New Derivatives of 1-(3-Methyl-1-Benzofuran-2-yl)Ethan-1-one: Synthesis and Preliminary Studies of Biological Activity
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Biological Evaluation
2.2.1. MTT Cytotoxicity Studies
2.2.2. Antiproliferative Activity
2.2.3. Reactive Oxygen Species (ROS) Generation
2.2.4. Inhibition of IL-6 Release
2.2.5. Activation of Apoptosis
2.3. Antibacterial Studies
3. Material and Methods
3.1. Chemistry
3.2. Synthetic Procedures
3.2.1. Synthesis of Starting Benzofurans 1–4
1-(5-Methoxy-3-Methyl-1-Benzofuran-2-yl)Ethanone (1)
1-(4,6-Dimethoxy-3-Methyl-1-Benzofuran-2-yl)Ethanone (2)
1-(4-Ethoxy-3-Methyl-1-Benzofuran-2-yl)Ethanone (3)
1-(6-Ethoxy-3-Methyl-1-Benzofuran-2-yl)Ethanone (4)
3.2.2. Procedure for Bromination by Using N-Bromosuccinimide (NBS)
1-[3-(Bromomethyl)-5-Methoxy-1-Benzofuran-2-yl]Ethanone (5)
1-[3-(Bromomethyl)-4,6-Dimethoxy-1-Benzofuran-2-yl]Ethanone (6)
1-[5-Bromo-3-(Bromomethyl)-4,6-Dimethoxy-1-Benzofuran-2-yl]Ethanone (7)
1-[3-(Bromomethyl)-4-Ethoxy-1-Benzofuran-2-yl]Ethanone (8)
1-[3-(Bromomethyl)-6-Ethoxy-1-Benzofuran-2-yl]Ethanone (9)
3.3. Biological Studies
3.3.1. MTT Cytotoxicity Studies
3.3.2. Trypan Blue Exclusion Assay
3.3.3. Annexin V-FITC Binding Apoptosis Determination Assay
3.3.4. Caspases 3 and 7 Activity Assay
3.3.5. ROS Detection: DHR 123 and DCFH-DA
3.3.6. Interleukin-6
3.4. Antimicrobial Activity
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faderl, S.; Talpaz, M.; Estrov, Z.; O’Brien, S.; Kurzrock, R.; Kantarjian, H.M. The biology of chronic myeloid leukemia. N. Engl. J. Med. 1999, 341, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, D.; Weinberg, O.K. How I investigate acute myeloid leukemia. Int. J. Lab. Hematol. 2020, 42, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Iqbal, N. Imatinib: A breakthrough of targeted therapy in cancer. Chemother. Res. Pract. 2014, 2014, 357027. [Google Scholar] [CrossRef] [PubMed]
- Trela, E.; Glowacki, S.; Blasiak, J. Therapy of chronic myeloid leukemia: Twilight of the imatinib era? ISRN Oncol. 2014, 2014, 596483. [Google Scholar] [CrossRef]
- Savage, D.G.; Antman, K.H. Imatinib mesylate—A new oral targeted therapy. N. Engl. J. Med. 2002, 346, 683–693. [Google Scholar] [CrossRef]
- Deininger, M.W.; Druker, B.J. Specific targeted therapy of chronic myelogenous leukemia with imatinib. Pharmacol. Rev. 2003, 55, 401–423. [Google Scholar] [CrossRef]
- Irvine, E.; Williams, C. Treatment-, patient-, and disease-related factors and the emergence of adverse events with tyrosine kinase inhibitors for the treatment of chronic myeloid leukemia. Pharmacotherapy 2013, 33, 868–881. [Google Scholar] [CrossRef]
- Cortes-Funes, H.; Coronado, C. Role of anthracyclines in the era of targeted therapy. Cardiovasc. Toxicol. 2007, 7, 56–60. [Google Scholar] [CrossRef]
- Fraczkowska, K.; Bacia, M.; Przybylo, M.; Drabik, D.; Kaczorowska, A.; Rybka, J.; Stefanko, E.; Drobczynski, S.; Masajada, J.; Podbielska, H.; et al. Alterations of biomechanics in cancer and normal cells induced by doxorubicin. Biomed. Pharmacother. 2018, 97, 1195–1203. [Google Scholar] [CrossRef]
- O’Hare, T.; Corbin, A.S.; Druker, B.J. Targeted CML therapy: Controlling drug resistance, seeking cure. Curr. Opin. Genet. Dev. 2006, 16, 92–99. [Google Scholar] [CrossRef]
- Apperley, J.F. Part I: Mechanisms of resistance to imatinib in chronic myeloid leukaemia. Lancet Oncol. 2007, 8, 1018–1029. [Google Scholar] [CrossRef] [PubMed]
- Apperley, J.F. Part II: Management of resistance to imatinib in chronic myeloid leukaemia. Lancet Oncol. 2007, 8, 1116–1128. [Google Scholar] [CrossRef]
- Kantarjian, H.M.; Talpaz, M.; Giles, F.; O’Brien, S.; Cortes, J. New insights into the pathophysiology of chronic myeloid leukemia and imatinib resistance. Ann. Intern. Med. 2006, 145, 913–923. [Google Scholar] [CrossRef]
- Piperdi, B.; Ling, Y.H.; Liebes, L.; Muggia, F.; Perez-Soler, R. Bortezomib: Understanding the mechanism of action. Mol. Cancer Ther. 2011, 10, 2029–2030. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, H.; Khayat, D.; Giaccone, G.; Facon, T. Proteasome inhibition and its clinical prospects in the treatment of hematologic and solid malignancies. Cancer 2005, 104, 1794–1807. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.G.; Barlogie, B.; Berenson, J.; Singhal, S.; Jagannath, S.; Irwin, D.; Rajkumar, S.V.; Srkalovic, G.; Alsina, M.; Alexanian, R.; et al. A Phase 2 Study of Bortezomib in Relapsed, Refractory Myeloma. N. Engl. J. Med. 2003, 348, 2609–2617. [Google Scholar] [CrossRef]
- Habtemariam, S. Antiinflammatory activity of the antirheumatic herbal drug, gravel root (Eupatorium purpureum): Further biological activities and constituents. Phytother. Res. 2001, 15, 687–690. [Google Scholar] [CrossRef]
- Pauletti, P.M.; Araujo, A.R.; Young, M.C.; Giesbrecht, A.M.; Bolzani, V.D. nor-Lignans from the leaves of Styrax ferrugineus (Styracaceae) with antibacterial and antifungal activity. Phytochemistry 2000, 55, 597–601. [Google Scholar] [CrossRef]
- Masubuchi, M.; Kawasaki, K.; Ebiike, H.; Ikeda, Y.; Tsujii, S.; Sogabe, S.; Fujii, T.; Sakata, K.; Shiratori, Y.; Aoki, Y.; et al. Design and synthesis of novel benzofurans as a new class of antifungal agents targeting fungal N-myristoyltransferase. Part 1. Bioorg. Med. Chem. Lett. 2001, 11, 1833–1837. [Google Scholar] [CrossRef]
- Kayser, O.; Chen, M.; Kharazmi, A.; Kiderlen, A.F. Aurones interfere with Leishmania major mitochondrial fumarate reductase. Z. Naturforsch C J. Biosci. 2002, 57, 717–720. [Google Scholar] [CrossRef]
- Hayakawa, I.; Shioya, R.; Agatsuma, T.; Furukawa, H.; Naruto, S.; Sugano, Y. 4-Hydroxy-3-methyl-6-phenylbenzofuran-2-carboxylic acid ethyl ester derivatives as potent anti-tumor agents. Bioorg. Med. Chem. Lett. 2004, 14, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Dawood, K.M. Benzofuran derivatives: A patent review. Expert Opin. Ther. Pat. 2013, 23, 1133–1156. [Google Scholar] [CrossRef] [PubMed]
- Khanam, H.; Shamsuzzaman. Bioactive Benzofuran derivatives: A review. Eur. J. Med. Chem. 2015, 97, 483–504. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zheng, X.; Gao, H.; Wan, C.; Rao, G.; Mao, Z. Design, Synthesis, and Biological Evaluation of Novel Benzofuran Derivatives Bearing N-Aryl Piperazine Moiety. Molecules 2016, 21, 1684. [Google Scholar] [CrossRef] [PubMed]
- Eldehna, W.M.; Al-Rashood, S.T.; Al-Warhi, T.; Eskandrani, R.O.; Alharbi, A.; El Kerdawy, A.M. Novel oxindole/benzofuran hybrids as potential dual CDK2/GSK-3beta inhibitors targeting breast cancer: Design, synthesis, biological evaluation, and in silico studies. J. Enzym. Inhib. Med. Chem. 2021, 36, 270–285. [Google Scholar] [CrossRef] [PubMed]
- Mokenapelli, S.; Thalari, G.; Vadiyaala, N.; Yerrabelli, J.R.; Irlapati, V.K.; Gorityala, N.; Sagurthi, S.R.; Chitneni, P.R. Synthesis, cytotoxicity, and molecular docking of substituted 3-(2-methylbenzofuran-3-yl)-5-(phenoxymethyl)-1,2,4-oxadiazoles. Arch. Pharm. 2020, 353, e2000006. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.P.; Xie, Q.; Huang, E.F.; Wang, L.; Zhang, B.Q.; Hu, J.S.; Wan, D.C.; Jin, Z.; Hu, C. Design, synthesis, and biological activity of a novel series of benzofuran derivatives against oestrogen receptor-dependent breast cancer cell lines. Bioorg. Chem. 2020, 95, 103566. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.K.; SahayaSheela, V.J.; Kolluru, S.; Pandian, G.N.; Santhoshkumar, T.R.; Dan, V.M.; Ramana, C.V. Discovery of 3-(benzofuran-2-ylmethyl)-1H-indole derivatives as potential autophagy inducers in cervical cancer cells. Bioorganic Med. Chem. Lett. 2020, 30, 127431. [Google Scholar] [CrossRef]
- Li, Q.; Jian, X.E.; Chen, Z.R.; Chen, L.; Huo, X.S.; Li, Z.H.; You, W.W.; Rao, J.J.; Zhao, P.L. Synthesis and biological evaluation of benzofuran-based 3,4,5-trimethoxybenzamide derivatives as novel tubulin polymerization inhibitors. Bioorg. Chem. 2020, 102, 104076. [Google Scholar] [CrossRef]
- Qi, Z.Y.; Hao, S.Y.; Tian, H.Z.; Bian, H.L.; Hui, L.; Chen, S.W. Synthesis and biological evaluation of 1-(benzofuran-3-yl)-4-(3,4,5-trimethoxyphenyl)-1H-1,2,3-triazole derivatives as tubulin polymerization inhibitors. Bioorg. Chem. 2020, 94, 103392. [Google Scholar] [CrossRef]
- Coskun, D.; Erkisa, M.; Ulukaya, E.; Coskun, M.F.; Ari, F. Novel 1-(7-ethoxy-1-benzofuran-2-yl) substituted chalcone derivatives: Synthesis, characterization and anticancer activity. Eur. J. Med. Chem. 2017, 136, 212–222. [Google Scholar] [CrossRef]
- Kossakowski, J.; Krawiecka, M.; Kuran, B.; Stefanska, J.; Wolska, I. Synthesis and preliminary evaluation of the antimicrobial activity of selected 3-benzofurancarboxylic acid derivatives. Molecules 2010, 15, 4737–4749. [Google Scholar] [CrossRef]
- Krawiecka, M.; Kuran, B.; Kossakowski, J.; Cieslak, M.; Kazmierczak-Baranska, J.; Krolewska, K.; Nawrot, B. Synthesis and Cytotoxic Properties of Halogen and Aryl-/Heteroarylpiperazinyl Derivatives of Benzofurans. Anti-Cancer Agents Med. Chem. 2015, 15, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Krolewska-Golinska, K.; Cieslak, M.J.; Sobczak, M.; Dolot, R.; Radzikowska-Cieciura, E.; Napiorkowska, M.; Wybranska, I.; Nawrot, B. Novel Benzo[B]Furans with Anti-Microtubule Activity Upregulate Expression of Apoptotic Genes and Arrest Leukemia Cells in G2/M Phase. Anti-Cancer Agents Med. Chem. 2019, 19, 375–388. [Google Scholar] [CrossRef]
- Napiórkowska, M.; Cieślak, M.; Kaźmierczak-Barańska, J.; Królewska-Golińska, K.; Nawrot, B. Synthesis of new derivatives of benzofuran as potential anticancer agents. Molecules 2019, 24, 1529. [Google Scholar] [CrossRef]
- Gupta, R.; Rajpoot, K.; Tekade, M.; Sharma, M.C.; Tekade, R.K. Methods and models for in vitro toxicity. In Pharmacokinetics and Toxicokinetic Considerations; Academic Press: Cambridge, MA, USA, 2022; Volume 2, pp. 145–174. [Google Scholar] [CrossRef]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 1997, 21, A–3B. [Google Scholar] [CrossRef]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2015, 111, A3. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Rafique, R.; Khan, K.M.; Chigurupati, S.; Ji, X.; Wadood, A.; Rehman, A.U.; Salar, U.; Iqbal, M.S.; Taha, M.; et al. Potent α-amylase inhibitors and radical (DPPH and ABTS) scavengers based on benzofuran-2-yl(phenyl)methanone derivatives: Syntheses, in vitro, kinetics, and in silico studies. Bioorg. Chem. 2020, 104, 104238. [Google Scholar] [CrossRef] [PubMed]
- Bouchmaa, N.; Ben Mrid, R.; Boukharsa, Y.; Nhiri, M.; Ait Mouse, H.; Taoufik, J.; Ansar, M.h.; Zyad, A. Cytotoxicity of new pyridazin-3(2 H)-one derivatives orchestrating oxidative stress in human triple-negative breast cancer (MDA-MB-468). Arch. Pharm. 2018, 351, 1800128. [Google Scholar] [CrossRef] [PubMed]
- Chand, K.; Rajeshwari; Hiremathad, A.; Singh, M.; Santos, M.A.; Keri, R.S. A review on antioxidant potential of bioactive heterocycle benzofuran: Natural and synthetic derivatives. Pharmacol. Rep. 2017, 69, 281–295. [Google Scholar] [CrossRef]
- Zhou, Y.; Hileman, E.O.; Plunkett, W.; Keating, M.J.; Huang, P. Free radical stress in chronic lymphocytic leukemia cells and its role in cellular sensitivity to ROS-generating anticancer agents. Blood 2003, 101, 4098–4104. [Google Scholar] [CrossRef]
- Jitschin, R.; Hofmann, A.D.; Bruns, H.; Gießl, A.; Bricks, J.; Berger, J.; Saul, D.; Eckart, M.J.; Mackensen, A.; Mougiakakos, D. Mitochondrial metabolism contributes to oxidative stress and reveals therapeutic targets in chronic lymphocytic leukemia. Blood 2014, 123, 2663–2672. [Google Scholar] [CrossRef]
- Nakamura, H.; Takada, K. Reactive oxygen species in cancer: Current findings and future directions. Cancer Sci. 2021, 112, 3945–3952. [Google Scholar] [CrossRef]
- Okon, I.S.; Zou, M.-H. Mitochondrial ROS and cancer drug resistance: Implications for therapy. Pharmacol. Res. 2015, 100, 170–174. [Google Scholar] [CrossRef]
- Sena, L.A.; Chandel, N.S. Physiological Roles of Mitochondrial Reactive Oxygen Species. Mol. Cell 2012, 48, 158–167. [Google Scholar] [CrossRef]
- Wiseman, H.; Halliwell, B. Damage to DNA by reactive oxygen and nitrogen species: Role in inflammatory disease and progression to cancer. Biochem. J. 1996, 313, 17–29. [Google Scholar] [CrossRef]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef]
- Aguilar-Hernandez, M.M.; Blunt, M.D.; Dobson, R.; Yeomans, A.; Thirdborough, S.; Larrayoz, M.; Smith, L.D.; Linley, A.; Strefford, J.C.; Davies, A.; et al. IL-4 enhances expression and function of surface IgM in CLL cells. Blood 2016, 127, 3015–3025. [Google Scholar] [CrossRef] [PubMed]
- Antosz, H.; Wojciechowska, K.; Sajewicz, J.; Choroszyńska, D.; Marzec-Kotarska, B.; Osiak, M.; Pająk, N.; Tomczak, W.; Jargiełło-Baszak, M.; Baszak, J. IL-6, IL-10, c-Jun and STAT3 expression in B-CLL. Blood Cells Mol. Dis. 2015, 54, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Drennan, S.; D’Avola, A.; Gao, Y.; Weigel, C.; Chrysostomou, E.; Steele, A.J.; Zenz, T.; Plass, C.; Johnson, P.W.; Williams, A.P.; et al. IL-10 production by CLL cells is enhanced in the anergic IGHV mutated subset and associates with reduced DNA methylation of the IL10 locus. Leukemia 2017, 31, 1686–1694. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Q.; Jia, L.; Li, Y.T.; Farren, T.; Agrawal, S.G.; Liu, F.T. Increased autocrine interleukin-6 production is significantly associated with worse clinical outcome in patients with chronic lymphocytic leukemia. J. Cell. Physiol. 2019, 234, 13994–14006. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Karin, M. Dangerous liaisons: STAT3 and NF-κB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 2010, 21, 11–19. [Google Scholar] [CrossRef]
- Nursal, A.F.; Pehlivan, M.; Sahin, H.H.; Pehlivan, S. The Associations of IL-6, IFN-γ, TNF-α, IL-10, and TGF-β1 Functional Variants with Acute Myeloid Leukemia in Turkish Patients. Genet. Test. Mol. Biomark. 2016, 20, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Correa, B.; Bergua, J.M.; Campos, C.; Gayoso, I.; Arcos, M.J.; Bañas, H.; Morgado, S.; Casado, J.G.; Solana, R.; Tarazona, R. Cytokine profiles in acute myeloid leukemia patients at diagnosis: Survival is inversely correlated with IL-6 and directly correlated with IL-10 levels. Cytokine 2013, 61, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Stevens, A.M.; Miller, J.M.; Munoz, J.O.; Gaikwad, A.S.; Redell, M.S. Interleukin-6 levels predict event-free survival in pediatric AML and suggest a mechanism of chemotherapy resistance. Blood Adv. 2017, 1, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, L.; Feng, Y.; Khadka, B.; Fang, Z.; Liu, J. HDAC8 promotes daunorubicin resistance of human acute myeloid leukemia cells via regulation of IL-6 and IL-8. Biol. Chem. 2021, 402, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ye, Y.; Wang, X.; Lu, B.; Guo, Z.; Wu, S. JMJD3-regulated expression of IL-6 is involved in the proliferation and chemosensitivity of acute myeloid leukemia cells. Biol. Chem. 2021, 402, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Navarro, S.; Mitjavila, M.T.; Katz, A.; Doly, J.; Vainchenker, W. Expression of interleukin 6 and its specific receptor by untreated and PMA-stimulated human erythroid and megakaryocytic cell lines. Exp. Hematol. 1991, 19, 11–17. [Google Scholar]
- Schuringa, J.-J.; Wierenga, A.T.J.; Kruijer, W.; Vellenga, E. Constitutive Stat3, Tyr705, and Ser727 phosphorylation in acute myeloid leukemia cells caused by the autocrine secretion of interleukin-6. Blood 2000, 95, 3765–3770. [Google Scholar] [CrossRef]
- Liang, X.; Wang, P.; Yang, C.; Huang, F.; Wu, H.; Shi, H.; Wu, X. Galangin Inhibits Gastric Cancer Growth through Enhancing STAT3 Mediated ROS Production. Front. Pharmacol. 2021, 12, 646628. [Google Scholar] [CrossRef]
- Leanza, L.; Romio, M.; Becker, K.A.; Azzolini, M.; Trentin, L.; Managò, A.; Venturini, E.; Zaccagnino, A.; Mattarei, A.; Carraretto, L.; et al. Direct Pharmacological Targeting of a Mitochondrial Ion Channel Selectively Kills Tumor Cells In Vivo. Cancer Cell 2017, 31, 516–531. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K. Reactive oxygen species (ROS) and cancer: Role of antioxidative nutraceuticals. Cancer Lett. 2017, 387, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Kudryavtseva, A.V.; Krasnov, G.S.; Dmitriev, A.A.; Alekseev, B.Y.; Kardymon, O.L.; Sadritdinova, A.F.; Fedorova, M.S.; Pokrovsky, A.V.; Melnikova, N.V.; Kaprin, A.D.; et al. Mitochondrial dysfunction and oxidative stress in aging and cancer. Oncotarget 2016, 7, 44879–44905. [Google Scholar] [CrossRef] [PubMed]
- Su, C.-M.; Chen, C.-Y.; Lu, T.; Sun, Y.; Li, W.; Huang, Y.-L.; Tsai, C.-H.; Chang, C.-S.; Tang, C.-H. A novel benzofuran derivative, ACDB, induces apoptosis of human chondrosarcoma cells through mitochondrial dysfunction and endoplasmic reticulum stress. Oncotarget 2016, 7, 83530–83543. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-F.; Chen, C.-Y.; Chen, H.-T.; Chang, C.-S.; Tang, C.-H. BL-038, a Benzofuran Derivative, Induces Cell Apoptosis in Human Chondrosarcoma Cells through Reactive Oxygen Species/Mitochondrial Dysfunction and the Caspases Dependent Pathway. Int. J. Mol. Sci. 2016, 17, 1491. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.C.; Borutaite, V. Regulation of apoptosis by the redox state of cytochrome c. Biochim. Biophys. Acta (BBA)—Bioenerg. 2008, 1777, 877–881. [Google Scholar] [CrossRef] [PubMed]
- Lakhani, S.A.; Masud, A.; Kuida, K.; Porter, G.A., Jr.; Booth, C.J.; Mehal, W.Z.; Inayat, I.; Flavell, R.A. Caspases 3 and 7: Key mediators of mitochondrial events of apoptosis. Science 2006, 311, 847–851. [Google Scholar] [CrossRef]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef]
- Chrzanowska, A.; Roszkowski, P.; Bielenica, A.; Olejarz, W.; Stepien, K.; Struga, M. Anticancer and antimicrobial effects of novel ciprofloxacin fatty acids conjugates. Eur. J. Med. Chem. 2020, 185, 111810. [Google Scholar] [CrossRef]
- M7-A7: Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2018; Volume 13, pp. 965–975.
- Opatrilova, R. Synthesis, Characterization and Physico-chemical Properties of New 2-(4-Arylpiperazine-1-yl)-1-(3-methylbenzofuran-2-yl)ethanoles as Potential Antihypertensive Agents. Curr. Org. Chem. 2009, 13, 965–967. [Google Scholar] [CrossRef]
- Prabst, K.; Engelhardt, H.; Ringgeler, S.; Hubner, H. Basic Colorimetric Proliferation Assays: MTT, WST, and Resazurin. Methods Mol. Biol. 2017, 1601, 1–17. [Google Scholar] [CrossRef] [PubMed]



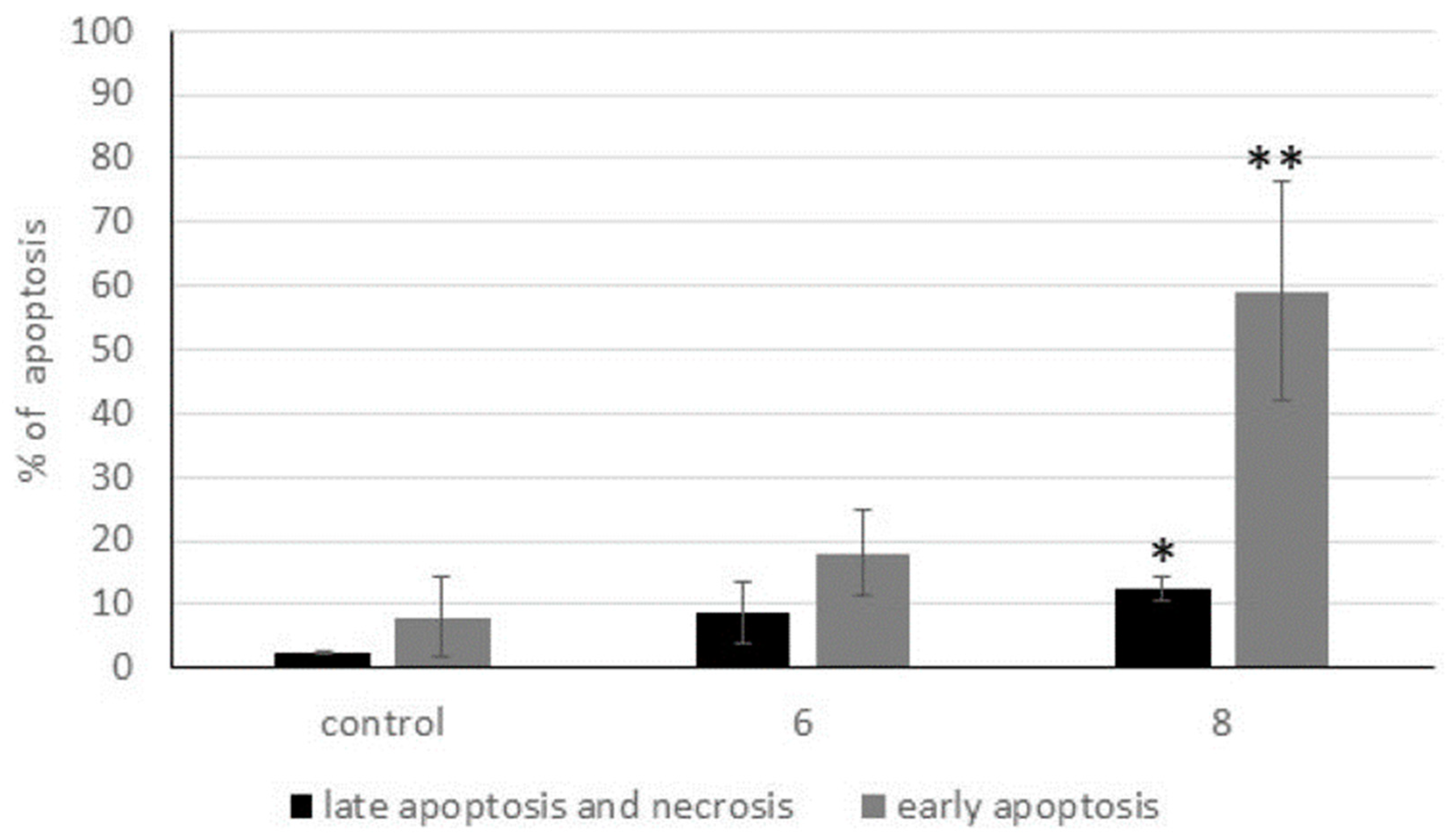
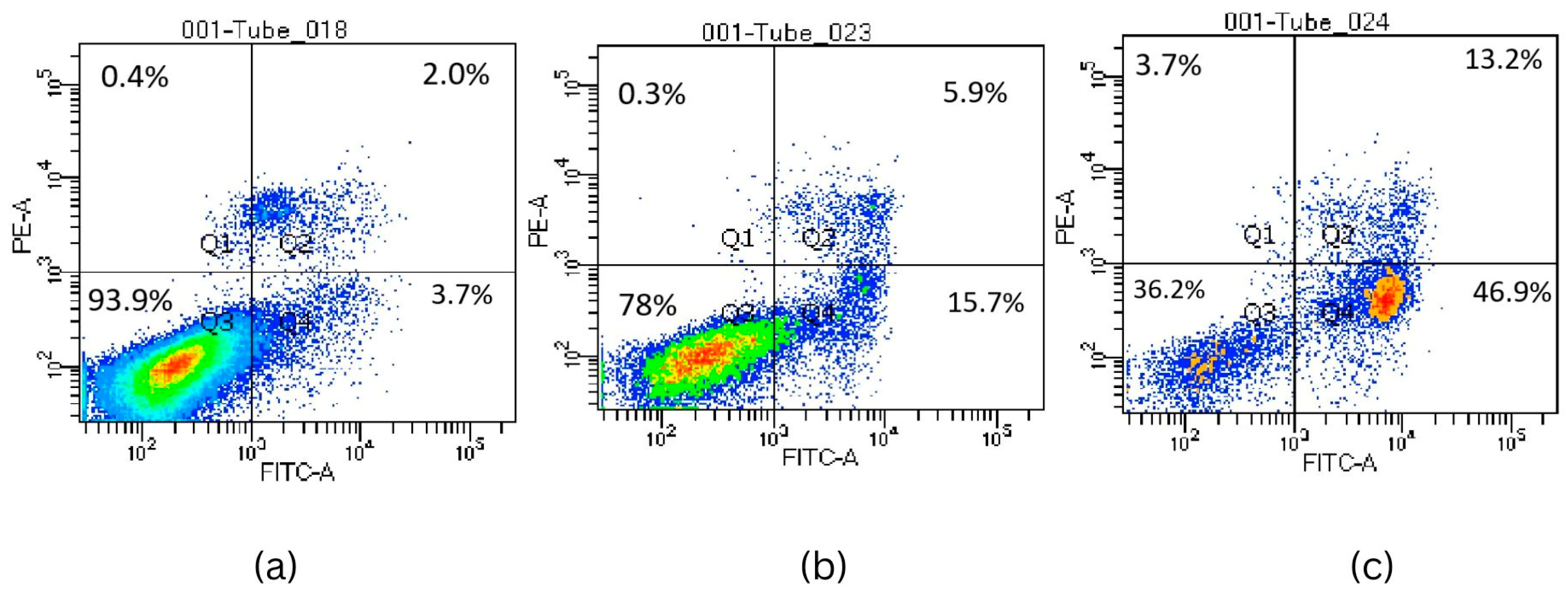
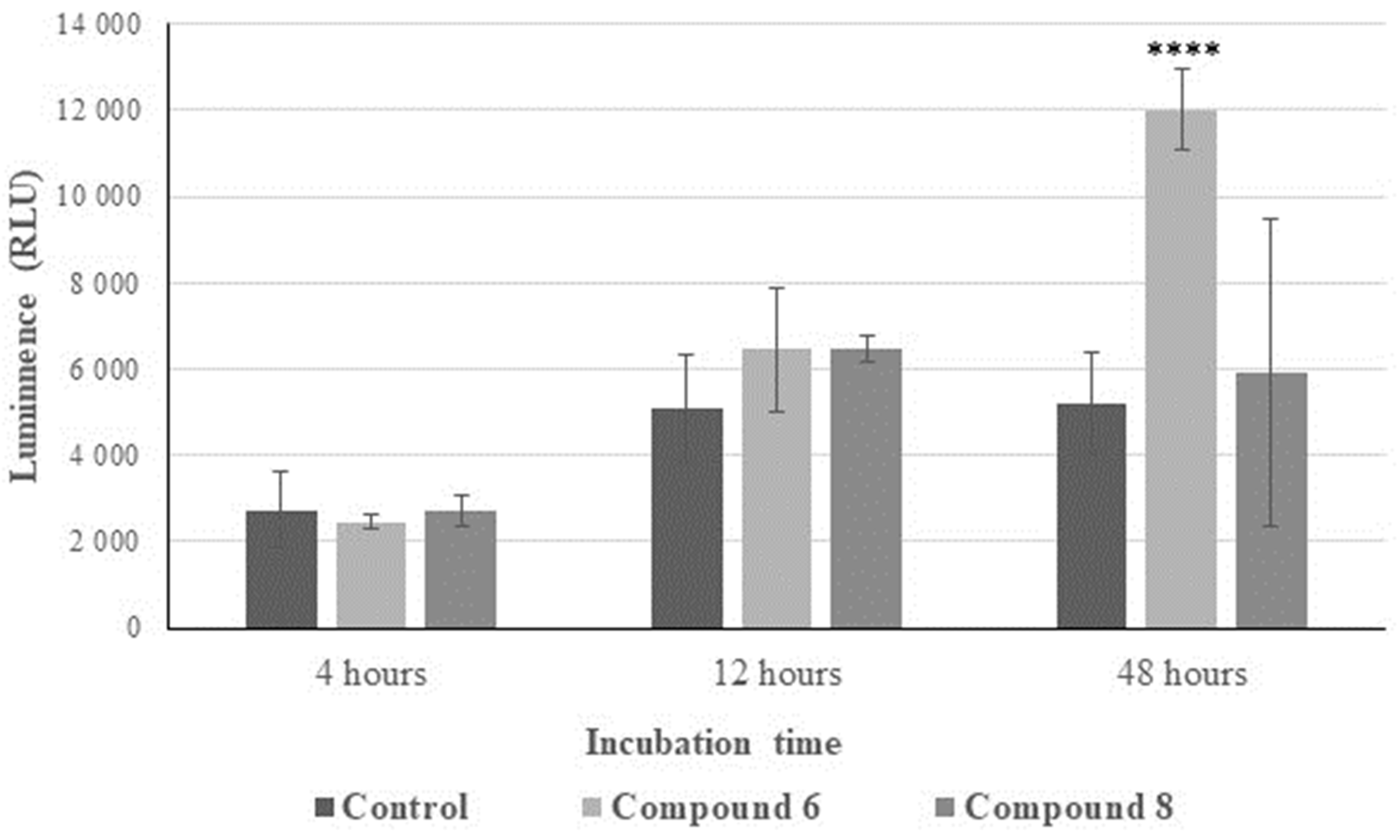
| Compound | K562 a | PC3 b | SW620 c | Cacki-1 d | HaCaT e | TI f |
|---|---|---|---|---|---|---|
| 1 | 57.00 ± 3.50 | 80 ± 20.0 | >100 | >100 | >100 | >100 |
| 2 | 36.32 ± 4.5 | >100 | >100 | >100 | 67.30 ± 29.68 | 1.853 |
| 3 | 58.00 ± 21.12 | 66.41 ± 28.72 | 52.33 ± 22.5 | >100 | >100 | 1.724 |
| 4 | 60.00 ± 9.0 | >100 | >100 | >100 | 55.29 ± 33.44 | 0.925 |
| 5 | 10.30 ± 0.55 | 17.50 ± 3.5 | 15.60 ± 4.1 | >100 | 15.00 ± 0.80 | 1.450 |
| 6 | 3.83 ± 0.60 | 7.02 ± 2.22 | >100 | >100 | 12.44 ± 1.27 | 3.248 |
| 7 | 50.00 ± 10.0 | 40.00 ± 15.0 | 47.15 ± 22.25 | >100 | 5.69 ± 5.67 | 0.114 |
| 8 | 2.59 ± 0.88 | 7.86 ± 2.62 | >100 | >100 | 23.57 ± 10.7 0 | 9.100 |
| 9 | 14.40 ± 3.70 | 40.00 ± 20.0 | >100 | >100 | 22.80 ± 1.51 | 1.583 |
| Bortezomib | 0.04 ± 0.01 | nd | nd | nd | nd | nd |
| Doxorubicin | 0.21 ± 0.10 | nd | nd | nd | nd | nd |
| Compound | Cell Number (×106) | Percentage of Viability (%) | |
|---|---|---|---|
| HaCaT a | Control | 2.70 ± 0.10 | 93.0 ± 0.27 |
| 6 | 2.45 ± 1.63 | 80.5 ± 19.09 | |
| 8 | 2.00 ± 0.42 | 87.0 ± 8.70 | |
| K562 b | Control | 0.75 ± 0.09 | 91.5 ± 3.50 |
| 6 | 0.62 ± 0.12 | 94.0 ± 1.00 | |
| 8 | 0.83 ± 0.12 | 96.0 ± 1.41 |
| Il-6 Concentration in K562 Cells in 72 h | |
|---|---|
| Control | 11.616 ± 1.730997 |
| 6 | 5.791 ± 2.047781 *** |
| 8 | 6.958 ± 3.622508 *** |
| Strain | Compound 7 (µg/mL) | Ciprofloxacine (µg/mL) |
|---|---|---|
| S.aureus NCTC 4163 | >512 | 0.25 |
| S.aureus ATCC 25923 | 16 | 0.5 |
| S.aureus ATCC 6538 | 64 | 0.125 |
| S.aureus NCTC 29213 | 32 | 0.5 |
| S. epiderminis ATCC 122228 | 16 | 0.125 |
| S. epidermidis ATCC 35984 | 16 | 0.125 |
| E. coli ATCC 25922 | >512 | 0.015 |
| P. aeruginosa ATCC 15442 | >512 | 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Napiórkowska, M.; Kumaravel, P.; Amboo Mahentheran, M.; Kiernozek-Kalińska, E.; Grosicka-Maciąg, E. New Derivatives of 1-(3-Methyl-1-Benzofuran-2-yl)Ethan-1-one: Synthesis and Preliminary Studies of Biological Activity. Int. J. Mol. Sci. 2024, 25, 1999. https://doi.org/10.3390/ijms25041999
Napiórkowska M, Kumaravel P, Amboo Mahentheran M, Kiernozek-Kalińska E, Grosicka-Maciąg E. New Derivatives of 1-(3-Methyl-1-Benzofuran-2-yl)Ethan-1-one: Synthesis and Preliminary Studies of Biological Activity. International Journal of Molecular Sciences. 2024; 25(4):1999. https://doi.org/10.3390/ijms25041999
Chicago/Turabian StyleNapiórkowska, Mariola, Pratheeba Kumaravel, Mithulya Amboo Mahentheran, Ewelina Kiernozek-Kalińska, and Emilia Grosicka-Maciąg. 2024. "New Derivatives of 1-(3-Methyl-1-Benzofuran-2-yl)Ethan-1-one: Synthesis and Preliminary Studies of Biological Activity" International Journal of Molecular Sciences 25, no. 4: 1999. https://doi.org/10.3390/ijms25041999
APA StyleNapiórkowska, M., Kumaravel, P., Amboo Mahentheran, M., Kiernozek-Kalińska, E., & Grosicka-Maciąg, E. (2024). New Derivatives of 1-(3-Methyl-1-Benzofuran-2-yl)Ethan-1-one: Synthesis and Preliminary Studies of Biological Activity. International Journal of Molecular Sciences, 25(4), 1999. https://doi.org/10.3390/ijms25041999








