The Role of Transglutaminase 2 in Cancer: An Update
Abstract
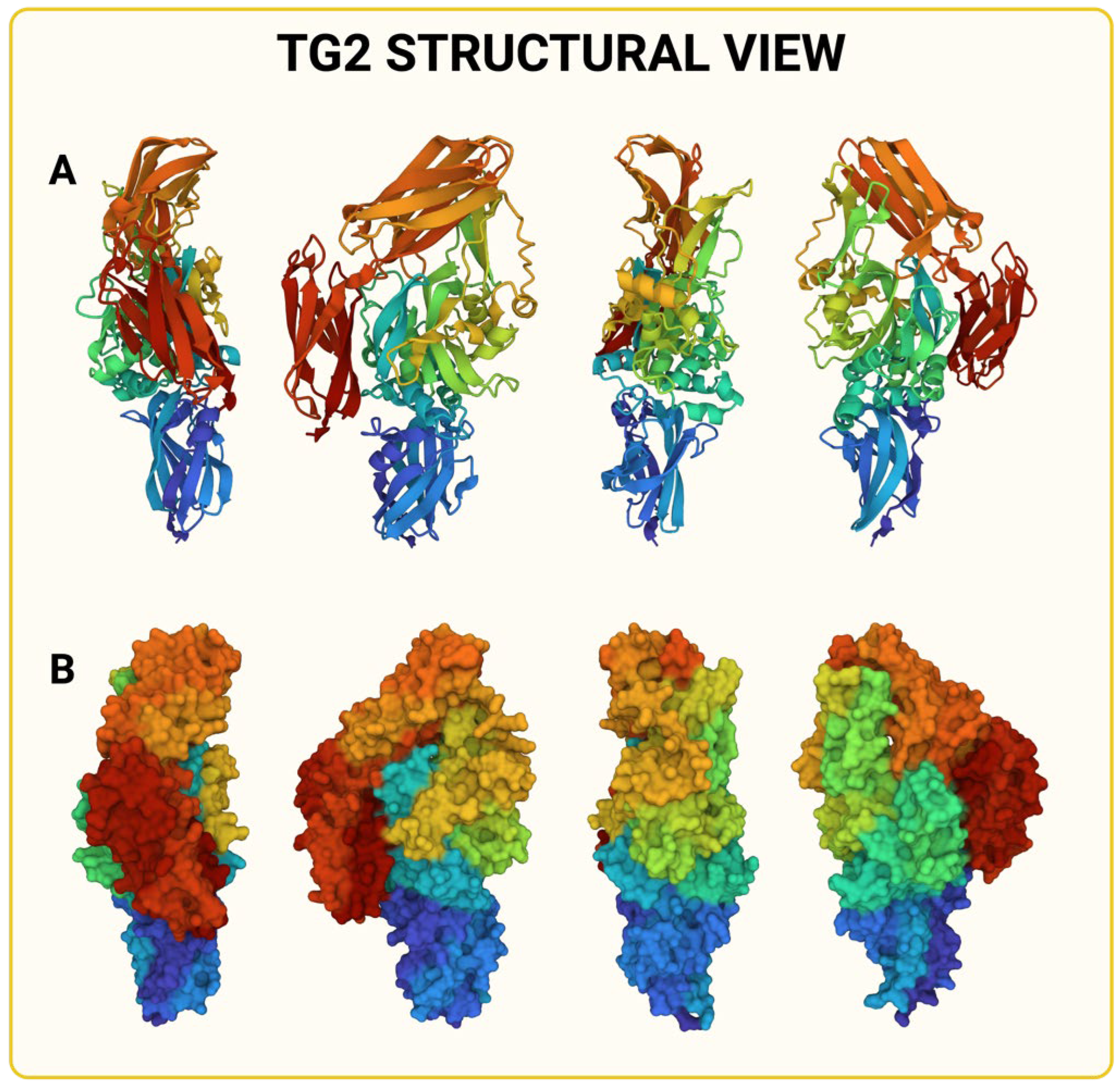
1. Introduction
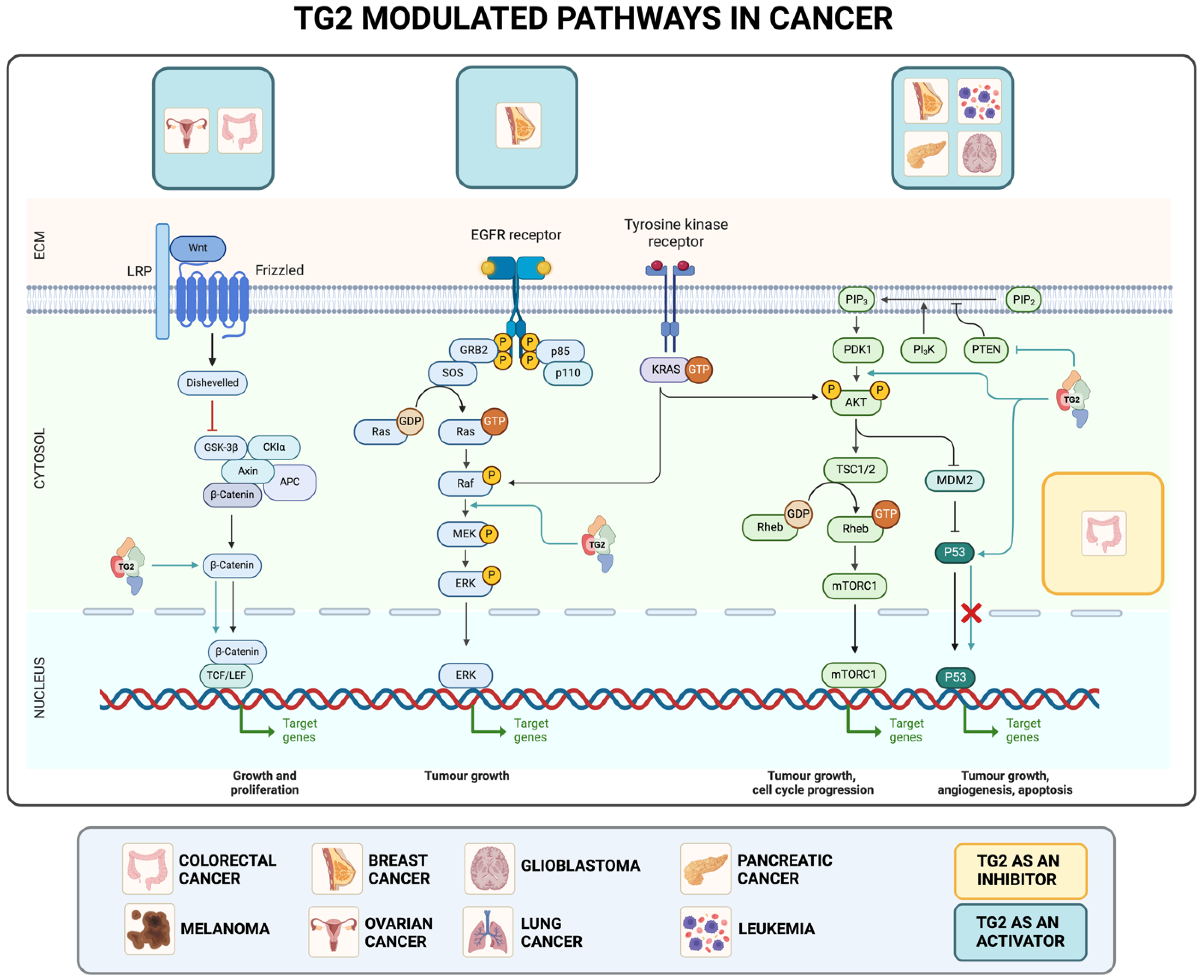
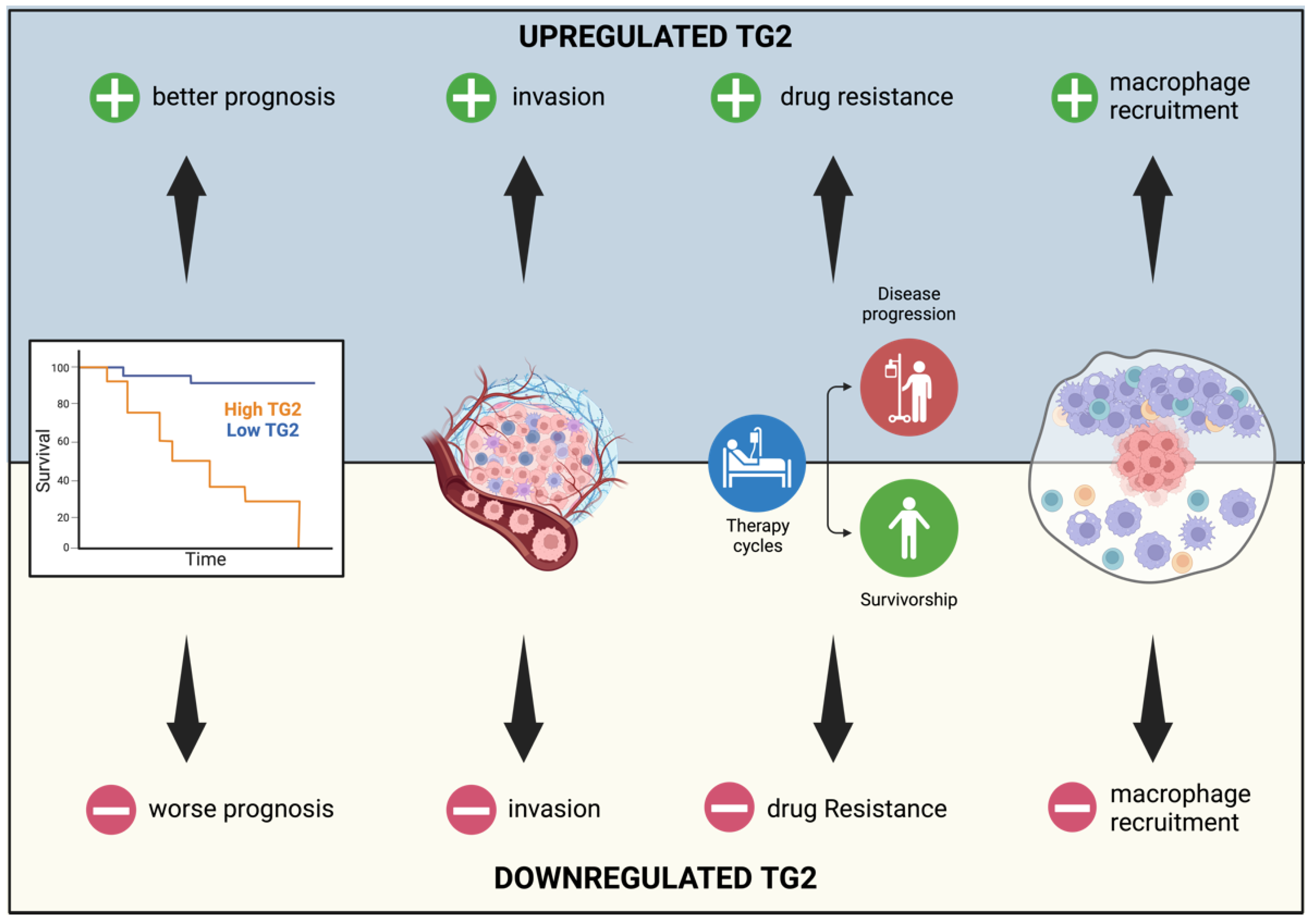
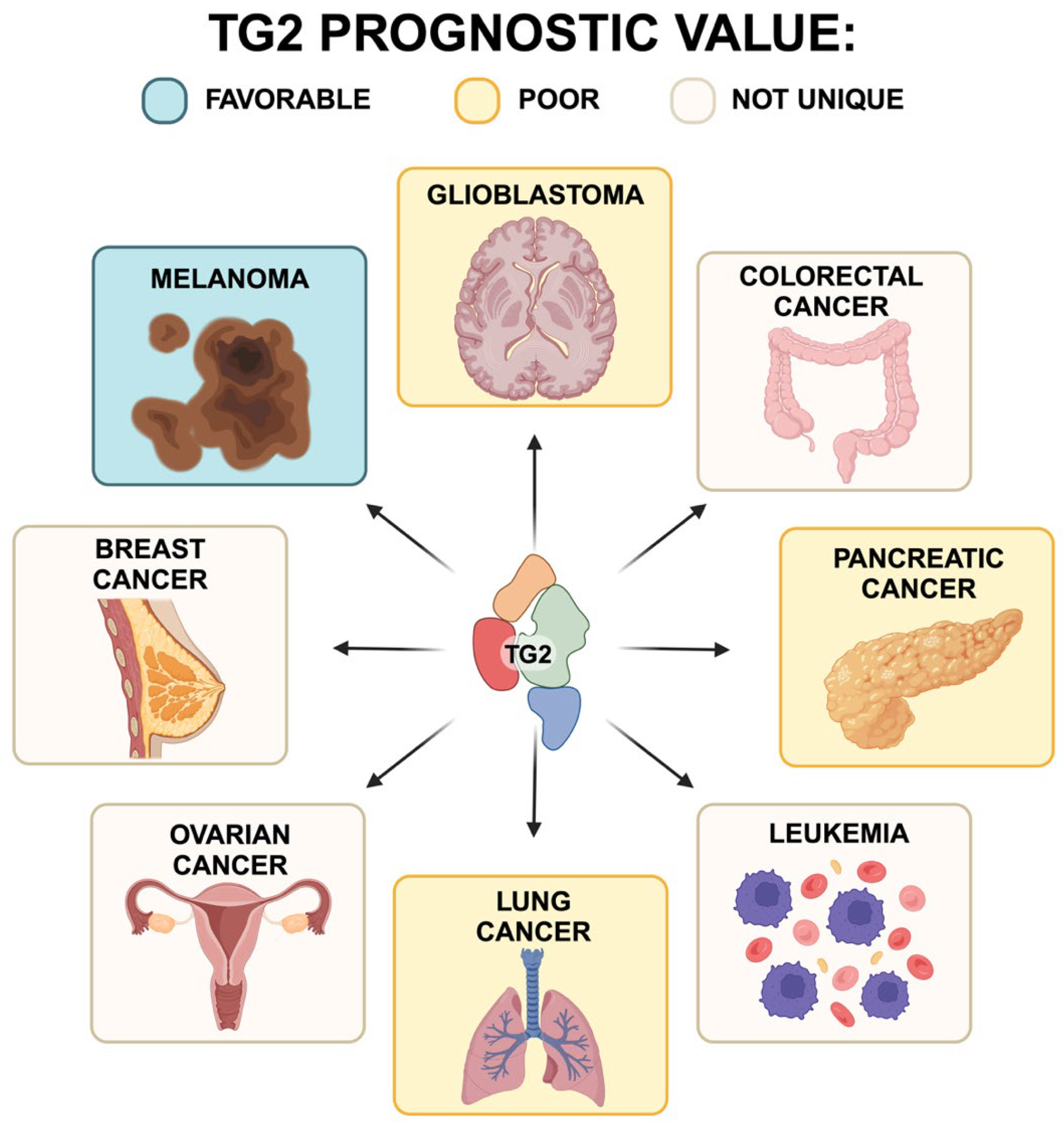
2. TG2 in Cancer
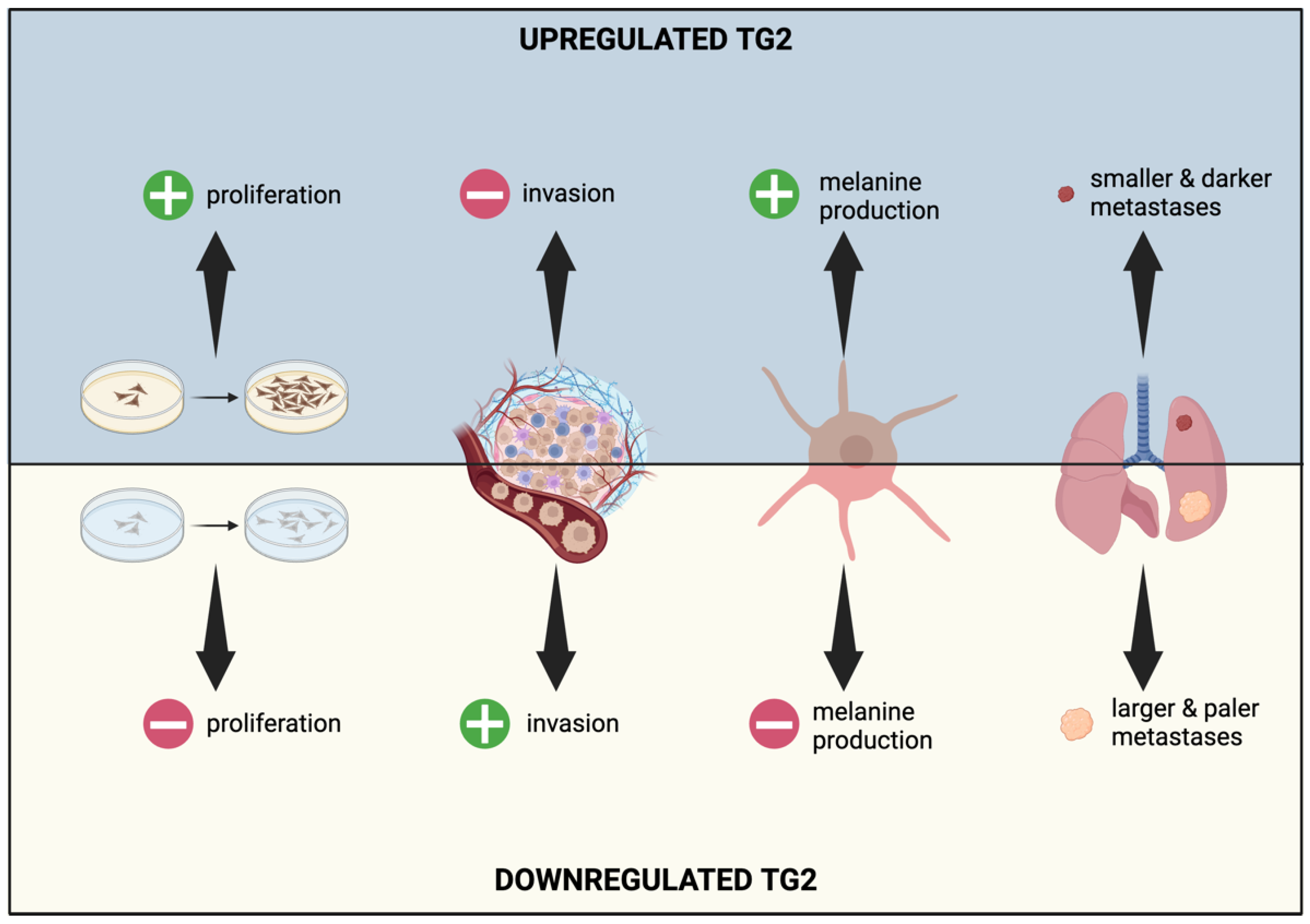
2.1. TG2 and Melanoma
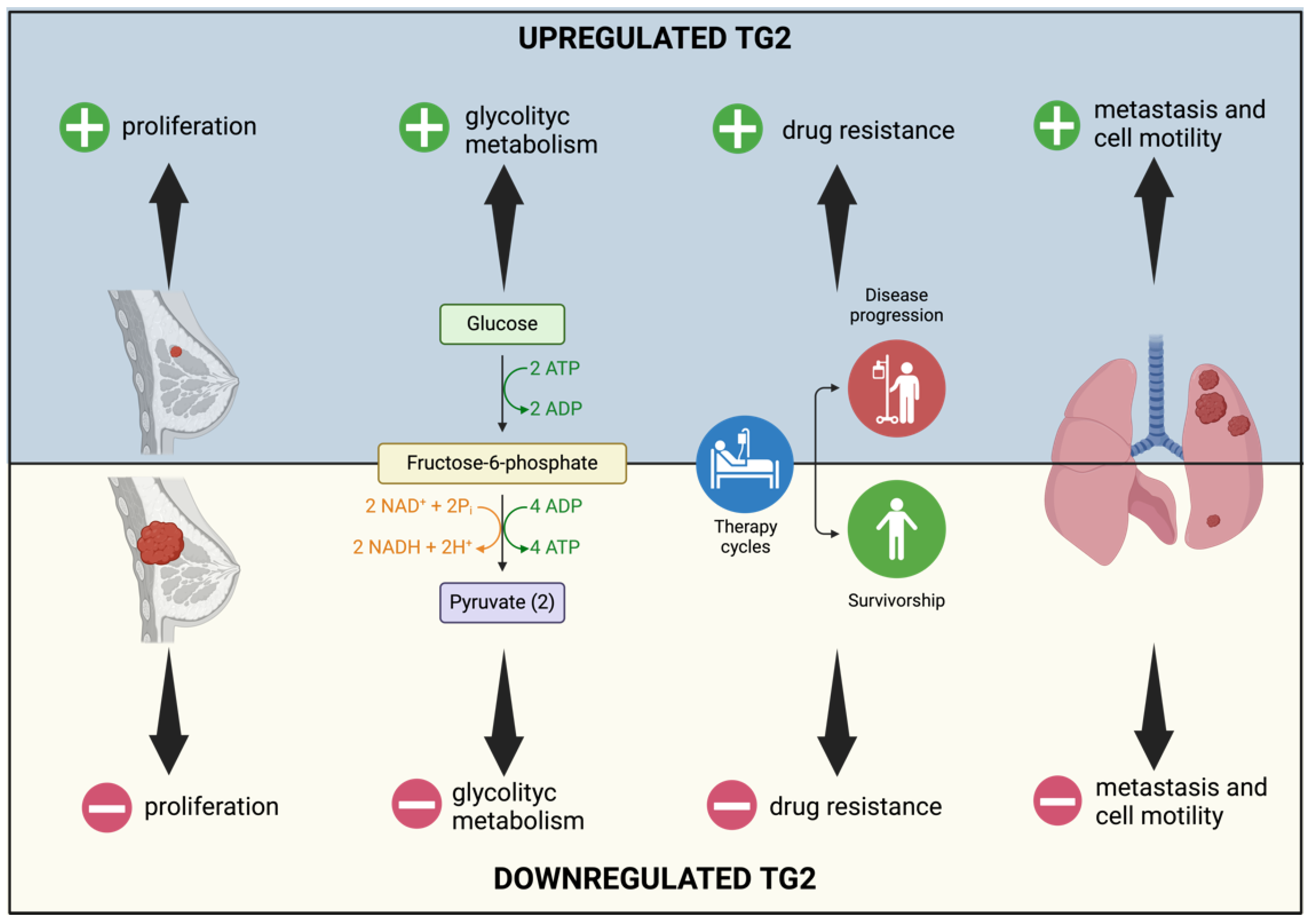
2.2. TG2 and Breast Cancer
2.3. TG2 and Glioblastoma
2.4. TG2 and Ovarian Cancer
2.5. TG2 and Colorectal Cancer
2.6. TG2 and Leukemia
2.7. TG2 and Pancreatic Cancer
2.8. TG2 and Lung Cancer
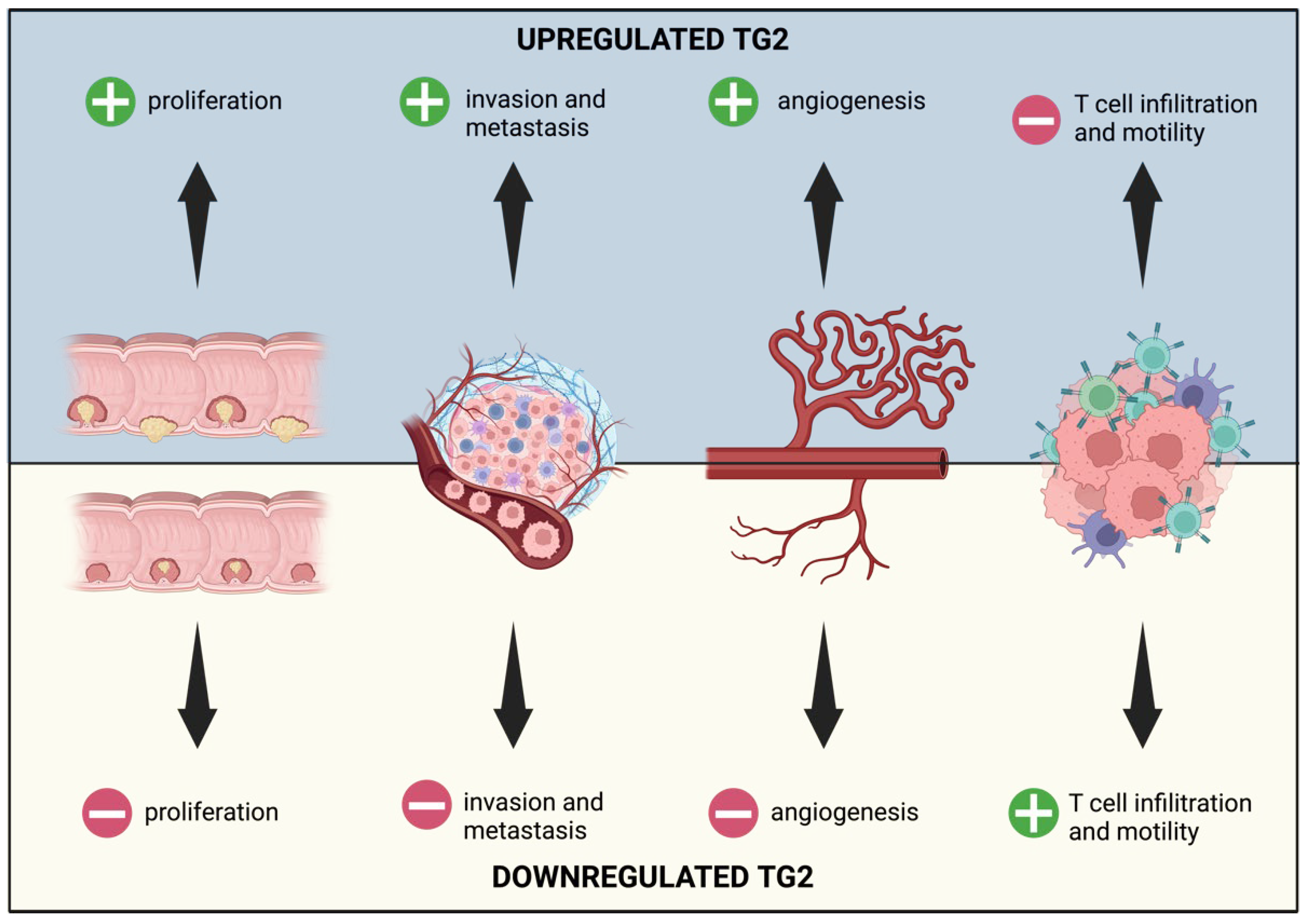
3. TG2 and Microenvironment
3.1. TG2 and Angiogenesis
3.2. TG2 and Cancer Associated Fibroblast (CAFs)
3.3. TG2 and Immune Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tabolacci, C.; De Martino, A.; Mischiati, C.; Feriotto, G.; Beninati, S. The Role of Tissue Transglutaminase in Cancer Cell Initiation, Survival and Progression. Med. Sci. 2019, 7, 19. [Google Scholar] [CrossRef]
- Tatsukawa, H.; Hitomi, K. Role of Transglutaminase 2 in Cell Death, Survival, and Fibrosis. Cells 2021, 10, 1842. [Google Scholar] [CrossRef] [PubMed]
- Lorand, L.; Iismaa, S.E. Transglutaminase Diseases: From Biochemistry to the Bedside. FASEB J. 2019, 33, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Satchwell, T.J.; Shoemark, D.K.; Sessions, R.B.; Toye, A.M. Protein 4.2: A Complex Linker. Blood Cells Mol. Dis. 2009, 42, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Csobán-Szabó, Z.; Fésüs, L.; Király, R. Protein-Peptide Based Assay for the Characterization of Human Blood Coagulation Factor XIII-A Isopeptidase Activity: Protein-Based Isopeptidase Assay for FXIII-A. Anal. Biochem. 2020, 600, 113699. [Google Scholar] [CrossRef] [PubMed]
- Aepler, J.; Wodtke, J.; Wodtke, R.; Haase-Kohn, C.; Löser, R.; Pietzsch, J.; Hauser, S. The Role of Transglutaminase 2 in the Radioresistance of Melanoma Cells. Cells 2022, 11, 1342. [Google Scholar] [CrossRef]
- Bianchi, N.; Beninati, S.; Bergamini, C.M. Spotlight on the Transglutaminase 2 Gene: A Focus on Genomic and Transcriptional Aspects. Biochem. J. 2018, 475, 1643–1667. [Google Scholar] [CrossRef]
- Odii, B.O.; Coussons, P. Biological Functionalities of Transglutaminase 2 and the Possibility of Its Compensation by Other Members of the Transglutaminase Family. Sci. World J. 2014, 2014, 714561. [Google Scholar] [CrossRef]
- Beninati, S.; Piacentini, M. The Transglutaminase Family: An Overview. Minireview Article. Amino Acids 2004, 26, C885–C889. [Google Scholar] [CrossRef]
- Eckert, R.L.; Kaartinen, M.T.; Nurminskaya, M.; Belkin, A.M.; Colak, G.; Johnson, G.V.W.; Mehta, K. Transglutaminase Regulation of Cell Function. Physiol. Rev. 2014, 94, 383–417. [Google Scholar] [CrossRef]
- Gundemir, S.; Colak, G.; Tucholski, J.; Johnson, G.V.W. Transglutaminase 2: A Molecular Swiss Army Knife. Biochim. Biophys. Acta Mol. Cell Res. 2012, 1823, 406–419. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.J.; Kwon, S.; Jeong, E.M.; Kim, C.M.; Lee, K.B.; Kim, I.G.; Park, H.H. Structure of Natural Variant Transglutaminase 2 Reveals Molecular Basis of Gaining Stability and Higher Activity. PLoS ONE 2018, 13, e0204707. [Google Scholar] [CrossRef] [PubMed]
- Rossin, F.; D’Eletto, M.; Farrace, M.G.; Piacentini, M. Transglutaminase Type 2: A Multifunctional Protein Chaperone? Mol. Cell Oncol. 2014, 1, e968506. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Palucci, I.; Matic, I.; Falasca, L.; Minerva, M.; Maulucci, G.; De Spirito, M.; Petruccioli, E.; Goletti, D.; Rossin, F.; Piacentini, M.; et al. Transglutaminase Type 2 Plays a Key Role in the Pathogenesis of Mycobacterium Tuberculosis Infection. J. Intern. Med. 2018, 283, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Jang, T.H.; Lee, D.S.; Choi, K.; Jeong, E.M.; Kim, I.G.; Kim, Y.W.; Chun, J.N.; Jeon, J.H.; Park, H.H. Crystal Structure of Transglutaminase 2 with GTP Complex and Amino Acid Sequence Evidence of Evolution of GTP Binding Site. PLoS ONE 2014, 9, e107005. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.M.; Lee, K.B.; Kim, G.E.; Kim, C.M.; Lee, J.H.; Kim, H.J.; Shin, J.W.; Kwon, M.A.; Park, H.H.; Kim, I.G. Competitive Binding of Magnesium to Calcium Binding Sites Reciprocally Regulates Transamidase and GTP Hydrolysis Activity of Transglutaminase 2. Int. J. Mol. Sci. 2020, 21, 791. [Google Scholar] [CrossRef]
- Sima, L.E.; Matei, D.; Condello, S. The Outside-In Journey of Tissue Transglutaminase in Cancer. Cells 2022, 11, 1779. [Google Scholar] [CrossRef]
- Martucciello, S.; Paolella, G.; Esposito, C.; Lepretti, M.; Caputo, I. Anti-Type 2 Transglutaminase Antibodies as Modulators of Type 2 Transglutaminase Functions: A Possible Pathological Role in Celiac Disease. Cell. Mol. Life Sci. 2018, 75, 4107–4124. [Google Scholar] [CrossRef]
- Facchiano, F.; D’Arcangelo, D.; Lentini, A.; Rossi, S.; Senatore, C.; Pannellini, T.; Tabolacci, C.; Facchiano, A.M.; Facchiano, A.; Beninati, S. Tissue Transglutaminase Activity Protects from Cutaneous Melanoma Metastatic Dissemination: An in Vivo Study. Amino Acids 2013, 44, 53–61. [Google Scholar] [CrossRef]
- Muccioli, S.; Brillo, V.; Varanita, T.; Rossin, F.; Zaltron, E.; Velle, A.; Alessio, G.; Angi, B.; Severin, F.; Tosi, A.; et al. Transglutaminase Type 2-MITF Axis Regulates Phenotype Switching in Skin Cutaneous Melanoma. Cell Death Dis. 2023, 14, 704. [Google Scholar] [CrossRef]
- Gates, E.W.J.; Calvert, N.D.; Cundy, N.J.; Brugnoli, F.; Navals, P.; Kirby, A.; Bianchi, N.; Adhikary, G.; Shuhendler, A.J.; Eckert, R.L.; et al. Cell-Impermeable Inhibitors Confirm That Intracellular Human Transglutaminase 2 Is Responsible for the Transglutaminase-Associated Cancer Phenotype. Int. J. Mol. Sci. 2023, 24, 12546. [Google Scholar] [CrossRef] [PubMed]
- D’Eletto, M.; Rossin, F.; Occhigrossi, L.; Farrace, M.G.; Faccenda, D.; Desai, R.; Marchi, S.; Refolo, G.; Falasca, L.; Antonioli, M.; et al. Transglutaminase Type 2 Regulates ER-Mitochondria Contact Sites by Interacting with GRP75. Cell Rep. 2018, 25, 3573–3581. [Google Scholar] [CrossRef] [PubMed]
- Rossin, F.; Villella, V.R.; D’Eletto, M.; Farrace, M.G.; Esposito, S.; Ferrari, E.; Monzani, R.; Occhigrossi, L.; Pagliarini, V.; Sette, C.; et al. TG2 Regulates the Heat-shock Response by the Post-translational Modification of HSF1. EMBO Rep. 2018, 19, e45067. [Google Scholar] [CrossRef] [PubMed]
- Rossin, F.; Costa, R.; Bordi, M.; D’Eletto, M.; Occhigrossi, L.; Farrace, M.G.; Barlev, N.; Ciccosanti, F.; Muccioli, S.; Chieregato, L.; et al. Transglutaminase Type 2 Regulates the Wnt/β-Catenin Pathway in Vertebrates. Cell Death Dis. 2021, 12, 249. [Google Scholar] [CrossRef]
- Bordeleau, F.; Wang, W.; Simmons, A.; Antonyak, M.A.; Cerione, R.A.; Reinhart-King, C.A. Tissue Transglutaminase 2 Regulates Tumor Cell Tensional Homeostasis by Increasing Contractility. J. Cell Sci. 2020, 133, 231134. [Google Scholar] [CrossRef]
- Hettasch, J.M.; Bandarenko, N.; Burchette, J.L.; Lai, T.S.; Marks, J.R.; Haroon, Z.A.; Peters, K.; Dewhirst, M.W.; Iglehart, J.D.; Greenberg, C.S. Tissue Transglutaminase Expression in Human Breast Cancer. Lab. Investig. 1996, 75, 637–645. [Google Scholar]
- Tang, Z.; Li, C.; Kang, B.; Gao, G.; Li, C.; Zhang, Z. GEPIA: A Web Server for Cancer and Normal Gene Expression Profiling and Interactive Analyses. Nucleic Acids Res. 2017, 45, 98–102. [Google Scholar] [CrossRef]
- Occhigrossi, L.; D’eletto, M.; Barlev, N.; Rossin, F. The Multifaceted Role of HSF1 in Pathophysiology: Focus on Its Interplay with TG2. Int. J. Mol. Sci. 2021, 22, 6366. [Google Scholar] [CrossRef]
- Boehm, J.E.; Singh, U.; Combs, C.; Antonyak, M.A.; Cerione, R.A. Tissue Transglutaminase Protects against Apoptosis by Modifying the Tumor Suppressor Protein P110 Rb. J. Biol. Chem. 2002, 277, 20127–20130. [Google Scholar] [CrossRef]
- Liu, B.; Wang, D.; Luo, E.; Hou, J.; Qiao, Y.; Yan, G.; Wang, Q.; Tang, C. Role of TG2-Mediated SERCA2 Serotonylation on Hypoxic Pulmonary Vein Remodeling. Front. Pharmacol. 2020, 10, 1611. [Google Scholar] [CrossRef]
- Ballestar, E.; Abad, C.; Franco, L. Core Histones Are Glutaminyl Substrates for Tissue Transglutaminase. J. Biol. Chem. 1996, 271, 18817–18824. [Google Scholar] [CrossRef]
- Fisher, M.L.; Kerr, C.; Adhikary, G.; Grun, D.; Xu, W.; Keillor, J.W.; Eckert, R.L. Transglutaminase Interaction with A6/Β4-Integrin Stimulates YAP1-Dependent ΔNp63α Stabilization and Leads to Enhanced Cancer Stem Cell Survival and Tumor Formation. Cancer Res. 2016, 76, 7265–7276. [Google Scholar] [CrossRef]
- Gagliardi, M.; Saverio, V.; Rossin, F.; D’Eletto, M.; Corazzari, M. Transglutaminase 2 and Ferroptosis: A New Liaison? Cell Death Discov. 2023, 9, 88. [Google Scholar] [CrossRef]
- Rossin, F.; Ciccosanti, F.; D’Eletto, M.; Occhigrossi, L.; Fimia, G.M.; Piacentini, M. Type 2 Transglutaminase in the Nucleus: The New Epigenetic Face of a Cytoplasmic Enzyme. Cell. Mol. Life Sci. 2023, 80, 52. [Google Scholar] [CrossRef]
- Fujimura, T.; Muto, Y.; Asano, Y. Immunotherapy for Melanoma: The Significance of Immune Checkpoint Inhibitors for the Treatment of Advanced Melanoma. Int. J. Mol. Sci. 2022, 23, 15720. [Google Scholar] [CrossRef]
- Aguiari, G.; Crudele, F.; Taccioli, C.; Minotti, L.; Corrà, F.; Keillor, J.W.; Grassilli, S.; Cervellati, C.; Volinia, S.; Bergamini, C.M.; et al. Dysregulation of Transglutaminase Type 2 through GATA3 Defines Aggressiveness and Doxorubicin Sensitivity in Breast Cancer. Int. J. Biol. Sci. 2022, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Shinde, A.; Kulkoyluoglu Cotul, E.; Chen, H.; Smith, A.; Libring, S.; Solorio, L.; Wendt, M.K. Transglutaminase-2 Mediates Acquisition of Neratinib Resistance in Metastatic Breast Cancer. Mol. Biomed. 2022, 3, 19. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Lee, H.J.; Yoon, S.; Ryu, H.-M.; Lee, E.; Jo, Y.; Seo, S.; Kim, D.; Lee, C.H.; Kim, W.; et al. Blockade of CCL2 Expression Overcomes Intrinsic PD-1/PD-L1 Inhibitor-Resistance in Transglutaminase 2-Induced PD-L1 Positive Triple Negative Breast Cancer. Am. J. Cancer Res. 2020, 10, 2878–2894. [Google Scholar]
- Zhao, X.; Kim, I.K.; Kallakury, B.; Chahine, J.J.; Iwama, E.; Pierobon, M.; Petricoin, E.; McCutcheon, J.N.; Zhang, Y.W.; Umemura, S.; et al. Acquired Small Cell Lung Cancer Resistance to Chk1 Inhibitors Involves Wee1 Up-Regulation. Mol. Oncol. 2021, 15, 1130–1145. [Google Scholar] [CrossRef]
- Han, J.A.; Park, S.C. Reduction of Transglutaminase 2 Expression Is Associated with an Induction of Drug Sensitivity in the PC-14 Human Lung Cancer Cell Line. J. Cancer Res. Clin. Oncol. 1999, 125, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.T.; Huang, C.H.; Chen, W.C.; Tsai, C.S.; Chao, Y.L.; Liu, S.H.; Chen, J.H.; Wu, Y.Y.; Lee, Y.J. TransglutaMinase 2 Promotes Migration and Invasion of Lung Cancer Cells. Oncol. Res. 2018, 26, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Budillon, A.; Carbone, C.; Di Gennaro, E. Tissue Transglutaminase: A New Target to Reverse Cancer Drug Resistance. Amino Acids 2013, 44, 63–72. [Google Scholar] [CrossRef]
- Muccioli, S.; Ciaccio, R.; Brillo, V.; Leanza, L. Promising Prognostic Value of Transglutaminase Type 2 and Its Correlation with Tumor-Infiltrating Immune Cells in Skin Cutaneous Melanoma. Cell Death Discov. 2022, 8, 294. [Google Scholar] [CrossRef]
- Tempest, R.; Guarnerio, S.; Maani, R.; Cooper, J.; Peake, N. The Biological and Biomechanical Role of Transglutaminase-2 in the Tumour Microenvironment. Cancers 2021, 13, 2788. [Google Scholar] [CrossRef]
- Szondy, Z.; Korponay-Szabó, I.; Király, R.; Sarang, Z.; Tsay, G.J. Transglutaminase 2 in Human Diseases. BioMedicine 2017, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.O.; Lim, Y.C.; Kim, Y.M.; Ha, K.S. Transglutaminase 2 Promotes Both Caspase-Dependent and Caspase-Independent Apoptotic Cell Death via the Calpain/Bax Protein Signaling Pathway. J. Biol. Chem. 2012, 287, 14377–14388. [Google Scholar] [CrossRef]
- Rossin, F.; D’Eletto, M.; MacDonald, D.; Farrace, M.G.; Piacentini, M. TG2 Transamidating Activity Acts as a Reostat Controlling the Interplay between Apoptosis and Autophagy. Amino Acids 2012, 42, 1793–1802. [Google Scholar] [CrossRef]
- Bianchi, N.; Brugnoli, F.; Grassilli, S.; Bourgeois, K.; Keillor, J.W.; Bergamini, C.M.; Aguiari, G.; Volinia, S.; Bertagnolo, V. The Motility and Mesenchymal Features of Breast Cancer Cells Correlate with the Levels and Intracellular Localization of Transglutaminase Type 2. Cells 2021, 10, 3059. [Google Scholar] [CrossRef]
- Kumar, S.; Mehta, K. Tissue Transglutaminase, Inflammation, and Cancer: How Intimate Is the Relationship? Amino Acids 2013, 44, 81–88. [Google Scholar] [CrossRef]
- Wang, K.; Zu, C.; Zhang, Y.; Wang, X.; Huan, X.; Wang, L. Blocking TG2 Attenuates Bleomycin-Induced Pulmonary Fibrosis in Mice through Inhibiting EMT. Respir. Physiol. Neurobiol. 2020, 276, 103402. [Google Scholar] [CrossRef]
- Ma, H.; Xie, L.; Zhang, L.; Yin, X.; Jiang, H.; Xie, X.; Chen, R.; Lu, H.; Ren, Z. Activated Hepatic Stellate Cells Promote Epithelial-to-Mesenchymal Transition in Hepatocellular Carcinoma through Transglutaminase 2-Induced Pseudohypoxia. Commun. Biol. 2018, 1, 168. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Adhikary, G.; Newland, J.J.; Xu, W.; Keillor, J.W.; Weber, D.J.; Eckert, R.L. Transglutaminase 2 Binds to the CD44v6 Cytoplasmic Domain to Stimulate CD44v6/ERK1/2 Signaling and Maintain an Aggressive Cancer Phenotype. Mol. Cancer Res. 2023, 21, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.L.; Adhikary, G.; Kerr, C.; Grun, D.; Eckert, R.L. Transglutaminase 2 Is a Direct Target Gene of YAP-TAZ—Response. Cancer Res. 2017, 77, 4736. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.D.; Ruiz-Gómez, G.; Cazzonelli, S.; Möller, S.; Wodtke, R.; Löser, R.; Freyse, J.; Dürig, J.N.; Rademann, J.; Hempel, U.; et al. Sulfated Glycosaminoglycans Inhibit Transglutaminase 2 by Stabilizing Its Closed Conformation. Sci. Rep. 2022, 12, 13326. [Google Scholar] [CrossRef] [PubMed]
- Stamnaes, J.; Cardoso, I.; Iversen, R.; Sollid, L.M. Transglutaminase 2 Strongly Binds to an Extracellular Matrix Component Other than Fibronectin via Its Second C-Terminal Beta-Barrel Domain. FEBS J. 2016, 283, 3994–4010. [Google Scholar] [CrossRef]
- Espitia Pinzón, N.; Brevé, J.J.P.; Bol, J.G.J.M.; Drukarch, B.; Baron, W.; van Dam, A.M. Tissue Transglutaminase in Astrocytes Is Enhanced by Inflammatory Mediators and Is Involved in the Formation of Fibronectin Fibril-like Structures. J. Neuroinflamm. 2017, 14, 260. [Google Scholar] [CrossRef]
- Kim Young Woo Park AE Yun-Sil Lee AE Dooil Jeoung, Y.A. Hyaluronic Acid Induces Transglutaminase II to Enhance Cell Motility; Role of Rac1 and FAK in the Induction of Transglutaminase II. Biotechnol. Lett. 2008, 30, 31–39. [Google Scholar] [CrossRef]
- Severin, F.; Mouawad, N.; Ruggeri, E.; Visentin, A.; Martinello, L.; Pagnin, E.; Trimarco, V.; Pravato, S.; Angotzi, F.; Facco, M.; et al. Focal Adhesion Kinase Activation by Calcium-Dependent Calpain Is Involved in Chronic Lymphocytic Leukaemia Cell Aggressiveness. Br. J. Haematol. 2023, 203, 224–236. [Google Scholar] [CrossRef]
- Lee, J.; Condello, S.; Yakubov, B.; Emerson, R.; Caperell-Grant, A.; Hitomi, K.; Xie, J.; Matei, D. Tissue Transglutaminase Mediated Tumor-Stroma Interaction Promotes Pancreatic Cancer Progression. Clin. Cancer Res. 2015, 21, 4482–4493. [Google Scholar] [CrossRef]
- Long, G.V.; Swetter, S.M.; Menzies, A.M.; Gershenwald, J.E.; Scolyer, R.A. Cutaneous Melanoma. Lancet 2023, 402, 485–502. [Google Scholar] [CrossRef]
- Di, G.; Ae, G.; Lentini, A.; Beninati, A.S.; Piacentini, A.M.; Rodolfo, A.C. In Vivo Evaluation of Type 2 Transglutaminase Contribution to the Metastasis Formation in Melanoma. Amino Acids 2009, 36, 717–724. [Google Scholar] [CrossRef]
- Fok, J.Y.; Ekmekcioglu, S.; Mehta, K. Implications of Tissue Transglutaminase Expression in Malignant Melanoma. Mol. Cancer Ther. 2006, 5, 1493–1503. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Friedland, S.; Corson, N.; Xu, L. GPR56 Inhibits Melanoma Growth by Internalizing and Degrading Its Ligand TG2. Cancer Res. 2014, 74, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Xu, N.; Sun, L.; Yang, Z.; He, M.; Li, Y. TG2 as a Novel Breast Cancer Prognostic Marker Promotes Cell Proliferation and Glycolysis by Activating the MEK/ERK/LDH Pathway. BMC Cancer 2022, 22, 1267. [Google Scholar] [CrossRef]
- Schwager, S.C.; Young, K.M.; Hapach, L.A.; Carlson, C.M.; Mosier, J.A.; McArdle, T.J.; Wang, W.; Schunk, C.; Jayathilake, A.L.; Bates, M.E.; et al. Weakly Migratory Metastatic Breast Cancer Cells Activate Fibroblasts via Microvesicle-Tg2 to Facilitate Dissemination and Metastasis. Elife 2022, 11, e74433. [Google Scholar] [CrossRef]
- Shinde, A.; Paez, J.S.; Libring, S.; Hopkins, K.; Solorio, L.; Wendt, M.K. Transglutaminase-2 Facilitates Extracellular Vesicle-Mediated Establishment of the Metastatic Niche. Oncogenesis 2020, 9, 16. [Google Scholar] [CrossRef]
- Seo, S.; Moon, Y.; Choi, J.; Yoon, S.; Jung, K.H.; Cheon, J.; Kim, W.; Kim, D.; Lee, C.H.; Kim, S.-W.; et al. The GTP Binding Activity of Transglutaminase 2 Promotes Bone Metastasis of Breast Cancer Cells by Downregulating MicroRNA-205. Am. J. Cancer Res. 2019, 9, 597–607. [Google Scholar]
- Canella, R.; Brugnoli, F.; Gallo, M.; Keillor, J.W.; Terrazzan, A.; Ferrari, E.; Grassilli, S.; Gates, E.W.J.; Volinia, S.; Bertagnolo, V.; et al. A Multidisciplinary Approach Establishes a Link between Transglutaminase 2 and the Kv10.1 Voltage-Dependent K+ Channel in Breast Cancer. Cancers 2023, 15, 178. [Google Scholar] [CrossRef]
- Yin, J.; Oh, Y.T.; Kim, J.Y.; Kim, S.S.; Choi, E.; Kim, T.H.; Hong, J.H.; Chang, N.; Cho, H.J.; Sa, J.K.; et al. Transglutaminase 2 Inhibition Reverses Mesenchymal Transdifferentiation of Glioma Stem Cells by Regulating C/EBPβ Signaling. Cancer Res. 2017, 77, 4973–4984. [Google Scholar] [CrossRef]
- Rong, L.; Li, N.; Zhang, Z. Emerging Therapies for Glioblastoma: Current State and Future Directions. J. Exp. Clin. Cancer Res. 2022, 41, 142. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Chen, Q.; Liu, H.; Zeng, L.; Zhou, Y.; Liu, X.; Bai, Y.; Zhang, J.; Pan, Y.; Shao, C. SDC1-Dependent TGM2 Determines Radiosensitivity in Glioblastoma by Coordinating EPG5-Mediated Fusion of Autophagosomes with Lysosomes. Autophagy 2023, 19, 839–857. [Google Scholar] [CrossRef]
- Gundemir, S.; Monteagudo, A.; Akbar, A.; Keillor, J.W.; Johnson, G.V.W. The Complex Role of Transglutaminase 2 in Glioblastoma Proliferation. Neuro Oncol. 2017, 19, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Katt, W.P.; Aplin, C.; Cerione, R.A. Exploring the Role of Transglutaminase in Patients with Glioblastoma: Current Perspectives. Onco Targets Ther. 2022, 15, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.C.; Yang, J.; Ng, M.C.; Ng, S.K.; Welch, W.R.; Muto, M.G.; Berkowitz, R.S.; Ng, S.W. Cyclin A1 Expression and Paclitaxel Resistance in Human Ovarian Cancer Cells. Eur. J. Cancer 2016, 67, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Eckert, R.L.; Fisher, M.L.; Grun, D.; Adhikary, G.; Xu, W.; Kerr, C. Transglutaminase Is a Tumor Cell and Cancer Stem Cell Survival Factor. Mol. Carcinog. 2015, 54, 947–958. [Google Scholar] [CrossRef]
- Condello, S.; Sima, L.; Ivan, C.; Cardenas, H.; Schiltz, G.; Mishra, R.K.; Matei, D. Tissue Tranglutaminase Regulates Interactions between Ovarian Cancer Stem Cells and the Tumor Niche. Cancer Res. 2018, 78, 2990–3001. [Google Scholar] [CrossRef]
- Yakubov, B.; Chelladurai, B.; Schmitt, J.; Emerson, R.; Turchi, J.J.; Matei, D. Extracellular Tissue Transglutaminase Activates Noncanonical NF-ΚB Signaling and Promotes Metastasis in Ovarian Cancer. Neoplasia 2013, 15, 609–819. [Google Scholar] [CrossRef] [PubMed]
- Condello, S.; Prasad, M.; Atwani, R.; Matei, D. Tissue Transglutaminase Activates Integrin-Linked Kinase and β-Catenin in Ovarian Cancer. J. Biol. Chem. 2022, 298, 102242. [Google Scholar] [CrossRef]
- Sima, L.E.; Chen, S.; Cardenas, H.; Zhao, G.; Wang, Y.; Ivan, C.; Huang, H.; Zhang, B.; Matei, D. Loss of Host Tissue Transglutaminase Boosts Antitumor T Cell Immunity by Altering STAT1/STAT3 Phosphorylation in Ovarian Cancer. J. Immunother. Cancer 2021, 9, e002682. [Google Scholar] [CrossRef]
- Kim, E.-B.; Jeon, H.Y.; Ouh, Y.T.; Lee, A.J.; Moon, C.H.; Na, S.H.; Ha, K.S. Proinsulin C-Peptide Inhibits High Glucose-Induced Migration and Invasion of Ovarian Cancer Cells. Biomed. Pharmacother. 2024, 172, 116232. [Google Scholar] [CrossRef]
- Su, D.; Liu, C.; Cui, J.; Tang, J.; Ruan, Y.; Zhang, Y. Advances and Prospects of Drug Clinical Research in Colorectal Cancer in 2022. Cancer Innov. 2023, 2, 99–113. [Google Scholar] [CrossRef]
- Malkomes, P.; Lunger, I.; Oppermann, E.; Abou-El-Ardat, K.; Oellerich, T.; Günther, S.; Canbulat, C.; Bothur, S.; Schnütgen, F.; Yu, W.; et al. Transglutaminase 2 Promotes Tumorigenicity of Colon Cancer Cells by Inactivation of the Tumor Suppressor P53. Oncogene 2021, 40, 4352–4367. [Google Scholar] [CrossRef]
- Yang, P.; Yu, D.; Zhou, J.; Zhuang, S.; Jiang, T. TGM2 Interference Regulates the Angiogenesis and Apoptosis of Colorectal Cancer via Wnt/β-Catenin Pathway. Cell Cycle 2019, 18, 1122–1134. [Google Scholar] [CrossRef]
- Fernández-Aceñero, M.J.; Torres, S.; Garcia-Palmero, I.; Díaz del Arco, C.; Casal, J.I. Prognostic Role of Tissue Transglutaminase 2 in Colon Carcinoma. Virchows Arch. 2016, 469, 611–619. [Google Scholar] [CrossRef]
- Ayinde, O.; Wang, Z.; Pinton, G.; Moro, L.; Griffin, M. Transglutaminase 2 Maintains a Colorectal Cancer Stem Phenotype by Regulating Epithelial-Mesenchymal Transition. Oncotarget 2019, 10, 4556–4569. [Google Scholar] [CrossRef]
- Malkomes, P.; Lunger, I.; Oppermann, E.; Lorenz, J.; Faqar-Uz-Zaman, S.F.; Han, J.; Bothur, S.; Ziegler, P.; Bankov, K.; Wild, P.; et al. Transglutaminase 2 Is Associated with Adverse Colorectal Cancer Survival and Represents a Therapeutic Target. Cancer Gene Ther. 2023, 30, 1346–1354. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Cai, J.; Xu, Z.; Zhou, S.; Ye, L.; Yan, Q.; Zhang, Y.; Fang, Y.; Liu, Y.; Tu, C.; et al. MiR-532-3p Suppresses Colorectal Cancer Progression by Disrupting the ETS1/TGM2 Axis-Mediated Wnt/β-Catenin Signaling. Cell Death Dis. 2019, 10, 739. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Oh, S.C.; Min, B.W.; Lee, D.H. Transglutaminase 2 Regulates Self-Renewal and Stem Cell Marker of Human Colorectal Cancer Stem Cells. Anticancer Res. 2018, 38, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Moresco, P.; Yan, R.; Li, J.; Gao, Y.; Biasci, D.; Yao, M.; Pearson, J.; Hechtman, J.F.; Janowitz, T.; et al. Carcinomas Assemble a Filamentous CXCL12-Keratin-19 Coating That Suppresses T Cell-Mediated Immune Attack. Proc. Natl. Acad. Sci. USA 2022, 119, e2119463119. [Google Scholar] [CrossRef] [PubMed]
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; de Oliveira Araujo, I.B.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th Edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 413–429. [Google Scholar] [CrossRef] [PubMed]
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.C.; et al. The 5th Edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef]
- Pierce, A.; Whetton, A.D.; Meyer, S.; Ravandi-Kashani, F.; Borthakur, G.; Coombes, K.R.; Zhang, N.; Kornblau, S. Transglutaminase 2 Expression in Acute Myeloid Leukemia: Association with Adhesion Molecule Expression and Leukemic Blast Motility. Proteomics 2013, 13, 2216–2222. [Google Scholar] [CrossRef]
- Coombs, C.C.; Tavakkoli, M.; Tallman, M.S. Acute Promyelocytic Leukemia: Where Did We Start, Where Are We Now, and the Future. Blood Cancer J. 2015, 5, e304. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadeh, Z.; Omidkhoda, A.; Chahardouli, B.; Hoseinzadeh, G.; Moghaddam, K.A.; Mousavi, S.A.; Rostami, S. The Impact of ICAM-1, CCL2 and TGM2 Gene Polymorphisms on Differentiation Syndrome in Acute Promyelocytic Leukemia. BMC Cancer 2021, 21, 46. [Google Scholar] [CrossRef] [PubMed]
- Jambrovics, K.; Póliska, S.; Scholtz, B.; Uray, I.P.; Balajthy, Z. ATO Increases ROS Production and Apoptosis of Cells by Enhancing Calpain-Mediated Degradation of the Cancer Survival Protein TG2. Int. J. Mol. Sci. 2023, 24, 10938. [Google Scholar] [CrossRef] [PubMed]
- Balajthy, Z.; Csomós, K.; Vámosi, G.; Szántó, A.; Lanotte, M.; Fésüs, L. Tissue-Transglutaminase Contributes to Neutrophil Granulocyte Differentiation and Functions. Blood 2006, 108, 2045–2054. [Google Scholar] [CrossRef] [PubMed]
- Jambrovics, K.; Botó, P.; Pap, A.; Sarang, Z.; Kolostyák, Z.; Czimmerer, Z.; Szatmari, I.; Fésüs, L.; Uray, I.P.; Balajthy, Z. Transglutaminase 2 Associated with PI3K and PTEN in a Membrane-Bound Signalosome Platform Blunts Cell Death. Cell Death Dis. 2023, 14, 217. [Google Scholar] [CrossRef] [PubMed]
- Belver, L.; Ferrando, A. The Genetics and Mechanisms of T Cell Acute Lymphoblastic Leukaemia. Nat. Rev. Cancer 2016, 16, 494–507. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.J.; Han, E.H.; Jang, I.K.; Yoon, S.H.; Park, J.E. Effect of Tissue Transglutaminase on Steroid Resistance in T-Cell Acute Lymphoblastic Leukemia. Anticancer Res. 2019, 39, 6165–6173. [Google Scholar] [CrossRef]
- Han, X.; Bueso-Ramos, C.E. Precursor T-Cell Acute Lymphoblastic Leukemia/Lymphoblastic Lymphoma and Acute Biphenotypic Leukemias. Am. J. Clin. Pathol. 2007, 127, 528–544. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, N.; Sun, T.; Zhao, H.; Chen, Y.; Liu, C. Role of TGM2 in T-cell Lymphoblastic Lymphoma via Regulation of IL-6/JAK/STAT3 Signalling. Mol. Med. Rep. 2022, 25, 76. [Google Scholar] [CrossRef]
- Silkenstedt, E.; Dreyling, M. Mantle Cell Lymphoma—Update on Molecular Biology, Prognostication and Treatment Approaches. Hematol. Oncol. 2023, 41, 36–42. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Z.; Miranda, R.N.; Jeffrey Medeiros, L.; McCarty, N. TG2 and NF-ΚB Signaling Coordinates the Survival of Mantle Cell Lymphoma Cells via Il6-Mediated Autophagy. Cancer Res. 2016, 76, 6410–6423. [Google Scholar] [CrossRef]
- Chereda, B.; Melo, J.V. Natural Course and Biology of CML. Ann. Hematol. 2015, 94, 107–121. [Google Scholar] [CrossRef]
- Kang, S.K.; Lee, J.Y.; Chung, T.W.; Kim, C.H. Overexpression of Transglutaminase 2 Accelerates the Erythroid Differentiation of Human Chronic Myelogenous Leukemia K562 Cell Line through PI3K/Akt Signaling Pathway. FEBS Lett. 2004, 577, 361–366. [Google Scholar] [CrossRef]
- Ha, S.H.; Kang, S.K.; Choi, H.; Kwak, C.H.; Abekura, F.; Park, J.Y.; Kwon, K.M.; Chang, H.W.; Lee, Y.C.; Ha, K.T.; et al. Induction of GD3/A1-Adrenergic Receptor/Transglutaminase 2-Mediated Erythroid Differentiation in Chronic Myelogenous Leukemic K562 Cells. Oncotarget 2017, 8, 72205–72210. [Google Scholar] [CrossRef] [PubMed]
- Feriotto, G.; Tagliati, F.; Brunello, A.; Beninati, S.; Tabolacci, C.; Mischiati, C. A Central Contribution of TG2 Activity to the Antiproliferative and Pro-Apoptotic Effects of Caffeic Acid in K562 Cells of Human Chronic Myeloid Leukemia. Int. J. Mol. Sci. 2022, 23, 15004. [Google Scholar] [CrossRef]
- Park, W.; Chawla, A.; O’Reilly, E.M. Pancreatic Cancer: A Review. JAMA—J. Am. Med. Assoc. 2021, 326, 851–862. [Google Scholar] [CrossRef]
- Mehta, K.; Han, A. Tissue Transglutaminase (TG2)-Induced Inflammation in Initiation, Progression, and Pathogenesis of Pancreatic Cancer. Cancers 2011, 3, 897–912. [Google Scholar] [CrossRef]
- Lee, J.; Yakubov, B.; Ivan, C.; Jones, D.R.; Caperell-Grant, A.; Fishel, M.; Cardenas, H.; Matei, D. Tissue Transglutaminase Activates Cancer-Associated Fibroblasts and Contributes to Gemcitabine Resistance in Pancreatic Cancer. Neoplasia 2016, 18, 689–698. [Google Scholar] [CrossRef]
- Zhang, S.; Yao, H.F.; Li, H.; Su, T.; Jiang, S.H.; Wang, H.; Zhang, Z.G.; Dong, F.Y.; Yang, Q.; Yang, X.M. Transglutaminases Are Oncogenic Biomarkers in Human Cancers and Therapeutic Targeting of TGM2 Blocks Chemoresistance and Macrophage Infiltration in Pancreatic Cancer. Cell. Oncol. 2023, 46, 1473–1492. [Google Scholar] [CrossRef]
- Verma, A.; Guha, S.; Wang, H.; Fok, J.Y.; Koul, D.; Abbruzzese, J.; Mehta, K. Tissue Transglutaminase Regulates Focal Adhesion Kinase/AKT Activation by Modulating PTEN Expression in Pancreatic Cancer Cells. Clin. Cancer Res. 2008, 14, 1997–2005. [Google Scholar] [CrossRef]
- Nicholson, A.G.; Tsao, M.S.; Beasley, M.B.; Borczuk, A.C.; Brambilla, E.; Cooper, W.A.; Dacic, S.; Jain, D.; Kerr, K.M.; Lantuejoul, S.; et al. The 2021 WHO Classification of Lung Tumors: Impact of Advances Since 2015. J. Thorac. Oncol. 2022, 17, 362–387. [Google Scholar] [CrossRef]
- Choi, C.M.; Jang, S.J.; Park, S.Y.; Choi, Y.B.; Jeong, J.H.; Kim, D.S.; Kim, H.K.; Park, K.S.; Nam, B.H.; Kim, H.R.; et al. Transglutaminase 2 as an Independent Prognostic Marker for Survival of Patients with Non-Adenocarcinoma Subtype of Non-Small Cell Lung Cancer. Mol. Cancer 2011, 10, 119. [Google Scholar] [CrossRef]
- Chihong, Z.; Yutian, L.; Danying, W.; Ruibin, J.; Huaying, S.; Linhui, G.; Jianguo, F. Prognostic Value of Transglutaminase 2 in Non-Small Cell Lung Cancer Patients. Oncotarget 2017, 8, 45577–45584. [Google Scholar] [CrossRef]
- Lee, M.Y.; Wu, M.F.; Cherng, S.H.; Chiu, L.Y.; Yang, T.Y.; Sheu, G.T. Tissue Transglutaminase 2 Expression Is Epigenetically Regulated in Human Lung Cancer Cells and Prevents Reactive Oxygen Species-Induced Apoptosis. Cancer Manag. Res. 2018, 10, 2835–2848. [Google Scholar] [CrossRef]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor Angiogenesis: Causes, Consequences, Challenges and Opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef]
- Potente, M.; Gerhardt, H.; Carmeliet, P. Basic and Therapeutic Aspects of Angiogenesis. Cell 2011, 146, 873–887. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Eelen, G.; Treps, L.; Li, X.; Carmeliet, P. Basic and Therapeutic Aspects of Angiogenesis Updated. Circ. Res. 2020, 127, 310–329. [Google Scholar] [CrossRef] [PubMed]
- Viallard, C.; Larrivée, B. Tumor Angiogenesis and Vascular Normalization: Alternative Therapeutic Targets. Angiogenesis 2017, 20, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Chen, Y.; Tan, S.; Wu, S.; Huang, Y.; Fu, S.; Luo, F.; He, J. The Research Progress of Antiangiogenic Therapy, Immune Therapy and Tumor Microenvironment. Front. Immunol. 2022, 13, 802846. [Google Scholar] [CrossRef] [PubMed]
- Sane, D.C.; Kontos, J.L.; Greenberg, C.S. Roles of Transglutaminases in Cardiac and Vascular Diseases. Front. Biosci. 2007, 12, 2530–2545. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, Z.A.; Barsigian, C.; Chalupowicz, G.D.; Bach, T.L.; Garcia-Manero, G.; Martinez, J. Colocalization of Tissue Transglutaminase and Stress Fibers in Human Vascular Smooth Muscle Cells and Human Umbilical Vein Endothelial Cells. Exp. Cell Res. 1997, 231, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Nadalutti, C.; Viiri, K.M.; Kaukinen, K.; Mäki, M.; Lindfors, K. Extracellular Transglutaminase 2 Has a Role in Cell Adhesion, Whereas Intracellular Transglutaminase 2 Is Involved in Regulation of Endothelial Cell Proliferation and Apoptosis. Cell Prolif. 2011, 44, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Collighan, R.J.; Pytel, K.; Rathbone, D.L.; Li, X.; Griffin, M. Characterization of Heparin-Binding Site of Tissue Transglutaminase: Its Importance in Cell Surface Targeting, Matrix Deposition, and Cell Signaling. J. Biol. Chem. 2012, 287, 13063–13083. [Google Scholar] [CrossRef]
- Gaudry, C.A.; Verderio, E.; Jones, R.A.; Smith, C.; Griffin, M. Tissue Transglutaminase Is an Important Player at the Surface of Human Endothelial Cells: Evidence for Its Externalization and Its Colocalization with the Β1 Integrin. Exp. Cell Res. 1999, 252, 104–113. [Google Scholar] [CrossRef]
- Wang, Z.; Perez, M.; Caja, S.; Melino, G.; Johnson, T.S.; Lindfors, K.; Griffin, M. A Novel Extracellular Role for Tissue Transglutaminase in Matrix-Bound VEGF-Mediated Angiogenesis. Cell Death Dis. 2013, 4, e808. [Google Scholar] [CrossRef]
- Lei, Z.; Chai, N.; Tian, M.; Zhang, Y.; Wang, G.; Liu, J.; Tian, Z.; Yi, X.; Chen, D.; Li, X.; et al. Novel Peptide GX1 Inhibits Angiogenesis by Specifically Binding to Transglutaminase-2 in the Tumorous Endothelial Cells of Gastric Cancer Article. Cell Death Dis. 2018, 9, 579. [Google Scholar] [CrossRef]
- Nezir, A.E.; Ulukan, B.; Telci, D. Transglutaminase 2: The Maestro of the Oncogenic Mediators in Renal Cell Carcinoma. Med. Sci. 2019, 7, 24. [Google Scholar] [CrossRef]
- Lee, S.H.; Kang, J.H.; Ha, J.S.; Lee, J.S.; Oh, S.J.; Choi, H.J.; Song, J.; Kim, S.Y. Transglutaminase 2-Mediated P53 Depletion Promotes Angiogenesis by Increasing Hif-1α-P300 Binding in Renal Cell Carcinoma. Int. J. Mol. Sci. 2020, 21, 5042. [Google Scholar] [CrossRef] [PubMed]
- Min, B.; Park, H.; Lee, S.; Li, Y.; Choi, J.M.; Lee, J.Y.; Kim, J.; Choi, Y.D.; Kwon, Y.G.; Lee, H.W.; et al. CHIP-Mediated Degradation of Transglutaminase 2 Negatively Regulates Tumor Growth and Angiogenesis in Renal Cancer. Oncogene 2016, 35, 3718–3728. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.A.; Kotsakis, P.; Johnson, T.S.; Chau, D.Y.S.; Ali, S.; Melino, G.; Griffin, M. Matrix Changes Induced by Transglutaminase 2 Lead to Inhibition of Angiogenesis and Tumor Growth. Cell Death Differ. 2006, 13, 1442–1453. [Google Scholar] [CrossRef] [PubMed]
- Spurlin, T.A.; Bhadriraju, K.; Chung, K.H.; Tona, A.; Plant, A.L. The Treatment of Collagen Fibrils by Tissue Transglutaminase to Promote Vascular Smooth Muscle Cell Contractile Signaling. Biomaterials 2009, 30, 5486–5489. [Google Scholar] [CrossRef] [PubMed]
- Stephens, P.; Grenard, P.; Aeschlimann, P.; Langley, M.; Blain, E.; Errington, R.; Kipling, D.; Thomas, D.; Aeschlimann, D. Crosslinking and G-Protein Functions of Transglutaminase 2 Contribute Differentially to Fibroblast Wound Healing Responses. J. Cell Sci. 2004, 117, 3389–3403. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.P.; Lygoe, K.A.; Nystrom, M.L.; Anderson, W.P.; Speight, P.M.; Marshall, J.F.; Thomas, G.J. Tumour-Derived TGF-Β1 Modulates Myofibroblast Differentiation and Promotes HGF/SF-Dependent Invasion of Squamous Carcinoma Cells. Br. J. Cancer 2004, 90, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Jakubek, Y.A.; Chang, K.; Sivakumar, S.; Yu, Y.; Giordano, M.R.; Fowler, J.; Huff, C.D.; Kadara, H.; Vilar, E.; Scheet, P. Large-Scale Analysis of Acquired Chromosomal Alterations in Non-Tumor Samples from Patients with Cancer. Nat. Biotechnol. 2020, 38, 90–96. [Google Scholar] [CrossRef]
- Grauel, A.L.; Nguyen, B.; Ruddy, D.; Laszewski, T.; Schwartz, S.; Chang, J.; Chen, J.; Piquet, M.; Pelletier, M.; Yan, Z.; et al. TGFβ-Blockade Uncovers Stromal Plasticity in Tumors by Revealing the Existence of a Subset of Interferon-Licensed Fibroblasts. Nat. Commun. 2020, 11, 6315. [Google Scholar] [CrossRef]
- Torres, S.; Garcia-Palmero, I.; Herrera, M.; Bartolomé, R.A.; Peña, C.; Fernandez-Aceñero, M.J.; Padilla, G.; Peláez-García, A.; Lopez-Lucendo, M.; Rodriguez-Merlo, R.; et al. LOXL2 Is Highly Expressed in Cancer-Associated Fibroblasts and Associates to Poor Colon Cancer Survival. Clin. Cancer Res. 2015, 21, 4892–4902. [Google Scholar] [CrossRef]
- Jia, C.; Wang, G.; Wang, T.; Fu, B.; Zhang, Y.; Huang, L.; Deng, Y.; Chen, G.; Wu, X.; Chen, J.; et al. Cancer-Associated Fibroblasts Induce Epithelial-Mesenchymal Transition via the Transglutaminase 2-Dependent Il-6/Il6r/Stat3 Axis in Hepatocellular Carcinoma. Int. J. Biol. Sci. 2020, 16, 2542–2558. [Google Scholar] [CrossRef]
- Zou, W. Immunosuppressive Networks in the Tumour Environment and Their Therapeutic Relevance. Nat. Rev. Cancer 2005, 5, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Chrobok, N.L.; Sestito, C.; Wilhelmus, M.M.M.; Drukarch, B.; van Dam, A.M. Is Monocyte- and Macrophage-Derived Tissue Transglutaminase Involved in Inflammatory Processes? Amino Acids 2017, 49, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Seiving, B.; Ohlsson, K.; Linder, C.; Stenberg, P. Transglutaminase Differentiation during Maturation of Human Blood Monocytes to Macrophages. Eur. J. Haematol. 1991, 46, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Matic, I.; Sacchi, A.; Rinaldi, A.; Melino, G.; Khosla, C.; Falasca, L.; Piacentini, M. Characterization of Transglutaminase Type II Role in Dendritic Cell Differentiation and Function. J. Leukoc. Biol. 2010, 88, 181–188. [Google Scholar] [CrossRef]
- Hodrea, J.; Demény, M.Á.; Majai, G.; Sarang, Z.; Korponay-Szabó, I.R.; Fésüs, L. Transglutaminase 2 Is Expressed and Active on the Surface of Human Monocyte-Derived Dendritic Cells and Macrophages. Immunol. Lett. 2010, 130, 74–81. [Google Scholar] [CrossRef]
- Kim, J.H.; Jeong, E.M.; Jeong, Y.J.; Lee, W.J.; Kang, J.S.; Kim, I.G.; Hwang, Y. il Transglutaminase 2 on the Surface of Dendritic Cells Is Proposed to Be Involved in Dendritic Cell-T Cell Interaction. Cell Immunol. 2014, 289, 55–62. [Google Scholar] [CrossRef]
- Sun, H.; Kaartinen, M.T. Transglutaminases in Monocytes and Macrophages. Med. Sci. 2018, 6, 115. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Q.; Zhang, X.; Cui, M.; Li, T.; Zhang, Y.; Liao, Q. Immune Subtyping for Pancreatic Cancer with Implication in Clinical Outcomes and Improving Immunotherapy. Cancer Cell Int. 2021, 21, 137. [Google Scholar] [CrossRef]
- Cho, S.Y.; Oh, Y.; Jeong, E.M.; Park, S.; Lee, D.; Wang, X.; Zeng, Q.; Qin, H.; Hu, F.; Gong, H.; et al. Amplification of Transglutaminase 2 Enhances Tumor-Promoting Inflammation in Gastric Cancers. Exp. Mol. Med. 2020, 52, 854–864. [Google Scholar] [CrossRef]
- Suzuki, A.S.; Yagi, R.; Kimura, M.Y.; Iwamura, C.; Shinoda, K.; Onodera, A.; Hirahara, K.; Tumes, D.J.; Koyama-Nasu, R.; Iismaa, S.E.; et al. Essential Role for CD30-Transglutaminase 2 Axis in Memory Th1 and Th17 Cell Generation. Front. Immunol. 2020, 11, 1536. [Google Scholar] [CrossRef]
- Kim, J.H.; Jeong, E.M.; Jeong, Y.J.; Lee, W.J.; Kang, J.S.; Kim, I.G.; Hwang, Y. il Transglutaminase 2 Modulates Antigen-Specific Antibody Response by Suppressing Blimp-1 and AID Expression of B Cells in Mice. Immunol. Lett. 2012, 147, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Jiang, M.; Chen, P.; Chen, P.; Wang, L.; Li, W.; Chen, B.; Liu, Y.; Liu, Y.; Wang, H.; et al. CGAS-STING, an Important Pathway in Cancer Immunotherapy. J. Hematol. Oncol. 2020, 13, 81. [Google Scholar] [CrossRef] [PubMed]
- Occhigrossi, L.; Rossin, F.; D’Eletto, M.; Farrace, M.G.; Ciccosanti, F.; Petrone, L.; Sacchi, A.; Nardacci, R.; Falasca, L.; Del Nonno, F.; et al. Transglutaminase 2 Regulates Innate Immunity by Modulating the STING/TBK1/IRF3 Axis. J. Immunol. 2021, 206, 2420–2429. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaltron, E.; Vianello, F.; Ruzza, A.; Palazzo, A.; Brillo, V.; Celotti, I.; Scavezzon, M.; Rossin, F.; Leanza, L.; Severin, F. The Role of Transglutaminase 2 in Cancer: An Update. Int. J. Mol. Sci. 2024, 25, 2797. https://doi.org/10.3390/ijms25052797
Zaltron E, Vianello F, Ruzza A, Palazzo A, Brillo V, Celotti I, Scavezzon M, Rossin F, Leanza L, Severin F. The Role of Transglutaminase 2 in Cancer: An Update. International Journal of Molecular Sciences. 2024; 25(5):2797. https://doi.org/10.3390/ijms25052797
Chicago/Turabian StyleZaltron, Elisabetta, Federica Vianello, Alessia Ruzza, Alberta Palazzo, Valentina Brillo, Ilaria Celotti, Matteo Scavezzon, Federica Rossin, Luigi Leanza, and Filippo Severin. 2024. "The Role of Transglutaminase 2 in Cancer: An Update" International Journal of Molecular Sciences 25, no. 5: 2797. https://doi.org/10.3390/ijms25052797
APA StyleZaltron, E., Vianello, F., Ruzza, A., Palazzo, A., Brillo, V., Celotti, I., Scavezzon, M., Rossin, F., Leanza, L., & Severin, F. (2024). The Role of Transglutaminase 2 in Cancer: An Update. International Journal of Molecular Sciences, 25(5), 2797. https://doi.org/10.3390/ijms25052797







