Associations between Cerebrovascular Function and the Expression of Genes Related to Endothelial Function in Hormonal Migraine
Abstract
1. Introduction
2. Results
3. Discussion
4. Methods and Materials
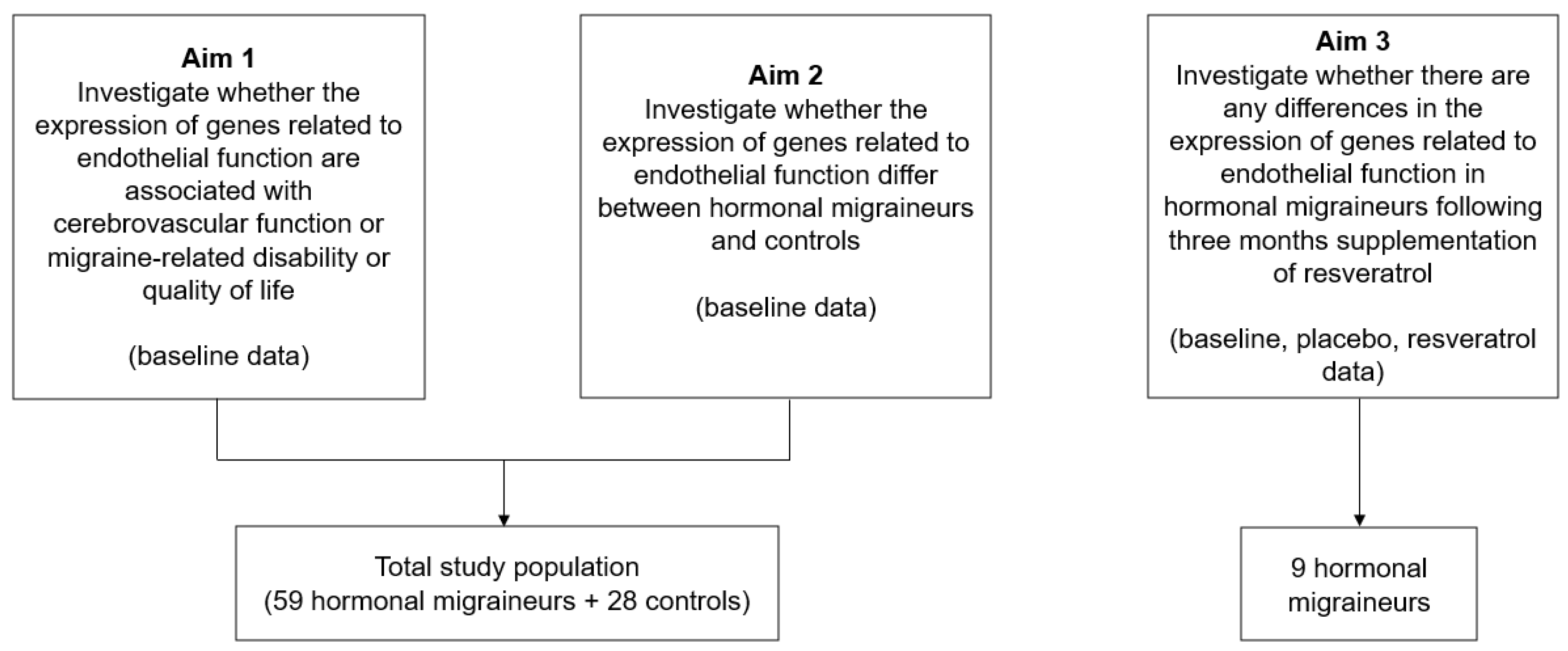
4.1. Study Design and Population
4.2. Study Outcomes
- Cerebrovascular Function
- b.
- Migraine Disability and Quality of Life
- c.
- Gene Expression Analysis
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sacco, S.; Ricci, S.; Degan, D.; Carolei, A. Migraine in women: The role of hormones and their impact on vascular diseases. J. Headache Pain 2012, 13, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Macgregor, E.A. Menstrual migraine: Therapeutic approaches. Ther. Adv. Neurol. Disord. 2009, 2, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Krause, D.N.; Duckles, S.P.; Pelligrino, D.A. Influence of sex steroid hormones on cerebrovascular function. J. Appl. Physiol. 2006, 101, 1252–1261. [Google Scholar] [CrossRef]
- Novella, S.; Dantas, A.P.; Segarra, G.; Medina, P.; Hermenegildo, C. Vascular Aging in Women: Is Estrogen the Fountain of Youth? Front. Physiol. 2012, 3, 165. [Google Scholar] [CrossRef]
- Dzator, J.S.A.; Howe, P.R.C.; Griffiths, L.R.; Coupland, K.G.; Wong, R.H.X. Cerebrovascular Function in Hormonal Migraine: An Exploratory Study. Front. Neurol. 2021, 12, 694980. [Google Scholar] [CrossRef]
- Tietjen, G.E.; Herial, N.A.; White, L.; Utley, C.; Kosmyna, J.M.; Khuder, S.A. Migraine and biomarkers of endothelial activation in young women. Stroke 2009, 40, 2977–2982. [Google Scholar] [CrossRef] [PubMed]
- Dzator, J.S.A.; Coupland, K.G.; Howe, P.R.C. Exploring the Effects of Resveratrol Supplementation on Cerebrovascular Function in Hormonal Migraineurs: A Pilot Study. IBRO Neurosci. Rep. 2023, in press. [CrossRef] [PubMed]
- Li, H.; Xia, N.; Hasselwander, S.; Daiber, A. Resveratrol and Vascular Function. Int. J. Mol. Sci. 2019, 20, 2155. [Google Scholar] [CrossRef]
- Rodriguez-Acevedo, A.J.; Smith, R.A.; Roy, B.; Sutherland, H.; Lea, R.A.; Frith, A.; MacGregor, E.A.; Griffiths, L.R. Genetic association and gene expression studies suggest that genetic variants in the SYNE1 and TNF genes are related to menstrual migraine. J. Headache Pain 2014, 15, 62. [Google Scholar] [CrossRef] [PubMed]
- Wattiez, A.S.; Sowers, L.P.; Russo, A.F. Calcitonin gene-related peptide (CGRP): Role in migraine pathophysiology and therapeutic targeting. Expert. Opin. Ther. Targets 2020, 24, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Russell, F.A.; King, R.; Smillie, S.J.; Kodji, X.; Brain, S.D. Calcitonin gene-related peptide: Physiology and pathophysiology. Physiol. Rev. 2014, 94, 1099–1142. [Google Scholar] [CrossRef]
- Fila, M.; Sobczuk, A.; Pawlowska, E.; Blasiak, J. Epigenetic Connection of the Calcitonin Gene-Related Peptide and Its Potential in Migraine. Int. J. Mol. Sci. 2022, 23, 6151. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Mancilla, E.; Villalón, C.M.; MaassenVanDenBrink, A. CGRP inhibitors for migraine prophylaxis: A safety review. Expert. Opin. Drug Saf. 2020, 19, 1237–1250. [Google Scholar] [CrossRef] [PubMed]
- Urits, I.; Jones, M.R.; Gress, K.; Charipova, K.; Fiocchi, J.; Kaye, A.D.; Viswanath, O. CGRP Antagonists for the Treatment of Chronic Migraines: A Comprehensive Review. Curr. Pain Headache Rep. 2019, 23, 29. [Google Scholar] [CrossRef] [PubMed]
- Visočnik, D.; Žvan, B.; Zaletel, M.; Zupan, M. αCGRP-Induced Changes in Cerebral and Systemic Circulation; A TCD Study. Front. Neurol. 2020, 11, 578103. [Google Scholar] [CrossRef]
- Schifter, S.; Krusell, L.R.; Sehested, J. Normal Serum Levels of Calcitonin Gene-Related Peptide (CGRP) in Mild to Moderate Essential Hypertension. Am. J. Hypertens. 1991, 4, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Masuda, A.; Shimamoto, K.; Mori, Y.; Nakagawa, M.; Ura, N.; Iimura, O. Plasma calcitonin gene-related peptide levels in patients with various hypertensive diseases. J. Hypertens. 1992, 10, 1499–1504. [Google Scholar] [CrossRef]
- Rijnberg, F.M.; Hazekamp, M.G.; Wentzel, J.J.; de Koning, P.J.H.; Westenberg, J.J.M.; Jongbloed, M.R.M.; Blom, N.A.; Roest, A.A.W. Energetics of Blood Flow in Cardiovascular Disease: Concept and Clinical Implications of Adverse Energetics in Patients with a Fontan Circulation. Circulation 2018, 137, 2393–2407. [Google Scholar] [CrossRef]
- Dunne, F.P.; Barry, D.G.; Ferriss, J.B.; Grealy, G.; Murphy, D. Changes in blood pressure during the normal menstrual cycle. Clin. Sci. 1991, 81, 515–518. [Google Scholar] [CrossRef]
- Choi, H.M.; Stebbins, C.L.; Nho, H.; Kim, M.S.; Chang, M.J.; Kim, J.K. Effects of Ovarian Cycle on Hemodynamic Responses during Dynamic Exercise in Sedentary Women. Korean J. Physiol. Pharmacol. 2013, 17, 499–503. [Google Scholar] [CrossRef]
- Greenberg, G.; Imeson, J.D.; Thompson, S.G.; Meade, T.W. Blood pressure and the menstrual cycle. Br. J. Obs. Gynaecol. 1985, 92, 1010–1014. [Google Scholar] [CrossRef]
- Wang, Y.F.; Wang, S.J. Hypertension and Migraine: Time to Revisit the Evidence. Curr. Pain Headache Rep. 2021, 25, 58. [Google Scholar] [CrossRef]
- Zhang, J.; Mao, Y.; Li, Y.; Zhao, K.; Xie, Q.; Wang, K.; Shi, J. Association between migraine or severe headache and hypertension among US adults: A cross-sectional study. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 350–358. [Google Scholar] [CrossRef]
- Jackson, J.L.; Kuriyama, A.; Kuwatsuka, Y.; Nickoloff, S.; Storch, D.; Jackson, W.; Zhang, Z.J.; Hayashino, Y. Beta-blockers for the prevention of headache in adults, a systematic review and meta-analysis. PLoS ONE 2019, 14, e0212785. [Google Scholar] [CrossRef]
- Carcel, C.; Haghdoost, F.; Shen, J.; Nanda, P.; Bai, Y.; Atkins, E.; Torii-Yoshimura, T.; Clough, A.J.; Davies, L.; Cordato, D.; et al. The effect of blood pressure lowering medications on the prevention of episodic migraine: A systematic review and meta-analysis. Cephalalgia 2023, 43, 03331024231183166. [Google Scholar] [CrossRef]
- Niu, G.; Chen, X. Vascular endothelial growth factor as an anti-angiogenic target for cancer therapy. Curr. Drug Targets 2010, 11, 1000–1017. [Google Scholar] [CrossRef]
- Jozkowicz, A.; Cooke, J.P.; Guevara, I.; Huk, I.; Funovics, P.; Pachinger, O.; Weidinger, F.; Dulak, J. Genetic augmentation of nitric oxide synthase increases the vascular generation of VEGF. Cardiovasc. Res. 2001, 51, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Mäki-Petäjä, K.M.; McGeoch, A.; Yang, L.L.; Hubsch, A.; McEniery, C.M.; Meyer, P.A.R.; Mir, F.; Gajendragadkar, P.; Ramenatte, N.; Anandappa, G.; et al. Mechanisms Underlying Vascular Endothelial Growth Factor Receptor Inhibition-Induced Hypertension: The HYPAZ Trial. Hypertension 2021, 77, 1591–1599. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Singhi, E.K.; Arroyo, J.P.; Ikizler, T.A.; Gould, E.R.; Brown, J.; Beckman, J.A.; Harrison, D.G.; Moslehi, J. Mechanisms of VEGF (Vascular Endothelial Growth Factor) Inhibitor-Associated Hypertension and Vascular Disease. Hypertension 2018, 71, e1–e8. [Google Scholar] [CrossRef] [PubMed]
- Troncoso, M.F.; Ortiz-Quintero, J.; Garrido-Moreno, V.; Sanhueza-Olivares, F.; Guerrero-Moncayo, A.; Chiong, M.; Castro, P.F.; García, L.; Gabrielli, L.; Corbalán, R.; et al. VCAM-1 as a predictor biomarker in cardiovascular disease. Biochim. Et Biophys. Acta (BBA)—Mol. Basis Dis. 2021, 1867, 166170. [Google Scholar] [CrossRef] [PubMed]
- de Faria, A.P.; Ritter, A.M.; Sabbatini, A.R.; Corrêa, N.B.; Brunelli, V.; Modolo, R.; Moreno, H. Deregulation of Soluble Adhesion Molecules in Resistant Hypertension and Its Role in Cardiovascular Remodeling. Circ. J. 2016, 80, 1196–1201. [Google Scholar] [CrossRef]
- Kilic, I.D.; Findikoglu, G.; Alihanoglu, Y.I.; Yildiz, B.S.; Uslu, S.; Rota, S.; Evrengul, H. Circulating adhesion molecules and arterial stiffness. Cardiovasc. J. Afr. 2015, 26, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Szeghy, R.E.; Stute, N.L.; Province, V.M.; Augenreich, M.A.; Stickford, J.L.; Stickford, A.S.L.; Ratchford, S.M. Six-month longitudinal tracking of arterial stiffness and blood pressure in young adults following SARS-CoV-2 infection. J. Appl. Physiol. 2022, 132, 1297–1309. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Bakker, S.J.L.; Dullaart, R.P.F. Soluble Vascular Cell Adhesion Molecules May be Protective of Future Cardiovascular Disease Risk: Findings from the PREVEND Prospective Cohort Study. J. Atheroscler. Thromb. 2017, 24, 804–818. [Google Scholar] [CrossRef]
- Ulyanova, T.; Scott, L.M.; Priestley, G.V.; Jiang, Y.; Nakamoto, B.; Koni, P.A.; Papayannopoulou, T. VCAM-1 expression in adult hematopoietic and nonhematopoietic cells is controlled by tissue-inductive signals and reflects their developmental origin. Blood 2005, 106, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Hsue, P.Y.; Scherzer, R.; Grunfeld, C.; Imboden, J.; Wu, Y.; Del Puerto, G.; Nitta, E.; Shigenaga, J.; Schnell Heringer, A.; Ganz, P.; et al. Depletion of B-cells with rituximab improves endothelial function and reduces inflammation among individuals with rheumatoid arthritis. J. Am. Heart Assoc. 2014, 3, e001267. [Google Scholar] [CrossRef] [PubMed]
- Henein, M.Y.; Vancheri, S.; Longo, G.; Vancheri, F. The Role of Inflammation in Cardiovascular Disease. Int. J. Mol. Sci. 2022, 23, 12906. [Google Scholar] [CrossRef] [PubMed]
- Mueller, L.; Gupta, A.K.; Stein, T.P. Deficiency of tumor necrosis factor alpha in a subclass of menstrual migraineurs. Headache 2001, 41, 129–137. [Google Scholar] [CrossRef]
- Ibrahimi, K.; Vermeersch, S.; Frederiks, P.; Geldhof, V.; Draulans, C.; Buntinx, L.; Lesaffre, E.; MaassenVanDenBrink, A.; de Hoon, J. The influence of migraine and female hormones on capsaicin-induced dermal blood flow. Cephalalgia 2017, 37, 1164–1172. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Robinson, J.F.; Khan, H.M.R.; Carter, D.E.; McKinney, J.; Miskie, B.A.; Hegele, R.A. Optimizing RNA extraction yield from whole blood for microarray gene expression analysis. Clin. Biochem. 2004, 37, 741–744. [Google Scholar] [CrossRef]
- Brkljacić, J.; Tanić, N.; Milutinović, D.V.; Elaković, I.; Jovanović, S.M.; Perisić, T.; Dundjerski, J.; Matić, G. Validation of endogenous controls for gene expression studies in peripheral lymphocytes from war veterans with and without PTSD. BMC Mol. Biol. 2010, 11, 26. [Google Scholar] [CrossRef]
- Xiao, J.; Li, X.; Liu, J.; Fan, X.; Lei, H.; Li, C. Identification of reference genes in blood before and after entering the plateau for SYBR green RT-qPCR studies. PeerJ 2017, 5, e3726. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dzator, J.S.A.; Howe, P.R.C.; Coupland, K.G.; Wong, R.H.X. A Randomised, Double-Blind, Placebo-Controlled Crossover Trial of Resveratrol Supplementation for Prophylaxis of Hormonal Migraine. Nutrients 2022, 14, 1763. [Google Scholar] [CrossRef]
- Olesen, J. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef]
- Yang, M.; Rendas-Baum, R.; Varon, S.F.; Kosinski, M. Validation of the Headache Impact Test (HIT-6™) across episodic and chronic migraine. Cephalalgia 2011, 31, 357–367. [Google Scholar] [CrossRef]
- Stewart, W.F.; Lipton, R.B.; Kolodner, K.B.; Sawyer, J.; Lee, C.; Liberman, J.N. Validity of the Migraine Disability Assessment (MIDAS) score in comparison to a diary-based measure in a population sample of migraine sufferers. Pain 2000, 88, 41–52. [Google Scholar] [CrossRef]
- Martin, B.C.; Pathak, D.S.; Sharfman, M.I.; Adelman, J.U.; Taylor, F.; Kwong, W.J.; Jhingran, P. Validity and reliability of the migraine-specific quality of life questionnaire (MSQ Version 2.1). Headache 2000, 40, 204–215. [Google Scholar] [CrossRef]
- Rodríguez-Osorio, X.; Sobrino, T.; Brea, D.; Martínez, F.; Castillo, J.; Leira, R. Endothelial progenitor cells: A new key for endothelial dysfunction in migraine. Neurology 2012, 79, 474–479. [Google Scholar] [CrossRef]
- Fidan, I.; Yüksel, S.; Ýmir, T.; İrkeç, C.; Aksakal, F.N. The importance of cytokines, chemokines and nitric oxide in pathophysiology of migraine. J. Neuroimmunol. 2006, 171, 184–188. [Google Scholar] [CrossRef]
- Kursun, O.; Yemisci, M.; van den Maagdenberg, A.M.J.M.; Karatas, H. Migraine and neuroinflammation: The inflammasome perspective. J. Headache Pain 2021, 22, 55. [Google Scholar] [CrossRef] [PubMed]
- Mason, B.N.; Russo, A.F. Vascular Contributions to Migraine: Time to Revisit? Front. Cell. Neurosci. 2018, 12, 233. [Google Scholar] [CrossRef]
- Sabri, M.R.; Dehghan, B.; Yaghini, O.; Nasiri, J.; Mansourian, M.; Khalifehsoltani, S. Endothelial dysfunction state in migraine headache and neutrally mediated syncope in children and young adults. J. Res. Med. Sci. 2015, 20, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhang, X.; Zhao, D.X.; Yin, J.; Hu, G.; Evans, C.E.; Zhao, Y.Y. Endothelial Hypoxia-Inducible Factor-1α Is Required for Vascular Repair and Resolution of Inflammatory Lung Injury through Forkhead Box Protein M1. Am. J. Pathol. 2019, 189, 1664–1679. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Park, Y.; Wu, J.; Chen, X.; Lee, S.; Yang, J.; Dellsperger, K.C.; Zhang, C. Role of TNF-α in vascular dysfunction. Clin. Sci. 2009, 116, 219–230. [Google Scholar] [CrossRef]
- Galea, J.; Armstrong, J.; Gadsdon, P.; Holden, H.; Francis, S.E.; Holt, C.M. Interleukin-1β in Coronary Arteries of Patients with Ischemic Heart Disease. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 1000–1006. [Google Scholar] [CrossRef]
- Böhm, F.; Pernow, J. The importance of endothelin-1 for vascular dysfunction in cardiovascular disease. Cardiovasc. Res. 2007, 76, 8–18. [Google Scholar] [CrossRef]
- Didion, S.P. Cellular and Oxidative Mechanisms Associated with Interleukin-6 Signaling in the Vasculature. Int. J. Mol. Sci. 2017, 18, 2563. [Google Scholar] [CrossRef]

| Hormonal Migraineurs (N = 59) | Controls (N = 28) | p Value | |
|---|---|---|---|
| Age (years) a | 38.63 + 1.05 | 35.89 + 1.83 | 0.208 |
| Body mass index (kg/m2) a | 25.58 + 0.63 | 25.12 + 1.18 | 0.443 |
| Systolic blood pressure (mmHg) b | 116.35 + 1.52 | 114.61 + 2.52 | 0.536 |
| Diastolic blood pressure (mmHg) b | 70.75 + 1.11 | 67.68 + 1.56 | 0.116 |
| Primer Name | Primer Sequence (5′-3′) | Transcript | Exon |
|---|---|---|---|
| CALCA Forward | TCAGCATCTTGGTCCTGTTG | NM_001741.3 | 2 |
| CALCA Reverse | CTGCACATAGTCCTGCACCA | NM_001741.3 | 3 |
| EDN1 Forward | TGGGAAAAAGTGTATTTATCAGCA | NM_001955.5 | 4 |
| EDN1 Reverse | TTTGACGCTGTTTCTCATGG | NM_001955.5 | 5 |
| HIF1A Forward | GCTTGGTGCTGATTTGTGAA | NM_001530.4 | 6 |
| HIF1A Reverse | TTCTGGCTCATATCCCATCA | NM_001530.4 | 7 |
| ICAM1 Forward | CTTGAGGGCACCTACCTCTG | NM_000201.3 | 6 |
| ICAM1 Reverse | CATTATGACTGCGGCTGCTA | NM_000201.3 | 7 |
| IL1B Forward | CTGTCCTGCGTGTTGAAAGA | NM_000576.3 | 6 |
| IL1B Reverse | ACTGGGCAGACTCAAATTCC | NM_000576.3 | 7 |
| IL6 Forward | GGCTGAAAAAGATGGATGCT | NM_000600.5 | 3 |
| IL6 Reverse | GCTCTGGCTTGTTCCTCACT | NM_000600.5 | 4 |
| NOS3 Forward | TGTCTGCATGGACCTGGATA | NM_000603.5 | 10 |
| NOS3 Reverse | CACGATGGTGACTTTGGCTA | NM_000603.5 | 11 |
| VCAM1 Forward | ATGGAATTCGAACCCAAACA | NM_001078.4 | 6 |
| VCAM1 Reverse | CCTGGCTCAAGCATGTCATA | NM_001078.4 | 7 |
| VEGF Forward | CCCACTGAGGAGTCCAACAT | NM_001025366.3 | 3 |
| VEGF Reverse | TGCATTCACATTTGTTGTGC | NM_001025366.3 | 4 |
| TNF Forward | GACAAGCCTGTAGCCCATGT | NM_000594.4 | 3 |
| TNF Reverse | GAGGTACAGGCCCTCTGATG | NM_000594.4 | 4 |
| GAPDH Forward | CGACCACTTTGTCAAGCTCA | NM_002046.7 | 8 |
| GAPDH Reverse | GGTGGTCCAGGGGTCTTACT | NM_002046.7 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dzator, J.S.A.; Smith, R.A.; Coupland, K.G.; Howe, P.R.C.; Griffiths, L.R. Associations between Cerebrovascular Function and the Expression of Genes Related to Endothelial Function in Hormonal Migraine. Int. J. Mol. Sci. 2024, 25, 1694. https://doi.org/10.3390/ijms25031694
Dzator JSA, Smith RA, Coupland KG, Howe PRC, Griffiths LR. Associations between Cerebrovascular Function and the Expression of Genes Related to Endothelial Function in Hormonal Migraine. International Journal of Molecular Sciences. 2024; 25(3):1694. https://doi.org/10.3390/ijms25031694
Chicago/Turabian StyleDzator, Jemima S. A., Robert A. Smith, Kirsten G. Coupland, Peter R. C. Howe, and Lyn R. Griffiths. 2024. "Associations between Cerebrovascular Function and the Expression of Genes Related to Endothelial Function in Hormonal Migraine" International Journal of Molecular Sciences 25, no. 3: 1694. https://doi.org/10.3390/ijms25031694
APA StyleDzator, J. S. A., Smith, R. A., Coupland, K. G., Howe, P. R. C., & Griffiths, L. R. (2024). Associations between Cerebrovascular Function and the Expression of Genes Related to Endothelial Function in Hormonal Migraine. International Journal of Molecular Sciences, 25(3), 1694. https://doi.org/10.3390/ijms25031694








