An In Vitro Study Evaluating the Safety of Mesalazine on Human Nasoepithelial Cells
Abstract
1. Introduction
2. Results
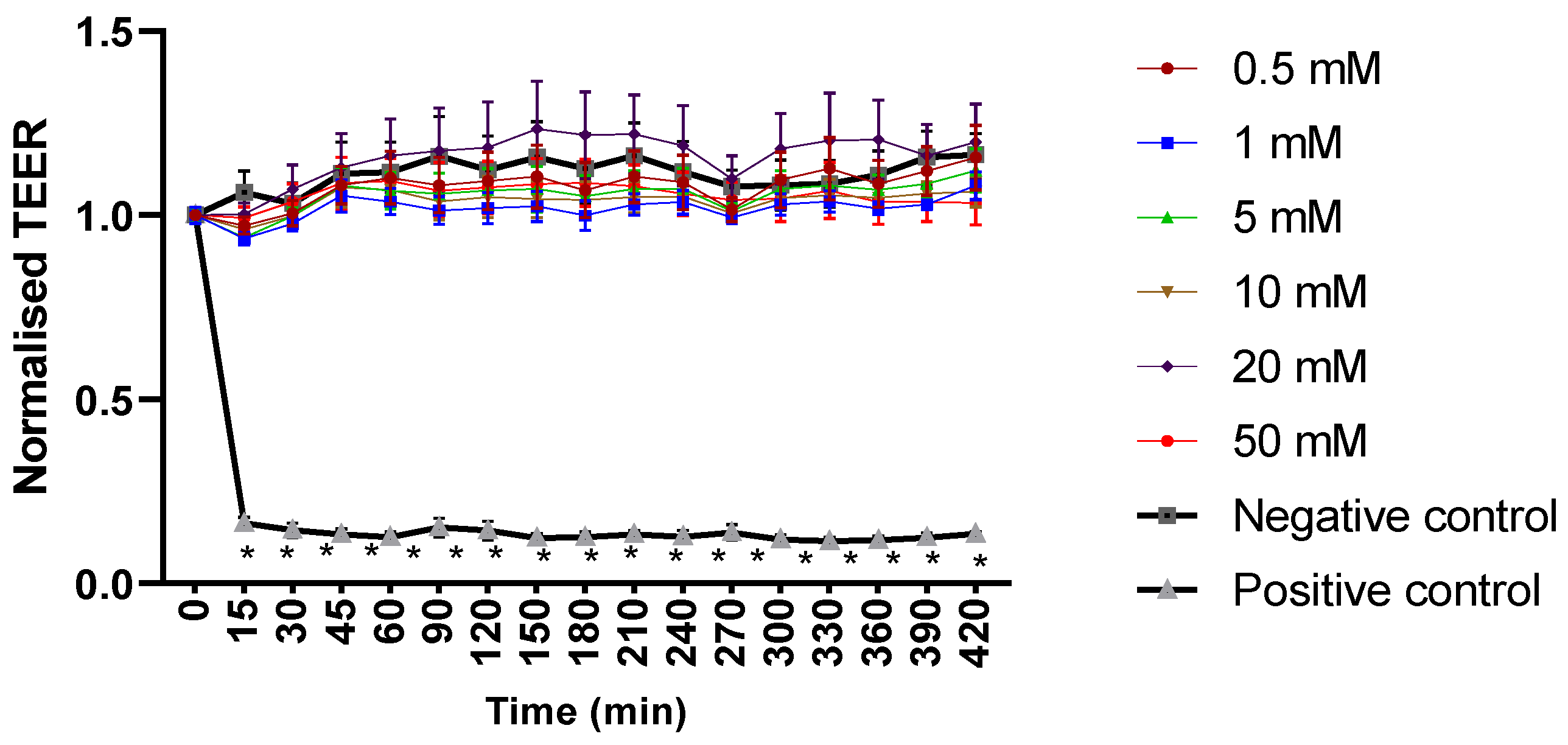
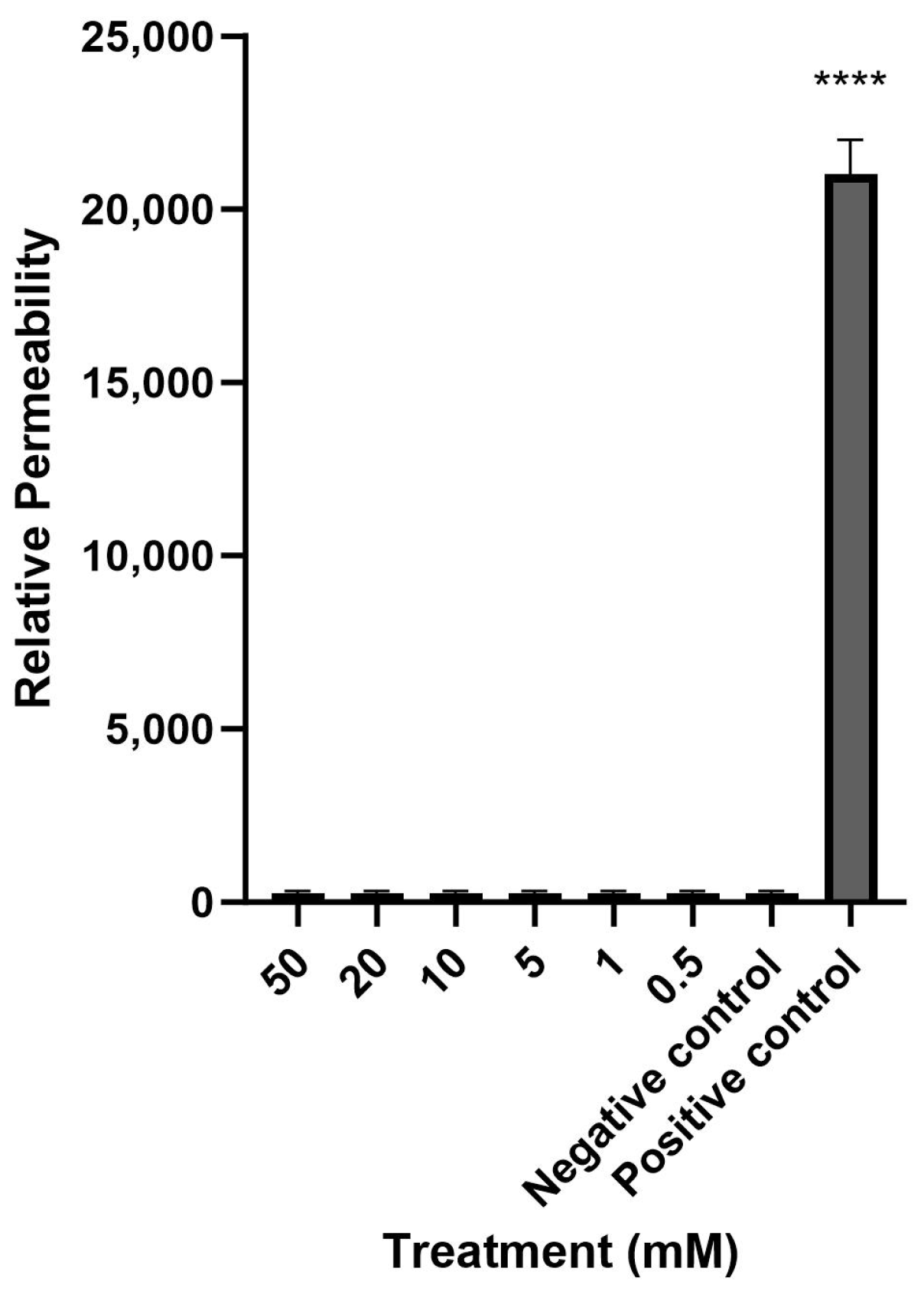
2.1. Mesalazine Does Not Appear to Affect the Mucosal Barrier Function of Primary Human Nasal Epithelial Cell Cultures
2.2. Mesalazine Does Not Appear to Be Cytotoxic to HNECs in Concentrations < 50 mM
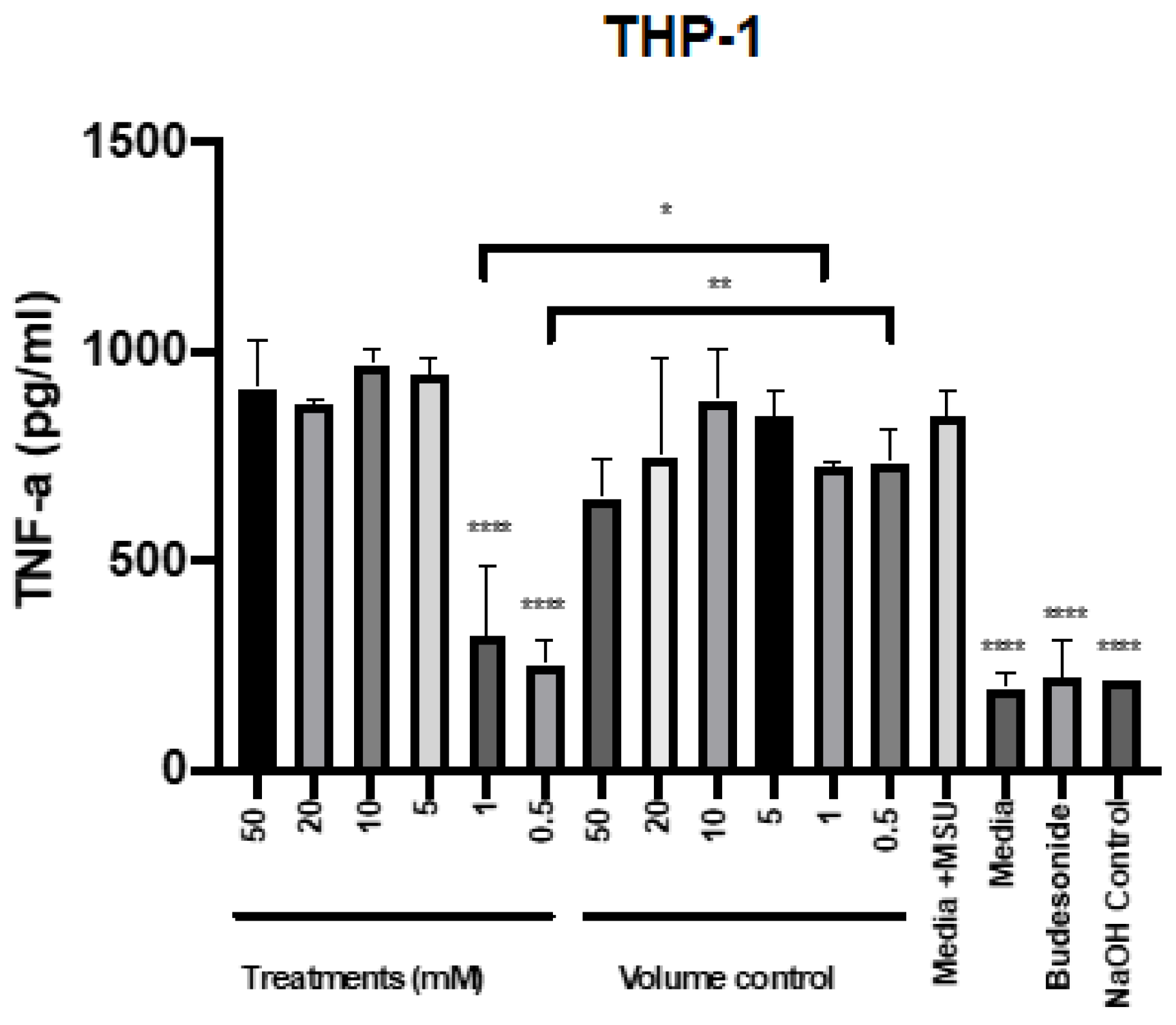
2.3. Mesalazine Reduces the Release of TNF-α by THP-1 Cells
2.4. Mesalazine Has No Effects on the Release of IL-6 by HNEC-ALI Cultures
3. Discussion
4. Materials and Methods
4.1. Mesalazine Preparation
4.2. Human Ethics Approval and Participant Recruitment
4.3. Harvesting and Culturing HNECs In Vitro
4.4. ALI Culture
4.5. Measurement of TEER
4.6. Measurement of Paracellular Permeability Using Fluorescently Labelled Dextrans
4.7. Measuring Cytotoxicity with LDH Assay
4.8. Measuring Cytotoxicity with MTT
4.9. Enzyme-Linked Immunosorbent Assay (ELISA) of IL-6
4.10. Enzyme-Linked Immunosorbent Assay TNF-α—Cells Inflammation Model
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benninger, M.S.; Ferguson, B.J.; Hadley, J.A.; Hamilos, D.L.; Jacobs, M.; Kennedy, D.W.; Lanza, D.C.; Marple, B.F.; Osguthorpe, J.D.; Stankiewicz, J.A.; et al. Adult Chronic Rhinosinusitis: Definitions, Diagnosis, Epidemiology, and Pathophysiology. Otolaryngol. Head Neck Surg. 2003, 129, S1–S32. [Google Scholar] [CrossRef] [PubMed]
- Habib, A.-R.; Campbell, R.; Kalish, L.; Wong, E.H.; Grayson, J.; Alvarado, R.; Sacks, R.; Harvey, R.J. The burden of chronic upper airway disorders in Australia: A population-based cross-sectional study. Aust. J. Otolaryngol. 2019, 2, 28. [Google Scholar] [CrossRef]
- Hamilos, D.L. Chronic rhinosinusitis: Epidemiology and medical management. J. Allergy Clin. Immunol. 2011, 128, 693–707. [Google Scholar] [CrossRef] [PubMed]
- Hastan, D.; Fokkens, W.J.; Bachert, C.; Newson, R.B.; Bislimovska, J.; Bockelbrink, A.; Bousquet, P.J.; Brozek, G.; Bruno, A.; Dahlen, S.E.; et al. Chronic rhinosinusitis in Europe–an underestimated disease. A GA2LEN study. Allergy 2011, 66, 1216–1223. [Google Scholar] [CrossRef]
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Anselmo-Lima, W.T.; et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology 2020, 58, 1–464. [Google Scholar] [CrossRef] [PubMed]
- Rudmik, L.; Smith, T.L. Quality of Life in Patients with Chronic Rhinosinusitis. Curr. Allergy Asthma Rep. 2011, 11, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.; Lanza, D.C.; Kennedy, D.W. Antimicrobial Resistance in Bacterial Chronic Sinusitis. Am. J. Rhinol. 1998, 12, 243–248. [Google Scholar] [CrossRef]
- Young, L.C.; Stow, N.W.; Zhou, L.; Douglas, R.G. Efficacy of Medical Therapy in Treatment of Chronic Rhinosinusitis. Allergy Rhinol. 2012, 3, e8–e12. [Google Scholar] [CrossRef]
- Matsuwaki, Y.; Ookushi, T.; Asaka, D.; Mori, E.; Nakajima, T.; Yoshida, T.; Kojima, J.; Chiba, S.; Ootori, N.; Moriyama, H. Chronic Rhinosinusitis: Risk Factors for the Recurrence of Chronic Rhinosinusitis Based on 5-Year Follow-Up after Endoscopic Sinus Surgery. Int. Arch. Allergy Immunol. 2008, 146, 77–81. [Google Scholar] [CrossRef]
- Daines, S.M.; Orlandi, R.R. Inflammatory cytokines in allergy and rhinosinusitis. Curr. Opin. Otolaryngol. Head Neck Surg. 2010, 18, 187–190. [Google Scholar] [CrossRef]
- Phan, N.T.; Cabot, P.J.; Wallwork, B.D.; Cervin, A.U.; Panizza, B.J. Cellular and molecular mechanisms of chronic rhinosinusitis and potential therapeutic strategies: Review on cytokines, nuclear factor kappa B and transforming growth factor beta. J. Laryngol. Otol. 2015, 129, S2–S7. [Google Scholar] [CrossRef]
- Ocaña, A.; Reglero, G. Effects of thyme extract oils (from Thymus vulgaris, Thymus zygis, and Thymus hyemalis) on cytokine production and gene expression of oxLDL-stimulated THP-1-macrophages. J. Obes. 2012, 2012, 104706. [Google Scholar] [CrossRef] [PubMed]
- Lennard, C.M.; Sun, L.L.; Chang, A.S.; Bolger, W.E. Interleukin-1β, interleukin-5, interleukin-6, interleukin-8, and tumor necrosis factor-α in chronic sinusitis: Response to systemic corticosteroids. Am. J. Rhinol. 2000, 14, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Xu, G.; Wen, W. A correlative study of NF-κB activity and cytokines expression in human chronic nasal sinusitis. J. Laryngol. Otol. 2007, 121, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Polasky, C.; Loyal, K.; Idel, C.; Wetterauer, D.; Heidemann, M.; Bruchhage, K.; Pries, R. Alteration of blood monocyte subsets in chronic rhinosinusitis with regard to anti-inflammatory 1,8-Cineol treatment. Rhinol. Online 2021, 4, 194–202. [Google Scholar] [CrossRef]
- Banks, C.A.; Schlosser, R.J.; Wang, E.W.; Casey, S.E.; Mulligan, R.M.; Mulligan, J.K. Macrophage Infiltrate Is Elevated in CRSwNP Sinonasal Tissue Regardless of Atopic Status. Otolaryngol. Neck Surg. 2014, 151, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Shaghayegh, G.; Cooksley, C.; Bouras, G.S.; Panchatcharam, B.S.; Idrizi, R.; Jana, M.; Ellis, S.; Psaltis, A.J.; Wormald, P.-J.; Vreugde, S. Chronic rhinosinusitis patients display an aberrant immune cell localization with enhanced S aureus biofilm metabolic activity and biomass. J. Allergy Clin. Immunol. 2022, 151, 723–736.e16. [Google Scholar] [CrossRef]
- Ramanathan, M., Jr.; Lee, W.-K.; Spannhake, E.W.; Lane, A.P. Th2 cytokines associated with chronic rhinosinusitis with polyps down-regulate the antimicrobial immune function of human sinonasal epithelial cells. Am. J. Rhinol. 2008, 22, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Böhm, S.K.; Kruis, W. Long-term efficacy and safety of once-daily mesalazine granules for the treatment of active ulcerative colitis. Clin. Exp. Gastroenterol. 2014, 7, 369–383. [Google Scholar] [CrossRef]
- Marteau, P.; Probert, C.S.; Lindgren, S.; Gassul, M.; Tan, T.G.; Dignass, A.; Befrits, R.; Midhagen, G.; Rademaker, J.; Foldager, M. Combined oral and enema treatment with Pentasa (mesalazine) is superior to oral therapy alone in patients with extensive mild/moderate active ulcerative colitis: A randomised, double blind, placebo controlled study. Gut 2005, 54, 960–965. [Google Scholar] [CrossRef]
- Kaiser, G.C.; Yan, F.; Polk, D. Mesalamine blocks tumor necrosis factor growth inhibition and nuclear factor κB activation in mouse colonocytes. Gastroenterology 1999, 116, 602–609. [Google Scholar] [CrossRef]
- Mahida, Y.R.; Lamming, C.E.; Gallagher, A.; Hawthorne, A.B.; Hawkey, C.J. 5-Aminosalicylic acid is a potent inhibitor of interleukin 1 beta production in organ culture of colonic biopsy specimens from patients with inflammatory bowel disease. Gut 1991, 32, 50–54. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rachmilewitz, D.; Karmeli, F.; Schwartz, L.W.; Simon, P.L. Effect of aminophenols (5-ASA and 4-ASA) on colonic interleukin-1 generation. Gut 1992, 33, 929–932. [Google Scholar] [CrossRef] [PubMed]
- Bell, C.M.; Habal, F.M. Safety of topical 5-aminosalicylic acid in pregnancy. Am. J. Gastroenterol. 1997, 92, 2201–2202. [Google Scholar]
- Dignass, A.; Schnabel, R.; Romatowski, J.; Pavlenko, V.; Dorofeyev, A.; Derova, J.; Jonaitis, L.; Dilger, K.; Nacak, T.; Greinwald, R.; et al. Efficacy and safety of a novel high-dose mesalazine tablet in mild to moderate active ulcerative colitis: A double-blind, multicentre, randomised trial. United Eur. Gastroenterol. J. 2017, 6, 138–147. [Google Scholar] [CrossRef]
- Kumar, V.; Abbas, A.; Aster, J. Robbins Basic Pathology, 9th ed.; Saunders: Philadelphia, PA, USA, 2013. [Google Scholar]
- Chanput, W.; Mes, J.J.; Wichers, H.J. THP-1 cell line: An in vitro cell model for immune modulation approach. Int. Immunopharmacol. 2014, 23, 37–45. [Google Scholar] [CrossRef]
- Maspero, J.; Adir, Y.; Al-Ahmad, M.; Celis-Preciado, C.A.; Colodenco, F.D.; Giavina-Bianchi, P.; Lababidi, H.; Ledanois, O.; Mahoub, B.; Perng, D.-W.; et al. Type 2 inflammation in asthma and other airway diseases. ERJ Open Res. 2022, 8, 576. [Google Scholar] [CrossRef]
- Wee, J.H.; Ko, Y.-K.; Khalmuratova, R.; Shin, H.-W.; Kim, D.W.; Rhee, C.-S. Effect of lipopolysaccharide and polyinosinic:polycytidylic acid in a murine model of nasal polyp. Sci. Rep. 2021, 11, 1021. [Google Scholar] [CrossRef]
- Mayadas, T.N.; Cullere, X.; Lowell, C.A. The Multifaceted Functions of Neutrophils. Annu. Rev. Pathol. Mech. Dis. 2014, 9, 181–218. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhou, B.; Wang, C.; Huang, Q.; Zhang, Q.; Han, Y.; Dai, W.; Fan, E.; Li, Y. Biofilm formation and Toll-like receptor 2, Toll-like receptor 4, and NF-kappaB expression in sinus tissues of patients with chronic rhinosinusitis. Am. J. Rhinol. Allergy 2012, 26, 104–109. [Google Scholar] [CrossRef] [PubMed]
- MacDermott, R.P. Progress in understanding the mechanisms of action of 5-aminosalicylic acid. Am. J. Gastroenterol. 2000, 95, 3343–3345. [Google Scholar] [CrossRef]
- Lim, W.C.; Wang, Y.; MacDonald, J.K.; Hanauer, S. Aminosalicylates for induction of remission or response in Crohn’s disease. Cochrane Database Syst. Rev. 2016, 7, CD008870. [Google Scholar] [CrossRef]
- Allgayer, H. Sulfasalazine and 5-ASA compounds. Gastroenterol. Clin. North Am. 1992, 21, 643–658. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ramezanpour, M.; Bolt, H.; Psaltis, A.J.; Wormald, P.-J.; Vreugde, S. Primary human nasal epithelial cells: A source of poly (I:C) LMW-induced IL-6 production. Sci. Rep. 2018, 8, 11325. [Google Scholar] [CrossRef]
- Ramezanpour, M.; Moraitis, S.; Smith, J.L.P.; Wormald, P.J.; Vreugde, S. Th17 Cytokines Disrupt the Airway Mucosal Barrier in Chronic Rhinosinusitis. Mediat. Inflamm. 2016, 2016, 9798206. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murphy, W.; Liu, S.; Javadiyan, S.; Vyskocil, E.; Feizi, S.; Callejas, C.; Wormald, P.-J.; Vreugde, S.; Psaltis, A.J. An In Vitro Study Evaluating the Safety of Mesalazine on Human Nasoepithelial Cells. Int. J. Mol. Sci. 2024, 25, 2796. https://doi.org/10.3390/ijms25052796
Murphy W, Liu S, Javadiyan S, Vyskocil E, Feizi S, Callejas C, Wormald P-J, Vreugde S, Psaltis AJ. An In Vitro Study Evaluating the Safety of Mesalazine on Human Nasoepithelial Cells. International Journal of Molecular Sciences. 2024; 25(5):2796. https://doi.org/10.3390/ijms25052796
Chicago/Turabian StyleMurphy, William, Sha Liu, Shari Javadiyan, Erich Vyskocil, Sholeh Feizi, Claudio Callejas, Peter-John Wormald, Sarah Vreugde, and Alkis J. Psaltis. 2024. "An In Vitro Study Evaluating the Safety of Mesalazine on Human Nasoepithelial Cells" International Journal of Molecular Sciences 25, no. 5: 2796. https://doi.org/10.3390/ijms25052796
APA StyleMurphy, W., Liu, S., Javadiyan, S., Vyskocil, E., Feizi, S., Callejas, C., Wormald, P.-J., Vreugde, S., & Psaltis, A. J. (2024). An In Vitro Study Evaluating the Safety of Mesalazine on Human Nasoepithelial Cells. International Journal of Molecular Sciences, 25(5), 2796. https://doi.org/10.3390/ijms25052796







