Sustained Extracellular Electrical Stimulation Modulates the Permeability of Gap Junctions in rd1 Mouse Retina with Photoreceptor Degeneration
Abstract
1. Introduction
2. Results
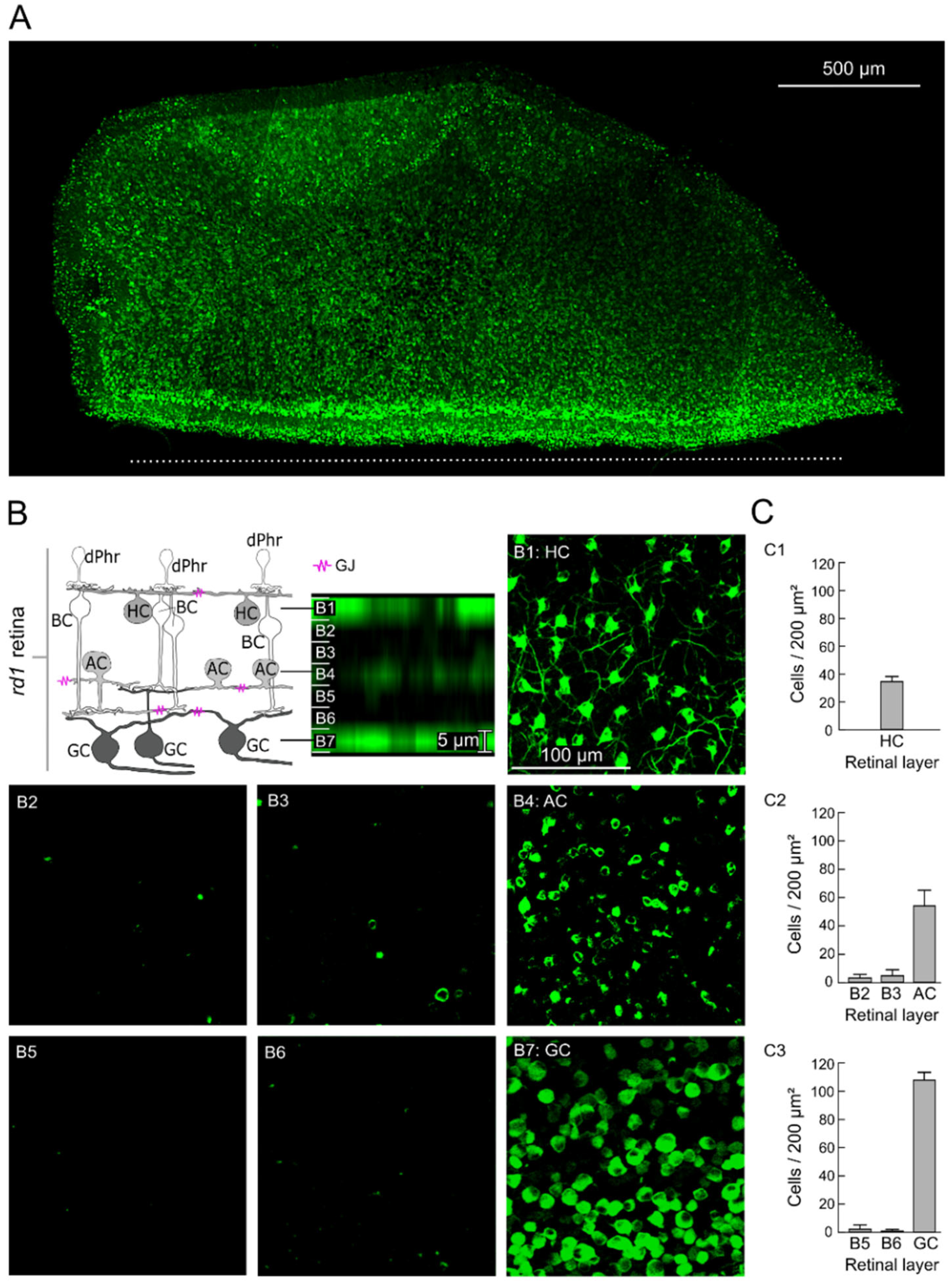
2.1. GJ-Nets of Degenerated rd1 Mouse Retinas
2.2. GJs of Degenerated rd1 Mouse Retinas Are Functional
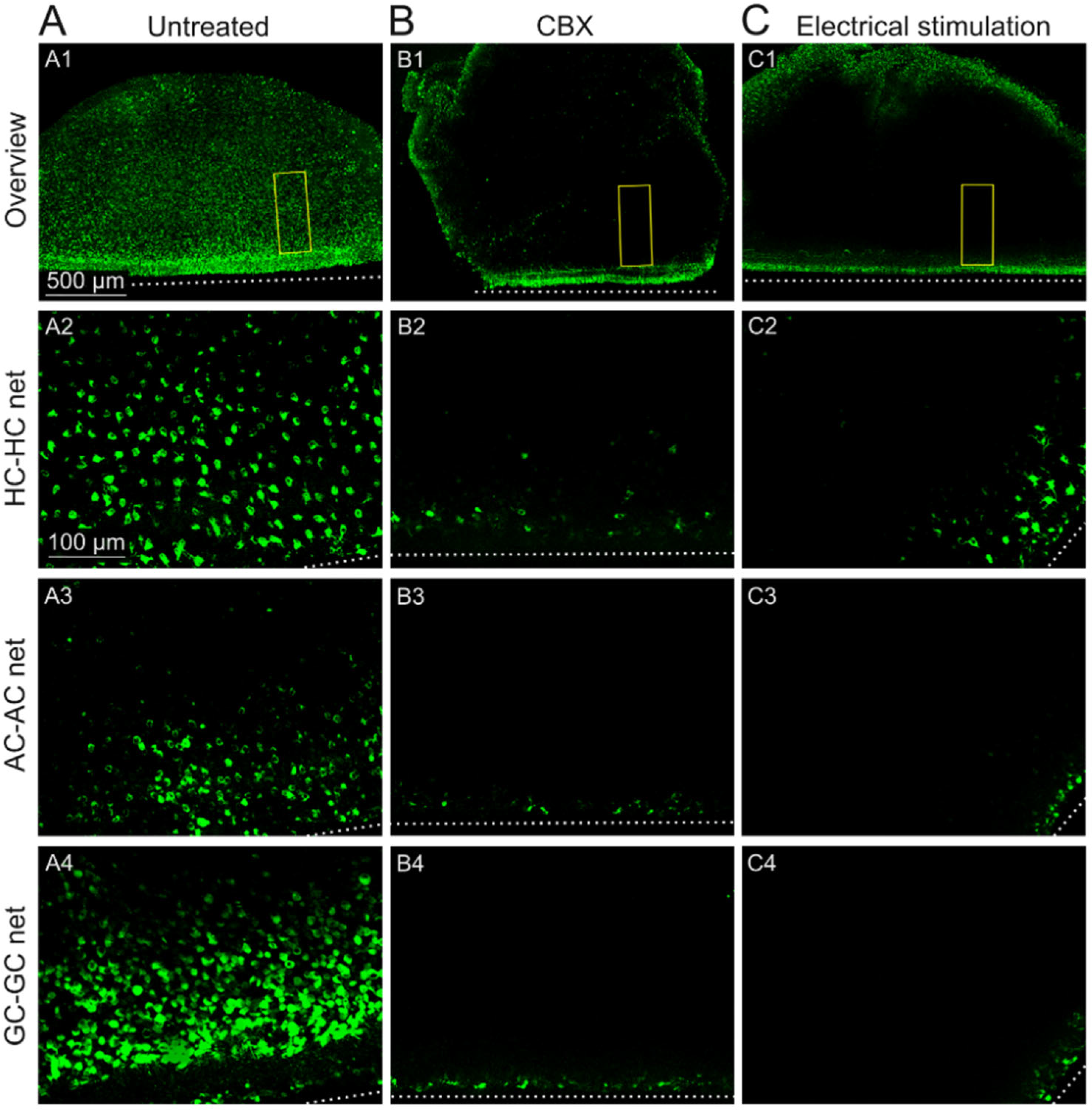
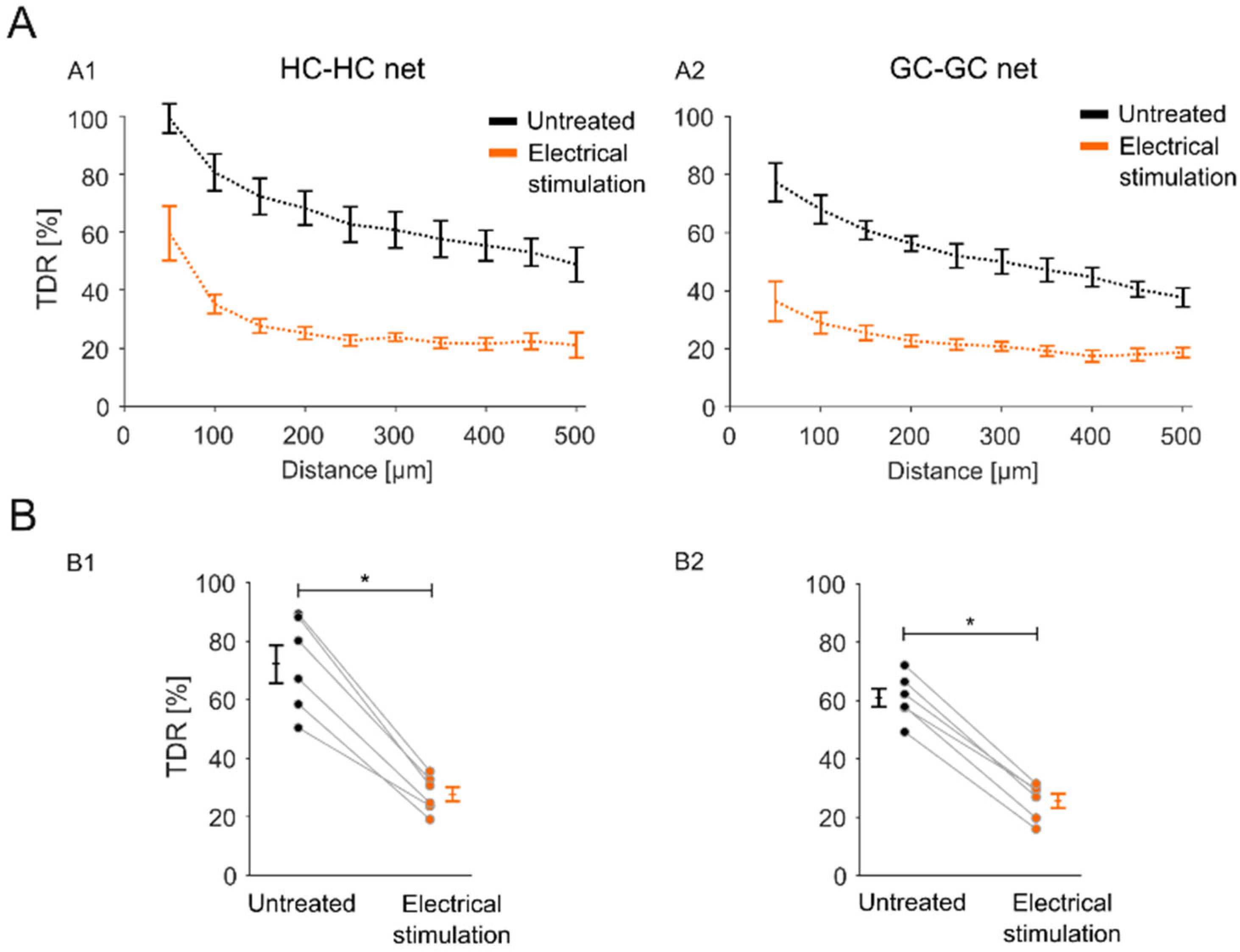
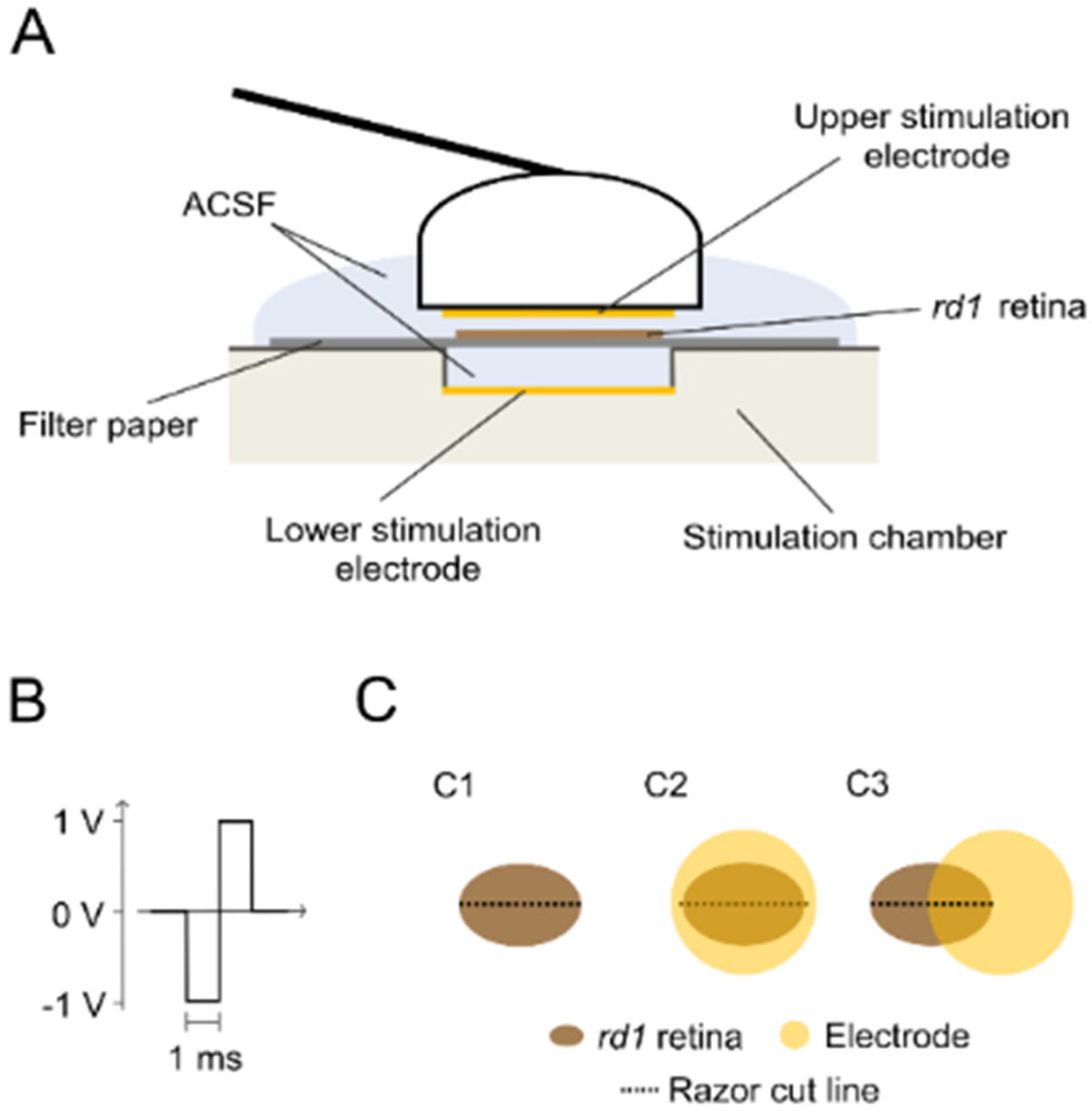
2.3. Sustained Electrical Stimulation Affects the GJ Permeability of Degenerated rd1 Mouse Retinas
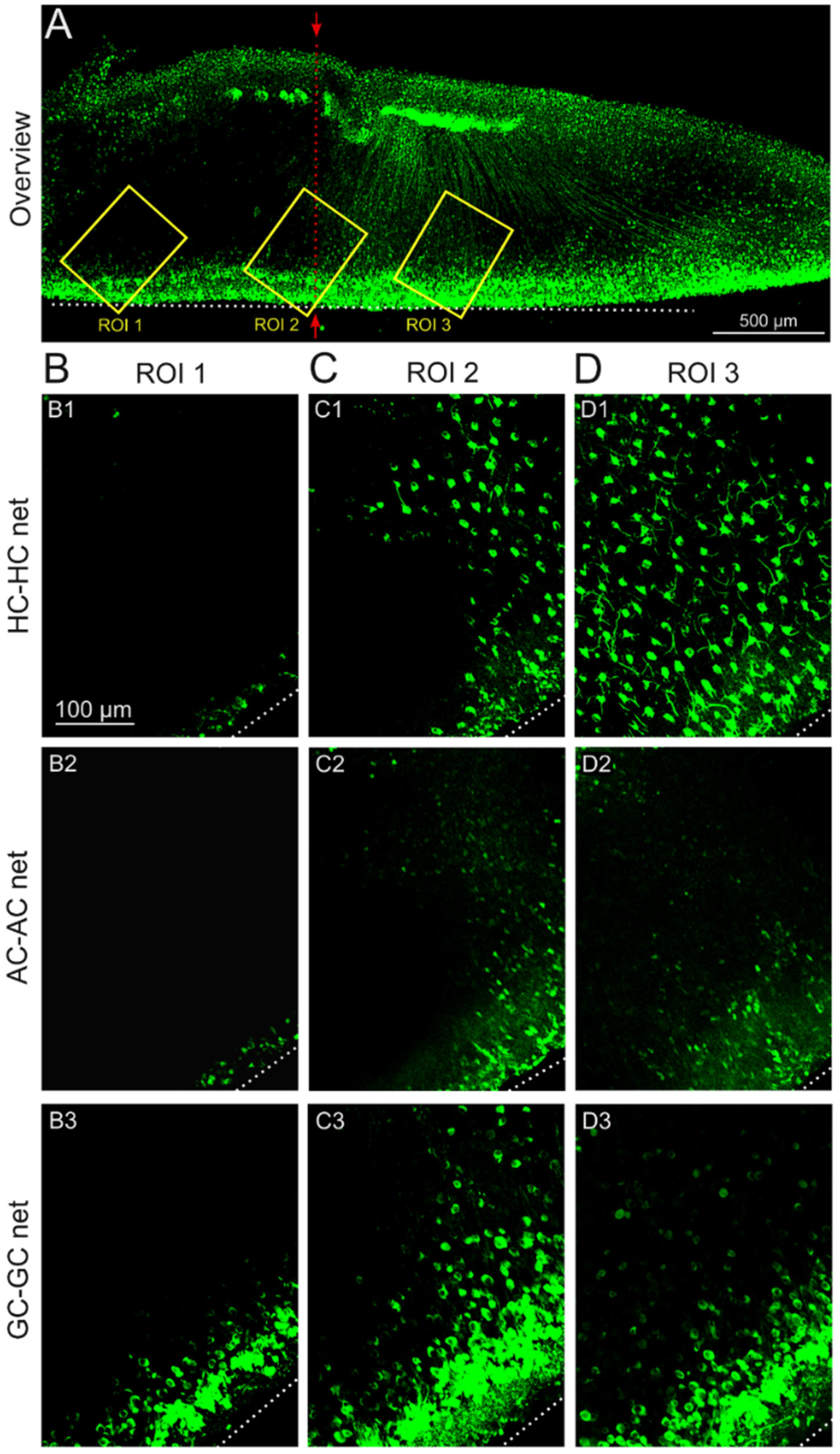
2.4. Impact of the Stimulation-Induced Reduction in GJ Permeability Is Local to the Stimulation Electrode Position
3. Discussion
3.1. Lateral Signal Transduction in Degenerated rd1 Mouse Retinas
3.2. Electrical Modulation of Retinal GJ-Nets
3.3. Implications of Electrical Stimulation-Mediated Modulation of GJ-Nets for the Development of Strategies for Electrical Implants
4. Materials and Methods
4.1. Animals
4.2. Retinal Tissue Preparation
4.3. Experimental Conditions
4.4. Retinal Tracer Loading
4.5. Fluorescence Imaging
4.6. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GJs | gap junctions |
| GJ-net | gap junction-coupled network |
| rd1 | retinal degeneration 1 |
| dPhr | degenerated photoreceptor |
| HC | horizontal cell |
| BC | bipolar cell |
| AC | amacrine cell |
| GC | ganglion cell |
| ACSF | artificial cerebrospinal fluid |
| PBS | phosphate-buffered saline |
| CBX | carbenoxolone |
| M | molar |
| mM | millimolar |
| µm | micrometer |
| mm | millimeter |
| µl | microliter |
| h | hour |
| min | minute |
| V | volt |
| ROI | region of interest |
| TDR | tracer diffusion rate |
| VGC | voltage gated channel |
| VGNaC | voltage gated sodium channel |
| VGCC | voltage gated calcium channel |
| ANOVA | analysis of variance |
| SEM | standard error of the mean |
| STD | standard deviation |
References
- Michalakis, S.; Gerhardt, M.; Rudolph, G.; Priglinger, S.; Priglinger, C. Achromatopsia: Genetics and Gene Therapy. Mol. Diagn. Ther. 2022, 26, 51–59. [Google Scholar] [CrossRef]
- Vighi, E.; Trifunovic, D.; Veiga-Crespo, P.; Rentsch, A.; Hoffmann, D.; Sahaboglu, A.; Strasser, T.; Kulkarni, M.; Bertolotti, E.; van den Heuvel, A.; et al. Combination of cGMP analogue and drug delivery system provides functional protection in hereditary retinal degeneration. Proc. Natl. Acad. Sci. USA 2018, 115, E2997–E3006. [Google Scholar] [CrossRef]
- Lagali, P.S.; Balya, D.; Awatramani, G.B.; Munch, T.A.; Kim, D.S.; Busskamp, V.; Cepko, C.L.; Roska, B. Light-activated channels targeted to ON bipolar cells restore visual function in retinal degeneration. Nat. Neurosci. 2008, 11, 667–675. [Google Scholar] [CrossRef]
- De Silva, S.R.; Moore, A.T. Optogenetic approaches to therapy for inherited retinal degenerations. J. Physiol. 2022, 600, 4623–4632. [Google Scholar] [CrossRef]
- Mathieson, K.; Loudin, J.; Goetz, G.; Huie, P.; Wang, L.; Kamins, T.I.; Galambos, L.; Smith, R.; Harris, J.S.; Sher, A.; et al. Photovoltaic Retinal Prosthesis with High Pixel Density. Nat. Photonics 2012, 6, 391–397. [Google Scholar] [CrossRef]
- da Cruz, L.; Coley, B.F.; Dorn, J.; Merlini, F.; Filley, E.; Christopher, P.; Chen, F.K.; Wuyyuru, V.; Sahel, J.; Stanga, P.; et al. The Argus II epiretinal prosthesis system allows letter and word reading and long-term function in patients with profound vision loss. Br. J. Ophthalmol. 2013, 97, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, E.; Alfaro, A.; Soto-Sanchez, C.; Gonzalez-Lopez, P.; Lozano, A.M.; Pena, S.; Grima, M.D.; Rodil, A.; Gomez, B.; Chen, X.; et al. Visual percepts evoked with an intracortical 96-channel microelectrode array inserted in human occipital cortex. J. Clin. Investig. 2021, 131, e151331. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Sugita, S.; Kurimoto, Y.; Takahashi, M. Trends of Stem Cell Therapies in Age-Related Macular Degeneration. J. Clin. Med. 2021, 10, 1785. [Google Scholar] [CrossRef] [PubMed]
- Barhoum, R.; Martinez-Navarrete, G.; Corrochano, S.; Germain, F.; Fernandez-Sanchez, L.; de la Rosa, E.J.; de la Villa, P.; Cuenca, N. Functional and structural modifications during retinal degeneration in the rd10 mouse. Neuroscience 2008, 155, 698–713. [Google Scholar] [CrossRef] [PubMed]
- Hoon, M.; Okawa, H.; Della Santina, L.; Wong, R.O. Functional architecture of the retina: Development and disease. Prog. Retin. Eye Res. 2014, 42, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Kalloniatis, M.; Nivison-Smith, L.; Chua, J.; Acosta, M.L.; Fletcher, E.L. Using the rd1 mouse to understand functional and anatomical retinal remodelling and treatment implications in retinitis pigmentosa: A review. Exp. Eye Res. 2016, 150, 106–121. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Bloomfield, S.A. Plasticity of Retinal Gap Junctions: Roles in Synaptic Physiology and Disease. Annu. Rev. Vis. Sci. 2018, 4, 79–100. [Google Scholar] [CrossRef] [PubMed]
- Trenholm, S.; Awatramani, G.B. Myriad Roles for Gap Junctions in Retinal Circuits. In Webvision: The Organization of the Retina and Visual System; University of Utah Health Sciences: Salt Lake City, UT, USA, 2019. [Google Scholar]
- Kolb, H.; Fernandez, E.; Nelson, R. The Organization of the Retina and Visual System. In Webvision: The Organization of the Retina and Visual System; University of Utah Health Sciences: Salt Lake City, UT, USA, 2005. [Google Scholar]
- Bloomfield, S.A.; Volgyi, B. The diverse functional roles and regulation of neuronal gap junctions in the retina. Nat. Rev. Neurosci. 2009, 10, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Pandarinath, C.; Bomash, I.; Victor, J.D.; Prusky, G.T.; Tschetter, W.; Nirenberg, S. A novel mechanism for switching a neural system from one state to another. Front. Comput. Neurosci. 2010, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Marc, R.E.; Jones, B.W.; Watt, C.B.; Strettoi, E. Neural remodeling in retinal degeneration. Progress. Retin. Eye Res. 2003, 22, 607–655. [Google Scholar] [CrossRef]
- Strettoi, E.; Pignatelli, V.; Rossi, C.; Porciatti, V.; Falsini, B. Remodeling of second-order neurons in the retina of rd/rd mutant mice. Vis. Res. 2003, 43, 867–877. [Google Scholar] [CrossRef]
- Pfeiffer, R.L.; Anderson, J.R.; Dahal, J.; Garcia, J.C.; Yang, J.H.; Sigulinsky, C.L.; Rapp, K.; Emrich, D.P.; Watt, C.B.; Johnstun, H.A.; et al. A pathoconnectome of early neurodegeneration: Network changes in retinal degeneration. Exp. Eye Res. 2020, 199, 108196. [Google Scholar] [CrossRef]
- Borowska, J.; Trenholm, S.; Awatramani, G.B. An intrinsic neural oscillator in the degenerating mouse retina. J. Neurosci. 2011, 31, 5000–5012. [Google Scholar] [CrossRef]
- Menzler, J.; Zeck, G. Network oscillations in rod-degenerated mouse retinas. J. Neurosci. 2011, 31, 2280–2291. [Google Scholar] [CrossRef]
- Haq, W.; Arango-Gonzalez, B.; Zrenner, E.; Euler, T.; Schubert, T. Synaptic remodeling generates synchronous oscillations in the degenerated outer mouse retina. Front. Neural Circuits 2014, 8, 108. [Google Scholar] [CrossRef]
- Goodenough, D.A.; Paul, D.L. Gap junctions. Cold Spring Harb. Perspect. Biol. 2009, 1, a002576. [Google Scholar] [CrossRef]
- Bowes, C.; Li, T.; Danciger, M.; Baxter, L.C.; Applebury, M.L.; Farber, D.B. Retinal degeneration in the rd mouse is caused by a defect in the β subunit of rod cGMP-phosphodiesterase. Nature 1990, 347, 677–680. [Google Scholar] [CrossRef]
- Choi, H.J.; Ribelayga, C.P.; Mangel, S.C. Cut-loading: A useful tool for examining the extent of gap junction tracer coupling between retinal neurons. J. Vis. Exp. 2012, 59, 3180. [Google Scholar] [CrossRef]
- Mills, S.L.; Massey, S.C. A series of biotinylated tracers distinguishes three types of gap junction in retina. J. Neurosci. 2000, 20, 8629–8636. [Google Scholar] [CrossRef]
- O’Brien, J.J.; Li, W.; Pan, F.; Keung, J.; O’Brien, J.; Massey, S.C. Coupling between A-Type Horizontal Cells Is Mediated by Connexin 50 Gap Junctions in the Rabbit Retina. J. Neurosci. 2006, 26, 11624. [Google Scholar] [CrossRef]
- Ivanova, E.; Yee, C.W.; Baldoni, R., Jr.; Sagdullaev, B.T. Aberrant activity in retinal degeneration impairs central visual processing and relies on Cx36-containing gap junctions. Exp. Eye Res. 2016, 150, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Prado, N.; Bukauskas, F.F. Heterotypic gap junction channels as voltage-sensitive valves for intercellular signaling. Proc. Natl. Acad. Sci. USA 2009, 106, 14855–14860. [Google Scholar] [CrossRef] [PubMed]
- Eickenscheidt, M.; Jenkner, M.; Thewes, R.; Fromherz, P.; Zeck, G. Electrical stimulation of retinal neurons in epiretinal and subretinal configuration using a multicapacitor array. J. Neurophysiol. 2012, 107, 2742–2755. [Google Scholar] [CrossRef]
- Haq, W.; Dietter, J.; Zrenner, E. Electrical activation of degenerated photoreceptors in blind mouse retina elicited network-mediated responses in different types of ganglion cells. Sci. Rep. 2018, 8, 16998. [Google Scholar] [CrossRef]
- Haq, W.; Zrenner, E.; Ueffing, M.; Paquet-Durand, F. Using Micro-Electrode-Array Recordings and Retinal Disease Models to Elucidate Visual Functions: Simultaneous Recording of Local Electroretinograms and Ganglion Cell Action Potentials Reveals the Origin of Retinal Oscillatory Potentials. Bioengineering 2023, 10, 725. [Google Scholar] [CrossRef] [PubMed]
- Lachapelle, P.; Benoit, J.; Little, J.M.; Faubert, J. The diagnostic use of the second oscillatory potential in clinical electroretinography. Doc. Ophthalmol. 1989, 73, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Bui, B.V.; Edmunds, B.; Cioffi, G.A.; Fortune, B. The gradient of retinal functional changes during acute intraocular pressure elevation. Investig. Ophthalmol. Vis. Sci. 2005, 46, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Shirao, Y.; Kawasaki, K. Electrical responses from diabetic retina. Prog. Retin. Eye Res. 1998, 17, 59–76. [Google Scholar] [CrossRef] [PubMed]
- Lachapelle, P. The human suprathreshold photopic oscillatory potentials: Method of analysis and clinical application. Doc. Ophthalmol. 1994, 88, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zemel, E.; Miller, B.; Perlman, I. Early retinal damage in experimental diabetes: Electroretinographical and morphological observations. Exp. Eye Res. 2002, 74, 615–625. [Google Scholar] [CrossRef]
- Hancock, H.A.; Kraft, T.W. Oscillatory potential analysis and ERGs of normal and diabetic rats. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1002–1008. [Google Scholar] [CrossRef]
- Pang, J.J.; Gao, F.; Lem, J.; Bramblett, D.E.; Paul, D.L.; Wu, S.M. Direct rod input to cone BCs and direct cone input to rod BCs challenge the traditional view of mammalian BC circuitry. Proc. Natl. Acad. Sci. USA 2010, 107, 395–400. [Google Scholar] [CrossRef]
- Garita-Hernandez, M.; Lampic, M.; Chaffiol, A.; Guibbal, L.; Routet, F.; Santos-Ferreira, T.; Gasparini, S.; Borsch, O.; Gagliardi, G.; Reichman, S.; et al. Restoration of visual function by transplantation of optogenetically engineered photoreceptors. Nat. Commun. 2019, 10, 4524. [Google Scholar] [CrossRef]
- Carlen, P.L.; Skinner, F.; Zhang, L.; Naus, C.; Kushnir, M.; Perez Velazquez, J.L. The role of gap junctions in seizures. Brain Res. Brain Res. Rev. 2000, 32, 235–241. [Google Scholar] [CrossRef]
- Nakase, T.; Naus, C.C. Gap junctions and neurological disorders of the central nervous system. Biochim. Biophys. Acta 2004, 1662, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Dere, E.; Zlomuzica, A. The role of gap junctions in the brain in health and disease. Neurosci. Biobehav. Rev. 2012, 36, 206–217. [Google Scholar] [CrossRef]
- Ivanova, E.; Yee, C.W.; Sagdullaev, B.T. Increased phosphorylation of Cx36 gap junctions in the AII amacrine cells of RD retina. Front. Cell Neurosci. 2015, 9, 390. [Google Scholar] [CrossRef]
- Hombach, S.; Janssen-Bienhold, U.; Sohl, G.; Schubert, T.; Bussow, H.; Ott, T.; Weiler, R.; Willecke, K. Functional expression of connexin57 in horizontal cells of the mouse retina. Eur. J. Neurosci. 2004, 19, 2633–2640. [Google Scholar] [CrossRef]
- Strettoi, E.; Pignatelli, V. Modifications of retinal neurons in a mouse model of retinitis pigmentosa. Proc. Natl. Acad. Sci. USA 2000, 97, 11020–11025. [Google Scholar] [CrossRef]
- Rossi, C.; Strettoi, E.; Galli-Resta, L. The spatial order of horizontal cells is not affected by massive alterations in the organization of other retinal cells. J. Neurosci. 2003, 23, 9924–9928. [Google Scholar] [CrossRef]
- Peichl, L.; Gonzalez-Soriano, J. Morphological types of horizontal cell in rodent retinae: A comparison of rat, mouse, gerbil, and guinea pig. Vis. Neurosci. 1994, 11, 501–517. [Google Scholar] [CrossRef]
- Famiglietti, E.V. Morphological identification and systematic classification of mammalian retinal ganglion cells. I. Rabbit retinal ganglion cells. J. Comp. Neurol. 2020, 528, 3305–3450. [Google Scholar] [CrossRef]
- Coombs, J.; van der List, D.; Wang, G.Y.; Chalupa, L.M. Morphological properties of mouse retinal ganglion cells. Neuroscience 2006, 140, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Laboulaye, M.A.; Tran, N.M.; Whitney, I.E.; Benhar, I.; Sanes, J.R. Mouse Retinal Cell Atlas: Molecular Identification of over Sixty Amacrine Cell Types. J. Neurosci. 2020, 40, 5177–5195. [Google Scholar] [CrossRef] [PubMed]
- Jeon, C.J.; Strettoi, E.; Masland, R.H. The major cell populations of the mouse retina. J. Neurosci. 1998, 18, 8936–8946. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Greferath, U.; Vessey, K.A.; Grayden, D.B.; Burkitt, A.N.; Fletcher, E.L. Changes in ganglion cells during retinal degeneration. Neuroscience 2016, 329, 1–11. [Google Scholar] [CrossRef]
- Anderson, E.E.; Greferath, U.; Fletcher, E.L. Changes in morphology of retinal ganglion cells with eccentricity in retinal degeneration. Cell Tissue Res. 2016, 364, 263–271. [Google Scholar] [CrossRef]
- Toychiev, A.H.; Ivanova, E.; Yee, C.W.; Sagdullaev, B.T. Block of gap junctions eliminates aberrant activity and restores light responses during retinal degeneration. J. Neurosci. 2013, 33, 13972–13977. [Google Scholar] [CrossRef]
- Trenholm, S.; Borowska, J.; Zhang, J.; Hoggarth, A.; Johnson, K.; Barnes, S.; Lewis, T.J.; Awatramani, G.B. Intrinsic oscillatory activity arising within the electrically coupled AII amacrine-ON cone bipolar cell network is driven by voltage-gated Na+ channels. J. Physiol. 2012, 590, 2501–2517. [Google Scholar] [CrossRef]
- Spray, D.C. Physiological Properties of Gap Junction Channels in the Nervous System. In Gap Junctions in the Nervous System; Springer: Berlin/Heidelberg, Germany, 1996; pp. 39–59. [Google Scholar]
- DeVries, S.; Schwartz, E. Hemi-gap-junction channels in solitary horizontal cells of the catfish retina. J. Physiol. 1992, 445, 201–230. [Google Scholar] [CrossRef]
- Lu, C.; McMahon, D.G. Modulation of hybrid bass retinal gap junctional channel gating by nitric oxide. J. Physiol. 1997, 499, 689–699. [Google Scholar] [CrossRef]
- Zhang, D.-Q.; McMahon, D.G. Gating of retinal horizontal cell hemi gap junction channels by voltage, Ca2+, and retinoic acid. Mol. Vis. 2001, 7, 247–252. [Google Scholar] [PubMed]
- Peiroten, L.; Zrenner, E.; Haq, W. Artificial Vision: The High-Frequency Electrical Stimulation of the Blind Mouse Retina Decay Spike Generation and Electrogenically Clamped Intracellular Ca(2+) at Elevated Levels. Bioengineering 2023, 10, 1208. [Google Scholar] [CrossRef] [PubMed]
- Peracchia, C. Chemical gating of gap junction channels; roles of calcium, pH and calmodulin. Biochim. Biophys. Acta 2004, 1662, 61–80. [Google Scholar] [CrossRef] [PubMed]
- Van Hook, M.J.; Nawy, S.; Thoreson, W.B. Voltage- and calcium-gated ion channels of neurons in the vertebrate retina. Prog. Retin. Eye Res. 2019, 72, 100760. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Riquelme, M.A.; Gu, S.; Jiang, J.X. Regulation of Connexin Gap Junctions and Hemichannels by Calcium and Calcium Binding Protein Calmodulin. Int. J. Mol. Sci. 2020, 21, 8194. [Google Scholar] [CrossRef]
- Pfahnl, A.; Dahl, G. Gating of cx46 gap junction hemichannels by calcium and voltage. Pflugers Arch. 1999, 437, 345–353. [Google Scholar] [CrossRef]
- Scemes, E.; Suadicani, S.O.; Spray, D.C. Chapter 7: Intercellular Calcium Wave Communication via Gap Junction Dependent and Independent Mechanisms. In Current Topics in Membranes; Peracchia, C., Ed.; Academic Press: Cambridge, MA, USA, 1999; Volume 49, pp. 145–173. [Google Scholar]
- Hampson, E.C.; Weiler, R.; Vaney, D.I. pH-gated dopaminergic modulation of horizontal cell gap junctions in mammalian retina. Proc. Biol. Sci. 1994, 255, 67–72. [Google Scholar] [CrossRef]
- He, S.; Weiler, R.; Vaney, D.I. Endogenous dopaminergic regulation of horizontal cell coupling in the mammalian retina. J. Comp. Neurol. 2000, 418, 33–40. [Google Scholar] [CrossRef]
- Masland, R.H. The fundamental plan of the retina. Nat. Neurosci. 2001, 4, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Tsai, D.; Morley, J.W.; Suaning, G.J.; Lovell, N.H. Survey of electrically evoked responses in the retina-stimulus preferences and oscillation among neurons. Sci. Rep. 2017, 7, 13802. [Google Scholar] [CrossRef] [PubMed]
- Bittner, A.K.; Haythornthwaite, J.A.; Diener-West, M.; Dagnelie, G. Photopsias are related in part to perceived stress and positive mood in retinitis pigmentosa. Eye 2012, 26, 101–108. [Google Scholar] [CrossRef]
- Barrett, J.M.; Degenaar, P.; Sernagor, E. Blockade of pathological retinal ganglion cell hyperactivity improves optogenetically evoked light responses in rd1 mice. Front. Cell Neurosci. 2015, 9, 330. [Google Scholar] [CrossRef] [PubMed]
- Kranz, K.; Paquet-Durand, F.; Weiler, R.; Janssen-Bienhold, U.; Dedek, K. Testing for a gap junction-mediated bystander effect in retinitis pigmentosa: Secondary cone death is not altered by deletion of connexin36 from cones. PLoS ONE 2013, 8, e57163. [Google Scholar] [CrossRef] [PubMed]
- Akopian, A.; Atlasz, T.; Pan, F.; Wong, S.; Zhang, Y.; Völgyi, B.; Paul, D.L.; Bloomfield, S.A. Gap junction-mediated death of retinal neurons is connexin and insult specific: A potential target for neuroprotection. J. Neurosci. 2014, 34, 10582–10591. [Google Scholar] [CrossRef] [PubMed]
- Jassim, A.H.; Cavanaugh, M.; Shah, J.S.; Willits, R.; Inman, D.M. Transcorneal Electrical Stimulation Reduces Neurodegenerative Process in a Mouse Model of Glaucoma. Ann. Biomed. Eng. 2021, 49, 858–870. [Google Scholar] [CrossRef] [PubMed]
- Stett, A.; Schatz, A.; Gekeler, F.; Franklin, J. Transcorneal Electrical Stimulation Dose-Dependently Slows the Visual Field Loss in Retinitis Pigmentosa. Transl. Vis. Sci. Technol. 2023, 12, 29. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, W.; Li, Y.; Lin, X. Structure and Function of Cochlear Gap Junctions and Implications for the Translation of Cochlear Gene Therapies. Front. Cell Neurosci. 2019, 13, 529. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Glueckert, R.; Linthicum, F.H.; Rieger, G.; Blumer, M.; Bitsche, M.; Pechriggl, E.; Rask-Andersen, H.; Schrott-Fischer, A. Possible role of gap junction intercellular channels and connexin 43 in satellite glial cells (SGCs) for preservation of human spiral ganglion neurons: A comparative study with clinical implications. Cell Tissue Res. 2014, 355, 267–278. [Google Scholar] [CrossRef]
- Harris, A.L. Connexin channel permeability to cytoplasmic molecules. Prog. Biophys. Mol. Biol. 2007, 94, 120–143. [Google Scholar] [CrossRef]
- Haq, W.; Basavaraju, S.; Speck, A.; Zrenner, E. Nature-inspired saccadic-like electrical stimulation paradigm promotes sustained retinal ganglion cell responses by spatiotemporally alternating activation of contiguous multi-electrode patterns. J. Neural. Eng. 2022, 19, 055001. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stürmer, S.; Bolz, S.; Zrenner, E.; Ueffing, M.; Haq, W. Sustained Extracellular Electrical Stimulation Modulates the Permeability of Gap Junctions in rd1 Mouse Retina with Photoreceptor Degeneration. Int. J. Mol. Sci. 2024, 25, 1616. https://doi.org/10.3390/ijms25031616
Stürmer S, Bolz S, Zrenner E, Ueffing M, Haq W. Sustained Extracellular Electrical Stimulation Modulates the Permeability of Gap Junctions in rd1 Mouse Retina with Photoreceptor Degeneration. International Journal of Molecular Sciences. 2024; 25(3):1616. https://doi.org/10.3390/ijms25031616
Chicago/Turabian StyleStürmer, Sophie, Sylvia Bolz, Eberhart Zrenner, Marius Ueffing, and Wadood Haq. 2024. "Sustained Extracellular Electrical Stimulation Modulates the Permeability of Gap Junctions in rd1 Mouse Retina with Photoreceptor Degeneration" International Journal of Molecular Sciences 25, no. 3: 1616. https://doi.org/10.3390/ijms25031616
APA StyleStürmer, S., Bolz, S., Zrenner, E., Ueffing, M., & Haq, W. (2024). Sustained Extracellular Electrical Stimulation Modulates the Permeability of Gap Junctions in rd1 Mouse Retina with Photoreceptor Degeneration. International Journal of Molecular Sciences, 25(3), 1616. https://doi.org/10.3390/ijms25031616





