Essential Role of Astrocytes in Learning and Memory
Abstract
1. Introduction
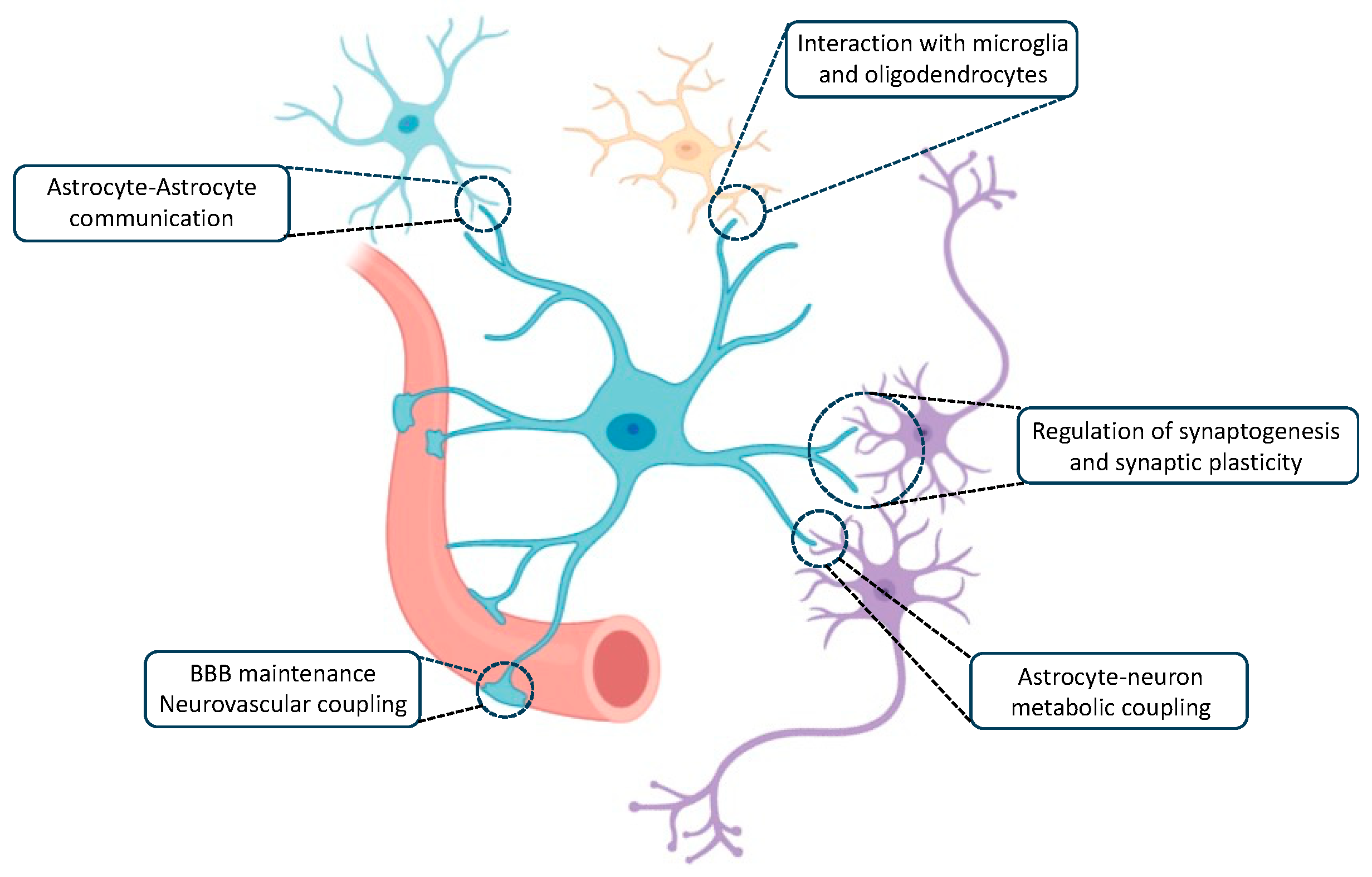
2. Role of Astrocytes in the Brain
2.1. Astrocyte–Astrocyte Communication
2.2. Interaction with Microglia and Oligodendrocytes
2.3. BBB Development and Maintenance and Neurovascular Coupling
2.4. Astrocyte–Neuron Metabolic Coupling
2.5. Regulation of Synaptogenesis
2.6. Regulation of Synaptic Plasticity and Activity
3. Bilateral Neuron–Astrocyte Interaction as a Potential Mechanism for Memory Modulation
3.1. Regulation of Synaptic Formation
3.2. Regulation of Synaptic Modulation
3.3. Neuromodulation
3.4. Adult Neurogenesis
4. Implication of Astrocytes on Learning and Memory Processes
4.1. Working and Spatial Memory
| Proposed Astrocytic Mechanism | Outcome of the Astrocytic Manipulation | Test | Reference |
|---|---|---|---|
| Melanopsin-induced astrocytic optogenetic activation | Improved working and spatial memory | T-maze Object in place preference test | [81] |
| Astrocytic Gq-GPCR signaling activation with hM3DGq | Improved working memory | T-maze | [83] |
| Melanopsin-induced astrocytic optogenetic activation | Improved spatial memory | Place novelty preference test | [82] |
| Genetic deletion of S100β | Improved spatial memory | MWM test | [84] |
| Astrocytic A2A receptor knockdown | Improved spatial memory | MWM test | [88] |
| Astrocytic Gq-GPCR inhibition with iβARK | Impaired spatial working memory | Y-maze Novel object placement | [85] |
| Genetic deletion of IP3R2 | Impaired spatial memory | Y-maze | [86] |
| Genetic deletion of astrocytic GABAB receptor | Impaired working memory | T-maze Object in place preference test | [81] |
| Astrocytic A2A receptor deletion | Impaired spatial memory | Y-maze Baited eight-radial arm maze | [87] |
| Inhibition of glycogenolysis | Impaired working memory | Spontaneous alternation in elevated plus-maze | [93] |
4.2. Recognition Memory
| Proposed Astrocytic Mechanism | Outcome of the Astrocytic Manipulation | Test | Reference |
|---|---|---|---|
| Decreased mitochondrial ROS in astrocytes | Impaired recognition memory | NOR task | [94] |
| Astrocyte deletion of proBDNF uptake carrier receptor (p75NTR) | Impaired recognition memory | NOR task | [95] |
| Inhibition of glycogenolysis | Impaired recognition memory | NOR task | [96] |
4.3. Contextual Memory
| Proposed Astrocytic Mechanism | Outcome of the Astrocytic Manipulation | Test | Reference |
|---|---|---|---|
| Activation of astrocytic Gq-GPCR signalling with hM3DGq or opto-α-1AR in CA1 | Improved fear memory | Fear conditioning | [83] |
| Gi-coupled µ-opioid receptor activation in astrocytes | Improved contextual memory | CPP test | [102] |
| Astrocytic α7-nAChRs deletion | Impaired fear memory | Fear conditioning | [97] |
| Elimination of astrocytic m1-AChRs | Impaired fear memory | Fear conditioning | [76] |
| Optogenetic activation of astrocytes | Impaired fear memory | Fear conditioning | [98] |
| Astrocytic Gq-GPCR signalling activation with hM3DGq in the amygdala | Impaired fear memory | Fear conditioning | [99] |
| Activation of astrocytic Gi-GPCR signalling with hM4DGi | Impaired fear memory | Fear conditioning | [103] |
| Astrocytic genetic deletion of IP3R2 | Impaired fear memory (and spatial memory) | Fear conditioning (and MWM test) | [104] |
| Astrocytic genetic deletion of IP3R2 | Impaired fear memory | Fear conditioning | [86] |
| Decrease in the generation of L-lactate | Impaired contextual memory | Inhibitory avoidance task | [61] |
| MTC4 or MCT1 deletion in the hippocampus | Impaired contextual memory | Inhibitory avoidance task | [105] |
| Astrocytic glucocorticoid receptor deletion | Impaired fear memory | Fear conditioning | [107] |
5. Astrocytic Calcium Signalling as a Key Element in AD Pathology
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Moscovitch, M.; Cabeza, R.; Winocur, G.; Nadel, L. Episodic Memory and Beyond: The Hippocampus and Neocortex in Transformation. Annu. Rev. Psychol. 2016, 67, 105–134. [Google Scholar] [CrossRef]
- Fields, R.D.; Araque, A.; Johansen-Berg, H.; Lim, S.S.; Lynch, G.; Nave, K.A.; Nedergaard, M.; Perez, R.; Sejnowski, T.; Wake, H. Glial Biology in Learning and Cognition. Neurosci. Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2014, 20, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Oberheim, N.A.; Takano, T.; Han, X.; He, W.; Lin, J.H.C.; Wang, F.; Xu, Q.; Wyatt, J.D.; Pilcher, W.; Ojemann, J.G.; et al. Uniquely Hominid Features of Adult Human Astrocytes. J. Neurosci. 2009, 29, 3276–3287. [Google Scholar] [CrossRef]
- Bushong, E.A.; Martone, M.E.; Jones, Y.Z.; Ellisman, M.H. Protoplasmic Astrocytes in CA1 Stratum Radiatum Occupy Separate Anatomical Domains. J. Neurosci. Off. J. Soc. Neurosci. 2002, 22, 183–192. [Google Scholar] [CrossRef]
- Genoud, C.; Quairiaux, C.; Steiner, P.; Hirling, H.; Welker, E.; Knott, G.W. Plasticity of Astrocytic Coverage and Glutamate Transporter Expression in Adult Mouse Cortex. PLoS Biol. 2006, 4, e343. [Google Scholar] [CrossRef]
- Houades, V.; Koulakoff, A.; Ezan, P.; Seif, I.; Giaume, C. Gap Junction-Mediated Astrocytic Networks in the Mouse Barrel Cortex. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 5207–5217. [Google Scholar] [CrossRef]
- Wilhelmsson, U.; Bushong, E.A.; Price, D.L.; Smarr, B.L.; Phung, V.; Terada, M.; Ellisman, M.H.; Pekny, M. Redefining the Concept of Reactive Astrocytes as Cells That Remain within Their Unique Domains upon Reaction to Injury. Proc. Natl. Acad. Sci. USA 2006, 103, 17513–17518. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and Pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef] [PubMed]
- Allaman, I.; Bélanger, M.; Magistretti, P.J. Astrocyte-Neuron Metabolic Relationships: For Better and for Worse. Trends Neurosci. 2011, 34, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Yang, T.; Cui, S.; Chen, G. Connexin Hemichannels in Astrocytes: Role in CNS Disorders. Front. Mol. Neurosci. 2019, 12, 23. [Google Scholar] [CrossRef]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef]
- Noble, M.; Murray, K. Purified Astrocytes Promote the in Vitro Division of a Bipotential Glial Progenitor Cell. EMBO J. 1984, 3, 2243–2247. [Google Scholar] [CrossRef]
- Richardson, W.D.; Pringle, N.; Mosley, M.J.; Westermark, B.; Dubois-Dalcq, M. A Role for Platelet-Derived Growth Factor in Normal Gliogenesis in the Central Nervous System. Cell 1988, 53, 309–319. [Google Scholar] [CrossRef]
- Ishibashi, T.; Dakin, K.A.; Stevens, B.; Lee, P.R.; Kozlov, S.V.; Stewart, C.L.; Fields, R.D. Astrocytes Promote Myelination in Response to Electrical Impulses. Neuron 2006, 49, 823–832. [Google Scholar] [CrossRef]
- Tognatta, R.; Karl, M.T.; Fyffe-Maricich, S.L.; Popratiloff, A.; Garrison, E.D.; Schenck, J.K.; Abu-Rub, M.; Miller, R.H. As-trocytes Are Required for Oligodendrocyte Survival and Maintenance of Myelin Compaction and Integrity. Front. Cell. Neurosci. 2020, 14, 74. [Google Scholar] [CrossRef] [PubMed]
- Cabezas, R.; Avila, M.; Gonzalez, J.; El-Bachá, R.S.; Báez, E.; García-Segura, L.M.; Jurado Coronel, J.C.; Capani, F.; Cardo-na-Gomez, G.P.; Barreto, G.E. Astrocytic Modulation of Blood Brain Barrier: Perspectives on Parkinson’s Disease. Front. Cell. Neurosci. 2014, 8, 211. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.I.; Katayama, T.; Prat, A. Glial Influence on the Blood Brain Barrier. Glia 2013, 61, 1939–1958. [Google Scholar] [CrossRef] [PubMed]
- Quintela-López, T.; Shiina, H.; Attwell, D. Neuronal Energy Use and Brain Evolution. Curr. Biol. CB 2022, 32, R650–R655. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Mendez, A.; Almeida, A.; Fernández, E.; Maestre, C.; Moncada, S.; Bolaños, J.P. The Bioenergetic and Antioxidant Status of Neurons Is Controlled by Continuous Degradation of a Key Glycolytic Enzyme by APC/C-Cdh1. Nat. Cell Biol. 2009, 11, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Magistretti, P.J.; Chatton, J.-Y. Relationship between L-Glutamate-Regulated Intracellular Na+ Dynamics and ATP Hydrolysis in Astrocytes. J. Neural Transm. Vienna Austria 1996 2005, 112, 77–85. [Google Scholar] [CrossRef]
- Porras, O.H.; Ruminot, I.; Loaiza, A.; Barros, L.F. Na(+)-Ca(2+) Cosignaling in the Stimulation of the Glucose Transporter GLUT1 in Cultured Astrocytes. Glia 2008, 56, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Pellerin, L.; Magistretti, P.J. Sweet Sixteen for ANLS. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2012, 32, 1152–1166. [Google Scholar] [CrossRef]
- Andersen, J.V.; Markussen, K.H.; Jakobsen, E.; Schousboe, A.; Waagepetersen, H.S.; Rosenberg, P.A.; Aldana, B.I. Glutamate Metabolism and Recycling at the Excitatory Synapse in Health and Neurodegeneration. Neuropharmacology 2021, 196, 108719. [Google Scholar] [CrossRef]
- Beard, E.; Lengacher, S.; Dias, S.; Magistretti, P.J.; Finsterwald, C. Astrocytes as Key Regulators of Brain Energy Metabolism: New Therapeutic Perspectives. Front. Physiol. 2021, 12, 825816. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.F.; San Martín, A.; Ruminot, I.; Sandoval, P.Y.; Fernández-Moncada, I.; Baeza-Lehnert, F.; Arce-Molina, R.; Con-treras-Baeza, Y.; Cortés-Molina, F.; Galaz, A.; et al. Near-Critical GLUT1 and Neurodegeneration. J. Neurosci. Res. 2017, 95, 2267–2274. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, G.; Wu, Z.; Farrell, R.J.; Ryan, T.A. GLUT4 Mobilization Supports Energetic Demands of Active Synapses. Neuron 2017, 93, 606–615.e3. [Google Scholar] [CrossRef]
- Baldwin, K.T.; Eroglu, C. Molecular Mechanisms of Astrocyte-Induced Synaptogenesis. Curr. Opin. Neurobiol. 2017, 45, 113–120. [Google Scholar] [CrossRef]
- Pfrieger, F.W.; Barres, B.A. Synaptic Efficacy Enhanced by Glial Cells in Vitro. Science 1997, 277, 1684–1687. [Google Scholar] [CrossRef]
- Ullian, E.M.; Sapperstein, S.K.; Christopherson, K.S.; Barres, B.A. Control of Synapse Number by Glia. Science 2001, 291, 657–661. [Google Scholar] [CrossRef]
- Madison, B.B. Srebp2: A Master Regulator of Sterol and Fatty Acid Synthesis. J. Lipid Res. 2016, 57, 333–335. [Google Scholar] [CrossRef]
- Shan, L.; Zhang, T.; Fan, K.; Cai, W.; Liu, H. Astrocyte-Neuron Signaling in Synaptogenesis. Front. Cell Dev. Biol. 2021, 9, 680301. [Google Scholar] [CrossRef]
- Sakers, K.; Lake, A.M.; Khazanchi, R.; Ouwenga, R.; Vasek, M.J.; Dani, A.; Dougherty, J.D. Astrocytes Locally Translate Transcripts in Their Peripheral Processes. Proc. Natl. Acad. Sci. USA 2017, 114, E3830–E3838. [Google Scholar] [CrossRef]
- Haydon, P.G. GLIA: Listening and Talking to the Synapse. Nat. Rev. Neurosci. 2001, 2, 185–193. [Google Scholar] [CrossRef]
- Volterra, A.; Meldolesi, J. Astrocytes, from Brain Glue to Communication Elements: The Revolution Continues. Nat. Rev. Neurosci. 2005, 6, 626–640. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, C.; Barres, B.A. Regulation of Synaptic Connectivity by Glia. Nature 2010, 468, 223–231. [Google Scholar] [CrossRef]
- Araque, A.; Carmignoto, G.; Haydon, P.G.; Oliet, S.H.R.; Robitaille, R.; Volterra, A. Gliotransmitters Travel in Time and Space. Neuron 2014, 81, 728–739. [Google Scholar] [CrossRef] [PubMed]
- Dudai, Y.; Karni, A.; Born, J. The Consolidation and Transformation of Memory. Neuron 2015, 88, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Frankland, P.W.; Bontempi, B. The Organization of Recent and Remote Memories. Nat. Rev. Neurosci. 2005, 6, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Vesce, S.; Bezzi, P.; Volterra, A. Synaptic Transmission with the Glia. News Physiol. Sci. Int. J. Physiol. Prod. Jointly Int. Union Physiol. Sci. Am. Physiol. Soc. 2001, 16, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Perea, G.; Navarrete, M.; Araque, A. Tripartite Synapses: Astrocytes Process and Control Synaptic Information. Trends Neurosci. 2009, 32, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Falcón-Moya, R.; Pérez-Rodríguez, M.; Prius-Mengual, J.; Andrade-Talavera, Y.; Arroyo-García, L.E.; Pérez-Artés, R.; Ma-teos-Aparicio, P.; Guerra-Gomes, S.; Oliveira, J.F.; Flores, G.; et al. Astrocyte-Mediated Switch in Spike Timing-Dependent Plasticity during Hippocampal Development. Nat. Commun. 2020, 11, 4388. [Google Scholar] [CrossRef] [PubMed]
- Kofuji, P.; Araque, A. Astrocytes and Behavior. Annu. Rev. Neurosci. 2021, 44, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Mauch, D.H.; Nägler, K.; Schumacher, S.; Göritz, C.; Müller, E.C.; Otto, A.; Pfrieger, F.W. CNS Synaptogenesis Promoted by Glia-Derived Cholesterol. Science 2001, 294, 1354–1357. [Google Scholar] [CrossRef] [PubMed]
- Kucukdereli, H.; Allen, N.J.; Lee, A.T.; Feng, A.; Ozlu, M.I.; Conatser, L.M.; Chakraborty, C.; Workman, G.; Weaver, M.; Sage, E.H.; et al. Control of Excitatory CNS Synaptogenesis by Astrocyte-Secreted Proteins Hevin and SPARC. Proc. Natl. Acad. Sci. USA 2011, 108, E440–E449. [Google Scholar] [CrossRef]
- Carmona, M.A.; Murai, K.K.; Wang, L.; Roberts, A.J.; Pasquale, E.B. Glial Ephrin-A3 Regulates Hippocampal Dendritic Spine Morphology and Glutamate Transport. Proc. Natl. Acad. Sci. USA 2009, 106, 12524–12529. [Google Scholar] [CrossRef]
- Chung, W.-S.; Clarke, L.E.; Wang, G.X.; Stafford, B.K.; Sher, A.; Chakraborty, C.; Joung, J.; Foo, L.C.; Thompson, A.; Chen, C.; et al. Astrocytes Mediate Synapse Elimination through MEGF10 and MERTK Pathways. Nature 2013, 504, 394–400. [Google Scholar] [CrossRef]
- Fu, M.; Zuo, Y. Experience-Dependent Structural Plasticity in the Cortex. Trends Neurosci. 2011, 34, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Yu, X.; Perlik, A.J.; Tobin, W.F.; Zweig, J.A.; Tennant, K.; Jones, T.; Zuo, Y. Rapid Formation and Selective Stabilization of Synapses for Enduring Motor Memories. Nature 2009, 462, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Pan, F.; Gan, W.-B. Stably Maintained Dendritic Spines Are Associated with Lifelong Memories. Nature 2009, 462, 920–924. [Google Scholar] [CrossRef]
- Hirrlinger, J.; Hülsmann, S.; Kirchhoff, F. Astroglial Processes Show Spontaneous Motility at Active Synaptic Terminals in Situ. Eur. J. Neurosci. 2004, 20, 2235–2239. [Google Scholar] [CrossRef]
- Danbolt, N.C. Glutamate Uptake. Prog. Neurobiol. 2001, 65, 1–105. [Google Scholar] [CrossRef] [PubMed]
- Araque, A.; Parpura, V.; Sanzgiri, R.P.; Haydon, P.G. Tripartite Synapses: Glia, the Unacknowledged Partner. Trends Neurosci. 1999, 22, 208–215. [Google Scholar] [CrossRef]
- Di Castro, M.A.; Chuquet, J.; Liaudet, N.; Bhaukaurally, K.; Santello, M.; Bouvier, D.; Tiret, P.; Volterra, A. Local Ca2+ Detection and Modulation of Synaptic Release by Astrocytes. Nat. Neurosci. 2011, 14, 1276–1284. [Google Scholar] [CrossRef] [PubMed]
- Panatier, A.; Theodosis, D.T.; Mothet, J.-P.; Touquet, B.; Pollegioni, L.; Poulain, D.A.; Oliet, S.H.R. Glia-Derived D-Serine Controls NMDA Receptor Activity and Synaptic Memory. Cell 2006, 125, 775–784. [Google Scholar] [CrossRef]
- Perea, G.; Araque, A. Astrocytes Potentiate Transmitter Release at Single Hippocampal Synapses. Science 2007, 317, 1083–1086. [Google Scholar] [CrossRef] [PubMed]
- Porter, J.T.; McCarthy, K.D. Hippocampal Astrocytes in Situ Respond to Glutamate Released from Synaptic Terminals. J. Neurosci. Off. J. Soc. Neurosci. 1996, 16, 5073–5081. [Google Scholar] [CrossRef] [PubMed]
- Schummers, J.; Yu, H.; Sur, M. Tuned Responses of Astrocytes and Their Influence on Hemodynamic Signals in the Visual Cortex. Science 2008, 320, 1638–1643. [Google Scholar] [CrossRef] [PubMed]
- Jourdain, P.; Bergersen, L.H.; Bhaukaurally, K.; Bezzi, P.; Santello, M.; Domercq, M.; Matute, C.; Tonello, F.; Gundersen, V.; Volterra, A. Glutamate Exocytosis from Astrocytes Controls Synaptic Strength. Nat. Neurosci. 2007, 10, 331–339. [Google Scholar] [CrossRef]
- Liu, Q.-S.; Xu, Q.; Kang, J.; Nedergaard, M. Astrocyte Activation of Presynaptic Metabotropic Glutamate Receptors Modu-lates Hippocampal Inhibitory Synaptic Transmission. Neuron Glia Biol. 2004, 1, 307–316. [Google Scholar] [CrossRef]
- Sibille, J.; Pannasch, U.; Rouach, N. Astroglial Potassium Clearance Contributes to Short-Term Plasticity of Synaptically Evoked Currents at the Tripartite Synapse. J. Physiol. 2014, 592, 87–102. [Google Scholar] [CrossRef]
- Suzuki, A.; Stern, S.A.; Bozdagi, O.; Huntley, G.W.; Walker, R.H.; Magistretti, P.J.; Alberini, C.M. Astrocyte-Neuron Lactate Transport Is Required for Long-Term Memory Formation. Cell 2011, 144, 810–823. [Google Scholar] [CrossRef] [PubMed]
- Nishida, H.; Okabe, S. Direct Astrocytic Contacts Regulate Local Maturation of Dendritic Spines. J. Neurosci. Off. J. Soc. Neurosci. 2007, 27, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Bernardinelli, Y.; Muller, D.; Nikonenko, I. Astrocyte-Synapse Structural Plasticity. Neural Plast. 2014, 2014, 232105. [Google Scholar] [CrossRef] [PubMed]
- Bernardinelli, Y.; Randall, J.; Janett, E.; Nikonenko, I.; König, S.; Jones, E.V.; Flores, C.E.; Murai, K.K.; Bochet, C.G.; Holtmaat, A.; et al. Activity-Dependent Structural Plasticity of Perisynaptic Astrocytic Domains Promotes Excitatory Synapse Stability. Curr. Biol. CB 2014, 24, 1679–1688. [Google Scholar] [CrossRef] [PubMed]
- Deemyad, T.; Lüthi, J.; Spruston, N. Astrocytes Integrate and Drive Action Potential Firing in Inhibitory Subnetworks. Nat. Commun. 2018, 9, 4336. [Google Scholar] [CrossRef] [PubMed]
- Araque, A.; Martín, E.D.; Perea, G.; Arellano, J.I.; Buño, W. Synaptically Released Acetylcholine Evokes Ca2+ Elevations in Astrocytes in Hippocampal Slices. J. Neurosci. Off. J. Soc. Neurosci. 2002, 22, 2443–2450. [Google Scholar] [CrossRef]
- Navarrete, M.; Perea, G.; Fernandez de Sevilla, D.; Gómez-Gonzalo, M.; Núñez, A.; Martín, E.D.; Araque, A. Astrocytes Mediate in Vivo Cholinergic-Induced Synaptic Plasticity. PLoS Biol. 2012, 10, e1001259. [Google Scholar] [CrossRef]
- Gould, E. How Widespread Is Adult Neurogenesis in Mammals? Nat. Rev. Neurosci. 2007, 8, 481–488. [Google Scholar] [CrossRef]
- Cameron, H.A.; Glover, L.R. Adult Neurogenesis: Beyond Learning and Memory. Annu. Rev. Psychol. 2015, 66, 53–81. [Google Scholar] [CrossRef]
- Cope, E.C.; Gould, E. Adult Neurogenesis, Glia, and the Extracellular Matrix. Cell Stem Cell 2019, 24, 690–705. [Google Scholar] [CrossRef]
- Mu, Y.; Gage, F.H. Adult Hippocampal Neurogenesis and Its Role in Alzheimer’s Disease. Mol. Neurodegener. 2011, 6, 85. [Google Scholar] [CrossRef]
- Araki, T.; Ikegaya, Y.; Koyama, R. The Effects of Microglia- and Astrocyte-Derived Factors on Neurogenesis in Health and Disease. Eur. J. Neurosci. 2021, 54, 5880–5901. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Li, L.-P.; Qin, X.-H.; Li, S.-J.; Zhang, M.; Wang, Q.; Hu, H.-H.; Fang, Y.-Y.; Gao, Y.-B.; Li, X.-W.; et al. Astrocytic Adenosine 5′-Triphosphate Release Regulates the Proliferation of Neural Stem Cells in the Adult Hippocampus. Stem Cells Dayt. Ohio 2013, 31, 1633–1643. [Google Scholar] [CrossRef] [PubMed]
- Platel, J.-C.; Dave, K.A.; Gordon, V.; Lacar, B.; Rubio, M.E.; Bordey, A. NMDA Receptors Activated by Subventricular Zone Astrocytic Glutamate Are Critical for Neuroblast Survival Prior to Entering a Synaptic Network. Neuron 2010, 65, 859–872. [Google Scholar] [CrossRef] [PubMed]
- Sultan, S.; Li, L.; Moss, J.; Petrelli, F.; Cassé, F.; Gebara, E.; Lopatar, J.; Pfrieger, F.W.; Bezzi, P.; Bischofberger, J.; et al. Synaptic Integration of Adult-Born Hippocampal Neurons Is Locally Controlled by Astrocytes. Neuron 2015, 88, 957–972. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-P.; Su, X.-H.; Hu, N.-Y.; Hu, J.; Li, X.-W.; Yang, J.-M.; Gao, T.-M. Astrocytes Mediate Cholinergic Regulation of Adult Hippocampal Neurogenesis and Memory Through M1 Muscarinic Receptor. Biol. Psychiatry 2022, 92, 984–998. [Google Scholar] [CrossRef] [PubMed]
- Hattiangady, B.; Shetty, A.K. Neural Stem Cell Grafting Counteracts Hippocampal Injury-Mediated Impairments in Mood, Memory, and Neurogenesis. Stem Cells Transl. Med. 2012, 1, 696–708. [Google Scholar] [CrossRef] [PubMed]
- Parihar, V.K.; Hattiangady, B.; Shuai, B.; Shetty, A.K. Mood and Memory Deficits in a Model of Gulf War Illness Are Linked with Reduced Neurogenesis, Partial Neuron Loss, and Mild Inflammation in the Hippocampus. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2013, 38, 2348–2362. [Google Scholar] [CrossRef] [PubMed]
- Xie, A.X.; Petravicz, J.; McCarthy, K.D. Molecular Approaches for Manipulating Astrocytic Signaling in Vivo. Front. Cell. Neurosci. 2015, 9, 144. [Google Scholar] [CrossRef]
- Yu, X.; Nagai, J.; Khakh, B.S. Improved Tools to Study Astrocytes. Nat. Rev. Neurosci. 2020, 21, 121–138. [Google Scholar] [CrossRef]
- Mederos, S.; Sánchez-Puelles, C.; Esparza, J.; Valero, M.; Ponomarenko, A.; Perea, G. GABAergic Signaling to Astrocytes in the Prefrontal Cortex Sustains Goal-Directed Behaviors. Nat. Neurosci. 2021, 24, 82–92. [Google Scholar] [CrossRef]
- Mederos, S.; Hernández-Vivanco, A.; Ramírez-Franco, J.; Martín-Fernández, M.; Navarrete, M.; Yang, A.; Boyden, E.S.; Perea, G. Melanopsin for Precise Optogenetic Activation of Astrocyte-Neuron Networks. Glia 2019, 67, 915–934. [Google Scholar] [CrossRef]
- Adamsky, A.; Kol, A.; Kreisel, T.; Doron, A.; Ozeri-Engelhard, N.; Melcer, T.; Refaeli, R.; Horn, H.; Regev, L.; Groysman, M.; et al. Astrocytic Activation Generates De Novo Neuronal Potentiation and Memory Enhancement. Cell 2018, 174, 59–71.e14. [Google Scholar] [CrossRef]
- Nishiyama, H.; Knopfel, T.; Endo, S.; Itohara, S. Glial Protein S100B Modulates Long-Term Neuronal Synaptic Plasticity. Proc. Natl. Acad. Sci. USA 2002, 99, 4037–4042. [Google Scholar] [CrossRef] [PubMed]
- Nagai, J.; Bellafard, A.; Qu, Z.; Yu, X.; Ollivier, M.; Gangwani, M.R.; Diaz-Castro, B.; Coppola, G.; Schumacher, S.M.; Gol-shani, P.; et al. Specific and Behaviorally Consequential Astrocyte Gq GPCR Signaling Attenuation in Vivo with iβARK. Neuron 2021, 109, 2256–2274.e9. [Google Scholar] [CrossRef]
- Pinto-Duarte, A.; Roberts, A.J.; Ouyang, K.; Sejnowski, T.J. Impairments in Remote Memory Caused by the Lack of Type 2 IP3 Receptors. Glia 2019, 67, 1976–1989. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.; Shen, H.-Y.; Augusto, E.; Wang, Y.; Wei, C.J.; Wang, Y.T.; Agostinho, P.; Boison, D.; Cunha, R.A.; Chen, J.-F. Deletion of Adenosine A2A Receptors from Astrocytes Disrupts Glutamate Homeostasis Leading to Psychomotor and Cog-nitive Impairment: Relevance to Schizophrenia. Biol. Psychiatry 2015, 78, 763–774. [Google Scholar] [CrossRef]
- Orr, A.G.; Hsiao, E.C.; Wang, M.M.; Ho, K.; Kim, D.H.; Wang, X.; Guo, W.; Kang, J.; Yu, G.-Q.; Adame, A.; et al. Astrocytic Adenosine Receptor A2A and Gs-Coupled Signaling Regulate Memory. Nat. Neurosci. 2015, 18, 423–434. [Google Scholar] [CrossRef]
- Shen, H.-Y.; Coelho, J.E.; Ohtsuka, N.; Canas, P.M.; Day, Y.-J.; Huang, Q.-Y.; Rebola, N.; Yu, L.; Boison, D.; Cunha, R.A.; et al. A Critical Role of the Adenosine A2A Receptor in Extrastriatal Neurons in Modulating Psychomotor Activity as Revealed by Opposite Phenotypes of Striatum and Forebrain A2A Receptor Knock-Outs. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 2970–2975. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Shen, H.-Y.; Coelho, J.E.; Araújo, I.M.; Huang, Q.-Y.; Day, Y.-J.; Rebola, N.; Canas, P.M.; Rapp, E.K.; Ferrara, J.; et al. Adenosine A2A Receptor Antagonists Exert Motor and Neuroprotective Effects by Distinct Cellular Mechanisms. Ann. Neurol. 2008, 63, 338–346. [Google Scholar] [CrossRef]
- Cunha, R.A. Adenosine as a Neuromodulator and as a Homeostatic Regulator in the Nervous System: Different Roles, Different Sources and Different Receptors. Neurochem. Int. 2001, 38, 107–125. [Google Scholar] [CrossRef] [PubMed]
- Pellerin, L.; Magistretti, P.J. Glutamate Uptake into Astrocytes Stimulates Aerobic Glycolysis: A Mechanism Coupling Neu-ronal Activity to Glucose Utilization. Proc. Natl. Acad. Sci. USA 1994, 91, 10625–10629. [Google Scholar] [CrossRef] [PubMed]
- Newman, L.A.; Korol, D.L.; Gold, P.E. Lactate Produced by Glycogenolysis in Astrocytes Regulates Memory Processing. PLoS ONE 2011, 6, e28427. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Gutierrez, C.; Bonora, N.; Bobo-Jimenez, V.; Jimenez-Blasco, D.; Lopez-Fabuel, I.; Fernandez, E.; Josephine, C.; Bonvento, G.; Enriquez, J.A.; Almeida, A.; et al. Astrocytic Mitochondrial ROS Modulate Brain Metabolism and Mouse Be-haviour. Nat. Metab. 2019, 1, 201–211. [Google Scholar] [CrossRef]
- Vignoli, B.; Battistini, G.; Melani, R.; Blum, R.; Santi, S.; Berardi, N.; Canossa, M. Peri-Synaptic Glia Recycles Brain-Derived Neurotrophic Factor for LTP Stabilization and Memory Retention. Neuron 2016, 92, 873–887. [Google Scholar] [CrossRef]
- Vezzoli, E.; Calì, C.; De Roo, M.; Ponzoni, L.; Sogne, E.; Gagnon, N.; Francolini, M.; Braida, D.; Sala, M.; Muller, D.; et al. Ultrastructural Evidence for a Role of Astrocytes and Glycogen-Derived Lactate in Learning-Dependent Synaptic Stabilization. Cereb. Cortex 2020, 30, 2114–2127. [Google Scholar] [CrossRef]
- Zhang, K.; Förster, R.; He, W.; Liao, X.; Li, J.; Yang, C.; Qin, H.; Wang, M.; Ding, R.; Li, R.; et al. Fear Learning Induces A7-Nicotinic Acetylcholine Receptor-Mediated Astrocytic Responsiveness That Is Required for Memory Persistence. Nat. Neurosci. 2021, 24, 1686–1698. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, L.; Wu, J.; Zhu, Z.; Feng, X.; Qin, L.; Zhu, Y.; Sun, L.; Liu, Y.; Qiu, Z.; et al. Activation of Astrocytes in Hippocampus Decreases Fear Memory through Adenosine A1 Receptors. eLife 2020, 9, e57155. [Google Scholar] [CrossRef]
- Martin-Fernandez, M.; Jamison, S.; Robin, L.M.; Zhao, Z.; Martin, E.D.; Aguilar, J.; Benneyworth, M.A.; Marsicano, G.; Araque, A. Synapse-Specific Astrocyte Gating of Amygdala-Related Behavior. Nat. Neurosci. 2017, 20, 1540–1548. [Google Scholar] [CrossRef]
- Durkee, C.A.; Covelo, A.; Lines, J.; Kofuji, P.; Aguilar, J.; Araque, A. Gi/o Protein-Coupled Receptors Inhibit Neurons but Activate Astrocytes and Stimulate Gliotransmission. Glia 2019, 67, 1076–1093. [Google Scholar] [CrossRef]
- Nagai, J.; Rajbhandari, A.K.; Gangwani, M.R.; Hachisuka, A.; Coppola, G.; Masmanidis, S.C.; Fanselow, M.S.; Khakh, B.S. Hyperactivity with Disrupted Attention by Activation of an Astrocyte Synaptogenic Cue. Cell 2019, 177, 1280–1292.e20. [Google Scholar] [CrossRef]
- Nam, M.-H.; Han, K.-S.; Lee, J.; Won, W.; Koh, W.; Bae, J.Y.; Woo, J.; Kim, J.; Kwong, E.; Choi, T.-Y.; et al. Activation of Astrocytic μ-Opioid Receptor Causes Conditioned Place Preference. Cell Rep. 2019, 28, 1154–1166.e5. [Google Scholar] [CrossRef]
- Kol, A.; Adamsky, A.; Groysman, M.; Kreisel, T.; London, M.; Goshen, I. Astrocytes Contribute to Remote Memory Formation by Modulating Hippocampal-Cortical Communication during Learning. Nat. Neurosci. 2020, 23, 1229–1239. [Google Scholar] [CrossRef]
- Liu, J.-H.; Zhang, M.; Wang, Q.; Wu, D.-Y.; Jie, W.; Hu, N.-Y.; Lan, J.-Z.; Zeng, K.; Li, S.-J.; Li, X.-W.; et al. Distinct Roles of Astroglia and Neurons in Synaptic Plasticity and Memory. Mol. Psychiatry 2022, 27, 873–885. [Google Scholar] [CrossRef]
- Descalzi, G.; Gao, V.; Steinman, M.Q.; Suzuki, A.; Alberini, C.M. Lactate from Astrocytes Fuels Learning-Induced mRNA Translation in Excitatory and Inhibitory Neurons. Commun. Biol. 2019, 2, 247. [Google Scholar] [CrossRef] [PubMed]
- Licznerski, P.; Duric, V.; Banasr, M.; Alavian, K.N.; Ota, K.T.; Kang, H.J.; Jonas, E.A.; Ursano, R.; Krystal, J.H.; Duman, R.S.; et al. Decreased SGK1 Expression and Function Contributes to Behavioral Deficits Induced by Traumatic Stress. PLoS Biol. 2015, 13, e1002282. [Google Scholar] [CrossRef] [PubMed]
- Tertil, M.; Skupio, U.; Barut, J.; Dubovyk, V.; Wawrzczak-Bargiela, A.; Soltys, Z.; Golda, S.; Kudla, L.; Wiktorowska, L.; Szklarczyk, K.; et al. Glucocorticoid Receptor Signaling in Astrocytes Is Required for Aversive Memory Formation. Transl. Psychiatry 2018, 8, 255. [Google Scholar] [CrossRef] [PubMed]
- Leng, K.; Li, E.; Eser, R.; Piergies, A.; Sit, R.; Tan, M.; Neff, N.; Li, S.H.; Rodriguez, R.D.; Suemoto, C.K.; et al. Molecular Characterization of Selectively Vulnerable Neurons in Alzheimer’s Disease. Nat. Neurosci. 2021, 24, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Habib, N.; McCabe, C.; Medina, S.; Varshavsky, M.; Kitsberg, D.; Dvir-Szternfeld, R.; Green, G.; Dionne, D.; Nguyen, L.; Marshall, J.L.; et al. Disease-Associated Astrocytes in Alzheimer’s Disease and Aging. Nat. Neurosci. 2020, 23, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Busche, M.A.; Kekuš, M.; Adelsberger, H.; Noda, T.; Förstl, H.; Nelken, I.; Konnerth, A. Rescue of Long-Range Circuit Dysfunction in Alzheimer’s Disease Models. Nat. Neurosci. 2015, 18, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A. Astroglial Calcium Signaling in Aging and Alzheimer’s Disease. Cold Spring Harb. Perspect. Biol. 2019, 11, a035188. [Google Scholar] [CrossRef] [PubMed]
- Busche, M.A.; Eichhoff, G.; Adelsberger, H.; Abramowski, D.; Wiederhold, K.-H.; Haass, C.; Staufenbiel, M.; Konnerth, A.; Garaschuk, O. Clusters of Hyperactive Neurons near Amyloid Plaques in a Mouse Model of Alzheimer’s Disease. Science 2008, 321, 1686–1689. [Google Scholar] [CrossRef] [PubMed]
- Busche, M.A.; Chen, X.; Henning, H.A.; Reichwald, J.; Staufenbiel, M.; Sakmann, B.; Konnerth, A. Critical Role of Soluble Amyloid-β for Early Hippocampal Hyperactivity in a Mouse Model of Alzheimer’s Disease. Proc. Natl. Acad. Sci. USA 2012, 109, 8740–8745. [Google Scholar] [CrossRef] [PubMed]
- Zott, B.; Simon, M.M.; Hong, W.; Unger, F.; Chen-Engerer, H.-J.; Frosch, M.P.; Sakmann, B.; Walsh, D.M.; Konnerth, A. A Vicious Cycle of β Amyloid-Dependent Neuronal Hyperactivation. Science 2019, 365, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Lerdkrai, C.; Asavapanumas, N.; Brawek, B.; Kovalchuk, Y.; Mojtahedi, N.; Olmedillas Del Moral, M.; Garaschuk, O. In-tracellular Ca2+ Stores Control in Vivo Neuronal Hyperactivity in a Mouse Model of Alzheimer’s Disease. Proc. Natl. Acad. Sci. USA 2018, 115, E1279–E1288. [Google Scholar] [CrossRef]
- Kuchibhotla, K.V.; Lattarulo, C.R.; Hyman, B.T.; Bacskai, B.J. Synchronous Hyperactivity and Intercellular Calcium Waves in Astrocytes in Alzheimer Mice. Science 2009, 323, 1211–1215. [Google Scholar] [CrossRef] [PubMed]
- Delekate, A.; Füchtemeier, M.; Schumacher, T.; Ulbrich, C.; Foddis, M.; Petzold, G.C. Metabotropic P2Y1 Receptor Signalling Mediates Astrocytic Hyperactivity in Vivo in an Alzheimer’s Disease Mouse Model. Nat. Commun. 2014, 5, 5422. [Google Scholar] [CrossRef] [PubMed]
- Reichenbach, N.; Delekate, A.; Breithausen, B.; Keppler, K.; Poll, S.; Schulte, T.; Peter, J.; Plescher, M.; Hansen, J.N.; Blank, N.; et al. P2Y1 Receptor Blockade Normalizes Network Dysfunction and Cognition in an Alzheimer’s Disease Model. J. Exp. Med. 2018, 215, 1649–1663. [Google Scholar] [CrossRef]
- Lines, J.; Baraibar, A.M.; Fang, C.; Martin, E.D.; Aguilar, J.; Lee, M.K.; Araque, A.; Kofuji, P. Astrocyte-Neuronal Network Interplay Is Disrupted in Alzheimer’s Disease Mice. Glia 2022, 70, 368–378. [Google Scholar] [CrossRef]
- Åbjørsbråten, K.S.; Skaaraas, G.H.E.S.; Cunen, C.; Bjørnstad, D.M.; Binder, K.M.G.; Bojarskaite, L.; Jensen, V.; Nilsson, L.N.G.; Rao, S.B.; Tang, W.; et al. Impaired Astrocytic Ca2+ Signaling in Awake-Behaving Alzheimer’s Disease Transgenic Mice. eLife 2022, 11, e75055. [Google Scholar] [CrossRef]
- Shah, D.; Gsell, W.; Wahis, J.; Luckett, E.S.; Jamoulle, T.; Vermaercke, B.; Preman, P.; Moechars, D.; Hendrickx, V.; Jaspers, T.; et al. Astrocyte Calcium Dysfunction Causes Early Network Hyperactivity in Alzheimer’s Disease. Cell Rep. 2022, 40, 111280. [Google Scholar] [CrossRef] [PubMed]
- Lia, A.; Sansevero, G.; Chiavegato, A.; Sbrissa, M.; Pendin, D.; Mariotti, L.; Pozzan, T.; Berardi, N.; Carmignoto, G.; Fasolato, C.; et al. Rescue of Astrocyte Activity by the Calcium Sensor STIM1 Restores Long-Term Synaptic Plasticity in Female Mice Modelling Alzheimer’s Disease. Nat. Commun. 2023, 14, 1590. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escalada, P.; Ezkurdia, A.; Ramírez, M.J.; Solas, M. Essential Role of Astrocytes in Learning and Memory. Int. J. Mol. Sci. 2024, 25, 1899. https://doi.org/10.3390/ijms25031899
Escalada P, Ezkurdia A, Ramírez MJ, Solas M. Essential Role of Astrocytes in Learning and Memory. International Journal of Molecular Sciences. 2024; 25(3):1899. https://doi.org/10.3390/ijms25031899
Chicago/Turabian StyleEscalada, Paula, Amaia Ezkurdia, María Javier Ramírez, and Maite Solas. 2024. "Essential Role of Astrocytes in Learning and Memory" International Journal of Molecular Sciences 25, no. 3: 1899. https://doi.org/10.3390/ijms25031899
APA StyleEscalada, P., Ezkurdia, A., Ramírez, M. J., & Solas, M. (2024). Essential Role of Astrocytes in Learning and Memory. International Journal of Molecular Sciences, 25(3), 1899. https://doi.org/10.3390/ijms25031899






