Modification of Glassy Carbon Electrodes with Complexes of Manganese(II) with Some Phenanthroline Derivatives Immobilized in Nafion Layer
Abstract
1. Introduction
2. Results and Discussion
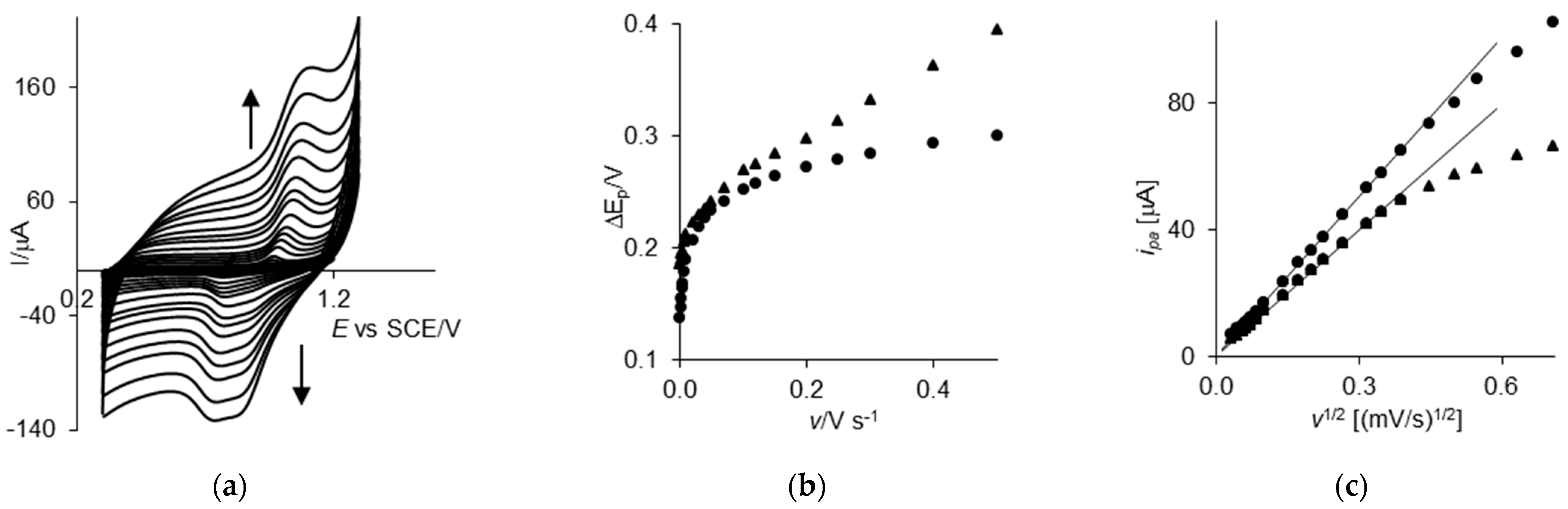
2.1. Cyclic Voltammetry
Disproportionation Constants
2.2. Chronocoulometry and Chronopotentiometry
2.3. Diffusion Coefficients Discussion
2.4. Electrocatalytic Activity against Reducing Agents
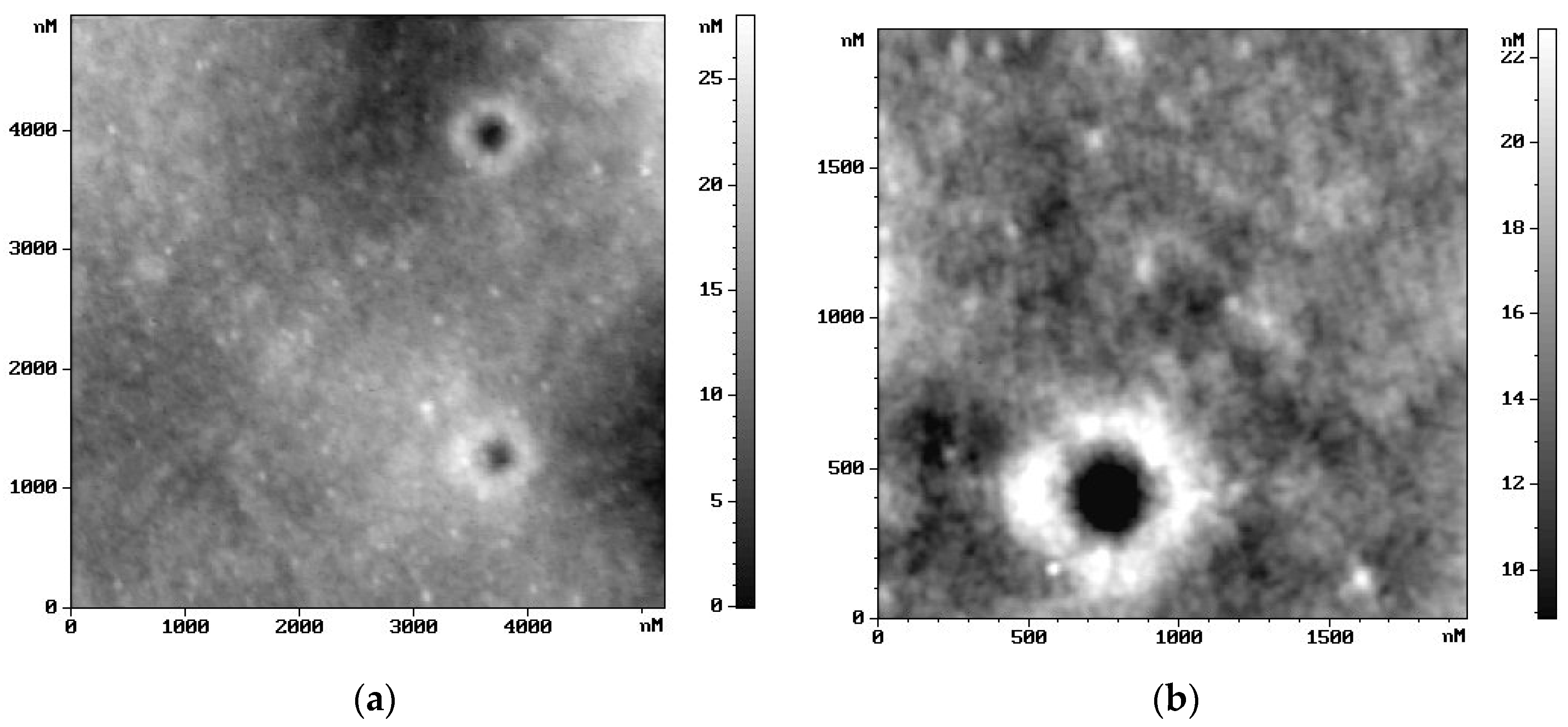
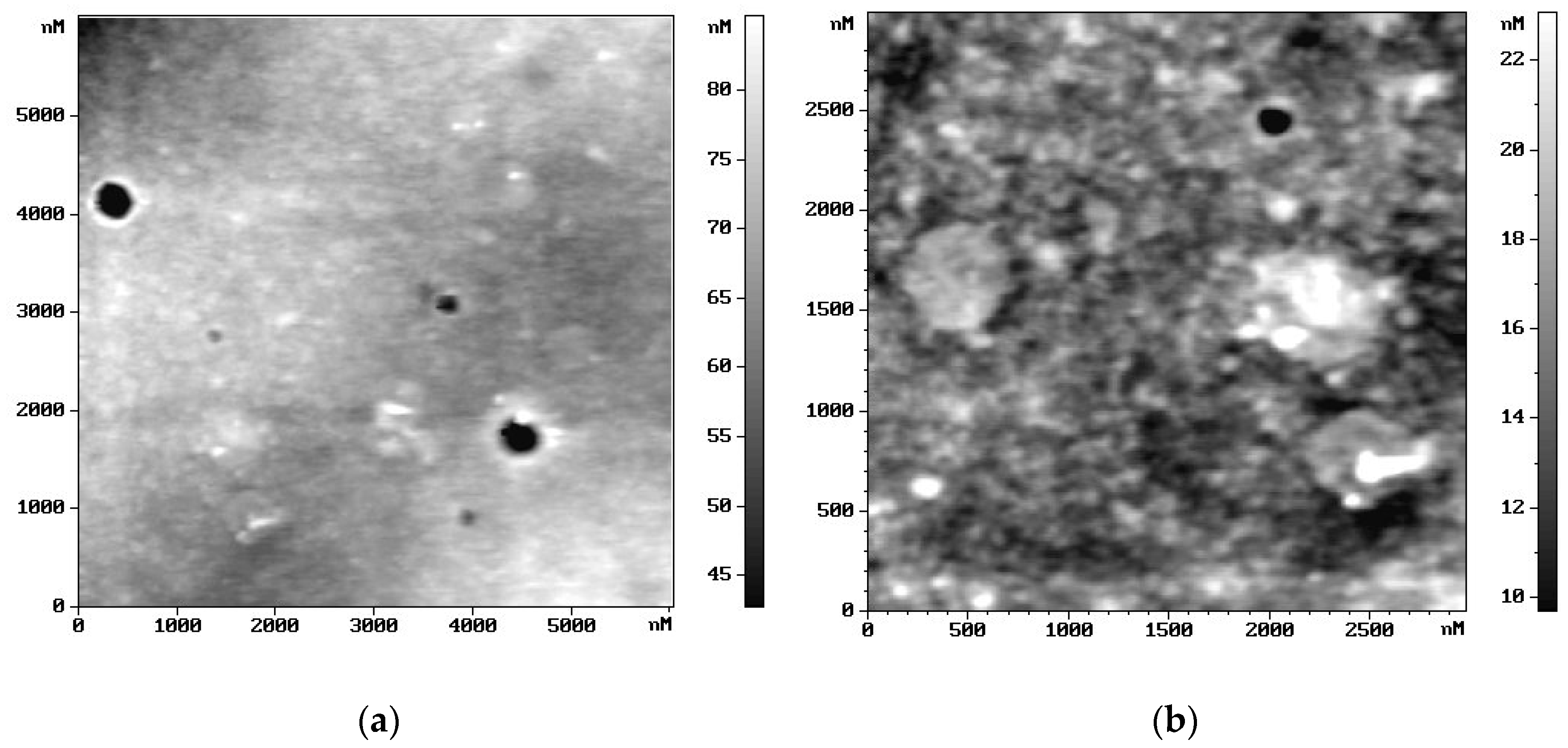
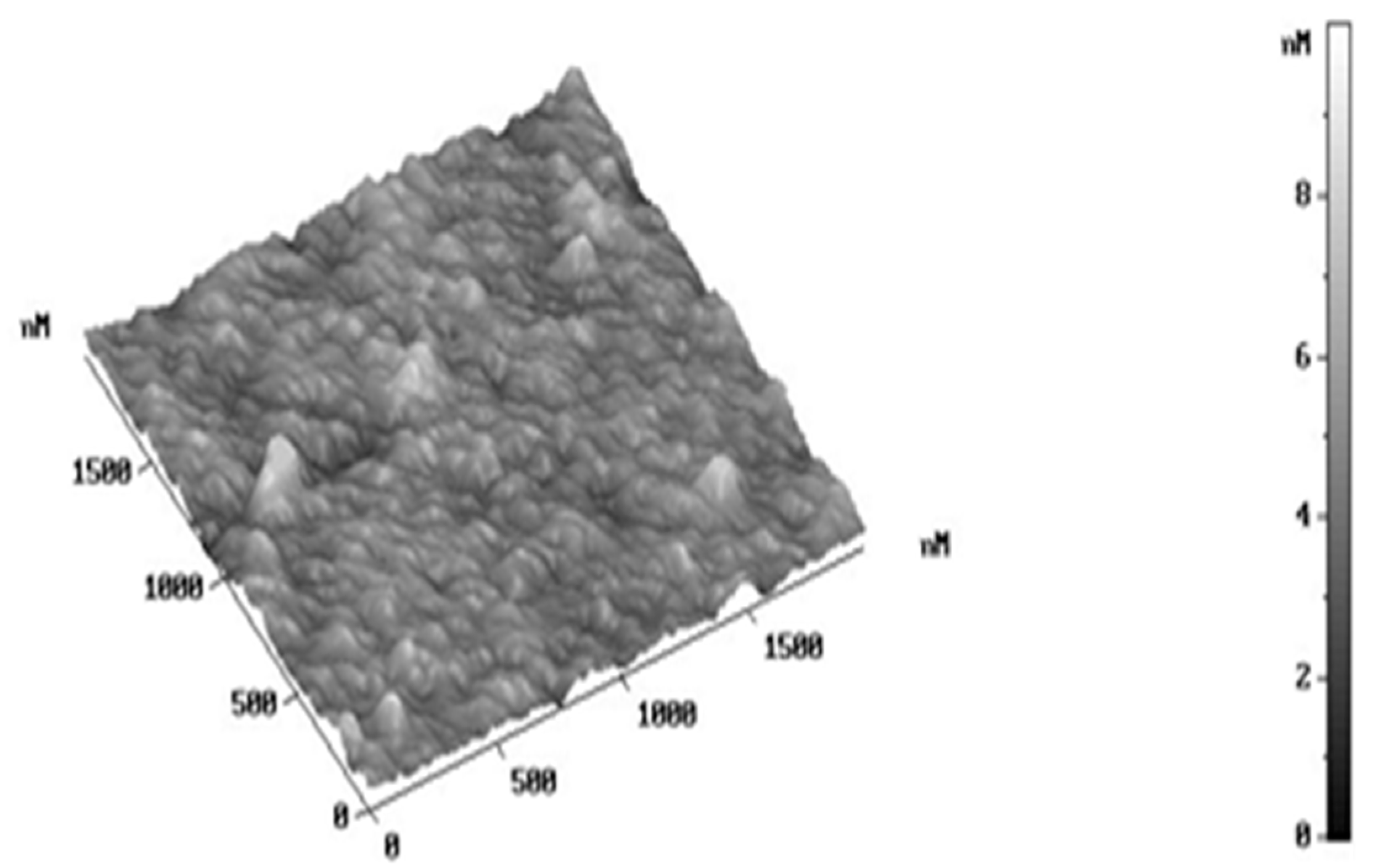
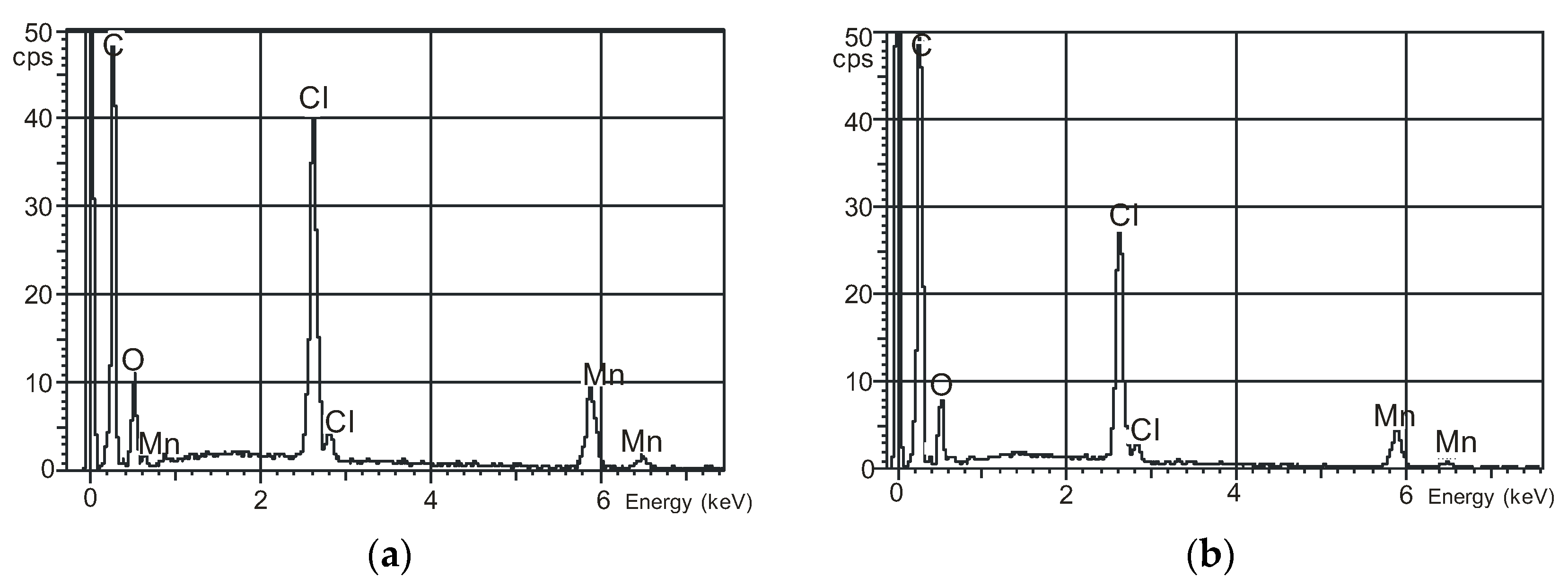
2.5. Microscopic Surface Characteristic
3. Materials and Methods
3.1. Apparatus
3.2. Reagents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Das, K.; Barman, M.K.; Maji, B. Advancements in multifunctional manganese complexes for catalytic hydrogen transfer reactions. Chem. Commun. 2021, 57, 8534–8549. [Google Scholar] [CrossRef]
- Friães, S.; Realista, S.; Gomes, C.S.B.; Martinho, P.N.; Royo, B. Click-Derived Triazoles and Triazolylidenes of Manganese for Electrocatalytic Reduction of CO2. Molecules 2021, 26, 6325. [Google Scholar] [CrossRef]
- Rohit, K.R.; Radhika, S.; Saranya, S.; Anilkumar, G. Manganese-Catalysed Dehydrogenative Coupling—An Overview. Adv. Synth. Catal. 2020, 362, 1602–1650. [Google Scholar] [CrossRef]
- Ballistreri, F.P.; Gangemi, C.M.A.; Pappalardo, A.; Tomaselli, G.A.; Toscano, R.M.; Sfrazzetto, G.T. (Salen)Mn(III) Catalyzed Asymmetric Epoxidation Reactions by Hydrogen Peroxide in Water: A Green Protocol. Int. J. Mol. Sci. 2016, 17, 1112. [Google Scholar] [CrossRef]
- Papanikolaou, M.G.; Hadjithoma, S.; Gallos, J.K.; Miras, H.N.; Plakatouras, J.C.; Keramidas, A.D.; Kabanos, T.A. Synthesis, structural and physicochemical properties of a series of manganese(II) complexes with a novel N5 tripodal-amidate ligand and their potential use as water oxidation catalysts. Polyhedron 2021, 204, 115260. [Google Scholar] [CrossRef]
- Egekenze, R.N.; Gultneh, Y.; Butcher, R. Mn(III) and Mn(II) complexes of tridentate Schiff base ligands; synthesis, characterization, structure, electrochemistry and catalytic activity. Inorg. Chim. Acta 2018, 478, 232–242. [Google Scholar] [CrossRef]
- Rigamonti, L.; Zardi, P.; Carlino, S.; Demartin, F.; Castellano, C.; Pigani, L.; Ponti, A.; Ferretti, A.M.; Pasini, A. Selective Formation, Reactivity, Redox and Magnetic Properties of MnIII and FeIII Dinuclear Complexes with Shortened Salen-Type Schiff Base Ligands. Int. J. Mol. Sci. 2020, 21, 7882. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wan, T.; Kong, X.; Shen, Q.; Li, K.; Wu, H. Electrocatalytic hydrogen evolution at carbon paste electrodes doped with a manganese(II) imidazoledicarboxylate complex. Z. Naturforschung B 2023, 78, 469–476. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, S.; Teng, J.; Zhao, S.; Li, Z.; Wu, M. Combination of Mn-Mo Oxide Nanoparticles on Carbon Nanotubes through Nitrogen Doping to Catalyze Oxygen Reduction. Molecules 2023, 28, 5544. [Google Scholar] [CrossRef] [PubMed]
- Sergienko, N.; Radjenovic, J. Manganese oxide coated TiO2 nanotube-based electrode for efficient and selective electrocatalytic sulfide oxidation to colloidal sulphur. Appl. Catal. B Environ. 2021, 296, 120383. [Google Scholar] [CrossRef]
- Keypour, H.; Kouhdareh, J.; Karimi-Nami, R.; Alavinia, S.; Karakaya, I.; Babaei, S.; Maryamabadi, A. Investigation of the electrocatalytic reaction for the oxidation of alcohols through the formation of a metal organic framework (Mn-MIL-100)/polymer matrix on the surface of an Au electrode. New J. Chem. 2023, 47, 6730–6738. [Google Scholar] [CrossRef]
- Hefnawy, M.A.; Medany, S.S.; El-Sherif, R.M.; Fadlallah, S.A. NiO-MnOx/Polyaniline/Graphite Electrodes for Urea Electrocatalysis: Synergetic Effect between Polymorphs of MnOx and NiO. ChemistrySelect 2022, 7, e202103735. [Google Scholar] [CrossRef]
- Soriano-López, J.; Elliott, R.; Kathalikkattil, A.C.; Ako, A.M.; Mulahmetović, M.; Venkatesan, M.; Schmitt, W. Bioinspired Water Oxidation Using a Mn-Oxo Cluster Stabilized by Non-Innocent Organic Tyrosine Y161 and Plastoquinone Mimics. ACS Sustain. Chem. Eng. 2020, 8, 13648–13659. [Google Scholar] [CrossRef]
- Peng, R.; Offenhäusser, A.; Ermolenko, Y.; Mourzina, Y. Biomimetic sensor based on Mn(III) meso-tetra(N-methyl-4-pyridyl) porphyrin for non-enzymatic electrocatalytic determination of hydrogen peroxide and as an electrochemical transducer in oxidase biosensor for analysis of biological media. Sens. Actuators B Chem. 2020, 321, 128437. [Google Scholar] [CrossRef]
- Yang, C.; Gu, Y.; Zhang, K.-L. Proton-Conductive and Electrochemical-Sensitive Sensing Behavior of a New Mn(II) Chain Coordination Polymer. Cryst. Growth Des. 2023, 23, 704–718. [Google Scholar] [CrossRef]
- Chen, T.; Huang, Z.; Liu, J.; Jiang, L.; Chu, J.; Song, C.; Kong, A. Mn-Pyridine N site-enriched Mn-N–C derived from covalent organic polymer for electrochemical oxygen reduction and capacitive storage. Ionics 2021, 27, 5229–5239. [Google Scholar] [CrossRef]
- Li, H.; Huang, C.; Li, Y.; Yang, W.; Liu, F. Electrocatalytic reduction of trace nitrobenzene using a graphene-oxide@polymerized-manganese-porphyrin composite. RSC Adv. 2019, 9, 22523–22530. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, N.; Mayuri, P.; Huang, S.-T.; Kumar, A.S. In-situ electrochemical immobilization of [Mn(bpy)2(H2O)2]2+ complex on MWCNT modified electrode and its electrocatalytic H2O2 oxidation and reduction reactions: A Mn-Pseudocatalase enzyme bio-mimicking electron-transfer functional model. J. Electroanal. Chem. 2018, 812, 10–21. [Google Scholar] [CrossRef]
- Singh, A.; Hocking, R.K.; Chang, S.L.-Y.; George, B.M.; Fehr, M.; Lips, K.; Schnegg, A.; Spiccia, L. Water Oxidation Catalysis by Nanoparticulate Manganese Oxide Thin Films: Probing the Effect of the Manganese Precursors. Chem. Mater. 2013, 25, 1098–1108. [Google Scholar] [CrossRef]
- Gagliardi, G.G.; Ibrahim, A.; Borello, D.; El-Kharouf, A. Composite Polymers Development and Application for Polymer Electrolyte Membrane Technologies—A Review. Molecules 2020, 25, 1712. [Google Scholar] [CrossRef]
- Malara, A.; Bonaccorsi, L.; Fotia, A.; Antonucci, P.L.; Frontera, P. Hybrid Fluoro-Based Polymers/Graphite Foil for H2/Natural Gas Separation. Materials 2023, 16, 2105. [Google Scholar] [CrossRef]
- Seen, A.J. Nafion: An excellent support for metal-complex catalysts. J. Mol. Catal. A Chem. 2001, 177, 105–112. [Google Scholar] [CrossRef]
- Shoji, M.; Oyaizu, K.; Nishide, H. Facilitated oxygen transport through a Nafion membrane containing cobaltporphyrin as a fixed oxygen carrier. Polymer 2008, 49, 5659–5664. [Google Scholar] [CrossRef]
- Peerce, P.J.; Bard, A.J. Polymer films on electrodes. Part III. Digital simulation model for cyclic voltammetry of electroactive polymer film and electrochemistry of poly(vinylferrocene) on platinum. J. Electroanal. Chem. 1980, 114, 89–115. [Google Scholar] [CrossRef]
- Rong, D.; Anson, F.C. Severe inhibition of the diffusion and electroactivity of Cu(bpy)2+2 complexes incorporated in Nafion® coatings on electrodes. J. Electroanal. Chem. 1996, 404, 171–177. [Google Scholar] [CrossRef]
- Sun, J.; Jordan, L.R.; Forsyth, M.; MacFarlane, D.R. Acid–Organic base swollen polymer membranes. Electrochim. Acta 2001, 46, 1703–1708. [Google Scholar] [CrossRef]
- Blauch, D.N.; Savéant, J.-M. Dynamics of electron hopping in assemblies of redox centers. Percolation and diffusion. J. Am. Chem. Soc. 1992, 114, 3323–3332. [Google Scholar] [CrossRef]
- Tomczyk, D.; Bukowski, W.; Bester, K.; Kaczmarek, M. Electrocatalytic Properties of Ni(II) Schiff Base Complex Polymer Films. Materials 2022, 15, 191. [Google Scholar] [CrossRef] [PubMed]
- Tomczyk, D.; Seliger, P.; Bukowski, W.; Bester, K. The Influence of Electrolyte Type on Kinetics of Redox Processes in the Polymer Films of Ni(II) Salen-Type Complexes. Molecules 2022, 27, 1812. [Google Scholar] [CrossRef]
- Nowak, L.; Andrijewski, G.; Tomczyk, D.; Cichomski, M. Modification of glassy carbon electrode with phenanthroline complexes of manganese(II) immobilized in nafion layer. Pol. J. Chem. 2007, 81, 493–503. [Google Scholar]
- Nowak, L.; Andrijewski, G.; Tomczyk, D.; Błaszczyk, T. Transport of phenanthroline complexes of manganese(II) ions through nafion layer on glassy carbon electrode. Pol. J. Chem. 2009, 83, 2195–2203. [Google Scholar]
- Andrijewski, G.; Nowak, L.; Tomczyk, D.; Różański, R.; Dałkowski, R. Complex formation equilibria and potentials of Mn(III)/Mn(II) couple in the presence of 1,10-phenanthroline derivatives in aqueous solutions. Pol. J. Chem. 2006, 80, 693–701. [Google Scholar]
- Zheng, S.; Gao, L.; Guo, J. Synthesis and Characterization of Functionalized MCM-41 with Copper- and Manganese-Phenanthroline Complexes. J. Solid State Chem. 2000, 152, 447–452. [Google Scholar] [CrossRef]
- Benton, D.J.; Moore, P. Rates of formation and dissociation of complexes of manganese(II) with the ligands 1,10-phenanthroline, 2,2′-bipyridine, and 2,2′2″-terpyridine in anhydrous methanol. J. Chem. Soc. Dalton Trans. 1973, 4, 399–404. [Google Scholar] [CrossRef]
- Ramalakshmi, D.; Reddy, K.R.; Padmavathy, d.; Rajasekharan, M.V.; Arulsamy, N.; Hodgson, D.J. Polyiodides of transition metal trischelate cations: Syntheses, structures, and spectral and electrical conductivity studies of [Mn(phen)3](I3)2 and [Mn(bpy)3](I3)1.5(I8)0.25. Inorg. Chim. Acta 1999, 284, 158–166. [Google Scholar] [CrossRef]
- Goldsmith, C.R.; Jiang, W. Alkene epoxidation catalyzed by manganese complexes with electronically modified 1,10-phenanthroline ligands. Inorg. Chim. Acta 2012, 384, 340–344. [Google Scholar] [CrossRef]
- Tikhonova, L.P.; Ivashchenko, T.S. Study of bromate oxidation of complexes of manganese(II) with 1,10 phenanthroline. Theor. Exp. Chem. 1991, 27, 43–48. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Zhang, H.; Ge, X. Screening of catalytic oxygen reduction reaction activity of 2, 9-dihalo-1, 10-phenanthroline metal complexes: The role of transition metals and halogen substitution. J. Colloid Interface Sci. 2022, 609, 130–138. [Google Scholar] [CrossRef]
- Hanaki, A. Copper-catalyzed Oxidation of Ascorbic Acid. Chem. Pharm. Bull. 1969, 17, 1839–1846. [Google Scholar] [CrossRef][Green Version]
- Pournaghi-Azar, M.H.; Ojani, R. Catalytic oxidation of ascorbic acid by some ferrocene derivative mediators at the glassy carbon electrode. Application to the voltammetric resolution of ascorbic acid and dopamine in the same sample. Talanta 1995, 42, 1839–1848. [Google Scholar] [CrossRef]
- Garakani, S.S.; Zhang, M.; Xie, D.; Sikdar, A.; Pang, K.; Yuan, J. Facile Fabrication of Wood-Derived Porous Fe3C/Nitrogen-Doped Carbon Membrane for Colorimetric Sensing of Ascorbic Acid. Nanomaterials 2023, 13, 2786. [Google Scholar] [CrossRef]
- Masihpour, N.; Hassaninejad-Darzi, S.K.; Sarvary, A. Nickel-Cobalt Salen Organometallic Complexes Encapsulated in Mesoporous NaA Nanozeolite for Electrocatalytic Quantification of Ascorbic Acid and Paracetamol. J. Inorg. Organomet. Polym. Mater. 2023, 33, 2661–2680. [Google Scholar] [CrossRef] [PubMed]
- Majsztrik, P.W.; Satterfield, M.B.; Bocarsly, A.B.; Benziger, J.B. Water sorption, desorption and transport in Nafion membranes. J. Membr. Sci. 2007, 301, 93–106. [Google Scholar] [CrossRef]
- Olmstead, M.L.; Nicholson, R.S. Cyclic voltammetry theory for the disproportionation reaction and spherical diffusion. Anal. Chem. 1969, 41, 862–864. [Google Scholar] [CrossRef]
- Galus, Z. Electroanalytical Methods of Physical and Chemical Parameters Determination; PWN: Warsaw, Poland, 1979. [Google Scholar]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods, 2nd ed.; John Willey & Sons: New York, NY, USA, 2001. [Google Scholar]
- Lyons, M.E.G. Annual Reports on the Progress of Chemistry, Section C, Physical Chemistry; The Royal Society of Chemistry: Cambridge, UK, 1990; Volume 87, p. 158. [Google Scholar]
- Yeager, H.L.; Steck, A. Cation and water diffusion in nafion ion exchange membranes: Influence of polymer structure. J. Electrochem. Soc. 1981, 128, 1880–1884. [Google Scholar] [CrossRef]
- Hsu, W.Y.; Gierke, T.D. Ion transport and clustering in nafion perfluorinated membranes. J. Membr. Sci. 1983, 13, 307–326. [Google Scholar] [CrossRef]
- Rubinstein, I. The influence of the polymer structure on electrochemical properties of uncharged molecules in nafion films on electrodes. J. Electroanal. Chem. Interfacial Electrochem. 1985, 188, 227–244. [Google Scholar] [CrossRef]
- Zook, L.A.; Leddy, J. Density and Solubility of Nafion: Recast, Annealed, and Commercial Films. Anal. Chem. 1996, 68, 3793–3796. [Google Scholar] [CrossRef]
- Oberbroeckling, K.J.; Dunwoody, D.C.; Minteer, S.D.; Leddy, J. Density of nafion exchanged with transition metal complexes and tetramethyl ammonium, ferrous, and hydrogen ions: Commercial and recast films. Anal. Chem. 2002, 74, 4794–4799. [Google Scholar] [CrossRef]
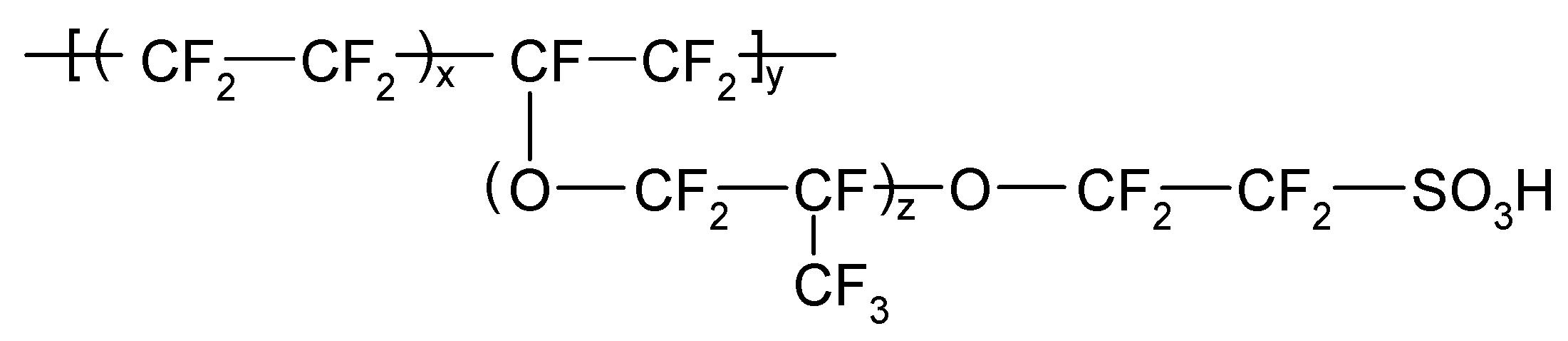

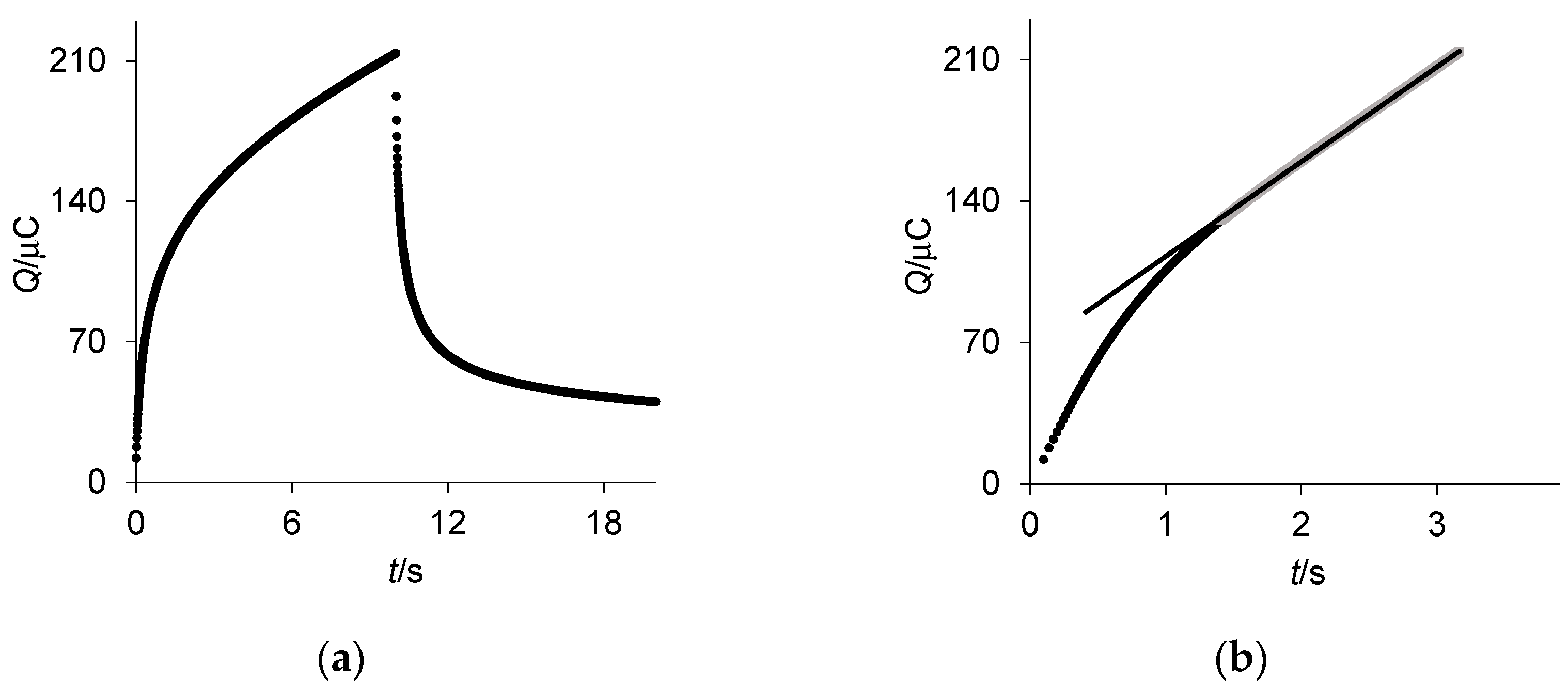


| Complex | k1/dm3 mol−1 s−1 | Complex | k1/dm3 mol−1 s−1 |
|---|---|---|---|
| [Mn(4-CH3-phen)2(H2O)2]3+ | 41.6 ± 0.8 | [Mn(4-CH3-phen)3]3+ | 21.7 ± 0.4 |
| [Mn(5-CH3-phen)2(H2O)2]3+ | 52.5 ± 0.8 | [Mn(5-CH3-phen)3]3+ | 34.8 ± 0.6 |
| [Mn(5-Cl-phen)2(H2O)2]3+ | 20.5 ± 0.4 | [Mn(5-Cl-phen)3]3+ | 4.6 ± 0.2 |
| [Mn(5-NO2-phen)2(H2O)2]3+ | 4.6 ± 0.2 | [Mn(5-NO2-phen)3]3+ | 7.3 ± 0.3 |
| [Mn(4,7-CH3-phen)2(H2O)2]3+ | 61.2 ± 0.9 | [Mn(4,7-CH3-phen)3]3+ | 54.8 ± 0.8 |
| [Mn(5,6-CH3-phen)2(H2O)2]3+ | 112.7 ± 1.2 | [Mn(5,6-CH3-phen)3]3+ | 33.3 ± 0.6 |
| Complex | cv Conventional Electrode | cc Conventional Electrode | cc Microelectrode Linear Diffusion | cc Microelectrode Mixed Diffusion |
|---|---|---|---|---|
| [Mn(4-CH3-phen)2(H2O)2]2+ | 4.8 × 10−8 | 3.3 × 10−8 | 2.3 × 10−8 | 4.5 × 10−8 |
| [Mn(4-CH3-phen)3]2+ | 4.1 × 10−8 | 2.4 × 10−8 | 5.7 × 10−8 | 4.4 × 10−8 |
| [Mn(5-CH3-phen)2(H2O)2]2+ | 4.7 × 10−8 | 2.8 × 10−8 | 4.9 × 10−8 | 3.8 × 10−8 |
| [Mn(5-CH3-phen)3]2+ | 4.0 × 10−8 | 2.7 × 10−8 | 5.9 × 10−8 | 2.6 × 10−8 |
| [Mn(5-Cl-phen)2(H2O)2]2+ | 2.5 × 10−8 | 2.7 × 10−8 | 3.3 × 10−8 | 2.7 × 10−8 |
| [Mn(5-Cl-phen)3]2+ | 1.7 × 10−8 | 3.8 × 10−8 | 3.0 × 10−8 | 2.3 × 10−8 |
| [Mn(5-NO2-phen)2(H2O)2]2+ | 1.6 × 10−8 | 2.1 × 10−8 | 3.1 × 10−8 | 2.1 × 10−8 |
| [Mn(5-NO2-phen)3]2+ | 0.6 × 10−8 | 3.0 × 10−8 | 3.0 × 10−8 | 2.6 × 10−8 |
| [Mn(4,7-CH3-phen)2(H2O)2]2+ | 2.4 × 10−8 | 2.6 × 10−8 | 2.5 × 10−8 | 3.4 × 10−8 |
| [Mn(4,7-CH3-phen)3]2+ | 2.2 × 10−8 | 2.4 × 10−8 | 2.8 × 10−8 | 3.1 × 10−8 |
| [Mn(5,6-CH3-phen)2(H2O)2]2+ | 2.3 × 10−8 | 3.2 × 10−8 | 2.3 × 10−8 | 4.7 × 10−8 |
| [Mn(5,6-CH3-phen)3]2+ | 2.1 × 10−8 | 4.1 × 10−8 | 2.1 × 10−8 | 4.1 × 10−8 |
| Complex | Increase in Anodic Peak High [%] in 10−3M Solution of Glycolic Acid Ascorbic Acid | Shift of Anodic Peak Potential [V] in 10−3M Solution of Glycolic Acid Ascorbic Acid | ||
|---|---|---|---|---|
| [Mn(4-CH3-phen)2(H2O)2]2+ | 112 | 125 | 0.018 | 0.093 |
| [Mn(4-CH3-phen)3]2+ | 116 | 139 | 0.025 | 0.081 |
| [Mn(5-CH3-phen)2(H2O)2]2+ | 111 | 123 | 0.035 | 0.045 |
| [Mn(5-CH3-phen)3]2+ | 112 | 129 | 0.018 | 0.044 |
| [Mn(5-Cl-phen)2(H2O)2]2+ | 115 | 128 | 0.013 | 0.048 |
| [Mn(5-Cl-phen)3]2+ | 120 | 164 | 0.045 | 0.040 |
| [Mn(5-NO2-phen)2(H2O)2]2+ | 119 | 130 | 0.088 | 0.026 |
| [Mn(5-NO2-phen)3]2+ | 123 | 173 | 0.004 | 0.013 |
| [Mn(4,7-CH3-phen)2(H2O)2]2+ | 102 | 113 | 0.013 | 0.057 |
| [Mn(4,7-CH3-phen)3]2+ | 106 | 131 | 0.027 | 0.044 |
| [Mn(5,6-CH3-phen)2(H2O)2]2+ | 104 | 121 | 0.099 | 0.084 |
| [Mn(5,6-CH3-phen)3]2+ | 108 | 134 | 0.034 | 0.045 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomczyk, D.; Seliger, P. Modification of Glassy Carbon Electrodes with Complexes of Manganese(II) with Some Phenanthroline Derivatives Immobilized in Nafion Layer. Int. J. Mol. Sci. 2024, 25, 2348. https://doi.org/10.3390/ijms25042348
Tomczyk D, Seliger P. Modification of Glassy Carbon Electrodes with Complexes of Manganese(II) with Some Phenanthroline Derivatives Immobilized in Nafion Layer. International Journal of Molecular Sciences. 2024; 25(4):2348. https://doi.org/10.3390/ijms25042348
Chicago/Turabian StyleTomczyk, Danuta, and Piotr Seliger. 2024. "Modification of Glassy Carbon Electrodes with Complexes of Manganese(II) with Some Phenanthroline Derivatives Immobilized in Nafion Layer" International Journal of Molecular Sciences 25, no. 4: 2348. https://doi.org/10.3390/ijms25042348
APA StyleTomczyk, D., & Seliger, P. (2024). Modification of Glassy Carbon Electrodes with Complexes of Manganese(II) with Some Phenanthroline Derivatives Immobilized in Nafion Layer. International Journal of Molecular Sciences, 25(4), 2348. https://doi.org/10.3390/ijms25042348






