Exploring the Importance of Differential Expression of Autophagy Markers in Term Placentas from Late-Onset Preeclamptic Pregnancies
Abstract
1. Introduction
2. Results
2.1. Clinical Characteristics of Patients
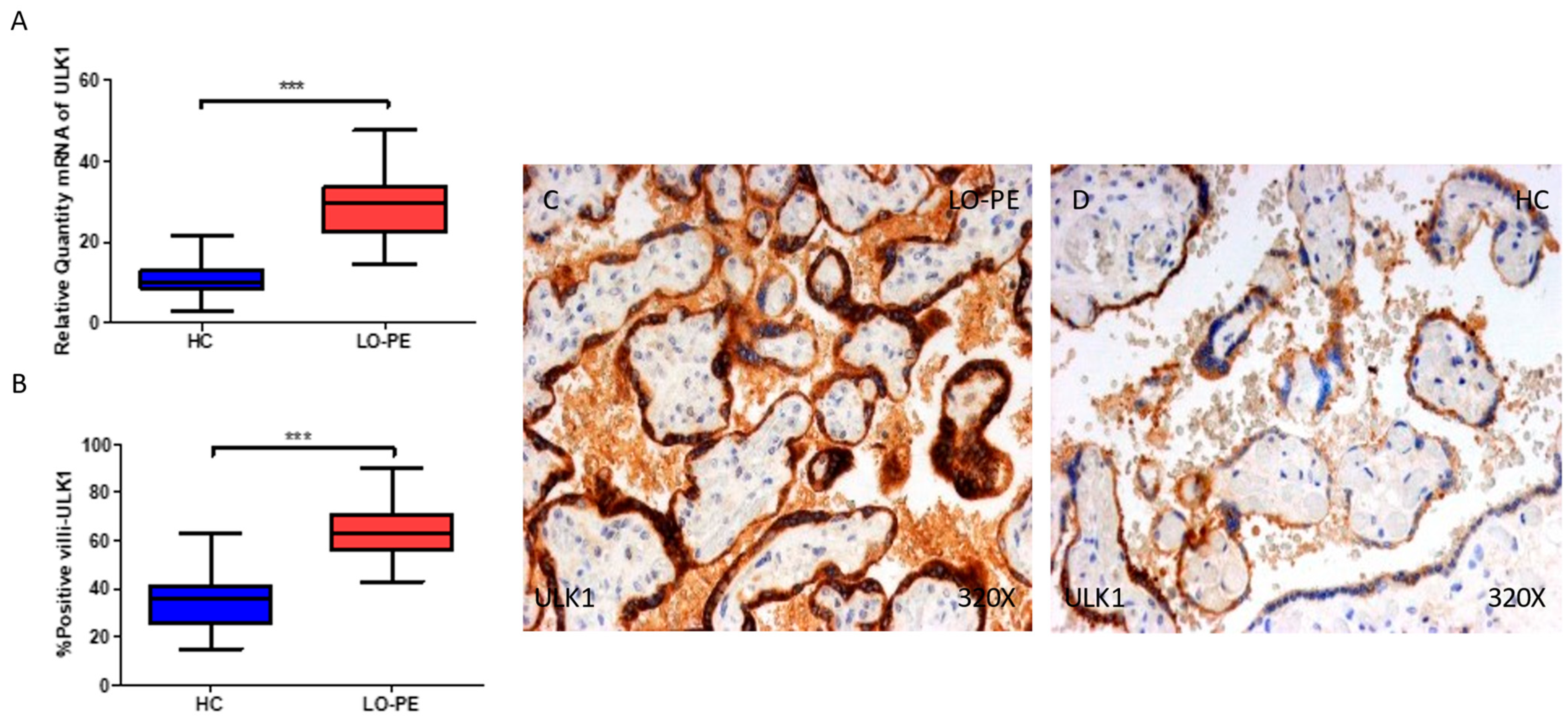
2.2. The Placentas of Women with Late-Onset Preeclampsia Exhibit Increased Expression of Autophagic Proteins Involved in the Initiation Phase, ULK-1 and ATG9A
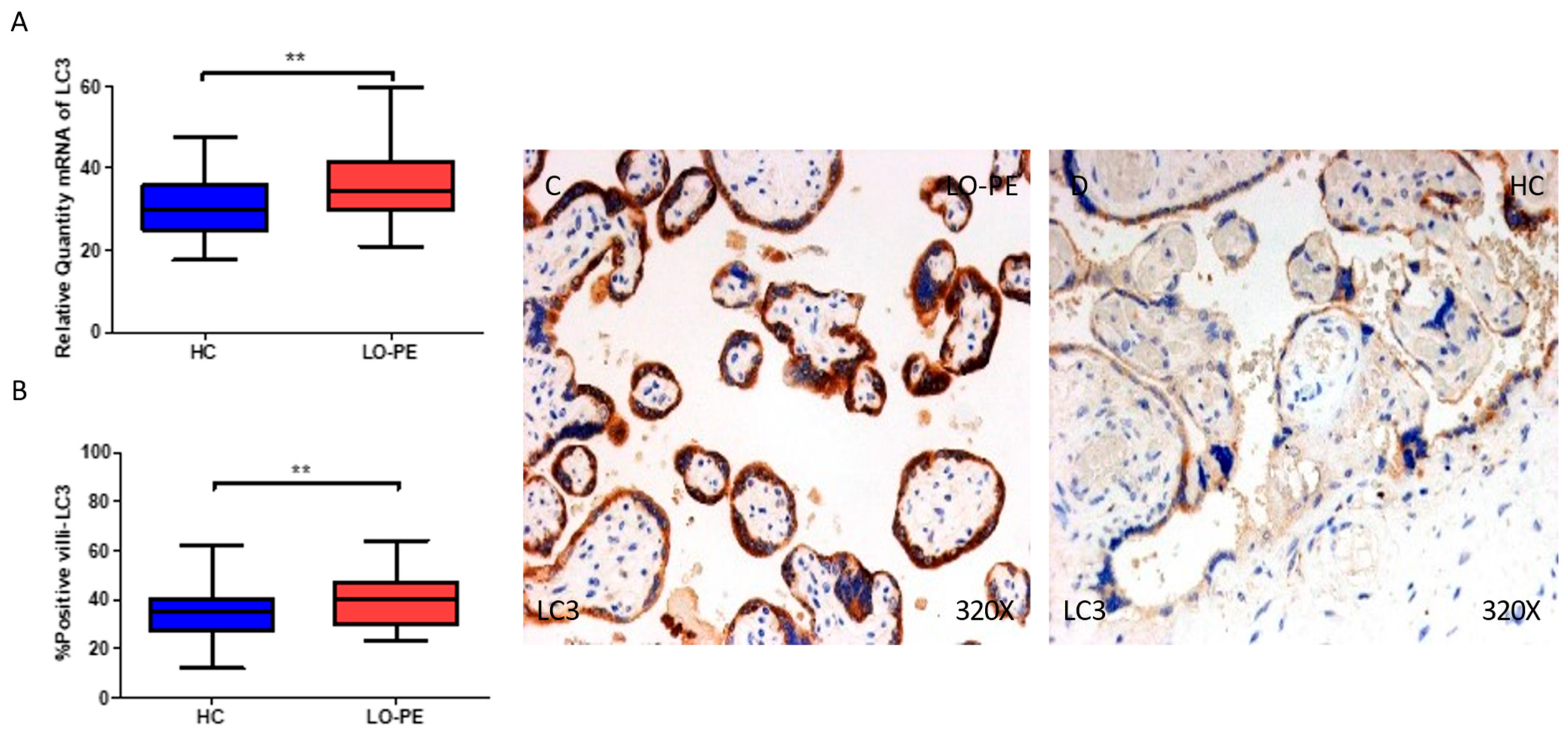
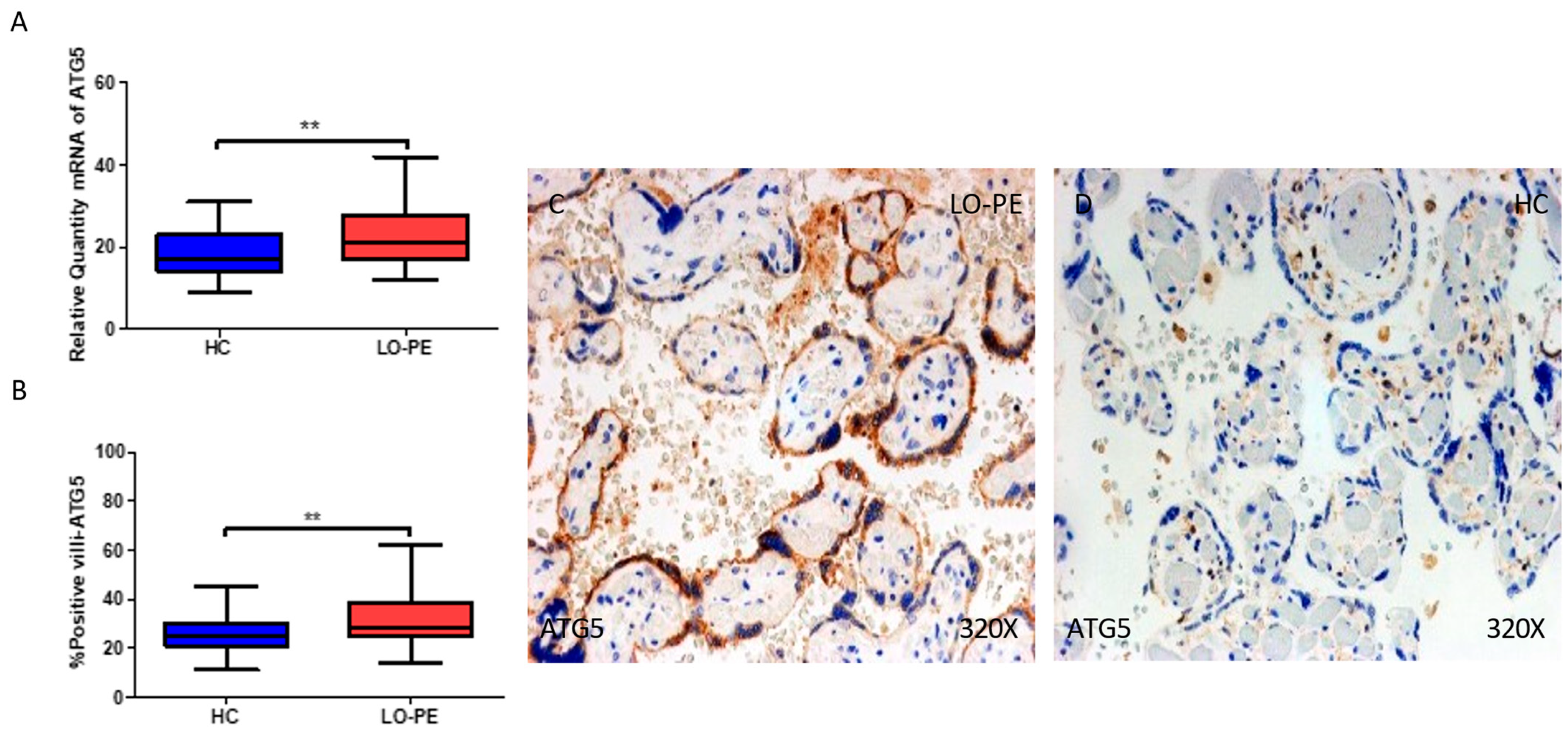
2.3. The Placental Tissue of Women with Late-Onset Preeclampsia Displays Augmented Expression of Autophagic Proteins Involved in the Elongation Phase, LC3 and ATG5
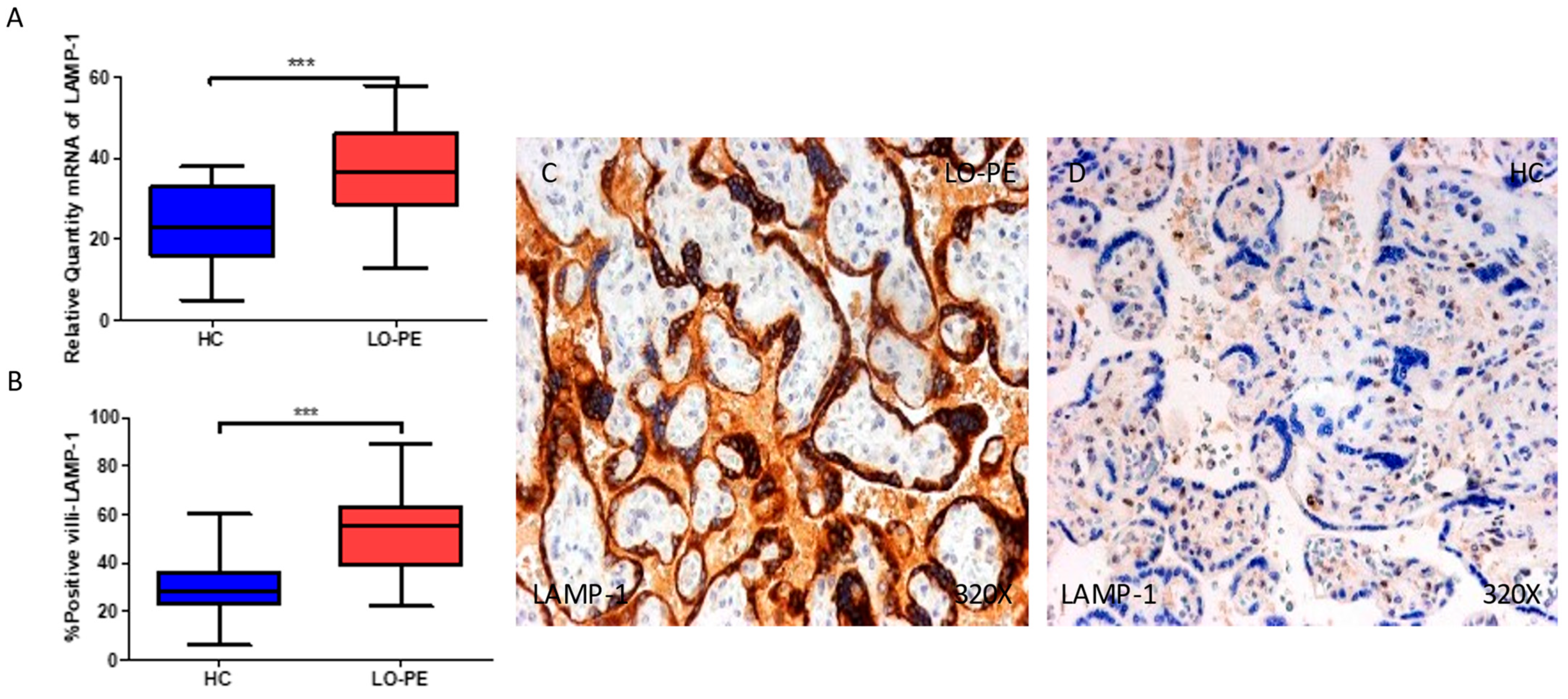
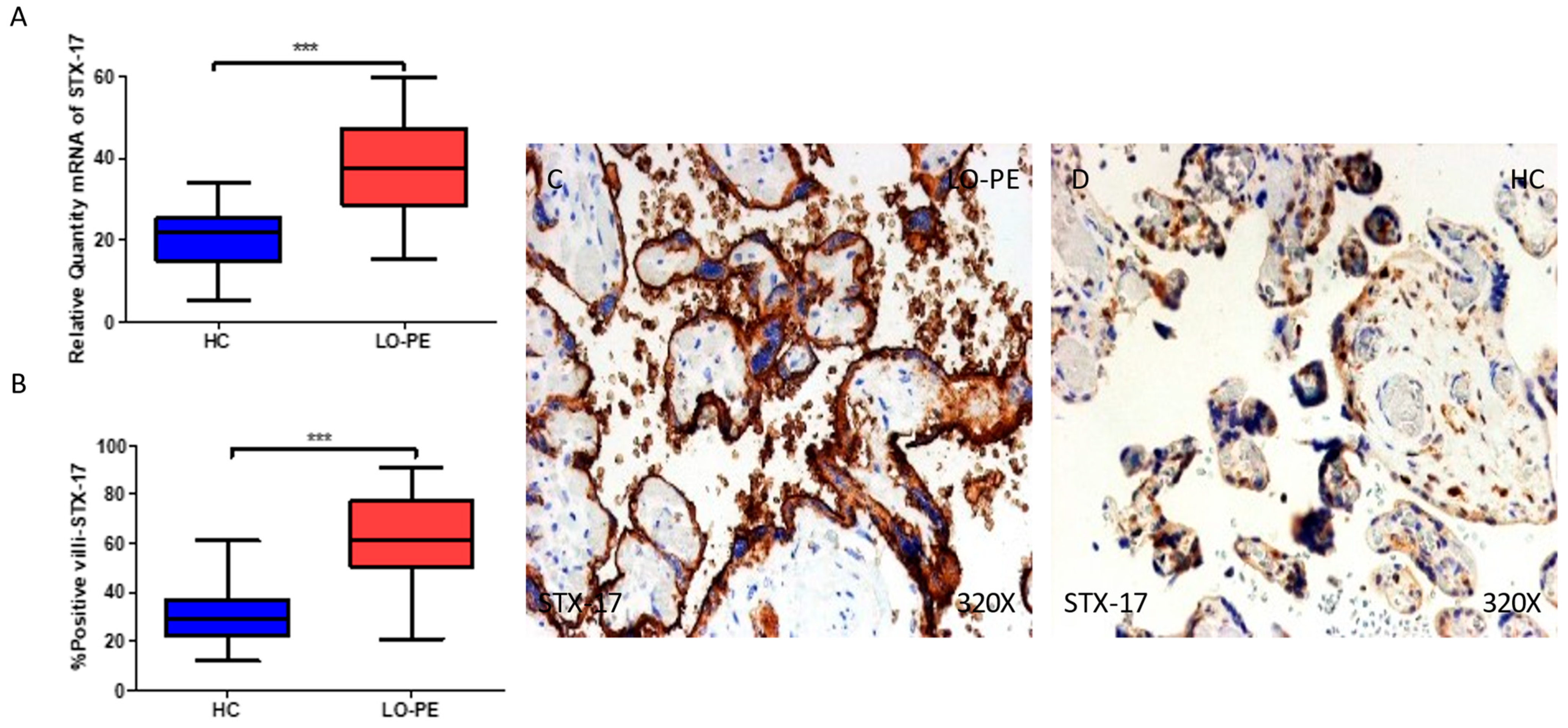
2.4. The Chorionic Villi of Women with Late-Onset Preeclampsia Show Augmented Expression of Autophagic Proteins Involved in the Fusion Phase, LAMP-1 and STX-17
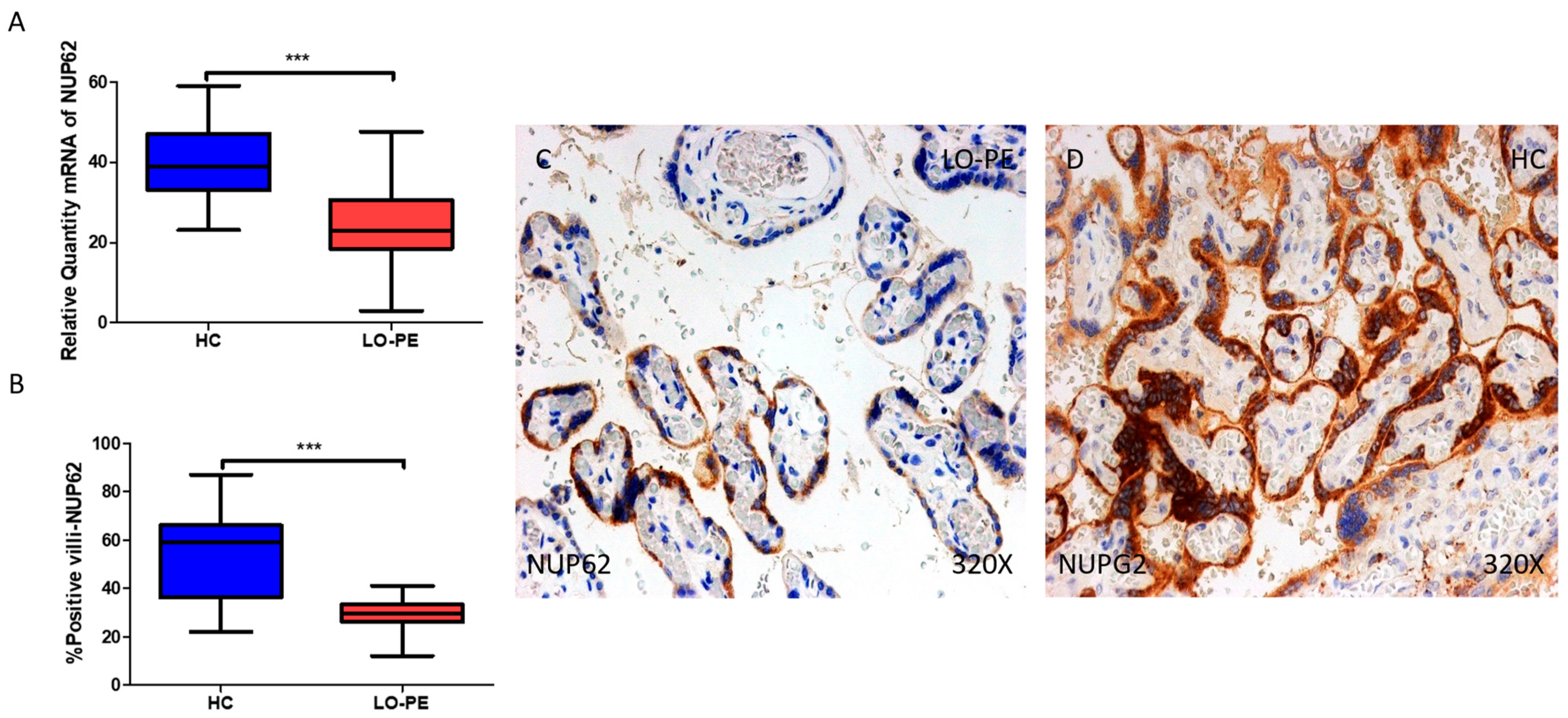
2.5. The Chorionic Villi of Women with Late-Onset Preeclampsia Report Diminished Expression of Autophagic Regulator NUP62
3. Discussion
4. Materials and Methods
4.1. Study Design and Participants
4.2. Sample Processing
4.3. Gene Expression Determination
4.4. Immunohistochemistry
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.; Le Ray, I.; Zhu, J.; Zhang, J.; Hua, J.; Reilly, M. Preeclampsia Prevalence, Risk Factors, and Pregnancy Outcomes in Sweden and China. JAMA Netw. Open 2021, 4, e218401. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martínez, O.; García-Montero, C.; Sáez, M.A.; Álvarez-Mon, M.A.; Torres-Carranza, D.; Álvarez-Mon, M.; Bujan, J.; García-Honduvilla, N.; Bravo, C.; et al. The Pivotal Role of the Placenta in Normal and Pathological Pregnancies: A Focus on Preeclampsia, Fetal Growth Restriction, and Maternal Chronic Venous Disease. Cells 2022, 11, 568. [Google Scholar] [CrossRef]
- Dimitriadis, E.; Rolnik, D.L.; Zhou, W.; Estrada-Gutierrez, G.; Koga, K.; Francisco, R.P.V.; Whitehead, C.; Hyett, J.; da Silva Costa, F.; Nicolaides, K.; et al. Pre-eclampsia. Nat. Rev. Dis. Primers 2023, 9, 8. [Google Scholar] [CrossRef]
- Roberge, S.; Bujold, E.; Nicolaides, K.H. Aspirin for the prevention of preterm and term preeclampsia: Systematic review and metaanalysis. Am. J. Obstet. Gynecol. 2018, 218, 287–293.e1. [Google Scholar] [CrossRef] [PubMed]
- English, F.A.; Kenny, L.C.; McCarthy, F.P. Risk factors and effective management of preeclampsia. Integr. Blood Press. Control 2015, 8, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Raymond, D.; Peterson, E. A critical review of early-onset and late-onset preeclampsia. Obstet. Gynecol. Surv. 2011, 66, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Wójtowicz, A.; Zembala-Szczerba, M.; Babczyk, D.; Kołodziejczyk-Pietruszka, M.; Lewaczyńska, O.; Huras, H. Early- and Late-Onset Preeclampsia: A Comprehensive Cohort Study of Laboratory and Clinical Findings according to the New ISHHP Criteria. Int. J. Hypertens. 2019, 2019, 4108271. [Google Scholar] [CrossRef]
- Teka, H.; Yemane, A.; Abraha, H.E.; Berhe, E.; Tadesse, H.; Gebru, F.; Yahya, M.; Tadesse, Y.; Gebre, D.; Abrha, M.; et al. Clinical presentation, maternal-fetal, and neonatal outcomes of early-onset versus late onset preeclampsia-eclampsia syndrome in a teaching hospital in a low-resource setting: A retrospective cohort study. PLoS ONE 2023, 18, e281952. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Puente, L.M.; Fraile-Martinez, O.; García-Montero, C.; Bujan, J.; De León-Luis, J.A.; Bravo, C.; Rodríguez-Benitez, P.; Pintado, P.; Ruiz-Labarta, F.J.; Álvarez-Mon, M.; et al. Placentas from Women with Late-Onset Preeclampsia Exhibit Increased Expression of the NLRP3 Inflammasome Machinery. Biomolecules 2023, 13, 1644. [Google Scholar] [CrossRef]
- Deretic, V. Autophagy in inflammation, infection, and immunometabolism. Immunity 2021, 54, 437–453. [Google Scholar] [CrossRef]
- Glick, D.; Barth, S.; Macleod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef]
- Nie, T.; Zhu, L.; Yang, Q. The Classification and Basic Processes of Autophagy. Adv. Exp. Med. Biol. 2021, 1208, 3–16. [Google Scholar] [CrossRef]
- Levine, B.; Kroemer, G. SnapShot: Macroautophagy. Cell 2008, 132, 162.e1. [Google Scholar] [CrossRef]
- Lee, Y.A.; Noon, L.A.; Akat, K.M.; Ybanez, M.D.; Lee, T.F.; Berres, M.L.; Fujiwara, N.; Goossens, N.; Chou, H.I.; Parvin-Nejad, F.P.; et al. Autophagy is a gatekeeper of hepatic differentiation and carcinogenesis by controlling the degradation of Yap. Nat. Commun. 2018, 9, 4962. [Google Scholar] [CrossRef]
- Staff, A.C. The two-stage placental model of preeclampsia: An update. J. Reprod. Immunol. 2019, 134–135, 1–10. [Google Scholar] [CrossRef]
- Marín, R.; Chiarello, D.I.; Abad, C.; Rojas, D.; Toledo, F.; Sobrevia, L. Oxidative stress and mitochondrial dysfunction in early-onset and late-onset preeclampsia. Biochim. Biophys. Acta. Mol. Basis Dis. 2020, 1866, 165961. [Google Scholar] [CrossRef] [PubMed]
- Aneman, I.; Pienaar, D.; Suvakov, S.; Simic, T.P.; Garovic, V.D.; McClements, L. Mechanisms of Key Innate Immune Cells in Early- and Late-Onset Preeclampsia. Front. Immunol. 2020, 11, 1864. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Virgilio, L.; Silva-Lucero, M.D.C.; Flores-Morelos, D.S.; Gallardo-Nieto, J.; Lopez-Toledo, G.; Abarca-Fernandez, A.M.; Zacapala-Gómez, A.E.; Luna-Muñoz, J.; Montiel-Sosa, F.; Soto-Rojas, L.O.; et al. Autophagy: A Key Regulator of Homeostasis and Disease: An Overview of Molecular Mechanisms and Modulators. Cells 2022, 11, 2262. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.y.; Roh, C.R. Autophagy in the placenta. Obstet. Gynecol. Sci. 2017, 60, 241. [Google Scholar] [CrossRef] [PubMed]
- Lokeswara, A.W.; Hiksas, R.; Irwinda, R.; Wibowo, N. Preeclampsia: From Cellular Wellness to Inappropriate Cell Death, and the Roles of Nutrition. Front. Cell Dev. Biol. 2021, 9, 726513. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Nguyen, T.N.; Lam, W.K.; Buffalo, C.Z.; Lazarou, M.; Yokom, A.L.; Hurley, J.H. Structural basis for ATG9A recruitment to the ULK1 complex in mitophagy initiation. Sci. Adv. 2023, 9, eadg2997. [Google Scholar] [CrossRef] [PubMed]
- Diceglie, C.; Anelli, G.M.; Martelli, C.; Serati, A.; Dico, A.L.; Lisso, F.; Parisi, F.; Novielli, C.; Paleari, R.; Cetin, I.; et al. Placental Antioxidant Defenses and Autophagy-Related Genes in Maternal Obesity and Gestational Diabetes Mellitus. Nutrients 2021, 13, 1303. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, M.A.; Manzor, N.F.M.; Zulkifli, N.F.; Zainal, N.; Hayati, A.R.; Asnawi, A.W.A. A Review of Candidate Genes and Pathways in Preeclampsia–An Integrated Bioinformatical Analysis. Biology 2020, 9, 62. [Google Scholar] [CrossRef]
- Nakashima, A.; Shima, T.; Tsuda, S.; Aoki, A.; Kawaguchi, M.; Yoneda, S.; Yamaki-Ushijima, A.; Cheng, S.B.; Sharma, S.; Saito, S. Disruption of Placental Homeostasis Leads to Preeclampsia. Int. J. Mol. Sci. 2020, 21, 3298. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, B.; Tian, J.; Teng, X.; Liu, T. Vital role of autophagy flux inhibition of placental trophoblast cells in pregnancy disorders induced by HEV infection. Emerg. Microbes Infect. 2023, 12, 2276336. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.Y.; Choi, S.J.; Kim, K.H.; Cho, E.; Kim, J.H.; Roh, C.R. Autophagy-related proteins, LC3 and Beclin-1, in placentas from pregnancies complicated by preeclampsia. Reprod. Sci. 2008, 15, 912–920. [Google Scholar] [CrossRef]
- Weel, I.C.; Ribeiro, V.R.; Romão-Veiga, M.; Fioratti, E.G.; Peraçoli, J.C.; Peraçoli, M.T.S. Down-regulation of autophagy proteins is associated with higher mTOR expression in the placenta of pregnant women with preeclampsia. Brazilian, J. Med. Biol. Res. = Rev. Bras. Pesqui. Med. Biol. 2023, 55, e12283. [Google Scholar] [CrossRef]
- Du, J.; Ji, Q.; Dong, L.; Meng, Y.; Xin, G. HDAC4 Knockdown Induces Preeclampsia Cell Autophagy and Apoptosis by miR-29b. Reprod. Sci. 2021, 28, 334–342. [Google Scholar] [CrossRef]
- Ji, L.; Chen, Z.; Xu, Y.; Xiong, G.; Liu, R.; Wu, C.; Hu, H.; Wang, L. Systematic Characterization of Autophagy in Gestational Diabetes Mellitus. Endocrinology 2017, 158, 2522–2532. [Google Scholar] [CrossRef]
- Alzubaidi, K.R.K.; Mahdavi, M.; Dolati, S.; Yousefi, M. Observation of increased levels of autophagy-related genes and proteins in women with preeclampsia: A clinical study. Mol. Biol. Rep. 2023, 50, 4831–4840. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, A.; Cheng, S.B.; Ikawa, M.; Yoshimori, T.; Huber, W.J.; Menon, R.; Huang, Z.; Fierce, J.; Padbury, J.F.; Sadovsky, Y.; et al. Evidence for lysosomal biogenesis proteome defect and impaired autophagy in preeclampsia. Autophagy 2020, 16, 1771–1785. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, A.; Cheng, S.B.; Kusabiraki, T.; Motomura, K.; Aoki, A.; Ushijima, A.; Ono, Y.; Tsuda, S.; Shima, T.; Yoshino, O.; et al. Endoplasmic reticulum stress disrupts lysosomal homeostasis and induces blockade of autophagic flux in human trophoblasts. Sci. Rep. 2019, 9, 11466. [Google Scholar] [CrossRef] [PubMed]
- Steegmaier, M.; Oorschot, V.; Klumperman, J.; Scheller, R.H. Syntaxin 17 Is Abundant in Steroidogenic Cells and Implicated in Smooth Endoplasmic Reticulum Membrane Dynamics. Mol. Biol. Cell 2000, 11, 2719. [Google Scholar] [CrossRef] [PubMed]
- Olvera-Sánchez, S.; Esparza-Perusquía, M.; Flores-Herrera, O.; Urban-Sosa, V.A.; Martínez, F.; Olvera-Sánchez, S.; Esparza-Perusquía, M.; Flores-Herrera, O.; Urban-Sosa, V.A.; Martínez, F. Aspectos generales del transporte de colesterol en la esteroidogénesis de la placenta humana. TIP Rev. Espec. Cienc. Químico-Biológicas 2019, 22, 1–9. [Google Scholar] [CrossRef]
- Bjørkøy, G.; Lamark, T.; Pankiv, S.; Øvervatn, A.; Brech, A.; Johansen, T. Chapter 12 Monitoring Autophagic Degradation of p62/SQSTM1. Methods Enzymol. 2009, 452, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Akram, K.M.; Frost, L.I.; Anumba, D.O.C. Impaired autophagy with augmented apoptosis in a Th1/Th2-imbalanced placental micromilieu is associated with spontaneous preterm birth. Front. Mol. Biosci. 2022, 9, 897228. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, V.R.; Romao-Veiga, M.; Nunes, P.R.; Peracoli, J.C.; Peracoli, M.T.S. Increase of autophagy marker p62 in the placenta from pregnant women with preeclampsia. Hum. Immunol. 2022, 83, 447–452. [Google Scholar] [CrossRef]
- Akaishi, R.; Yamada, T.; Nakabayashi, K.; Nishihara, H.; Furuta, I.; Kojima, T.; Morikawa, M.; Fujita, N.; Minakami, H. Autophagy in the placenta of women with hypertensive disorders in pregnancy. Placenta 2014, 35, 974–980. [Google Scholar] [CrossRef]
- Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet. Gynecol. 2020, 135, E237–E260. [CrossRef]
- Von Dadelszen, P.; Payne, B.; Li, J.; Ansermino, J.M.; Pipkin, F.B.; Côté, A.M.; Douglas, M.J.; Gruslin, A.; Hutcheon, J.A.; Joseph, K.S.; et al. Prediction of adverse maternal outcomes in pre-eclampsia: Development and validation of the fullPIERS model. Lancet 2011, 377, 219–227. [Google Scholar] [CrossRef]
- Sibai, B.M. Evaluation and management of severe preeclampsia before 34 weeks’ gestation. Am. J. Obstet. Gynecol. 2011, 205, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.A.; Sáez, M.A.; Fraile-Martínez, O.; Álvarez-Mon, M.A.; García-Montero, C.; Guijarro, L.G.; Asúnsolo, Á.; Álvarez-Mon, M.; Bujan, J.; García-Honduvilla, N.; et al. Overexpression of glycolysis markers in placental tissue of pregnant women with chronic venous disease: A histological study. Int. J. Med. Sci. 2022, 19, 186. [Google Scholar] [CrossRef]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- Vallone, P.M.; Butler, J.M. AutoDimer: A screening tool for primer-dimer and hairpin structures. Biotechniques 2004, 37, 226–231. [Google Scholar] [CrossRef] [PubMed]
- García-Montero, C.; Fraile-Martinez, O.; Rodriguez-Martín, S.; Funes Moñux, R.M.; Saz, J.V.; Bravo, C.; De Leon-Luis, J.A.; Ruiz-Minaya, M.; Pekarek, L.; Saez, M.A.; et al. Irregular Expression of Cellular Stress Response Markers in the Placenta of Women with Chronic Venous Disease. Antioxidants 2022, 11, 2277. [Google Scholar] [CrossRef]
- Fraile-Martinez, O.; García-Montero, C.; Alvarez-Mon, M.Á.; Gomez-Lahoz, A.M.; Monserrat, J.; Llavero-Valero, M.; Ruiz-Grande, F.; Coca, S.; Alvarez-Mon, M.; Buján, J.; et al. Venous Wall of Patients with Chronic Venous Disease Exhibits a Glycolytic Phenotype. J. Pers. Med. 2022, 12, 1642. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martinez, O.; García-Montero, C.; Funes Moñux, R.M.; Rodriguez-Martín, S.; Bravo, C.; De Leon-Luis, J.A.; Saz, J.V.; Saez, M.A.; Guijarro, L.G.; et al. The Placentas of Women Who Suffer an Episode of Psychosis during Pregnancy Have Increased Lipid Peroxidation with Evidence of Ferroptosis. Biomolecules 2023, 13, 120. [Google Scholar] [CrossRef]


| HC (n = 43) | LO-PE (n = 68) | p-Value | |
|---|---|---|---|
| Maternal age (years) mean ± SD | 31.4 ± 5.1 | 29 ± 4.8 | * p = 0.0154 |
| Nulliparous (%) total number | 14 (32.6) | 53 (77.9) | *** p < 0.0001 |
| Gestation (weeks) | 39.1 ± 1.5 | 38.6 ± 1.4 | NS (p = 0.075) |
| C-section (%) Total number | 8 (18.6) | 15 (22.1) | NS (p = 0.270) |
| Placental weight (g) | 501 ± 65.3 | 370.3 ± 61.7 | *** p < 0.0001 |
| GENE | SEQUENCE Fwd (5′ → 3′) | SEQUENCE Rev (5′ → 3′) | Temp |
|---|---|---|---|
| TBP | TGCACAGGAGCCAAGAGTGAA | CACATCACAGCTCCCCACCA | 60 °C |
| ULK1 | GTTCCAAACACCTCGGTCCT | CGATCTCCATGGGCTTCTCC | 59 °C |
| LC3 | GAGTTACCTCCCGCAGCC | ACCCAGAGGGACAACCCTAA | 60 °C |
| NUP62 | CGAGGTGGATGTCCGTCTTT | GTCTGCAGCCTTGGGAAGAT | 61 °C |
| STX17 | CCCGGCGGGAGGTTTTT | AAGTCAGTGACCAGTTGGCT | 60 °C |
| LAMP1 | GGCCTCTTGCGTCTGGTAAC | AAAGGTACGCCTGGATGGTG | 57 °C |
| ATG9A | GGCTGGAGAGGAGCACATAC | ACCAGCAATGACCAGGATGG | 60 °C |
| ATG5 | GCAACTCTGGATGGGATTGC | TTGCAGCAGCGAAGTGTTTC | 61 °C |
| Antigen | Species | Dilution | Provider | Protocol Specifications |
|---|---|---|---|---|
| ULK1 | Rabbit polyclonal | 1:100 | Abcam (ab203207) | 10 mM sodium citrate. pH = 6, before incubation with blocking solution |
| LC3 | Rabbit monoclonal | 1:150 | Abcam (ab192890) | ------ |
| NUP62 | Rabbit monoclonal | 1:1000 | Abcam (ab207305) | EDTA, pH = 9, before incubation with blocking solution |
| STX17 | Rabbit polyclonal | 1:200 | Abcam (ab229646) | ------ |
| LAMP1 | Rabbit polyclonal | 1:250 | Abcam (ab24170) | EDTA, pH = 9, before incubation with blocking solution |
| ATG9A | Rabbit monoclonal | 1:500 | Abcam (ab108338) | 100% Triton 0.1% in PBS for 10 min before incubation with blocking solution |
| ATG5 | Rabbit monoclonal | 1:100 | sc-133158 | 100% Triton 0.1% in PBS for 10 min before incubation with blocking solution |
| IgG (Rabbit) | Mouse | 1:1000 | Sigma-Aldrich (Saint Louis, MO, USA) (RG96/B5283) | ------ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Puente, L.M.; García-Montero, C.; Fraile-Martinez, O.; Bujan, J.; De León-Luis, J.A.; Bravo, C.; Rodríguez-Benitez, P.; López-González, L.; Díaz-Pedrero, R.; Álvarez-Mon, M.; et al. Exploring the Importance of Differential Expression of Autophagy Markers in Term Placentas from Late-Onset Preeclamptic Pregnancies. Int. J. Mol. Sci. 2024, 25, 2029. https://doi.org/10.3390/ijms25042029
Garcia-Puente LM, García-Montero C, Fraile-Martinez O, Bujan J, De León-Luis JA, Bravo C, Rodríguez-Benitez P, López-González L, Díaz-Pedrero R, Álvarez-Mon M, et al. Exploring the Importance of Differential Expression of Autophagy Markers in Term Placentas from Late-Onset Preeclamptic Pregnancies. International Journal of Molecular Sciences. 2024; 25(4):2029. https://doi.org/10.3390/ijms25042029
Chicago/Turabian StyleGarcia-Puente, Luis M., Cielo García-Montero, Oscar Fraile-Martinez, Julia Bujan, Juan A. De León-Luis, Coral Bravo, Patrocinio Rodríguez-Benitez, Laura López-González, Raul Díaz-Pedrero, Melchor Álvarez-Mon, and et al. 2024. "Exploring the Importance of Differential Expression of Autophagy Markers in Term Placentas from Late-Onset Preeclamptic Pregnancies" International Journal of Molecular Sciences 25, no. 4: 2029. https://doi.org/10.3390/ijms25042029
APA StyleGarcia-Puente, L. M., García-Montero, C., Fraile-Martinez, O., Bujan, J., De León-Luis, J. A., Bravo, C., Rodríguez-Benitez, P., López-González, L., Díaz-Pedrero, R., Álvarez-Mon, M., García-Honduvilla, N., Saez, M. A., & Ortega, M. A. (2024). Exploring the Importance of Differential Expression of Autophagy Markers in Term Placentas from Late-Onset Preeclamptic Pregnancies. International Journal of Molecular Sciences, 25(4), 2029. https://doi.org/10.3390/ijms25042029









