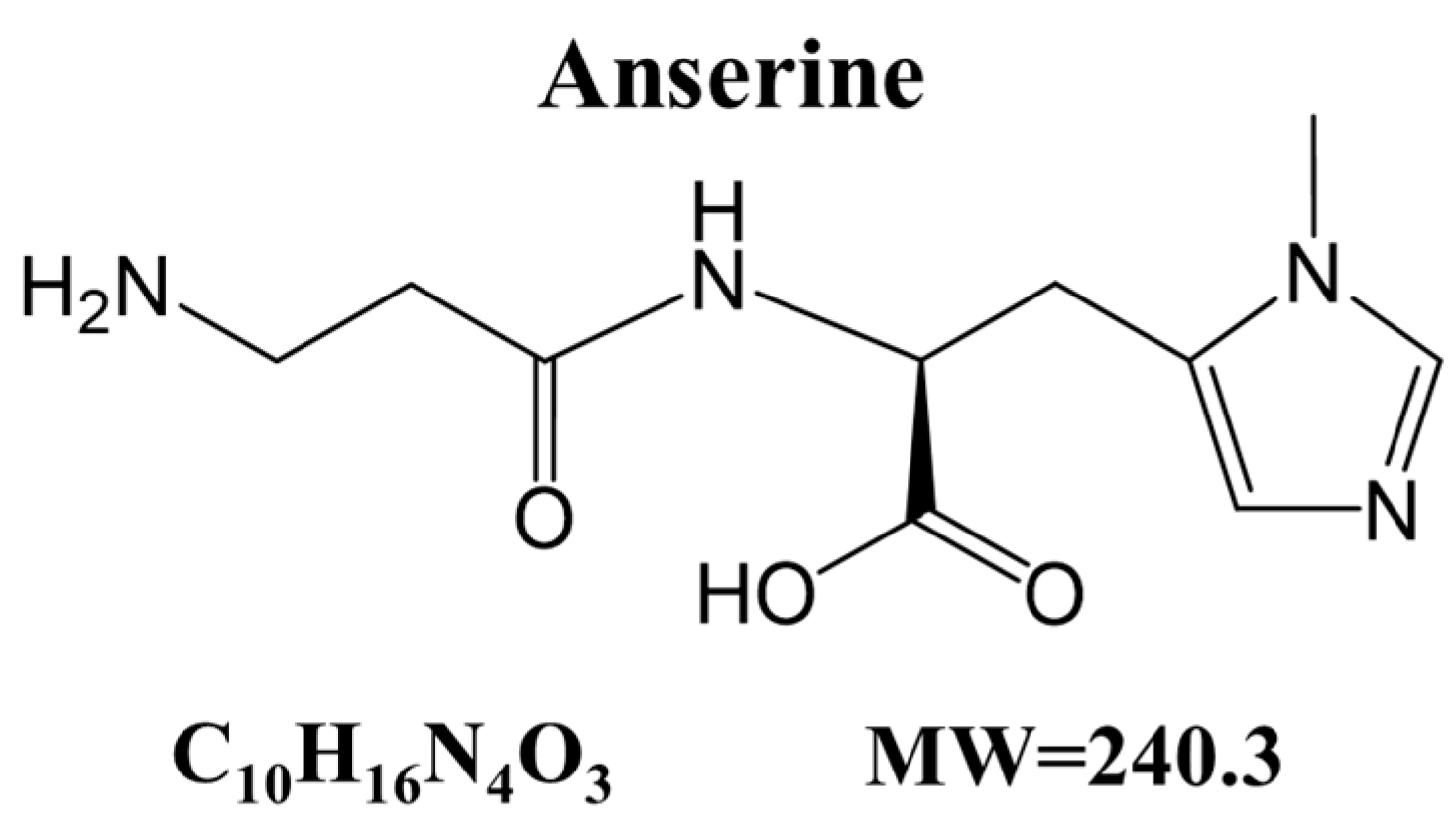
Anserine, a Histidine-Containing Dipeptide, Suppresses Pressure Overload-Induced Systolic Dysfunction by Inhibiting Histone Acetyltransferase Activity of p300 in Mice
Abstract
1. Introduction
2. Results
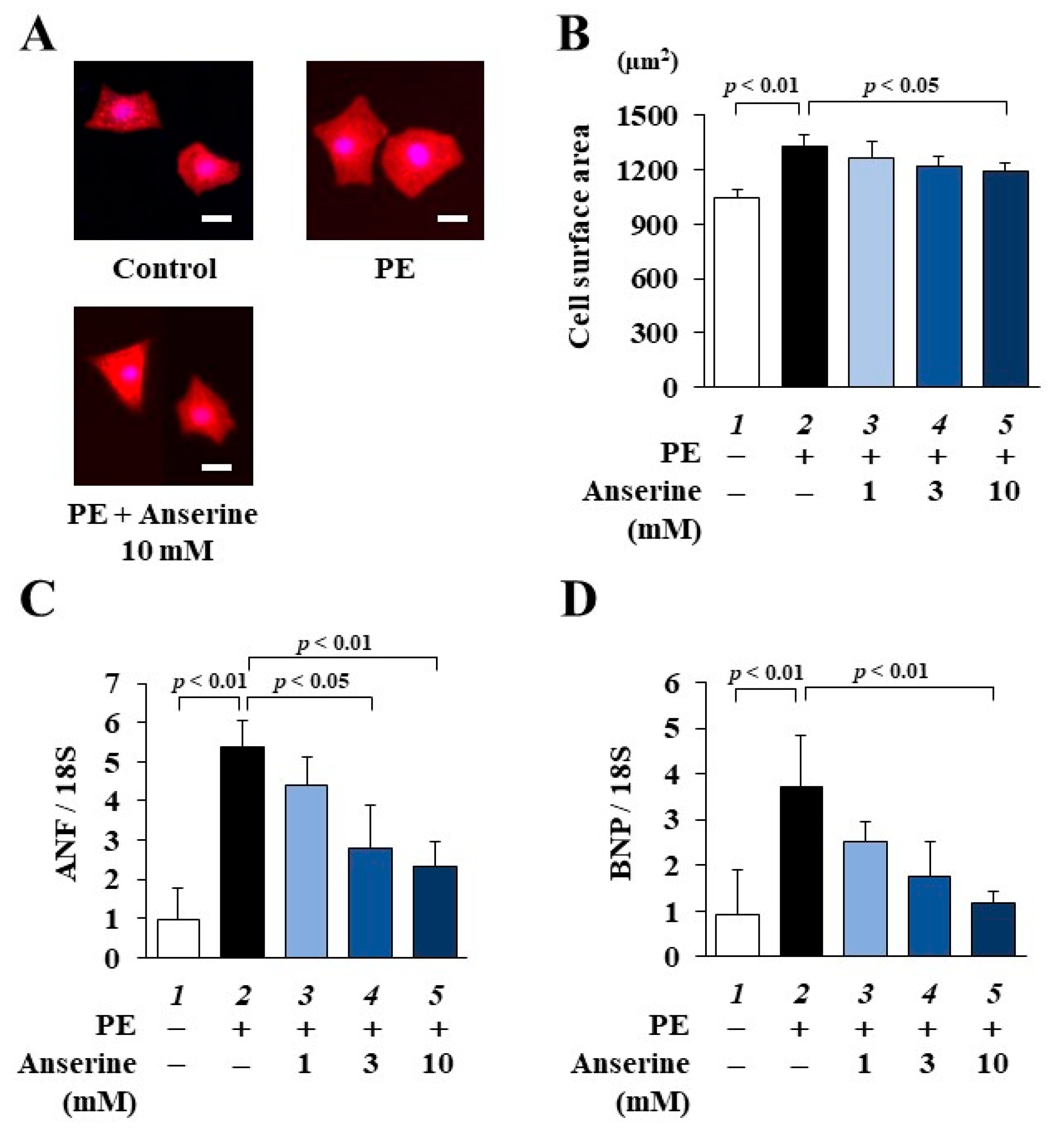
2.1. Anserine Suppressed Phenylephrine (PE)-Induced Cardiomyocyte Hypertrophic Responses
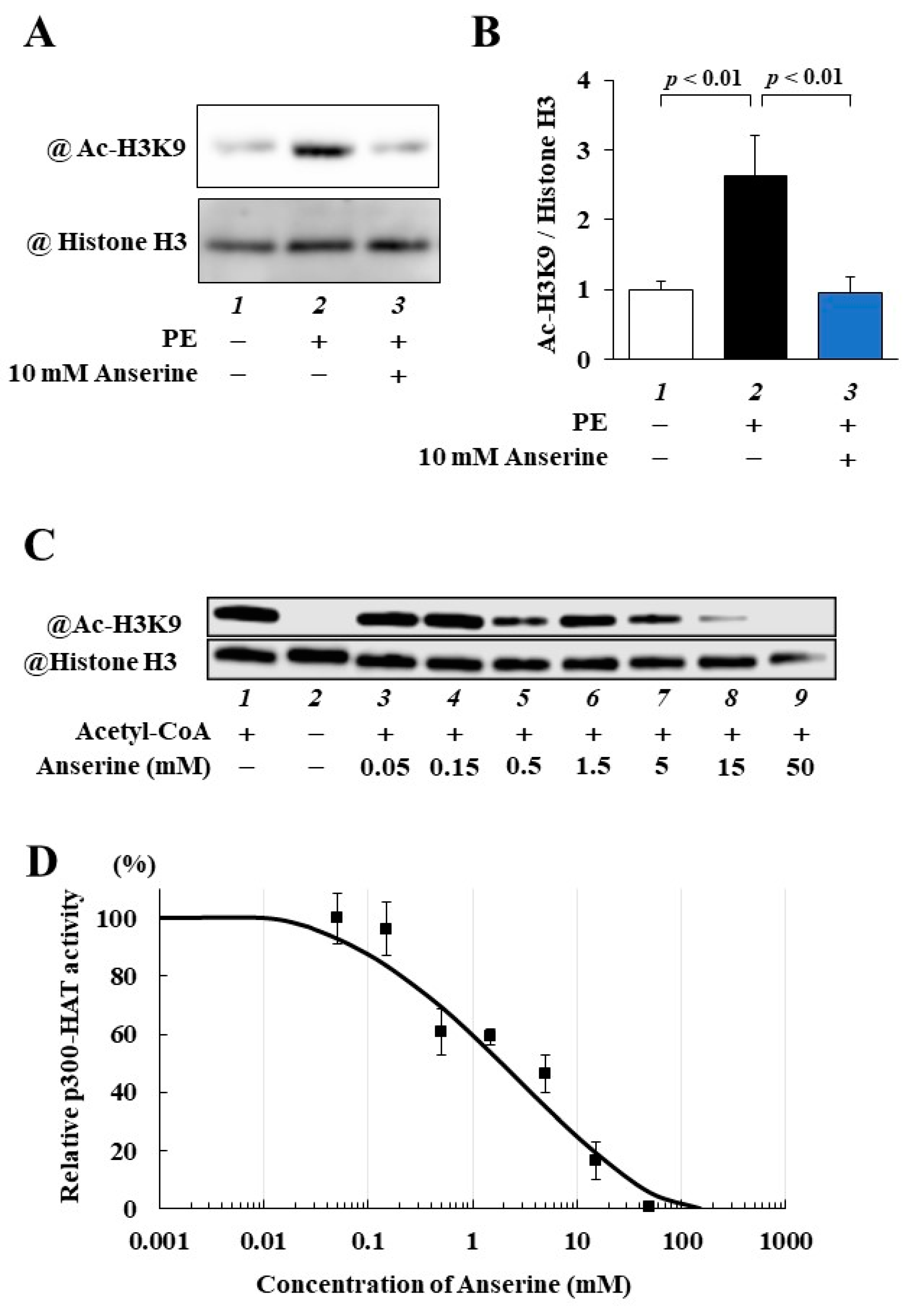
2.2. Anserine Suppressed p300-HAT Activity in Cultured Cardiomyocytes and In Vitro
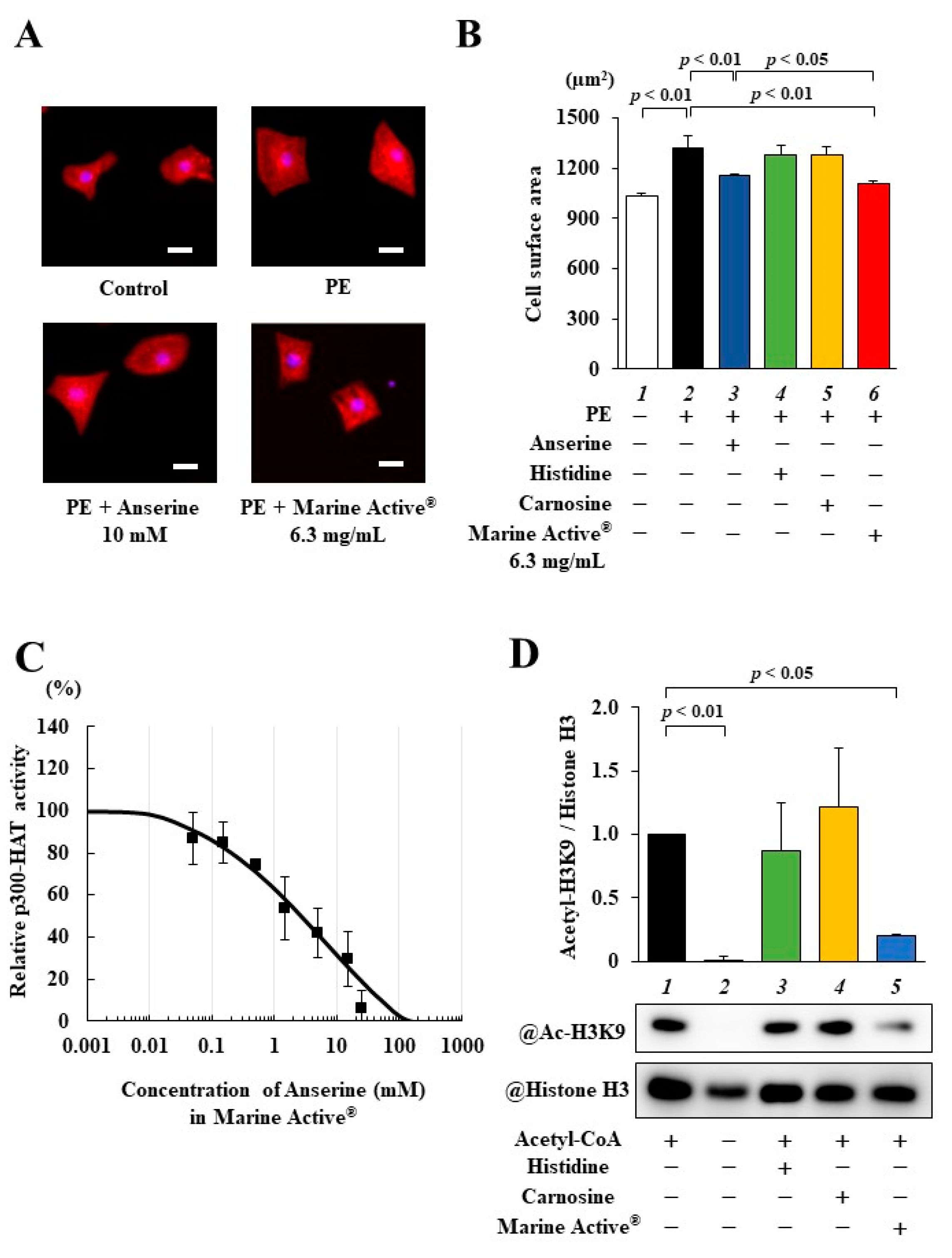
2.3. Marine Active® Suppressed PE-Induced Cardiomyocyte Hypertrophy
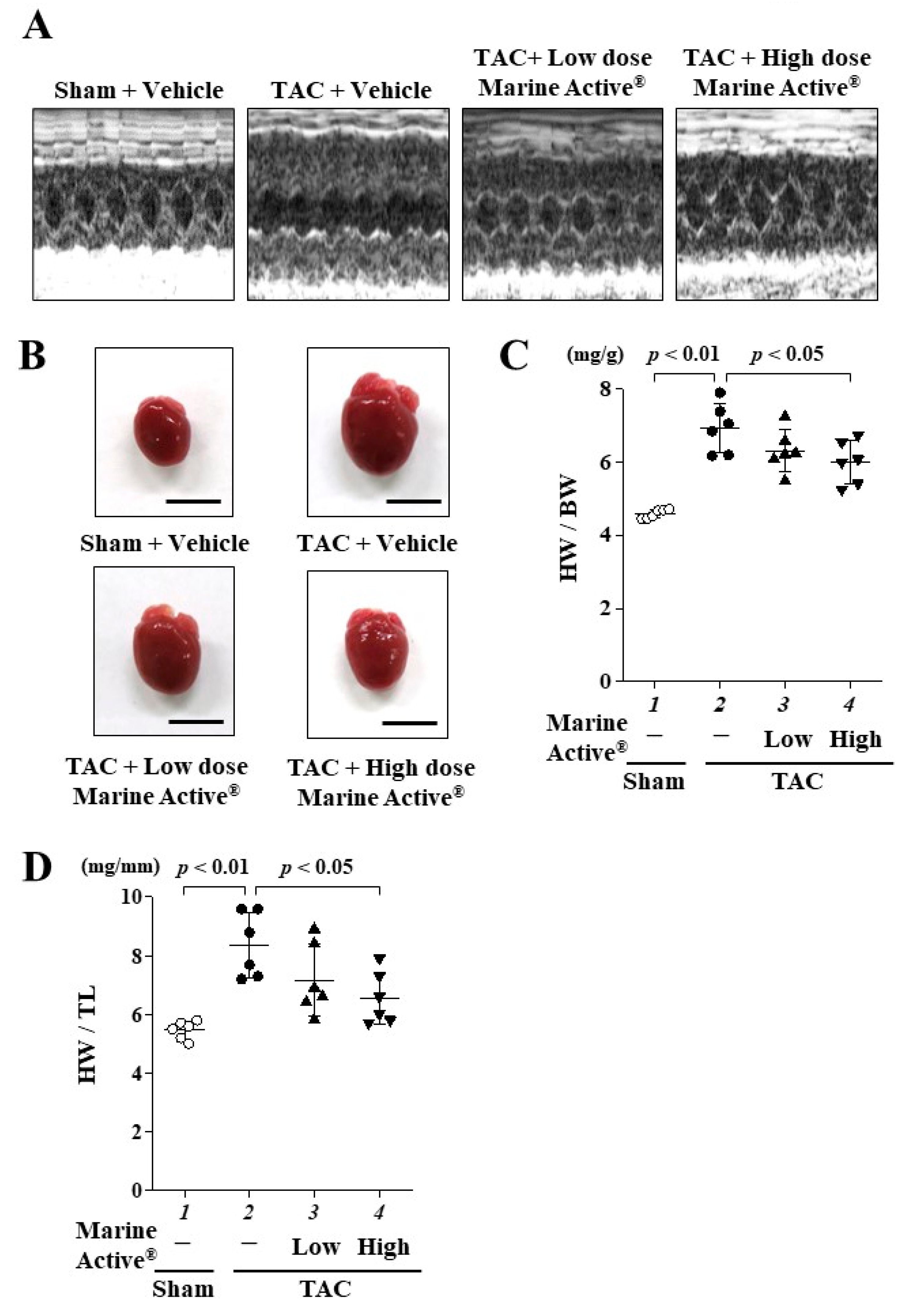
2.4. Marine Active® Suppressed Transverse Aortic Constriction (TAC)-Induced Systolic Dysfunction
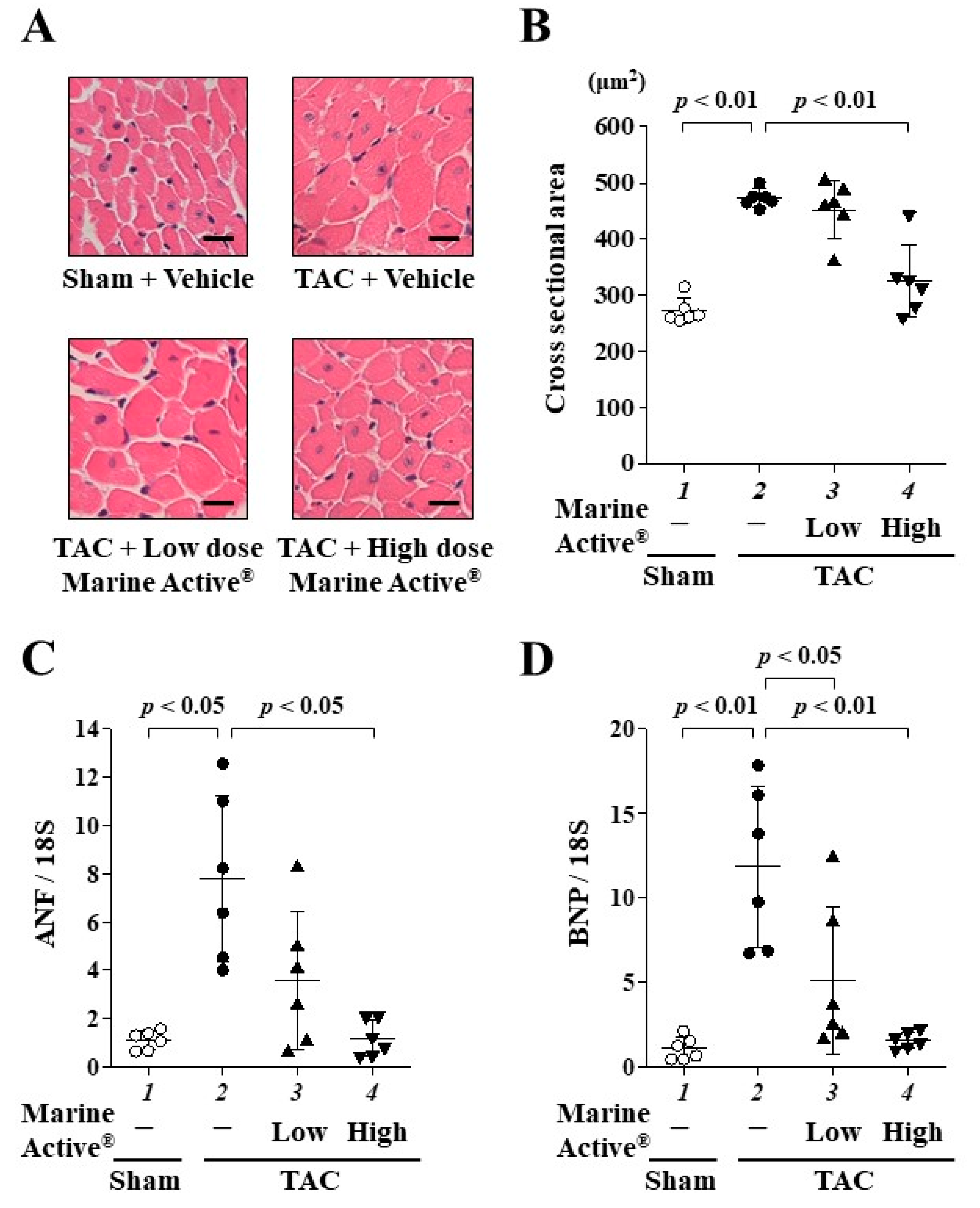
2.5. Marine Active® Suppressed TAC-Induced Cardiac Hypertrophy
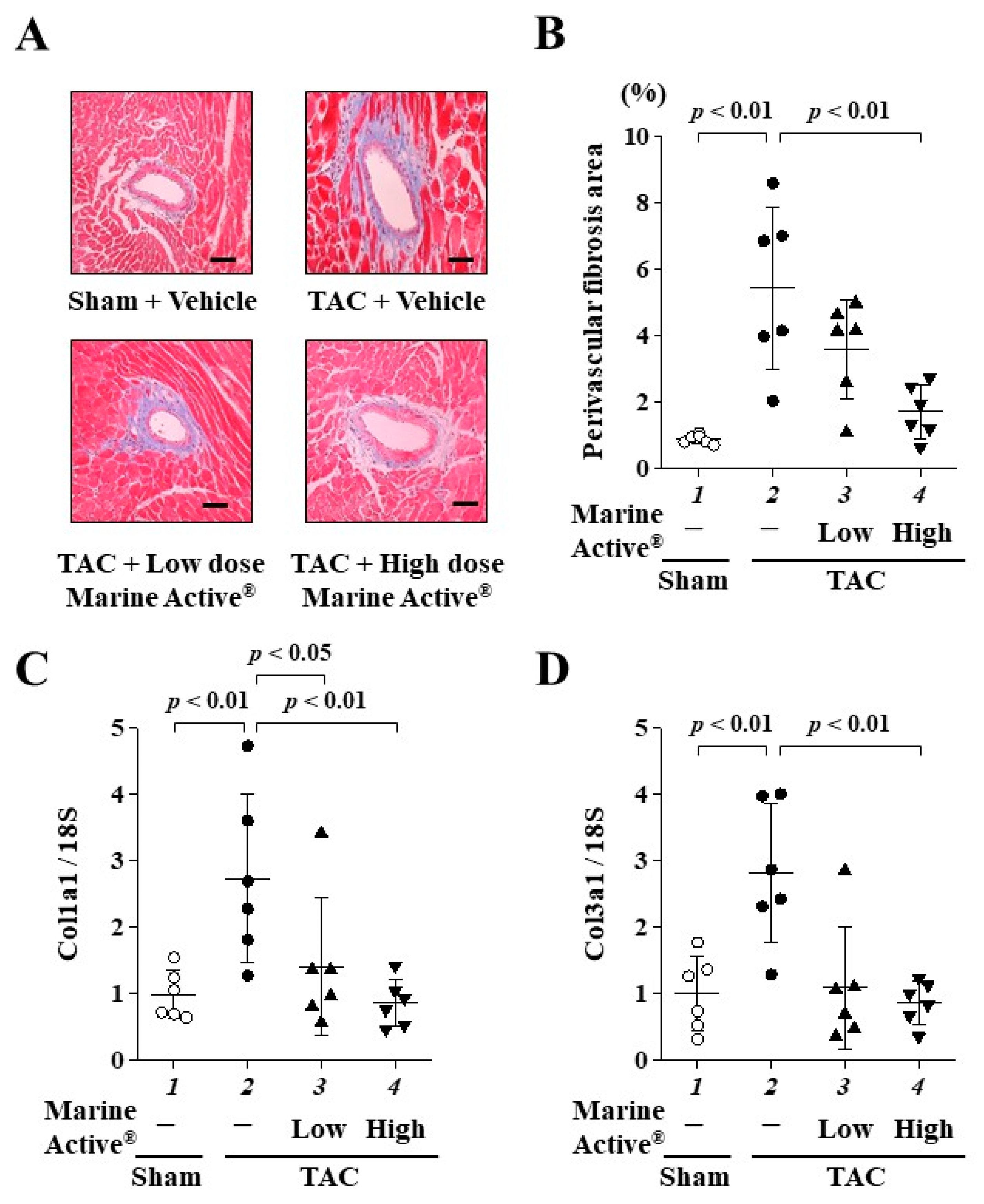
2.6. Marine Active® Suppressed TAC-Induced Cardiac Fibrosis
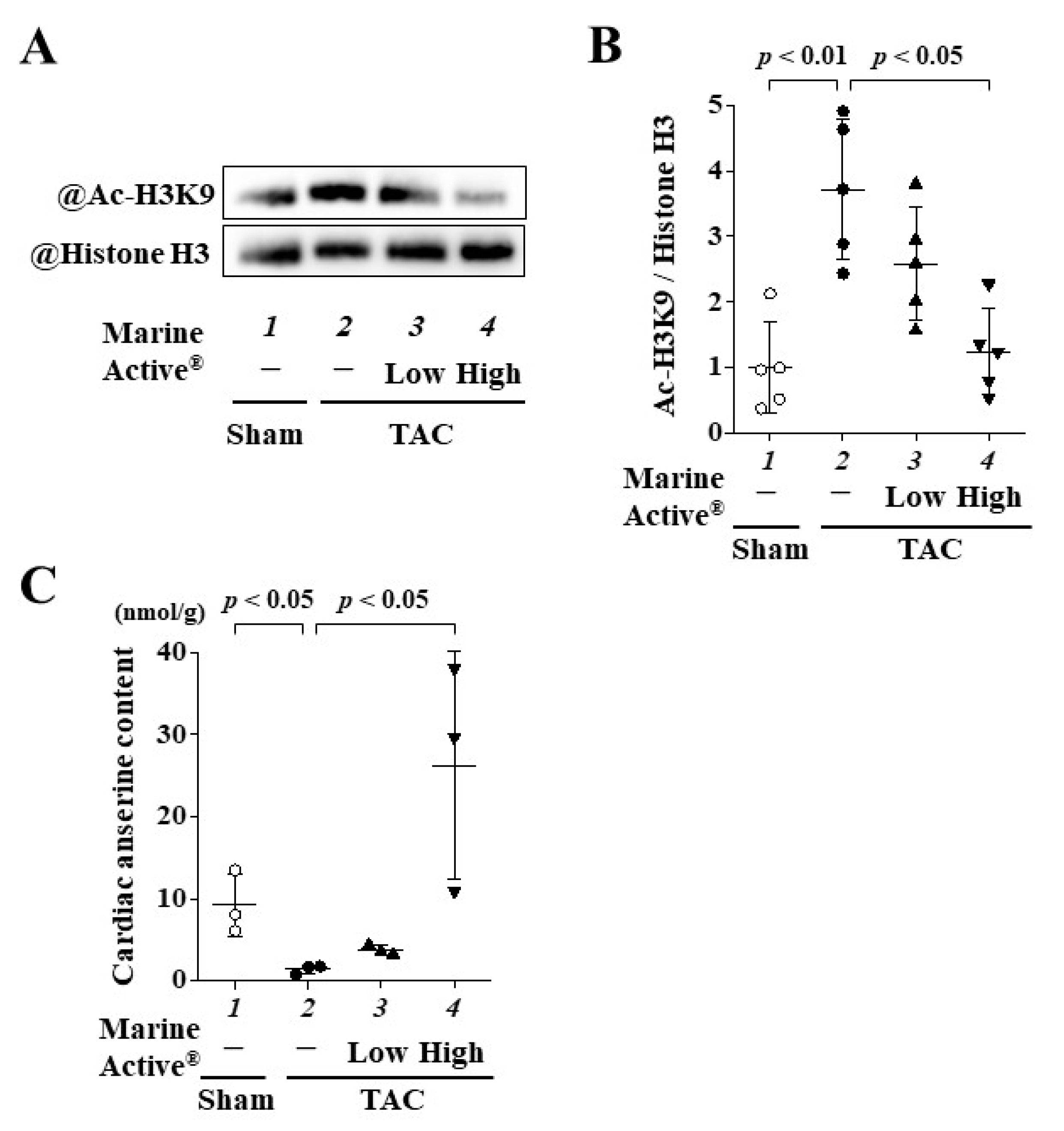
2.7. Marine Active® Suppressed TAC-Induced Acetylation of Histone H3K9
2.8. Oral Administration of Marine Active® Increased the Content of Anserine in Heart Tissue
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals
4.3. Neonatal Rat Cardiomyocytes
4.4. Fluorescence Staining and Confocal Microscopy
4.5. RNA Isolation and Quantitative Reverse Transcription PCR
4.6. Western Blotting
4.7. In Vitro p300-HAT Assay
4.8. TAC Surgery
4.9. Drug Treatments
4.10. Echocardiography
4.11. Histological Analysis
4.12. Measurement of Anserine Content in Heart Tissue
4.13. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oparil, S. Pathogenesis of Ventricular Hypertrophy. J. Am. Coll. Cardiol. 1985, 5, 57B–65B. [Google Scholar] [CrossRef]
- Wu, Q.-Q.; Xiao, Y.; Yuan, Y.; Ma, Z.-G.; Liao, H.-H.; Liu, C.; Zhu, J.-X.; Yang, Z.; Deng, W.; Tang, Q. Mechanisms Contributing to Cardiac Remodelling. Clin. Sci. 2017, 131, 2319–2345. [Google Scholar] [CrossRef]
- Yildiz, M.; Oktay, A.A.; Stewart, M.H.; Milani, R.V.; Ventura, H.O.; Lavie, C.J. Left Ventricular Hypertrophy and Hypertension. Prog. Cardiovasc. Dis. 2020, 63, 10–21. [Google Scholar] [CrossRef]
- Rossignol, P.; Hernandez, A.F.; Solomon, S.D.; Zannad, F. Heart Failure Drug Treatment. Lancet 2019, 393, 1034–1044. [Google Scholar] [CrossRef]
- Yanazume, T.; Hasegawa, K.; Morimoto, T.; Kawamura, T.; Wada, H.; Matsumori, A.; Kawase, Y.; Hirai, M.; Kita, T. Cardiac P300 Is Involved in Myocyte Growth with Decompensated Heart Failure. Mol. Cell. Biol. 2003, 23, 3593–3606. [Google Scholar] [CrossRef]
- Wei, J.Q.; Shehadeh, L.A.; Mitrani, J.M.; Pessanha, M.; Slepak, T.I.; Webster, K.A.; Bishopric, N.H. Quantitative Control of Adaptive Cardiac Hypertrophy by Acetyltransferase P300. Circulation 2008, 118, 934–946. [Google Scholar] [CrossRef]
- Balasubramanyam, K.; Varier, R.A.; Altaf, M.; Swaminathan, V.; Siddappa, N.B.; Ranga, U.; Kundu, T.K. Curcumin, a Novel P300/CREB-Binding Protein-Specific Inhibitor of Acetyltransferase, Represses the Acetylation of Histone/Nonhistone Proteins and Histone Acetyltransferase-Dependent Chromatin Transcription. J. Biol. Chem. 2004, 279, 51163–51171. [Google Scholar] [CrossRef]
- Morimoto, T.; Sunagawa, Y.; Kawamura, T.; Takaya, T.; Wada, H.; Nagasawa, A.; Komeda, M.; Fujita, M.; Shimatsu, A.; Kita, T.; et al. The Dietary Compound Curcumin Inhibits P300 Histone Acetyltransferase Activity and Prevents Heart Failure in Rats. J. Clin. Invest. 2008, 118, 868–878. [Google Scholar] [CrossRef] [PubMed]
- Sunagawa, Y.; Sono, S.; Katanasaka, Y.; Funamoto, M.; Hirano, S.; Miyazaki, Y.; Hojo, Y.; Suzuki, H.; Morimoto, E.; Marui, A.; et al. Optimal Dose-Setting Study of Curcumin for Improvement of Left Ventricular Systolic Function after Myocardial Infarction in Rats. J. Pharmacol. Sci. 2014, 126, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Boldyrev, A.A.; Aldini, G.; Derave, W. Physiology and Pathophysiology of Carnosine. Physiol. Rev. 2013, 93, 1803–1845. [Google Scholar] [CrossRef] [PubMed]
- Boldyrev, A.A. Carnosine: New Concept for the Function of an Old Molecule. Biochemistry 2012, 77, 313–326. [Google Scholar] [CrossRef]
- Abe, H. Role of Histidine-Related Compounds as Intracellular Proton Buffering Constituents in Vertebrate Muscle. Biochemistry 2000, 65, 757–765. [Google Scholar]
- Fontana, M.; Pinnen, F.; Lucente, G.; Pecci, L. Prevention of Peroxynitrite-Dependent Damage by Carnosine and Related Sulphonamido Pseudodipeptides. Cell Mol. Life Sci. 2002, 59, 546–551. [Google Scholar] [CrossRef]
- Szwergold, B.S. Carnosine and Anserine Act as Effective Transglycating Agents in Decomposition of Aldose-Derived Schiff Bases. Biochem. Biophys. Res. Commun. 2005, 336, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, V.H.; Oliveira, A.H.S.; de Oliveira, L.F.; da Silva, R.P.; Di Mascio, P.; Gualano, B.; Artioli, G.G.; Medeiros, M.H.G. Exercise and β-Alanine Supplementation on Carnosine-Acrolein Adduct in Skeletal Muscle. Redox Biol. 2018, 18, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Roberts, P.R.; Zaloga, G.P. Cardiovascular Effects of Carnosine. Biochemistry 2000, 65, 856–861. [Google Scholar] [PubMed]
- Zhao, J.; Conklin, D.J.; Guo, Y.; Zhang, X.; Obal, D.; Guo, L.; Jagatheesan, G.; Katragadda, K.; He, L.; Yin, X.; et al. Cardiospecific Overexpression of ATPGD1 (Carnosine Synthase) Increases Histidine Dipeptide Levels and Prevents Myocardial Ischemia Reperfusion Injury. J. Am. Heart Assoc. 2020, 9, e015222. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, L.d.S.; Sales, L.P.; Saito, T.R.; Campos, J.C.; Fernandes, A.L.; Natali, J.; Jensen, L.; Arnold, A.; Ramalho, L.; Bechara, L.R.G.; et al. Histidine Dipeptides Are Key Regulators of Excitation-Contraction Coupling in Cardiac Muscle: Evidence from a Novel CARNS1 Knockout Rat Model. Redox Biol. 2021, 44, 102016. [Google Scholar] [CrossRef] [PubMed]
- Sunagawa, Y.; Katayama, A.; Funamoto, M.; Shimizu, K.; Shimizu, S.; Sari, N.; Katanasaka, Y.; Miyazaki, Y.; Hosomi, R.; Hasegawa, K.; et al. The Polyunsaturated Fatty Acids, EPA and DHA, Ameliorate Myocardial Infarction-Induced Heart Failure by Inhibiting P300-HAT Activity in Rats. J. Nutr. Biochem. 2022, 106, 109031. [Google Scholar] [CrossRef] [PubMed]
- Sunagawa, Y.; Shimizu, K.; Katayama, A.; Funamoto, M.; Shimizu, K.; Nurmila, S.; Shimizu, S.; Miyazaki, Y.; Katanasaka, Y.; Hasegawa, K.; et al. Metformin Suppresses Phenylephrine-Induced Hypertrophic Responses by Inhibiting P300-HAT Activity in Cardiomyocytes. J. Pharmacol. Sci. 2021, 147, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.; Sun, T.; Ramirez, V.; Lux, E.; Eren, M.; Vaughan, D.E.; Ghosh, A.K. Acetyltransferase P300 Inhibitor Reverses Hypertension-Induced Cardiac Fibrosis. J. Cell Mol. Med. 2019, 23, 3026–3031. [Google Scholar] [CrossRef]
- Li, S.; Peng, B.; Luo, X.; Sun, H.; Peng, C. Anacardic Acid Attenuates Pressure-Overload Cardiac Hypertrophy through Inhibiting Histone Acetylases. J. Cell Mol. Med. 2019, 23, 2744–2752. [Google Scholar] [CrossRef]
- Kienesberger, P.C.; Pulinilkunnil, T.; Nagendran, J.; Dyck, J.R.B. Myocardial Triacylglycerol Metabolism. J. Mol. Cell Cardiol. 2013, 55, 101–110. [Google Scholar] [CrossRef]
- van der Vusse, G.J.; van Bilsen, M.; Glatz, J.F. Cardiac Fatty Acid Uptake and Transport in Health and Disease. Cardiovasc. Res. 2000, 45, 279–293. [Google Scholar] [CrossRef]
- Geissler, S.; Zwarg, M.; Knütter, I.; Markwardt, F.; Brandsch, M. The Bioactive Dipeptide Anserine Is Transported by Human Proton-Coupled Peptide Transporters. FEBS J. 2010, 277, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Gardner, M.L.; Illingworth, K.M.; Kelleher, J.; Wood, D. Intestinal Absorption of the Intact Peptide Carnosine in Man, and Comparison with Intestinal Permeability to Lactulose. J. Physiol. 1991, 439, 411–422. [Google Scholar] [CrossRef]
- Hisatsune, T.; Kaneko, J.; Kurashige, H.; Cao, Y.; Satsu, H.; Totsuka, M.; Katakura, Y.; Imabayashi, E.; Matsuda, H. Effect of Anserine/Carnosine Supplementation on Verbal Episodic Memory in Elderly People. J. Alzheimers Dis. 2016, 50, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Everaert, I.; Baron, G.; Barbaresi, S.; Gilardoni, E.; Coppa, C.; Carini, M.; Vistoli, G.; Bex, T.; Stautemas, J.; Blancquaert, L.; et al. Development and Validation of a Sensitive LC-MS/MS Assay for the Quantification of Anserine in Human Plasma and Urine and Its Application to Pharmacokinetic Study. Amino Acids 2019, 51, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Yeum, K.-J.; Orioli, M.; Regazzoni, L.; Carini, M.; Rasmussen, H.; Russell, R.M.; Aldini, G. Profiling Histidine Dipeptides in Plasma and Urine after Ingesting Beef, Chicken or Chicken Broth in Humans. Amino Acids 2010, 38, 847–858. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Hauske, S.J.; Zhang, S.; Rodriguez-Niño, A.; Albrecht, T.; Pastene, D.O.; van den Born, J.; van Goor, H.; Ruf, S.; Kohlmann, M.; et al. Identification and Characterisation of Carnostatine (SAN9812), a Potent and Selective Carnosinase (CN1) Inhibitor with in Vivo Activity. Amino Acids 2019, 51, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Kubomura, D.; Matahira, Y.; Masui, A.; Matsuda, H. Intestinal Absorption and Blood Clearance of L-Histidine-Related Compounds after Ingestion of Anserine in Humans and Comparison to Anserine-Containing Diets. J. Agric. Food Chem. 2009, 57, 1781–1785. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Kiersztan, A.; Drozak, J. Biosynthesis of Carnosine and Related Dipeptides in Vertebrates. Curr. Protein Pept. Sci. 2018, 19, 771–789. [Google Scholar] [CrossRef] [PubMed]
- Drozak, J.; Veiga-da-Cunha, M.; Vertommen, D.; Stroobant, V.; Van Schaftingen, E. Molecular Identification of Carnosine Synthase as ATP-Grasp Domain-Containing Protein 1 (ATPGD1). J. Biol. Chem. 2010, 285, 9346–9356. [Google Scholar] [CrossRef] [PubMed]
- Bellia, F.; Vecchio, G.; Rizzarelli, E. Carnosinases, Their Substrates and Diseases. Molecules 2014, 19, 2299–2329. [Google Scholar] [CrossRef]
- de Souza Gonçalves, L.; Pereira, W.R.; da Silva, R.P.; Yamaguchi, G.C.; Carvalho, V.H.; Vargas, B.S.; Jensen, L.; de Medeiros, M.H.G.; Roschel, H.; Artioli, G.G. Anserine Is Expressed in Human Cardiac and Skeletal Muscles. Physiol. Rep. 2023, 11, e15833. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Katanasaka, Y.; Sunagawa, Y.; Miyazaki, Y.; Funamoto, M.; Wada, H.; Hasegawa, K.; Morimoto, T. Tyrosine Phosphorylation of RACK1 Triggers Cardiomyocyte Hypertrophy by Regulating the Interaction between P300 and GATA4. Biochim. Biophys. Acta Mol. Basis Dis. 2016, 1862, 1544–1557. [Google Scholar] [CrossRef] [PubMed]
- Sunagawa, Y.; Morimoto, T.; Takaya, T.; Kaichi, S.; Wada, H.; Kawamura, T.; Fujita, M.; Shimatsu, A.; Kita, T.; Hasegawa, K. Cyclin-Dependent Kinase-9 Is a Component of the P300/GATA4 Complex Required for Phenylephrine-Induced Hypertrophy in Cardiomyocytes. J. Biol. Chem. 2010, 285, 9556–9568. [Google Scholar] [CrossRef] [PubMed]
- Sunagawa, Y.; Morimoto, T.; Wada, H.; Takaya, T.; Katanasaka, Y.; Kawamura, T.; Yanagi, S.; Marui, A.; Sakata, R.; Shimatsu, A.; et al. A Natural P300-Specific Histone Acetyltransferase Inhibitor, Curcumin, in Addition to Angiotensin-Converting Enzyme Inhibitor, Exerts Beneficial Effects on Left Ventricular Systolic Function after Myocardial Infarction in Rats. Circ. J. 2011, 75, 2151–2159. [Google Scholar] [CrossRef]
- Kawase, Y.; Sunagawa, Y.; Shimizu, K.; Funamoto, M.; Hamabe-Horiike, T.; Katanasaka, Y.; Shimizu, S.; Hawke, P.; Mori, K.; Komiyama, M.; et al. 6-Shogaol, an Active Component of Ginger, Inhibits P300 Histone Acetyltransferase Activity and Attenuates the Development of Pressure-Overload-Induced Heart Failure. Nutrients 2023, 15, 2232. [Google Scholar] [CrossRef]
- Sunagawa, Y.; Wada, H.; Suzuki, H.; Sasaki, H.; Imaizumi, A.; Fukuda, H.; Hashimoto, T.; Katanasaka, Y.; Shimatsu, A.; Kimura, T.; et al. A Novel Drug Delivery System of Oral Curcumin Markedly Improves Efficacy of Treatment for Heart Failure after Myocardial Infarction in Rats. Biol. Pharm. Bull. 2012, 35, 139–144. [Google Scholar] [CrossRef][Green Version]
- Shigemura, Y.; Iwasaki, Y.; Sato, Y.; Kato, T.; Seko, T.; Ishihara, K. Detection of Balenine in Mouse Plasma after Administration of Opah-Derived Balenine by HPLC with PITC Pre-Column Derivatization. Foods 2022, 11, 590. [Google Scholar] [CrossRef] [PubMed]
- Everaert, I.; Mooyaart, A.; Baguet, A.; Zutinic, A.; Baelde, H.; Achten, E.; Taes, Y.; De Heer, E.; Derave, W. Vegetarianism, Female Gender and Increasing Age, but Not CNDP1 Genotype, Are Associated with Reduced Muscle Carnosine Levels in Humans. Amino Acids 2011, 40, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Bando, K.; Shimotsuji, T.; Toyoshima, H.; Hayashi, C.; Miyai, K. Fluorometric Assay of Human Serum Carnosinase Activity in Normal Children, Adults and Patients with Myopathy. Ann. Clin. Biochem. 1984, 21 Pt 6, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Peters, V.; Jansen, E.E.W.; Jakobs, C.; Riedl, E.; Janssen, B.; Yard, B.A.; Wedel, J.; Hoffmann, G.F.; Zschocke, J.; Gotthardt, D.; et al. Anserine Inhibits Carnosine Degradation but in Human Serum Carnosinase (CN1) Is Not Correlated with Histidine Dipeptide Concentration. Clin. Chim. Acta 2011, 412, 263–267. [Google Scholar] [CrossRef]
| Sham | TAC | |||
|---|---|---|---|---|
| Vehicle | Vehicle | Low Dose of Marine Active® (Anserine 60 mg/kg) | High Dose of Marine Active® (Anserine 200 mg/kg) | |
| LVIDs (mm) | 1.33 ± 0.12 | 1.52 ± 0.17 | 1.39 ± 0.13 | 1.15 ± 0.09 ## |
| LVIDd (mm) | 2.50 ± 0.17 | 2.35 ± 0.21 | 2.24 ± 0.17 | 2.18 ± 0.21 * |
| PWd (mm) | 1.19 ± 0.10 | 1.77 ± 0.11 ** | 1.72 ± 0.14 ** | 1.38 ± 0.12 *## |
| IVSd (mm) | 1.21 ± 0.10 | 1.66 ± 0.15 ** | 1.63 ± 0.13 ** | 1.39 ± 0.10 *## |
| FS (%) | 46.6 ± 3.6 | 35.5 ± 3.2 ** | 38.1 ± 1.5 ** | 47.0 ± 2.2 ## |
| EF (%) | 83.9 ± 3.5 | 72.2 ± 3.7 ** | 75.4 ± 2.1 ** | 84.4 ± 2.4 ## |
| HR (bpm) | 573 ± 20 | 607 ± 17 | 620 ± 12 | 626 ± 10 |
| BW (g) | 27.0 ± 1.6 | 27.3 ± 1.9 | 26.8 ± 2.1 | 26.3 ± 1.4 |
| Target Gene | Forward | Reverse |
|---|---|---|
| Nppa (ANF) | 5′-AGGCCATATTGGAGCAAATC-3′ | 5′-CATCTTCTCCTCCAGGTGGT-3′ |
| Nppb (BNP) | 5′-GATTCTGCTCCTGCTTTTCC-3′ | 5′-CATCGTGGATTGTTCTGGAG-3′ |
| Col1a1 (Collagen alpha 1) | 5′-AAGAAGACATCCCTGAAGTCA-3′ | 5′-TTGTGGCAGATACAGATCAAG-3′ |
| Col3a1 (Collagen 3 alpha 1) | 5′-CCCAACCCAGAGATCCCATT-3′ | 5′-GAAGCACAGGAGCAGGTGTAGA-3′ |
| RNA18S1 (18S) | 5′-CTTAGAGGGACAAGTGGCG-3′ | 5′-GGACATCTAAGGGCATCACA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sunagawa, Y.; Tsukabe, R.; Irokawa, Y.; Funamoto, M.; Suzuki, Y.; Yamada, M.; Shimizu, S.; Katanasaka, Y.; Hamabe-Horiike, T.; Kawase, Y.; et al. Anserine, a Histidine-Containing Dipeptide, Suppresses Pressure Overload-Induced Systolic Dysfunction by Inhibiting Histone Acetyltransferase Activity of p300 in Mice. Int. J. Mol. Sci. 2024, 25, 2344. https://doi.org/10.3390/ijms25042344
Sunagawa Y, Tsukabe R, Irokawa Y, Funamoto M, Suzuki Y, Yamada M, Shimizu S, Katanasaka Y, Hamabe-Horiike T, Kawase Y, et al. Anserine, a Histidine-Containing Dipeptide, Suppresses Pressure Overload-Induced Systolic Dysfunction by Inhibiting Histone Acetyltransferase Activity of p300 in Mice. International Journal of Molecular Sciences. 2024; 25(4):2344. https://doi.org/10.3390/ijms25042344
Chicago/Turabian StyleSunagawa, Yoichi, Ryosuke Tsukabe, Yudai Irokawa, Masafumi Funamoto, Yuto Suzuki, Miho Yamada, Satoshi Shimizu, Yasufumi Katanasaka, Toshihide Hamabe-Horiike, Yuto Kawase, and et al. 2024. "Anserine, a Histidine-Containing Dipeptide, Suppresses Pressure Overload-Induced Systolic Dysfunction by Inhibiting Histone Acetyltransferase Activity of p300 in Mice" International Journal of Molecular Sciences 25, no. 4: 2344. https://doi.org/10.3390/ijms25042344
APA StyleSunagawa, Y., Tsukabe, R., Irokawa, Y., Funamoto, M., Suzuki, Y., Yamada, M., Shimizu, S., Katanasaka, Y., Hamabe-Horiike, T., Kawase, Y., Naruta, R., Shimizu, K., Mori, K., Hosomi, R., Komiyama, M., Hasegawa, K., & Morimoto, T. (2024). Anserine, a Histidine-Containing Dipeptide, Suppresses Pressure Overload-Induced Systolic Dysfunction by Inhibiting Histone Acetyltransferase Activity of p300 in Mice. International Journal of Molecular Sciences, 25(4), 2344. https://doi.org/10.3390/ijms25042344






