A Novel Tendon Injury Model, Induced by Collagenase Administration Combined with a Thermo-Responsive Hydrogel in Rats, Reproduces the Pathogenesis of Human Degenerative Tendinopathy
Abstract
1. Introduction
2. Results
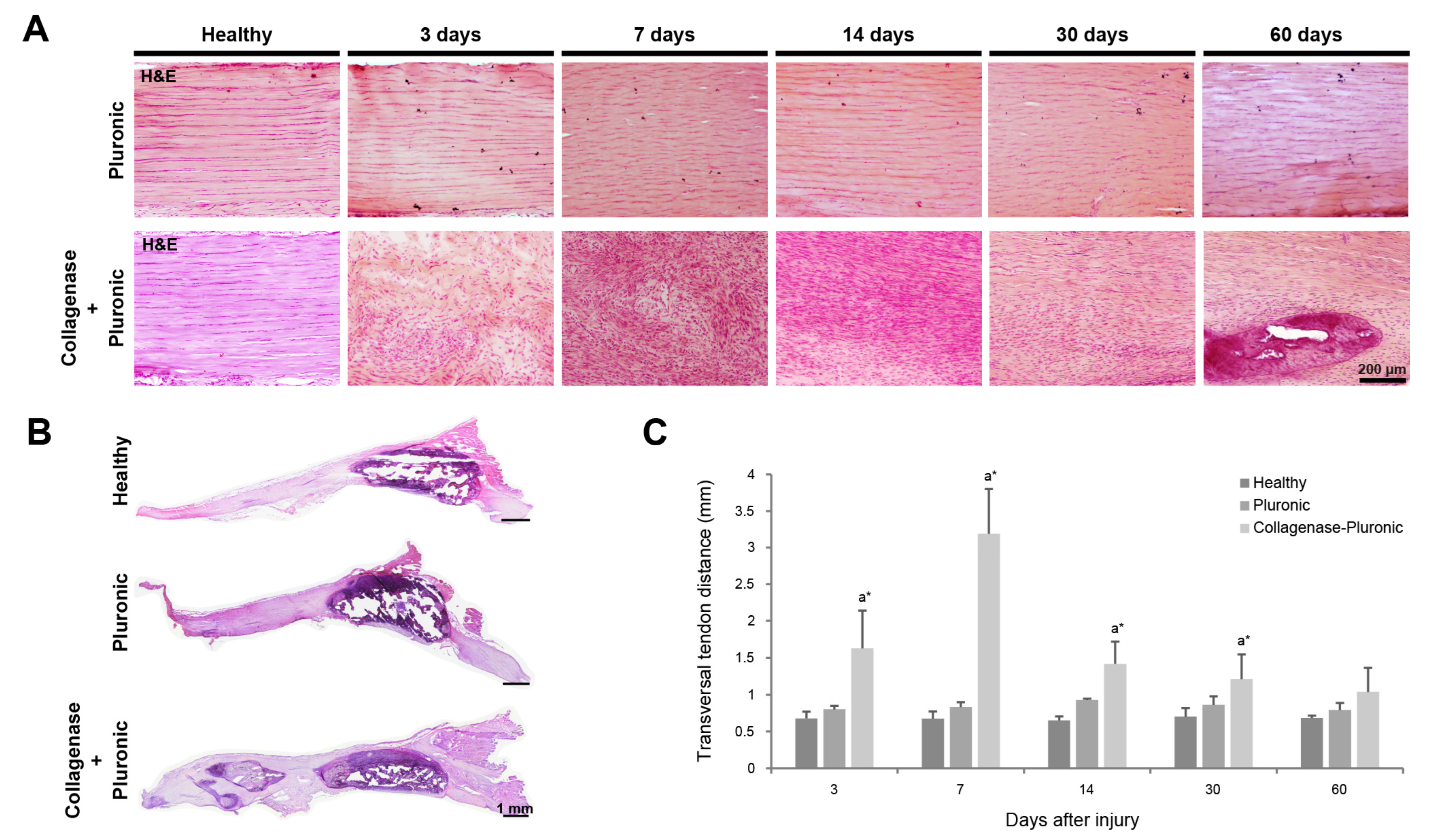
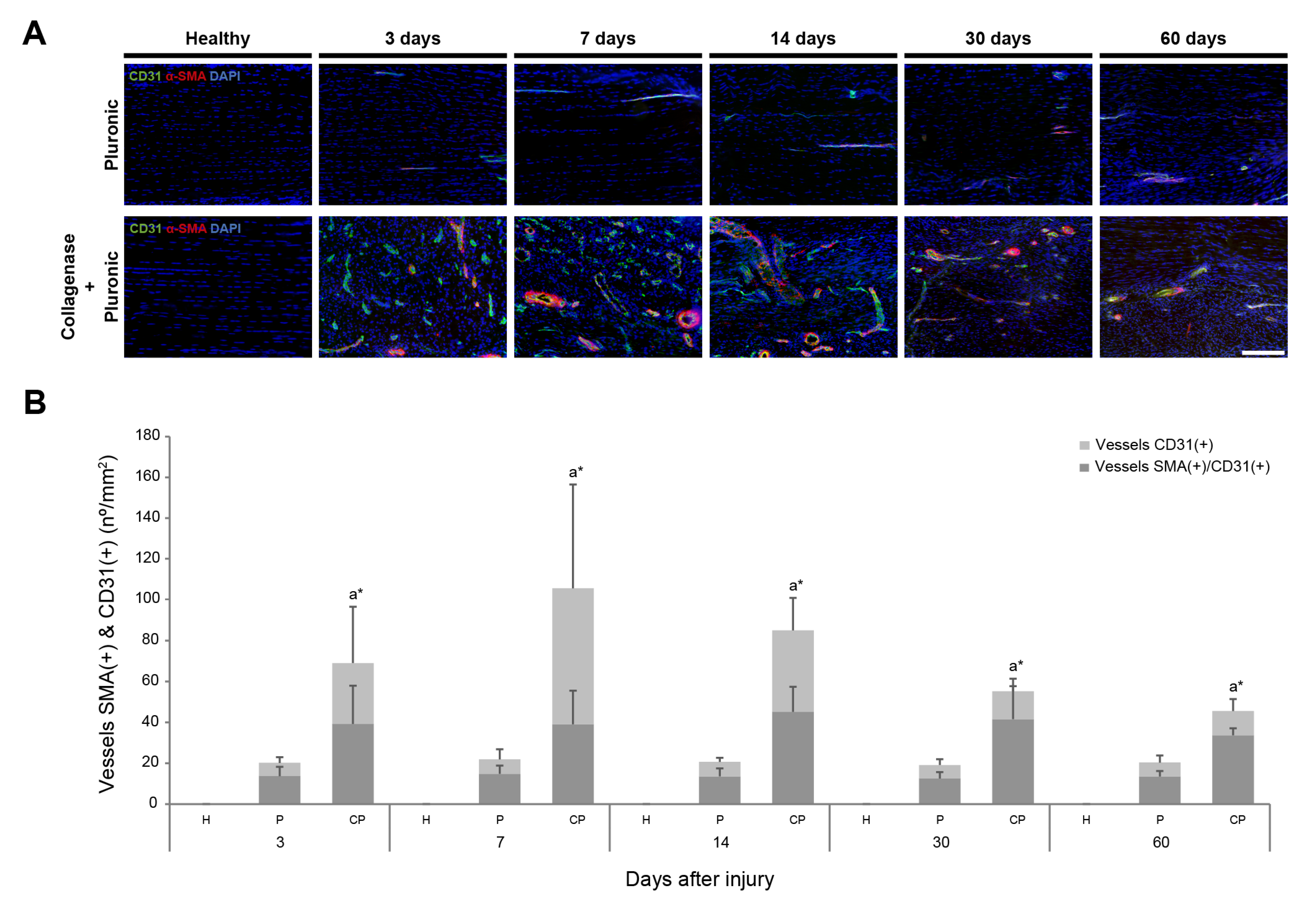
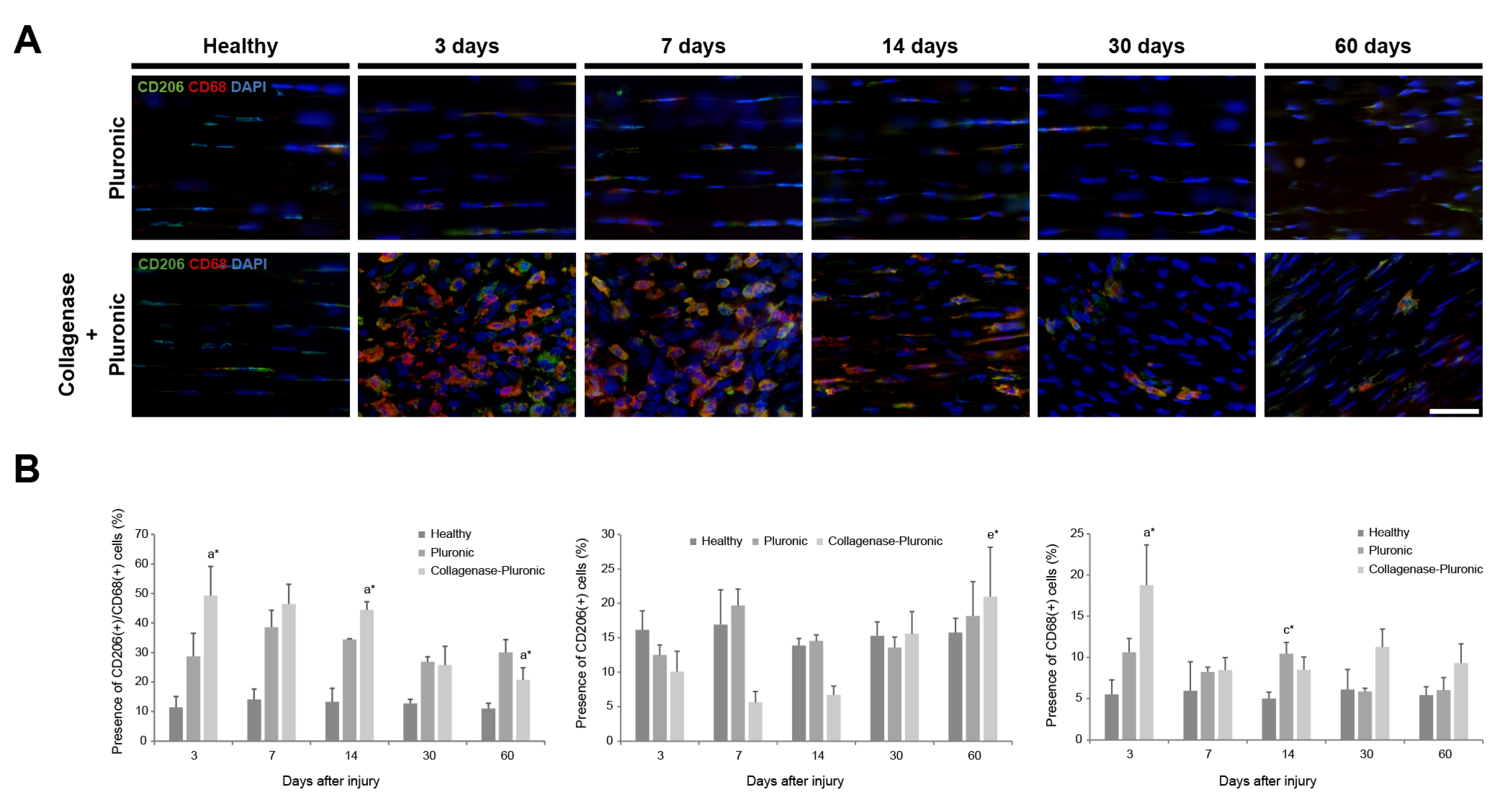
2.1. Histology and Immunofluorescence Analysis of Patellar Tendon Injury
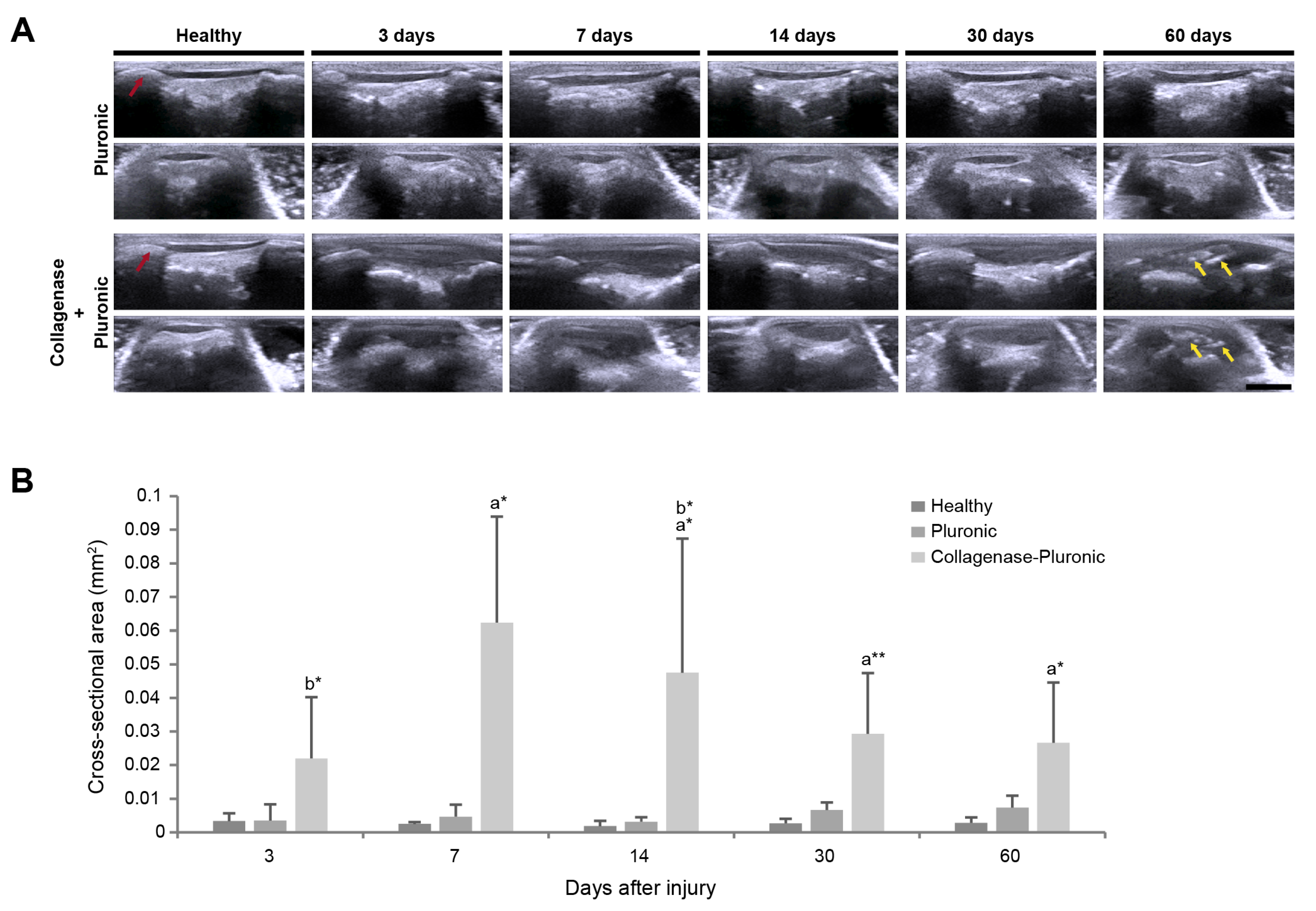
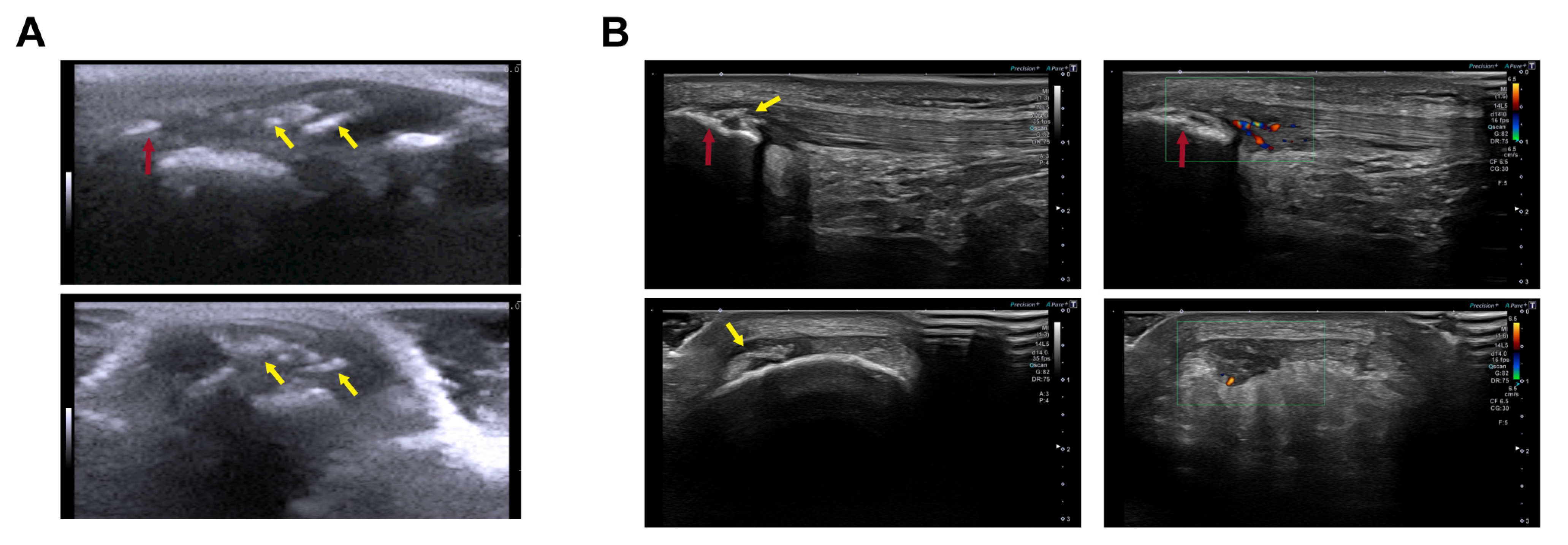
2.2. Ultrasound Clinical Imaging Analysis of the Longitudinal Evolution of Patellar Tendon Injury
2.3. Biomechanical Analysis of Patellar Tendon Injury
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Generation of Patellar Tendinopathy Model in Rats
4.3. Ultrasound Clinical Imaging Analysis of the Longitudinal Evolution of Patellar Tendon Injury
4.4. Histology and Immunofluorescence Analysis of Patellar Tendon Injury
4.5. Biomechanical Analysis of Patellar Tendon Injury
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Woolf, A.D.; Pfleger, B. Burden of Major Musculoskeletal Conditions. Bull. World Health Organ. 2003, 81, 646–656. [Google Scholar]
- Kaux, J.-F.; Forthomme, B.; Goff, C.L.; Crielaard, J.-M.; Croisier, J.-L. Current Opinions on Tendinopathy. J. Sports Sci. Med. 2011, 10, 238–253. [Google Scholar]
- Valle, X.; Alentorn-Geli, E.; Tol, J.L.; Hamilton, B.; Garrett, W.E.J.; Pruna, R.; Til, L.; Gutierrez, J.A.; Alomar, X.; Balius, R.; et al. Muscle Injuries in Sports: A New Evidence-Informed and Expert Consensus-Based Classification with Clinical Application. Sports Med. 2017, 47, 1241–1253. [Google Scholar] [CrossRef]
- Wu, F.; Nerlich, M.; Docheva, D. Tendon Injuries: Basic Science and New Repair Proposals. EFORT Open Rev. 2017, 2, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Nourissat, G.; Berenbaum, F.; Duprez, D. Tendon Injury: From Biology to Tendon Repair. Nat. Rev. Rheumatol. 2015, 11, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Millar, N.L.; Murrell, G.A.C.; McInnes, I.B. Inflammatory Mechanisms in Tendinopathy–towards Translation. Nat. Rev. Rheumatol. 2017, 13, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.T. The Patellar Tendon. Semin. Musculoskelet. Radiol. 2013, 17, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Timpka, T.; Jacobsson, J.; Bickenbach, J.; Finch, C.F.; Ekberg, J.; Nordenfelt, L. What Is a Sports Injury? Sports Med. 2014, 44, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Maffulli, N. Tendon Injury and Tendinopathy: Healing and Repair. J. Bone Jt. Surg. Am. 2005, 87, 187–202. [Google Scholar] [CrossRef]
- Cook, J.L.; Purdam, C.R. Is Tendon Pathology a Continuum? A Pathology Model to Explain the Clinical Presentation of Load-Induced Tendinopathy. Br. J. Sports Med. 2009, 43, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Riley, G. Tendinopathy--from Basic Science to Treatment. Nat. Clin. Pract. Rheumatol. 2008, 4, 82–89. [Google Scholar] [CrossRef]
- Liu, L.; Hindieh, J.; Leong, D.J.; Sun, H.B. Advances of Stem Cell Based-Therapeutic Approaches for Tendon Repair. J. Orthop. Transl. 2017, 9, 69–75. [Google Scholar] [CrossRef]
- Rudavsky, A.; Cook, J. Physiotherapy Management of Patellar Tendinopathy (Jumper’s Knee). J. Physiother. 2014, 60, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.; Ashe, M.C. Common Tendinopathies in the Upper and Lower Extremities. Curr. Sports Med. Rep. 2006, 5, 233–241. [Google Scholar] [CrossRef]
- Figueroa, D.; Figueroa, F.; Calvo, R. Patellar Tendinopathy: Diagnosis and Treatment. J. Am. Acad. Orthop. Surg. 2016, 24, e184–e192. [Google Scholar] [CrossRef]
- Zwerver, J.; Bredeweg, S.W.; van den Akker-Scheek, I. Prevalence of Jumper’s Knee among Nonelite Athletes from Different Sports: A Cross-Sectional Survey. Am. J. Sports Med. 2011, 39, 1984–1988. [Google Scholar] [CrossRef] [PubMed]
- van der Worp, H.; van Ark, M.; Roerink, S.; Pepping, G.-J.; van den Akker-Scheek, I.; Zwerver, J. Risk Factors for Patellar Tendinopathy: A Systematic Review of the Literature. Br. J. Sports Med. 2011, 45, 446–452. [Google Scholar] [CrossRef]
- Maffulli, N.; Wong, J.; Almekinders, L.C. Types and Epidemiology of Tendinopathy. Clin. Sports Med. 2003, 22, 675–692. [Google Scholar] [CrossRef]
- Chang, A.; Miller, T.T. Imaging of Tendons. Sports Health 2009, 1, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Abat-González, F.; Martín-Martínez, A.; de-Rus-Aznar, I.; Campos-Moraes, J. Patellar tendinopathy: Diagnosis by ultrasound and magnetic resonance imaging. Conservative and surgical management alternatives. Rev. Española De Artrosc. Y Cirugía Articul. 2022, 29, 13. [Google Scholar] [CrossRef]
- Fu, S.-C.; Rolf, C.; Cheuk, Y.-C.; Lui, P.P.; Chan, K.-M. Deciphering the Pathogenesis of Tendinopathy: A Three-Stages Process. Sports Med. Arthrosc. Rehabil. Ther. Technol. 2010, 2, 30. [Google Scholar] [CrossRef]
- Watson, J.; Barker-Davies, R.M.; Bennett, A.N.; Fong, D.T.P.; Wheeler, P.C.; Lewis, M.; Ranson, C. Sport and Exercise Medicine Consultants Are Reliable in Assessing Tendon Neovascularity Using Ultrasound Doppler. BMJ Open Sport Exerc. Med. 2018, 4, e000298. [Google Scholar] [CrossRef] [PubMed]
- Couppé, C.; Kongsgaard, M.; Aagaard, P.; Hansen, P.; Bojsen-Moller, J.; Kjaer, M.; Magnusson, S.P. Habitual Loading Results in Tendon Hypertrophy and Increased Stiffness of the Human Patellar Tendon. J. Appl. Physiol. 2008, 105, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.J.; Ng, G.Y.; Lee, W.C.; Fu, S.N. Changes in Morphological and Elastic Properties of Patellar Tendon in Athletes with Unilateral Patellar Tendinopathy and Their Relationships with Pain and Functional Disability. PLoS ONE 2014, 9, e108337. [Google Scholar] [CrossRef]
- Terslev, L.; Qvistgaard, E.; Torp-Pedersen, S.; Laetgaard, J.; Danneskiold-Samsøe, B.; Bliddal, H. Ultrasound and Power Doppler Findings in Jumper’s Knee–Preliminary Observations. Eur. J. Ultrasound 2001, 13, 183–189. [Google Scholar] [CrossRef]
- Warden, S.J.; Brukner, P. Patellar Tendinopathy. Clin. Sports Med. 2003, 22, 743–759. [Google Scholar] [CrossRef] [PubMed]
- Beebe, J.A.; Cross, P.S. Patellar Tendinopathy: Preliminary Surgical Results. Sports Health 2013, 5, 220–224. [Google Scholar] [CrossRef]
- Oliva, F.; Via, A.G.; Maffulli, N. Physiopathology of Intratendinous Calcific Deposition. BMC Med. 2012, 10, 95. [Google Scholar] [CrossRef]
- Butt, A.; Umaskanth, N.; Sahu, A. Image-Guided Intervention in the Management of Chronic Patellar Tendinopathy with Calcification: A Three-Pronged Approach. BMJ Case Rep. 2021, 14, e240553. [Google Scholar] [CrossRef]
- Fenwick, S.; Harrall, R.; Hackney, R.; Bord, S.; Horner, A.; Hazleman, B.; Riley, G. Endochondral Ossification in Achilles and Patella Tendinopathy. Rheumatology 2002, 41, 474–476. [Google Scholar] [CrossRef]
- Cook, J.L.; Rio, E.; Purdam, C.R.; Docking, S.I. Revisiting the Continuum Model of Tendon Pathology: What Is Its Merit in Clinical Practice and Research? Br. J. Sports Med. 2016, 50, 1187–1191. [Google Scholar] [CrossRef]
- Arvind, V.; Huang, A.H. Reparative and Maladaptive Inflammation in Tendon Healing. Front. Bioeng. Biotechnol. 2021, 9, 719047. [Google Scholar] [CrossRef]
- Millar, N.L.; Dean, B.J.; Dakin, S.G. Inflammation and the Continuum Model: Time to Acknowledge the Molecular Era of Tendinopathy. Br. J. Sports Med. 2016, 50, 1486. [Google Scholar] [CrossRef]
- Dakin, S.G.; Martinez, F.O.; Yapp, C.; Wells, G.; Oppermann, U.; Dean, B.J.F.; Smith, R.D.J.; Wheway, K.; Watkins, B.; Roche, L.; et al. Inflammation Activation and Resolution in Human Tendon Disease. Sci. Transl. Med. 2015, 7, 311ra173. [Google Scholar] [CrossRef]
- Dean, B.J.F.; Gettings, P.; Dakin, S.G.; Carr, A.J. Are Inflammatory Cells Increased in Painful Human Tendinopathy? A Systematic Review. Br. J. Sports Med. 2016, 50, 216–220. [Google Scholar] [CrossRef]
- Schubert, T.E.O.; Weidler, C.; Lerch, K.; Hofstädter, F.; Straub, R.H. Achilles Tendinosis Is Associated with Sprouting of Substance P Positive Nerve Fibres. Ann. Rheum. Dis. 2005, 64, 1083–1086. [Google Scholar] [CrossRef]
- Legerlotz, K.; Jones, E.R.; Screen, H.R.C.; Riley, G.P. Increased Expression of IL-6 Family Members in Tendon Pathology. Rheumatology 2012, 51, 1161–1165. [Google Scholar] [CrossRef]
- Gaida, J.E.; Bagge, J.; Purdam, C.; Cook, J.; Alfredson, H.; Forsgren, S. Evidence of the TNF-α System in the Human Achilles Tendon: Expression of TNF-α and TNF Receptor at Both Protein and MRNA Levels in the Tenocytes. Cells Tissues Organs 2012, 196, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Andarawis-Puri, N.; Flatow, E.L.; Soslowsky, L.J. Tendon Basic Science: Development, Repair, Regeneration, and Healing. J. Orthop. Res. 2015, 33, 780–784. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.K.W.; McIlwraith, C.W. “One Health” in Tendinopathy Research: Current Concepts. J. Orthop. Res. 2021, 39, 1596–1602. [Google Scholar] [CrossRef] [PubMed]
- Theodorou, A.; Komnos, G.; Hantes, M. Patellar Tendinopathy: An Overview of Prevalence, Risk Factors, Screening, Diagnosis, Treatment and Prevention. Arch. Orthop. Trauma Surg. 2023, 143, 6695–6705. [Google Scholar] [CrossRef] [PubMed]
- Challoumas, D.; Biddle, M.; Millar, N.L. Recent Advances in Tendinopathy. Fac. Rev. 2020, 9, 16. [Google Scholar] [CrossRef]
- Muñoz-Fernández, A.C.; Barragán-Carballar, C.; Villafañe, J.H.; Martín-Pérez, S.; Alonso-Pérez, J.L.; Díaz-Meco, R.; García-Jiménez, D.; Sánchez-Romero, E.A. A New Ultrasound-Guided Percutaneous Electrolysis and Exercise Treatment in Patellar Tendinopathy: Three Case Reports. Front. Biosci. 2021, 26, 1166–1175. [Google Scholar] [CrossRef]
- Dirks, R.C.; Warden, S.J. Models for the Study of Tendinopathy. J. Musculoskelet. Neuronal Interact. 2011, 11, 141–149. [Google Scholar]
- Wu, S.Y.; Kim, W.; Kremen, T.J.J. In Vitro Cellular Strain Models of Tendon Biology and Tenogenic Differentiation. Front. Bioeng. Biotechnol. 2022, 10, 826748. [Google Scholar] [CrossRef]
- Gomez-Florit, M.; Labrador-Rached, C.J.; Domingues, R.M.A.; Gomes, M.E. The Tendon Microenvironment: Engineered in Vitro Models to Study Cellular Crosstalk. Adv. Drug Deliv. Rev. 2022, 185, 114299. [Google Scholar] [CrossRef]
- Citeroni, M.R.; Ciardulli, M.C.; Russo, V.; Della Porta, G.; Mauro, A.; El Khatib, M.; Di Mattia, M.; Galesso, D.; Barbera, C.; Forsyth, N.R.; et al. In Vitro Innovation of Tendon Tissue Engineering Strategies. Int. J. Mol. Sci. 2020, 21, 6726. [Google Scholar] [CrossRef] [PubMed]
- Wunderli, S.L.; Blache, U.; Beretta Piccoli, A.; Niederöst, B.; Holenstein, C.N.; Passini, F.S.; Silván, U.; Bundgaard, L.; Auf dem Keller, U.; Snedeker, J.G. Tendon Response to Matrix Unloading Is Determined by the Patho-Physiological Niche. Matrix Biol. 2020, 89, 11–26. [Google Scholar] [CrossRef] [PubMed]
- de Mos, M.; Koevoet, W.; van Schie, H.T.M.; Kops, N.; Jahr, H.; Verhaar, J.A.N.; van Osch, G.J.V.M. In Vitro Model to Study Chondrogenic Differentiation in Tendinopathy. Am. J. Sports Med. 2009, 37, 1214–1222. [Google Scholar] [CrossRef]
- Monteiro, R.F.; Bakht, S.M.; Gomez-Florit, M.; Stievani, F.C.; Alves, A.L.G.; Reis, R.L.; Gomes, M.E.; Domingues, R.M.A. Writing 3D In Vitro Models of Human Tendon within a Biomimetic Fibrillar Support Platform. ACS Appl. Mater. Interfaces 2023, 15, 50598–50611. [Google Scholar] [CrossRef]
- Calejo, I.; Labrador-Rached, C.J.; Gomez-Florit, M.; Docheva, D.; Reis, R.L.; Domingues, R.M.A.; Gomes, M.E. Bioengineered 3D Living Fibers as In Vitro Human Tissue Models of Tendon Physiology and Pathology. Adv. Healthc. Mater. 2022, 11, e2102863. [Google Scholar] [CrossRef]
- Schwartz, A.; Watson, J.N.; Hutchinson, M.R. Patellar Tendinopathy. Sports Health 2015, 7, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Lake, S.P.; Ansorge, H.L.; Soslowsky, L.J. Animal Models of Tendinopathy. Disabil. Rehabil. 2008, 30, 1530–1541. [Google Scholar] [CrossRef] [PubMed]
- Oshita, T.; Tobita, M.; Tajima, S.; Mizuno, H. Adipose-Derived Stem Cells Improve Collagenase-Induced Tendinopathy in a Rat Model. Am. J. Sports Med. 2016, 44, 1983–1989. [Google Scholar] [CrossRef] [PubMed]
- Perucca Orfei, C.; Lovati, A.B.; Viganò, M.; Stanco, D.; Bottagisio, M.; Di Giancamillo, A.; Setti, S.; de Girolamo, L. Dose-Related and Time-Dependent Development of Collagenase-Induced Tendinopathy in Rats. PLoS ONE 2016, 11, e0161590. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.; Kim, S.E.; Shim, K.-S.; Kim, H.-J.; Song, M.H.; Park, K.; Song, H.-R. Exploring the In Vivo Anti-Inflammatory Actions of Simvastatin-Loaded Porous Microspheres on Inflamed Tenocytes in a Collagenase-Induced Animal Model of Achilles Tendinitis. Int. J. Mol. Sci. 2018, 19, 820. [Google Scholar] [CrossRef]
- Watts, A.E.; Nixon, A.J.; Yeager, A.E.; Mohammed, H.O. A Collagenase Gel/Physical Defect Model for Controlled Induction of Superficial Digital Flexor Tendonitis. Equine Vet. J. 2012, 44, 576–586. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wang, Z.; Tang, C.; Yin, Z.; Huang, J.; Ruan, D.; Fei, Y.; Wang, C.; Mo, X.; Li, J.; et al. Animal Model for Tendinopathy. J. Orthop. Transl. 2023, 42, 43–56. [Google Scholar] [CrossRef]
- de Cesar Netto, C.; Godoy-Santos, A.L.; Augusto Pontin, P.; Natalino, R.J.M.; Pereira, C.A.M.; Lima, F.D.d.O.; da Fonseca, L.F.; Staggers, J.R.; Cavinatto, L.M.; Schon, L.C.; et al. Novel Animal Model for Achilles Tendinopathy: Controlled Experimental Study of Serial Injections of Collagenase in Rabbits. PLoS ONE 2018, 13, e0192769. [Google Scholar] [CrossRef]
- Ghelfi, J.; Bacle, M.; Stephanov, O.; de Forges, H.; Soulairol, I.; Roger, P.; Ferretti, G.R.; Beregi, J.-P.; Frandon, J. Collagenase-Induced Patellar Tendinopathy with Neovascularization: First Results towards a Piglet Model of Musculoskeletal Embolization. Biomedicines 2021, 10, 2. [Google Scholar] [CrossRef]
- Ueda, Y.; Inui, A.; Mifune, Y.; Takase, F.; Kataoka, T.; Kurosawa, T.; Yamaura, K.; Kokubu, T.; Kuroda, R. Molecular Changes to Tendons after Collagenase-Induced Acute Tendon Injury in a Senescence-Accelerated Mouse Model. BMC Musculoskelet. Disord. 2019, 20, 120. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Chieh, H.-F.; Lin, C.-J.; Jou, I.-M.; Sun, Y.-N.; Kuo, L.-C.; Wu, P.-T.; Su, F.-C. Characteristics of Sonography in a Rat Achilles Tendinopathy Model: Possible Non-Invasive Predictors of Biomechanics. Sci. Rep. 2017, 7, 5100. [Google Scholar] [CrossRef]
- Lui, P.P.-Y.; Chan, L.-S.; Fu, S.-C.; Chan, K.-M. Expression of Sensory Neuropeptides in Tendon Is Associated with Failed Healing and Activity-Related Tendon Pain in Collagenase-Induced Tendon Injury. Am. J. Sports Med. 2010, 38, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Lui, P.P.; Fu, S.; Chan, L.; Hung, L.; Chan, K. Chondrocyte Phenotype and Ectopic Ossification in Collagenase-Induced Tendon Degeneration. J. Histochem. Cytochem. 2009, 57, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Sullo, A.; Maffulli, N.; Capasso, G.; Testa, V. The Effects of Prolonged Peritendinous Administration of PGE1 to the Rat Achilles Tendon: A Possible Animal Model of Chronic Achilles Tendinopathy. J. Orthop. Sci. 2001, 6, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Yamamoto, M.; Hirouchi, H.; Taniguchi, S.; Watanabe, G.; Matsunaga, S.; Abe, S. Regeneration Process of Myotendinous Junction Injury Induced by Collagenase Injection between Achilles Tendon and Soleus Muscle in Mice. Anat. Sci. Int. 2024, 99, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Zhang, J.; Zhao, G.; Zhou, Y.; Zhang, C.-Q.; Wang, J.H.-C. Creating an Animal Model of Tendinopathy by Inducing Chondrogenic Differentiation with Kartogenin. PLoS ONE 2016, 11, e0148557. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for Biomedical Applications. Adv. Drug Deliv. Rev. 2002, 54, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Gioffredi, E.; Boffito, M.; Calzone, S.; Giannitelli, S.M.; Rainer, A.; Trombetta, M.; Mozetic, P.; Chiono, V. Pluronic F127 Hydrogel Characterization and Biofabrication in Cellularized Constructs for Tissue Engineering Applications. Procedia CIRP 2016, 49, 125–132. [Google Scholar] [CrossRef]
- Cidade, M.T.; Ramos, D.J.; Santos, J.; Carrelo, H.; Calero, N.; Borges, J.P. Injectable Hydrogels Based on Pluronic/Water Systems Filled with Alginate Microparticles for Biomedical Applications. Materials 2019, 12, 1083. [Google Scholar] [CrossRef]
- Perumal, G.; Ramasamy, B.; Nandkumar, A.M.; Doble, M. Influence of Magnesium Particles and Pluronic F127 on Compressive Strength and Cytocompatibility of Nanocomposite Injectable and Moldable Beads for Bone Regeneration. J. Mech. Behav. Biomed. Mater. 2018, 88, 453–462. [Google Scholar] [CrossRef]
- Lee, J.; Kim, G. Three-Dimensional Hierarchical Nanofibrous Collagen Scaffold Fabricated Using Fibrillated Collagen and Pluronic F-127 for Regenerating Bone Tissue. ACS Appl. Mater. Interfaces 2018, 10, 35801–35811. [Google Scholar] [CrossRef]
- Brunet-Maheu, J.-M.; Fernandes, J.C.; de Lacerda, C.A.V.; Shi, Q.; Benderdour, M.; Lavigne, P. Pluronic F-127 as a Cell Carrier for Bone Tissue Engineering. J. Biomater. Appl. 2009, 24, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Suntornnond, R.; Tan, E.Y.S.; An, J.; Chua, C.K. A Highly Printable and Biocompatible Hydrogel Composite for Direct Printing of Soft and Perfusable Vasculature-like Structures. Sci. Rep. 2017, 7, 16902. [Google Scholar] [CrossRef] [PubMed]
- Akash, M.S.H.; Rehman, K.; Chen, S. Pluronic F127-Based Thermosensitive Gels for Delivery of Therapeutic Proteins and Peptides. Polym. Rev. 2014, 54, 573–597. [Google Scholar] [CrossRef]
- Andrade, F.; das Neves, J.; Gener, P.; Schwartz, S.J.; Ferreira, D.; Oliva, M.; Sarmento, B. Biological Assessment of Self-Assembled Polymeric Micelles for Pulmonary Administration of Insulin. Nanomedicine 2015, 11, 1621–1631. [Google Scholar] [CrossRef] [PubMed]
- Diniz, I.M.A.; Chen, C.; Xu, X.; Ansari, S.; Zadeh, H.H.; Marques, M.M.; Shi, S.; Moshaverinia, A. Pluronic F-127 Hydrogel as a Promising Scaffold for Encapsulation of Dental-Derived Mesenchymal Stem Cells. J. Mater. Sci. Mater. Med. 2015, 26, 153. [Google Scholar] [CrossRef] [PubMed]
- Rafael, D.; Andrade, F.; Montero, S.; Gener, P.; Seras-Franzoso, J.; Martínez, F.; González, P.; Florindo, H.; Arango, D.; Sayós, J.; et al. Rational Design of a SiRNA Delivery System: ALOX5 and Cancer Stem Cells as Therapeutic Targets. PRNANO 2018, 1, 86–105. [Google Scholar] [CrossRef]
- Rafael, D.; Gener, P.; Andrade, F.; Seras-Franzoso, J.; Montero, S.; Fernández, Y.; Hidalgo, M.; Arango, D.; Sayós, J.; Florindo, H.F.; et al. AKT2 SiRNA Delivery with Amphiphilic-Based Polymeric Micelles Show Efficacy against Cancer Stem Cells. Drug Deliv. 2018, 25, 961–972. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kwon, B.; Lee, K.; Son, Y.H.; Chung, S.G. Therapeutic Mechanisms of Human Adipose-Derived Mesenchymal Stem Cells in a Rat Tendon Injury Model. Am. J. Sports Med. 2017, 45, 1429–1439. [Google Scholar] [CrossRef]
- Domínguez, D.; Contreras-Muñoz, P.; Lope, S.; Rodas, G.; Marotta, M. Generation of a New Model of Patellar Tendinopathy in Rats Which Mimics the Human Sports Pathology: A Pilot Study. Apunt. Med. Esport 2017, 52, 53–59. [Google Scholar] [CrossRef]
- Pusztaszeri, M.P.; Seelentag, W.; Bosman, F.T. Immunohistochemical Expression of Endothelial Markers CD31, CD34, von Willebrand Factor, and Fli-1 in Normal Human Tissues. J. Histochem. Cytochem. 2006, 54, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Lertkiatmongkol, P.; Liao, D.; Mei, H.; Hu, Y.; Newman, P.J. Endothelial Functions of Platelet/Endothelial Cell Adhesion Molecule-1 (CD31). Curr. Opin. Hematol. 2016, 23, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Donadon, M.; Santoro, M.M. The Origin and Mechanisms of Smooth Muscle Cell Development in Vertebrates. Development 2021, 148, dev197384. [Google Scholar] [CrossRef] [PubMed]
- Bacakova, L.; Travnickova, M.; Filova, E.; Matějka, R.; Stepanovska, J.; Musilkova, J.; Zarubova, J.; Molitor, M.; Bacakova, L.; Travnickova, M.; et al. The Role of Vascular Smooth Muscle Cells in the Physiology and Pathophysiology of Blood Vessels. In Muscle Cell and Tissue—Current Status of Research Field; IntechOpen: London, UK, 2018; ISBN 978-1-78984-006-3. [Google Scholar]
- Klinge, U.; Dievernich, A.; Tolba, R.; Klosterhalfen, B.; Davies, L. CD68+ Macrophages as Crucial Components of the Foreign Body Reaction Demonstrate an Unconventional Pattern of Functional Markers Quantified by Analysis with Double Fluorescence Staining. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 3134–3146. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Killingsworth, M.C.; Myasoedova, V.A.; Orekhov, A.N.; Bobryshev, Y.V. CD68/Macrosialin: Not Just a Histochemical Marker. Lab. Investig. 2017, 97, 4–13. [Google Scholar] [CrossRef]
- Rőszer, T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediat. Inflamm. 2015, 2015, 816460. [Google Scholar] [CrossRef]
- Hodgson, R.J.; O’Connor, P.J.; Grainger, A.J. Tendon and Ligament Imaging. Br. J. Radiol. 2012, 85, 1157–1172. [Google Scholar] [CrossRef]
- Huang, Y.-M.; Lin, Y.-C.; Chen, C.-Y.; Hsieh, Y.-Y.; Liaw, C.-K.; Huang, S.-W.; Tsuang, Y.-H.; Chen, C.-H.; Lin, F.-H. Thermosensitive Chitosan-Gelatin-Glycerol Phosphate Hydrogels as Collagenase Carrier for Tendon-Bone Healing in a Rabbit Model. Polymers 2020, 12, 436. [Google Scholar] [CrossRef]
- Mallya, S.K.; Mookhtiar, K.A.; Van Wart, H.E. Accurate, Quantitative Assays for the Hydrolysis of Soluble Type I, II, and III 3H-Acetylated Collagens by Bacterial and Tissue Collagenases. Anal. Biochem. 1986, 158, 334–345. [Google Scholar] [CrossRef]
- Meng, X.; Liu, J.; Yu, X.; Li, J.; Lu, X.; Shen, T. Pluronic F127 and D-α-Tocopheryl Polyethylene Glycol Succinate (TPGS) Mixed Micelles for Targeting Drug Delivery across The Blood Brain Barrier. Sci. Rep. 2017, 7, 2964. [Google Scholar] [CrossRef]
- Dan, M.J.; Oliver, R.A.; Crowley, J.D.; Lovric, V.; Parr, W.C.H.; Broe, D.; Cross, M.; Walsh, W.R. The Effect of Surgery on Patellar Tendinopathy: Novel Use of MRI Questions the Exploitability of the Rat Collagenase Model to Humans. Knee 2019, 26, 1182–1191. [Google Scholar] [CrossRef]
- Allen, A.D.; Bassil, A.M.; Berkoff, D.J.; Al Maliki, M.; Draeger, R.W.; Weinhold, P.S. Minocycline Microspheres Did Not Significantly Improve Outcomes after Collagenase Injection of Tendon. J. Orthop. 2019, 16, 580–584. [Google Scholar] [CrossRef]
- Kader, D.; Saxena, A.; Movin, T.; Maffulli, N. Achilles Tendinopathy: Some Aspects of Basic Science and Clinical Management. Br. J. Sports Med. 2002, 36, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Lui, P.P.-Y.; Chan, L.-S.; Lee, Y.-W.; Fu, S.C.; Chan, K.-M. Sustained Expression of Proteoglycans and Collagen Type III/Type I Ratio in a Calcified Tendinopathy Model. Rheumatology 2010, 49, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Oshiro, W.; Lou, J.; Xing, X.; Tu, Y.; Manske, P.R. Flexor Tendon Healing in the Rat: A Histologic and Gene Expression Study. J. Hand Surg. Am. 2003, 28, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Riley, G. The Pathogenesis of Tendinopathy. A Molecular Perspective. Rheumatology 2004, 43, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.C.d.F.; Albertini, R.; Serra, A.J.; da Silva, E.A.P.; de Oliveira, V.L.C.; Silva, L.M.; Leal-Junior, E.C.P.; de Carvalho, P. de T.C. Photobiomodulation Therapy on Collagen Type I and III, Vascular Endothelial Growth Factor, and Metalloproteinase in Experimentally Induced Tendinopathy in Aged Rats. Lasers Med. Sci. 2016, 31, 1915–1923. [Google Scholar] [CrossRef] [PubMed]
- Bin, Z.; Liu, X.; Sheng, G.; Dingxuan, W.; Sen, L. Animal Models of Tendinopathy Induced by Chemicals. Cell. Mol. Biol. 2022, 68, 221. [Google Scholar] [CrossRef]
- Tom, S.; Parkinson, J.; Ilic, M.Z.; Cook, J.; Feller, J.A.; Handley, C.J. Changes in the Composition of the Extracellular Matrix in Patellar Tendinopathy. Matrix Biol. 2009, 28, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Knobloch, K. The Role of Tendon Microcirculation in Achilles and Patellar Tendinopathy. J. Orthop. Surg. Res. 2008, 3, 18. [Google Scholar] [CrossRef] [PubMed]
- Ohberg, L.; Alfredson, H. Ultrasound Guided Sclerosis of Neovessels in Painful Chronic Achilles Tendinosis: Pilot Study of a New Treatment. Br. J. Sports Med. 2002, 36, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Alfredson, H.; Ohberg, L. Neovascularisation in Chronic Painful Patellar Tendinosis--Promising Results after Sclerosing Neovessels Outside the Tendon Challenge the Need for Surgery. Knee Surg. Sports Traumatol. Arthrosc. 2005, 13, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Rees, J.D.; Stride, M.; Scott, A. Tendons--Time to Revisit Inflammation. Br. J. Sports Med. 2014, 48, 1553–1557. [Google Scholar] [CrossRef] [PubMed]
- Bautista, C.A.; Srikumar, A.; Tichy, E.D.; Qian, G.; Jiang, X.; Qin, L.; Mourkioti, F.; Dyment, N.A. CD206+ Tendon Resident Macrophages and Their Potential Crosstalk with Fibroblasts and the ECM during Tendon Growth and Maturation. Front. Physiol. 2023, 14, 1122348. [Google Scholar] [CrossRef]
- Russo, V.; El Khatib, M.; Prencipe, G.; Citeroni, M.R.; Faydaver, M.; Mauro, A.; Berardinelli, P.; Cerveró-Varona, A.; Haidar-Montes, A.A.; Turriani, M.; et al. Tendon Immune Regeneration: Insights on the Synergetic Role of Stem and Immune Cells during Tendon Regeneration. Cells 2022, 11, 434. [Google Scholar] [CrossRef]
- Lehner, C.; Spitzer, G.; Gehwolf, R.; Wagner, A.; Weissenbacher, N.; Deininger, C.; Emmanuel, K.; Wichlas, F.; Tempfer, H.; Traweger, A. Tenophages: A Novel Macrophage-like Tendon Cell Population Expressing CX3CL1 and CX3CR1. Dis. Model. Mech. 2019, 12, dmm041384. [Google Scholar] [CrossRef]
- Klatte-Schulz, F.; Minkwitz, S.; Schmock, A.; Bormann, N.; Kurtoglu, A.; Tsitsilonis, S.; Manegold, S.; Wildemann, B. Different Achilles Tendon Pathologies Show Distinct Histological and Molecular Characteristics. Int. J. Mol. Sci. 2018, 19, 404. [Google Scholar] [CrossRef]
- Kragsnaes, M.S.; Fredberg, U.; Stribolt, K.; Kjaer, S.G.; Bendix, K.; Ellingsen, T. Stereological Quantification of Immune-Competent Cells in Baseline Biopsy Specimens from Achilles Tendons: Results from Patients with Chronic Tendinopathy Followed for More Than 4 Years. Am. J. Sports Med. 2014, 42, 2435–2445. [Google Scholar] [CrossRef]
- de la Durantaye, M.; Piette, A.B.; van Rooijen, N.; Frenette, J. Macrophage Depletion Reduces Cell Proliferation and Extracellular Matrix Accumulation but Increases the Ultimate Tensile Strength of Injured Achilles Tendons. J. Orthop. Res. 2014, 32, 279–285. [Google Scholar] [CrossRef]
- Sugg, K.B.; Lubardic, J.; Gumucio, J.P.; Mendias, C.L. Changes in Macrophage Phenotype and Induction of Epithelial-to-Mesenchymal Transition Genes Following Acute Achilles Tenotomy and Repair. J. Orthop. Res. 2014, 32, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Sunwoo, J.Y.; Eliasberg, C.D.; Carballo, C.B.; Rodeo, S.A. The Role of the Macrophage in Tendinopathy and Tendon Healing. J. Orthop. Res. 2020, 38, 1666–1675. [Google Scholar] [CrossRef] [PubMed]
- Marsolais, D.; Côté, C.H.; Frenette, J. Neutrophils and Macrophages Accumulate Sequentially Following Achilles Tendon Injury. J. Orthop. Res. 2001, 19, 1203–1209. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Xu, Y.; Gan, Y.; Song, L.; Zhang, C.; Wang, L.; Zhou, Q. Role of the ERK1/2 Signaling Pathway in Osteogenesis of Rat Tendon-Derived Stem Cells in Normoxic and Hypoxic Cultures. Int. J. Med. Sci. 2016, 13, 629–637. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, G.; Deng, Y.; Li, K.; Liu, Y.; Wang, L.; Wu, Z.; Chen, C.; Zhang, K.; Yu, B. Hedgehog Signalling Contributes to Trauma-Induced Tendon Heterotopic Ossification and Regulates Osteogenesis through Antioxidant Pathway in Tendon-Derived Stem Cells. Antioxidants 2022, 11, 2265. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Cheng, L.; Zheng, Q.; Liu, W.; Wang, Y. Scavenging of Reactive Oxygen Species Can Adjust the Differentiation of Tendon Stem Cells and Progenitor Cells and Prevent Ectopic Calcification in Tendinopathy. Acta Biomater. 2022, 152, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, J.H.-C. BMP-2 Mediates PGE(2)-Induced Reduction of Proliferation and Osteogenic Differentiation of Human Tendon Stem Cells. J. Orthop. Res. 2012, 30, 47–52. [Google Scholar] [CrossRef]
- Rui, Y.; Lui, P.P.; Chan, L.; Chan, K.; Fu, S.; Li, G. Does Erroneous Differentiation of Tendon-Derived Stem Cells Contribute to the Pathogenesis of Calcifying Tendinopathy? Chin. Med. J. 2011, 124, 606–610. [Google Scholar]
- Breda, S.J.; van der Vlist, A.; de Vos, R.-J.; Krestin, G.P.; Oei, E.H.G. The Association between Patellar Tendon Stiffness Measured with Shear-Wave Elastography and Patellar Tendinopathy-a Case-Control Study. Eur. Radiol. 2020, 30, 5942–5951. [Google Scholar] [CrossRef]
- Arirachakaran, A.; Boonard, M.; Yamaphai, S.; Prommahachai, A.; Kesprayura, S.; Kongtharvonskul, J. Extracorporeal Shock Wave Therapy, Ultrasound-Guided Percutaneous Lavage, Corticosteroid Injection and Combined Treatment for the Treatment of Rotator Cuff Calcific Tendinopathy: A Network Meta-Analysis of RCTs. Eur. J. Orthop. Surg. Traumatol. 2017, 27, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Constantinou, A.P.; Georgiou, T. K Pre-Clinical and Clinical Applications of Thermoreversible Hydrogels in Biomedical Engineering: A Review. Polym. Int. 2021, 70, 1433–1448. [Google Scholar] [CrossRef]
- Hsiao, M.-Y.; Lin, A.-C.; Liao, W.-H.; Wang, T.-G.; Hsu, C.-H.; Chen, W.-S.; Lin, F.-H. Drug-Loaded Hyaluronic Acid Hydrogel as a Sustained-Release Regimen with Dual Effects in Early Intervention of Tendinopathy. Sci. Rep. 2019, 9, 4784. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Song, M.H.; Shim, K.-S.; Kim, H.-J.; Lim, Y.-M.; Song, H.-R.; Park, K.; Kim, S.E. Therapeutic Efficacy of Intratendinous Delivery of Dexamethasone Using Porous Microspheres for Amelioration of Inflammation and Tendon Degeneration on Achilles Tendinitis in Rats. Biomed. Res. Int. 2020, 2020, 5052028. [Google Scholar] [CrossRef] [PubMed]
- Ilaltdinov, A.W.; Gong, Y.; Leong, D.J.; Gruson, K.I.; Zheng, D.; Fung, D.T.; Sun, L.; Sun, H.B. Advances in the Development of Gene Therapy, Noncoding RNA, and Exosome-Based Treatments for Tendinopathy. Ann. N Y Acad. Sci. 2021, 1490, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-Y.; Jou, I.-M.; Ko, P.-Y.; Hsu, K.-L.; Su, W.-R.; Kuo, L.-C.; Lee, P.-Y.; Wu, C.-L.; Wu, P.-T. Amelioration of Experimental Tendinopathy by Lentiviral CD44 Gene Therapy Targeting Senescence-Associated Secretory Phenotypes. Mol. Ther. Methods Clin. Dev. 2022, 26, 157–168. [Google Scholar] [CrossRef]
- Kim, M.Y.; Farnebo, S.; Woon, C.Y.L.; Schmitt, T.; Pham, H.; Chang, J. Augmentation of Tendon Healing with an Injectable Tendon Hydrogel in a Rat Achilles Tendon Model. Plast. Reconstr. Surg. 2014, 133, 645e–653e. [Google Scholar] [CrossRef]
- Zuniga, K.; Gadde, M.; Scheftel, J.; Senecal, K.; Cressman, E.; Van Dyke, M.; Rylander, M.N. Collagen/Kerateine Multi-Protein Hydrogels as a Thermally Stable Extracellular Matrix for 3D in Vitro Models. Int. J. Hyperth. 2021, 38, 830–845. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidal, L.; Lopez-Garzon, M.; Venegas, V.; Vila, I.; Domínguez, D.; Rodas, G.; Marotta, M. A Novel Tendon Injury Model, Induced by Collagenase Administration Combined with a Thermo-Responsive Hydrogel in Rats, Reproduces the Pathogenesis of Human Degenerative Tendinopathy. Int. J. Mol. Sci. 2024, 25, 1868. https://doi.org/10.3390/ijms25031868
Vidal L, Lopez-Garzon M, Venegas V, Vila I, Domínguez D, Rodas G, Marotta M. A Novel Tendon Injury Model, Induced by Collagenase Administration Combined with a Thermo-Responsive Hydrogel in Rats, Reproduces the Pathogenesis of Human Degenerative Tendinopathy. International Journal of Molecular Sciences. 2024; 25(3):1868. https://doi.org/10.3390/ijms25031868
Chicago/Turabian StyleVidal, Laura, Maria Lopez-Garzon, Vanesa Venegas, Ingrid Vila, David Domínguez, Gil Rodas, and Mario Marotta. 2024. "A Novel Tendon Injury Model, Induced by Collagenase Administration Combined with a Thermo-Responsive Hydrogel in Rats, Reproduces the Pathogenesis of Human Degenerative Tendinopathy" International Journal of Molecular Sciences 25, no. 3: 1868. https://doi.org/10.3390/ijms25031868
APA StyleVidal, L., Lopez-Garzon, M., Venegas, V., Vila, I., Domínguez, D., Rodas, G., & Marotta, M. (2024). A Novel Tendon Injury Model, Induced by Collagenase Administration Combined with a Thermo-Responsive Hydrogel in Rats, Reproduces the Pathogenesis of Human Degenerative Tendinopathy. International Journal of Molecular Sciences, 25(3), 1868. https://doi.org/10.3390/ijms25031868






