The Effects of Fucoidan Derived from Sargassum filipendula and Fucus vesiculosus on the Survival and Mineralisation of Osteogenic Progenitors
Abstract
1. Introduction
2. Results
2.1. Characterisation of Fucoidans
2.2. Effect of Fucoidan on hES-MP Attachment
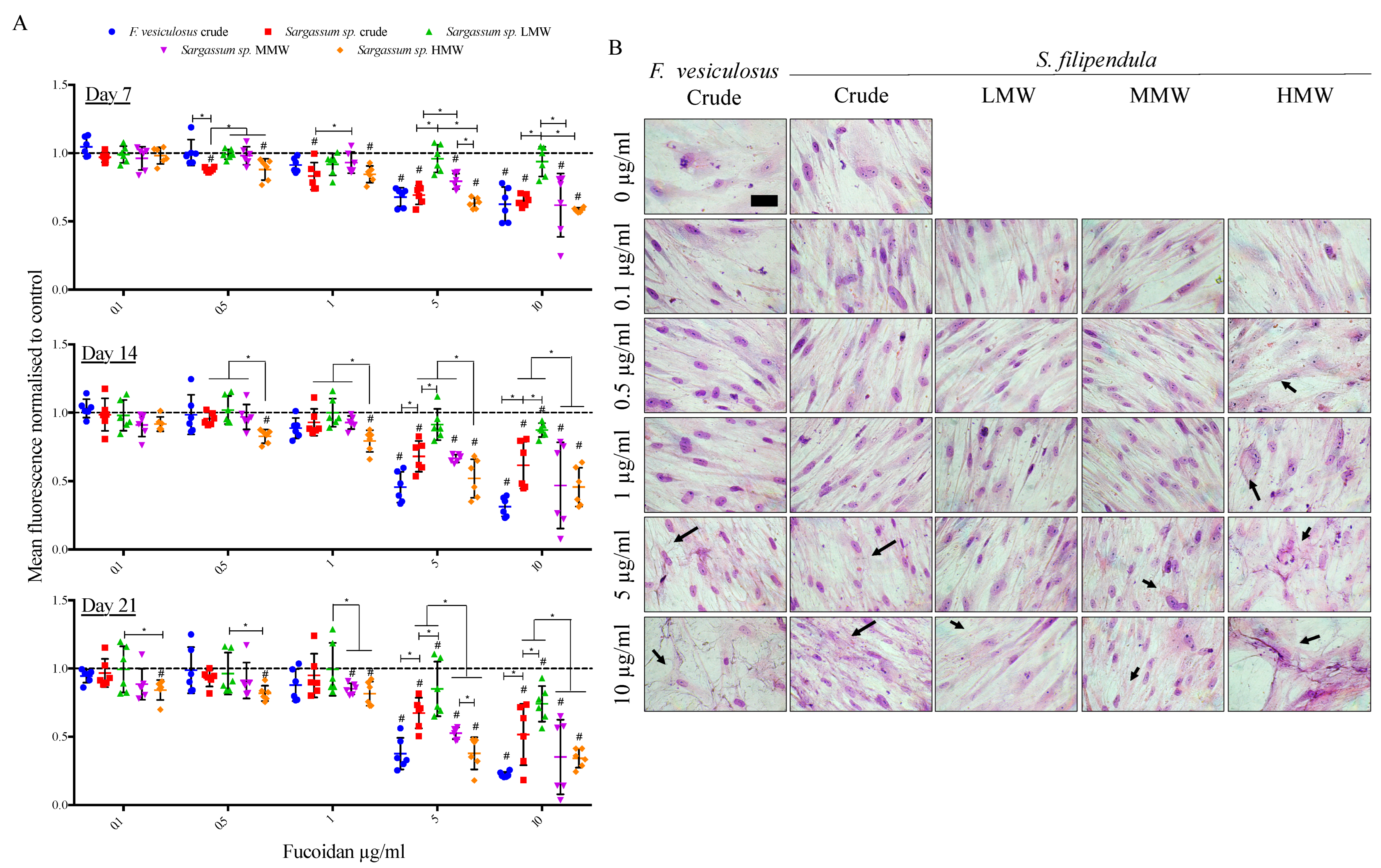
2.3. Effect of Fucoidan on hES-MP Proliferation and Morphology
2.4. Cell Cycle Analysis
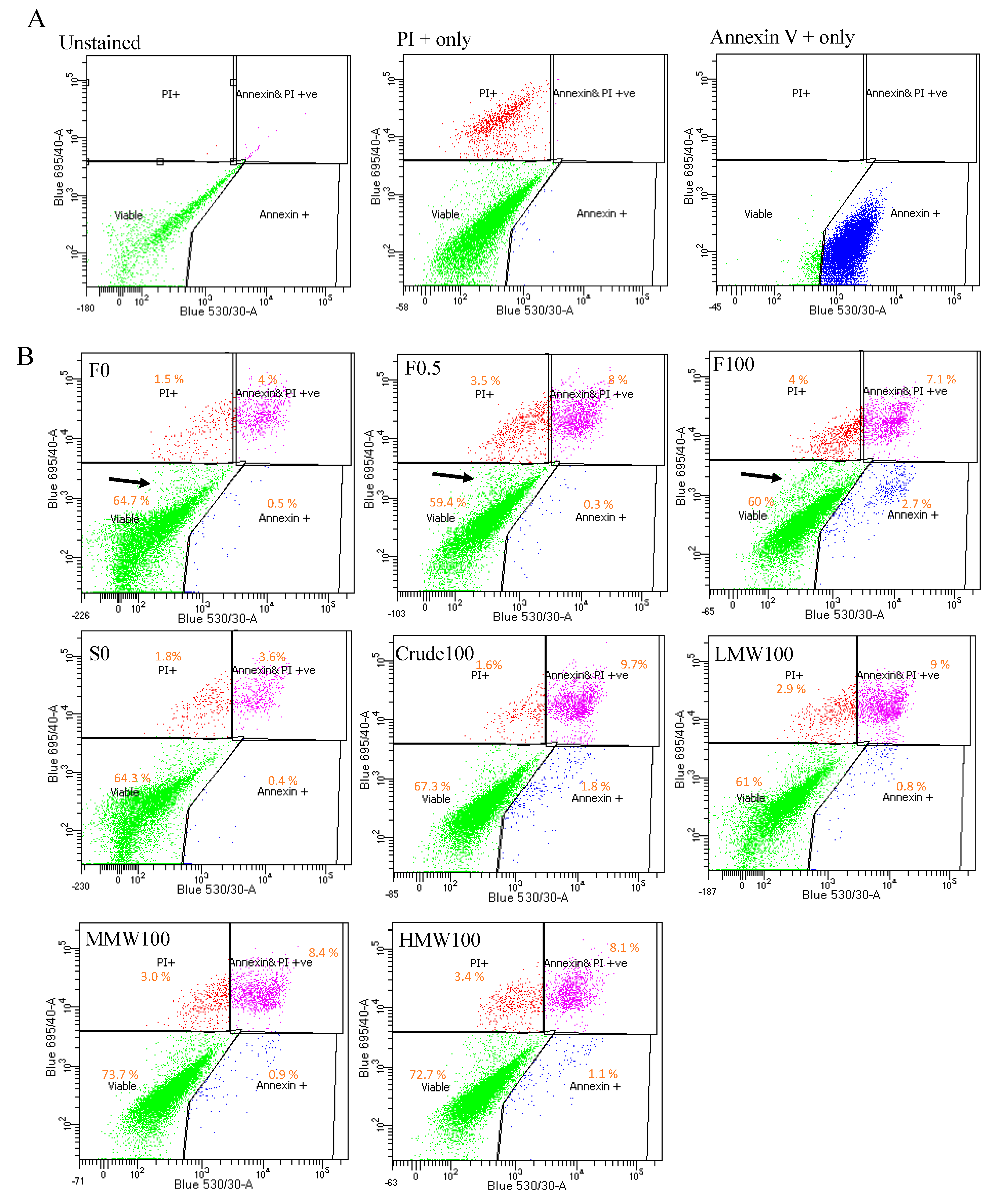
2.5. Cell Death Analysis
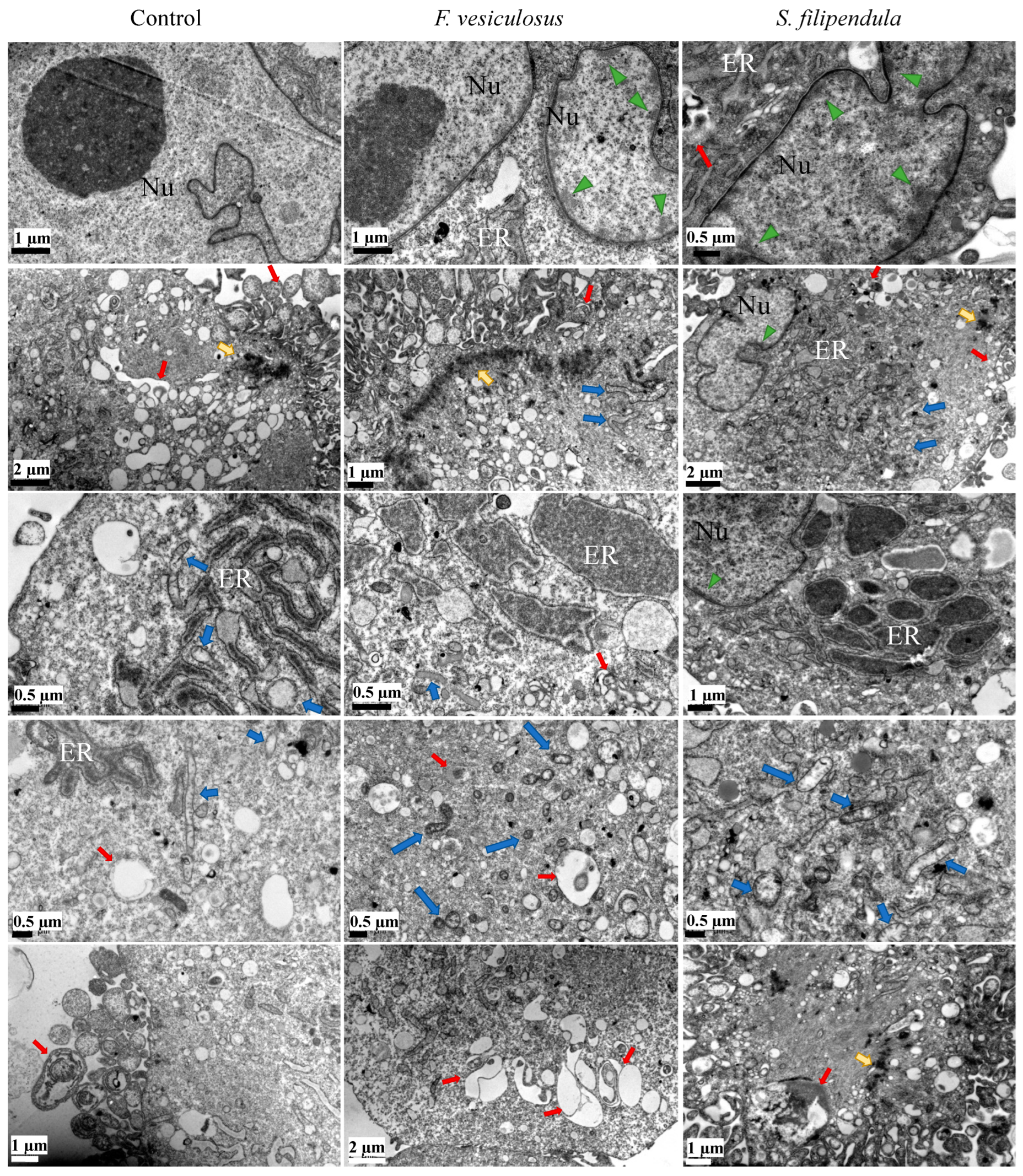
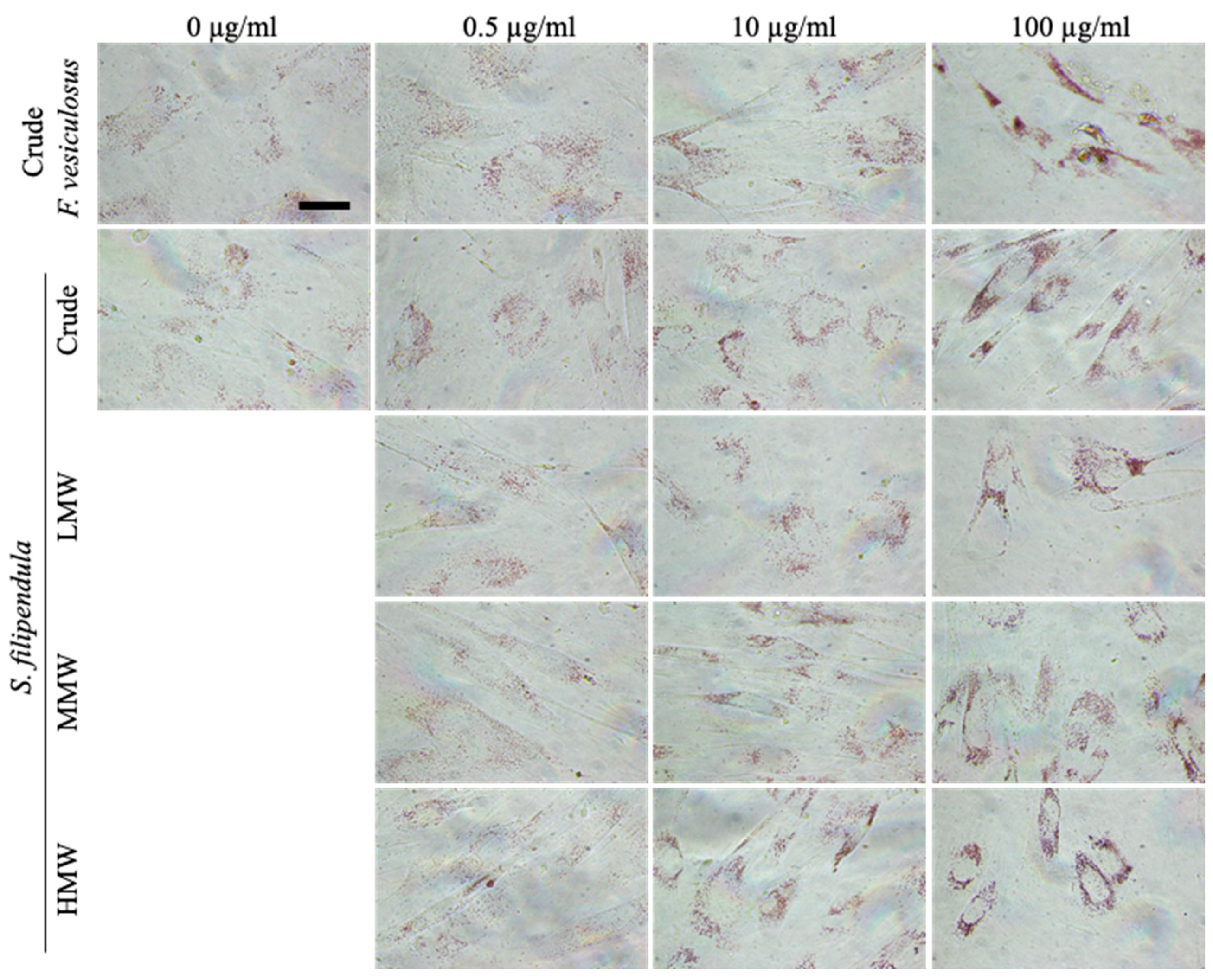
2.6. Ultrastructure Examination of hES-MPs Treated with Fucoidan
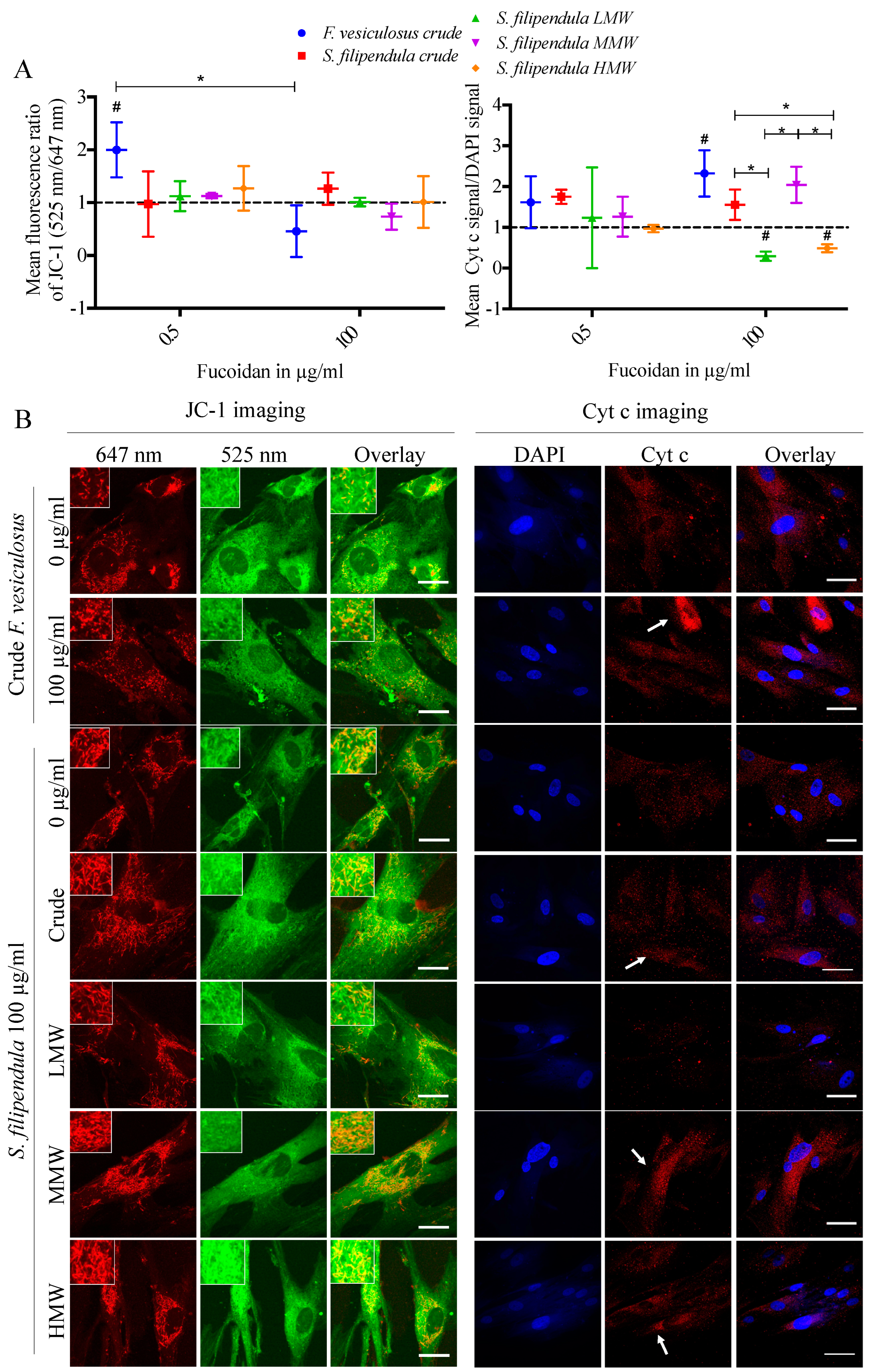
2.7. Effect of Fucoidan on Mitochondrial Health
2.8. Localisation of Fucoidan in hES-MPs
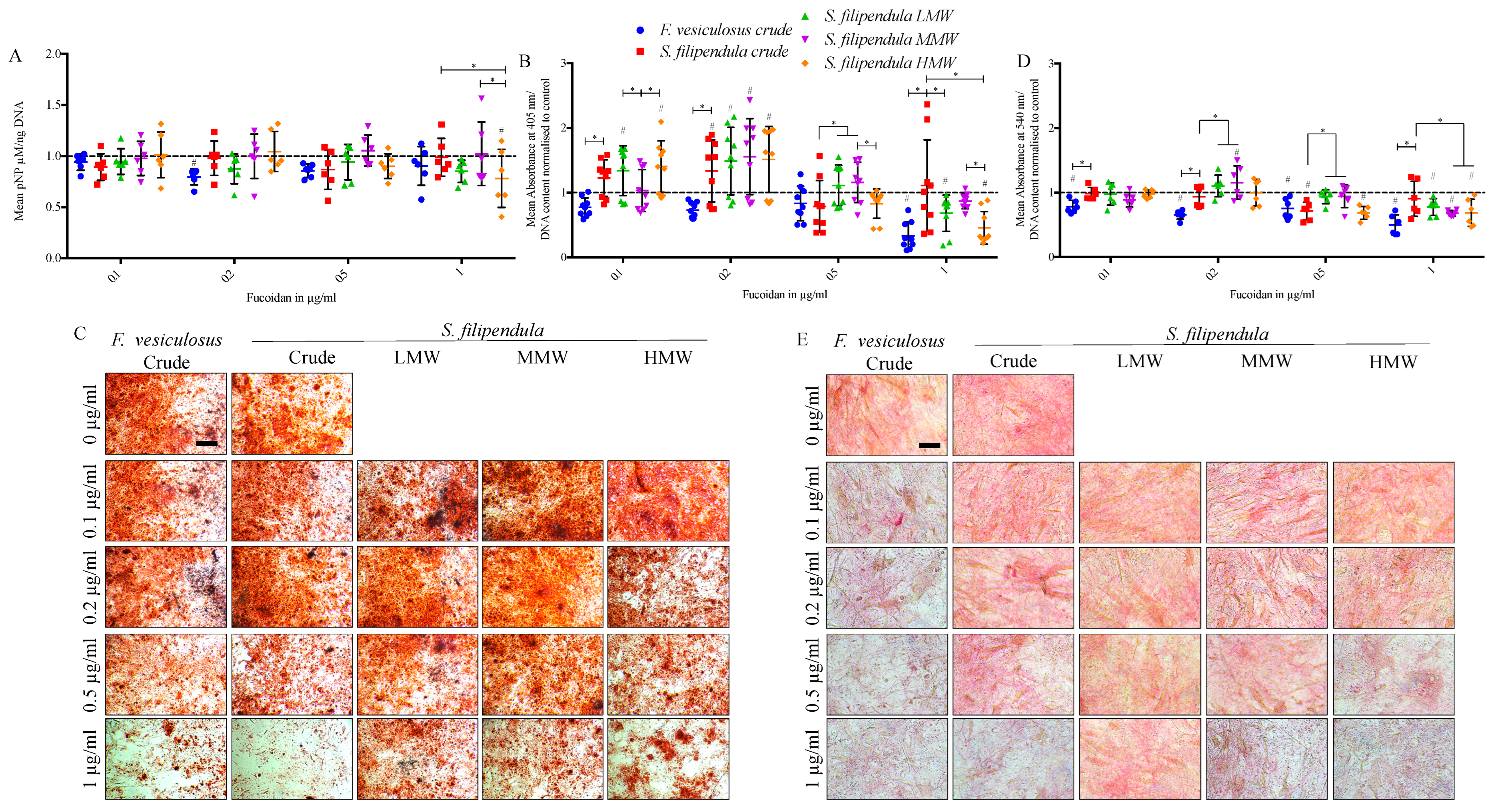
2.9. Effect of Fucoidan on Osteogenic Differentiation
3. Discussion
4. Materials and Methods
4.1. Extraction and Purification of Fucoidan
4.2. FTIR-ATR
4.3. Culturing of Human Embryonic Derived-Mesenchymal Progenitor (hES-MP) Cells
4.4. Cell Metabolic Activity Assay
4.5. Focal Adhesion Staining
4.6. DNA Content Assay
4.7. Giemsa Staining
4.8. Cell Cycle Analysis
4.9. Annexin V/PI Staining
4.10. TEM
4.11. Neutral Red Staining
4.12. JC-1 Staining
4.13. ALP Activity Assay
4.14. Sirius Red Assay
4.15. Alizarin Red S Staining
4.16. Immunostaining for Cytochrome c (cyt c) and Fucoidan
4.17. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ottaviani, G.; Jaffe, N. The Epidemiology of Osteosarcoma BT-Pediatric and Adolescent Osteosarcoma; Jaffe, N., Bruland, O.S., Bielack, S., Eds.; Springer: Boston, MA, USA, 2010; pp. 3–13. ISBN 978-1-4419-0284-9. [Google Scholar]
- Tiwari, A. Current Concepts in Surgical Treatment of Osteosarcoma. J. Clin. Orthop. Trauma 2012, 3, 4–9. [Google Scholar] [CrossRef]
- Luetke, A.; Meyers, P.A.; Lewis, I.; Juergens, H. Osteosarcoma Treatment-Where Do We Stand? A State of the Art Review. Cancer Treat. Rev. 2014, 40, 523–532. [Google Scholar] [CrossRef]
- Huang, J.; Ni, J.; Liu, K.; Yu, Y.; Xie, M.; Kang, R.; Vernon, P.; Cao, L.; Tang, D. HMGB1 Promotes Drug Resistance in Osteosarcoma. Cancer Res. 2012, 72, 230–238. [Google Scholar] [CrossRef]
- Graci, C.; Maccauro, G.; Muratorp, F.; Spinelli, M.S.; Rosa, M.A.; Fabbriciani, C. Infection Following Bone Tumor Resection and Reconstruction with Tumoral Prostheses: A Literature Review. Int. J. Immunopathol. Pharmacol. 2010, 23, 1005–1013. [Google Scholar] [CrossRef]
- Lim, J.S.; Kim, D.H.; Lee, J.A.; Kim, D.H.; Cho, J.; Cho, W.H.; Lee, S.Y.; Jeon, D.G. Young Age at Diagnosis, Male Sex, and Decreased Lean Mass Are Risk Factors of Osteoporosis in Long-Term Survivors of Osteosarcoma. J. Pediatr. Hematol. Oncol. 2013, 35, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Holzer, G.; Krepler, P.; Koschat, M.A.; Grampp, S.; Dominkus, M.; Kotz, R. Bone Mineral Density in Long-Term Survivors of Highly Malignant Osteosarcoma. J. Bone Jt. Surg.-Ser. B 2003, 85, 231–237. [Google Scholar] [CrossRef]
- Kanis, J.A.; McCloskey, E.V.; Johansson, H.; Cooper, C.; Rizzoli, R.; Reginster, J.Y. European Guidance for the Diagnosis and Management of Osteoporosis in Postmenopausal Women. Osteoporos. Int. 2013, 24, 23–57. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and Bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef]
- Senthilkumar, K.; Ramajayam, G.; Venkatesan, J.; Kim, S.K.; Ahn, B.C. Biomedical Applications of Fucoidan, Seaweed Polysaccharides. In Seaweed Polysaccharides: Isolation, Biological and Biomedical Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 269–281. ISBN 9780128098172. [Google Scholar]
- Jin, W.; Zhang, Q.; Wang, J.; Zhang, W. A Comparative Study of the Anticoagulant Activities of Eleven Fucoidans. Carbohydr. Polym. 2013, 91, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, W.A.J.P.; Jeon, Y.J. Biological Activities and Potential Industrial Applications of Fucose Rich Sulfated Polysaccharides and Fucoidans Isolated from Brown Seaweeds: A Review. Carbohydr. Polym. 2012, 88, 13–20. [Google Scholar] [CrossRef]
- Kimura, R.; Rokkaku, T.; Takeda, S.; Senba, M.; Mori, N. Cytotoxic Effects of Fucoidan Nanoparticles against Osteosarcoma. Mar. Drugs 2013, 11, 4267–4278. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jeon, T.J. Fucoidan Induces Cell Aggregation and Apoptosis in Osteosarcoma MG-63 Cells. Anim. Cells Syst. 2016, 20, 186–192. [Google Scholar] [CrossRef]
- Wang, F.; Schmidt, H.; Pavleska, D.; Wermann, T.; Seekamp, A.; Fuchs, S. Crude Fucoidan Extracts Impair Angiogenesis in Models Relevant for Bone Regeneration and Osteosarcoma via Reduction of VEGF and SDF-1. Mar. Drugs 2017, 15, 186. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Silva, M.; Radziun, K.; Martinez, D.C.; Hill, C.J.; Marshall, J.; Hearnden, V.; Puertas-Mejia, M.A.; Reilly, G.C. Fucoidan Inhibition of Osteosarcoma Cells Is Species and Molecular Weight Dependent. Mar. Drugs 2020, 18, 104. [Google Scholar] [CrossRef]
- Changotade, S.I.T.; Korb, G.; Bassil, J.; Barroukh, B.; Willig, C.; Colliec-Jouault, S.; Durand, P.; Godeau, G.; Senni, K. Potential Effects of a Low-Molecular-Weight Fucoidan Extracted from Brown Algae on Bone Biomaterial Osteoconductive Properties. J. Biomed. Mater. Res.-Part A 2008, 87, 666–675. [Google Scholar] [CrossRef]
- Hwang, P.A.; Hung, Y.L.; Phan, N.N.; Hieu, B.T.N.; Chang, P.M.; Li, K.L.; Lin, Y.C.; Cytotechnology, D.O.I. The In Vitro and In Vivo Effects of the Low Molecular Weight Fucoidan on the Bone Osteogenic Differentiation Properties. Cytotechnology 2016, 68, 1349–1359. [Google Scholar] [CrossRef]
- Pereira, J.; Portron, S.; Dizier, B.; Vinatier, C.; Masson, M.; Sourice, S.; Galy-Fauroux, I.; Corre, P.; Weiss, P.; Fischer, A.-M.; et al. The in Vitro and in Vivo Effects of a Low-Molecular-Weight Fucoidan on the Osteogenic Capacity of Human Adipose-Derived Stromal Cells. Tissue Eng. Part A 2014, 20, 275–284. [Google Scholar] [CrossRef]
- Kim, B.S.; Kang, H.J.; Park, J.Y.; Lee, J. Fucoidan Promotes Osteoblast Differentiation via JNK- and ERK-Dependent BMP2-Smad 1/5/8 Signaling in Human Mesenchymal Stem Cells. Exp. Mol. Med. 2015, 47, e128–e129. [Google Scholar] [CrossRef]
- Puvaneswary, S.; Talebian, S.; Raghavendran, H.B.; Murali, M.R.; Mehrali, M.; Afifi, A.M.; Kasim, N.H.B.A.; Kamarul, T. Fabrication and in Vitro Biological Activity of ΒTCP-Chitosan-Fucoidan Composite for Bone Tissue Engineering. Carbohydr. Polym. 2015, 134, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Ahn, T.Y.; Kang, J.H.; Kang, D.J.; Venkatesan, J.; Chang, H.K.; Bhatnagar, I.; Chang, K.-Y.; Hwang, J.-H.; Salameh, Z.; Kim, S.-K.; et al. Interaction of Stem Cells with Nano Hydroxyapatite-Fucoidan Bionanocomposites for Bone Tissue Regeneration. Int. J. Biol. Macromol. 2016, 93, 1488–1491. [Google Scholar] [CrossRef]
- Lee, J.S.; Jin, G.H.; Yeo, M.G.; Jang, C.H.; Lee, H.; Kim, G.H. Fabrication of Electrospun Biocomposites Comprising Polycaprolactone/ Fucoidan for Tissue Regeneration. Carbohydr. Polym. 2012, 90, 181–188. [Google Scholar] [CrossRef]
- Park, S.; Lee, K.W.; Lim, D.-S.; Lee, S. The Sulfated Polysaccharide Fucoidan Stimulates Osteogenic Differentiation of Human Adipose-Derived Stem Cells. Stem Cells Dev. 2012, 21, 2204–2211. [Google Scholar] [CrossRef]
- Jeong, H.; Venkatesan, J.; Kim, S. Hydroxyapatite-Fucoidan Nanocomposites for Bone Tissue Engineering. Int. J. Biol. Macromol. 2013, 57, 138–141. [Google Scholar] [CrossRef]
- Puvaneswary, S.; Raghavendran, H.B.; Talebian, S.; Murali, M.R.; Mahmod, S.A.; Singh, S.; Kamarul, T. Incorporation of Fucoidan in β-Tricalcium Phosphate-Chitosan Scaffold Prompts the Differentiation of Human Bone Marrow Stromal Cells into Osteogenic Lineage. Sci. Rep. 2016, 6, 24202. [Google Scholar] [CrossRef]
- Venkatesan, J.; Bhatnagar, I.; Kim, S.K. Chitosan-Alginate Biocomposite Containing Fucoidan for Bone Tissue Engineering. Mar. Drugs 2014, 12, 300–316. [Google Scholar] [CrossRef]
- Cho, Y.S.; Jung, W.K.; Kim, J.A.; Choi, I.W.; Kim, S.K. Beneficial Effects of Fucoidan on Osteoblastic MG-63 Cell Differentiation. Food Chem. 2009, 116, 990–994. [Google Scholar] [CrossRef]
- Jang, H.-O.; Park, Y.-S.; Lee, J.-H.; Seo, J.-B.; Koo, K.-I.; Jeong, S.-C.; Jin, S.-D.; Lee, Y.-H.; Eom, H.-S.; Yun, I. Effect of Extracts from Safflower Seeds on Osteoblast Differentiation and Intracellular Calcium Ion Concentration in MC3T3-E1 Cells. Nat. Prod. Res. 2007, 21, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.T.; Baek, S.H.; Jeong, S.C.; Yoon, Y.D.; Kim, O.H.; Oh, B.C.; Jung, J.W.; Kim, J.H. Osteoprotective Effects of Polysaccharide-Enriched Hizikia fusiforme Processing Byproduct In Vitro and In Vivo Models. J. Med. Food 2016, 19, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Luna-Pérez, Y.; Ríos-López, L.G.; Otero-Tejada, E.L.; Mejía-Giraldo, J.C.; Puertas-Mejía, M.Á. Sargassum Filipendula, a Source of Bioactive Compounds with Antioxidant and Matrix Metalloproteinases Inhibition Activities In Vitro with Potential Dermocosmetic Application. Antioxidants 2023, 12, 876. [Google Scholar] [CrossRef] [PubMed]
- Zayed, A.; El-Aasr, M.; Ibrahim, A.R.S.; Ulber, R. Fucoidan Characterization: Determination of Purity and Physicochemical and Chemical Properties. Mar. Drugs 2020, 18, 571. [Google Scholar] [CrossRef] [PubMed]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important Determinants for Fucoidan Bioactivity: A Critical Review of Structure-Function Relations and Extraction Methods for Fucose-Containing Sulfated Polysaccharides from Brown Seaweeds. Mar. Drugs 2011, 9, 2106–2130. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.L.; Lee, B.Y.; You, S.G. Relationship between Oversulfation and Conformation of Low and High Molecular Weight Fucoidans and Evaluation of Their in Vitro Anticancer Activity. Molecules 2011, 16, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Haroun-Bouhedja, F.; Ellouali, M.; Sinquin, C.; Boisson-Vidal, C. Relationship between Sulfate Groups and Biological Activities of Fucans. Thromb. Res. 2000, 100, 453–459. [Google Scholar] [CrossRef]
- Jang, H.; Kang, K.; El-Sayed, M.A. Real-Time Tracking of the Autophagy Process in Living Cells Using Plasmonically Enhanced Raman Spectroscopy of Fucoidan-Coated Gold Nanoparticles. J. Mater. Chem. B 2018, 6, 5460–5465. [Google Scholar] [CrossRef]
- Torode, T.A.; Marcus, S.E.; Jam, M.; Tonon, T.; Blackburn, R.S.; Hervé, C.; Knox, J.P. Monoclonal Antibodies Directed to Fucoidan Preparations from Brown Algae. PLoS ONE 2015, 10, e0118366. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, C.; Emanuelsson, K.; Wessberg, F.; Kajic, K.; Axell, M.Z.; Eriksson, P.S.; Lindahl, A.; Hyllner, J.; Strehl, R. Human Embryonic Stem Cell-Derived Mesenchymal Progenitors-Potential in Regenerative Medicine. Stem Cell Res. 2009, 3, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Grant, D.M.; Hossain, K.M.Z.; Ahmed, I.; Sottile, V. Role of Geometrical Cues in Bone Marrow-Derived Mesenchymal Stem Cell Survival, Growth and Osteogenic Differentiation. J. Biomater. Appl. 2018, 32, 906–919. [Google Scholar] [CrossRef]
- Gupta, D.; Hossain, K.M.Z.; Roe, M.; Smith, E.F.; Ahmed, I.; Sottile, V.; Grant, D.M. Long-Term Culture of Stem Cells on Phosphate-Based Glass Microspheres: Synergistic Role of Chemical Formulation and 3D Architecture. ACS Appl. Bio Mater. 2021, 4, 5987–6004. [Google Scholar] [CrossRef]
- Perelman, A.; Wachtel, C.; Cohen, M.; Haupt, S.; Shapiro, H.; Tzur, A. JC-1: Alternative Excitation Wavelengths Facilitate Mitochondrial Membrane Potential Cytometry. Cell Death Dis. 2012, 3, e430. [Google Scholar] [CrossRef]
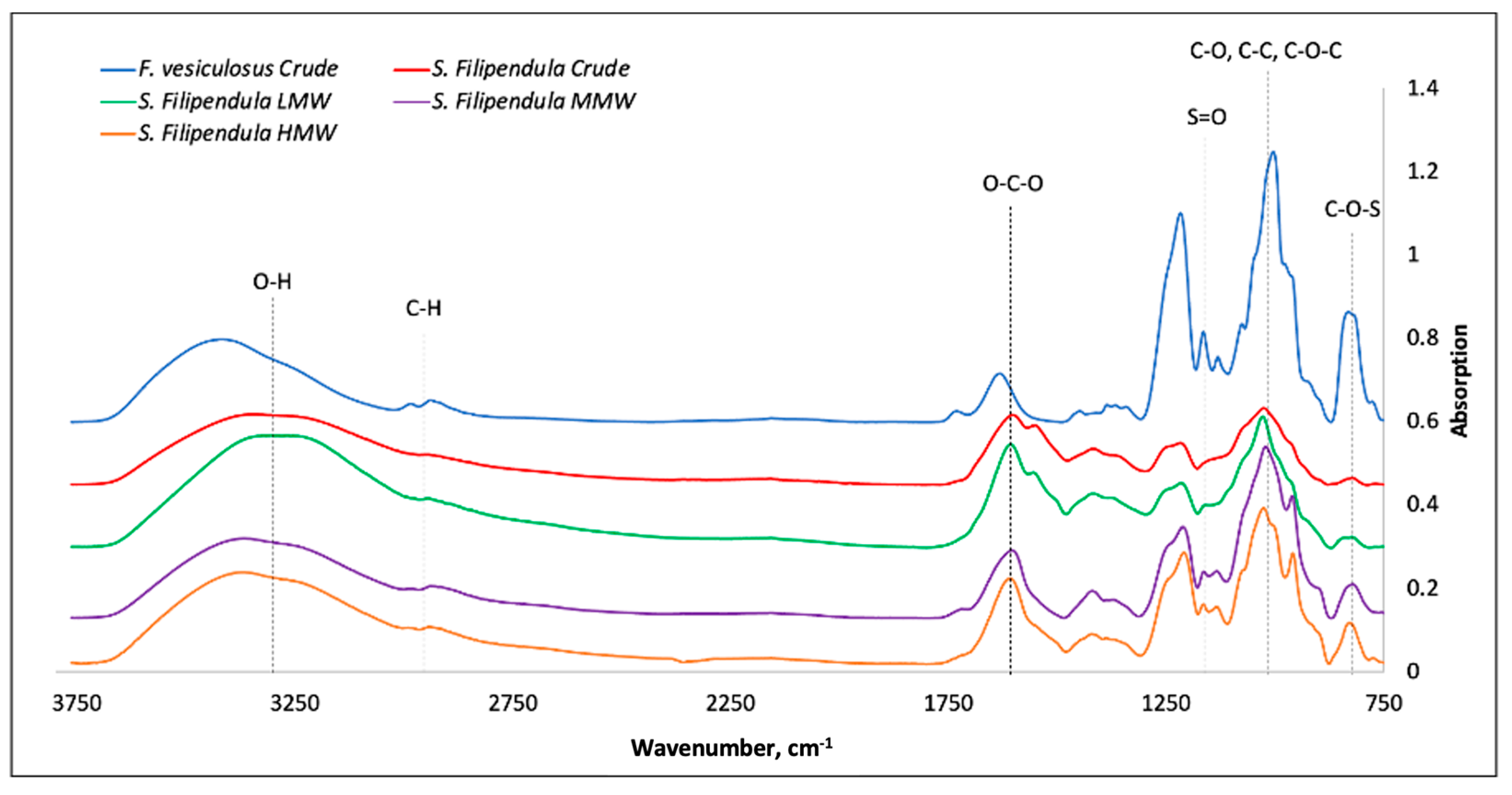




| Wavelength, cm−1 | Functional Group Assignment |
|---|---|
| 3400–1600 | O-H, C-H, O-C-O; C-OH |
| 1610–1430 | Uronic acid |
| 1240–1210 | Sulphated group extension |
| 900–950 | Glycosidic bonds |
| 810–840 | Sulphated group |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, D.; Martinez, D.C.; Puertas-Mejía, M.A.; Hearnden, V.L.; Reilly, G.C. The Effects of Fucoidan Derived from Sargassum filipendula and Fucus vesiculosus on the Survival and Mineralisation of Osteogenic Progenitors. Int. J. Mol. Sci. 2024, 25, 2085. https://doi.org/10.3390/ijms25042085
Gupta D, Martinez DC, Puertas-Mejía MA, Hearnden VL, Reilly GC. The Effects of Fucoidan Derived from Sargassum filipendula and Fucus vesiculosus on the Survival and Mineralisation of Osteogenic Progenitors. International Journal of Molecular Sciences. 2024; 25(4):2085. https://doi.org/10.3390/ijms25042085
Chicago/Turabian StyleGupta, Dhanak, Diana C. Martinez, Miguel Angel Puertas-Mejía, Vanessa L. Hearnden, and Gwendolen C. Reilly. 2024. "The Effects of Fucoidan Derived from Sargassum filipendula and Fucus vesiculosus on the Survival and Mineralisation of Osteogenic Progenitors" International Journal of Molecular Sciences 25, no. 4: 2085. https://doi.org/10.3390/ijms25042085
APA StyleGupta, D., Martinez, D. C., Puertas-Mejía, M. A., Hearnden, V. L., & Reilly, G. C. (2024). The Effects of Fucoidan Derived from Sargassum filipendula and Fucus vesiculosus on the Survival and Mineralisation of Osteogenic Progenitors. International Journal of Molecular Sciences, 25(4), 2085. https://doi.org/10.3390/ijms25042085







