Mitochondrial Unfolded Protein Response Gene Clpp Is Required for Oocyte Function and Female Fertility
Abstract
1. Introduction
2. Results
2.1. Oocyte-Specific Targeted Deletion of Clpp Results in Female Subfertility
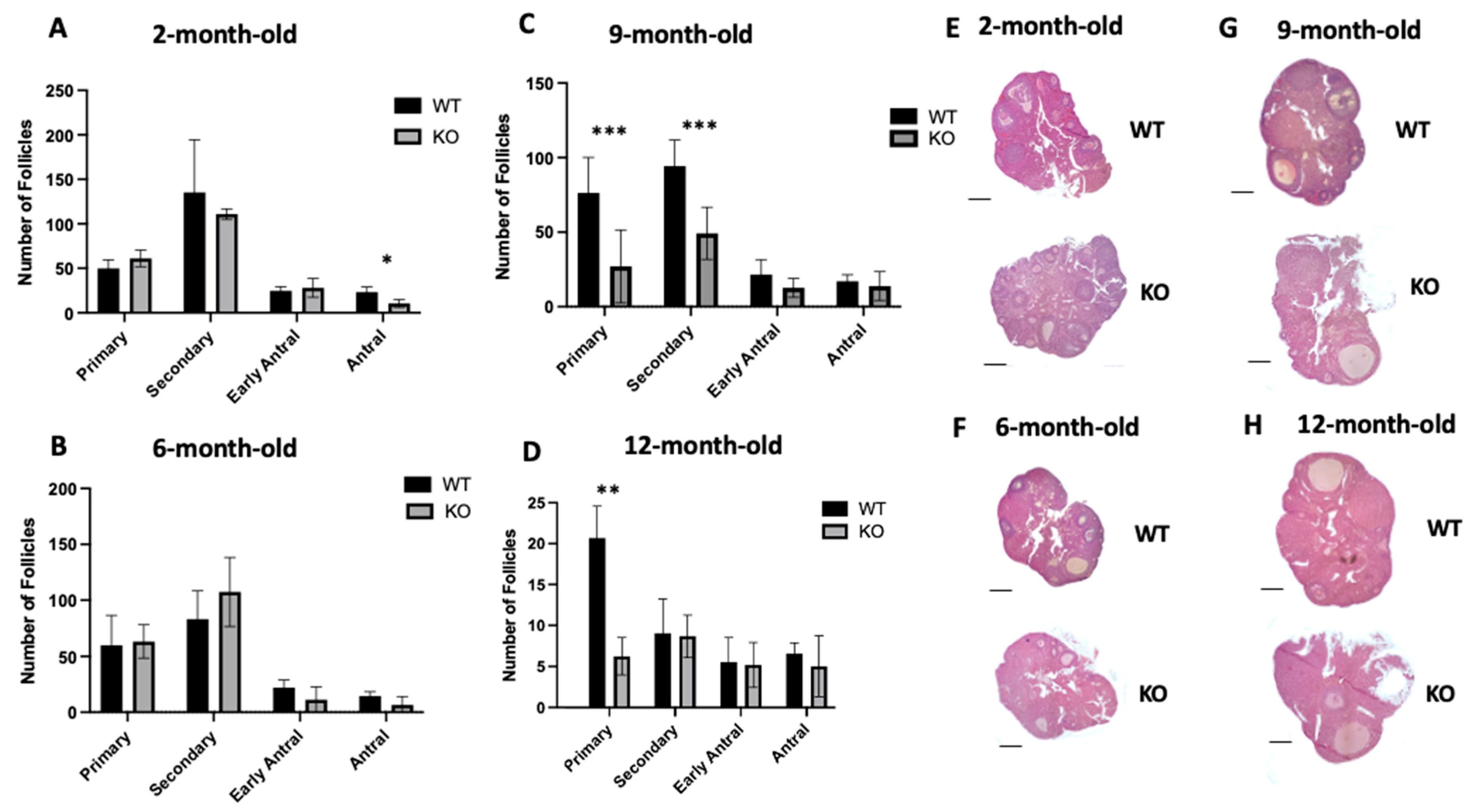
2.2. Lack of Clpp in Oocytes Alters Follicle Development
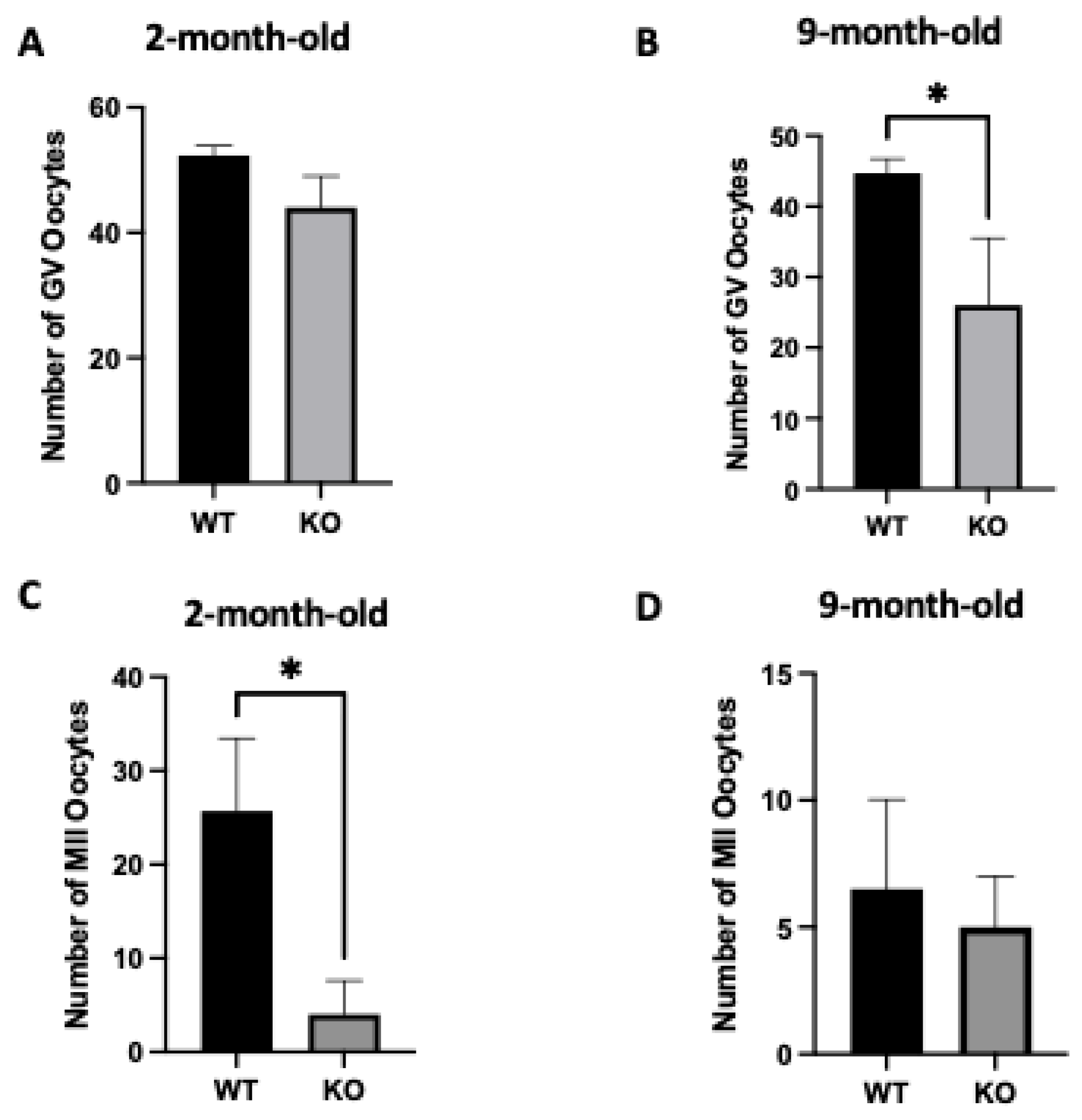
2.3. Oocyte-Specific Targeted Deletion of Clpp Results in Altered Oocyte Development
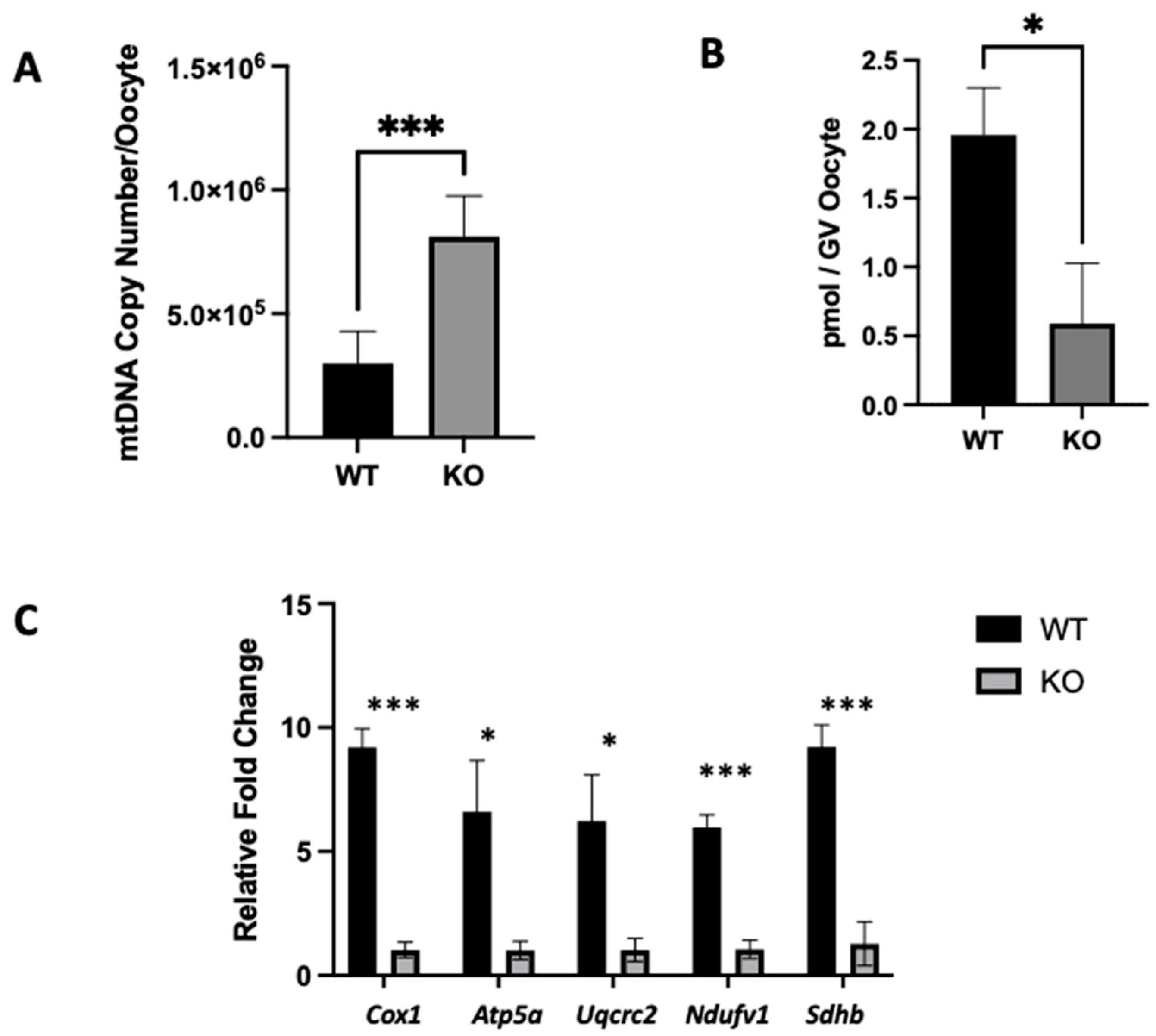
2.4. Clpp Depletion in Oocytes Results in Mitochondrial Dysfunction
3. Discussion
4. Material and Methods
4.1. Animals
4.2. Assessment of Fertility
4.3. Histomorphometric Analysis of Folliculogenesis in Ovaries
4.4. Oocyte Collection
4.5. Quantification of mtDNA Copy Number in Oocytes
4.6. Quantification of ATP
4.7. Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Blerkom, J. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion 2011, 11, 797–813. [Google Scholar] [CrossRef]
- Fragouli, E.; Wells, D. Mitochondrial DNA Assessment to Determine Oocyte and Embryo Viability. Semin. Reprod. Med. 2015, 33, 401–409. [Google Scholar] [CrossRef]
- BLledo, J.A.; Morales, E.; Garcia-Hernandez, J.; Ten, A.; Bernabeu, J.; Llacer, R. Bernabeu, Comprehensive mitochondrial DNA analysis and IVF outcome. Hum. Reprod. Open 2018, 2018, hoy023. [Google Scholar] [CrossRef]
- Reynier, P.; May-Panloup, P.; Chrétien, M.-F.; Morgan, C.J.; Jean, M.; Savagner, F.; Barrière, P.; Malthièry, Y. Mitochondrial DNA content affects the fertilizability of human oocytes. Mol. Hum. Reprod. 2001, 7, 425–429. [Google Scholar] [CrossRef]
- Van Blerkom, J.; Runner, M.N. Mitochondrial reorganization during resumption of arrested meiosis in the mouse oocyte. Am. J. Anat. 1984, 171, 335–355. [Google Scholar] [CrossRef]
- Nagai, S.; Mabuchi, T.; Hirata, S.; Shoda, T.; Kasai, T.; Yokota, S.; Shitara, H.; Yonekawa, H.; Hoshi, K. Correlation of Abnormal Mitochondrial Distribution in Mouse Oocytes with Reduced Developmental Competence. Tohoku J. Exp. Med. 2006, 210, 137–144. [Google Scholar] [CrossRef]
- Benedetti, C.; Haynes, C.M.; Yang, Y.; Harding, H.P.; Ron, D. Ubiquitin-like protein 5 positively regulates chaperone gene expression in the mitochondrial unfolded protein response. Genetics 2006, 174, 229–239. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, J.; Levichkin, I.V.; Stasinopoulos, S.; Ryan, M.T.; Hoogenraad, N.J. A mitochondrial specific stress response in mammalian cells. Embo J. 2002, 21, 4411–4419. [Google Scholar] [CrossRef]
- Nargund, A.M.; Fiorese, C.J.; Pellegrino, M.W.; Deng, P.; Haynes, C.M. Mitochondrial and Nuclear Accumulation of the Transcription Factor ATFS-1 Promotes OXPHOS Recovery during the UPRmt. Mol. Cell 2015, 58, 123–133. [Google Scholar] [CrossRef]
- Haynes, C.M.; Petrova, K.; Benedetti, C.; Yang, Y.; Ron, D. ClpP Mediates Activation of a Mitochondrial Unfolded Protein Response in C. elegans. Dev. Cell 2007, 13, 467–480. [Google Scholar] [CrossRef]
- Brodie, E.J.; Zhan, H.; Saiyed, T.; Truscott, K.N.; Dougan, D.A. Perrault syndrome type 3 caused by diverse molecular defects in CLPP. Sci. Rep. 2018, 8, 12862. [Google Scholar] [CrossRef]
- Key, J.; Gispert, S.; Koornneef, L.; Sleddens-Linkels, E.; Kohli, A.; Torres-Odio, S.; Koepf, G.; Amr, S.; Reichlmeir, M.; Harter, P.N.; et al. CLPP Depletion Causes Diplotene Arrest; Underlying Testis Mitochondrial Dysfunction Occurs with Accumulation of Perrault Proteins ERAL1, PEO1, and HARS2. Cells 2022, 12, 52. [Google Scholar] [CrossRef]
- Fiumara, A.; Sorge, G.; Toscano, A.; Parano, E.; Pavone, L.; Opitz, J.M. Perrault syndrome: Evidence for progressive nervous system involvement. Am. J. Med. Genet. A 2004, 128A, 246–249. [Google Scholar] [CrossRef]
- Wang, T.; Babayev, E.; Jiang, Z.; Li, G.; Zhang, M.; Esencan, E.; Horvath, T.; Seli, E. Mitochondrial unfolded protein response gene Clpp is required to maintain ovarian follicular reserve during aging, for oocyte competence, and development of pre-implantation embryos. Aging Cell 2018, 17, e12784. [Google Scholar] [CrossRef]
- Gispert, S.; Parganlija, D.; Klinkenberg, M.; Dröse, S.; Wittig, I.; Mittelbronn, M.; Grzmil, P.; Koob, S.; Hamann, A.; Walter, M.; et al. Loss of mitochondrial peptidase Clpp leads to infertility, hearing loss plus growth retardation via accumulation of CLPX, mtDNA and inflammatory factors. Hum. Mol. Genet. 2013, 22, 4871–4887. [Google Scholar] [CrossRef]
- Esencan, E.; Cozzolino, M.; Imamoglu, G.; Seli, E. Mitochondrial Stress Response Gene Clpp Is Not Required for Granulosa Cell Function. Antioxidants 2020, 10, 1. [Google Scholar] [CrossRef]
- Li, G.; Gu, J.; Zhou, X.; Wu, T.; Li, X.; Hua, R.; Hai, Z.; Xiao, Y.; Su, J.; Yeung, W.S.B.; et al. Mitochondrial stress response gene Clpp deficiency impairs oocyte competence and deteriorate cyclophosphamide-induced ovarian damage in young mice. Front. Endocrinol. 2023, 14, 1122012. [Google Scholar] [CrossRef]
- de Vries, W.N.; Binns, L.T.; Fancher, K.S.; Dean, J.; Moore, R.; Kemler, R.; Knowles, B.B. Expression of Cre recombinase in mouse oocytes: A means to study maternal effect genes. Genesis 2000, 26, 110–112. [Google Scholar] [CrossRef]
- Ren, Y.; Suzuki, H.; Jagarlamudi, K.; Golnoski, K.; McGuire, M.; Lopes, R.; Pachnis, V.; Rajkovic, A. Lhx8 regulates primordial follicle activation and postnatal folliculogenesis. BMC Biol. 2015, 13, 39. [Google Scholar] [CrossRef]
- Martinelli, P.; Rugarli, E.I. Emerging roles of mitochondrial proteases in neurodegeneration. Biochim. Biophys. Acta (BBA)-Bioenerg. 2010, 1797, 1–10. [Google Scholar] [CrossRef]
- Sheng, X.; Liu, C.; Yan, G.; Li, G.; Liu, J.; Yang, Y.; Li, S.; Li, Z.; Zhou, J.; Zhen, X.; et al. The mitochondrial protease LONP1 maintains oocyte development and survival by suppressing nuclear translocation of AIFM1 in mammals. eBioMedicine 2022, 75, 103790. [Google Scholar] [CrossRef]
- Lee, Y.G.; Kim, H.W.; Nam, Y.; Shin, K.J.; Lee, Y.J.; Park, D.H.; Rhee, H.W.; Seo, J.K.; Chae, Y.C. LONP1 and ClpP cooperatively regulate mitochondrial proteostasis for cancer cell survival. Oncogenesis 2021, 10, 18. [Google Scholar] [CrossRef]
- Rankin, T.; Soyal, S.; Dean, J. The mouse zona pellucida: Folliculogenesis, fertility and pre-implantation development. Mol. Cell. Endocrinol. 2000, 163, 21–25. [Google Scholar] [CrossRef]
- de Figueiredo, J.R.; de Lima, L.F.; Silva, J.R.V.; Santos, R.R. Control of growth and development of preantral follicle: Insights from in vitro culture. Anim. Reprod. 2018, 15, 648–659. [Google Scholar] [CrossRef]
- Kasapoglu, I.; Seli, E. Mitochondrial Dysfunction and Ovarian Aging. Endocrinology 2020, 161, bqaa001. [Google Scholar] [CrossRef]
- Zhang, M.; Bener, M.B.; Jiang, Z.; Wang, T.; Esencan, E.; Scott Iii, R.; Horvath, T.; Seli, E. Mitofusin 1 is required for female fertility and to maintain ovarian follicular reserve. Cell Death Dis. 2019, 10, 560. [Google Scholar] [CrossRef]
- Zhang, M.; Bener, M.B.; Jiang, Z.; Wang, T.; Esencan, E.; Scott, R.; Horvath, T.; Seli, E. Mitofusin 2 plays a role in oocyte and follicle development, and is required to maintain ovarian follicular reserve during reproductive aging. Aging 2019, 11, 3919–3938. [Google Scholar] [CrossRef]
- Ben-Meir, A.; Burstein, E.; Borrego-Alvarez, A.; Chong, J.; Wong, E.; Yavorska, T.; Naranian, T.; Chi, M.; Wang, Y.; Bentov, Y.; et al. Coenzyme Q10 restores oocyte mitochondrial function and fertility during reproductive aging. Aging Cell 2015, 14, 887–895. [Google Scholar] [CrossRef]
- Chiang, J.L.; Shukla, P.; Pagidas, K.; Ahmed, N.S.; Karri, S.; Gunn, D.D.; Hurd, W.W.; Singh, K.K. Mitochondria in Ovarian Aging and Reproductive Longevity. Ageing Res. Rev. 2020, 63, 101168. [Google Scholar] [CrossRef]
- Benkhalifa, M.; Ferreira, Y.J.; Chahine, H.; Louanjli, N.; Miron, P.; Merviel, P.; Copin, H. Mitochondria: Participation to infertility as source of energy and cause of senescence. Int. J. Biochem. Cell Biol. 2014, 55, 60–64. [Google Scholar] [CrossRef]
- Pang, W.; Zhang, Y.; Zhao, N.; Darwiche, S.S.; Fu, X.; Xiang, W. Low expression of Mfn2 is associated with mitochondrial damage and apoptosis in the placental villi of early unexplained miscarriage. Placenta 2013, 34, 613–618. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Y. Adenosine triphosphate content in human unfertilized oocytes, undivided zygotes and embryos unsuitable for transfer or cryopreservation. J. Int. Med. Res. 2012, 40, 734–739. [Google Scholar] [CrossRef]
- Purandhar, K.; Jena, P.K.; Prajapati, B.; Rajput, P.; Seshadri, S. Understanding the role of heat shock protein isoforms in male fertility, aging and apoptosis. World J. Men’s Health 2014, 32, 123–132. [Google Scholar] [CrossRef]
- Jee, B.; Dhar, R.; Singh, S.; Karmakar, S. Heat Shock Proteins and Their Role in Pregnancy: Redefining the Function of “Old Rum in a New Bottle”. Front. Cell Dev. Biol. 2021, 9, 648463. [Google Scholar] [CrossRef]
- Esfandiari, N.; Falcone, T.; Agarwal, A.; Goldberg, J.M.; Nelson, D.R.; Sharma, R.K. Are heat shock proteins acting as modulators of pre-implantation mouse embryo development and apoptosis? Fertil. Steril. 2002, 78, S108. [Google Scholar] [CrossRef]
- Salmeri, N.; Viganò, P.; Cavoretto, P.; Marci, R.; Candiani, M. The kisspeptin system in and beyond reproduction: Exploring intricate pathways and potential links between endometriosis and polycystic ovary syndrome. Rev. Endocr. Metab. Disord. 2023; ahead of print. [Google Scholar] [CrossRef]
- Cejudo Roman, A.; Pinto, F.M.; Dorta, I.; Almeida, T.A.; Hernández, M.; Illanes, M.; Tena-Sempere, M.; Candenas, L. Analysis of the expression of neurokinin B, kisspeptin, and their cognate receptors NK3R and KISS1R in the human female genital tract. Fertil. Steril. 2012, 97, 1213–1219. [Google Scholar] [CrossRef]
- León, S.; Fernandois, D.; Sull, A.; Sull, J.; Calder, M.; Hayashi, K.; Bhattacharya, M.; Power, S.; Vilos, G.A.; Vilos, A.G.; et al. Beyond the brain-Peripheral kisspeptin signaling is essential for promoting endometrial gland development and function. Sci. Rep. 2016, 6, 29073. [Google Scholar] [CrossRef]
- Mattam, U.; Talari, N.K.; Paripati, A.K.; Krishnamoorthy, T.; Sepuri, N.B.V. Kisspeptin preserves mitochondrial function by inducing mitophagy and autophagy in aging rat brain hippocampus and human neuronal cell line. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118852. [Google Scholar] [CrossRef]
- Hu, K.L.; Chang, H.M.; Zhao, H.C.; Yu, Y.; Li, R.; Qiao, J. Potential roles for the kisspeptin/kisspeptin receptor system in implantation and placentation. Hum. Reprod. Update 2019, 25, 326–343. [Google Scholar] [CrossRef]
- Evans, M.C.; Lord, R.A.; Anderson, G.M. Multiple Leptin Signalling Pathways in the Control of Metabolism and Fertility: A Means to Different Ends? Int. J. Mol. Sci. 2021, 22, 9210. [Google Scholar] [CrossRef]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef]
- Mounzih, K.; Lu, R.; Chehab, F.F. Leptin treatment rescues the sterility of genetically obese ob/ob males. Endocrinology 1997, 138, 1190–1193. [Google Scholar] [CrossRef]
- Kleinridders, A.; Lauritzen, H.P.; Ussar, S.; Christensen, J.H.; Mori, M.A.; Bross, P.; Kahn, C.R. Leptin regulation of Hsp60 impacts hypothalamic insulin signaling. J. Clin. Investig. 2013, 123, 4667–4680. [Google Scholar] [CrossRef] [PubMed]
- Hauffe, R.; Rath, M.; Schell, M.; Ritter, K.; Kappert, K.; Deubel, S.; Ott, C.; Jähnert, M.; Jonas, W.; Schürmann, A.; et al. HSP60 reduction protects against diet-induced obesity by modulating energy metabolism in adipose tissue. Mol. Metab. 2021, 53, 101276. [Google Scholar] [CrossRef] [PubMed]
- Seli, E.; Lalioti, M.D.; Flaherty, S.M.; Sakkas, D.; Terzi, N.; Steitz, J.A. An embryonic poly(A)-binding protein (ePAB) is expressed in mouse oocytes and early preimplantation embryos. Proc. Natl. Acad. Sci. USA 2005, 102, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Pascale May-Panloup, Lisa Boucret, Juan-Manuel Chao de la Barca, Valérie Desquiret-Dumas, Véronique Ferré-L’Hotellier, Catherine Morinière, Philippe Descamps, Vincent Procaccio, Pascal Reynier, Ovarian ageing: The role of mitochondria in oocytes and follicles. Hum. Reprod. Update 2016, 22, 725–743. [CrossRef]
- Cozzolino, M.; Ergun, Y.; Seli, E. Targeted Deletion of Mitofusin 1 and Mitofusin 2 Causes Female Infertility and Loss of Follicular Reserve. Reprod. Sci. 2022, 30, 560–568. [Google Scholar] [CrossRef]

| Assessment of Fertility in Oocyte Specific Clpp−/− Mice | |||||||
|---|---|---|---|---|---|---|---|
| Genotype | n | Litters | Pups | Pups per Litter | Litter per Female | Pups per Female | |
| 2–month-old | WT | 5 | 16 | 72 | 4.5 ± 0.11 | 3.2 ± 0.16 | 14.4 ± 3.76 |
| KO | 5 | 10 | 36 | 3.6 ± 0.79 | 2 ± 0.54 | 7.2 ± 1.68 * | |
| 6–month–old | WT | 4 | 12 | 69 | 5.75 ± 1.18 | 3 ± 0 | 17.25 ± 3.49 |
| KO | 4 | 5 | 24 | 4.8 ± 0.47 | 1.25 ± 1.19 * | 6 ± 2.44 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ergun, Y.; Imamoglu, A.G.; Cozzolino, M.; Demirkiran, C.; Basar, M.; Garg, A.; Yildirim, R.M.; Seli, E. Mitochondrial Unfolded Protein Response Gene Clpp Is Required for Oocyte Function and Female Fertility. Int. J. Mol. Sci. 2024, 25, 1866. https://doi.org/10.3390/ijms25031866
Ergun Y, Imamoglu AG, Cozzolino M, Demirkiran C, Basar M, Garg A, Yildirim RM, Seli E. Mitochondrial Unfolded Protein Response Gene Clpp Is Required for Oocyte Function and Female Fertility. International Journal of Molecular Sciences. 2024; 25(3):1866. https://doi.org/10.3390/ijms25031866
Chicago/Turabian StyleErgun, Yagmur, Aysegul Gizem Imamoglu, Mauro Cozzolino, Cem Demirkiran, Murat Basar, Akanksha Garg, Raziye Melike Yildirim, and Emre Seli. 2024. "Mitochondrial Unfolded Protein Response Gene Clpp Is Required for Oocyte Function and Female Fertility" International Journal of Molecular Sciences 25, no. 3: 1866. https://doi.org/10.3390/ijms25031866
APA StyleErgun, Y., Imamoglu, A. G., Cozzolino, M., Demirkiran, C., Basar, M., Garg, A., Yildirim, R. M., & Seli, E. (2024). Mitochondrial Unfolded Protein Response Gene Clpp Is Required for Oocyte Function and Female Fertility. International Journal of Molecular Sciences, 25(3), 1866. https://doi.org/10.3390/ijms25031866






