Wheat Transcriptional Corepressor TaTPR1 Suppresses Susceptibility Genes TaDND1/2 and Potentiates Post-Penetration Resistance against Blumeria graminis forma specialis tritici
Abstract
1. Introduction
2. Results
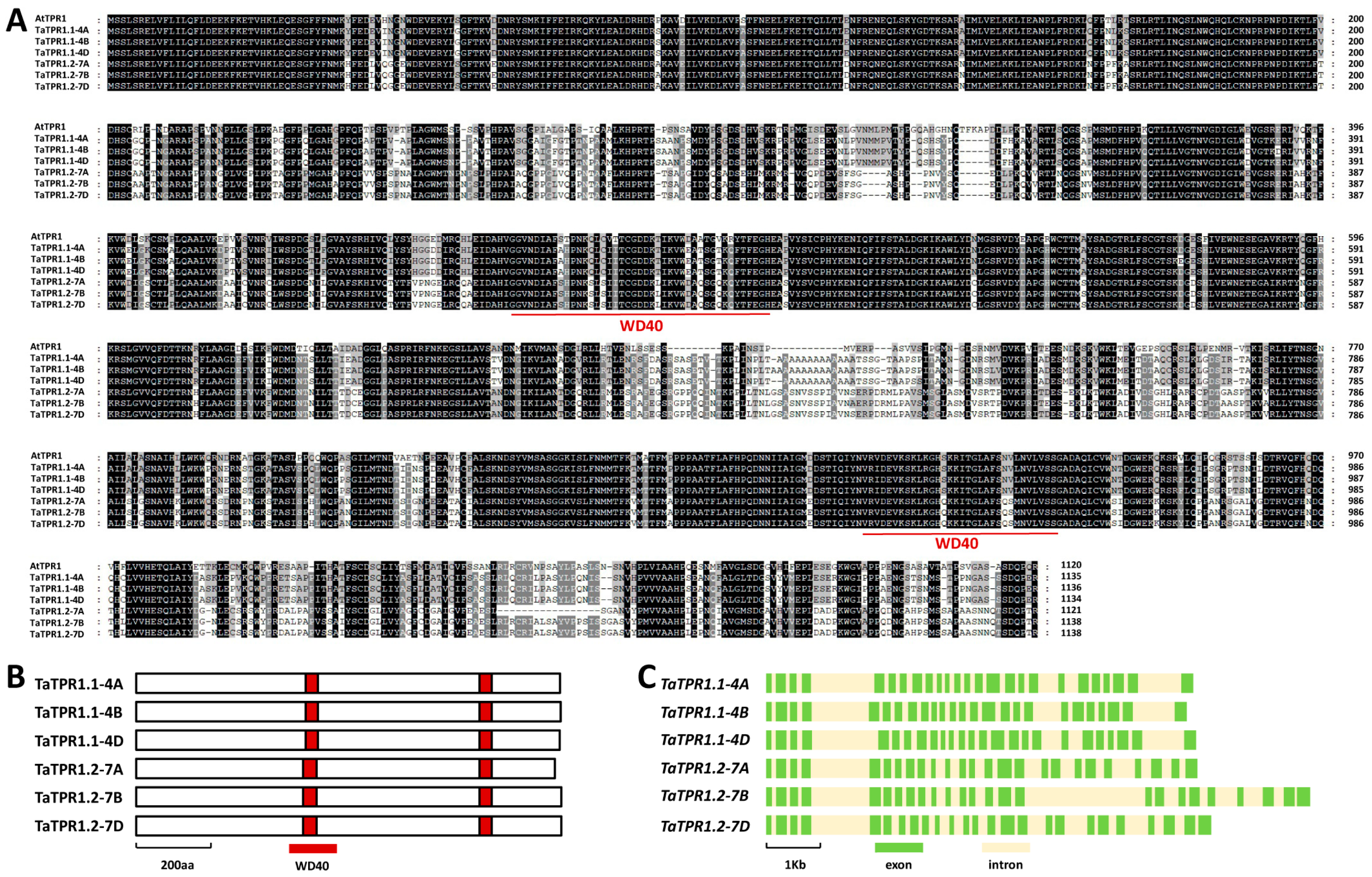
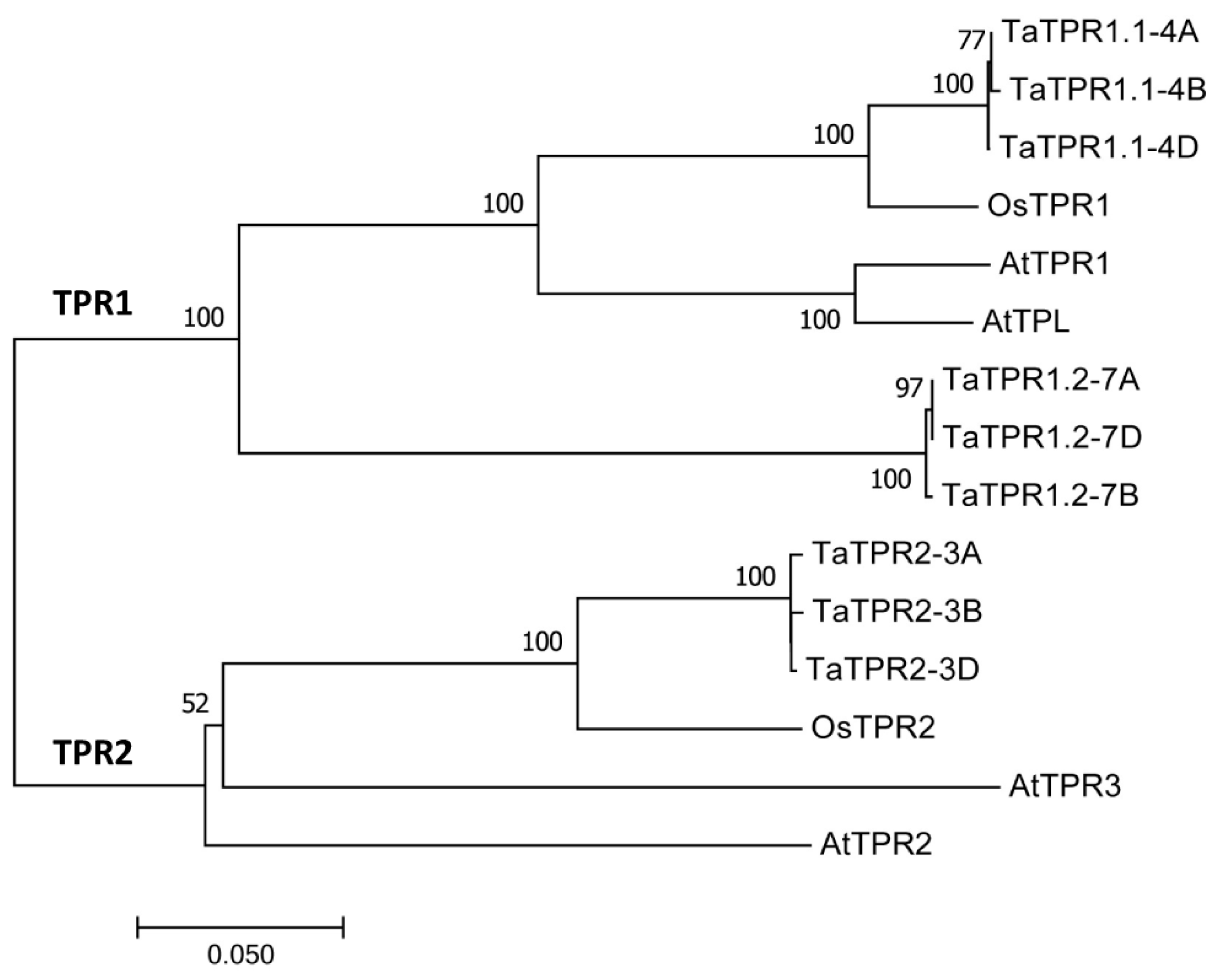
2.1. Homology-Based Identification of Wheat TaTPR1
2.2. TaTPR1 Potentiates Wheat Post-Penetration Resistance against Powdery Mildew
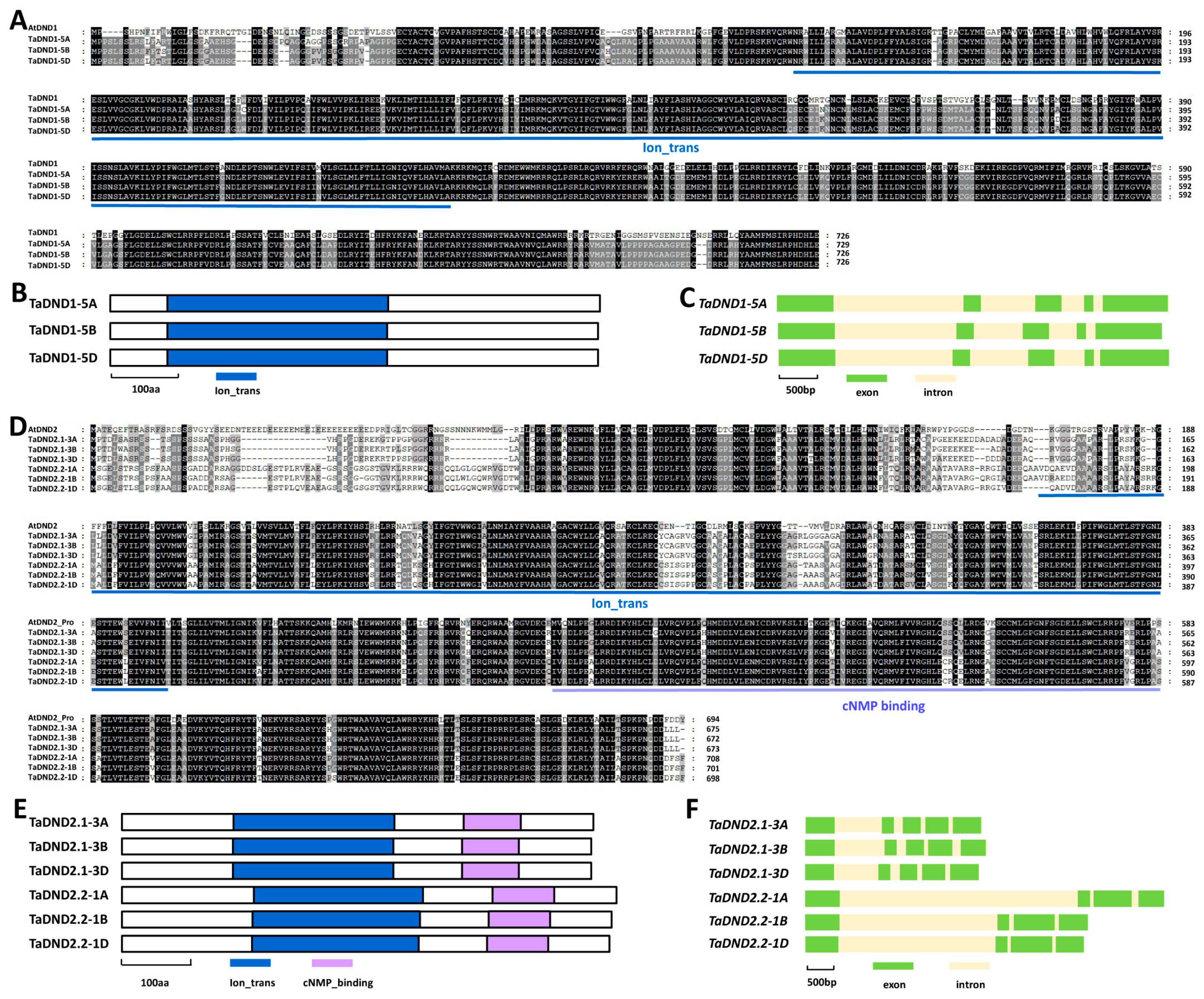
2.3. Homology-Based Identification of TaDND1 and TaDND2 in Bread Wheat
2.4. TaDND1 and TaDND2 Positively Contribute to the Wheat Susceptibility to B.g. tritici
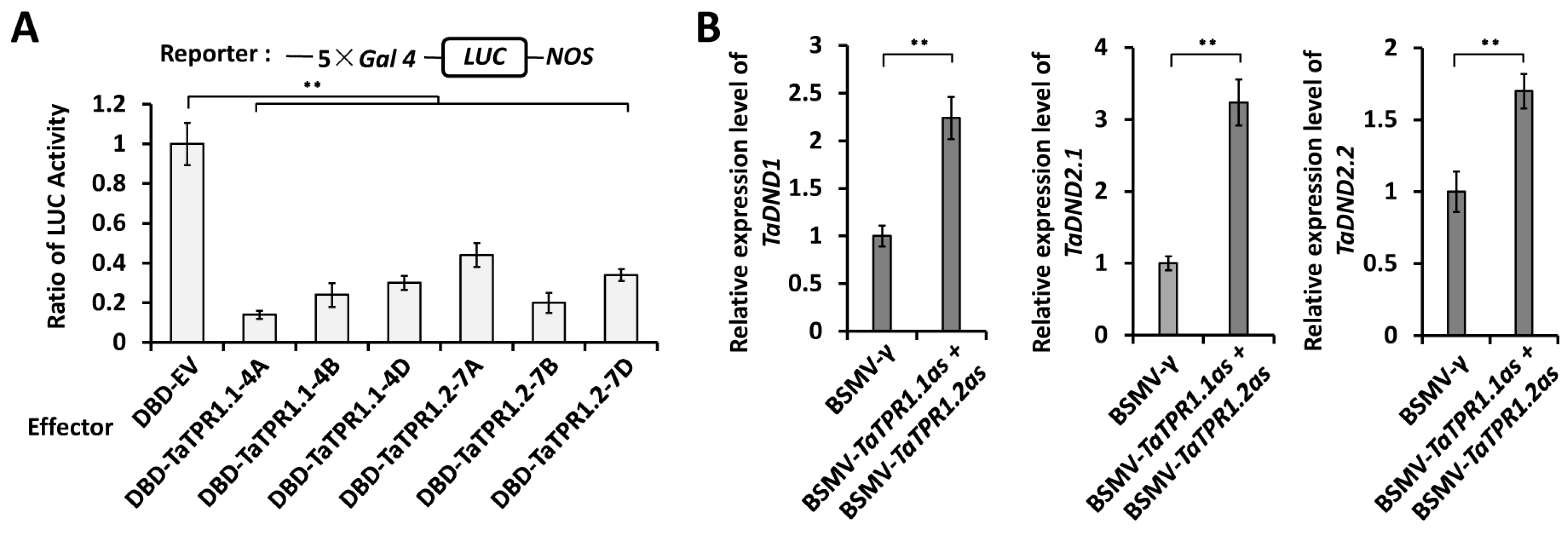
2.5. TaTPR1 Is a Transcriptional Corepressor and Suppresses the Expression of TaDND1 and TaDND2
3. Discussion
3.1. TaTPR1 Positively Regulates Wheat Powdery Mildew Immunity
3.2. TaDND1 and TaDND2 Contribute to Wheat Powdery Mildew Susceptibility
3.3. Transcriptional Corepressor TaTPR1 Suppresses Expression of TaDND1 and TaDND2
4. Materials and Methods
4.1. Plant and Pathogen Materials
4.2. Quantitative Reverse-Transcription PCR (qRT-PCR)
4.3. BSMV-Mediated Gene Silencing and Microcolony Index Analysis
4.4. Single-Cell Transient Gene Silencing/Overexpression Assays and Haustorium Index Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levy, A.A.; Feldman, M. Evolution and origin of bread wheat. Plant Cell 2022, 34, 2549–2567. [Google Scholar] [CrossRef]
- Lee, R. The outlook for population growth. Science 2011, 333, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Kusch, S.; Qian, J.; Loos, A.; Kümmel, F.; Spanu, P.D.; Panstruga, R. Long-term and rapid evolution in powdery mildew fungi. Mol. Ecol. 2023. [Google Scholar] [CrossRef]
- Mapuranga, J.; Chang, J.; Yang, W. Combating powdery mildew: Advances in molecular interactions between Blumeria graminis f. sp. tritici and wheat. Front. Plant Sci. 2022, 13, 1102908. [Google Scholar] [CrossRef] [PubMed]
- van Schie, C.C.; Takken, F.L. Susceptibility genes 101: How to be a good host. Annu. Rev. Phytopathol. 2014, 52, 551–581. [Google Scholar] [CrossRef]
- Li, M.; Yang, Z.; Chang, C. Susceptibility is new resistance: Wheat susceptibility genes and exploitation in resistance breeding. Agriculture 2022, 12, 1419. [Google Scholar] [CrossRef]
- Zhou, J.M.; Zhang, Y. Plant immunity: Danger perception and signaling. Cell 2020, 181, 978–989. [Google Scholar] [CrossRef]
- van der Burgh, A.M.; Joosten, M.H.A.J. Plant immunity: Thinking outside and inside the box. Trends Plant Sci. 2019, 24, 587–601. [Google Scholar] [CrossRef]
- Pruitt, R.N.; Gust, A.A.; Nürnberger, T. Plant immunity unified. Nat. Plants 2021, 7, 382–383. [Google Scholar] [CrossRef]
- Saijo, Y.; Loo, E.P.; Yasuda, S. Pattern recognition receptors and signaling in plant-microbe interactions. Plant J. 2018, 93, 592–613. [Google Scholar] [CrossRef]
- Li, L.; Yu, Y.; Zhou, Z.; Zhou, J.M. Plant pattern-recognition receptors controlling innate immunity. Sci. China Life Sci. 2016, 59, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Couto, D.; Zipfel, C. Regulation of pattern recognition receptor signaling in plants. Nat. Rev. Immunol. 2016, 16, 537–552. [Google Scholar] [CrossRef]
- Bjornson, M.; Pimprikar, P.; Nürnberger, T.; Zipfel, C. The transcriptional landscape of Arabidopsis thaliana pattern-triggered immunity. Nat. Plants 2021, 7, 579–586. [Google Scholar] [CrossRef]
- Yu, X.; Feng, B.; He, P.; Shan, L. From chaos to harmony: Responses and signaling upon microbial pattern recognition. Annu. Rev. Phytopathol. 2017, 55, 109–137. [Google Scholar] [CrossRef] [PubMed]
- Dangl, J.L.; Horvath, D.M.; Staskawicz, B.J. Pivoting the plant immune system from dissection to deployment. Science 2013, 341, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.G.; Vance, R.E.; Dangl, J.L. Intracellular innate immune surveillance devices in plants and animals. Science 2016, 354, 6316. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Tsuda, K.; Parker, J.E. Effector-triggered immunity: From pathogen perception to robust defense. Annu. Rev. Plant Biol. 2015, 66, 487–503. [Google Scholar] [CrossRef]
- Adachi, H.; Tsuda, K. Convergence of cell-surface and intracellular immune receptor signalling. New Phytol. 2019, 221, 1676–1678. [Google Scholar] [CrossRef]
- Birkenbihl, R.P.; Liu, S.; Somssich, I.E. Transcriptional events defining plant immune responses. Curr. Opin. Plant Biol. 2017, 38, 1–9. [Google Scholar] [CrossRef]
- Tsuda, K.; Somssich, I. Transcriptional networks in plant immunity. New Phytol. 2015, 206, 932–947. [Google Scholar] [CrossRef]
- Szemenyei, H.; Hannon, M.; Long, J.A. TOPLESS mediates auxin-dependent transcriptional repression during Arabidopsis embryogenesis. Science 2008, 319, 1384–1386. [Google Scholar] [CrossRef]
- Kim, H.; Shim, D.; Moon, S.; Lee, J.; Bae, W.; Choi, H.; Kim, K.; Ryu, H. Transcriptional network regulation of the brassinosteroid signaling pathway by the BES1-TPL-HDA19 co-repressor complex. Planta 2019, 250, 1371–1377. [Google Scholar] [CrossRef]
- Saini, R.; Nandi, A.K. TOPLESS in the regulation of plant immunity. Plant Mol. Biol. 2022, 109, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Xu, F.; Zhang, Y.; Cheng, Y.T.; Wiermer, M.; Li, X.; Zhang, Y. Arabidopsis resistance protein SNC1 activates immune responses through association with a transcriptional corepressor. Proc. Natl. Acad. Sci. USA 2010, 107, 13960–13965. [Google Scholar] [CrossRef] [PubMed]
- Griebel, T.; Lapin, D.; Locci, F.; Kracher, B.; Bautor, J.; Concia, L.; Benhamed, M.; Parker, J.E. Arabidopsis Topless-related 1 mitigates physiological damage and growth penalties of induced immunity. New Phytol. 2023, 239, 1404–1419. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, G.; Lal, N.K.; Nagalakshmi, U.; Li, Y.; Zheng, W.; Huang, P.J.; Branon, T.C.; Ting, A.Y.; Walley, J.W.; et al. TurboID-based proximity labeling reveals that UBR7 is a regulator of N NLR immune receptor-mediated immunity. Nat. Commun. 2019, 10, 3252. [Google Scholar] [CrossRef] [PubMed]
- Clough, S.J.; Fengler, K.A.; Yu, I.C.; Lippok, B.; Smith, R.K., Jr.; Bent, A.F. The Arabidopsis dnd1 “defense, no death” gene encodes a mutated cyclic nucleotide-gated ion channel. Proc. Natl. Acad. Sci. USA 2000, 97, 9323–9328. [Google Scholar] [CrossRef] [PubMed]
- Jurkowski, G.I.; Smith, R.K., Jr.; Yu, I.C.; Ham, J.H.; Sharma, S.B.; Klessig, D.F.; Fengler, K.A.; Bent, A.F. Arabidopsis DND2, a second cyclic nucleotide-gated ion channel gene for which mutation causes the “defense, no death” phenotype. Mol. Plant Microbe Interact. 2004, 17, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Hou, C.; Ren, Z.; Wang, C.; Zhao, F.; Dahlbeck, D.; Hu, S.; Zhang, L.; Niu, Q.; Li, L.; et al. A calmodulin-gated calcium channel links pathogen patterns to plant immunity. Nature 2019, 572, 131–135. [Google Scholar] [CrossRef]
- Sun, K.; van Tuinen, A.; van Kan, J.A.L.; Wolters, A.A.; Jacobsen, E.; Visser, R.G.F.; Bai, Y. Silencing of DND1 in potato and tomato impedes conidial germination, attachment and hyphal growth of Botrytis cinerea. BMC Plant Biol. 2017, 17, 235. [Google Scholar] [CrossRef]
- Sun, K.; Wolters, A.M.; Loonen, A.E.; Huibers, R.P.; van der Vlugt, R.; Goverse, A.; Jacobsen, E.; Visser, R.G.; Bai, Y. Down-regulation of Arabidopsis DND1 orthologs in potato and tomato leads to broad-spectrum resistance to late blight and powdery mildew. Transgenic Res. 2016, 25, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Wolters, A.M.; Vossen, J.H.; Rouwet, M.E.; Loonen, A.E.; Jacobsen, E.; Visser, R.G.; Bai, Y. Silencing of six susceptibility genes results in potato late blight resistance. Transgenic Res. 2016, 25, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhi, P.; Wang, X.; Fan, Q.; Chang, C. Wheat WD40-repeat protein TaHOS15 functions in a histone deacetylase complex to fine-tune defense responses to Blumeria graminis f.sp. tritici. J. Exp. Bot. 2019, 70, 255–268. [Google Scholar] [CrossRef]
- Zhi, P.; Kong, L.; Liu, J.; Zhang, X.; Wang, X.; Li, H.; Sun, M.; Li, Y.; Chang, C. Histone deacetylase TaHDT701 functions in TaHDA6-TaHOS15 complex to regulate wheat defense responses to Blumeria graminis f.sp. tritici. Int. J. Mol. Sci. 2020, 21, 2640. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yang, Z.; Liu, J.; Chang, C. Wheat susceptibility genes TaCAMTA2 and TaCAMTA3 negatively regulate post-penetration resistance against Blumeria graminis forma specialis tritici. Int. J. Mol. Sci. 2023, 24, 10224. [Google Scholar] [CrossRef]
- Moore, J.W.; Herrera-Foessel, S.; Lan, C.; Schnippenkoetter, W.; Ayliffe, M.; Huerta-Espino, J.; Lillemo, M.; Viccars, L.; Milne, R.; Periyannan, S.; et al. A recently evolved hexose transporter variant confers resistance to multiple pathogens in wheat. Nat. Genet. 2015, 47, 1494–1498. [Google Scholar] [CrossRef]
- Várallyay, E.; Giczey, G.; Burgyán, J. Virus-induced gene silencing of MLO genes induces powdery mildew resistance in Triticum aestivum. Arch. Virol. 2012, 157, 1345–1350. [Google Scholar] [CrossRef]
- Acevedo-Garcia, J.; Spencer, D.; Thieron, H.; Reinstädler, A.; Hammond-Kosack, K.; Phillips, A.L.; Panstruga, R. mlo-based powdery mildew resistance in hexaploid bread wheat generated by a non-transgenic TILLING approach. Plant Biotechnol. J. 2017, 15, 367–378. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, X.; Shan, Q.; Zhang, Y.; Liu, J.; Gao, C.; Qiu, J.L. Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat. Biotechnol. 2014, 32, 947–951. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bai, Y.; Wu, G.; Zou, S.; Chen, Y.; Gao, C.; Tang, D. Simultaneous modification of three homoeologs of TaEDR1 by genome editing enhances powdery mildew resistance in wheat. Plant J. 2017, 91, 714–724. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lin, D.; Zhang, Y.; Deng, M.; Chen, Y.; Lv, B.; Li, B.; Lei, Y.; Wang, Y.; Zhao, L.; et al. Genome-edited powdery mildew resistance in wheat without growth penalties. Nature 2022, 602, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhang, X.; Zhao, B.; Song, P.; Zhang, X.; Wang, B.; Li, Q. Wheat Class III Peroxidase TaPOD70 is a potential susceptibility factor negatively regulating wheat resistance to Blumeria graminis f. sp. tritici. Phytopathology 2023, 113, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Genger, R.K.; Jurkowski, G.I.; McDowell, J.M.; Lu, H.; Jung, H.W.; Greenberg, J.T.; Bent, A.F. Signaling pathways that regulate the enhanced disease resistance of Arabidopsis “defense, no death” mutants. Mol. Plant Microbe Interact. 2008, 21, 1285–1296. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.S.; Mukhtar, M.S.; Mansoor, S. Editing: Targeting susceptibility genes for plant disease resistance. Trends Biotechnol. 2018, 36, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Koseoglou, E.; van der Wolf, J.M.; Visser, R.; Bai, Y. Susceptibility reversed: Modified plant susceptibility genes for resistance to bacteria. Trends Plant Sci. 2022, 27, 69–79. [Google Scholar] [CrossRef]
- McCallum, C.M.; Comai, L.; Greene, E.A.; Henikoff, S. Targeting induced local lesions IN genomes (TILLING) for plant functional genomics. Plant Physiol. 2000, 123, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Kurowska, M.; Daszkowska-Golec, A.; Gruszka, D.; Marzec, M.; Szurman, M.; Szarejko, I.; Maluszynski, M. TILLING: A shortcut in functional genomics. J. Appl. Genet. 2011, 52, 371–390. [Google Scholar] [CrossRef]
- Manghwar, H.; Lindsey, K.; Zhang, X.; Jin, S. CRISPR/Cas system: Recent advances and future prospects for genome editing. Trends Plant Sci. 2019, 24, 1102–1125. [Google Scholar] [CrossRef]
- Schenke, D.; Cai, D. Applications of CRISPR/Cas to improve crop disease resistance: Beyond inactivation of susceptibility factors. iScience 2020, 23, 101478. [Google Scholar] [CrossRef]
- Gao, C. Genome engineering for crop improvement and future agriculture. Cell 2021, 184, 1621–1635. [Google Scholar] [CrossRef] [PubMed]
- Zhi, P.; Chang, C. Exploiting epigenetic variations for crop disease resistance improvement. Front. Plant Sci. 2021, 12, 692328. [Google Scholar] [CrossRef]
- Yang, Z.; Zhi, P.; Chang, C. Priming seeds for the future: Plant immune memory and application in crop protection. Front. Plant Sci. 2022, 13, 961840. [Google Scholar] [CrossRef]
- Khan, M.K.; Pandey, A.; Hamurcu, M.; Rajpal, V.R.; Vyhnanek, T.; Topal, A.; Raina, S.N.; Gezgin, S. Insight into the Boron Toxicity Stress-Responsive Genes in Boron-Tolerant Triticum dicoccum Shoots Using RNA Sequencing. Agronomy 2023, 13, 631. [Google Scholar] [CrossRef]
- Pandey, A.; Khan, M.K.; Hamurcu, M.; Brestic, M.; Topal, A.; Gezgin, S. Insight into the Root Transcriptome of a Boron-Tolerant Triticum zhukovskyi Genotype Grown under Boron Toxicity. Agronomy 2022, 12, 2421. [Google Scholar] [CrossRef]
- Liu, L.; Li, H.; Wang, X.; Chang, C. Transcription factor TaMYB30 activates wheat wax biosynthesis. Int. J. Mol. Sci. 2023, 24, 10235. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhi, P.; Gao, R.; Chen, W.; Chang, C. Wheat Transcriptional Corepressor TaTPR1 Suppresses Susceptibility Genes TaDND1/2 and Potentiates Post-Penetration Resistance against Blumeria graminis forma specialis tritici. Int. J. Mol. Sci. 2024, 25, 1695. https://doi.org/10.3390/ijms25031695
Zhi P, Gao R, Chen W, Chang C. Wheat Transcriptional Corepressor TaTPR1 Suppresses Susceptibility Genes TaDND1/2 and Potentiates Post-Penetration Resistance against Blumeria graminis forma specialis tritici. International Journal of Molecular Sciences. 2024; 25(3):1695. https://doi.org/10.3390/ijms25031695
Chicago/Turabian StyleZhi, Pengfei, Rongxin Gao, Wanzhen Chen, and Cheng Chang. 2024. "Wheat Transcriptional Corepressor TaTPR1 Suppresses Susceptibility Genes TaDND1/2 and Potentiates Post-Penetration Resistance against Blumeria graminis forma specialis tritici" International Journal of Molecular Sciences 25, no. 3: 1695. https://doi.org/10.3390/ijms25031695
APA StyleZhi, P., Gao, R., Chen, W., & Chang, C. (2024). Wheat Transcriptional Corepressor TaTPR1 Suppresses Susceptibility Genes TaDND1/2 and Potentiates Post-Penetration Resistance against Blumeria graminis forma specialis tritici. International Journal of Molecular Sciences, 25(3), 1695. https://doi.org/10.3390/ijms25031695




