El Sayed, K.A. The Maxi-K (BK) Channel Antagonist Penitrem A as a Novel Breast Cancer-Targeted Therapeutic. Mar. Drugs 2018, 16, 157. [Google Scholar] [CrossRef]
- Kraft, R.; Krause, P.; Jung, S.; Basrai, D.; Liebmann, L.; Bolz, J.; Patt, S. BK channel openers inhibit migration of human glioma cells. Pflug. Arch. 2003, 446, 248–255. [Google Scholar] [CrossRef]
- Sizemore, G.; McLaughlin, S.; Newman, M.; Brundage, K.; Ammer, A.; Martin, K.; Pugacheva, E.; Coad, J.; Mattes, M.D.; Yu, H.G. Opening large-conductance potassium channels selectively induced cell death of triple-negative breast cancer. BMC Cancer 2020, 20, 595. [Google Scholar] [CrossRef]
- Catacuzzeno, L.; Caramia, M.; Sforna, L.; Belia, S.; Guglielmi, L.; D’Adamo, M.C.; Pessia, M.; Franciolini, F. Reconciling the discrepancies on the involvement of large-conductance Ca2+-activated K channels in glioblastoma cell migration. Front. Cell Neurosci. 2015, 9, 152. [Google Scholar] [CrossRef]
- Rosa, P.; Catacuzzeno, L.; Sforna, L.; Mangino, G.; Carlomagno, S.; Mincione, G.; Petrozza, V.; Ragona, G.; Franciolini, F.; Calogero, A. BK channels blockage inhibits hypoxia-induced migration and chemoresistance to cisplatin in human glioblastoma cells. J. Cell Physiol. 2018, 233, 6866–6877. [Google Scholar] [CrossRef]
- Michelucci, A.; Sforna, L.; Franciolini, F.; Catacuzzeno, L. Hypoxia, Ion Channels and Glioblastoma Malignancy. Biomolecules 2023, 13, 1742. [Google Scholar] [CrossRef] [PubMed]
- Sallam, A.A.; Houssen, W.E.; Gissendanner, C.R.; Orabi, K.Y.; Foudah, A.I.; El Sayed, K.A. Bioguided discovery and pharmacophore modeling of the mycotoxic indole diterpene alkaloids penitrems as breast cancer proliferation, migration, and invasion inhibitors. Medchemcomm 2013, 4, 1360–1369. [Google Scholar] [CrossRef] [PubMed]
- Sallam, A.A.; Ayoub, N.M.; Foudah, A.I.; Gissendanner, C.R.; Meyer, S.A.; El Sayed, K.A. Indole diterpene alkaloids as novel inhibitors of the Wnt/β-catenin pathway in breast cancer cells. Eur. J. Med. Chem. 2013, 70, 594–606. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Lin, Y.; He, F.; Shao, L.; Ma, W.; He, F. Role for calcium-activated potassium channels (BK) in migration control of human hepatocellular carcinoma cells. J. Cell Mol. Med. 2021, 25, 9685–9696. [Google Scholar] [CrossRef]
- Lee, H.; Kwon, O.B.; Lee, J.E.; Jeon, Y.H.; Lee, D.S.; Min, S.H.; Kim, J.W. Repositioning Trimebutine Maleate as a Cancer Treatment Targeting Ovarian Cancer Stem Cells. Cells 2021, 10, 918. [Google Scholar] [CrossRef] [PubMed]
- Grover, N.D.; Limaye, R.P.; Gokhale, D.V.; Patil, T.R. Zonisamide: A review of the clinical and experimental evidence for its use in Parkinson’s disease. Indian. J. Pharmacol. 2013, 45, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Stocker, M. Ca2+-activated K+ channels: Molecular determinants and function of the SK family. Nat. Rev. Neurosci. 2004, 5, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Adelman, J.P. SK channels and calmodulin. Channels 2016, 10, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.A.; Rivard, A.F.; Bächinger, H.P.; Adelman, J.P. Structure of the gating domain of a Ca2+-activated K+ channel complexed with Ca2+/calmodulin. Nature 2001, 410, 1120–1124. [Google Scholar] [CrossRef] [PubMed]
- Halling, D.B.; Kenrick, S.A.; Riggs, A.F.; Aldrich, R.W. Calcium-dependent stoichiometries of the KCa2.2 (SK) intracellular domain/calmodulin complex in solution. J. Gen. Physiol. 2014, 143, 231–252. [Google Scholar] [CrossRef]
- Hoffman, J.F.; Joiner, W.; Nehrke, K.; Potapova, O.; Foye, K.; Wickrema, A. The hSK4 (KCNN4) isoform is the Ca2+-activated K+ channel (Gardos channel) in human red blood cells. Proc. Natl. Acad. Sci. USA 2003, 100, 7366–7371. [Google Scholar] [CrossRef]
- Wulff, H.; Knaus, H.G.; Pennington, M.; Chandy, K.G. K+ channel expression during B cell differentiation: Implications for immunomodulation and autoimmunity. J. Immunol. 2004, 173, 776–786. [Google Scholar] [CrossRef] [PubMed]
- Mark Duffy, S.; Berger, P.; Cruse, G.; Yang, W.; Bolton, S.J.; Bradding, P. The K+ channel iKCA1 potentiates Ca2+ influx and degranulation in human lung mast cells. J. Allergy Clin. Immunol. 2004, 114, 66–72. [Google Scholar] [CrossRef]
- Lin, Y.; Zhao, Y.J.; Zhang, H.L.; Hao, W.J.; Zhu, R.D.; Wang, Y.; Hu, W.; Zhou, R.P. Regulatory role of KCa3.1 in immune cell function and its emerging association with rheumatoid arthritis. Front. Immunol. 2022, 13, 997621. [Google Scholar] [CrossRef]
- Ghanshani, S.; Wulff, H.; Miller, M.J.; Rohm, H.; Neben, A.; Gutman, G.A.; Cahalan, M.D.; Chandy, K.G. Up-regulation of the IKCa1 potassium channel during T-cell activation. Molecular mechanism and functional consequences. J. Biol. Chem. 2000, 275, 37137–37149. [Google Scholar] [CrossRef]
- Feske, S.; Wulff, H.; Skolnik, E.Y. Ion channels in innate and adaptive immunity. Annu. Rev. Immunol. 2015, 33, 291–353. [Google Scholar] [CrossRef]
- Zheng, F.; Tao, Y.; Liu, J.; Geng, Z.; Wang, Y.; Wang, Y.; Fu, S.; Wang, W.; Xie, C.; Zhang, Y.; et al. KCa3.1 Inhibition of Macrophages Suppresses Inflammatory Response Leading to Endothelial Damage in a Cell Model of Kawasaki Disease. J. Inflamm. Res. 2021, 14, 719–735. [Google Scholar] [CrossRef] [PubMed]
- Bernard, K.; Bogliolo, S.; Soriani, O.; Ehrenfeld, J. Modulation of calcium-dependent chloride secretion by basolateral SK4-like channels in a human bronchial cell line. J. Membr. Biol. 2003, 196, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Heitzmann, D.; Warth, R. Physiology and pathophysiology of potassium channels in gastrointestinal epithelia. Physiol. Rev. 2008, 88, 1119–1182. [Google Scholar] [CrossRef] [PubMed]
- Takahata, T.; Hayashi, M.; Ishikawa, T. SK4/IK1-like channels mediate TEA-insensitive, Ca2+-activated K+ currents in bovine parotid acinar cells. Am. J. Physiol. Cell Physiol. 2003, 284, C127–C144. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, V.; Koeberle, P.D.; Wang, Y.; Schlichter, L.C. The Ca2+-activated K+ channel KCNN4/KCa3.1 contributes to microglia activation and nitric oxide-dependent neurodegeneration. J. Neurosci. 2007, 27, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.M.; Grössinger, E.M.; Horiuchi, M.; Davis, K.W.; Jin, L.W.; Maezawa, I.; Wulff, H. Differential Kv1.3, KCa3.1, and Kir2.1 expression in “classically” and “alternatively” activated microglia. Glia 2017, 65, 106–121. [Google Scholar] [CrossRef]
- King, B.; Rizwan, A.P.; Asmara, H.; Heath, N.C.; Engbers, J.D.; Dykstra, S.; Bartoletti, T.M.; Hameed, S.; Zamponi, G.W.; Turner, R.W. IKCa channels are a critical determinant of the slow AHP in CA1 pyramidal neurons. Cell Rep. 2015, 11, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Roshchin, M.V.; Ierusalimsky, V.N.; Balaban, P.M.; Nikitin, E.S. Ca2+-activated KCa3.1 potassium channels contribute to the slow afterhyperpolarization in L5 neocortical pyramidal neurons. Sci. Rep. 2020, 10, 14484. [Google Scholar] [CrossRef]
- Wang, K.; Mateos-Aparicio, P.; Hönigsperger, C.; Raghuram, V.; Wu, W.W.; Ridder, M.C.; Sah, P.; Maylie, J.; Storm, J.F.; Adelman, J.P. IK1 channels do not contribute to the slow afterhyperpolarization in pyramidal neurons. Elife 2016, 5, e11206. [Google Scholar] [CrossRef]
- Sahu, G.; Turner, R.W. The Molecular Basis for the Calcium-Dependent Slow Afterhyperpolarization in CA1 Hippocampal Pyramidal Neurons. Front. Physiol. 2021, 12, 759707. [Google Scholar] [CrossRef]
- Fioretti, B.; Pietrangelo, T.; Catacuzzeno, L.; Franciolini, F. Intermediate-conductance Ca2+-activated K+ channel is expressed in C2C12 myoblasts and is downregulated during myogenesis. Am. J. Physiol. Cell Physiol. 2005, 289, C89–C96. [Google Scholar] [CrossRef]
- Pietrangelo, T.; Fioretti, B.; Mancinelli, R.; Catacuzzeno, L.; Franciolini, F.; Fanò, G.; Fulle, S. Extracellular guanosine-5’-triphosphate modulates myogenesis via intermediate Ca2+-activated K+ currents in C2C12 mouse cells. J. Physiol. 2006, 572, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Ono, Y.; Sakamoto, K. DCEBIO facilitates myogenic differentiation via intermediate conductance Ca2+ activated K+ channel activation in C2C12 myoblasts. J. Pharmacol. Sci. 2017, 133, 276–279. [Google Scholar] [CrossRef]
- McNaughton-Smith, G.A.; Burns, J.F.; Stocker, J.W.; Rigdon, G.C.; Creech, C.; Arrington, S.; Shelton, T.; de Franceschi, L. Novel inhibitors of the Gardos channel for the treatment of sickle cell disease. J. Med. Chem. 2008, 51, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Ataga, K.I.; Reid, M.; Ballas, S.K.; Yasin, Z.; Bigelow, C.; James, L.S.; Smith, W.R.; Galacteros, F.; Kutlar, A.; Hull, J.H.; et al. Improvements in haemolysis and indicators of erythrocyte survival do not correlate with acute vaso-occlusive crises in patients with sickle cell disease: A phase III randomized, placebo-controlled, double-blind study of the Gardos channel blocker senicapoc (ICA-17043). Br. J. Haematol. 2011, 153, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Wulff, H.; Castle, N.A. Therapeutic potential of KCa3.1 blockers: Recent advances and promising trends. Expert. Rev. Clin. Pharmacol. 2010, 3, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Rivera, A.; Vandorpe, D.H.; Shmukler, B.E.; Gallagher, D.R.; Fikry, C.C.; Kuypers, F.A.; Brugnara, C.; Snyder, L.M.; Alper, S.L. Erythrocytes from hereditary xerocytosis patients heterozygous for KCNN4 V282M exhibit increased spontaneous Gardos channel-like activity inhibited by senicapoc. Am. J. Hematol. 2017, 92, E108–E110. [Google Scholar] [CrossRef]
- An Explananatory, Proof-of-Concept Study of Senicapoc in Patients with Familial Dehydrated Stomatocytosis Caused by the V282M Mutation in the Gardos (KCNN4) Channel. NCT04372498. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04372498 (accessed on 6 January 2024).
- Yu, Z.H.; Xu, J.R.; Wang, Y.X.; Xu, G.N.; Xu, Z.P.; Yang, K.; Wu, D.Z.; Cui, Y.Y.; Chen, H.Z. Targeted inhibition of KCa3.1 channel attenuates airway inflammation and remodeling in allergic asthma. Am. J. Respir. Cell Mol. Biol. 2013, 48, 685–693. [Google Scholar] [CrossRef]
- Lin, H.; Zheng, C.; Li, J.; Yang, C.; Hu, L. Ca2+ -activated K+ channel-3.1 blocker TRAM-34 alleviates murine allergic rhinitis. Int. Immunopharmacol. 2014, 23, 642–648. [Google Scholar] [CrossRef]
- Strøbæk, D.; Brown, D.T.; Jenkins, D.P.; Chen, Y.J.; Coleman, N.; Ando, Y.; Chiu, P.; Jørgensen, S.; Demnitz, J.; Wulff, H.; et al. NS6180, a new KCa 3.1 channel inhibitor prevents T-cell activation and inflammation in a rat model of inflammatory bowel disease. Br. J. Pharmacol. 2013, 168, 432–444. [Google Scholar] [CrossRef]
- Friebel, K.; Schönherr, R.; Kinne, R.W.; Kunisch, E. Functional role of the KCa3.1 potassium channel in synovial fibroblasts from rheumatoid arthritis patients. J. Cell Physiol. 2015, 230, 1677–1688. [Google Scholar] [CrossRef]
- Agarwal, J.J.; Zhu, Y.; Zhang, Q.Y.; Mongin, A.A.; Hough, L.B. TRAM-34, a putatively selective blocker of intermediate-conductance, calcium-activated potassium channels, inhibits cytochrome P450 activity. PLoS ONE 2013, 8, e63028. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, Y.; Chen, L.; Zhu, J. Effects of Intermediate-Conductance Ca2+-Activated K+ Channels on Human Endometrial Carcinoma Cells. Cell Biochem. Biophys. 2015, 72, 515–525. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, X.; Yin, Q.; Yi, J.; Shen, W.; Zhao, L.; Zhu, Z.; Liu, J. Inhibition of SK4 Potassium Channels Suppresses Cell Proliferation, Migration and the Epithelial-Mesenchymal Transition in Triple-Negative Breast Cancer Cells. PLoS ONE 2016, 11, e0154471. [Google Scholar] [CrossRef]
- Wang, Z.H.; Shen, B.; Yao, H.L.; Jia, Y.C.; Ren, J.; Feng, Y.J.; Wang, Y.Z. Blockage of intermediate-conductance-Ca2+ -activated K+ channels inhibits progression of human endometrial cancer. Oncogene 2007, 26, 5107–5114. [Google Scholar] [CrossRef]
- Lee, E.L.; Hasegawa, Y.; Shimizu, T.; Okada, Y. IK1 channel activity contributes to cisplatin sensitivity of human epidermoid cancer cells. Am. J. Physiol. Cell Physiol. 2008, 294, C1398–C1406. [Google Scholar] [CrossRef]
- Lai, W.; Liu, L.; Zeng, Y.; Wu, H.; Xu, H.; Chen, S.; Chu, Z. KCNN4 channels participate in the EMT induced by PRL-3 in colorectal cancer. Med. Oncol. 2013, 30, 566. [Google Scholar] [CrossRef]
- Deng, X.L.; Lau, C.P.; Lai, K.; Cheung, K.F.; Lau, G.K.; Li, G.R. Cell cycle-dependent expression of potassium channels and cell proliferation in rat mesenchymal stem cells from bone marrow. Cell Prolif. 2007, 40, 656–670. [Google Scholar] [CrossRef]
- Xu, H.; Lai, W.; Zhang, Y.; Liu, L.; Luo, X.; Zeng, Y.; Wu, H.; Lan, Q.; Chu, Z. Tumor-associated macrophage-derived IL-6 and IL-8 enhance invasive activity of LoVo cells induced by PRL-3 in a KCNN4 channel-dependent manner. BMC Cancer 2014, 14, 330. [Google Scholar] [CrossRef]
- Sciaccaluga, M.; Fioretti, B.; Catacuzzeno, L.; Pagani, F.; Bertollini, C.; Rosito, M.; Catalano, M.; D’Alessandro, G.; Santoro, A.; Cantore, G.; et al. CXCL12-induced glioblastoma cell migration requires intermediate conductance Ca2+-activated K+ channel activity. Am. J. Physiol. Cell Physiol. 2010, 299, C175–C184. [Google Scholar] [CrossRef]
- Catacuzzeno, L.; Aiello, F.; Fioretti, B.; Sforna, L.; Castigli, E.; Ruggieri, P.; Tata, A.M.; Calogero, A.; Franciolini, F. Serum-activated K and Cl currents underlay U87-MG glioblastoma cell migration. J. Cell Physiol. 2011, 226, 1926–1933. [Google Scholar] [CrossRef]
- Ruggieri, P.; Mangino, G.; Fioretti, B.; Catacuzzeno, L.; Puca, R.; Ponti, D.; Miscusi, M.; Franciolini, F.; Ragona, G.; Calogero, A. The inhibition of KCa3.1 channels activity reduces cell motility in glioblastoma derived cancer stem cells. PLoS ONE 2012, 7, e47825. [Google Scholar] [CrossRef]
- D’Alessandro, G.; Monaco, L.; Catacuzzeno, L.; Antonangeli, F.; Santoro, A.; Esposito, V.; Franciolini, F.; Wulff, H.; Limatola, C. Radiation Increases Functional KCa3.1 Expression and Invasiveness in Glioblastoma. Cancers 2019, 11, 279. [Google Scholar] [CrossRef]
- Michelucci, A.; Sforna, L.; Di Battista, A.; Franciolini, F.; Catacuzzeno, L. Ca2+ -activated K+ channels regulate cell volume in human glioblastoma cells. J. Cell Physiol. 2023, 238, 2120–2134. [Google Scholar] [CrossRef]
- Fioretti, B.; Franciolini, F.; Catacuzzeno, L. A model of intracellular Ca2+ oscillations based on the activity of the intermediate-conductance Ca2+-activated K+ channels. Biophys. Chem. 2005, 113, 17–23. [Google Scholar] [CrossRef]
- Catacuzzeno, L.; Fioretti, B.; Franciolini, F. A theoretical study on the role of Ca2+-activated K+ channels in the regulation of hormone-induced Ca2+ oscillations and their synchronization in adjacent cells. J. Theor. Biol. 2012, 309, 103–112. [Google Scholar] [CrossRef]
- Catacuzzeno, L.; Franciolini, F. Role of KCa3.1 Channels in Modulating Ca2+ Oscillations during Glioblastoma Cell Migration and Invasion. Int. J. Mol. Sci. 2018, 19, 2970. [Google Scholar] [CrossRef]
- Roy, J.W.; Cowley, E.A.; Blay, J.; Linsdell, P. The intermediate conductance Ca2+-activated K+ channel inhibitor TRAM-34 stimulates proliferation of breast cancer cells via activation of oestrogen receptors. Br. J. Pharmacol. 2010, 159, 650–658. [Google Scholar] [CrossRef]
- Bonito, B.; Sauter, D.R.; Schwab, A.; Djamgoz, M.B.; Novak, I. KCa3.1 (IK) modulates pancreatic cancer cell migration, invasion and proliferation: Anomalous effects on TRAM-34. Pflug. Arch. 2016, 468, 1865–1875. [Google Scholar] [CrossRef]
- Song, P.; Du, Y.; Song, W.; Chen, H.; Xuan, Z.; Zhao, L.; Chen, J.; Chen, J.; Guo, D.; Jin, C.; et al. KCa3.1 as an Effective Target for Inhibition of Growth and Progression of Intrahepatic Cholangiocarcinoma. J. Cancer 2017, 8, 1568–1578. [Google Scholar] [CrossRef]
- Pillozzi, S.; D’Amico, M.; Bartoli, G.; Gasparoli, L.; Petroni, G.; Crociani, O.; Marzo, T.; Guerriero, A.; Messori, L.; Severi, M.; et al. The combined activation of KCa3.1 and inhibition of Kv11.1/hERG1 currents contribute to overcome Cisplatin resistance in colorectal cancer cells. Br. J. Cancer 2018, 118, 200–212. [Google Scholar] [CrossRef]
- Miller, R.G.; Mitchell, J.D.; Moore, D.H. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Cochrane Database Syst. Rev. 2012, 2012, Cd001447. [Google Scholar] [CrossRef]
- Reich, E.P.; Cui, L.; Yang, L.; Pugliese-Sivo, C.; Golovko, A.; Petro, M.; Vassileva, G.; Chu, I.; Nomeir, A.A.; Zhang, L.K.; et al. Blocking ion channel KCNN4 alleviates the symptoms of experimental autoimmune encephalomyelitis in mice. Eur. J. Immunol. 2005, 35, 1027–1036. [Google Scholar] [CrossRef]
- Chen, Y.J.; Nguyen, H.M.; Maezawa, I.; Grössinger, E.M.; Garing, A.L.; Köhler, R.; Jin, L.W.; Wulff, H. The potassium channel KCa3.1 constitutes a pharmacological target for neuroinflammation associated with ischemia/reperfusion stroke. J. Cereb. Blood Flow. Metab. 2016, 36, 2146–2161. [Google Scholar] [CrossRef]
- Yi, M.; Yu, P.; Lu, Q.; Geller, H.M.; Yu, Z.; Chen, H. KCa3.1 constitutes a pharmacological target for astrogliosis associated with Alzheimer’s disease. Mol. Cell Neurosci. 2016, 76, 21–32. [Google Scholar] [CrossRef]
- Jin, L.W.; Lucente, J.D.; Nguyen, H.M.; Singh, V.; Singh, L.; Chavez, M.; Bushong, T.; Wulff, H.; Maezawa, I. Repurposing the KCa3.1 inhibitor senicapoc for Alzheimer’s disease. Ann. Clin. Transl. Neurol. 2019, 6, 723–738. [Google Scholar] [CrossRef]
- Staal, R.G.W.; Khayrullina, T.; Zhang, H.; Davis, S.; Fallon, S.M.; Cajina, M.; Nattini, M.E.; Hu, A.; Zhou, H.; Poda, S.B.; et al. Inhibition of the potassium channel KCa3.1 by senicapoc reverses tactile allodynia in rats with peripheral nerve injury. Eur. J. Pharmacol. 2017, 795, 1–7. [Google Scholar] [CrossRef]
- Proof of Mechanism Study of Senicapoc in Mild or Prodromal Alzheimer’s Disease. NCT04804241. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04804241?term=NCT04804241&draw=2&rank=1 (accessed on 6 January 2024).
- DosSantos, M.F.; Filha, L.G.A.; Veríssimo, C.P.; Sanz, C.K.; Gazerani, P. Presence of Small-Conductance Calcium-Activated Potassium (SK) Channels in the Central and Peripheral Nervous Systems and Their Role in Health and Disease. J. Integr. Neurosci. 2023, 22, 69. [Google Scholar] [CrossRef]
- Ikonen, S.; Riekkinen, P., Jr. Effects of apamin on memory processing of hippocampal-lesioned mice. Eur. J. Pharmacol. 1999, 382, 151–156. [Google Scholar] [CrossRef]
- Faber, E.S.; Sah, P. Functions of SK channels in central neurons. Clin. Exp. Pharmacol. Physiol. 2007, 34, 1077–1083. [Google Scholar] [CrossRef]
- Adelman, J.P.; Maylie, J.; Sah, P. Small-conductance Ca2+-activated K+ channels: Form and function. Annu. Rev. Physiol. 2012, 74, 245–269. [Google Scholar] [CrossRef]
- Cangiano, L.; Wallén, P.; Grillner, S. Role of apamin-sensitive KCa channels for reticulospinal synaptic transmission to motoneuron and for the afterhyperpolarization. J. Neurophysiol. 2002, 88, 289–299. [Google Scholar] [CrossRef]
- Young, S.M., Jr.; Veeraraghavan, P. Presynaptic voltage-gated calcium channels in the auditory brainstem. Mol. Cell Neurosci. 2021, 112, 103609. [Google Scholar] [CrossRef]
- Faber, E.S.; Delaney, A.J.; Sah, P. SK channels regulate excitatory synaptic transmission and plasticity in the lateral amygdala. Nat. Neurosci. 2005, 8, 635–641. [Google Scholar] [CrossRef]
- Deschaux, O.; Bizot, J.C.; Goyffon, M. Apamin improves learning in an object recognition task in rats. Neurosci. Lett. 1997, 222, 159–162. [Google Scholar] [CrossRef]
- Tuteja, D.; Xu, D.; Timofeyev, V.; Lu, L.; Sharma, D.; Zhang, Z.; Xu, Y.; Nie, L.; Vázquez, A.E.; Young, J.N.; et al. Differential expression of small-conductance Ca2+-activated K+ channels SK1, SK2, and SK3 in mouse atrial and ventricular myocytes. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H2714–H2723. [Google Scholar] [CrossRef]
- Gu, M.; Zhu, Y.; Yin, X.; Zhang, D.M. Small-conductance Ca2+-activated K+ channels: Insights into their roles in cardiovascular disease. Exp. Mol. Med. 2018, 50, 1–7. [Google Scholar] [CrossRef]
- Terentyev, D.; Rochira, J.A.; Terentyeva, R.; Roder, K.; Koren, G.; Li, W. Sarcoplasmic reticulum Ca²⁺ release is both necessary and sufficient for SK channel activation in ventricular myocytes. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H738–H746. [Google Scholar] [CrossRef]
- Zhang, Q.; Timofeyev, V.; Lu, L.; Li, N.; Singapuri, A.; Long, M.K.; Bond, C.T.; Adelman, J.P.; Chiamvimonvat, N. Functional roles of a Ca2+-activated K+ channel in atrioventricular nodes. Circ. Res. 2008, 102, 465–471. [Google Scholar] [CrossRef]
- Ozgen, N.; Dun, W.; Sosunov, E.A.; Anyukhovsky, E.P.; Hirose, M.; Duffy, H.S.; Boyden, P.A.; Rosen, M.R. Early electrical remodeling in rabbit pulmonary vein results from trafficking of intracellular SK2 channels to membrane sites. Cardiovasc. Res. 2007, 75, 758–769. [Google Scholar] [CrossRef]
- Qi, X.Y.; Diness, J.G.; Brundel, B.J.; Zhou, X.B.; Naud, P.; Wu, C.T.; Huang, H.; Harada, M.; Aflaki, M.; Dobrev, D.; et al. Role of small-conductance calcium-activated potassium channels in atrial electrophysiology and fibrillation in the dog. Circulation 2014, 129, 430–440. [Google Scholar] [CrossRef]
- Tsai, W.C.; Chan, Y.H.; Hsueh, C.H.; Everett, T.H.t.; Chang, P.C.; Choi, E.K.; Olaopa, M.A.; Lin, S.F.; Shen, C.; Kudela, M.A.; et al. Small conductance calcium-activated potassium current and the mechanism of atrial arrhythmia in mice with dysfunctional melanocyte-like cells. Heart Rhythm. 2016, 13, 1527–1535. [Google Scholar] [CrossRef]
- Rahm, A.K.; Gramlich, D.; Wieder, T.; Müller, M.E.; Schoeffel, A.; El Tahry, F.A.; Most, P.; Heimberger, T.; Sandke, S.; Weis, T.; et al. Trigger-Specific Remodeling of KCa2 Potassium Channels in Models of Atrial Fibrillation. Pharmgenom. Pers. Med. 2021, 14, 579–590. [Google Scholar] [CrossRef]
- Darkow, E.; Nguyen, T.T.; Stolina, M.; Kari, F.A.; Schmidt, C.; Wiedmann, F.; Baczkó, I.; Kohl, P.; Rajamani, S.; Ravens, U.; et al. Small Conductance Ca2+-Activated K+ (SK) Channel mRNA Expression in Human Atrial and Ventricular Tissue: Comparison Between Donor, Atrial Fibrillation and Heart Failure Tissue. Front. Physiol. 2021, 12, 650964. [Google Scholar] [CrossRef]
- Katherine, Z.; Shawn, K.; Frank, S.; Jun, F. SK Channels and Heart Disease. In Ion Transporters—From Basic Properties to Medical Treatment; Zuzana Sevcikova, T., Ed.; IntechOpen: Rijeka, Croatia, 2022. [Google Scholar]
- Hsieh, Y.C.; Chang, P.C.; Hsueh, C.H.; Lee, Y.S.; Shen, C.; Weiss, J.N.; Chen, Z.; Ai, T.; Lin, S.F.; Chen, P.S. Apamin-sensitive potassium current modulates action potential duration restitution and arrhythmogenesis of failing rabbit ventricles. Circ. Arrhythm. Electrophysiol. 2013, 6, 410–418. [Google Scholar] [CrossRef]
- Bonilla, I.M.; Long, V.P., 3rd; Vargas-Pinto, P.; Wright, P.; Belevych, A.; Lou, Q.; Mowrey, K.; Yoo, J.; Binkley, P.F.; Fedorov, V.V.; et al. Calcium-activated potassium current modulates ventricular repolarization in chronic heart failure. PLoS ONE 2014, 9, e108824. [Google Scholar] [CrossRef]
- Chua, S.K.; Chang, P.C.; Maruyama, M.; Turker, I.; Shinohara, T.; Shen, M.J.; Chen, Z.; Shen, C.; Rubart-von der Lohe, M.; Lopshire, J.C.; et al. Small-conductance calcium-activated potassium channel and recurrent ventricular fibrillation in failing rabbit ventricles. Circ. Res. 2011, 108, 971–979. [Google Scholar] [CrossRef]
- Chang, P.C.; Chen, P.S. SK channels and ventricular arrhythmias in heart failure. Trends Cardiovasc. Med. 2015, 25, 508–514. [Google Scholar] [CrossRef]
- Stowe, D.F.; Gadicherla, A.K.; Zhou, Y.; Aldakkak, M.; Cheng, Q.; Kwok, W.M.; Jiang, M.T.; Heisner, J.S.; Yang, M.; Camara, A.K. Protection against cardiac injury by small Ca2+-sensitive K+ channels identified in guinea pig cardiac inner mitochondrial membrane. Biochim. Biophys. Acta 2013, 1828, 427–442. [Google Scholar] [CrossRef]
- Khandai, P.; Forcelli, P.A.; N’Gouemo, P. Activation of small conductance calcium-activated potassium channels suppresses seizure susceptibility in the genetically epilepsy-prone rats. Neuropharmacology 2020, 163, 107865. [Google Scholar] [CrossRef]
- Lappin, S.C.; Dale, T.J.; Brown, J.T.; Trezise, D.J.; Davies, C.H. Activation of SK channels inhibits epileptiform bursting in hippocampal CA3 neurons. Brain Res. 2005, 1065, 37–46. [Google Scholar] [CrossRef]
- Anderson, N.J.; Slough, S.; Watson, W.P. In vivo characterisation of the small-conductance KCa (SK) channel activator 1-ethyl-2-benzimidazolinone (1-EBIO) as a potential anticonvulsant. Eur. J. Pharmacol. 2006, 546, 48–53. [Google Scholar] [CrossRef]
- Criado-Marrero, M.; Santini, E.; Porter, J.T. Modulating fear extinction memory by manipulating SK potassium channels in the infralimbic cortex. Front. Behav. Neurosci. 2014, 8, 96. [Google Scholar] [CrossRef]
- Chen, T.; Zhu, J.; Hang, C.H.; Wang, Y.H. The Potassium SK Channel Activator NS309 Protects Against Experimental Traumatic Brain Injury Through Anti-Inflammatory and Immunomodulatory Mechanisms. Front. Pharmacol. 2019, 10, 1432. [Google Scholar] [CrossRef]
- Dolga, A.M.; de Andrade, A.; Meissner, L.; Knaus, H.G.; Höllerhage, M.; Christophersen, P.; Zischka, H.; Plesnila, N.; Höglinger, G.U.; Culmsee, C. Subcellular expression and neuroprotective effects of SK channels in human dopaminergic neurons. Cell Death Dis. 2014, 5, e999. [Google Scholar] [CrossRef]
- Stackman, R.W.; Hammond, R.S.; Linardatos, E.; Gerlach, A.; Maylie, J.; Adelman, J.P.; Tzounopoulos, T. Small conductance Ca2+-activated K+ channels modulate synaptic plasticity and memory encoding. J. Neurosci. 2002, 22, 10163–10171. [Google Scholar] [CrossRef]
- Inan, S.Y.; Aksu, F.; Baysal, F. The effects of some K+ channel blockers on scopolamine- or electroconvulsive shock-induced amnesia in mice. Eur. J. Pharmacol. 2000, 407, 159–164. [Google Scholar] [CrossRef]
- Mpari, B.; Regaya, I.; Escoffier, G.; Mourre, C. Differential effects of two blockers of small conductance Ca2+-activated K+ channels, apamin and lei-Dab7, on learning and memory in rats. J. Integr. Neurosci. 2005, 4, 381–396. [Google Scholar] [CrossRef]
- Losin, S.; McKean, C.M. Chlorzoxazone (paraflex) in the treatment of severe spasticity. Dev. Med. Child. Neurol. 1966, 8, 768–769. [Google Scholar] [CrossRef]
- Cao, Y.; Dreixler, J.C.; Roizen, J.D.; Roberts, M.T.; Houamed, K.M. Modulation of recombinant small-conductance Ca2+-activated K+ channels by the muscle relaxant chlorzoxazone and structurally related compounds. J. Pharmacol. Exp. Ther. 2001, 296, 683–689. [Google Scholar]
- Thakore, K.N. Chlorzoxazone. In Encyclopedia of Toxicology, 4th ed.; Wexler, P., Ed.; Academic Press: Oxford, UK, 2024; pp. 23–26. [Google Scholar]
- Hopf, F.W.; Simms, J.A.; Chang, S.J.; Seif, T.; Bartlett, S.E.; Bonci, A. Chlorzoxazone, an SK-type potassium channel activator used in humans, reduces excessive alcohol intake in rats. Biol. Psychiatry 2011, 69, 618–624. [Google Scholar] [CrossRef]
- Alviña, K.; Khodakhah, K. KCa channels as therapeutic targets in episodic ataxia type-2. J. Neurosci. 2010, 30, 7249–7257. [Google Scholar] [CrossRef]
- Dimitriadi, M.; Kye, M.J.; Kalloo, G.; Yersak, J.M.; Sahin, M.; Hart, A.C. The neuroprotective drug riluzole acts via small conductance Ca2+-activated K+ channels to ameliorate defects in spinal muscular atrophy models. J. Neurosci. 2013, 33, 6557–6562. [Google Scholar] [CrossRef]
- Thompson, J.M.; Ji, G.; Neugebauer, V. Small-conductance calcium-activated potassium (SK) channels in the amygdala mediate pain-inhibiting effects of clinically available riluzole in a rat model of arthritis pain. Mol. Pain. 2015, 11, 51. [Google Scholar] [CrossRef]
- Kasumu, A.; Bezprozvanny, I. Deranged calcium signaling in Purkinje cells and pathogenesis in spinocerebellar ataxia 2 (SCA2) and other ataxias. Cerebellum 2012, 11, 630–639. [Google Scholar] [CrossRef]
- Durr, A. Autosomal dominant cerebellar ataxias: Polyglutamine expansions and beyond. Lancet Neurol. 2010, 9, 885–894. [Google Scholar] [CrossRef]
- Skibsbye, L.; Wang, X.; Axelsen, L.N.; Bomholtz, S.H.; Nielsen, M.S.; Grunnet, M.; Bentzen, B.H.; Jespersen, T. Antiarrhythmic Mechanisms of SK Channel Inhibition in the Rat Atrium. J. Cardiovasc. Pharmacol. 2015, 66, 165–176. [Google Scholar] [CrossRef]
- Kirchhoff, J.E.; Diness, J.G.; Abildgaard, L.; Sheykhzade, M.; Grunnet, M.; Jespersen, T. Antiarrhythmic effect of the Ca2+-activated K+ (SK) channel inhibitor ICA combined with either amiodarone or dofetilide in an isolated heart model of atrial fibrillation. Pflug. Arch. 2016, 468, 1853–1863. [Google Scholar] [CrossRef]
- Skibsbye, L.; Bengaard, A.K.; Uldum-Nielsen, A.M.; Boddum, K.; Christ, T.; Jespersen, T. Inhibition of Small Conductance Calcium-Activated Potassium (SK) Channels Prevents Arrhythmias in Rat Atria During β-Adrenergic and Muscarinic Receptor Activation. Front. Physiol. 2018, 9, 510. [Google Scholar] [CrossRef]
- Kirchhoff, J.E.; Diness, J.G.; Sheykhzade, M.; Grunnet, M.; Jespersen, T. Synergistic antiarrhythmic effect of combining inhibition of Ca²⁺-activated K⁺ (SK) channels and voltage-gated Na⁺ channels in an isolated heart model of atrial fibrillation. Heart Rhythm. 2015, 12, 409–418. [Google Scholar] [CrossRef]
- Simó-Vicens, R.; Kirchhoff, J.E.; Dolce, B.; Abildgaard, L.; Speerschneider, T.; Sørensen, U.S.; Grunnet, M.; Diness, J.G.; Bentzen, B.H. A new negative allosteric modulator, AP14145, for the study of small conductance calcium-activated potassium (KCa2) channels. Br. J. Pharmacol. 2017, 174, 4396–4408. [Google Scholar] [CrossRef]
- Diness, J.G.; Skibsbye, L.; Simó-Vicens, R.; Santos, J.L.; Lundegaard, P.; Citerni, C.; Sauter, D.R.P.; Bomholtz, S.H.; Svendsen, J.H.; Olesen, S.P.; et al. Termination of Vernakalant-Resistant Atrial Fibrillation by Inhibition of Small-Conductance Ca2+-Activated K+ Channels in Pigs. Circ. Arrhythm. Electrophysiol. 2017, 10, e005125. [Google Scholar] [CrossRef]
- Linz, B.; Hesselkilde, E.M.; Skarsfeldt, M.A.; Hertel, J.N.; Sattler, S.M.; Yan, Y.; Tfelt-Hansen, J.; Diness, J.G.; Bentzen, B.H.; Linz, D.; et al. Pharmacological inhibition of SK-channels with AP14145 prevents atrial arrhythmogenic changes in a porcine model for obstructive respiratory events. J. Cardiovasc. Electrophysiol. 2023, 34, 126–134. [Google Scholar] [CrossRef]
- Diness, J.G.; Sørensen, U.S.; Nissen, J.D.; Al-Shahib, B.; Jespersen, T.; Grunnet, M.; Hansen, R.S. Inhibition of small-conductance Ca2+-activated K+ channels terminates and protects against atrial fibrillation. Circ. Arrhythm. Electrophysiol. 2010, 3, 380–390. [Google Scholar] [CrossRef]
- Burashnikov, A.; Barajas-Martinez, H.; Hu, D.; Robinson, V.M.; Grunnet, M.; Antzelevitch, C. The Small Conductance Calcium-Activated Potassium Channel Inhibitors NS8593 and UCL1684 Prevent the Development of Atrial Fibrillation Through Atrial-Selective Inhibition of Sodium Channel Activity. J. Cardiovasc. Pharmacol. 2020, 76, 164–172. [Google Scholar] [CrossRef]
- Springer, J.P.; Clardy, J.; Wells, J.M.; Cole, R.J.; Kirksey, J.W. The structure of paxilline, a tremorgenic metabolite of penicillium paxilli bainier. Tetrahedron Lett. 1975, 16, 2531–2534. [Google Scholar] [CrossRef]
- Munday-Finch, S.C.; Wilkins, A.L.; Miles, C.O.; Tomoda, H.; Ōmura, S. Isolation and Structure Elucidation of Lolilline, a Possible Biosynthetic Precursor of the Lolitrem Family of Tremorgenic Mycotoxins. J. Agric. Food Chem. 1997, 45, 199–204. [Google Scholar] [CrossRef]
- Cole, R.J.; Cox, R.H. 8—Tremorgen Group. In Handbook of Toxic Fungal Metabolites; Cole, R.J., Cox, R.H., Eds.; Academic Press: San Diego, CA, USA, 1981; pp. 355–509. [Google Scholar]
- McLeay, L.M.; Smith, B.L.; Munday-Finch, S.C. Tremorgenic mycotoxins paxilline, penitrem and lolitrem B, the non-tremorgenic 31-epilolitrem B and electromyographic activity of the reticulum and rumen of sheep. Res. Vet. Sci. 1999, 66, 119–127. [Google Scholar] [CrossRef]
- Miles, C.O.; Wilkins, A.L.; Gallagher, R.T.; Hawkes, A.D.; Munday, S.C.; Towers, N.R. Synthesis and tremorgenicity of paxitriols and lolitriol: Possible biosynthetic precursors of lolitrem B. J. Agric. Food Chem. 1992, 40, 234–238. [Google Scholar] [CrossRef]
- Strong, P.N.; Mukherjee, S.; Shah, N.; Chowdhary, A.; Jeyaseelan, K. Scorpion Venom Research Around the World: Indian Red Scorpion. In Toxinology: Scorpion Venoms; Gopalakrishnakone, P., Ed.; Springer: Dordrecht, The Netherlands, 2021; pp. 1–13. [Google Scholar]
- Bawaskar, H.S.; Bawaskar, P.H. Management of the cardiovascular manifestations of poisoning by the Indian red scorpion (Mesobuthus tamulus). Br. Heart J. 1992, 68, 478–480. [Google Scholar] [CrossRef]
- Vincent, J.P.; Schweitz, H.; Lazdunski, M. Structure-function relationships and site of action of apamin, a neurotoxic polypeptide of bee venom with an action on the central nervous system. Biochemistry 1975, 14, 2521–2525. [Google Scholar] [CrossRef]
- Cheng-Raude, D.; Treloar, M.; Habermann, E. Preparation and pharmacokinetics of labeled derivatives of apamin. Toxicon 1976, 14, 467–476. [Google Scholar] [CrossRef]
- Habermann, E. Neurotoxicity of apamin and MCD peptide upon central application. Naunyn Schmiedebergs Arch. Pharmacol. 1977, 300, 189–191. [Google Scholar] [CrossRef]
- Lallement, G.; Fosbraey, P.; Baille-Le-Crom, V.; Tattersall, J.E.; Blanchet, G.; Wetherell, J.R.; Rice, P.; Passingham, S.L.; Sentenac-Roumanou, H. Compared toxicity of the potassium channel blockers, apamin and dendrotoxin. Toxicology 1995, 104, 47–52. [Google Scholar] [CrossRef]
- Apamin. Material Safety Data Sheet. Available online: https://datasheets.scbt.com/sc-200994.pdf (accessed on 6 January 2024).
- Kountouras, J.; Chatzopoulos, D.; Zavos, C.; Boura, P.; Venizelos, J.; Kalis, A. Efficacy of trimebutine therapy in patients with gastroesophageal reflux disease and irritable bowel syndrome. Hepatogastroenterology 2002, 49, 193–197. [Google Scholar]
- Roy, D.; Talajic, M.; Dorian, P.; Connolly, S.; Eisenberg, M.J.; Green, M.; Kus, T.; Lambert, J.; Dubuc, M.; Gagné, P.; et al. Amiodarone to prevent recurrence of atrial fibrillation. Canadian Trial of Atrial Fibrillation Investigators. N. Engl. J. Med. 2000, 342, 913–920. [Google Scholar] [CrossRef]
- Singh, S.; Zoble, R.G.; Yellen, L.; Brodsky, M.A.; Feld, G.K.; Berk, M.; Billing, C.B., Jr. Efficacy and safety of oral dofetilide in converting to and maintaining sinus rhythm in patients with chronic atrial fibrillation or atrial flutter: The symptomatic atrial fibrillation investigative research on dofetilide (SAFIRE-D) study. Circulation 2000, 102, 2385–2390. [Google Scholar] [CrossRef]
- Yu, H.B.; Li, M.; Wang, W.P.; Wang, X.L. High throughput screening technologies for ion channels. Acta Pharmacol. Sin. 2016, 37, 34–43. [Google Scholar] [CrossRef]
- Zhang, G.; Xu, X.; Jia, Z.; Geng, Y.; Liang, H.; Shi, J.; Marras, M.; Abella, C.; Magleby, K.L.; Silva, J.R.; et al. An allosteric modulator activates BK channels by perturbing coupling between Ca2+ binding and pore opening. Nat. Commun. 2022, 13, 6784. [Google Scholar] [CrossRef]

| BK Channel | IK Channel | SK Channel (SK1, SK2, SK3) | |
|---|---|---|---|
| Opening mechanism | Voltage-dependent and Ca2+-dependent | Only Ca2+-dependent | Only Ca2+-dependent |
| Conductance (pS) | 200–300 | 30–40 | 4–14 |
| Aliases | KCa1.1, Maxi-K, Slo1 | KCa3.1, SK4, IK1 | KCa2.1, KCa2.2, KCa2.3 |
| Ca2+-binding affinity (μM) | 1–11 | 0.2–0.5 | 0.3–0.6 |
| Ca2+-binding site | RCK domain | Calmodulin-binding domain | Calmodulin-binding domain |
| Gene encoding α-subunit (human) | KCNMA1 | KCNN4 | KCNN1, KCNN2, KCNN3 |
| Modulator/Drug | Structure | Characteristics of Modulator/Drug | Effects on Diseases or Disorders | References |
|---|---|---|---|---|
| Zonisamide |  | BK activator Nav and T-type Ca2+ blocker Approved drug used to treat symptoms of epilepsy and Parkinson’s disease | Combined with other medicines to manage convulsions The main anticonvulsant mechanism via BK channel is unknown | [76,77,124] |
| Resveratrol | 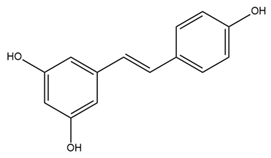 | BK activator, Nav inhibitor Phytoalexin naturally occurring in grapes and red wine | Reduces nerve tissue damage Prevents the onset of seizures Decreases ET-1 and increases NO through mitoBK Protects cardiovascular system | [64,79,80,101] |
| Paxilline (PAX) | 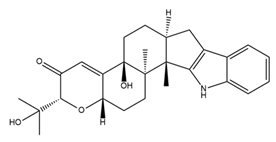 | BK channel blocker Tremorgenic fungal alkaloid | Reduces seizure duration and intensity in epilepsy Reduces heart rate Reduces firing rate in SANC and heart rate Induces cell cycle arrest and reduces cell size, migration, invasion, and induction of apoptosis | [62,81,82,97,98,112,113] |
| Iberiotoxin (IbTX) |  | BK channel blocker Purified from scorpions | Inhibitory effects on burst activity in primary cultured mouse neurons Reduces heart rate Induces cell cycle arrest and decreases cell proliferation, migration, and invasion | [62,83,84,97,98,112,113,122] |
| Isopimaric acid (ISO) |  | BK activator Toxin derived from conifers | Cognitive recovery in mouse AD model | [86] |
| BMS-204352 (MaxiPost) |  | BK and Kv7 activator Phase III clinical trial for acute ischemic stroke treatment | Restored glutamate balance in the hippocampus and improved spatial memory in a rat FXS model Restores habits that are impaired due to illness Improves ischemia in nerves | [91,92,93,94,95] |
| Lolitrem B | 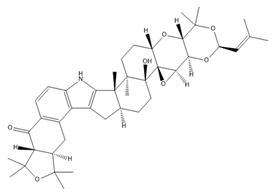 | BK inhibitor | Reduces heart rate | [62] |
| NS1619 | 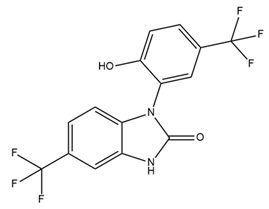 | BK activator Benzimidazole derivative L-type Ca2+ channel and Kv channel inhibitor | Dilates blood vessels through mitoBK Cardioprotective effects against ischemia/reperfusion injury Reduces cell proliferation, migration, and induction of apoptosis | [36,102,104,115] |
| NS11021 | 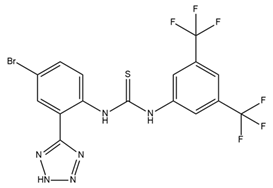 | BK activator | Increases channel opening likelihood Extends the survival of cardiac cells under locally ischemic conditions | [103,111] |
| BMS 191011 | 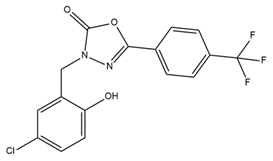 | BK activator | Suppresses tumor growth | [116] |
| Penitrem A | 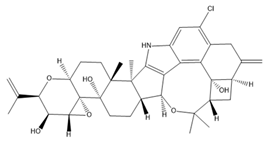 | BK inhibitor | Reduces cell proliferation and invasion | [114,120,121] |
| Tetra-ethylammonium |  | BK inhibitor | Decreases cell proliferation, migration, and invasion | [122] |
| Trimebutine maleate |  | BK and voltage-gated calcium channel inhibitor | Suppresses tumor growth | [123] |
| Modulator/Drug | Structure | Characteristics of Modulator/Drug | Effects on Diseases or Disorders | References |
|---|---|---|---|---|
| Senicapoc (ICA-107043) |  | IK-selective inhibitor Developed based on clotrimazole structure Phase III clinical trial in sickle cell anemia patients | Reduces red blood cell dehydration and hemolysis and increases hemoglobin concentration in sickle cell disease without decreasing clinical pain Attenuates the increase in airway resistance and decreases an exhaled inflammation marker (NO) Reduces tactile allodynia without any effect on motor activity Suppresses the growth of intrahepatic cholangiocarcinoma cells | [149,150,175,182] |
| TRAM-34 |  | IK-selective inhibitor | Reduces the activation of microglia/macrophages, leading to a reduction in neuroinflammation related to ischemic/reperfusion stroke Improves memory deficits in AD Decreases sneezing, nose rubbing, epithelial cell proliferation, eosinophil infiltration in allergic rhinitis Diminishes cell proliferation and the secretion of proinflammatory cytokines in synovial fibroblasts from rheumatoid arthritis patients Inhibits cancer cell proliferation, cell cycle, invasion, metastasis, and resistance | [154,156,160,161,179,180] |
| NS6180 | IK-selective inhibitor | Decreases IK expression in human, rat, and mouse red blood cells Reduces cytokines (IL-2, IL-4, TNF-α, and IFN-γ) | [155] | |
| 1-EBIO |  | IK and SK activator | Promotes the apoptosis process of cisplatin-resistant cancer cells | [161] |
| SKA-31 | 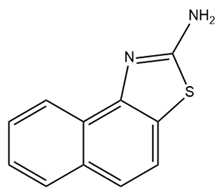 | IK activator | Combined with E4031 and cisplatin, promotes apoptosis and inhibits the proliferation of colorectal cancer cells | [176] |
| Riluzole | 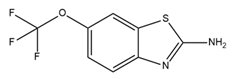 | IK and SK activator | Combined with cisplatin, overcomes drug resistance in colorectal cancer cells | [176] |
| Modulator/ Drug | Structure | Characteristics of Modulator/Drug | Effects on Diseases or Disorders | References |
|---|---|---|---|---|
| 1-EBIO | 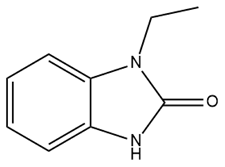 | SK and IK activator | Reduces acoustically evoked seizures Suppresses epileptiform activity in the acute hippocampus Decreases seizure probability in mice | [207,208,209] |
| DCEBIO |  | SK and IK activator | Modulates fear extinction memory | [210] |
| NS309 |  | SK and IK activator | Neuron protection role Decreases brain edema, traumatic brain injury-induced deficits in neurological function, and neuronal apoptosis Improves dopaminergic cell depletion | [211,212] |
| Apamin | 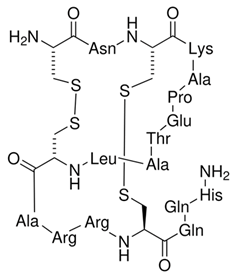 | SK-selective inhibitor Derived from bee venom | Increases the excitability of hippocampal neurons and induces synaptic plasticity Enhances object recognition memory and improves the retrieval of extinction memories | [210,213,214,215] |
| Chlorzoxazone (CZX) | 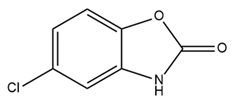 | SK2 activator Approved drug to treat muscle pain | Reduces neuronal activity and ultimately results in decreased alcohol consumption Episodic ataxia type 2 treatment | [216,219,220] |
| Riluzole |  | SK and IK activator Approved for amyotrophic lateral sclerosis treatment | Improves disease-related loss-of-function defects in a mouse model of joint pain Used in spinocerebellar ataxia type 2 treatment | [221,222,223] |
| ICA |  | SK inhibitor | Prolongs AP duration and reduces excitability Combined with dofetilide or aminodarone to treat AF, reduces the risk of ventricular arrhythmias with fewer side effects Combined with flecainide or ranolazine, reduces AF at sub-efficacious doses | [225,226,227] |
| AP14145 | 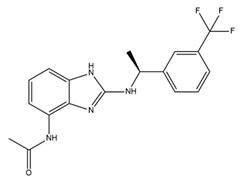 | SK2 and SK3 inhibitor | Extends the atrial effective refractory period Attenuates vernakalant-resistant AF in a pig model Improves obstructive respiratory events | [229,230,231] |
| NS8593 |  | SK inhibitor | Prolongs the atrial effective refractory period and decreases AF | [233] |
| UCL1684 | 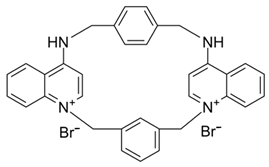 | SK inhibitor | Prolongs the atrial effective refractory period and decreases AF | [233] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van, N.T.H.; Kim, W.K.; Nam, J.H. Challenges in the Therapeutic Targeting of KCa Channels: From Basic Physiology to Clinical Applications. Int. J. Mol. Sci. 2024, 25, 2965. https://doi.org/10.3390/ijms25052965
Van NTH, Kim WK, Nam JH. Challenges in the Therapeutic Targeting of KCa Channels: From Basic Physiology to Clinical Applications. International Journal of Molecular Sciences. 2024; 25(5):2965. https://doi.org/10.3390/ijms25052965
Chicago/Turabian StyleVan, Nhung Thi Hong, Woo Kyung Kim, and Joo Hyun Nam. 2024. "Challenges in the Therapeutic Targeting of KCa Channels: From Basic Physiology to Clinical Applications" International Journal of Molecular Sciences 25, no. 5: 2965. https://doi.org/10.3390/ijms25052965
APA StyleVan, N. T. H., Kim, W. K., & Nam, J. H. (2024). Challenges in the Therapeutic Targeting of KCa Channels: From Basic Physiology to Clinical Applications. International Journal of Molecular Sciences, 25(5), 2965. https://doi.org/10.3390/ijms25052965







