The Impacts of Periconceptional Alcohol on Neonatal Ovaries and Subsequent Adult Fertility in the Rat
Abstract
1. Introduction
2. Results
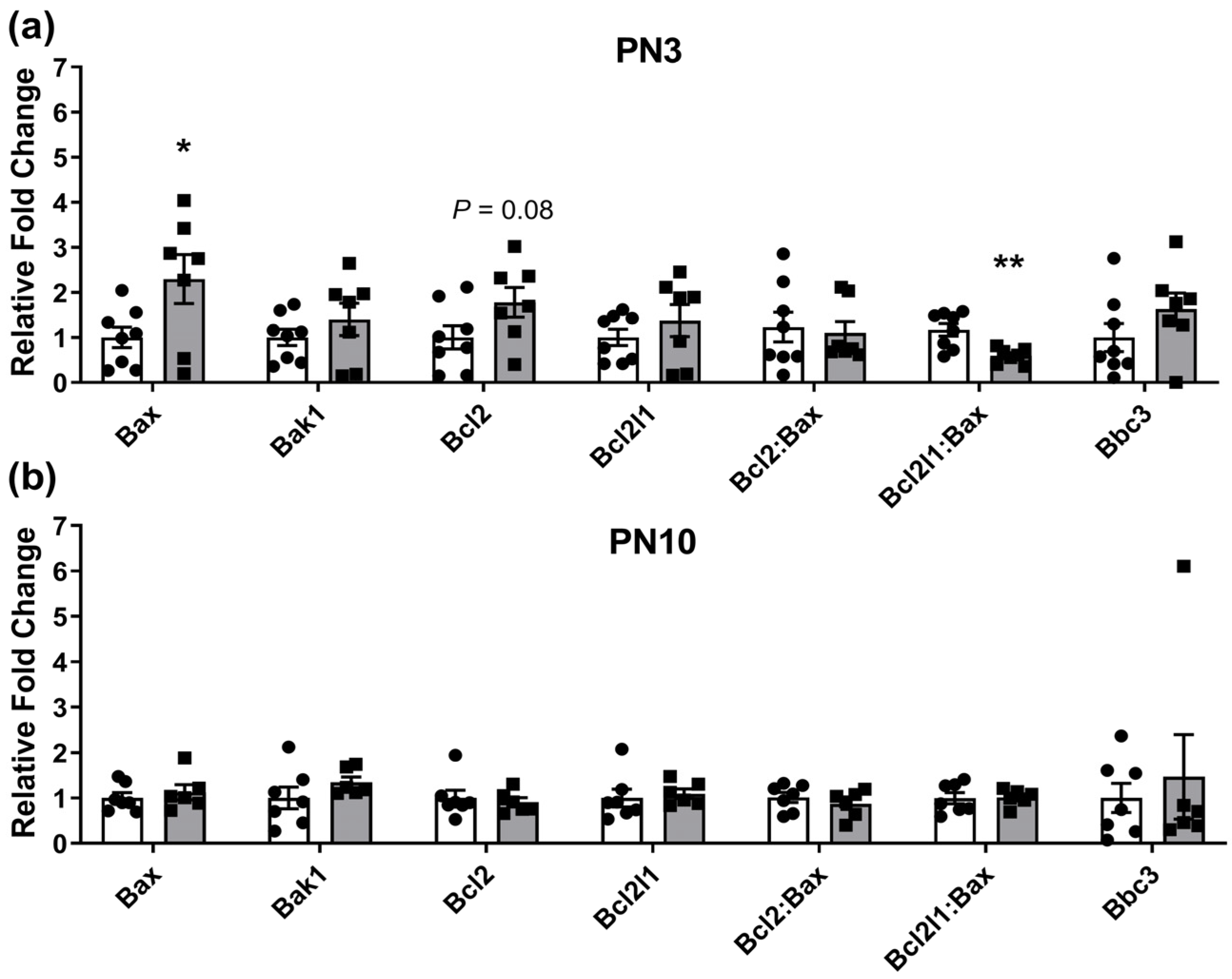
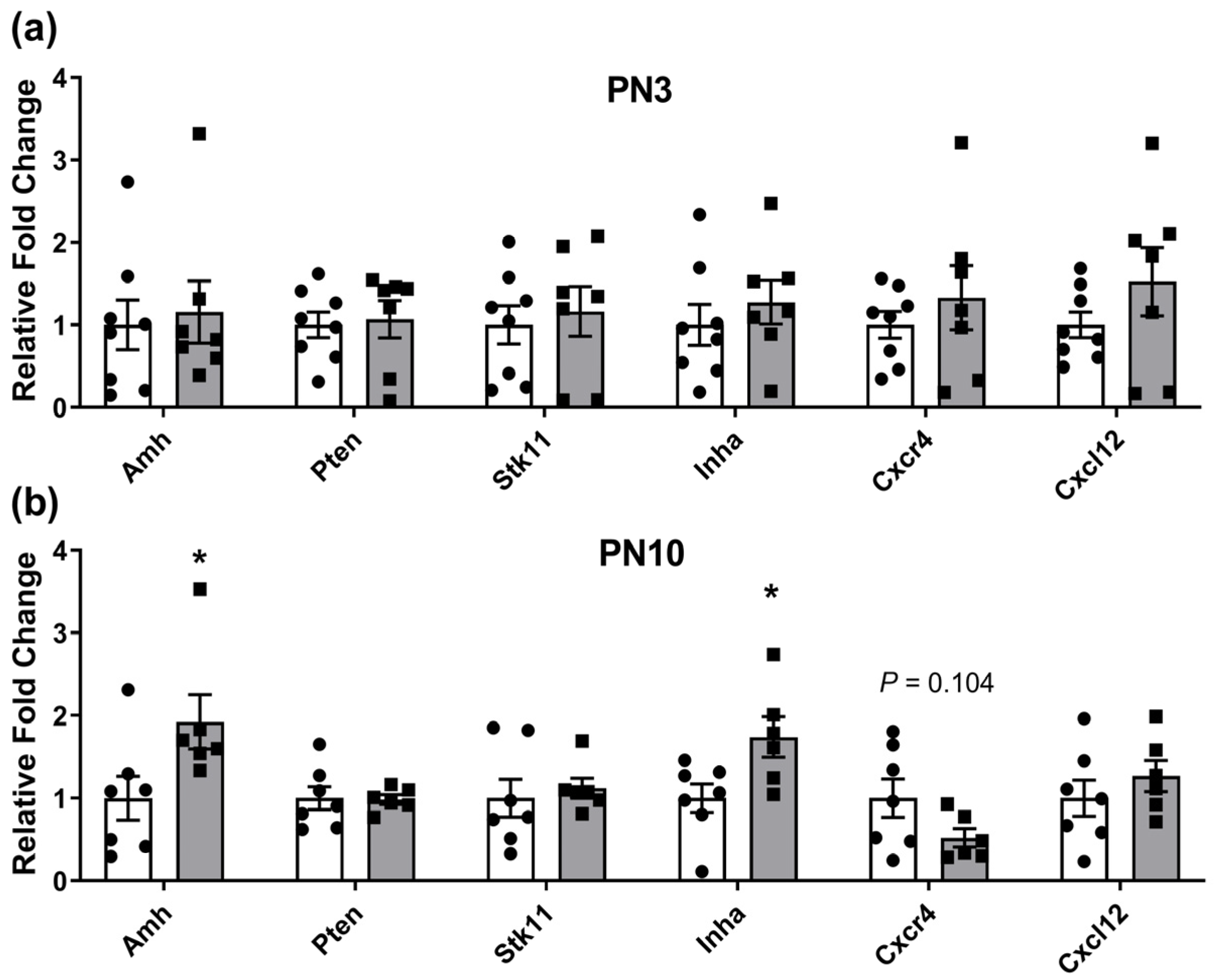
2.1. Ovarian Gene Expression Changes in Neonates Exposed to Periconceptional Alcohol
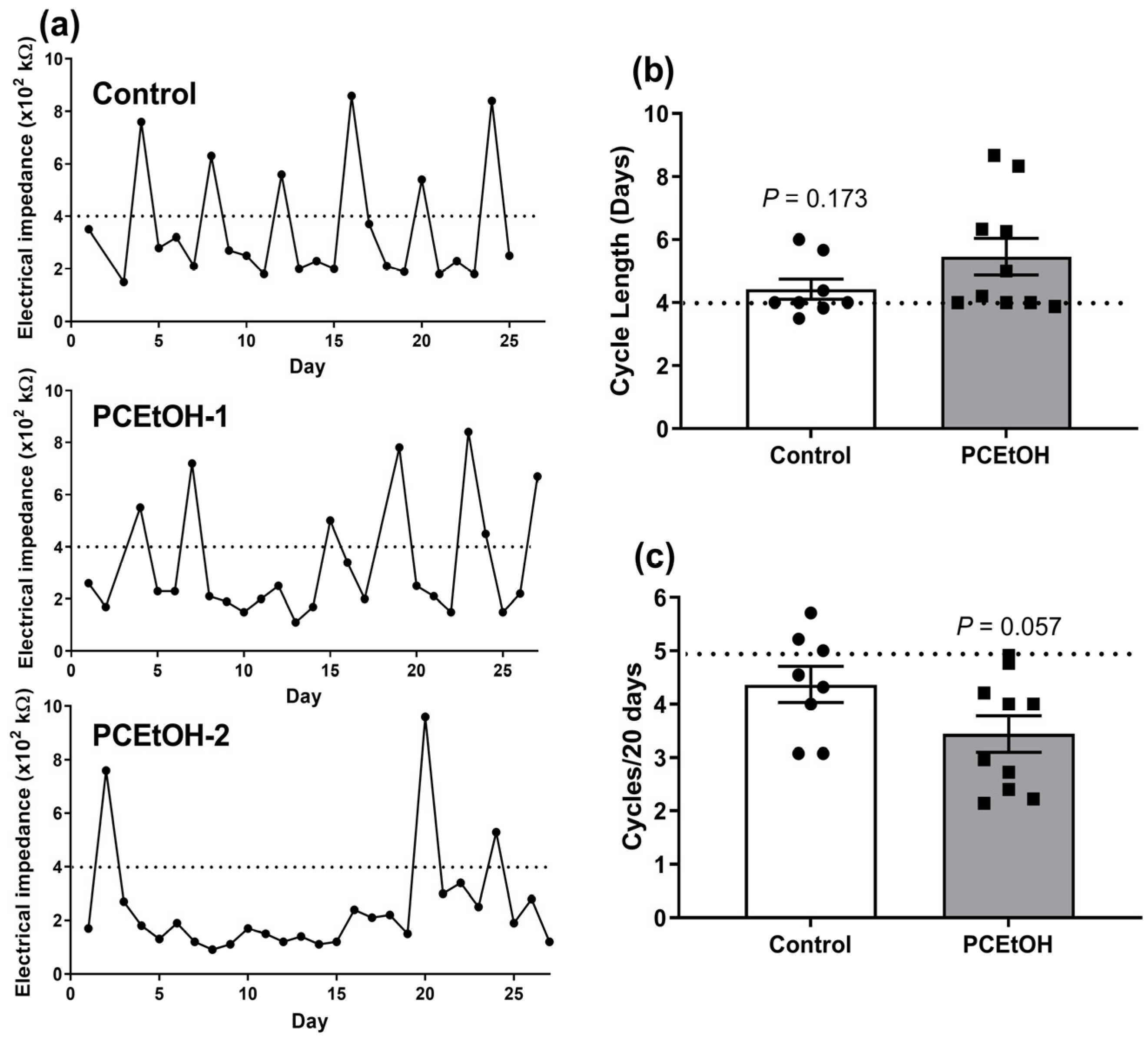
2.2. Estrous Cyclicity and Breeding Performance in Adult Offspring
3. Discussion
4. Materials and Methods
4.1. Animal Model and Tissue Collection
4.2. Analysis of Gene Expression for Regulators of Ovarian Reserve in F1 Neonatal Ovaries
4.3. Measurement of Estrous Cyclicity and Fertility in F1 Females
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alviggi, C.; Humaidan, P.; Howles, C.M.; Tredway, D.; Hillier, S.G. Biological versus chronological ovarian age: Implications for assisted reproductive technology. Reprod. Biol. Endocrinol. 2009, 7, 101. [Google Scholar] [CrossRef] [PubMed]
- Richardson, M.C.; Guo, M.; Fauser, B.C.; Macklon, N.S. Environmental and developmental origins of ovarian reserve. Hum. Reprod. Update 2014, 20, 353–369. [Google Scholar] [CrossRef]
- Findlay, J.K.; Hutt, K.J.; Hickey, M.; Anderson, R.A. What is the “ovarian reserve”? Fertil. Steril. 2015, 103, 628–630. [Google Scholar] [CrossRef]
- Childs, A.J.; Kinnell, H.L.; Collins, C.S.; Hogg, K.; Bayne, R.A.; Green, S.J.; McNeilly, A.S.; Anderson, R.A. BMP signaling in the human fetal ovary is developmentally regulated and promotes primordial germ cell apoptosis. Stem Cells 2010, 28, 1368–1378. [Google Scholar] [CrossRef]
- Picut, C.A.; Dixon, D.; Simons, M.L.; Stump, D.G.; Parker, G.A.; Remick, A.K. Postnatal ovary development in the rat: Morphologic study and correlation of morphology to neuroendocrine parameters. Toxicol. Pathol. 2015, 43, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.R.; Knowlton, N.S.; Thyer, A.C.; Charleston, J.S.; Soules, M.R.; Klein, N.A. A new model of reproductive aging: The decline in ovarian non-growing follicle number from birth to menopause. Hum. Reprod. 2008, 23, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.A.; Beckett, E.L.; Roman, S.D.; McLaughlin, E.A.; Sutherland, J.M. Advances in human primordial follicle activation and premature ovarian insufficiency. Reproduction 2020, 159, R15–R29. [Google Scholar] [CrossRef] [PubMed]
- Wallace, W.H.; Kelsey, T.W. Human ovarian reserve from conception to the menopause. PLoS ONE 2010, 5, e8772. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, Y.; Ma, J.; Ma, H.; Liang, X. Potential factors result in diminished ovarian reserve: A comprehensive review. J. Ovarian Res. 2023, 16, 208. [Google Scholar] [CrossRef]
- Monniaux, D. Factors influencing establishment of the ovarian reserve and their effects on fertility. Anim. Reprod. 2018, 15, 635–647. [Google Scholar] [CrossRef]
- McCormack, C.; Hutchinson, D.; Burns, L.; Wilson, J.; Elliott, E.; Allsop, S.; Najman, J.; Jacobs, S.; Rossen, L.; Olsson, C.; et al. Prenatal Alcohol Consumption Between Conception and Recognition of Pregnancy. Alcohol. Clin. Exp. Res. 2017, 41, 369–378. [Google Scholar] [CrossRef]
- Muggli, E.; O’Leary, C.; Donath, S.; Orsini, F.; Forster, D.; Anderson, P.J.; Lewis, S.; Nagle, C.; Craig, J.M.; Elliott, E.; et al. “Did you ever drink more?” A detailed description of pregnant women’s drinking patterns. BMC Public Health 2016, 16, 683. [Google Scholar] [CrossRef]
- Ishitsuka, K.; Hanada-Yamamoto, K.; Mezawa, H.; Saito-Abe, M.; Konishi, M.; Ohya, Y.; Japan, E.; Children’s Study, G. Determinants of Alcohol Consumption in Women Before and After Awareness of Conception. Matern. Child Health J. 2020, 24, 165–176. [Google Scholar] [CrossRef]
- Gardebjer, E.M.; Cuffe, J.S.M.; Ward, L.C.; Steane, S.; Anderson, S.T.; Dorey, E.S.; Kalisch-Smith, J.I.; Pantaleon, M.; Chong, S.; Yamada, L.; et al. Effects of periconceptional maternal alcohol intake and a postnatal high-fat diet on obesity and liver disease in male and female rat offspring. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E694–E704. [Google Scholar] [CrossRef] [PubMed]
- Kalisch-Smith, J.I.; Steane, S.E.; Simmons, D.G.; Pantaleon, M.; Anderson, S.T.; Akison, L.K.; Wlodek, M.E.; Moritz, K.M. Periconceptional alcohol exposure causes female-specific perturbations to trophoblast differentiation and placental formation in the rat. Development 2019, 146, dev.172205. [Google Scholar] [CrossRef] [PubMed]
- Steane, S.E.; Fielding, A.M.; Kent, N.L.; Andersen, I.; Browne, D.J.; Tejo, E.N.; Gardebjer, E.M.; Kalisch-Smith, J.I.; Sullivan, M.A.; Moritz, K.M.; et al. Maternal choline supplementation in a rat model of periconceptional alcohol exposure: Impacts on the fetus and placenta. Alcohol. Clin. Exp. Res. 2021, 45, 2130–2146. [Google Scholar] [CrossRef] [PubMed]
- Gardebjer, E.M.; Anderson, S.T.; Pantaleon, M.; Wlodek, M.E.; Moritz, K.M. Maternal alcohol intake around the time of conception causes glucose intolerance and insulin insensitivity in rat offspring, which is exacerbated by a postnatal high-fat diet. FASEB J. 2015, 29, 2690–2701. [Google Scholar] [CrossRef]
- Dorey, E.S.; Cullen, C.L.; Lucia, D.; Mah, K.M.; Manchadi, M.R.; Muhlhausler, B.S.; Moritz, K.M. The impact of periconceptional alcohol exposure on fat preference and gene expression in the mesolimbic reward pathway in adult rat offspring. J. Dev. Orig. Health Dis. 2018, 9, 223–231. [Google Scholar] [CrossRef]
- Dorey, E.S.; Walton, S.L.; Kalisch-Smith, J.I.; Paravicini, T.M.; Gardebjer, E.M.; Weir, K.A.; Singh, R.R.; Bielefeldt-Ohmann, H.; Anderson, S.T.; Wlodek, M.E.; et al. Periconceptional ethanol exposure induces a sex specific diuresis and increase in AQP2 and AVPR2 in the kidneys of aged rat offspring. Physiol. Rep. 2019, 7, e14273. [Google Scholar] [CrossRef]
- Chang, C.W.; Sung, Y.W.; Hsueh, Y.W.; Chen, Y.Y.; Ho, M.; Hsu, H.C.; Yang, T.C.; Lin, W.C.; Chang, H.M. Growth hormone in fertility and infertility: Mechanisms of action and clinical applications. Front. Endocrinol. 2022, 13, 1040503. [Google Scholar] [CrossRef]
- Kaur, S.; Kurokawa, M. Regulation of Oocyte Apoptosis: A View from Gene Knockout Mice. Int. J. Mol. Sci. 2023, 24, 1345. [Google Scholar] [CrossRef]
- Frost, E.R.; Ford, E.A.; Peters, A.E.; Lovell-Badge, R.; Taylor, G.; McLaughlin, E.A.; Sutherland, J.M. A New Understanding, Guided by Single-Cell Sequencing, of the Establishment and Maintenance of the Ovarian Reserve in Mammals. Sex. Dev. 2023, 17, 145–155. [Google Scholar] [CrossRef]
- Kristensen, S.G.; Andersen, K.; Clement, C.A.; Franks, S.; Hardy, K.; Andersen, C.Y. Expression of TGF-beta superfamily growth factors, their receptors, the associated SMADs and antagonists in five isolated size-matched populations of pre-antral follicles from normal human ovaries. Mol. Hum. Reprod. 2014, 20, 293–308. [Google Scholar] [CrossRef] [PubMed]
- McReight, E.K.; Liew, S.H.; Steane, S.E.; Hutt, K.J.; Moritz, K.M.; Akison, L.K. Moderate episodic prenatal alcohol does not impact female offspring fertility in rats. Reproduction 2020, 159, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Acuna, E.; Fornes, R.; Fernandois, D.; Garrido, M.P.; Greiner, M.; Lara, H.E.; Paredes, A.H. Increases in norepinephrine release and ovarian cyst formation during ageing in the rat. Reprod. Biol. Endocrinol. 2009, 7, 64. [Google Scholar] [CrossRef] [PubMed]
- Akison, L.K.; Moritz, K.M.; Reid, N. Adverse reproductive outcomes associated with fetal alcohol exposure: A systematic review. Reproduction 2019, 157, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Himelreich Peric, M.; Takahashi, M.; Jezek, D.; Cunha, G.R. Early development of the human embryonic testis. Differentiation 2023, 129, 4–16. [Google Scholar] [CrossRef] [PubMed]
- McGivern, R.F.; Raum, W.J.; Handa, R.J.; Sokol, R.Z. Comparison of two weeks versus one week of prenatal ethanol exposure in the rat on gonadal organ weights, sperm count, and onset of puberty. Neurotoxicol. Teratol. 1992, 14, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Udani, M.; Parker, S.; Gavaler, J.; Van Thiel, D.H. Effects of in utero exposure to alcohol upon male rats. Alcohol. Clin. Exp. Res. 1985, 9, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Lan, N.; Vogl, A.W.; Weinberg, J. Prenatal ethanol exposure delays the onset of spermatogenesis in the rat. Alcohol. Clin. Exp. Res. 2013, 37, 1074–1081. [Google Scholar] [CrossRef]
- Boggan, W.O.; Randall, C.L.; Dodds, H.M. Delayed sexual maturation in female C57BL/6J mice prenatally exposed to alcohol. Res. Commun. Chem. Pathol. Pharmacol. 1979, 23, 117–125. [Google Scholar]
- Gardebjer, E.M.; Cuffe, J.S.; Pantaleon, M.; Wlodek, M.E.; Moritz, K.M. Periconceptional alcohol consumption causes fetal growth restriction and increases glycogen accumulation in the late gestation rat placenta. Placenta 2014, 35, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Touquet, R.; Csipke, E.; Holloway, P.; Brown, A.; Patel, T.; Seddon, A.J.; Gulati, P.; Moore, H.; Batrick, N.; Crawford, M.J. Resuscitation room blood alcohol concentrations: One-year cohort study. Emerg. Med. J. 2008, 25, 752–756. [Google Scholar] [CrossRef]
- Gray, S.P.; Denton, K.M.; Cullen-McEwen, L.; Bertram, J.F.; Moritz, K.M. Prenatal exposure to alcohol reduces nephron number and raises blood pressure in progeny. J. Am. Soc. Nephrol. 2010, 21, 1891–1902. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Nyomba, B.L. Effects of prenatal alcohol exposure on glucose tolerance in the rat offspring. Metabolism 2003, 52, 454–462. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.; Cuthill, I.C.; Emerson, M.; Altman, D.G.; NC3Rs Reporting Guidelines Working Group. Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Br. J. Pharmacol. 2010, 160, 1577–1579. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]
- Greenfeld, C.R.; Pepling, M.E.; Babus, J.K.; Furth, P.A.; Flaws, J.A. BAX regulates follicular endowment in mice. Reproduction 2007, 133, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Chen, Q.; Song, Y.; Xiang, Y.; Li, Q.; Sang, Y.; Zhang, L.; Bai, L.; Zhu, Y. Downregulation of homeobox A1 in human granulosa cells is involved in diminished ovarian reserve through promoting cell apoptosis and mitochondrial dysfunction. Mol. Cell. Endocrinol. 2024, 580, 112084. [Google Scholar] [CrossRef]
- Hutt, K.J. The role of BH3-only proteins in apoptosis within the ovary. Reproduction 2015, 149, R81–R89. [Google Scholar] [CrossRef]
- Liew, S.H.; Vaithiyanathan, K.; Hutt, K.J. Taking control of the female fertile lifespan: A key role for Bcl-2 family proteins. Reprod. Fertil. Dev. 2016, 28, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Flaws, J.A.; Hirshfield, A.N.; Hewitt, J.A.; Babus, J.K.; Furth, P.A. Effect of bcl-2 on the primordial follicle endowment in the mouse ovary. Biol. Reprod. 2001, 64, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- Ratts, V.S.; Flaws, J.A.; Kolp, R.; Sorenson, C.M.; Tilly, J.L. Ablation of bcl-2 gene expression decreases the numbers of oocytes and primordial follicles established in the post-natal female mouse gonad. Endocrinology 1995, 136, 3665–3668. [Google Scholar] [CrossRef] [PubMed]
- Rucker, E.B., 3rd; Dierisseau, P.; Wagner, K.U.; Garrett, L.; Wynshaw-Boris, A.; Flaws, J.A.; Hennighausen, L. Bcl-x and Bax regulate mouse primordial germ cell survival and apoptosis during embryogenesis. Mol. Endocrinol. 2000, 14, 1038–1052. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, E.E.; Stanfield, J.; Skinner, M.K. Interactions between progesterone and tumor necrosis factor-alpha in the regulation of primordial follicle assembly. Reproduction 2006, 132, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Myers, M.; Morgan, F.H.; Liew, S.H.; Zerafa, N.; Gamage, T.U.; Sarraj, M.; Cook, M.; Kapic, I.; Sutherland, A.; Scott, C.L.; et al. PUMA regulates germ cell loss and primordial follicle endowment in mice. Reproduction 2014, 148, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.N.; Zerafa, N.; Liew, S.H.; Morgan, F.H.; Strasser, A.; Scott, C.L.; Findlay, J.K.; Hickey, M.; Hutt, K.J. Loss of PUMA protects the ovarian reserve during DNA-damaging chemotherapy and preserves fertility. Cell Death Dis. 2018, 9, 618. [Google Scholar] [CrossRef] [PubMed]
- Pangas, S.A. Regulation of the ovarian reserve by members of the transforming growth factor beta family. Mol. Reprod. Dev. 2012, 79, 666–679. [Google Scholar] [CrossRef]
- Durlinger, A.L.; Visser, J.A.; Themmen, A.P. Regulation of ovarian function: The role of anti-Mullerian hormone. Reproduction 2002, 124, 601–609. [Google Scholar] [CrossRef]
- Durlinger, A.L.; Kramer, P.; Karels, B.; de Jong, F.H.; Uilenbroek, J.T.; Grootegoed, J.A.; Themmen, A.P. Control of primordial follicle recruitment by anti-Mullerian hormone in the mouse ovary. Endocrinology 1999, 140, 5789–5796. [Google Scholar] [CrossRef]
- Reddy, P.; Liu, L.; Adhikari, D.; Jagarlamudi, K.; Rajareddy, S.; Shen, Y.; Du, C.; Tang, W.; Hamalainen, T.; Peng, S.L.; et al. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science 2008, 319, 611–613. [Google Scholar] [CrossRef]
- Jagarlamudi, K.; Liu, L.; Adhikari, D.; Reddy, P.; Idahl, A.; Ottander, U.; Lundin, E.; Liu, K. Oocyte-specific deletion of Pten in mice reveals a stage-specific function of PTEN/PI3K signaling in oocytes in controlling follicular activation. PLoS ONE 2009, 4, e6186. [Google Scholar] [CrossRef]
- Jiang, Z.Z.; Hu, M.W.; Ma, X.S.; Schatten, H.; Fan, H.Y.; Wang, Z.B.; Sun, Q.Y. LKB1 acts as a critical gatekeeper of ovarian primordial follicle pool. Oncotarget 2016, 7, 5738–5753. [Google Scholar] [CrossRef] [PubMed]
- Myers, M.; Middlebrook, B.S.; Matzuk, M.M.; Pangas, S.A. Loss of inhibin alpha uncouples oocyte-granulosa cell dynamics and disrupts postnatal folliculogenesis. Dev. Biol. 2009, 334, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Chand, A.L.; Harrison, C.A.; Shelling, A.N. Inhibin and premature ovarian failure. Hum. Reprod. Update 2010, 16, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, L.M.; Balcazar, I.B.; Duran, C. Using vaginal wall impedance to determine estrous cycle phase in Lewis rats. Lab Anim. 2012, 41, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Korsmeyer, S.J.; Shutter, J.R.; Veis, D.J.; Merry, D.E.; Oltvai, Z.N. Bcl-2/Bax: A rheostat that regulates an anti-oxidant pathway and cell death. Semin. Cancer Biol. 1993, 4, 327–332. [Google Scholar]


| Parameter—Mean (SD) | Control (n = 7) 1 | PCEtOH (n = 7) 1 | p Value 2 |
|---|---|---|---|
| F1 age at 1st mating (weeks) | 14.71 (1.9) | 14.63 (2.1) | 0.937 |
| Pups per litter (Litter 1) 3 | 16 (2.4) | 16 (2.2) | 0.648 |
| Pups per litter (Litter 2) 3,4 | 16 (3.1) | 14 (2.4) | 0.364 |
| Survival of F2 pups to PN10 (%) 5 | 96.79 (3.2) | 89.86 (27.3) | 0.837 |
| F2 Pup weight at PN3 (g)—females 6 | 8.19 (1.1) | 8.34 (0.9) | 0.794 |
| F2 Pup weight at PN3 (g)—males 6 | 8.83 (1.4) | 8.85 (1.1) | 0.973 |
| F2 Pup weight at weaning (g)—females 7 | 44.36 (3.0) | 40.06 (2.9) | 0.085 |
| F2 Pup weight at weaning (g)—males 7 | 47.22 (2.3) | 44.27 (2.6) | 0.140 |
| Gene Name | Gene Symbol | Function Related to Ovarian Reserve | Reference(s) |
|---|---|---|---|
| Apoptotic pathway | |||
| BCL2 associated X, apoptosis regulator | Bax | Pro-apoptotic factor | [38,39] |
| BCL2 antagonist/killer 1 | Bak1 | Pro-apoptotic factor | [40,41] |
| BCL2 apoptosis regulator | Bcl2 | Pro-survival factor | [39,42,43] |
| BCL2 like 1 | Bcl2l1 (Bclx) | Pro-survival factor | [44,45] |
| BCL2 binding component3 | Bbc3 (Puma) | Pro-apoptotic via activation of Bax/Bak1 or inhibition of pro-survival BCL2 family members | [46,47] |
| Follicle growth and recruitment | |||
| Anti-mullerian hormone | Amh | Maintenance of ovarian reserve by inhibiting recruitment to the growing follicle pool | [48,49,50] |
| Phosphatase and tensin homolog | Pten | Maintenance of quiescent state of primordial follicles via the PI3K/AKT pathway | [51,52] |
| Serine/threonine kinase 11 | Stk11 (Lkb1) | Maintenance of quiescent state of primordial follicles via mTOR | [53] |
| Inhibin subunit alpha | Inha | Indirectly impacts on follicle growth and recruitment via negative feedback of FSH | [54,55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steane, S.E.; Burgess, D.J.; Moritz, K.M.; Akison, L.K. The Impacts of Periconceptional Alcohol on Neonatal Ovaries and Subsequent Adult Fertility in the Rat. Int. J. Mol. Sci. 2024, 25, 2471. https://doi.org/10.3390/ijms25052471
Steane SE, Burgess DJ, Moritz KM, Akison LK. The Impacts of Periconceptional Alcohol on Neonatal Ovaries and Subsequent Adult Fertility in the Rat. International Journal of Molecular Sciences. 2024; 25(5):2471. https://doi.org/10.3390/ijms25052471
Chicago/Turabian StyleSteane, Sarah E., Danielle J. Burgess, Karen M. Moritz, and Lisa K. Akison. 2024. "The Impacts of Periconceptional Alcohol on Neonatal Ovaries and Subsequent Adult Fertility in the Rat" International Journal of Molecular Sciences 25, no. 5: 2471. https://doi.org/10.3390/ijms25052471
APA StyleSteane, S. E., Burgess, D. J., Moritz, K. M., & Akison, L. K. (2024). The Impacts of Periconceptional Alcohol on Neonatal Ovaries and Subsequent Adult Fertility in the Rat. International Journal of Molecular Sciences, 25(5), 2471. https://doi.org/10.3390/ijms25052471






