Abstract
Long-term oral ingestion of unheated yuzu seed oil in humans reduces lipid peroxides in the blood. Moreover, yuzu seed oil contains limonin, which can induce antioxidant and anti-inflammatory effects by activating the transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2). Previously, Nrf2 has been shown to reduce atopic dermatitis (AD). Therefore, we hypothesized that ingesting unheated yuzu seed oil can regulate AD through Nrf2. An AD model was established using NC/Nga mice through repeated local exposure to mite antigens. Unheated and purified yuzu seed oil (100 µL/mice) or water (control, 100 µL/mice) was administered orally once a day using a gastric cannula for rodents for 28 days. On day 28, mice in the unheated yuzu seed oil group exhibited significantly lower clinical skin severity scores and ear thickness than those in the purified yuzu seed oil and water groups. Serum histamine levels remained unaltered among the three AD-induced groups. Serum Dermatophagoides farina body (Dfb)-specific immunoglobulin E (IgE) levels were significantly lower in the unheated yuzu seed oil group. Oral ingestion of yuzu seed oil in NC/Nga AD model mice significantly suppressed dermatitis deterioration and decreased serum IgE levels. Clinical trials (n = 41) have already confirmed that unheated yuzu oil is safe for long-term intake, further suggesting its potential use in improving AD symptoms.
1. Introduction
Yuzu (Citrus junos Sieb. ex Tanaka) is a typical Japanese citrus fruit with a desirable aroma. Yuzu juice and peel, which are originally from China and arrived in Japan via Korea 1000 years ago, are characterized by a sour taste and refreshing aroma and are used as raw materials for vinegar and seasonings in Japan. Although yuzu seeds constitute up to 9% of the fruit weight, they are typically discarded after juice extraction because of their perceived lack of utility. However, recognizing the richness of active ingredients in the seeds, we extracted unheated yuzu seed oil from yuzu seeds using the cold-press method, subsequently exploring its effect. Component analysis of citrus seed oil showed that it contains a large amount of limonoids (e.g., limonin, nomilin, and obacunone) [1,2,3]. Therefore, research is being conducted on the effects of limonoids contained in yuzu seed oil with respect to their anti-oxidant activity [4,5,6].
We have previously demonstrated that long-term oral intake of unheated yuzu seed oil in humans effectively reduced lipid peroxides in the blood [7], showcasing its antioxidant effect. The transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2), which plays an important role in mitigating lipid peroxidation, is a key regulator of cellular antioxidant responses and controls the expression of genes that counteract oxidative and electrophilic stresses [8,9]. In the nucleus, Nrf2 binds to antioxidant response element (ARE) sequences along with the small musculoaponeurotic fibrosarcoma oncogene homologue (sMaf) to regulate the expression of more than 200 antioxidant and anti-inflammatory genes [10,11,12]. Therefore, it has been suggested that unheated yuzu seed oil contains certain components that activate Nrf2 and suppress reactive oxygen species (ROS) production.
Atopic dermatitis (AD) is a chronic inflammatory skin disease disorder characterized by eczema and repeatedly exhibits exacerbation and remission of recurrent eczematous lesions [13]. AD pathology comprises three factors: (1) skin barrier dysfunction, (2) immune dysregulation (type-2 helper T (Th2) dominance or interleukin (IL)-4/IL-13 axis inflammation), and (3) intense itching, which occur independently or mutually [14,15,16]. IL-4 and IL-13 activate dual oxidase protein 1 (DUOX1) and generate ROS [17]. Oxidative stress is one of the most important pathogenic factors of AD [18]. Patients with AD are more susceptible to damage caused by ROS or oxidizers than controls, which is evident from an increase in malondialdehyde and a decrease in enzymatic and non-enzymatic antioxidants [19]. Oxidative stress and changes in antioxidant defenses are involved in the pathophysiology of acute exacerbations of AD [20]. These results suggest that antioxidant supplements may be beneficial in treating AD [18].
Studies using AD mouse models and analyses of human AD samples have revealed that Nrf2 improves AD inflammation [21,22,23,24,25,26]. Activating Nrf2 by oral intake of a natural or chemical substance improved inflammatory signaling in human keratinocytes in vitro as well as sensitizer-induced skin inflammation in a mouse model of human AD.
In this study, we evaluated the effects of consuming yuzu seed oil in NC/Nga mice, an established animal model for human AD.
2. Results
2.1. Effects of Yuzu Seed Oil on Inflammation Score and Ear Thickness
The non-sensitized group, in which the mite antigen was not applied, showed no development of human AD-like skin lesions, resulting in a clinical skin severity score of zero.
Conversely, in the three AD-induced groups, topical application of the antigen immediately induced clinical symptoms, such as erythema, edema, and hemorrhage on the ears and back. This was followed by superficial erosion, deep excoriation, scarring, and skin dryness, all of which were markedly reduced in both unheated and purified yuzu seed oil groups (Figure 1). On day 28, the clinical skin severity score of the water, unheated, and purified yuzu oil groups were 4.0 ± 0.71 (n = 5), 0.4 ± 0.89 (n = 5; p < 0.001 vs. water group), and 2.0 ± 1.00 (n = 5; p = 0.0064 vs. water group) points, respectively. A significant difference was observed between the unheated and purified yuzu seed oil group (p = 0.028; Figure 2).
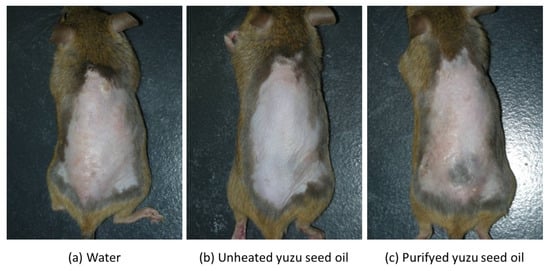
Figure 1.
Macroscopic features of Dermatophagoides farina body (Dfb)-induced human AD-like skin lesions on the dorsal skin of NC/Nga mice on day 28 following the initiation of sensitization. After completely shaving the dorsal hair and depilation using a hair removal cream, the mice backs were sensitized by applying mite antigen. “Water” is the negative control group. (a) The water group (n = 5) received 100 µL/mice of water via an oral gastric cannula for rodents once a day for 28 days after sensitization. (b,c) The unheated and purified yuzu seed oil groups (n = 5 each) received 100 µL/mice of unheated and purified yuzu seed oil, respectively, in the same manner.
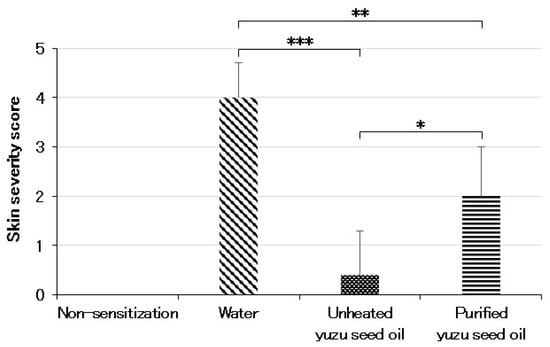
Figure 2.
Skin severity score evaluated on day 28 after initiating sensitization by an AD-scoring method. The degree of each symptom (erythema, edema/thickness, hemorrhage/excoriation, and dryness) was scored as 0 (absent), 1 (mild), 2 (moderate), or 3 (severe). The sum of the individual scores was taken as the dermatitis score. In the case of no skin lesion, the score was 0, and the maximum score attainable was 12. Every group represents an average of the scores of five mice. Data are expressed as the means ± SD. The unpaired Student’s t-test was used to analyze differences between the two groups. *** p < 0.001, ** p < 0.01, and * p < 0.05. The skin sensitivity score of the non-sensitized group is that of normal mice.
The ear thicknesses in the different groups were as follows: 0.27 ± 0.019, 0.41 ± 0.073, 0.28 ± 0.017 (p < 0.001 vs. water group), and 0.37 ± 0.057 mm (each n = 5), for non-sensitized, water, and unheated and purified yuzu seed oil groups, respectively. A significant difference was also observed between the unheated and purified yuzu seed oil groups (p < 0.001). In contrast, no differences were observed between the purified yuzu seed oil and water groups (Figure 3).
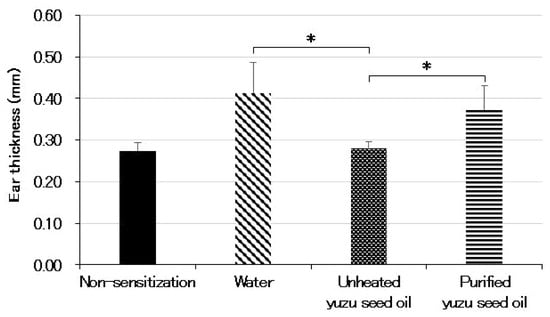
Figure 3.
The thickness of the left and right ears were measured five times each with a micrometer equipped with a constant pressure device on day 28 after the initiation of sensitization. The average thickness of both ears was calculated as ear swelling. Every group represents an average of five mice. Data are expressed as the means ± SD. The unpaired Student’s t-test was used to analyze differences between the two groups. * p < 0.001. The ear thickness of the non-sensitized group is that of normal mouse ears. “Water” is the negative control group. The thickness of the ear indicates the intensity of dermatitis.
2.2. Yuzu Seed Oil Did Not Inhibit an Increase in Serum Histamine Levels
The serum histamine level (in nM) of the non-sensitization group was 0.02 ± 0.007 (n = 5). Conversely, the histamine level of the water group, which was sensitized with the antigen under specific pathogen-free (SPF) conditions, increased to 0.97 ± 0.360 (p < 0.001). Among the three sensitized groups, the unheated yuzu seed oil group showed lower values (0.66 ± 0.212), but no significant differences were observed among the three groups (Figure 4).
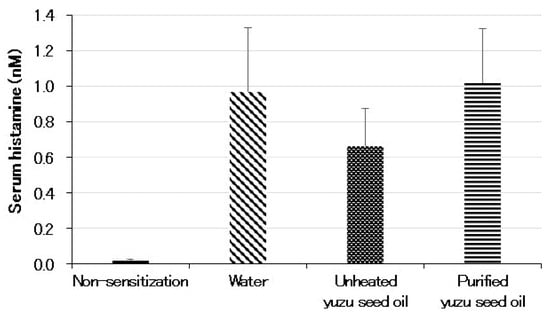
Figure 4.
Serum levels of histamine measured using ELISA on day 28 following the initiation of sensitization. Every group represents an average of the data of five mice. Data are expressed as the means ± SD. Histamine levels in the non-sensitized group correspond to those of normal mice.
2.3. Inhibitory Effect of Yuzu Seed Oil on Serum Dfb-Specific IgE Levels
The serum Dermatophagoides farina body (Dfb)-specific IgE levels demonstrated a significant correlation with dermatitis progression. The oral administration of yuzu seed oil to NC/Nga mice reduced the elevation of serum Dfb-specific IgE levels. The level of IgE was represented by the absorbance at A450. The serum Dfb-specific IgE level of the water group was 1.05 ± 0.442 (n = 5), which was significantly higher than that of the non-sensitized group, 0.14 ± 0.035 (n = 5; p < 0.001). Furthermore, the serum Dfb-specific IgE levels of the unheated and purified yuzu seed oil groups were 0.34 ± 0.136 (n = 5; p = 0.0018 vs. water group) and 0.51 ± 0.162 (n = 5; p = 0.011 vs. water group), respectively. Moreover, unheated yuzu seed oil was significantly more effective than purified yuzu seed oil (p = 0.048; Figure 5).
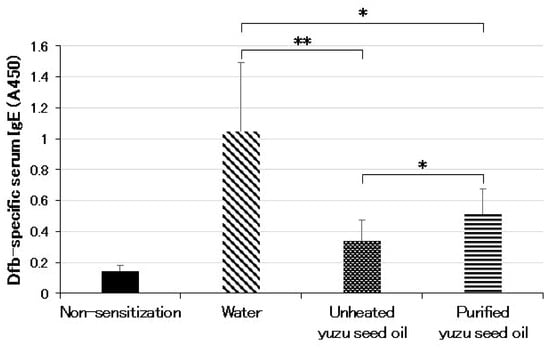
Figure 5.
Serum levels of Dfb-specific IgE measured using ELISA on day 28 post the initiation of sensitization. The level of IgE is shown as absorbance at A450. Every group represents an average of the serum Dfb-specific IgE level of five mice. Data are expressed as the means ± SD. The unpaired Student’s t-test was used to analyze differences between the two groups. ** p < 0.01 and * p < 0.05. Serum levels of the Dfb-specific IgE in the non-sensitized group correspond to those of normal mice.
3. Discussion
Limonin, a limonoid belonging to the triterpenoid group, is a bitter component of citrus seeds and fruit tissues. The unheated yuzu seed oil used in this study contained limonin, which has been shown to regulate the expression of various cytoprotective enzymes such as heme oxygenase-1 (HO-1) and NAD(P)H quinone oxidoreductase 1 (NQO1) by activating the Nrf2/ARE pathway [27,28,29]. Moreover, inducing the Nrf2/HO-1 signaling pathway inhibits inflammatory AD in skin keratinocytes [30]. Therefore, we hypothesized that limonin-containing unheated yuzu seed oil plays a regulatory role in AD via the Nrf2/ARE pathway.
In the present study, the clinical skin severity score and ear thickness of the water group (negative control), which was sensitized with the antigen under SPF conditions, increased from sensitization onset. In contrast, oral ingestion of unheated yuzu seed oil significantly suppressed the visual appearance of dermatitis and increased the clinical skin severity score, ear thickness, and serum Dfb-specific IgE levels compared with the water and purified yuzu seed oil groups. Yamamoto et al. [31] have investigated the role of scratching behavior in a Dfb-induced dermatitis model in NC/Nga mice. They revealed that the severity of dermatitis correlated with increasing scratching behavior. Itch is an unpleasant sensation that may prompt desire to scratch the affected area [32]. Therefore, an increase in scratching behavior is accompanied by intense itching. Therefore, the results of this study indicate that oral intake of unheated yuzu seed oil reduces itching, which is one of the etiological factors of AD.
Regarding itch, there are at least two types of afferents, histamine-dependent and histamine-independent types, which indicates that the effect of antihistamines in treating AD is limited [33,34]. In this study, the unheated yuzu seed oil group had good skin scores and auricle thickness; however, no significant difference was observed in blood histamine levels compared with the control and purified yuzu seed oil groups. These findings suggest that unheated yuzu seed oil suppresses non-histaminergic pathways in atopic itching.
Th2 cells release IL-4 and IL-13, leading to IgE class switching in B cells and production of antigen-specific IgE via the signal transducer and activator of transcription (STAT) pathway [13,35]. In this study, serum IgE production was suppressed in the unheated yuzu seed oil group compared to that in the control and purified yuzu seed oil groups. Th2 cytokine production is negatively regulated by Nrf2 [36], suggesting that limonin in unheated yuzu seed oil activates Nrf2, thereby suppressing IL-4 and IL-13. Moreover, IL-4 and IL-13 have been implicated in chronic itch [37]. Histamine, which has been reported to cause itching, did not differ among the three groups in this study. Limonin-activated Nrf2 suppressed IL-4 and IL-13 production, potentially suppressing itching.
Moreover, dorsal skin symptoms that could not be scratched were significantly alleviated in the unheated yuzu seed oil group. ROS play an important role in AD development. Oxidative stress may be an intrinsic mediator of amplification and chronicity in AD [38]. Limonin activates Nrf2 and exerts antioxidant effects. Qin et al. [39] have shown that limonin extracted from pomelo seeds dose-dependently enhanced the transcription of Nrf2 and its downstream genes HO-1 and NQO1 in HepG2 cells, and also stimulated the expression of Nrf2, HO-1, and NQO1. Based on the above results, we conclude that limonin exerts its antioxidant properties by activating the Nrf2/ARE pathway. Moreover, Li et al. [40] have established a model of NAFLD in zebrafish larvae and shown that limonin treatment induced antioxidant effects by upregulating the Nrf2/HO-1 signaling pathway in the liver, showing a protective effect against NAFLD. We have previously demonstrated that long-term oral intake of unheated yuzu seed oil in humans reduces lipid peroxides in the blood [7]. Therefore, it was further confirmed that unheated yuzu seed oil has an antioxidant effect. This antioxidant effect was attributed to Nrf2 activation by the limonin contained in unheated yuzu seed oil, and this antioxidant effect improved the skin lesions on the back.
Activation of the Nrf2-ARE pathway has been reported to improve the symptoms of dermatitis in an AD model. Choi et al. [21] have shown that saponins derived from Platycodon grandiflorus root attenuated AD-like skin lesions by activating Nrf1/ARE-mediated OH-1 in a 2,4-dinitrochlorobenzene (DNCB)-induced AD mouse model. Park et al. [23] have reported that 6-Shogalo, an active compound in ginger, alleviates DNCB-induced AD-like skin lesions and scratching behavior by inhibiting immune mediators, including IL-4 and IL-13, via regulation of the ROS/mitogen-activated protein kinase (MAPKs)/Nrf2 signaling pathway. Karuppagounder et al. [22] have demonstrated that quercetin treatment in NC/Nga mice attenuated D. farinae extract-induced AD-like clinical symptoms via the induction of Nrf2 signaling pathways. As a result, quercetin inhibited IL-4, an inflammatory cytokine. Choi et al. [26] have reported that oral administration of miquelianin, which activates Nrf2 ex vivo, suppresses ear thickening and inflammatory cell infiltration, reduces serum IgE production in trimellitic anhydride-induced AD model mice, and alleviates IL-13 production in lymph nodes. As described above, Nrf2 stimulation suppresses type 2 immunity or IL-4/IL-13 axis inflammation, a significant etiological factor in AD.
Although the purified yuzu seed oil used in this study contained limonin below the detection limit, the skin severity scores and Dfb-specific serum IgE levels were significantly lower than those of the control (water group). This suggests that yuzu seed oil may contain active ingredients other than limonin that are not lost during volatile purification. However, additional studies are required to confirm their presence.
4. Materials and Methods
4.1. Oils Used for Intervention
The yuzu seed oil used in this study was extracted from yuzu seeds using a type AP 10 Reinartz screw press (Maschinenfabrik Reinartz, Neuss, Germany), with the operating frequency set to 62 Hz for the cold-pressing of oil seeds. The extract was filtered and used as unheated yuzu seed oil. Unheated yuzu seed oil was purified by using vaporization to generate purified yuzu seed oil. Both oils were bottled without any additives and stored in unlabeled 200 mL brown glass bottles at +5 °C until use.
The unheated yuzu seed oil exhibited a fatty acid composition of 36.8% oleic acid, 35.6% linoleic acid, and 20.1% palmitic acid. The purified yuzu seed oil contained 37.0% oleic acid, 35.4% linoleic acid, and 19.9% palmitic acid (measured by using gas chromatography, Japan Food Research Laboratories, Tokyo, Japan). Furthermore, an X-LC system (JASCO Corporation, Tokyo, Japan) at the Kochi Prefectural Industrial Technology Center was used to reveal that 100 mL of unheated yuzu seed oil contained 49.4 mg or more of nomilin, 10.4 mg or more of limonin, and 1.88 mg or more of obakunon. However, the purified yuzu seed oil had nomilin, limonin, and obakunon contents below the detection limit.
4.2. Animals and Ethics
For the experiments, 10-week-old NC/Nga mice were used as the mouse model of AD [41]. NC/Nga mice developed human AD-like skin lesions with elevated serum IgE levels when bred under conventional conditions [42]. They were purchased from Charles River Laboratories Japan (Yokohama, Japan).
The animals were maintained in HEPA-filtered air at our facility. All animals were healthy; five mice were housed per cage in an air-conditioned animal room at 23 ± 2 °C, a relative humidity of 50 ± 5%, in light and dark cycles of 12 h each, and fed with a laboratory diet and water ad libitum.
All animal experiments were approved by the Animal Study Committee of Kochi Medical School according to the government guidelines for animal care (protocol code: E-00010).
4.3. Sensitization and Development of AD Mouse Model
In vivo experiments on AD-like skin lesions were induced by repeated topical application of Dfb extract [31] (Biostir AD, Biostir Inc. 20, Kobe, Japan). After complete shaving of the dorsal hair and depilation using a hair removal cream, the backs and ears of the mice were sensitized by applying 100 mg Biostir AD (Biostir Inc. 20), except for the non-sensitized group, three times a week for 2 weeks following the first challenge (Figure 6).
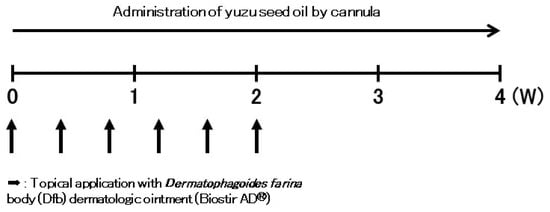
Figure 6.
Effects of yuzu seed oil on Dfb-induced AD-like skin lesions in NC/Nga mice. This figure illustrates the experimental schedule of the Dfb-induced human AD-like mouse model. Biostir AD (100 mg) was repeatedly applied to the back and ears three times a week for 2 weeks following the first challenge.
4.4. Oral Administration Method of Yuzu Seed Oil
We divided NC/Nga mice into four groups: a normal healthy group (non-sensitized group, n = 5) and three AD-induced groups followed by orally administering water, yuzu juice, or yuzu seed oil (100 µL/mice by an oral gastric cannula for rodents once a day for 28 days); a negative control (water group, n = 5), unheated yuzu seed oil (n = 5), and purified yuzu seed oil groups (n = 5).
4.5. Clinical Estimation of Skin Lesions in NC/Nga Mice
The backs and ears of the NC/Nga mice were clinically observed for 4 weeks. The clinical severity of dermatitis was recorded using macroscopic diagnostic criteria. Briefly, dermatitis severity was evaluated on day 28 by assessing four specific criteria: the severity of dermatitis was assessed macroscopically by an AD-scoring method [41], in which the degree of each symptom (erythema, edema/thickness, hemorrhage/excoriation, and dryness) was scored as 0 (absent), 1 (mild), 2 (moderate), or 3 (severe). The sum of individual scores was used as the dermatitis score.
4.6. Measurement of Ear Thickness
On day 28, the thicknesses of the left and right ears were measured five times using a micrometer equipped with a constant-pressure device (MDQ-30, Mitsutoyo Corporation, Kanagawa, Japan) under light ether anesthesia. The average thickness of both ears was calculated and considered as ear swelling.
4.7. Measurement of Histamine in the Serum
Serum histamine levels were measured using ELISA (Histamine enzyme immunosorbent kit). The kit was purchased from BIO connect (Montigny Le Bretonneux, France).
4.8. Measurement of Serum Dfb-Specific IgE Levels
The serum levels of Dfb-specific immunoglobulin E (IgE) were examined on day 28. For the Dfb-specific IgE antibodies, a 96-well plate was coated with 50 μL/well antigen solution containing 2.5 μg/mL Biostir AD (Biostir Inc. 20) in 50 mM carbonate–bicarbonate buffer (pH 9.6) overnight at 4 °C. The chemical reagent of the mouse IgE ELISA kit (Mouse IgE ELISA Quantitation Set: Set Bethyl Laboratories, Inc., Montgomery, TX, USA) was used for the enzyme reaction, followed by a labeled antibody reaction according to the manufacturer’s instructions.
4.9. Statistical Analysis
Data were analyzed using Microsoft Excel 2019 (MS Excel 2019: Microsoft Corporation, WA, USA). The data were expressed as means ± SD. The unpaired Student’s t-test was used to analyze differences between two groups. Values of p < 0.05 were considered statistically significant.
5. Conclusions
Oral ingestion of unheated yuzu seed oil in NC/Nga AD model mice led to the striking suppression of dermatitis development and elevated serum IgE levels. We suggest that the component involved is limonin, which is present in unheated yuzu seed oil and is below the detection limit in purified yuzu seed oil. Continuous intake of unheated yuzu seed oil, a plant-derived natural product, in healthy adults has been determined as safe after long-term administration [7], underscoring its potential as a viable candidate for treating AD.
Author Contributions
Conceptualization, K.A.; methodology, K.A.; software, Y.W. and M.M.; validation, Y.W. and M.M.; formal analysis, K.A. and S.M.; investigation, K.A.; data curation, Y.W. and M.M.; writing—original draft preparation, K.A.; writing—review and editing, K.A., Y.W. and S.M.; supervision, S.M.; project administration, S.M.; funding acquisition, M.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Umajimura Agricultural Cooperative (funding number: 7002-72).
Institutional Review Board Statement
The animal study protocol was approved by the Animal Study Committee of Kochi Medical School (protocol code: E-00010 and date of approval: 22 April 2011).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hashinaga, F.; Herman, Z.; Hasegawa, S. Limonoids in seeds of yuzu (Citrus junos Sieb. ex Tanaka). Nippon Shokuhin Kogyo Gakkaishi 1990, 37, 380–382. [Google Scholar] [CrossRef][Green Version]
- Minamisawa, M.; Yoshida, S.; Uzawa, A. The functional evaluation of waste yuzu (Citrus junos) seeds. Food Funct. 2014, 5, 330–336. [Google Scholar] [CrossRef]
- Suzuki, S.; Yoshikane, Y.; Nakashima, E.; Kinoshita-Kitagawa, A.; Toutani, M.; Sawamura, M. Determination of flavonoids and limonoids in yuzu seed oils and various carrier oils. Jpn. J. Aromather. 2019, 20, 13–21. [Google Scholar]
- Kim, S.Y.; Shin, K.S. Evaluation of physiological activities of the citron (Citrus junos Sieb. ex Tanaka) Seed Extracts. Prev. Nutr. Food Sci. 2013, 18, 196–202. [Google Scholar] [CrossRef]
- Minamisawa, M.; Suzumura, T.; Bose, S.; Taniai, T.; Kawai, G.; Suzuki, K.; Yamaguchi, A.; Yamanaka, S. Effect of Yuzu (Citrus junos) Seed Limonoids and spermine on intestinal microbiota and Hypothalamic Tissue in the Sandhoff disease Mouse Model. Med. Sci. 2021, 9, 17. [Google Scholar] [CrossRef]
- Choi, M.H.; Yang, S.H.; Kim, N.D.; Shin, H.J. Nomilin from Yuzu Seed Has in vitro antioxidant activity and downregulates melanogenesis in B16F10 melanoma cells through the PKA/CREB signaling pathway. Antioxidants 2022, 11, 1636. [Google Scholar] [CrossRef]
- Asano, K.; Miyamoto, M.; Watanabe, Y.; Kawamura, K.; Kitazoe, N.; Matsuura, A.; Kamada, M.; Sumitani, Y.; Iyoki, M.; Watanabe, N.; et al. Effect of unheated Yuzu Seed oil intake on oxidative stress in humans-A randomized, dose-controlled, parallel-group comparative trial. Jpn. Pharmacol. Ther. 2022, 50, 347–357. [Google Scholar]
- Dodson, M.; Castro-Portuguez, R.; Zhang, D.D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019, 23, 101107. [Google Scholar] [CrossRef]
- Ishii, T.; Itoh, K.; Takahashi, S.; Sato, H.; Yanagawa, T.; Katoh, Y.; Bannai, S.; Yamamoto, M. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J. Biol. Chem. 2000, 275, 16023–16029. [Google Scholar] [CrossRef]
- Itoh, K.; Chiba, T.; Takahashi, S.; Ishii, T.; Igarashi, K.; Katoh, Y.; Oyake, T.; Hayashi, N.; Satoh, K.; Hatayama, I.; et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997, 236, 313–322. [Google Scholar] [CrossRef]
- Cuadrado, A. Structural and functional characterization of Nrf2 degradation by glycogen synthase kinase 3/β-TrCP. Free Radic. Biol. Med. 2015, 88, 147–157. [Google Scholar] [CrossRef]
- Shilovsky, G.A.; Putyatina, T.S.; Morgunova, G.V.; Seliverstov, A.V.; Ashapkin, V.V.; Sorokina, E.V.; Markov, A.V.; Skulachev, V.P. A crosstalk between the biorhythms and gatekeepers of longevity: Dual role of glycogen synthase Kinase-3. Biochemistry 2021, 86, 433–448. [Google Scholar] [CrossRef]
- Langan, S.M.; Irvine, A.D.; Weidinger, S. Atopic dermatitis. Lancet 2020, 396, 345–360. [Google Scholar] [CrossRef]
- Howard, M.; Farrar, J.; Hilfiker, M.; Johnson, B.; Takatsu, K.; Hamaoka, T.; Paul, W.E. Identification of a T cell-derived B cell growth factor distinct from interleukin 2. J. Exp. Med. 1982, 155, 914–923. [Google Scholar] [CrossRef]
- Paul, W.E.; Ohara, J. B-cell stimulatory factor-1/interleukin 4. Annu. Rev. Immunol. 1987, 5, 429–459. [Google Scholar] [CrossRef]
- Schmitz, J.; Owyang, A.; Oldham, E.; Song, Y.; Murphy, E.; McClanahan, T.K.; Zurawski, G.; Moshrefi, M.; Qin, J.; Li, X.; et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 2005, 23, 479–490. [Google Scholar] [CrossRef]
- Hirakawa, S.; Saito, R.; Ohara, H.; Okuyama, R.; Aiba, S. Dual oxidase 1 induced by Th2 cytokines promotes STAT6 phosphorylation via oxidative inactivation of protein tyrosine phosphatase 1B in human epidermal keratinocytes. J. Immunol. 2011, 186, 4762–4770. [Google Scholar] [CrossRef]
- Ji, H.; Li, X.K. Oxidative stress in atopic dermatitis. Oxid. Med. Cell. Longev. 2016, 2016, 2721469. [Google Scholar] [CrossRef]
- Sivaranjani, N.; Rao, S.V.; Rajeev, G. Role of reactive oxygen species and antioxidants in atopic dermatitis. J. Clin. Diagn. Res. 2013, 7, 2683–2685. [Google Scholar] [CrossRef]
- Tsukahara, H.; Shibata, R.; Ohshima, Y.; Todoroki, Y.; Sato, S.; Ohta, N.; Hiraoka, M.; Yoshida, A.; Nishima, S.; Mayumi, M. Oxidative stress and altered antioxidant defenses in children with acute exacerbation of atopic dermatitis. Life Sci. 2003, 72, 2509–2516. [Google Scholar] [CrossRef]
- Choi, J.H.; Jin, S.W.; Han, E.H.; Park, B.H.; Kim, H.G.; Khanal, T.; Hwang, Y.P.; Do, M.T.; Lee, H.S.; Chung, Y.C.; et al. Platycodon grandiflorum root-derived saponins attenuate atopic dermatitis-like skin lesions via suppression of NF-κB and STAT1 and activation of Nrf2/ARE-mediated heme oxygenase-1. Phytomedicine 2014, 21, 1053–1061. [Google Scholar] [CrossRef]
- Karuppagounder, V.; Arumugam, S.; Thandavarayan, R.A.; Pitchaimani, V.; Sreedhar, R.; Afrin, R.; Harima, M.; Suzuki, H.; Nomoto, M.; Miyashita, S.; et al. Modulation of HMGB1 translocation and RAGE/NFκB cascade by quercetin treatment mitigates atopic dermatitis in NC/Nga transgenic mice. Exp. Dermatol. 2015, 24, 418–423. [Google Scholar] [CrossRef]
- Park, G.; Oh, D.S.; Lee, M.G.; Lee, C.E.; Kim, Y.U. 6-Shogaol, an active compound of ginger, alleviates allergic dermatitis-like skin lesions via cytokine inhibition by activating the Nrf2 pathway. Toxicol. Appl. Pharmacol. 2016, 310, 51–59. [Google Scholar] [CrossRef]
- Lee, J.H.; Jo, E.H.; Lee, B.; Noh, H.M.; Park, S.; Lee, Y.M.; Kim, D.K.; Park, M.C. Soshiho-Tang, a traditional herbal medicine, alleviates atopic dermatitis symptoms via regulation of inflammatory mediators. Front. Pharmacol. 2019, 10, 742. [Google Scholar] [CrossRef]
- Lee, J.H.; Lim, J.Y.; Jo, E.H.; Noh, H.M.; Park, S.; Park, M.C.; Kim, D.K. Chijabyukpi-tang inhibits pro-inflammatory cytokines and chemokines via the Nrf2/HO-1 signaling pathway in TNF-α/IFN-γ-stimulated HaCaT cells and ameliorates 2,4-dinitrochlorobenzene-induced atopic dermatitis-like skin lesions in mice. Front. Pharmacol. 2020, 11, 1018. [Google Scholar] [CrossRef]
- Choi, D.W.; Jung, S.Y.; Kim, G.D.; Lee, S.Y.; Shin, H.S. Miquelianin inhibits allergic responses in mice by suppressing CD4+ T cell proliferation. Antioxidants 2021, 10, 1120. [Google Scholar] [CrossRef]
- Jin, J.; Lv, X.; Wang, B.; Ren, C.; Jiang, J.; Chen, H.; Chen, X.; Gu, M.; Pan, Z.; Tian, N.; et al. Limonin inhibits IL-1β-induced inflammation and catabolism in chondrocytes and ameliorates osteoarthritis by activating Nrf2. Oxid. Med. Cell. Longev. 2021, 2021, 7292512. [Google Scholar] [CrossRef]
- Yang, R.; Song, C.; Chen, J.; Zhou, L.; Jiang, X.; Cao, X.; Sun, Y.; Zhang, Q. Limonin ameliorates acetaminophen-induced hepatotoxicity by activating Nrf2 antioxidative pathway and inhibiting NF-κB inflammatory response via upregulating Sirt1. Phytomedicine 2020, 69, 153211. [Google Scholar] [CrossRef]
- Jia, B.; Zhao, L.; Liu, P.; Li, M.; Tian, Z. Limonin ameliorates indomethacin-induced intestinal damage and ulcers through Nrf2/ARE pathway. Immun. Inflamm. Dis. 2023, 11, e787. [Google Scholar] [CrossRef]
- Shim, K.S.; Park, M.; Yang, W.K.; Lee, H.; Kim, S.H.; Choo, B.K.; Chae, S.; Kim, H.K.; Kim, T.; Kimm, K.M. Veronica persica ethanol extract ameliorates dinitrochlorobenzene-induced atopic dermatitis-like skin inflammation in mice, likely by inducing Nrf2/HO-1 signaling. Antioxidants 2023, 12, 1267. [Google Scholar] [CrossRef]
- Yamamoto, M.; Haruna, T.; Yasui, K.; Takahashi, H.; Iduhara, M.; Takaki, S.; Deguchi, M.; Arimura, A. A novel atopic dermatitis model induced by topical application with Dermatophagoides farinae extract in NC/Nga mice. Allergol. Int. 2007, 56, 139–148. [Google Scholar] [CrossRef]
- Andersen, H.H.; Elberling, J.; Arendt-Nielsen, L. Human surrogate models of histaminergic and non-histaminergic itch. Acta Derm. Venereol. 2015, 95, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Ikoma, A.; Steinhoff, M.; Ständer, S.; Yosipovitch, G.; Schmelz, M. The neurobiology of itch. Nat. Rev. Neurosci. 2006, 7, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, M.; Takamori, K.; Takamori, K. Itch and nerve fibers with special reference to atopic dermatitis: Therapeutic implications. J. Dermatol. 2014, 41, 205–212. [Google Scholar] [CrossRef]
- Gandhi, N.A.; Bennett, B.L.; Graham, N.M.; Pirozzi, G.; Stahl, N.; Yancopoulos, G.D. Targeting key proximal drivers of type 2 inflammation in disease. Nat. Rev. Drug Discov. 2016, 15, 35–50. [Google Scholar] [CrossRef]
- Yoo, O.K.; Choi, W.J.; Keum, Y.S. Cardamonin inhibits oxazolone-induced atopic dermatitis by the induction of NRF2 and the inhibition of Th2 cytokine production. Antioxidants 2020, 9, 834. [Google Scholar] [CrossRef]
- Oetjen, L.K.; Mack, M.R.; Feng, J.; Whelan, T.M.; Niu, H.; Guo, C.J.; Chen, S.; Trier, A.M.; Xu, A.Z.; Tripathi, S.V.; et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell 2017, 171, 217–228.e13. [Google Scholar] [CrossRef]
- Borgia, F.; Li Pomi, F.; Vaccaro, M.; Alessandrello, C.; Papa, V.; Gangemi, S. Oxidative stress and phototherapy in atopic dermatitis: Mechanisms, role, and future perspectives. Biomolecules 2022, 12, 1904. [Google Scholar] [CrossRef]
- Qin, S.; Lv, C.; Wang, Q.; Zheng, Z.; Sun, X.; Tang, M.; Deng, F. Extraction, identification, and antioxidant property evaluation of limonin from pummelo seeds. Anim. Nutr. 2018, 4, 281–287. [Google Scholar] [CrossRef]
- Li, Y.; Yang, M.; Lin, H.; Yan, W.; Deng, G.; Ye, H.; Shi, H.; Wu, C.; Ma, G.; Xu, S.; et al. Limonin alleviates non-alcoholic fatty liver disease by reducing lipid accumulation, suppressing inflammation and oxidative stress. Front. Pharmacol. 2021, 12, 801730. [Google Scholar] [CrossRef] [PubMed]
- Suto, H.; Matsuda, H.; Mitsuishi, K.; Hira, K.; Uchida, T.; Unno, T.; Ogawa, H.; Ra, C. NC/Nga mice: A mouse model for atopic dermatitis. Int. Arch. Allergy Immunol. 1999, 120 (Suppl. 1), 70–75. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Watanabe, N.; Geba, G.P.; Sperl, J.; Tsudzuki, M.; Hiroi, J.; Matsumoto, M.; Ushio, H.; Saito, S.; Askenase, P.W.; et al. Development of atopic dermatitis-like skin lesion with IgE hyperproduction in NC/Nga mice. Int. Immunol. 1997, 9, 461–466. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).