Role of Oxygen and Its Radicals in Peripheral Nerve Regeneration: From Hypoxia to Physoxia to Hyperoxia
Abstract
1. Introduction
2. Role of Oxygen in Normal Nerve Function
3. Role of Oxygen and Its Radicals in Nerve Regeneration after Injury under Physiological (Physoxic) Conditions
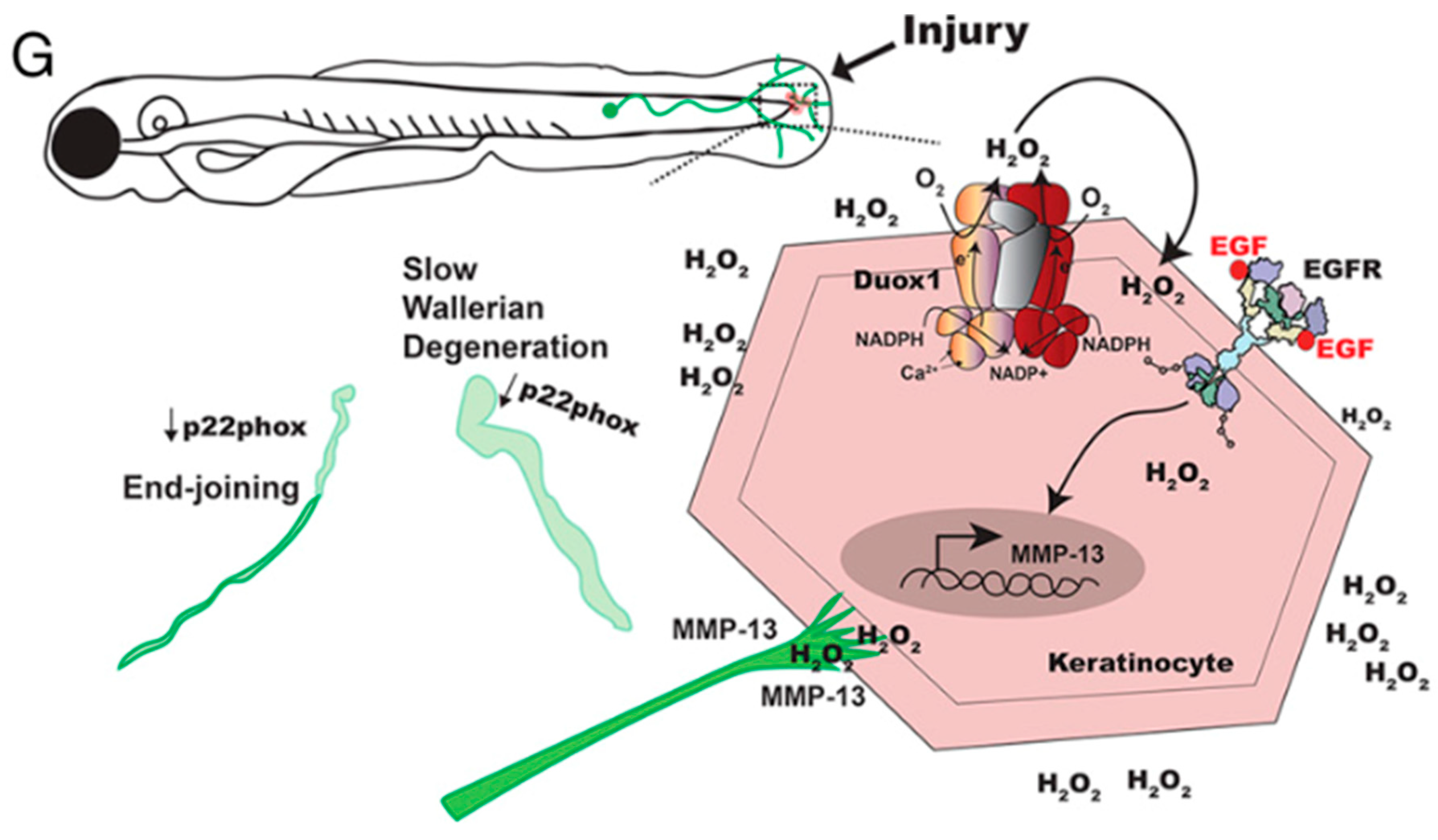
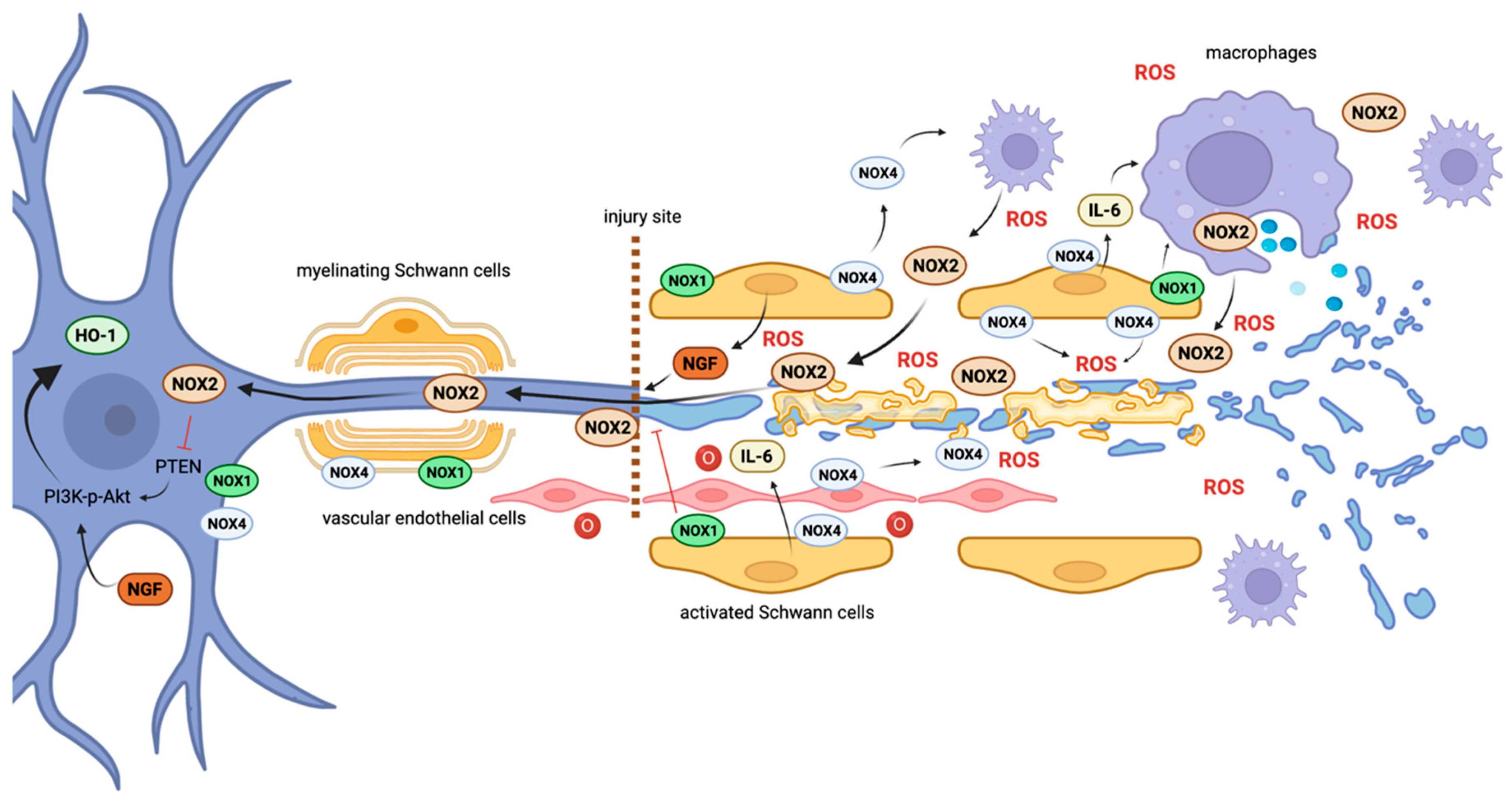
3.1. Distal Axon Degeneration and Schwann Cell Function
3.2. ROS and Redox Signaling during Inflammation
3.3. Retrograde Injury Signaling and Axonal Regeneration
4. Repercussions of Ischemia/Hypoxia on Nerve Regeneration and Redox Signaling
4.1. Prolonged Ischemia
4.2. Effects of Ischemia-Reperfusion after Injury
| Oxygenation State | Observed Phenomenon | Reported Nox-Related Mechanism | Reference |
|---|---|---|---|
| Acute ischemia | Neoangiogenesis, inflammation, cell proliferation. Repeated brief ischemia stimulates nerve regeneration and increases levels of VEGF and Schwann cell proliferation. | Nox4/Nox2/pSer36-p66Shc feed-forward loop leading to VEGFR2 activation. | [100,101,102,103] |
| Prolonged ischemia | Energy depletion, conduction failure, necrosis. | NOX4 acts as an energy sensor, inducing autophagy and cell survival via the protein kinase RNA-activated-like ER kinase signaling pathway. | [104,105,110] |
| Diabetes increases susceptibility to ischemic conduction failure and necrosis. | ROS overexpression in diabetic nerves is well established, and specific ROS sources are unknown. | [106,107,136] | |
| Ischemia-reperfusion | Endoneuronal edema, fiber degeneration. | Excessive oxidative stress established as main pathomechanism. | [114,115,134,135] |
| Increased expression/activity of Nox1 and Nox2, but not Nox4 in myocardial reperfusion. | [122,123,124,125,126,127,128,134] | ||
| Neuroprotective effect of inhibition of Nox in reperfusion injury. | [121,125,129,130,131,132,133] |
5. Effects of Hyperbaric Oxygen on Peripheral Nerve Regeneration
- It induces the antioxidant response;
- It limits detrimental Nox-mediated ROS production during reperfusion-injury and the modulation of the inflammatory response;
- It induces relative hypoxia-inducing neoangiogenesis;
- It has a pro-regenerative effect through redox-mediated growth factor activation.
5.1. Induction of Antioxidants by HBOT
5.2. HBOT, Reperfusion Injury, and Excessive Inflammation
5.3. Relative Hypoxia after HBOT and Angiogenic Effect
5.4. Pro-Regenerative Effects of HBOT
| Mechanism of Action | Reported Redox-Related Effects | References |
|---|---|---|
| Antioxidant induction | Decrease in overall Nox activity and levels of oxidative stress | [153] |
| Higher glutathione peroxidase production | [144,145] | |
| Increased activity of superoxide dismutase and catalase and decreased lipid peroxidation levels | [146] | |
| Upregulation of IL-10, BCL-2, heme oxygenase-1, and heat-shock proteins 70 and 72 | [147,148,149,150,151,152] | |
| Inhibition of inducible and neuronal nitric oxide synthase | [147,148,149,150,151,152] | |
| NGF-mediated protection against oxidative stress via HO-1 | [48,49,154] | |
| Ischemia-reperfusion injury and modulation of inflammation | Reducing cell death during ischemia | [155] |
| Reduced post-perfusion edema, higher post-perfusion glutathione level | [144] | |
| Reduced neutrophil recruitment and activation | [156] | |
| Reduced reperfusion injury via inhibition of ICAM-1 expression and integrin β2 | [157,158,159] | |
| Inhibition of pro-inflammatory factors, including nuclear transcription factor κB, IL-1, IL-6, IL-8, TNF-α, interferon γ, and platelet-activating factor | [160,161,162,163,164,165,166,167,168,169,170,171] | |
| Relative hypoxia, induction of neoangenenesis | Increases in HIF-1α, VEGFA, SDF-1, VEGFR2, and CXCR4 | [173] |
| Nox4/Nox2/pSer36-p66Shc feed-forward loop, providing yet another compelling example of the interplay | [94] | |
| Pro-regenerative effects | Decreased Nogo-A and reticulon receptors | [174] |
| Increased neurotrophin-3 | [175] | |
| Decrease in apoptosis-promoting genes c-fos, c-jun, Ba, and Caspase-3 | [154] | |
| Higher NGF expression | [176] | |
| Higher expression of neurotrophic factors NGF, BDNF, GDNF, and NF-3 | [154] |
5.5. Evidence of the Efficacy of HBOT in Improving Functional Outcome in Peripheral Nerve Injury
5.6. Conclusions and Clinical Relevance
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scheib, J.; Hoke, A. Advances in peripheral nerve regeneration. Nat. Rev. Neurol. 2013, 9, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Wood, M.D.; Kemp, S.W.; Weber, C.; Borschel, G.H.; Gordon, T. Outcome measures of peripheral nerve regeneration. Ann. Anat. 2011, 193, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Hunt, T.K.; Zederfeldt, B.; Goldstick, T.K. Oxygen and healing. Am. J. Surg. 1969, 118, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Tandara, A.A.; Mustoe, T.A. Oxygen in wound healing--more than a nutrient. World J. Surg. 2004, 28, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Roy, S. Redox signals in wound healing. Biochim. Biophys. Acta 2008, 1780, 1348–1361. [Google Scholar] [CrossRef] [PubMed]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef] [PubMed]
- Beckman, K.B.; Ames, B.N. The free radical theory of aging matures. Physiol. Rev. 1998, 78, 547–581. [Google Scholar] [CrossRef]
- Scott, T.L.; Rangaswamy, S.; Wicker, C.A.; Izumi, T. Repair of oxidative DNA damage and cancer: Recent progress in DNA base excision repair. Antioxid. Redox Signal. 2014, 20, 708–726. [Google Scholar] [CrossRef]
- Fukui, K. Reactive oxygen species induce neurite degeneration before induction of cell death. J. Clin. Biochem. Nutr. 2016, 59, 155–159. [Google Scholar] [CrossRef]
- Vincent, A.M.; Kato, K.; McLean, L.L.; Soules, M.E.; Feldman, E.L. Sensory neurons and schwann cells respond to oxidative stress by increasing antioxidant defense mechanisms. Antioxid. Redox Signal. 2009, 11, 425–438. [Google Scholar] [CrossRef]
- Inoguchi, T.; Sonta, T.; Tsubouchi, H.; Etoh, T.; Kakimoto, M.; Sonoda, N.; Sato, N.; Sekiguchi, N.; Kobayashi, K.; Sumimoto, H.; et al. Protein kinase C-dependent increase in reactive oxygen species (ROS) production in vascular tissues of diabetes: Role of vascular NAD(P)H oxidase. J. Am. Soc. Nephrol. JASN 2003, 14 (Suppl. 3), S227–S232. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- D’Autreaux, B.; Toledano, M.B. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007, 8, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Holmstrom, K.M.; Finkel, T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Spencer, N.Y.; Engelhardt, J.F. The basic biology of redoxosomes in cytokine-mediated signal transduction and implications for disease-specific therapies. Biochemistry 2014, 53, 1551–1564. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Khanna, S.; Nallu, K.; Hunt, T.K.; Sen, C.K. Dermal wound healing is subject to redox control. Mol. Ther. J. Am. Soc. Gene Ther. 2006, 13, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K. The general case for redox control of wound repair. Wound Repair Regen. Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc. 2003, 11, 431–438. [Google Scholar] [CrossRef]
- Andre-Levigne, D.; Modarressi, A.; Pepper, M.S.; Pittet-Cuenod, B. Reactive Oxygen Species and NOX Enzymes Are Emerging as Key Players in Cutaneous Wound Repair. Int. J. Mol. Sci. 2017, 18, 2149. [Google Scholar] [CrossRef] [PubMed]
- Rieger, S.; Sagasti, A. Hydrogen peroxide promotes injury-induced peripheral sensory axon regeneration in the zebrafish skin. PLoS Biol. 2011, 9, e1000621. [Google Scholar] [CrossRef] [PubMed]
- Min, J.Y.; Park, M.H.; Park, M.K.; Park, K.W.; Lee, N.W.; Kim, T.; Kim, H.J.; Lee, D.H. Staurosporin induces neurite outgrowth through ROS generation in HN33 hippocampal cell lines. J. Neural Transm. 2006, 113, 1821–1826. [Google Scholar] [CrossRef]
- Pirotte, N.; Stevens, A.S.; Fraguas, S.; Plusquin, M.; Van Roten, A.; Van Belleghem, F.; Paesen, R.; Ameloot, M.; Cebria, F.; Artois, T.; et al. Reactive Oxygen Species in Planarian Regeneration: An Upstream Necessity for Correct Patterning and Brain Formation. Oxid. Med. Cell. Longev. 2015, 2015, 392476. [Google Scholar] [CrossRef]
- Hervera, A.; De Virgiliis, F.; Palmisano, I.; Zhou, L.; Tantardini, E.; Kong, G.; Hutson, T.; Danzi, M.C.; Perry, R.B.; Santos, C.X.C.; et al. Reactive oxygen species regulate axonal regeneration through the release of exosomal NADPH oxidase 2 complexes into injured axons. Nat. Cell Biol. 2018, 20, 307–319. [Google Scholar] [CrossRef]
- Eid, S.A.; Savelieff, M.G.; Eid, A.A.; Feldman, E.L. Nox, Nox, Are You There? The Role of NADPH Oxidases in the Peripheral Nervous System. Antioxid. Redox Signal. 2021, 37, 613–630. [Google Scholar] [CrossRef]
- Rishal, I.; Fainzilber, M. Axon-soma communication in neuronal injury. Nat. Rev. Neurosci. 2014, 15, 32–42. [Google Scholar] [CrossRef]
- Saito, A.; Cavalli, V. Signaling Over Distances. Mol. Cell. Proteom. 2016, 15, 382–393. [Google Scholar] [CrossRef] [PubMed]
- McKeown, S.R. Defining normoxia, physoxia and hypoxia in tumours-implications for treatment response. Br. J. Radiol. 2014, 87, 20130676. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Sheng, Z.H. Mitochondrial transport and docking in axons. Exp. Neurol. 2009, 218, 257–267. [Google Scholar] [CrossRef]
- Zhu, X.H.; Qiao, H.; Du, F.; Xiong, Q.; Liu, X.; Zhang, X.; Ugurbil, K.; Chen, W. Quantitative imaging of energy expenditure in human brain. Neuroimage 2012, 60, 2107–2117. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y. Metabolic aspects of neuronal degeneration: From a NAD(+) point of view. Neurosci. Res. 2019, 139, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Vervust, W.; Ghysels, A. Oxygen Storage in Stacked Phospholipid Membranes Under an Oxygen Gradient as a Model for Myelin Sheaths. Adv. Exp. Med. Biol. 2022, 1395, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Nayernia, Z.; Jaquet, V.; Krause, K.H. New insights on NOX enzymes in the central nervous system. Antioxid. Redox Signal. 2014, 20, 2815–2837. [Google Scholar] [CrossRef]
- De Logu, F.; Nassini, R.; Materazzi, S.; Carvalho Goncalves, M.; Nosi, D.; Rossi Degl’Innocenti, D.; Marone, I.M.; Ferreira, J.; Li Puma, S.; Benemei, S.; et al. Schwann cell TRPA1 mediates neuroinflammation that sustains macrophage-dependent neuropathic pain in mice. Nat. Commun. 2017, 8, 1887. [Google Scholar] [CrossRef]
- Kallenborn-Gerhardt, W.; Schroder, K.; Geisslinger, G.; Schmidtko, A. NOXious signaling in pain processing. Pharmacol. Ther. 2013, 137, 309–317. [Google Scholar] [CrossRef]
- Ibi, M.; Matsuno, K.; Shiba, D.; Katsuyama, M.; Iwata, K.; Kakehi, T.; Nakagawa, T.; Sango, K.; Shirai, Y.; Yokoyama, T.; et al. Reactive oxygen species derived from NOX1/NADPH oxidase enhance inflammatory pain. J. Neurosci. 2008, 28, 9486–9494. [Google Scholar] [CrossRef]
- Vincent, A.M.; Hayes, J.M.; McLean, L.L.; Vivekanandan-Giri, A.; Pennathur, S.; Feldman, E.L. Dyslipidemia-induced neuropathy in mice: The role of oxLDL/LOX-1. Diabetes 2009, 58, 2376–2385. [Google Scholar] [CrossRef]
- Cao, X.; Demel, S.L.; Quinn, M.T.; Galligan, J.J.; Kreulen, D. Localization of NADPH oxidase in sympathetic and sensory ganglion neurons and perivascular nerve fibers. Auton. Neurosci. 2009, 151, 90–97. [Google Scholar] [CrossRef]
- Puntambekar, P.; Mukherjea, D.; Jajoo, S.; Ramkumar, V. Essential role of Rac1/NADPH oxidase in nerve growth factor induction of TRPV1 expression. J. Neurochem. 2005, 95, 1689–1703. [Google Scholar] [CrossRef] [PubMed]
- Coyoy, A.; Valencia, A.; Guemez-Gamboa, A.; Moran, J. Role of NADPH oxidase in the apoptotic death of cultured cerebellar granule neurons. Free Radic. Biol. Med. 2008, 45, 1056–1064. [Google Scholar] [CrossRef] [PubMed]
- Tammariello, S.P.; Quinn, M.T.; Estus, S. NADPH oxidase contributes directly to oxidative stress and apoptosis in nerve growth factor-deprived sympathetic neurons. J. Neurosci. 2000, 20, RC53. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.A.; Johnson, D.A.; Kraft, A.D.; Calkins, M.J.; Jakel, R.J.; Vargas, M.R.; Chen, P.C. The Nrf2-ARE pathway: An indicator and modulator of oxidative stress in neurodegeneration. Ann. N. Y. Acad. Sci. 2008, 1147, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Kratsovnik, E.; Bromberg, Y.; Sperling, O.; Zoref-Shani, E. Oxidative stress activates transcription factor NF-kB-mediated protective signaling in primary rat neuronal cultures. J. Mol. Neurosci. 2005, 26, 27–32. [Google Scholar] [CrossRef]
- Gamper, N.; Ooi, L. Redox and nitric oxide-mediated regulation of sensory neuron ion channel function. Antioxid. Redox Signal. 2015, 22, 486–504. [Google Scholar] [CrossRef]
- Eftekharpour, E.; Fernyhough, P. Oxidative Stress and Mitochondrial Dysfunction Associated with Peripheral Neuropathy in Type 1 Diabetes. Antioxid. Redox Signal. 2022, 37, 578–596. [Google Scholar] [CrossRef]
- Zajaczkowska, R.; Kocot-Kepska, M.; Leppert, W.; Wrzosek, A.; Mika, J.; Wordliczek, J. Mechanisms of Chemotherapy-Induced Peripheral Neuropathy. Int. J. Mol. Sci. 2019, 20, 1451. [Google Scholar] [CrossRef]
- Hichor, M.; Sundaram, V.K.; Eid, S.A.; Abdel-Rassoul, R.; Petit, P.X.; Borderie, D.; Bastin, J.; Eid, A.A.; Manuel, M.; Grenier, J.; et al. Liver X Receptor exerts a protective effect against the oxidative stress in the peripheral nerve. Sci. Rep. 2018, 8, 2524. [Google Scholar] [CrossRef]
- Kallenborn-Gerhardt, W.; Lu, R.; Syhr, K.M.; Heidler, J.; von Melchner, H.; Geisslinger, G.; Bangsow, T.; Schmidtko, A. Antioxidant activity of sestrin 2 controls neuropathic pain after peripheral nerve injury. Antioxid. Redox Signal. 2013, 19, 2013–2023. [Google Scholar] [CrossRef]
- Lv, W.; Deng, B.; Duan, W.; Li, Y.; Liu, Y.; Li, Z.; Xia, W.; Li, C. Schwann Cell Plasticity is Regulated by a Weakened Intrinsic Antioxidant Defense System in Acute Peripheral Nerve Injury. Neuroscience 2018, 382, 1–13. [Google Scholar] [CrossRef]
- Salinas, M.; Diaz, R.; Abraham, N.G.; Ruiz de Galarreta, C.M.; Cuadrado, A. Nerve growth factor protects against 6-hydroxydopamine-induced oxidative stress by increasing expression of heme oxygenase-1 in a phosphatidylinositol 3-kinase-dependent manner. J. Biol. Chem. 2003, 278, 13898–13904. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Xiong, J.; Wang, Y.; Shen, J.; Velkov, T.; Xiao, X. Nerve Growth Factor Confers Neuroprotection against Colistin-Induced Peripheral Neurotoxicity. ACS Infect. Dis. 2020, 6, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Hor, C.P.; Fung, W.Y.; Ang, H.A.; Lim, S.C.; Kam, L.Y.; Sim, S.W.; Lim, L.H.; Choon, W.Y.; Wong, J.W. Efficacy of Oral Mixed Tocotrienols in Diabetic Peripheral Neuropathy: A Randomized Clinical Trial. JAMA Neurol. 2018, 75, 444–452. [Google Scholar] [CrossRef]
- Saklayen, M.G.; Yap, J.; Vallyathan, V. Effect of month-long treatment with oral N-acetylcysteine on the oxidative stress and proteinuria in patients with diabetic nephropathy: A pilot study. J. Investig. Med. 2010, 58, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Elbatreek, M.H.; Pachado, M.P.; Cuadrado, A.; Jandeleit-Dahm, K.; Schmidt, H. Reactive Oxygen Comes of Age: Mechanism-Based Therapy of Diabetic End-Organ Damage. Trends Endocrinol. Metab. 2019, 30, 312–327. [Google Scholar] [CrossRef] [PubMed]
- McElroy, T.; Zeidan, R.S.; Rathor, L.; Han, S.M.; Xiao, R. The role of mitochondria in the recovery of neurons after injury. Neural Regen. Res. 2023, 18, 317–318. [Google Scholar] [CrossRef] [PubMed]
- Clapham, D.E. Calcium signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, C.; Agnoletto, C.; Bononi, A.; Bonora, M.; De Marchi, E.; Marchi, S.; Missiroli, S.; Patergnani, S.; Poletti, F.; Rimessi, A.; et al. Mitochondrial calcium homeostasis as potential target for mitochondrial medicine. Mitochondrion 2012, 12, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Han, S.M.; Baig, H.S.; Hammarlund, M. Mitochondria Localize to Injured Axons to Support Regeneration. Neuron 2016, 92, 1308–1323. [Google Scholar] [CrossRef]
- Zhou, B.; Yu, P.; Lin, M.Y.; Sun, T.; Chen, Y.; Sheng, Z.H. Facilitation of axon regeneration by enhancing mitochondrial transport and rescuing energy deficits. J. Cell Biol. 2016, 214, 103–119. [Google Scholar] [CrossRef]
- Horvath, R.; Medina, J.; Reilly, M.M.; Shy, M.E.; Zuchner, S. Peripheral neuropathy in mitochondrial disease. Handb. Clin. Neurol. 2023, 194, 99–116. [Google Scholar] [CrossRef]
- Gold, R.; Archelos, J.J.; Hartung, H.P. Mechanisms of immune regulation in the peripheral nervous system. Brain Pathol. 1999, 9, 343–360. [Google Scholar] [CrossRef]
- Giridharan, S.S.; Caplan, S. MICAL-family proteins: Complex regulators of the actin cytoskeleton. Antioxid. Redox Signal. 2014, 20, 2059–2073. [Google Scholar] [CrossRef]
- Kim, M.; Kim, H.; Kim, D.; Kim, D.; Huh, Y.; Park, C.; Chung, H.J.; Jung, J.; Jeong, N.Y. Heme Oxygenase 1 in Schwann Cells Regulates Peripheral Nerve Degeneration Against Oxidative Stress. ASN Neuro 2019, 11, 1759091419838949. [Google Scholar] [CrossRef]
- Feldman, E.L.; Callaghan, B.C.; Pop-Busui, R.; Zochodne, D.W.; Wright, D.E.; Bennett, D.L.; Bril, V.; Russell, J.W.; Viswanathan, V. Diabetic neuropathy. Nat. Rev. Dis. Primers 2019, 5, 41. [Google Scholar] [CrossRef]
- Viader, A.; Sasaki, Y.; Kim, S.; Strickland, A.; Workman, C.S.; Yang, K.; Gross, R.W.; Milbrandt, J. Aberrant Schwann cell lipid metabolism linked to mitochondrial deficits leads to axon degeneration and neuropathy. Neuron 2013, 77, 886–898. [Google Scholar] [CrossRef]
- Meda, F.; Gauron, C.; Rampon, C.; Teillon, J.; Volovitch, M.; Vriz, S. Nerves Control Redox Levels in Mature Tissues Through Schwann Cells and Hedgehog Signaling. Antioxid. Redox Signal. 2016, 24, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Babetto, E.; Wong, K.M.; Beirowski, B. A glycolytic shift in Schwann cells supports injured axons. Nat. Neurosci. 2020, 23, 1215–1228. [Google Scholar] [CrossRef] [PubMed]
- Kallenborn-Gerhardt, W.; Schroder, K.; Del Turco, D.; Lu, R.; Kynast, K.; Kosowski, J.; Niederberger, E.; Shah, A.M.; Brandes, R.P.; Geisslinger, G.; et al. NADPH oxidase-4 maintains neuropathic pain after peripheral nerve injury. J. Neurosci. 2012, 32, 10136–10145. [Google Scholar] [CrossRef]
- Cadiz Diaz, A.; Schmidt, N.A.; Yamazaki, M.; Hsieh, C.J.; Lisse, T.S.; Rieger, S. Coordinated NADPH oxidase/hydrogen peroxide functions regulate cutaneous sensory axon de- and regeneration. Proc. Natl. Acad. Sci. USA 2022, 119, e2115009119. [Google Scholar] [CrossRef] [PubMed]
- Schafer, M.; Werner, S. Oxidative stress in normal and impaired wound repair. Pharmacol. Res. Off. J. Ital. Pharmacol. Soc. 2008, 58, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Brooker, R.J. Genetics: Analysis & Principles, 4th ed.; McGraw-Hill: New York, NY, USA, 2012; Volume xviii, pp. 761–785. [Google Scholar]
- Eckert, J.W.; Abramson, S.L.; Starke, J.; Brandt, M.L. The surgical implications of chronic granulomatous disease. Am. J. Surg. 1995, 169, 320–323. [Google Scholar] [CrossRef]
- Deffert, C.; Cachat, J.; Krause, K.H. Phagocyte NADPH oxidase, chronic granulomatous disease and mycobacterial infections. Cell. Microbiol. 2014, 16, 1168–1178. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, C.E.; Carroll, K.S. Cysteine-mediated redox signaling: Chemistry, biology, and tools for discovery. Chem. Rev. 2013, 113, 4633–4679. [Google Scholar] [CrossRef]
- de Oliveira-Marques, V.; Cyrne, L.; Marinho, H.S.; Antunes, F. A quantitative study of NF-kappaB activation by H2O2: Relevance in inflammation and synergy with TNF-alpha. J. Immunol. 2007, 178, 3893–3902. [Google Scholar] [CrossRef]
- Klyubin, I.V.; Kirpichnikova, K.M.; Gamaley, I.A. Hydrogen peroxide-induced chemotaxis of mouse peritoneal neutrophils. Eur. J. Cell Biol. 1996, 70, 347–351. [Google Scholar] [PubMed]
- Nakamura, H.; Herzenberg, L.A.; Bai, J.; Araya, S.; Kondo, N.; Nishinaka, Y.; Herzenberg, L.A.; Yodoi, J. Circulating thioredoxin suppresses lipopolysaccharide-induced neutrophil chemotaxis. Proc. Natl. Acad. Sci. USA 2001, 98, 15143–15148. [Google Scholar] [CrossRef] [PubMed]
- Fraticelli, A.; Serrano, C.V., Jr.; Bochner, B.S.; Capogrossi, M.C.; Zweier, J.L. Hydrogen peroxide and superoxide modulate leukocyte adhesion molecule expression and leukocyte endothelial adhesion. Biochim. Biophys. Acta 1996, 1310, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Ogura, M.; Kitamura, M. Oxidant stress incites spreading of macrophages via extracellular signal-regulated kinases and p38 mitogen-activated protein kinase. J. Immunol. 1998, 161, 3569–3574. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Youker, K.; Ballantyne, C.; Entman, M.; Smith, C.W. Hydrogen peroxide induces LFA-1-dependent neutrophil adherence to cardiac myocytes. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H835–H842. [Google Scholar] [CrossRef] [PubMed]
- Haddad, J.J.; Saade, N.E.; Safieh-Garabedian, B. Redox regulation of TNF-alpha biosynthesis: Augmentation by irreversible inhibition of gamma-glutamylcysteine synthetase and the involvement of an IkappaB-alpha/NF-kappaB-independent pathway in alveolar epithelial cells. Cell. Signal. 2002, 14, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.; Wang, L.; Shin, Y.K.; Lee, K.Y.; Suh, D.J.; Park, H.T. Interleukin-6 induces proinflammatory signaling in Schwann cells: A high-throughput analysis. Biochem. Biophys. Res. Commun. 2009, 382, 410–414. [Google Scholar] [CrossRef]
- Li, B.; Bedard, K.; Sorce, S.; Hinz, B.; Dubois-Dauphin, M.; Krause, K.H. NOX4 expression in human microglia leads to constitutive generation of reactive oxygen species and to constitutive IL-6 expression. J. Innate Immun. 2009, 1, 570–581. [Google Scholar] [CrossRef]
- Vilhardt, F.; Haslund-Vinding, J.; Jaquet, V.; McBean, G. Microglia antioxidant systems and redox signalling. Br. J. Pharmacol. 2017, 174, 1719–1732. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lan, T.; Zhang, C.; Zeng, C.; Hou, J.; Yang, Z.; Zhang, M.; Liu, J.; Liu, B. Reciprocal activation between IL-6/STAT3 and NOX4/Akt signalings promotes proliferation and survival of non-small cell lung cancer cells. Oncotarget 2015, 6, 1031–1048. [Google Scholar] [CrossRef] [PubMed]
- Eid, S.A.; El Massry, M.; Hichor, M.; Haddad, M.; Grenier, J.; Dia, B.; Barakat, R.; Boutary, S.; Chanal, J.; Aractingi, S.; et al. Targeting the NADPH Oxidase-4 and Liver X Receptor Pathway Preserves Schwann Cell Integrity in Diabetic Mice. Diabetes 2020, 69, 448–464. [Google Scholar] [CrossRef] [PubMed]
- Levigne, D.; Modarressi, A.; Krause, K.H.; Pittet-Cuenod, B. NADPH oxidase 4 deficiency leads to impaired wound repair and reduced dityrosine-crosslinking, but does not affect myofibroblast formation. Free Radic. Biol. Med. 2016, 96, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Le Belle, J.E.; Orozco, N.M.; Paucar, A.A.; Saxe, J.P.; Mottahedeh, J.; Pyle, A.D.; Wu, H.; Kornblum, H.I. Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell Stem Cell 2011, 8, 59–71. [Google Scholar] [CrossRef] [PubMed]
- De Virgiliis, F.; Hutson, T.H.; Palmisano, I.; Amachree, S.; Miao, J.; Zhou, L.; Todorova, R.; Thompson, R.; Danzi, M.C.; Lemmon, V.P.; et al. Enriched conditioning expands the regenerative ability of sensory neurons after spinal cord injury via neuronal intrinsic redox signaling. Nat. Commun. 2020, 11, 6425. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Qiu, Y.; Gao, Y.; Wan, D.; Zhu, H. A Subtle Network Mediating Axon Guidance: Intrinsic Dynamic Structure of Growth Cone, Attractive and Repulsive Molecular Cues, and the Intermediate Role of Signaling Pathways. Neural Plast. 2019, 2019, 1719829. [Google Scholar] [CrossRef]
- Suzukawa, K.; Miura, K.; Mitsushita, J.; Resau, J.; Hirose, K.; Crystal, R.; Kamata, T. Nerve growth factor-induced neuronal differentiation requires generation of Rac1-regulated reactive oxygen species. J. Biol. Chem. 2000, 275, 13175–13178. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; An, J.M.; Lee, H.G.; Seo, S.R.; Kim, S.S.; Kim, J.Y.; Kang, J.W.; Bae, Y.S.; Seo, J.T. Activation of Rac1-dependent redox signaling is critically involved in staurosporine-induced neurite outgrowth in PC12 cells. Free Radic. Res. 2013, 47, 95–103. [Google Scholar] [CrossRef]
- Ibi, M.; Katsuyama, M.; Fan, C.; Iwata, K.; Nishinaka, T.; Yokoyama, T.; Yabe-Nishimura, C. NOX1/NADPH oxidase negatively regulates nerve growth factor-induced neurite outgrowth. Free Radic. Biol. Med. 2006, 40, 1785–1795. [Google Scholar] [CrossRef]
- Munnamalai, V.; Weaver, C.J.; Weisheit, C.E.; Venkatraman, P.; Agim, Z.S.; Quinn, M.T.; Suter, D.M. Bidirectional interactions between NOX2-type NADPH oxidase and the F-actin cytoskeleton in neuronal growth cones. J. Neurochem. 2014, 130, 526–540. [Google Scholar] [CrossRef]
- Feldman, E.L.; Nave, K.A.; Jensen, T.S.; Bennett, D.L.H. New Horizons in Diabetic Neuropathy: Mechanisms, Bioenergetics, and Pain. Neuron 2017, 93, 1296–1313. [Google Scholar] [CrossRef]
- Montezano, A.C.; Burger, D.; Ceravolo, G.S.; Yusuf, H.; Montero, M.; Touyz, R.M. Novel Nox homologues in the vasculature: Focusing on Nox4 and Nox5. Clin. Sci. 2011, 120, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Burger, D.; Montezano, A.C.; Nishigaki, N.; He, Y.; Carter, A.; Touyz, R.M. Endothelial microparticle formation by angiotensin II is mediated via Ang II receptor type I/NADPH oxidase/ Rho kinase pathways targeted to lipid rafts. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1898–1907. [Google Scholar] [CrossRef] [PubMed]
- Mezentsev, A.; Merks, R.M.; O’Riordan, E.; Chen, J.; Mendelev, N.; Goligorsky, M.S.; Brodsky, S.V. Endothelial microparticles affect angiogenesis in vitro: Role of oxidative stress. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H1106–H1114. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.; Biswas, B.; Manhas, A.; Singh, A.; Goyal, D.; Gaestel, M.; Jagavelu, K. Proinflammatory Effect of Endothelial Microparticles Is Mitochondria Mediated and Modulated Through MAPKAPK2 (MAPK-Activated Protein Kinase 2) Leading to Attenuation of Cardiac Hypertrophy. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1100–1112. [Google Scholar] [CrossRef]
- Burger, D.; Turner, M.; Munkonda, M.N.; Touyz, R.M. Endothelial Microparticle-Derived Reactive Oxygen Species: Role in Endothelial Signaling and Vascular Function. Oxid. Med. Cell. Longev. 2016, 2016, 5047954. [Google Scholar] [CrossRef]
- Jansen, F.; Yang, X.; Franklin, B.S.; Hoelscher, M.; Schmitz, T.; Bedorf, J.; Nickenig, G.; Werner, N. High glucose condition increases NADPH oxidase activity in endothelial microparticles that promote vascular inflammation. Cardiovasc. Res. 2013, 98, 94–106. [Google Scholar] [CrossRef]
- Burnett, M.G.; Zager, E.L. Pathophysiology of peripheral nerve injury: A brief review. Neurosurg. Focus 2004, 16, E1. [Google Scholar] [CrossRef]
- Smaila, B.D.; Holland, S.D.; Babaeijandaghi, F.; Henderson, H.G.; Rossi, F.M.V.; Ramer, M.S. Systemic hypoxia mimicry enhances axonal regeneration and functional recovery following peripheral nerve injury. Exp. Neurol. 2020, 334, 113436. [Google Scholar] [CrossRef]
- Zhou, X.B.; Zou, D.X.; Gu, W.; Wang, D.; Feng, J.S.; Wang, J.Y.; Zhou, J.L. An Experimental Study on Repeated Brief Ischemia in Promoting Sciatic Nerve Repair and Regeneration in Rats. World Neurosurg. 2018, 114, e11–e21. [Google Scholar] [CrossRef]
- Kim, Y.M.; Kim, S.J.; Tatsunami, R.; Yamamura, H.; Fukai, T.; Ushio-Fukai, M. ROS-induced ROS release orchestrated by Nox4, Nox2, and mitochondria in VEGF signaling and angiogenesis. Am. J. Physiol. Cell Physiol. 2017, 312, C749–C764. [Google Scholar] [CrossRef]
- Schmelzer, J.D.; Zochodne, D.W.; Low, P.A. Ischemic and reperfusion injury of rat peripheral nerve. Proc. Natl. Acad. Sci. USA 1989, 86, 1639–1642. [Google Scholar] [CrossRef]
- Zollman, P.J.; Awad, O.; Schmelzer, J.D.; Low, P.A. Effect of ischemia and reperfusion in vivo on energy metabolism of rat sciatic-tibial and caudal nerves. Exp. Neurol. 1991, 114, 315–320. [Google Scholar] [CrossRef]
- Baba, M.; Nukada, H.; McMorran, D.; Takahashi, K.; Wada, R.; Yagihashi, S. Prolonged ischemic conduction failure after reperfusion in diabetic nerve. Muscle Nerve 2006, 33, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Levigne, D.; Tobalem, M.; Modarressi, A.; Pittet-Cuenod, B. Hyperglycemia Increases Susceptibility to Ischemic Necrosis. BioMed Res. Int. 2013, 2013, 490964. [Google Scholar] [CrossRef] [PubMed]
- Low, P.A.; Schmelzer, J.D.; Ward, K.K. The effect of age on energy metabolism and resistance to ischaemic conduction failure in rat peripheral nerve. J. Physiol. 1986, 374, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Shanmugasundaram, K.; Nayak, B.K.; Friedrichs, W.E.; Kaushik, D.; Rodriguez, R.; Block, K. NOX4 functions as a mitochondrial energetic sensor coupling cancer metabolic reprogramming to drug resistance. Nat. Commun. 2017, 8, 997. [Google Scholar] [CrossRef] [PubMed]
- Sciarretta, S.; Zhai, P.; Shao, D.; Zablocki, D.; Nagarajan, N.; Terada, L.S.; Volpe, M.; Sadoshima, J. Activation of NADPH oxidase 4 in the endoplasmic reticulum promotes cardiomyocyte autophagy and survival during energy stress through the protein kinase RNA-activated-like endoplasmic reticulum kinase/eukaryotic initiation factor 2alpha/activating transcription factor 4 pathway. Circ. Res. 2013, 113, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Sanchez, J.A.; Carty, L.; Iruarrizaga-Lejarreta, M.; Palomo-Irigoyen, M.; Varela-Rey, M.; Griffith, M.; Hantke, J.; Macias-Camara, N.; Azkargorta, M.; Aurrekoetxea, I.; et al. Schwann cell autophagy, myelinophagy, initiates myelin clearance from injured nerves. J. Cell Biol. 2015, 210, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.B.; Maguire, J.J.; Mahdavian, M.; Wicke, C.; Marcocci, L.; Scheuenstuhl, H.; Chang, M.; Le, A.X.; Hopf, H.W.; Hunt, T.K. Wound hypoxia and acidosis limit neutrophil bacterial killing mechanisms. Arch. Surg. 1997, 132, 991–996. [Google Scholar] [CrossRef]
- Soneja, A.; Drews, M.; Malinski, T. Role of nitric oxide, nitroxidative and oxidative stress in wound healing. Pharmacol. Rep. PR 2005, 57, 108–119. [Google Scholar]
- Anderson, G.M.; Nukada, H.; McMorran, P.D. Carbonyl histochemistry in rat reperfusion nerve injury. Brain Res. 1997, 772, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Nagamatsu, M.; Schmelzer, J.D.; Zollman, P.J.; Smithson, I.L.; Nickander, K.K.; Low, P.A. Ischemic reperfusion causes lipid peroxidation and fiber degeneration. Muscle Nerve 1996, 19, 37–47. [Google Scholar] [CrossRef]
- Mitsui, Y.; Schmelzer, J.D.; Zollman, P.J.; Kihara, M.; Low, P.A. Hypothermic neuroprotection of peripheral nerve of rats from ischaemia-reperfusion injury. Brain 1999, 122 Pt 1, 161–169. [Google Scholar] [CrossRef]
- Iida, H.; Schmelzer, J.D.; Schmeichel, A.M.; Wang, Y.; Low, P.A. Peripheral nerve ischemia: Reperfusion injury and fiber regeneration. Exp. Neurol. 2003, 184, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Carden, D.L.; Granger, D.N. Pathophysiology of ischaemia-reperfusion injury. J. Pathol. 2000, 190, 255–266. [Google Scholar] [CrossRef]
- Raedschelders, K.; Ansley, D.M.; Chen, D.D. The cellular and molecular origin of reactive oxygen species generation during myocardial ischemia and reperfusion. Pharmacol. Ther. 2012, 133, 230–255. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, T.H.; Reynolds, C.A.; Kumar, R.; Przyklenk, K.; Huttemann, M. Molecular mechanisms of ischemia-reperfusion injury in brain: Pivotal role of the mitochondrial membrane potential in reactive oxygen species generation. Mol. Neurobiol. 2013, 47, 9–23. [Google Scholar] [CrossRef]
- Granger, D.N.; Kvietys, P.R. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol. 2015, 6, 524–551. [Google Scholar] [CrossRef]
- Simone, S.; Rascio, F.; Castellano, G.; Divella, C.; Chieti, A.; Ditonno, P.; Battaglia, M.; Crovace, A.; Staffieri, F.; Oortwijn, B.; et al. Complement-dependent NADPH oxidase enzyme activation in renal ischemia/reperfusion injury. Free Radic. Biol. Med. 2014, 74, 263–273. [Google Scholar] [CrossRef]
- Nakagiri, A.; Sunamoto, M.; Murakami, M. NADPH oxidase is involved in ischaemia/reperfusion-induced damage in rat gastric mucosa via ROS production--role of NADPH oxidase in rat stomachs. Inflammopharmacology 2007, 15, 278–281. [Google Scholar] [CrossRef]
- Choi, D.H.; Kim, J.H.; Lee, K.H.; Kim, H.Y.; Kim, Y.S.; Choi, W.S.; Lee, J. Role of neuronal NADPH oxidase 1 in the peri-infarct regions after stroke. PLoS ONE 2015, 10, e0116814. [Google Scholar] [CrossRef]
- Miller, A.A.; Dusting, G.J.; Roulston, C.L.; Sobey, C.G. NADPH-oxidase activity is elevated in penumbral and non-ischemic cerebral arteries following stroke. Brain Res. 2006, 1111, 111–116. [Google Scholar] [CrossRef]
- Yokota, H.; Narayanan, S.P.; Zhang, W.; Liu, H.; Rojas, M.; Xu, Z.; Lemtalsi, T.; Nagaoka, T.; Yoshida, A.; Brooks, S.E.; et al. Neuroprotection from retinal ischemia/reperfusion injury by NOX2 NADPH oxidase deletion. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8123–8131. [Google Scholar] [CrossRef] [PubMed]
- Doerries, C.; Grote, K.; Hilfiker-Kleiner, D.; Luchtefeld, M.; Schaefer, A.; Holland, S.M.; Sorrentino, S.; Manes, C.; Schieffer, B.; Drexler, H.; et al. Critical role of the NAD(P)H oxidase subunit p47phox for left ventricular remodeling/dysfunction and survival after myocardial infarction. Circ. Res. 2007, 100, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Gan, X.; Su, G.; Zhao, W.; Huang, P.; Luo, G.; Hei, Z. The mechanism of sevoflurane preconditioning-induced protections against small intestinal ischemia reperfusion injury is independent of mast cell in rats. Mediat. Inflamm. 2013, 2013, 378703. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Song, Y.S.; Chan, P.H. Inhibition of NADPH oxidase is neuroprotective after ischemia-reperfusion. J. Cereb. Blood Flow Metab. 2009, 29, 1262–1272. [Google Scholar] [CrossRef] [PubMed]
- Uysal, A.; Sahna, E.; Ozguler, I.M.; Burma, O.; Ilhan, N. Effects of apocynin, an NADPH oxidase inhibitor, on levels of ADMA, MPO, iNOS and TLR4 induced by myocardial ischemia reperfusion. Perfusion 2015, 30, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Dodia, C.; Chatterjee, S.; Zagorski, J.; Mesaros, C.; Blair, I.A.; Feinstein, S.I.; Jain, M.; Fisher, A.B. A novel nontoxic inhibitor of the activation of NADPH oxidase reduces reactive oxygen species production in mouse lung. J. Pharmacol. Exp. Ther. 2013, 345, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Paterniti, I.; Galuppo, M.; Mazzon, E.; Impellizzeri, D.; Esposito, E.; Bramanti, P.; Cuzzocrea, S. Protective effects of apocynin, an inhibitor of NADPH oxidase activity, in splanchnic artery occlusion and reperfusion. J. Leukoc. Biol. 2010, 88, 993–1003. [Google Scholar] [CrossRef] [PubMed]
- Korthuis, R.J.; Gute, D.C.; Blecha, F.; Ross, C.R. PR-39, a proline/arginine-rich antimicrobial peptide, prevents postischemic microvascular dysfunction. Am. J. Physiol. 1999, 277, H1007–H1013. [Google Scholar] [CrossRef]
- Braunersreuther, V.; Montecucco, F.; Asrih, M.; Pelli, G.; Galan, K.; Frias, M.; Burger, F.; Quindere, A.L.; Montessuit, C.; Krause, K.H.; et al. Role of NADPH oxidase isoforms NOX1, NOX2 and NOX4 in myocardial ischemia/reperfusion injury. J. Mol. Cell. Cardiol. 2013, 64, 99–107. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, T.; Yang, K.; Xu, J.; Wu, J.M.; Liu, W.L. NADPH oxidase 2 does not contribute to early reperfusion-associated reactive oxygen species generation following transient focal cerebral ischemia. Neural Regen. Res. 2016, 11, 1773–1778. [Google Scholar] [CrossRef]
- Urner, S.; Ho, F.; Jha, J.C.; Ziegler, D.; Jandeleit-Dahm, K. NADPH Oxidase Inhibition: Preclinical and Clinical Studies in Diabetic Complications. Antioxid. Redox Signal. 2020, 33, 415–434. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.J.; Frykberg, R.G.; Oropallo, A.; Sen, C.K.; Armstrong, D.G.; Nair, H.K.R.; Serena, T.E. Efficacy of Topical Wound Oxygen Therapy in Healing Chronic Diabetic Foot Ulcers: Systematic Review and Meta-Analysis. Adv. Wound Care 2023, 12, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K. Wound healing essentials: Let there be oxygen. Wound Repair Regen. Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc. 2009, 17, 1–18. [Google Scholar] [CrossRef]
- Sanchez, E.C. Hyperbaric oxygenation in peripheral nerve repair and regeneration. Neurol. Res. 2007, 29, 184–198. [Google Scholar] [CrossRef]
- Andre-Levigne, D.; Modarressi, A.; Pignel, R.; Bochaton-Piallat, M.L.; Pittet-Cuenod, B. Hyperbaric oxygen therapy promotes wound repair in ischemic and hyperglycemic conditions, increasing tissue perfusion and collagen deposition. Wound Repair Regen. Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc. 2016, 24, 954–965. [Google Scholar] [CrossRef]
- Nazario, J.; Kuffler, D.P. Hyperbaric oxygen therapy and promoting neurological recovery following nerve trauma. Undersea Hyperb. Med. 2011, 38, 345–366. [Google Scholar]
- Dong, H.; Xiong, L.; Zhu, Z.; Chen, S.; Hou, L.; Sakabe, T. Preconditioning with hyperbaric oxygen and hyperoxia induces tolerance against spinal cord ischemia in rabbits. Anesthesiology 2002, 96, 907–912. [Google Scholar] [CrossRef]
- Nie, H.; Xiong, L.; Lao, N.; Chen, S.; Xu, N.; Zhu, Z. Hyperbaric oxygen preconditioning induces tolerance against spinal cord ischemia by upregulation of antioxidant enzymes in rabbits. J. Cereb. Blood Flow Metab. 2006, 26, 666–674. [Google Scholar] [CrossRef]
- Haapaniemi, T.; Sirsjo, A.; Nylander, G.; Larsson, J. Hyperbaric oxygen treatment attenuates glutathione depletion and improves metabolic restitution in postischemic skeletal muscle. Free Radic. Res. 1995, 23, 91–101. [Google Scholar] [CrossRef]
- Yasar, M.; Yildiz, S.; Mas, R.; Dundar, K.; Yildirim, A.; Korkmaz, A.; Akay, C.; Kaymakcioglu, N.; Ozisik, T.; Sen, D. The effect of hyperbaric oxygen treatment on oxidative stress in experimental acute necrotizing pancreatitis. Physiol. Res. 2003, 52, 111–116. [Google Scholar] [CrossRef]
- Shams, Z.; Khalatbary, A.R.; Ahmadvand, H.; Zare, Z.; Kian, K. Neuroprotective effects of hyperbaric oxygen (HBO) therapy on neuronal death induced by sciatic nerve transection in rat. BMC Neurol. 2017, 17, 220. [Google Scholar] [CrossRef]
- Kurata, S.; Yamashita, U.; Nakajima, H. Hyperbaric oxygenation reduces the cytostatic activity and transcription of nitric oxide synthetase gene of mouse peritoneal macrophages. Biochim. Biophys. Acta 1995, 1263, 35–38. [Google Scholar] [CrossRef]
- Rothfuss, A.; Speit, G. Investigations on the mechanism of hyperbaric oxygen (HBO)-induced adaptive protection against oxidative stress. Mutat. Res. 2002, 508, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Wada, K.; Miyazawa, T.; Nomura, N.; Tsuzuki, N.; Nawashiro, H.; Shima, K. Preferential conditions for and possible mechanisms of induction of ischemic tolerance by repeated hyperbaric oxygenation in gerbil hippocampus. Neurosurgery 2001, 49, 160–166, discussion 166–167. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, R.E.; Silbergleit, R.; Hof, P.R.; Haywood, Y.; Fiskum, G. Hyperbaric oxygen reduces neuronal death and improves neurological outcome after canine cardiac arrest. Stroke 2003, 34, 1311–1316. [Google Scholar] [CrossRef]
- Shyu, W.C.; Lin, S.Z.; Saeki, K.; Kubosaki, A.; Matsumoto, Y.; Onodera, T.; Chiang, M.F.; Thajeb, P.; Li, H. Hyperbaric oxygen enhances the expression of prion protein and heat shock protein 70 in a mouse neuroblastoma cell line. Cell. Mol. Neurobiol. 2004, 24, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Matsuyama, Y.; Yanase, M.; Ito, S.; Adachi, K.; Satake, K.; Ishiguro, N.; Kiuchi, K. Effects of hyperbaric oxygen on GDNF expression and apoptosis in spinal cord injury. Neuroreport 2004, 15, 2369–2373. [Google Scholar] [CrossRef]
- Ostrowski, R.P.; Tang, J.; Zhang, J.H. Hyperbaric oxygen suppresses NADPH oxidase in a rat subarachnoid hemorrhage model. Stroke 2006, 37, 1314–1318. [Google Scholar] [CrossRef]
- Xing, P.; Ma, K.; Li, L.; Wang, D.; Hu, G.; Long, W. The protection effect and mechanism of hyperbaric oxygen therapy in rat brain with traumatic injury. Acta Cir. Bras. 2018, 33, 341–353. [Google Scholar] [CrossRef]
- Garcia-Covarrubias, L.; Cuauhtemoc Sanchez-Rodriguez, E. Hyperbaric oxygenation therapy, basic concepts. Gac. Med. Mex. 2000, 136, 45–56. [Google Scholar]
- Tjarnstrom, J.; Wikstrom, T.; Bagge, U.; Risberg, B.; Braide, M. Effects of hyperbaric oxygen treatment on neutrophil activation and pulmonary sequestration in intestinal ischemia-reperfusion in rats. Eur. Surg. Res. 1999, 31, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Buras, J.A.; Stahl, G.L.; Svoboda, K.K.; Reenstra, W.R. Hyperbaric oxygen downregulates ICAM-1 expression induced by hypoxia and hypoglycemia: The role of NOS. Am. J. Physiol. Cell Physiol. 2000, 278, C292–C302. [Google Scholar] [CrossRef]
- Chen, Q.; Banick, P.D.; Thom, S.R. Functional inhibition of rat polymorphonuclear leukocyte B2 integrins by hyperbaric oxygen is associated with impaired cGMP synthesis. J. Pharmacol. Exp. Ther. 1996, 276, 929–933. [Google Scholar]
- Thom, S.R.; Mendiguren, I.; Hardy, K.; Bolotin, T.; Fisher, D.; Nebolon, M.; Kilpatrick, L. Inhibition of human neutrophil beta2-integrin-dependent adherence by hyperbaric O2. Am. J. Physiol. 1997, 272, C770–C777. [Google Scholar] [CrossRef] [PubMed]
- Sakoda, M.; Ueno, S.; Kihara, K.; Arikawa, K.; Dogomori, H.; Nuruki, K.; Takao, S.; Aikou, T. A potential role of hyperbaric oxygen exposure through intestinal nuclear factor-kappaB. Crit. Care Med. 2004, 32, 1722–1729. [Google Scholar] [CrossRef]
- Weisz, G.; Lavy, A.; Adir, Y.; Melamed, Y.; Rubin, D.; Eidelman, S.; Pollack, S. Modification of in vivo and in vitro TNF-alpha, IL-1, and IL-6 secretion by circulating monocytes during hyperbaric oxygen treatment in patients with perianal Crohn’s disease. J. Clin. Immunol. 1997, 17, 154–159. [Google Scholar] [CrossRef]
- Bitterman, N.; Bitterman, H.; Kinarty, A.; Melamed, Y.; Lahat, N. Effect of a single exposure to hyperbaric oxygen on blood mononuclear cells in human subjects. Undersea Hyperb. Med. 1993, 20, 197–204. [Google Scholar]
- Inamoto, Y.; Okuno, F.; Saito, K.; Tanaka, Y.; Watanabe, K.; Morimoto, I.; Yamashita, U.; Eto, S. Effect of hyperbaric oxygenation on macrophage function in mice. Biochem. Biophys. Res. Commun. 1991, 179, 886–891. [Google Scholar] [CrossRef]
- Yamashita, M.; Yamashita, M. Hyperbaric oxygen treatment attenuates cytokine induction after massive hemorrhage. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E811–E816. [Google Scholar] [CrossRef] [PubMed]
- Benson, R.M.; Minter, L.M.; Osborne, B.A.; Granowitz, E.V. Hyperbaric oxygen inhibits stimulus-induced proinflammatory cytokine synthesis by human blood-derived monocyte-macrophages. Clin. Exp. Immunol. 2003, 134, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Rocco, M.; Antonelli, M.; Letizia, V.; Alampi, D.; Spadetta, G.; Passariello, M.; Conti, G.; Serio, P.; Gasparetto, A. Lipid peroxidation, circulating cytokine and endothelin 1 levels in healthy volunteers undergoing hyperbaric oxygenation. Minerva Anestesiol. 2001, 67, 393–400. [Google Scholar] [PubMed]
- Granowitz, E.V.; Skulsky, E.J.; Benson, R.M.; Wright, J.; Garb, J.L.; Cohen, E.R.; Smithline, E.C.; Brown, R.B. Exposure to increased pressure or hyperbaric oxygen suppresses interferon-gamma secretion in whole blood cultures of healthy humans. Undersea Hyperb. Med. 2002, 29, 216–225. [Google Scholar] [PubMed]
- Yang, Z.J.; Bosco, G.; Montante, A.; Ou, X.I.; Camporesi, E.M. Hyperbaric O2 reduces intestinal ischemia-reperfusion-induced TNF-alpha production and lung neutrophil sequestration. Eur. J. Appl. Physiol. 2001, 85, 96–103. [Google Scholar] [CrossRef] [PubMed]
- van den Blink, B.; van der Kleij, A.J.; Versteeg, H.H.; Peppelenbosch, M.P. Immunomodulatory effect of oxygen and pressure. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002, 132, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.C.; Wan, F.J.; Wu, C.C.; Tung, C.S.; Wu, T.H. Hyperbaric oxygen protects against lipopolysaccharide-stimulated oxidative stress and mortality in rats. Eur. J. Pharmacol. 2005, 508, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.M.; Gao, C.J.; Li, W.X.; Lin, M.T.; Niu, K.C. Resuscitation from experimental heatstroke by hyperbaric oxygen therapy. Crit. Care Med. 2005, 33, 813–818. [Google Scholar] [CrossRef]
- Nadeau, J.R.; Arnold, B.M.; Johnston, J.M.; Muir, G.D.; Verge, V.M.K. Acute intermittent hypoxia enhances regeneration of surgically repaired peripheral nerves in a manner akin to electrical stimulation. Exp. Neurol. 2021, 341, 113671. [Google Scholar] [CrossRef]
- Huang, X.; Liang, P.; Jiang, B.; Zhang, P.; Yu, W.; Duan, M.; Guo, L.; Cui, X.; Huang, M.; Huang, X. Hyperbaric oxygen potentiates diabetic wound healing by promoting fibroblast cell proliferation and endothelial cell angiogenesis. Life Sci. 2020, 259, 118246. [Google Scholar] [CrossRef]
- Zhou, C.; Li, Y.; Nanda, A.; Zhang, J.H. HBO suppresses Nogo-A, Ng-R, or RhoA expression in the cerebral cortex after global ischemia. Biochem. Biophys. Res. Commun. 2003, 309, 368–376. [Google Scholar] [CrossRef]
- Yang, J.T.; Chang, C.N.; Lee, T.H.; Lin, T.N.; Hsu, J.C.; Hsu, Y.H.; Wu, J.H. Hyperbaric oxygen treatment decreases post-ischemic neurotrophin-3 mRNA down-regulation in the rat hippocampus. Neuroreport 2001, 12, 3589–3592. [Google Scholar] [CrossRef]
- Muller, A.; Tal, R.; Donohue, J.F.; Akin-Olugbade, Y.; Kobylarz, K.; Paduch, D.; Cutter, S.C.; Mehrara, B.J.; Scardino, P.T.; Mulhall, J.P. The effect of hyperbaric oxygen therapy on erectile function recovery in a rat cavernous nerve injury model. J. Sex. Med. 2008, 5, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, P.O.; Nelson, A.G.; Fanton, J.W.; Yates, T.; Kagan-Hallet, K.S. Effect of hyperbaric oxygenation on peripheral nerve regeneration in adult male rabbits. Undersea Hyperb. Med. 1996, 23, 107–113. [Google Scholar]
- Haapaniemi, T.; Nylander, G.; Kanje, M.; Dahlin, L. Hyperbaric oxygen treatment enhances regeneration of the rat sciatic nerve. Exp. Neurol. 1998, 149, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Haapaniemi, T.; Nishiura, Y.; Dahlin, L.B. Effects of hyperbaric oxygen treatment on axonal outgrowth in sciatic nerve grafts in rats. Scand. J. Plast. Reconstr. Surg. Hand Surg. 2001, 35, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Haapaniemi, T.; Nishiura, Y.; Dahlin, L.B. Functional evaluation after rat sciatic nerve injury followed by hyperbaric oxygen treatment. J. Peripher. Nerv. Syst. 2002, 7, 149–154. [Google Scholar] [CrossRef]
- Tuma Junior, P.; Dias, M.D.; Arrunategui, G.; Duarte, G.G.; Wada, A.; Cunha, A.S.; Ferreira, M.C. Effect of hyperbaric oxygen on the regeneration of experimental crush injuries of nerves. Rev. Hosp. Clin. Fac. Med. Sao Paulo 1999, 54, 81–84. [Google Scholar] [CrossRef][Green Version]
- Nishiura, Y.; Haapaniemi, T.; Dahlin, L.B. Hyperbaric oxygen treatment has different effects on nerve regeneration in acellular nerve and muscle grafts. J. Peripher. Nerv. Syst. 2001, 6, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Eguiluz-Ordonez, R.; Sanchez, C.E.; Venegas, A.; Figueroa-Granados, V.; Hernandez-Pando, R. Effects of hyperbaric oxygen on peripheral nerves. Plast. Reconstr. Surg. 2006, 118, 350–357, discussion 358–359. [Google Scholar] [CrossRef] [PubMed]
- Toledo, G.L.; Sangalette, B.S.; Passerotti, L.C.; Nascimento, J.A.; Shinohara, A.L.; Oliveira, A.L.R.; Buzalaf, M.A.R.; Rodrigues, A.C. Guided neural regeneration with autologous fat grafting and oxygen hyperbaric therapy. Braz. Oral Res. 2021, 35, e138. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, W.A.; Brown, R.E.; Roth, A.C.; Mathur, A.; Stephenson, L.L. Functional evaluation of peripheral-nerve repair and the effect of hyperbaric oxygen. J. Reconstr. Microsurg. 1995, 11, 27–29, discussion 29–30. [Google Scholar] [CrossRef]
- Brenna, C.T.; Khan, S.; Katznelson, R.; Brull, R. The role of hyperbaric oxygen therapy in the management of perioperative peripheral nerve injury: A scoping review of the literature. Reg. Anesth. Pain Med. 2023, 48, 443–453. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
André-Lévigne, D.; Pignel, R.; Boet, S.; Jaquet, V.; Kalbermatten, D.F.; Madduri, S. Role of Oxygen and Its Radicals in Peripheral Nerve Regeneration: From Hypoxia to Physoxia to Hyperoxia. Int. J. Mol. Sci. 2024, 25, 2030. https://doi.org/10.3390/ijms25042030
André-Lévigne D, Pignel R, Boet S, Jaquet V, Kalbermatten DF, Madduri S. Role of Oxygen and Its Radicals in Peripheral Nerve Regeneration: From Hypoxia to Physoxia to Hyperoxia. International Journal of Molecular Sciences. 2024; 25(4):2030. https://doi.org/10.3390/ijms25042030
Chicago/Turabian StyleAndré-Lévigne, Dominik, Rodrigue Pignel, Sylvain Boet, Vincent Jaquet, Daniel F. Kalbermatten, and Srinivas Madduri. 2024. "Role of Oxygen and Its Radicals in Peripheral Nerve Regeneration: From Hypoxia to Physoxia to Hyperoxia" International Journal of Molecular Sciences 25, no. 4: 2030. https://doi.org/10.3390/ijms25042030
APA StyleAndré-Lévigne, D., Pignel, R., Boet, S., Jaquet, V., Kalbermatten, D. F., & Madduri, S. (2024). Role of Oxygen and Its Radicals in Peripheral Nerve Regeneration: From Hypoxia to Physoxia to Hyperoxia. International Journal of Molecular Sciences, 25(4), 2030. https://doi.org/10.3390/ijms25042030







