Mesenchymal Stem Cells from Familial Alzheimer’s Patients Express MicroRNA Differently
Abstract
1. Introduction
2. Results
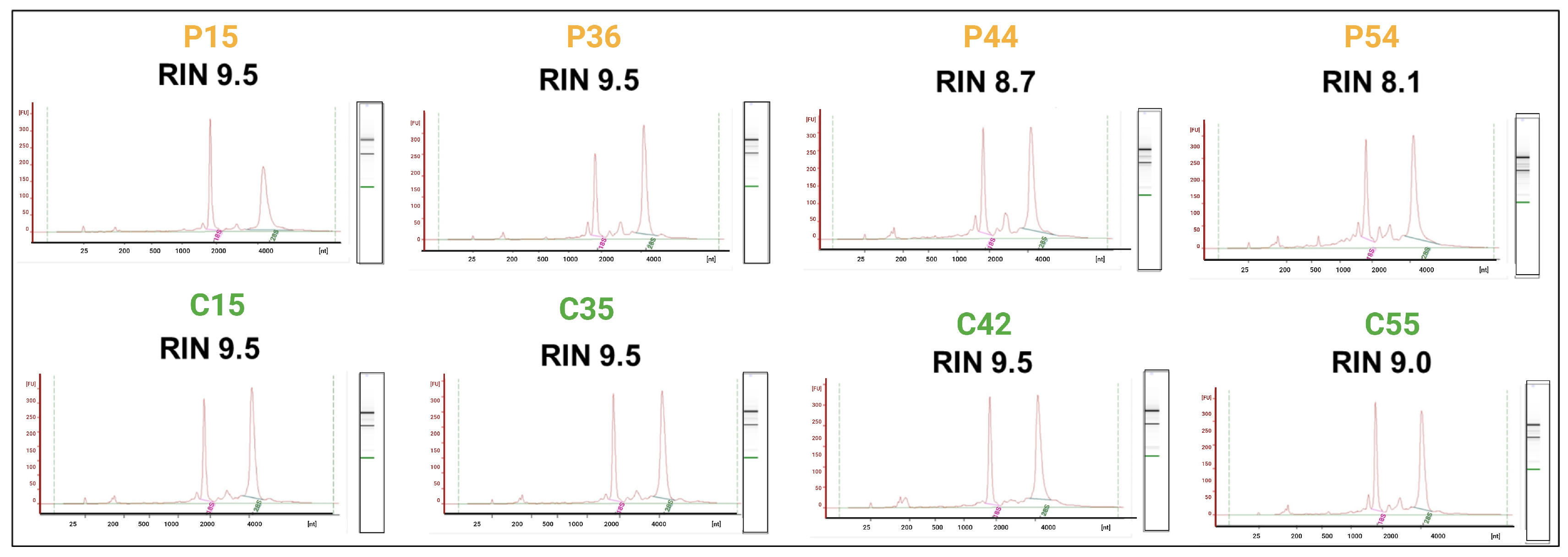
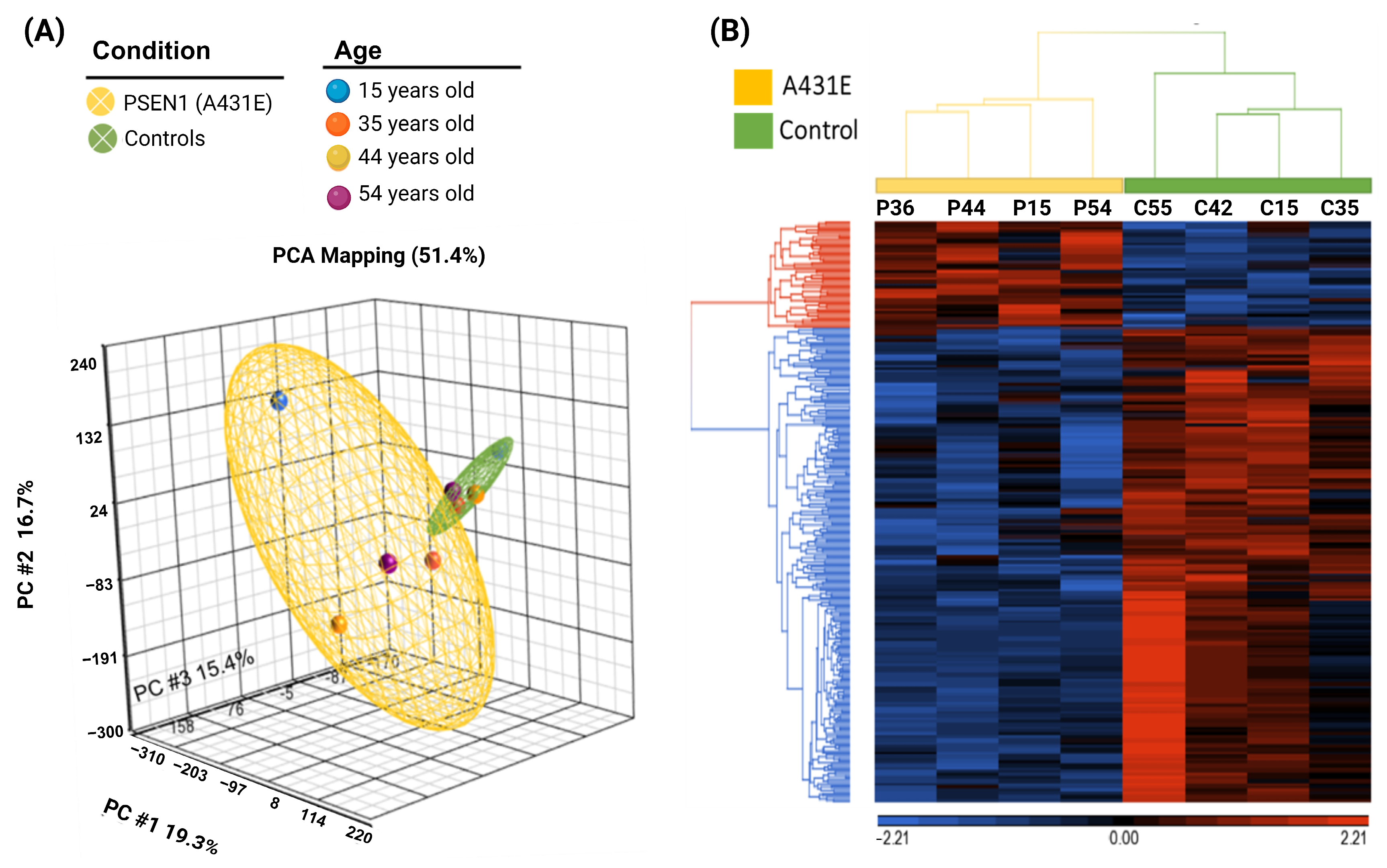
2.1. RNA Quality and Integrity
2.2. Altered Transcriptomic Profile of MSCs Derived from PSEN1(A431E) Mutation Carriers by Disease Stage
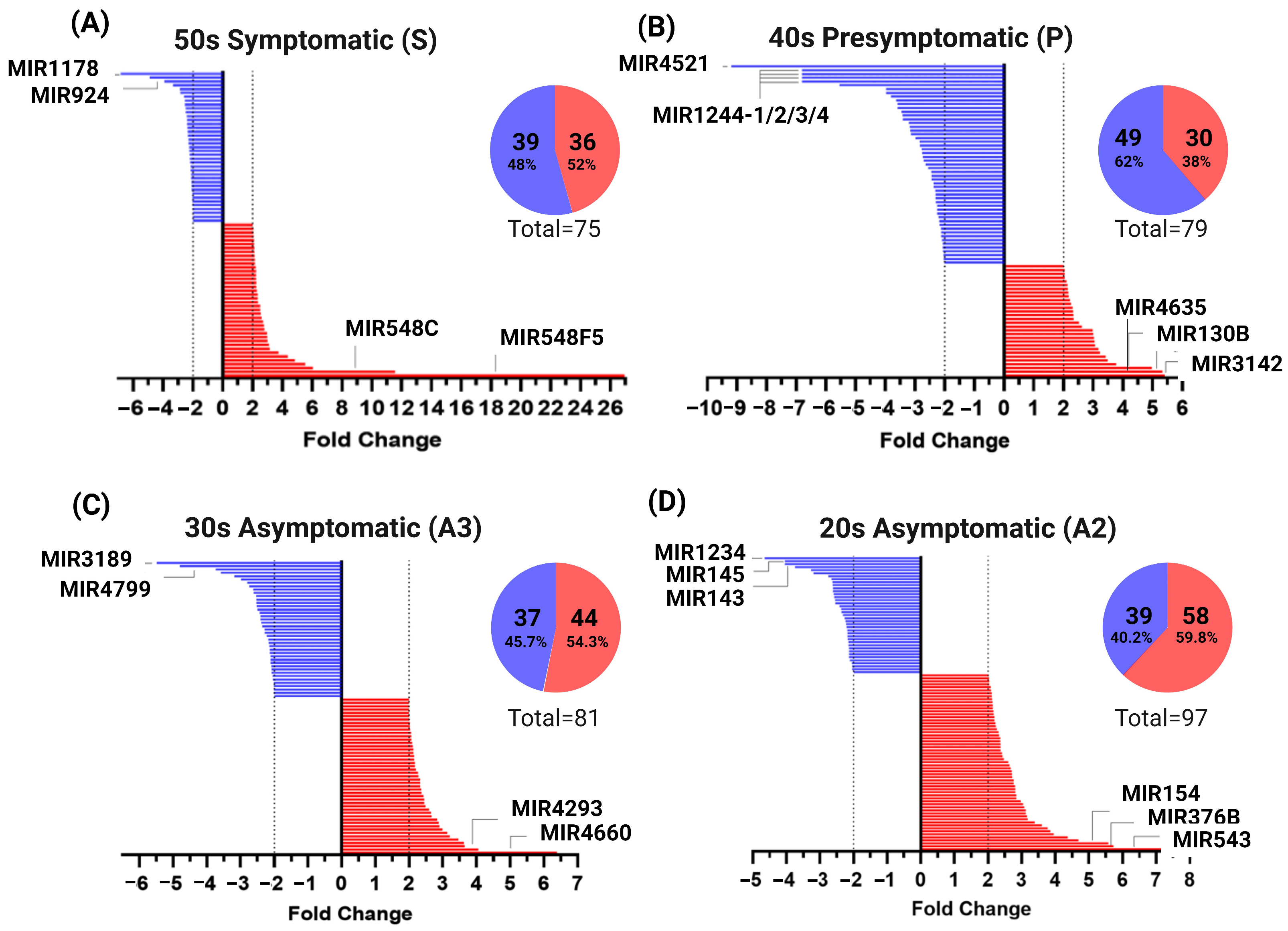
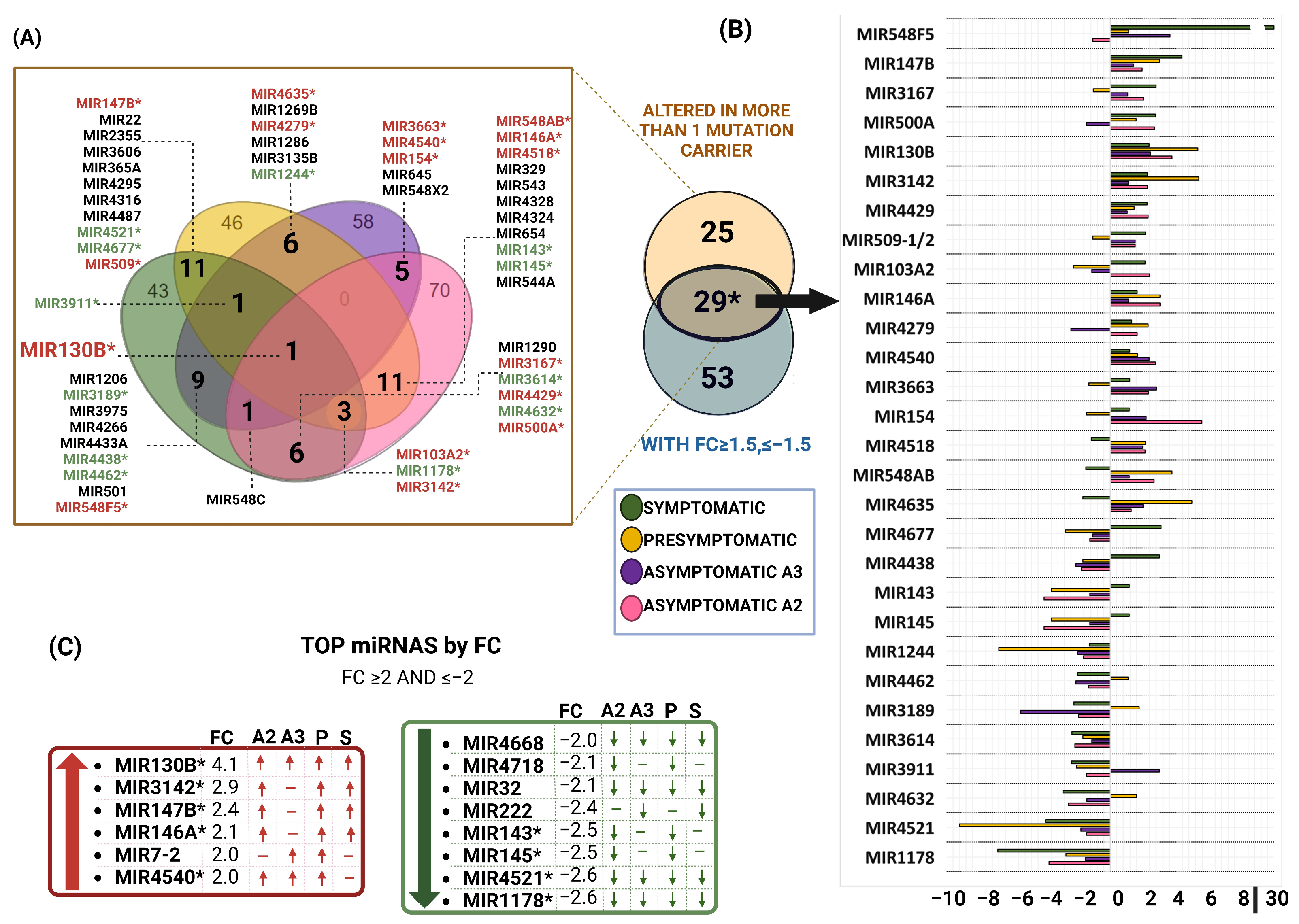
2.3. miRNAs Profile of MSCs Derived from PSEN1(A431E) Mutation Carriers by Disease Stage
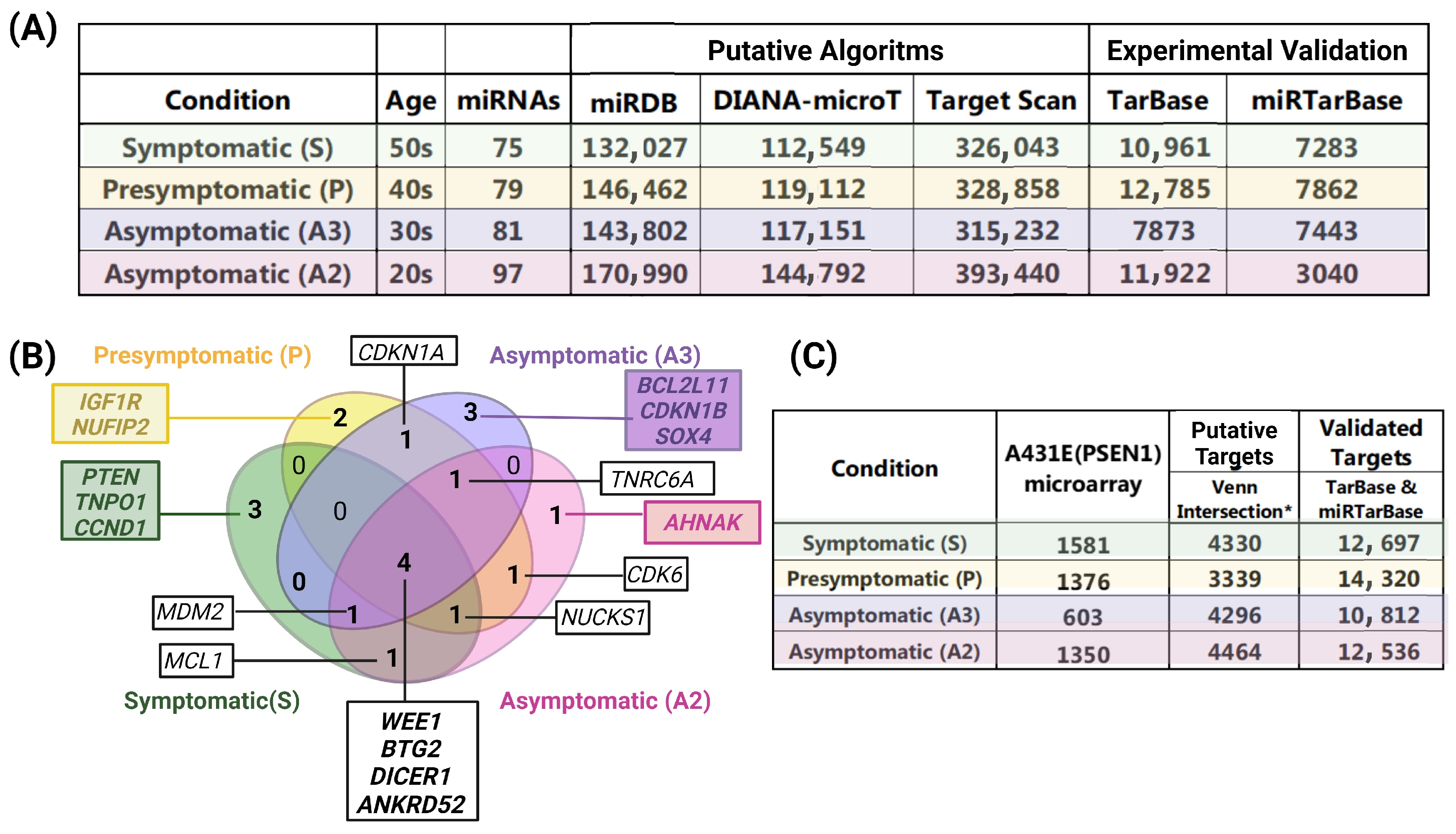
2.4. Target Interactions and Genes Affected by Altered miRNAs in Individuals with the PSEN1(A431E) Mutation According to the Stage of the Disease
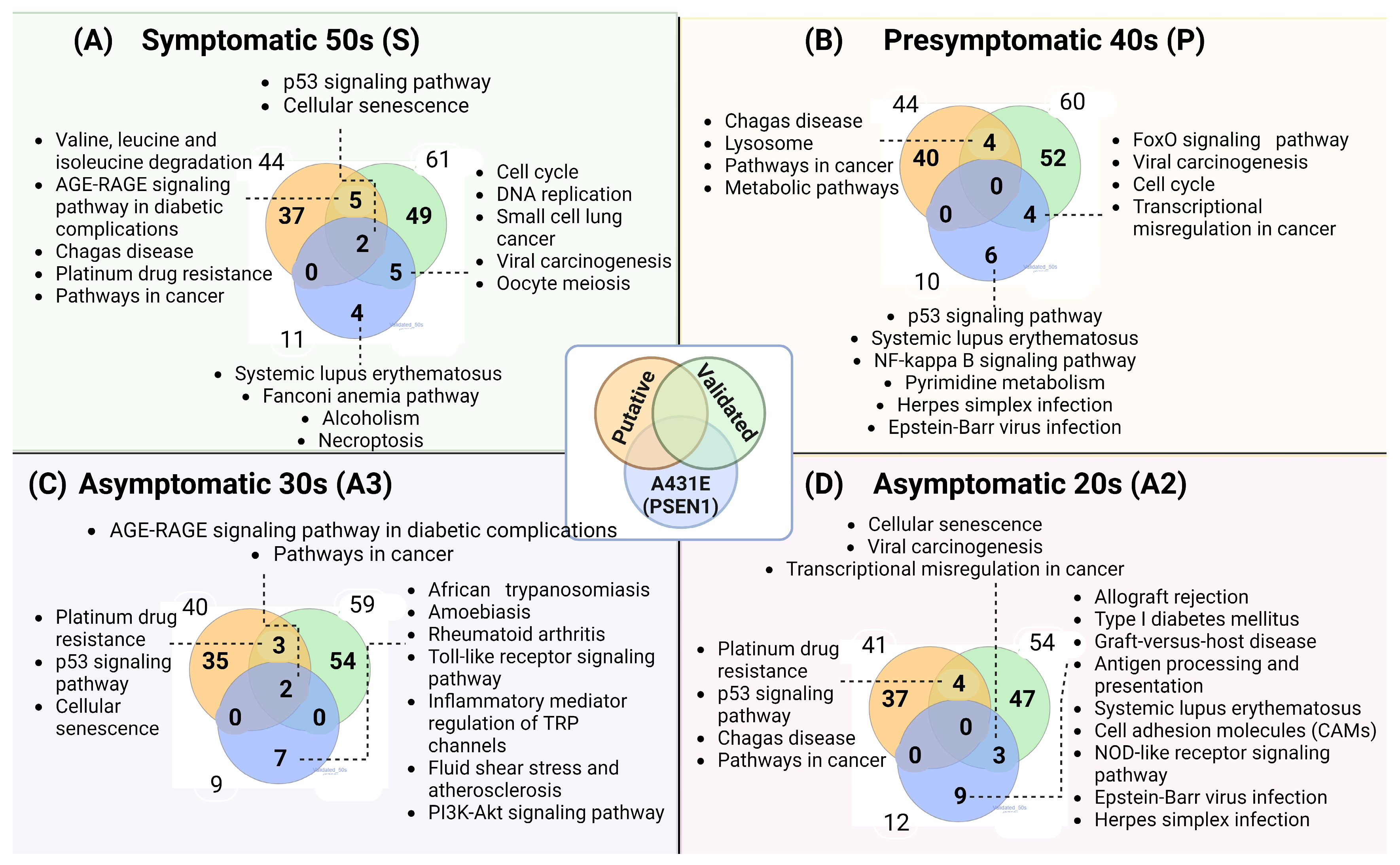
2.5. The Gene Ontology and Signaling Pathways Are Disrupted in Individuals Carrying the PSEN1(A431E) Mutation at Different Stages of the Disease
2.6. PSEN1(A431E) Mutation Transcriptomic Profile
2.7. PSEN1(A431E) Mutation miRNA Profile
2.8. Functional Analysis of mRNAs in PSEN1(A431E) Mutation Carriers vs. Controls
2.9. Comparision between miRNAs Altered in MSCs Derived from PSEN1(A431E) Mutation Carriers and Studies Previously Reported
3. Discussion
3.1. miRNAs Altered in MSCs Derived from the PSEN1(A431E) Symptomatic Mutation Carrier
3.2. miRNAs Altered in MSCs Derived from the PSEN1(A431E) Presymptomatic Mutation Carrier
3.3. miRNAs Altered in MSCs Derived from the PSEN1(A431E) Asymptomatic Mutation Carriers (A2 and A3)
3.4. miRNAs Altered in PSEN1(A431E) Mutation Carriers Compared to Controls
3.5. PSEN1(A431E) Mutation Carriers Enriched Signaling Pathways and Deregulated miRNAs’ Target Genes
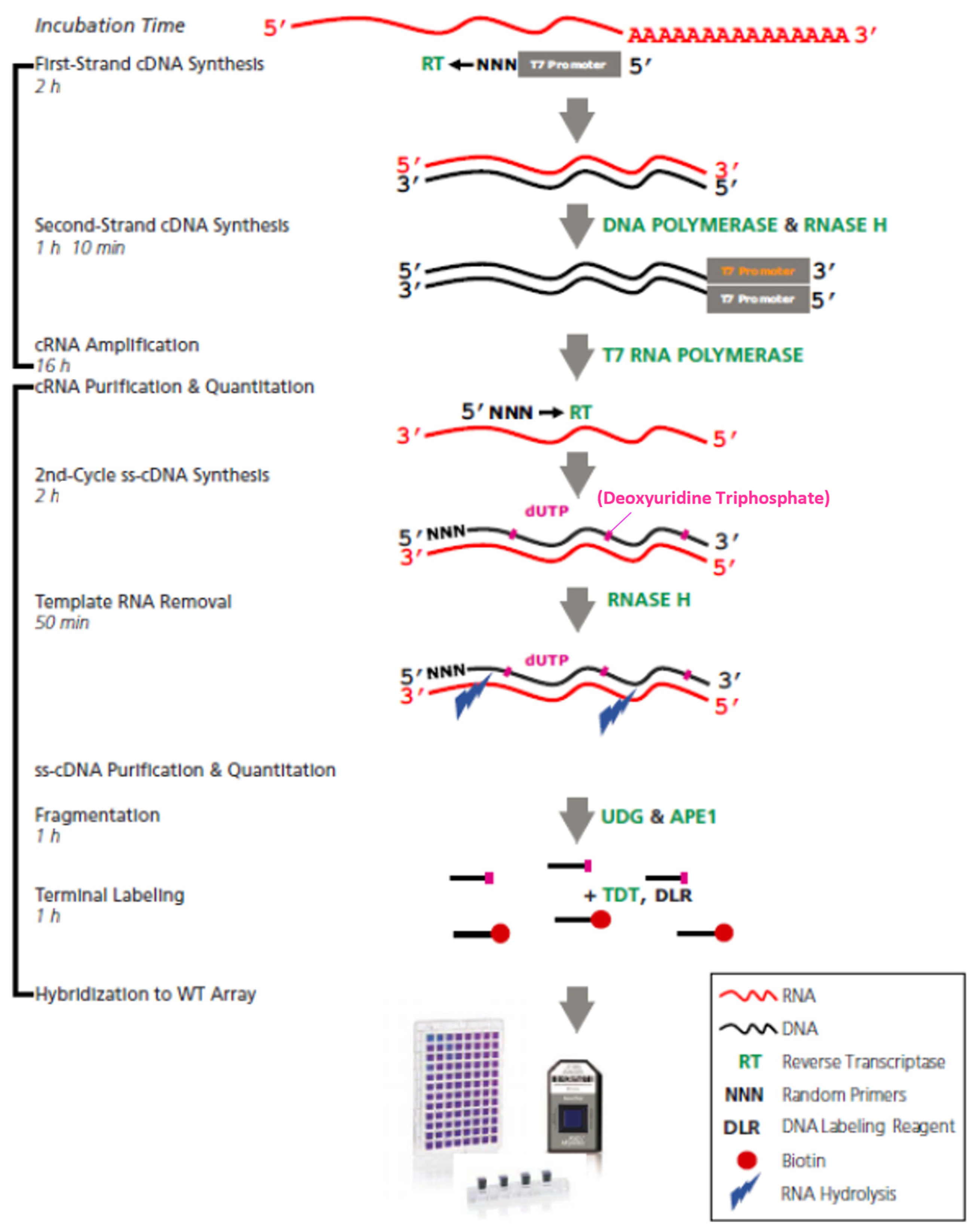
4. Materials and Methods
4.1. Subjects of Study
4.2. Sample Collection and RNA Extraction
4.3. Microarray Assays
4.4. Data Analysis
4.5. Prediction and Validation of Target mRNAs
4.6. Bioinformatic Analysis
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prince, M.J. World Alzheimer Report 2015: The Global Impact of Dementia. 25 August 2015. Available online: https://www.alz.co.uk/research/world-report-2015 (accessed on 23 July 2020).
- Chouraki, V.; Seshadri, S. Genetics of Alzheimer’s Disease. In Advances in Genetics; Elsevier: Amsterdam, The Netherlands, 2014; Volume 87, pp. 245–294. [Google Scholar] [CrossRef]
- Masters, C.L.; Bateman, R.; Blennow, K.; Rowe, C.C.; Sperling, R.A.; Cummings, J.L. Alzheimer’s disease. Nat. Rev. Dis. Primers 2015, 1, 15056. [Google Scholar] [CrossRef]
- Ringman, J.M.; Casado, M.; Van Berlo, V.; Pa, J.; Joseph-Mathurin, N.; Fagan, A.M.; Benzinger, T.; Bateman, R.J.; Morris, J.C. A novel PSEN1 (S230N) mutation causing early-onset Alzheimer’s Disease associated with prosopagnosia, hoarding, and Parkinsonism. Neurosci. Lett. 2017, 657, 11–15. [Google Scholar] [CrossRef]
- Rudzinski, L.A.; Fletcher, R.M.; Dickson, D.W.; Crook, R.; Hutton, M.L.; Adamson, J.; Graff-Radford, N.R. Early Onset Familial Alzheimer’s Disease with Spastic Paraparesis, Dysarthria, and Seizures and N135S Mutation in PSEN1. Alzheimer Dis. Assoc. Disord. 2008, 22, 299–307. [Google Scholar] [CrossRef]
- Soosman, S.K.; Joseph-Mathurin, N.; Braskie, M.N.; Bordelon, Y.M.; Wharton, D.; Casado, M.; Coppola, G.; McCallum, H.; Nuwer, M.; Coutin-Churchman, P.; et al. Widespread white matter and conduction defects in PSEN1- related spastic paraparesis. Neurobiol. Aging 2016, 47, 201–209. [Google Scholar] [CrossRef]
- Yescas, P.; Huertas-Vazquez, A.; Villarreal-Molina, M.T.; Rasmussen, A.; Tusié-Luna, M.T.; López, M.; Canizales-Quinteros, S.; Alonso, M.E. Founder effect for the Ala431Glu mutation of the presenilin 1 gene causing early-onset Alzheimer’s disease in Mexican families. Neurogenetics 2006, 7, 195–200. [Google Scholar] [CrossRef]
- Orozco-Barajas, M.; Oropeza-Ruvalcaba, Y.; Canales-Aguirre, A.A.; Sánchez-González, V.J. PSEN1 c.1292C<A Variant and Early-Onset Alzheimer’s Disease: A Scoping Review. Front. Aging Neurosci. 2022, 14, 860529. [Google Scholar] [CrossRef] [PubMed]
- Panizza, E. DeepOmicsAE: Representing Signaling Modules in Alzheimer’s Disease with Deep Learning Analysis of Proteomics, Metabolomics, and Clinical Data. J. Vis. Exp. JoVE 2023, 202. [Google Scholar] [CrossRef]
- Subramanian, I.; Verma, S.; Kumar, S.; Jere, A.; Anamika, K. Multi-omics Data Integration, Interpretation, and Its Application. Bioinform. Biol. Insights 2020, 14, 1177932219899051. [Google Scholar] [CrossRef]
- Li, P.; Marshall, L.; Oh, G.; Jakubowski, J.L.; Groot, D.; He, Y.; Wang, T.; Petronis, A.; Labrie, V. Epigenetic dysregulation of enhancers in neurons is associated with Alzheimer’s disease pathology and cognitive symptoms. Nat. Commun. 2019, 10, 2246. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jiao, B.; Shen, L. The Epigenetics of Alzheimer’s Disease: Factors and Therapeutic Implications. Front. Genet. 2018, 9, 579. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, K.; Samson, W.; Ji, H. Decoding noncoding RNA: The long and short of it. Circ. Res. 2013, 113, 240–241. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target Recognition and Regulatory Functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef]
- Dragomir, M.P.; Knutsen, E.; Calin, G.A. SnapShot: Unconventional miRNA Functions. Cell 2018, 174, 1038–1038.e1. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. Metazoan MicroRNAs. Cell 2018, 173, 20–51. [Google Scholar] [CrossRef] [PubMed]
- Takousis, P.; Sadlon, A.; Schulz, J.; Wohlers, I.; Dobricic, V.; Middleton, L.; Lill, C.M.; Perneczky, R.; Bertram, L. Differential expression of microRNAs in Alzheimer’s disease brain, blood, and cerebrospinal fluid. Alzheimer’s Dement. 2019, 15, 1468–1477. [Google Scholar] [CrossRef]
- Lv, Z.; Hu, L.; Yang, Y.; Zhang, K.; Sun, Z.; Zhang, J.; Zhang, L.; Hao, Y. Comparative study of microRNA profiling in one Chinese Family with PSEN1 G378E mutation. Metab. Brain Dis. 2018, 33, 1711–1720. [Google Scholar] [CrossRef]
- Fung, S.; Amos, P.; Case, A.; Ramakrishnan, A.; Neumann, H.; Garden, G.A.; Jayadev, S. O2-05-03: Familial Alzheimer’s disease PSEN2 mutations regulate inflammatory microRNAs in microglia. Alzheimer’s Dement. 2015, 11, P184. [Google Scholar] [CrossRef]
- Saraceno, C.; Musardo, S.; Marcello, E.; Pelucchi, S.; Diluca, M. Modeling Alzheimer’s Disease: From Past to Future. Front. Pharmacol. 2013, 4, 77. Available online: https://www.frontiersin.org/articles/10.3389/fphar.2013.00077 (accessed on 23 July 2020). [CrossRef]
- Miya Shaik, M.; Tamargo, I.A.; Abubakar, M.B.; Kamal, M.A.; Greig, N.H.; Gan, S.H. The Role of microRNAs in Alzheimer’s Disease and Their Therapeutic Potentials. Genes 2018, 9, 174. [Google Scholar] [CrossRef]
- Dursun, E.; Candaş, E.; Yılmazer, S.; Gezen-Ak, D. Amyloid Beta 1–42 Alters the Expression of miRNAs in Cortical Neurons. J. Mol. Neurosci. 2019, 67, 181–192. [Google Scholar] [CrossRef]
- Aloi, M.S.; Prater, K.E.; Sánchez, R.E.A.; Beck, A.; Pathan, J.L.; Davidson, S.; Jayadev, S.; Garden, G.A. Microglia specific deletion of miR-155 in Alzheimer’s disease mouse models reduces amyloid-β pathology but causes hyperexcitability and seizures. J. Neuroinflamm. 2023, 20, 60. [Google Scholar] [CrossRef]
- Benítez-King, G.; Riquelme, A.; Ortíz-López, L.; Berlanga, C.; Rodríguez-Verdugo, M.S.; Romo, F.; Calixto, E.; Solís-Chagoyán, H.; Jímenez, M.A.; Montaño, L.M.; et al. A non-invasive method to isolate the neuronal linage from the nasal epithelium from schizophrenic and bipolar diseases. J. Neurosci. Methods 2011, 201, 35–45. [Google Scholar] [CrossRef]
- Veron, A.D.; Bienboire-Frosini, C.; Feron, F.; Codecasa, E.; Deveze, A.; Royer, D.; Watelet, P.; Asproni, P.; Sadelli, K.; Chabaud, C.; et al. Isolation and characterization of olfactory ecto-mesenchymal stem cells from eight mammalian genera. BMC Vet. Res. 2018, 14, 17. [Google Scholar] [CrossRef]
- Delorme, B.; Nivet, E.; Gaillard, J.; Häupl, T.; Ringe, J.; Devèze, A.; Magnan, J.; Sohier, J.; Khrestchatisky, M.; Roman, F.S.; et al. The human nose harbors a niche of olfactory ectomesenchymal stem cells displaying neurogenic and osteogenic properties. Stem Cells Dev. 2010, 19, 853–866. [Google Scholar] [CrossRef]
- Jiménez-Vaca, A.L.; Benitez-King, G.; Ruiz, V.; Ramírez-Rodríguez, G.B.; Hernández-de la Cruz, B.; Salamanca-Gómez, F.A.; González-Márquez, H.; Ramírez-Sánchez, I.; Ortíz-López, L.; Vélez-Del Valle, C.; et al. Exfoliated Human Olfactory Neuroepithelium: A Source of Neural Progenitor Cells. Mol. Neurobiol. 2018, 55, 2516–2523. [Google Scholar] [CrossRef]
- Jiménez-Acosta, M.A.; Hernández, L.J.R.; Cristerna, M.L.P.; Meraz-Ríos, M.A. Mini-Review: Mesenchymal stem cells. New alternatives for nervous system disorders. Curr. Stem Cell Res. Ther. 2022, 17, 299–321. [Google Scholar] [CrossRef]
- Dan, X.; Wechter, N.; Gray, S.; Mohanty, J.G.; Croteau, D.L.; Bohr, V.A. Olfactory dysfunction in aging and neurodegenerative diseases. Ageing Res. Rev. 2021, 70, 101416. [Google Scholar] [CrossRef]
- Kotecha, A.; Corrêa, A.; Fisher, K.; Rushworth, J. Olfactory Dysfunction as a Global Biomarker for Sniffing out Alzheimer’s Disease: A Meta-Analysis. Biosensors 2018, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Talamo, B.R.; Rudel, R.; Kosik, K.S.; Lee, V.M.-Y.; Neff, S.; Adelman, L.; Kauer, J.S. Pathological changes in olfactory neurons in patients with Alzheimer’s disease. Nature 1989, 337, 736–739. [Google Scholar] [CrossRef] [PubMed]
- Kovács, T.; Cairns, N.J.; Lantos, P.L. Olfactory centres in Alzheimer’s disease: Olfactory bulb is involved in early Braak’s stages. Neuroreport 2001, 12, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Scopa, C.; Marrocco, F.; Latina, V.; Ruggeri, F.; Corvaglia, V.; La Regina, F.; Ammassari-Teule, M.; Middei, S.; Amadoro, G.; Meli, G.; et al. Impaired adult neurogenesis is an early event in Alzheimer’s disease neurodegeneration mediated by intracellular Aβ oligomers. Cell Death Differ. 2020, 27, 934–948. [Google Scholar] [CrossRef] [PubMed]
- Rochín-Hernández, L.J.; Jiménez-Acosta, M.A.; Ramírez-Reyes, L.; Figueroa-Corona, M.d.P.; Sánchez-González, V.J.; Orozco-Barajas, M.; Meraz-Ríos, M.A. The Proteome Profile of Olfactory Ecto-Mesenchymal Stem Cells-Derived from Patients with Familial Alzheimer’s Disease Reveals New Insights for AD Study. Int. J. Mol. Sci. 2023, 24, 12606. [Google Scholar] [CrossRef] [PubMed]
- Nowak, J.S.; Michlewski, G. miRNAs in development and pathogenesis of the nervous system. Biochem. Soc. Trans. 2013, 41, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lei, Z.; Sun, T. The role of microRNAs in neurodegenerative diseases: A review. Cell Biol. Toxicol. 2023, 39, 53–83. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.J.; Liang, R.; Li, N.; Wang, E. The impact of noncoding RNA on the biochemical and molecular mechanisms of aging. Biochim. Biophys. Acta 2009, 1790, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Fan, M.; Zheng, Q.; Hao, S.; Yang, L.; Xia, Q.; Qi, C.; Ge, J. MicroRNAs in Alzheimer’s disease: Potential diagnostic markers and therapeutic targets. Biomed. Pharmacother. 2022, 148, 112681. [Google Scholar] [CrossRef]
- Zoltowska, K.M.; Laskowska-Kaszub, K.; Nagaraj, S.; Wojda, U. Chapter 9-Implication of microRNAs in Alzheimer’s disease pathogenesis. In Genetics, Neurology, Behavior, and Diet in Dementia; Martin, C.R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 131–145. [Google Scholar] [CrossRef]
- Wang, L.; Shui, X.; Diao, Y.; Chen, D.; Zhou, Y.; Lee, T.H. Potential Implications of miRNAs in the Pathogenesis, Diagnosis, and Therapeutics of Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 16259. [Google Scholar] [CrossRef]
- Abdelmaksoud, N.M.; Sallam, A.-A.M.; Abulsoud, A.I.; El-Dakroury, W.A.; Abdel Mageed, S.S.; Al-Noshokaty, T.M.; Elrebehy, M.A.; Elshaer, S.S.; Mahmoud, N.A.; Fathi, D.; et al. Unraveling the role of miRNAs in the diagnosis, progression, and therapeutic intervention of Alzheimer’s disease. Pathol. Res. Pract. 2023, 253, 155007. [Google Scholar] [CrossRef]
- Guévremont, D.; Tsui, H.; Knight, R.; Fowler, C.J.; Masters, C.L.; Martins, R.N.; Abraham, W.C.; Tate, W.P.; Cutfield, N.J.; Williams, J.M. Plasma microRNA vary in association with the progression of Alzheimer’s disease. Alzheimer’s Dement. 2022, 14, e12251. [Google Scholar] [CrossRef]
- Cosín-Tomás, M.; Antonell, A.; Lladó, A.; Alcolea, D.; Fortea, J.; Ezquerra, M.; Lleó, A.; Martí, M.J.; Pallàs, M.; Sanchez-Valle, R.; et al. Plasma miR-34a-5p and miR-545-3p as Early Biomarkers of Alzheimer’s Disease: Potential and Limitations. Mol. Neurobiol. 2017, 54, 5550–5562. [Google Scholar] [CrossRef]
- Aharon, A.; Spector, P.; Ahmad, R.S.; Horrany, N.; Sabbach, A.; Brenner, B.; Aharon-Peretz, J. Extracellular Vesicles of Alzheimer’s Disease Patients as a Biomarker for Disease Progression. Mol. Neurobiol. 2020, 57, 4156–4169. [Google Scholar] [CrossRef]
- Visconte, C.; Fenoglio, C.; Serpente, M.; Muti, P.; Sacconi, A.; Rigoni, M.; Arighi, A.; Borracci, V.; Arcaro, M.; Arosio, B.; et al. Altered Extracellular Vesicle miRNA Profile in Prodromal Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 14749. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, S.; Laskowska-Kaszub, K.; Dębski, K.J.; Wojsiat, J.; Dąbrowski, M.; Gabryelewicz, T.; Kuźnicki, J.; Wojda, U. Profile of 6 microRNA in blood plasma distinguish early stage Alzheimer’s disease patients from non-demented subjects. Oncotarget 2017, 8, 16122–16143. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Guo, L.; Liu, C. Genome-wide analysis of mir-548 gene family reveals evolutionary and functional implications. J. Biomed. Biotechnol. 2012, 2012, 679563. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhang, M.; Yang, X.; Cui, T.; Dai, J. MicroRNA-548c-3p inhibits T98G glioma cell proliferation and migration by downregulating c-Myb. Oncol. Lett. 2017, 13, 3866–3872. [Google Scholar] [CrossRef] [PubMed]
- Feng, X. miR-548b Suppresses Melanoma Cell Growth, Migration, and Invasion by Negatively Regulating Its Target Gene HMGB1. Cancer Biother. Radiopharm. 2021, 36, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Salazar, E.G.; Almeraya, E.V.; López-Perez, T.V.; Patiño, N.; Salmeron, J.; Velázquez-Cruz, R. MicroRNA-548-3p overexpression inhibits proliferation, migration and invasion in osteoblast-like cells by targeting STAT1 and MAFB. J. Biochem. 2020, 168, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.-L.; Ma, Y.-L.; Hsieh, D.-Y.; Liu, Y.-C.; Lee, E.H. STAT1 Negatively Regulates Spatial Memory Formation and Mediates the Memory-Impairing Effect of Aβ. Neuropsychopharmacology 2014, 39, 746–758. [Google Scholar] [CrossRef]
- Anderson, A.G.; Rogers, B.B.; Loupe, J.M.; Rodriguez-Nunez, I.; Roberts, S.C.; White, L.M.; Brazell, J.N.; Bunney, W.E.; Bunney, B.G.; Watson, S.J.; et al. Single nucleus multiomics identifies ZEB1 and MAFB as candidate regulators of Alzheimer’s disease-specific cis-regulatory elements. Cell Genom. 2023, 3, 100263. [Google Scholar] [CrossRef]
- Zhang, J.; Shu, Y.; Qu, Y.; Zhang, L.; Chu, T.; Zheng, Y.; Zhao, H. C-myb Plays an Essential Role in the Protective Function of IGF-1 on Cytotoxicity Induced by Aβ25–35 via the PI3K/Akt Pathway. J. Mol. Neurosci. 2017, 63, 412–418. [Google Scholar] [CrossRef]
- Gaikwad, S.; Kayed, R. HMGB1-Mediated Senescence and Brain Inflammation Contributes to Cognitive Dysfunctions. Alzheimer’s Dement. 2022, 18, e068747. [Google Scholar] [CrossRef]
- Saberiyan, M.; Ghasemi, Z.; Yaghoobi, H. MiR-548 K regulatory effect on the ABCG2 gene expression in MDR breast cancer cells. Cancer Rep. 2023, 6, e1816. [Google Scholar] [CrossRef]
- Pereira, C.D.; Martins, F.; Wiltfang, J.; da Cruz E Silva, O.A.B.; Rebelo, S. ABC Transporters Are Key Players in Alzheimer’s Disease. J. Alzheimer’s Dis. JAD 2018, 61, 463–485. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Meng, G.; Zhang, M.; Zhao, Y.; Zhou, Y.; Zhao, Y.; Liu, X. Exploring Potential Biomarkers Underlying the Pathogenesis of Alzheimer’s Disease by Bioinformatics Gene Analysis. Ann. Clin. Lab. Sci. 2021, 51, 198–205. [Google Scholar] [PubMed]
- Ansari, A.; Maffioletti, E.; Milanesi, E.; Marizzoni, M.; Frisoni, G.B.; Blin, O.; Richardson, J.C.; Bordet, R.; Forloni, G.; Gennarelli, M.; et al. miR-146a and miR-181a are involved in the progression of mild cognitive impairment to Alzheimer’s disease. Neurobiol. Aging 2019, 82, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Burgos, K.; Malenica, I.; Metpally, R.; Courtright, A.; Rakela, B.; Beach, T.; Shill, H.; Adler, C.; Sabbagh, M.; Villa, S.; et al. Profiles of Extracellular miRNA in Cerebrospinal Fluid and Serum from Patients with Alzheimer’s and Parkinson’s Diseases Correlate with Disease Status and Features of Pathology. PLoS ONE 2014, 9, e94839. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Ortiz, C.J.; Baglietto-Vargas, D.; Martinez-Coria, H.; LaFerla, F.M.; Kitazawa, M. Upregulation of miR-181 decreases c-Fos and SIRT-1 in the hippocampus of 3xTg-AD mice. J. Alzheimer’s Dis. JAD 2014, 42, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Boissonneault, V.; Plante, I.; Rivest, S.; Provost, P. MicroRNA-298 and microRNA-328 regulate expression of mouse beta-amyloid precursor protein-converting enzyme 1. J. Biol. Chem. 2009, 284, 1971–1981. [Google Scholar] [CrossRef] [PubMed]
- Bekris, L.M.; Lutz, F.; Montine, T.J.; Yu, C.E.; Tsuang, D.; Peskind, E.R.; Leverenz, J.B. MicroRNA in Alzheimer’s disease: An exploratory study in brain, cerebrospinal fluid and plasma. Biomarkers 2013, 18, 455–466. [Google Scholar] [CrossRef]
- Li, Y.; Han, X.; Fan, H.; Sun, J.; Ni, M.; Zhang, L.; Fang, F.; Zhang, W.; Ma, P. Circular RNA AXL increases neuron injury and inflammation through targeting microRNA-328 mediated BACE1 in Alzheimer’s disease. Neurosci. Lett. 2022, 776, 136531. [Google Scholar] [CrossRef]
- Lau, P.; Bossers, K.; Janky, R.; Salta, E.; Frigerio, C.S.; Barbash, S.; … De Strooper, B. Alteration of the microRNA network during the progression of Alzheimer’s disease. EMBO Mol. Med. 2013, 5, 1613–1634. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Xu, J.; Huang, H.; Shen, P.; You, L.; Zhou, L.; Zheng, L.; Zhang, T.; Zhao, Y. MiR-1178 promotes the proliferation, G1/S transition, migration and invasion of pancreatic cancer cells by targeting CHIP. PLoS ONE 2015, 10, e0116934. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, Z.; Du, Y.; Hao, S.; Zhao, B. Hsa_circ_0043603 promotes the progression of esophageal squamous cell carcinoma by sponging miR-1178–3p and regulating AADAC expression. Heliyon 2023, 9, e19807. [Google Scholar] [CrossRef]
- Wang, L.Q.; Deng, A.C.; Zhao, L.; Li, Q.; Wang, M.; Zhang, Y. MiR-1178-3p promotes the proliferation, migration and invasion of nasopharyngeal carcinoma Sune-1 cells by targeting STK4. J. Biol. Regul. Homeost. Agents 2019, 33, 321–330. [Google Scholar]
- Bi, J.; Liu, H.; Dong, W.; Xie, W.; He, Q.; Cai, Z.; Huang, J.; Lin, T. Circular RNA circ-ZKSCAN1 inhibits bladder cancer progression through miR-1178-3p/p21 axis and acts as a prognostic factor of recurrence. Mol. Cancer 2019, 18, 133. [Google Scholar] [CrossRef]
- Fan, H.; Lv, P.; Mu, T.; Zhao, X.; Liu, Y.; Feng, Y.; Lv, J.; Liu, M.; Tang, H. LncRNA n335586/miR-924/CKMT1A axis contributes to cell migration and invasion in hepatocellular carcinoma cells. Cancer Lett. 2018, 429, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, S.; Pan, S.; Yan, Q.; Li, Y.; Zhao, Y. Circ_0000463 contributes to the progression and glutamine metabolism of non-small-cell lung cancer by targeting miR-924/SLC1A5 signaling. J. Clin. Lab. Anal. 2021, 36, e24116. [Google Scholar] [CrossRef]
- Wang, H.; Chen, X.; Yang, B.; Xia, Z.; Chen, Q. MiR-924 as a tumor suppressor inhibits non-small cell lung cancer by inhibiting RHBDD1/Wnt/β-catenin signaling pathway. Cancer Cell Int. 2020, 20, 491. [Google Scholar] [CrossRef]
- Rastegar-Moghaddam, S.H.; Ebrahimzadeh-Bideskan, A.; Shahba, S.; Malvandi, A.M.; Mohammadipour, A. Roles of the miR-155 in Neuroinflammation and Neurological Disorders: A Potent Biological and Therapeutic Target. Cell. Mol. Neurobiol. 2023, 43, 455–467. [Google Scholar] [CrossRef]
- Guedes, J.R.; Custódia, C.M.; Silva, R.J.; de Almeida, L.P.; Pedroso de Lima, M.C.; Cardoso, A.L. Early miR-155 upregulation contributes to neuroinflammation in Alzheimer’s disease triple transgenic mouse model. Hum. Mol. Genet. 2014, 23, 6286–6301. [Google Scholar] [CrossRef]
- Sierksma, A.; Lu, A.; Salta, E.; Vanden Eynden, E.; Callaerts-Vegh, Z.; D’Hooge, R.; … De Strooper, B. Deregulation of neuronal miRNAs induced by amyloid-β or TAU pathology. Mol. Neurodegener. 2018, 13, 54. [Google Scholar] [CrossRef]
- Wang, X.; Huang, T.; Zhao, Y.; Zheng, Q.; Thompson, R.C.; Bu, G.; Zhang, Y.; Hong, W.; Xu, H. Sorting Nexin 27 Regulates Aβ Production through Modulating γ-Secretase Activity. Cell Rep. 2014, 9, 1023–1033. [Google Scholar] [CrossRef]
- Garcia, G.; Pinto, S.; Ferreira, S.; Lopes, D.; Serrador, M.J.; Fernandes, A.; Vaz, A.R.; Mendonça, A.D.; Edenhofer, F.; Malm, T.; et al. Emerging Role of miR-21-5p in Neuron-Glia Dysregulation and Exosome Transfer Using Multiple Models of Alzheimer’s Disease. Cells 2022, 11, 3377. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, H.; Rodriguez, S.; Cao, L.; Parish, J.; Mumaw, C.; Zollman, A.; Kamoka, M.M.; Mu, J.; Chen, D.Z.; et al. Notch-dependent repression of miR-155 in the bone marrow niche regulates hematopoiesis in an NF-κB-dependent manner. Cell Stem Cell 2014, 15, 51–65. [Google Scholar] [CrossRef]
- Hurley, E.M.; Mozolewski, P.; Dobrowolski, R.; Hsieh, J. Familial Alzheimer’s disease-associated PSEN1 mutations affect neurodevelopment through increased Notch signaling. Stem Cell Rep. 2023, 18, 1516–1533. [Google Scholar] [CrossRef]
- Han, L.; Yue, X.; Zhou, X.; Lan, F.; You, G.; Zhang, W.; Zhang, K.; Zhang, C.; Cheng, J.; Yu, S.; et al. MicroRNA-21 Expression is regulated by β-catenin/STAT3 Pathway and Promotes Glioma Cell Invasion by Direct Targeting RECK. CNS Neurosci. Ther. 2012, 18, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Gámez-Valero, A.; Campdelacreu, J.; Vilas, D.; Ispierto, L.; Reñé, R.; Álvarez, R.; Armengol, M.P.; Borràs, F.E.; Beyer, K. Exploratory study on microRNA profiles from plasma-derived extracellular vesicles in Alzheimer’s disease and dementia with Lewy bodies. Transl. Neurodegener. 2019, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Garcia, G.; Pinto, S.; Cunha, M.; Fernandes, A.; Koistinaho, J.; Brites, D. Neuronal Dynamics and miRNA Signaling Differ between SH-SY5Y APPSwe and PSEN1 Mutant iPSC-Derived AD Models upon Modulation with miR-124 Mimic and Inhibitor. Cells 2021, 10, 2424. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, J.; Li, L.; Xue, L.; Zhang, Y.; Wang, P. MicroRNA-135a and -200b, potential Biomarkers for Alzheimer׳s disease, regulate β secretase and amyloid precursor protein. Brain Res. 2014, 1583, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-G.; Song, J.; Zhang, Y.-Q.; Wang, P.-C. MicroRNA-193b is a regulator of amyloid precursor protein in the blood and cerebrospinal fluid derived exosomal microRNA-193b is a biomarker of Alzheimer’s disease. Mol. Med. Rep. 2014, 10, 2395–2400. [Google Scholar] [CrossRef] [PubMed]
- Nie, C.; Sun, Y.; Zhen, H.; Guo, M.; Ye, J.; Liu, Z.; Yang, Y.; Zhang, X. Differential Expression of Plasma Exo-miRNA in Neurodegenerative Diseases by Next-Generation Sequencing. Front. Neurosci. 2020, 14, 438. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.T.; Liu, C.G.; Gao, S.C.; Zhang, Y.; Wang, P.C. The Serum Exosome Derived MicroRNA-135a, -193b, and -384 Were Potential Alzheimer’s Disease Biomarkers. Biomed. Environ. Sci. BES 2018, 31, 87–96. [Google Scholar] [CrossRef]
- Manzine, P.R.; Pelucchi, S.; Horst, M.A.; Vale, F.A.C.; Pavarini, S.C.I.; Audano, M.; Mitro, N.; Di Luca, M.; Marcello, E.; Cominetti, M.R. microRNA 221 Targets ADAM10 mRNA and is Downregulated in Alzheimer’s Disease. J. Alzheimer’s Dis. JAD 2018, 61, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-W.; Pyun, J.-M.; Bice, P.J.; Bennett, D.A.; Saykin, A.J.; Kim, S.; Park, Y.H.; Nho, K. miR-129-5p as a biomarker for pathology and cognitive decline in Alzheimer’s disease. Alzheimer’s Res. Ther. 2024, 16, 5. [Google Scholar] [CrossRef] [PubMed]
- Kuthethur, R.; Adiga, D.; Kandettu, A.; Jerome, M.S.; Mallya, S.; Mumbrekar, K.D.; Kabekkodu, S.P.; Chakrabarty, S. MiR-4521 perturbs FOXM1-mediated DNA damage response in breast cancer. Front. Mol. Biosci. 2023, 10, 1131433. [Google Scholar] [CrossRef] [PubMed]
- Ayesha, M.; Majid, A.; Zhao, D.; Greenaway, F.T.; Yan, N.; Liu, Q.; Liu, S.; Sun, M.-Z. MiR-4521 plays a tumor repressive role in growth and metastasis of hepatocarcinoma cells by suppressing phosphorylation of FAK/AKT pathway via targeting FAM129A. J. Adv. Res. 2021, 36, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Yanbin, Z.; Jing, Z. CircSAMD4A accelerates cell proliferation of osteosarcoma by sponging miR-1244 and regulating MDM2 mRNA expression. Biochem. Biophys. Res. Commun. 2019, 516, 102–111. [Google Scholar] [CrossRef]
- Liu, Y.; He, X.; Chen, Y.; Cao, D. Long non-coding RNA LINC00504 regulates the Warburg effect in ovarian cancer through inhibition of miR-1244. Mol. Cell. Biochem. 2020, 464, 39–50. [Google Scholar] [CrossRef]
- Xie, W.; Liu, L.U.; He, C.; Zhao, M.; Ni, R.; Zhang, Z.; Shui, C. Circ_0002711 knockdown suppresses cell growth and aerobic glycolysis by modulating miR-1244/ROCK1 axis in ovarian cancer. J. Biosci. 2021, 46, 21. [Google Scholar] [CrossRef]
- Banzhaf-Strathmann, J.; Benito, E.; May, S.; Arzberger, T.; Tahirovic, S.; Kretzschmar, H.; Fischer, A.; Edbauer, D. MicroRNA-125b induces tau hyperphosphorylation and cognitive deficits in Alzheimer’s disease. EMBO J. 2014, 33, 1667–1680. [Google Scholar] [CrossRef]
- Lugli, G.; Cohen, A.M.; Bennett, D.A.; Shah, R.C.; Fields, C.J.; Hernandez, A.G.; Smalheiser, N.R. Plasma Exosomal miRNAs in Persons with and without Alzheimer Disease: Altered Expression and Prospects for Biomarkers. PLoS ONE 2015, 10, e0139233. [Google Scholar] [CrossRef]
- Butovsky, O.; Jedrychowski, M.P.; Moore, C.S.; Cialic, R.; Lanser, A.J.; Gabriely, G.; Koeglsperger, T.; Dake, B.; Wu, P.M.; Doykan, C.E.; et al. Identification of a unique TGF-β–dependent molecular and functional signature in microglia. Nat. Neurosci. 2014, 17, 131–143. [Google Scholar] [CrossRef]
- Cogswell, J.P.; Ward, J.; Taylor, I.A.; Waters, M.; Shi, Y.; Cannon, B.; Kelnar, K.; Kemppainen, J.; Brown, D.; Chen, C.; et al. Identification of miRNA Changes in Alzheimer’s Disease Brain and CSF Yields Putative Biomarkers and Insights into Disease Pathways. J. Alzheimer’s Dis. 2008, 14, 27–41. [Google Scholar] [CrossRef]
- Sethi, P.; Lukiw, W.J. Micro-RNA abundance and stability in human brain: Specific alterations in Alzheimer’s disease temporal lobe neocortex. Neurosci. Lett. 2009, 459, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Gu, H.; Guo, Q.; Liang, S.; Xue, J.; Yao, F.; Liu, X.; Li, F.; Liu, H.; Sun, L.; et al. Profiling of Serum Exosome MiRNA Reveals the Potential of a MiRNA Panel as Diagnostic Biomarker for Alzheimer’s Disease. Mol. Neurobiol. 2021, 58, 3084–3094. [Google Scholar] [CrossRef] [PubMed]
- McKeever, P.M.; Schneider, R.; Taghdiri, F.; Weichert, A.; Multani, N.; Brown, R.A.; Boxer, A.L.; Karydas, A.; Miller, B.; Robertson, J.; et al. MicroRNA Expression Levels Are Altered in the Cerebrospinal Fluid of Patients with Young-Onset Alzheimer’s Disease. Mol. Neurobiol. 2018, 55, 8826–8841. [Google Scholar] [CrossRef] [PubMed]
- Guedes, J.R.; Santana, I.; Cunha, C.; Duro, D.; Almeida, M.R.; Cardoso, A.M.; de Lima, M.C.P.; Cardoso, A.L. MicroRNA deregulation and chemotaxis and phagocytosis impairment in Alzheimer’s disease. Alzheimer’s Dement. 2016, 3, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Qi, C.; Liu, Y.; Shen, Y.; Zhao, X.; Qin, H.; Zhang, Y.; Yu, T. MicroRNA miR-27b-3p regulate microglial inflammation response and cell apoptosis by inhibiting A20 (TNF-α-induced protein 3). Bioengineered 2021, 12, 9902–9913. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Shao, W.; Li, Y.; Liu, L.; Chen, W.; Wang, C.; Li, B. Long non-coding RNA LINC01128 affects proliferation, migration, and invasion of glioma cells by regulating miR-27b-3p. Folia Neuropathol. 2022, 60, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Bazrgar, M.; Khodabakhsh, P.; Dargahi, L.; Mohagheghi, F.; Ahmadiani, A. MicroRNA modulation is a potential molecular mechanism for neuroprotective effects of intranasal insulin administration in amyloid βeta oligomer induced Alzheimer’s like rat model. Exp. Gerontol. 2022, 164, 111812. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, J.; Guo, S.; Zeng, L.; Cai, Z.; Zhang, J.; Wang, L.; Li, Z.; Liu, R. miR-23b-3p rescues cognition in Alzheimer’s disease by reducing tau phosphorylation and apoptosis via GSK-3β signaling pathways. Mol. Ther. Nucleic Acids 2022, 28, 539–557. [Google Scholar] [CrossRef]
- Sun, L.; Liu, A.; Zhang, J.; Ji, W.; Li, Y.; Yang, X.; Wu, Z.; Guo, J. miR-23b improves cognitive impairments in traumatic brain injury by targeting ATG12-mediated neuronal autophagy. Behav. Brain Res. 2018, 340, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Li, L.; Cui, C.; Zhao, Z.; Song, G. Overexpression of let-7a increases neurotoxicity in a PC12 cell model of Alzheimer’s disease via regulating autophagy. Exp. Ther. Med. 2017, 14, 3688–3698. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.Y.; Chang, M.N.V.; Lei, J.X.; Koukiekolo, R.; Smith, B.; Zhang, D.; Ghribi, O. Identification of microRNAs involved in Alzheimer’s progression using a rabbit model of the disease. Am. J. Neurodegener. Dis. 2014, 3, 33–44. [Google Scholar] [PubMed]
- Han, L.; Zhou, Y.; Zhang, R.; Wu, K.; Lu, Y.; Li, Y.; Duan, R.; Yao, Y.; Zhu, D.; Jia, Y. MicroRNA Let-7f-5p Promotes Bone Marrow Mesenchymal Stem Cells Survival by Targeting Caspase-3 in Alzheimer Disease Model. Front. Neurosci. 2018, 12, 333. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Wang, B.; Li, W. CircAXL Knockdown Alleviates Aβ1-42-Induced Neurotoxicity in Alzheimer’s Disease via Repressing PDE4A by Releasing miR-1306-5p. Neurochem. Res. 2022, 47, 1707–1720. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Xie, X.-Y.; Sui, X.-F.; Wang, P.; Chen, Z.; Zhang, J.-B. Profile of Pathogenic Proteins and MicroRNAs in Plasma-derived Extracellular Vesicles in Alzheimer’s Disease: A Pilot Study. Neuroscience 2020, 432, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Hébert, S.S.; Wang, W.-X.; Zhu, Q.; Nelson, P.T. A Study of Small RNAs from Cerebral Neocortex of Pathology-Verified Alzheimer’s Disease, Dementia with Lewy Bodies, Hippocampal Sclerosis, Frontotemporal Lobar Dementia, and Non-Demented Human Controls. J. Alzheimer’s Dis. JAD 2013, 35, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Serpente, M.; Fenoglio, C.; D’Anca, M.; Arcaro, M.; Sorrentino, F.; Visconte, C.; Arighi, A.; Fumagalli, G.G.; Porretti, L.; Cattaneo, A.; et al. MiRNA Profiling in Plasma Neural-Derived Small Extracellular Vesicles from Patients with Alzheimer’s Disease. Cells 2020, 9, 1443. [Google Scholar] [CrossRef]
- Satoh, J.-I.; Kino, Y.; Niida, S. MicroRNA-Seq Data Analysis Pipeline to Identify Blood Biomarkers for Alzheimer’s Disease from Public Data. Biomark. Insights 2015, 10, 21–31. [Google Scholar] [CrossRef]
- Ye, X.; Luo, H.; Chen, Y.; Wu, Q.; Xiong, Y.; Zhu, J.; Diao, Y.; Wu, Z.; Miao, J.; Wan, J. MicroRNAs 99b-5p/100-5p Regulated by Endoplasmic Reticulum Stress are Involved in Abeta-Induced Pathologies. Front. Aging Neurosci. 2015, 7, 210. [Google Scholar] [CrossRef]
- Tang, Z.-B.; Chen, H.-P.; Zhong, D.; Song, J.-H.; Cao, J.-W.; Zhao, M.-Q.; Han, B.-C.; Duan, Q.; Sheng, X.-M.; Yao, J.-L.; et al. LncRNA RMRP accelerates autophagy-mediated neurons apoptosis through miR-3142/TRIB3 signaling axis in alzheimer’s disease. Brain Res. 2022, 1785, 147884. [Google Scholar] [CrossRef]
- Luo, Q.; Wang, H.; Li, J. Serum miR-3142 could be Used as a Potential Biomarker to Screen Cervical Cancer Patients from Healthy Controls. Clin. Lab. 2019, 65, 597. [Google Scholar] [CrossRef] [PubMed]
- Man, W.; Cui, Y.; Li, J.; Li, Y.; Jin, J.; Jin, Y.; Wu, X.; Zhong, R.; Li, X.; Yao, H.; et al. circTAB2 inhibits lung cancer proliferation, migration and invasion by sponging miR-3142 to upregulate GLIS2. Apoptosis 2023, 28, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, X.; Wu, L.; Chen, H.; Xu, C.; Wei, J.; Du, K. MicroRNA expression profile in stage IA lung adenocarcinoma and miR-940 target prediction. Int. J. Clin. Exp. Med. 2019, 12, 1443–1452. [Google Scholar]
- Hu, Y.-B.; Zhang, Y.-F.; Ren, R.-J.; Dammer, E.B.; Xie, X.-Y.; Chen, S.-W.; Huang, Q.; Huang, W.-Y.; Zhang, R.; Chen, H.-Z.; et al. microRNA-425 loss mediates amyloid plaque microenvironment heterogeneity and promotes neurodegenerative pathologies. Aging Cell 2021, 20, e13454. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Wu, Y.; Li, L.; Liu, C. MicroRNA-425-5p promotes tau phosphorylation and cell apoptosis in Alzheimer’s disease by targeting heat shock protein B8. J. Neural Transm. 2020, 127, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de Frutos, M.; Galán-Chilet, I.; Goedeke, L.; Kim, B.; Pardo-Marqués, V.; Pérez-García, A.; Herrero, J.I.; Fernández-Hernando, C.; Kim, J.; Ramírez, C.M. MicroRNA 7 Impairs Insulin Signaling and Regulates Aβ Levels through Posttranscriptional Regulation of the Insulin Receptor Substrate 2, Insulin Receptor, Insulin-Degrading Enzyme, and Liver X Receptor Pathway. Mol. Cell. Biol. 2019, 39, e00170-19. [Google Scholar] [CrossRef] [PubMed]
- Frutos, M.F.; Pardo-Marqués, V.; Torrecilla-Parra, M.; Rada, P.; Pérez-García, A.; Martín-Martín, Y.; de la Peña, G.; Gómez, A.; Toledano-Zaragoza, A.; Gómez-Coronado, D.; et al. MiR-7 controls cholesterol biosynthesis through posttranscriptional regulation of DHCR24 expression. Biochim. Biophys. Acta. Gene Regul. Mech. 2023, 1866, 194938. [Google Scholar] [CrossRef]
- Sempere, L.F.; Freemantle, S.; Pitha-Rowe, I.; Moss, E.; Dmitrovsky, E.; Ambros, V. Expression profiling of mammalian microRNAs uncovers a subset of brain-expressed microRNAs with possible roles in murine and human neuronal differentiation. Genome Biol. 2004, 5, R13. [Google Scholar] [CrossRef]
- Bak, M.; Silahtaroglu, A.; Møller, M.; Christensen, M.; Rath, M.F.; Skryabin, B.; Tommerup, N.; Kauppinen, S. MicroRNA expression in the adult mouse central nervous system. RNA 2008, 14, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Mathew, L.K.; Skuli, N.; Mucaj, V.; Lee, S.S.; Zinn, P.O.; Sathyan, P.; Imtiyaz, H.Z.; Zhang, Z.; Davuluri, R.V.; Rao, S.; et al. miR-218 opposes a critical RTK-HIF pathway in mesenchymal glioblastoma. Proc. Natl. Acad. Sci. USA 2014, 111, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Qu, Y.; Xu, Y.; Zhang, L.; Zhou, L.; Han, Y.; Zhao, W.; Yu, P.; Zhang, Y.; Li, X.; et al. Microarray microRNA profiling of urinary exosomes in a 5XFAD mouse model of Alzheimer’s disease. Anim. Models Exp. Med. 2021, 4, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Han, G.; Li, F.; Li, W.; Bu, P.; Zhang, H.; Xie, J. (R)-9bMS Inhibited the Protein Synthesis and Autophagy of Triple Negative Breast Cancer Cells via Regulating miR-4660/mTOR Axis. Protein Pept. Lett. 2023, 30, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yan, Y.-F.; Lv, Q.; Li, Y.-J.; Wang, R.-R.; Sun, G.-B.; Pan, L.; Hu, J.-X.; Xie, N.; Zhang, C.; et al. miR-4293 upregulates lncRNA WFDC21P by suppressing mRNA-decapping enzyme 2 to promote lung carcinoma proliferation. Cell Death Dis. 2021, 12, 735. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, J.; Sun, X.; Ma, G.; Luo, G.; Miao, Z.; Song, L. miR-132 improves the cognitive function of rats with Alzheimer’s disease by inhibiting the MAPK1 signal pathway. Exp. Ther. Med. 2020, 20, 159. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Cong, Y.; Feng, N.; Liang, W.; Wu, Y. Up-regulated microRNA-132 reduces the cognition-damaging effect of sevoflurane on Alzheimer’s disease rats by inhibiting FOXA1. Genomics 2021, 113, 3644–3652. [Google Scholar] [CrossRef]
- Dong, J.; Liu, Y.; Zhan, Z.; Wang, X. MicroRNA-132 is associated with the cognition improvement following voluntary exercise in SAMP8 mice. Brain Res. Bull. 2018, 140, 80–87. [Google Scholar] [CrossRef]
- Cha, D.J.; Mengel, D.; Mustapic, M.; Liu, W.; Selkoe, D.J.; Kapogiannis, D.; Galasko, D.; Rissman, R.A.; Bennett, D.A.; Walsh, D.M. miR-212 and miR-132 Are Downregulated in Neurally Derived Plasma Exosomes of Alzheimer’s Patients. Front. Neurosci. 2019, 13, 1208. [Google Scholar] [CrossRef]
- Denk, J.; Boelmans, K.; Siegismund, C.; Lassner, D.; Arlt, S.; Jahn, H. MicroRNA Profiling of CSF Reveals Potential Biomarkers to Detect Alzheimer’s Disease. PLoS ONE 2015, 10, e0126423. [Google Scholar] [CrossRef]
- Li, D.; Chen, Y.; Zhang, T.; Lv, Z.; Zhang, L.; Li, X.; Zhang, A. Profiling microRNA from peripheral blood mononuclear cells in early-onset familial Alzheimer’s disease. Neuroreport 2023, 34, 178–183. [Google Scholar] [CrossRef]
- Yu, Y.; Chang, Z.; Han, C.; Zhuang, L.; Zhou, C.; Qi, X.; Peng, Z. Long non-coding RNA MINCR aggravates colon cancer via regulating miR-708-5p-mediated Wnt/β-catenin pathway. Biomed. Pharmacother. 2020, 129, 110292. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Y.; Zhu, H.; Ma, C.; Dai, X.; Qin, C. Downregulated microRNA-222 is correlated with increased p27Kip1 expression in a double transgenic mouse model of Alzheimer’s disease. Mol. Med. Rep. 2015, 12, 7687–7692. [Google Scholar] [CrossRef]
- Duan, Q.; Si, E. MicroRNA-25 aggravates Aβ1-42-induced hippocampal neuron injury in Alzheimer’s disease by downregulating KLF2 via the Nrf2 signaling pathway in a mouse model. J. Cell. Biochem. 2019, 120, 15891–15905. [Google Scholar] [CrossRef]
- Tan, S.; Ohtsuka, T.; González, A.; Kageyama, R. Micro RNA 9 regulates neural stem cell differentiation by controlling H es1 expression dynamics in the developing brain. Genes Cells 2012, 17, 952–961. [Google Scholar] [CrossRef]
- Ryu, I.S.; Kim, D.H.; Cho, H.-J.; Ryu, J.-H. The role of microRNA-485 in neurodegenerative diseases. Rev. Neurosci. 2023, 34, 49–62. [Google Scholar] [CrossRef]
- Kim, J.; Yoon, H.; Horie, T.; Burchett, J.M.; Restivo, J.L.; Rotllan, N.; Ramírez, C.M.; Verghese, P.B.; Ihara, M.; Hoe, H.-S.; et al. microRNA-33 Regulates ApoE Lipidation and Amyloid-β Metabolism in the Brain. J. Neurosci. Off. J. Soc. Neurosci. 2015, 35, 14717–14726. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Huang, B.; Yang, L.; Chen, K.; Zhao, D.; Luo, X.; Wang, Y. Downregulation of miR-33 Has Protective Effect Against Aβ25–35-Induced Injury in SH-SH-SY5Y Cells. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e921026. [Google Scholar] [CrossRef]
- Li, W.; Zheng, Y. MicroRNAs in Extracellular Vesicles of Alzheimer’s Disease. Cells 2023, 12, 1378. [Google Scholar] [CrossRef] [PubMed]
- Jahangard, Y.; Monfared, H.; Moradi, A.; Zare, M.; Mirnajafi-Zadeh, J.; Mowla, S.J. Therapeutic Effects of Transplanted Exosomes Containing miR-29b to a Rat Model of Alzheimer’s Disease. Front. Neurosci. 2020, 14, 564. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, M.; Bosco, P.; Tamburello, L.; Barbagallo, C.; Condorelli, A.G.; Tornitore, M.; Spada, R.S.; Barbagallo, D.; Scalia, M.; Elia, M.; et al. miRNAs Plasma Profiles in Vascular Dementia: Biomolecular Data and Biomedical Implications. Front. Cell. Neurosci. 2016, 10, 51. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Q.; Niu, J.; Lu, K.; Xie, B.; Cui, D.; Xu, S. Screening of microRNAs associated with Alzheimer’s disease using oxidative stress cell model and different strains of senescence accelerated mice. J. Neurol. Sci. 2014, 338, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.G.; Li, Y.Y.; Zhao, Y.; Bhattacharjee, S.; Lukiw, W.J. Differential Regulation of Interleukin-1 Receptor-associated Kinase-1 (IRAK-1) and IRAK-2 by MicroRNA-146a and NF-κB in Stressed Human Astroglial Cells and in Alzheimer Disease. J. Biol. Chem. 2010, 285, 38951–38960. [Google Scholar] [CrossRef]
- Zhan-Qiang, H.; Hai-Hua, Q.; Chi, Z.; Miao, W.; Cui, Z.; Zi-Yin, L.; Jing, H.; Yi-Wei, W. miR-146a aggravates cognitive impairment and Alzheimer disease-like pathology by triggering oxidative stress through MAPK signaling. Neurologia 2023, 38, 486–494. [Google Scholar] [CrossRef]
- Lei, B.; Liu, J.; Yao, Z.; Xiao, Y.; Zhang, X.; Zhang, Y.; Xu, J. NF-κB-Induced Upregulation of miR-146a-5p Promoted Hippocampal Neuronal Oxidative Stress and Pyroptosis via TIGAR in a Model of Alzheimer’s Disease. Front. Cell. Neurosci. 2021, 15, 653881. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Kuiperij, H.B.; Claassen, J.A.; Küsters, B.; Verbeek, M.M. MicroRNAs in Alzheimer’s disease: Differential expression in hippocampus and cell-free cerebrospinal fluid. Neurobiol. Aging 2014, 35, 152–158. [Google Scholar] [CrossRef]
- Sarkar, S.; Jun, S.; Rellick, S.; Quintana, D.D.; Cavendish, J.Z.; Simpkins, J.W. Expression of microRNA-34a in Alzheimer’s disease brain targets genes linked to synaptic plasticity, energy metabolism, and resting state network activity. Brain Res. 2016, 1646, 139–151. [Google Scholar] [CrossRef]
- Sun, C.; Jia, N.; Li, R.; Zhang, Z.; Zhong, Y.; Han, K. miR-143-3p inhibition promotes neuronal survival in an Alzheimer’s disease cell model by targeting neuregulin-1. Folia Neuropathol. 2020, 58, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Tomashevski, A.; Husseman, J.; Jin, L.-W.; Nochlin, D.; Vincent, I. Constitutive Wee1 activity in adult brain neurons with M phase-type alterations in Alzheimer neurodegeneration. J. Alzheimer’s Dis. JAD 2001, 3, 195–207. [Google Scholar] [CrossRef]
- Chow, N.; Cox, C.; Callahan, L.M.; Weimer, J.M.; Guo, L.; Coleman, P.D. Expression profiles of multiple genes in single neurons of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 1998, 95, 9620–9625. [Google Scholar] [CrossRef]
- Rouault, J.-P.; Falette, N.; Guéhenneux, F.; Guillot, C.; Rimokh, R.; Wang, Q.; Berthet, C.; Moyret-Lalle, C.; Savatier, P.; Pain, B.; et al. Identification of BTG2, an antiproliferative p53–dependent component of the DNA damage cellular response pathway. Nat. Genet. 1996, 14, 482–486. [Google Scholar] [CrossRef]
- Yuniati, L.; Scheijen, B.; Van Der Meer, L.T.; Van Leeuwen, F.N. Tumor suppressors BTG1 and BTG2: Beyond growth control. J. Cell. Physiol. 2019, 234, 5379–5389. [Google Scholar] [CrossRef] [PubMed]
- Wheaton, K.; Muir, J.; Ma, W.; Benchimol, S. BTG2 antagonizes Pin1 in response to mitogens and telomere disruption during replicative senescence. Aging Cell 2010, 9, 747–760. [Google Scholar] [CrossRef]
- Wang, Y.; Lian, M.; Zhou, J.; Wu, S. Brain Dicer1 Is Down-Regulated in a Mouse Model of Alzheimer’s Disease Via Aβ42-Induced Repression of Nuclear Factor Erythroid 2-Related Factor 2. Mol. Neurobiol. 2020, 57, 4417–4437. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-F.; Liu, Y.-P.; Lin, Y.-F.; Hsu, C.-F.; Lin, H.; Chang, W.-C.; Pan, C.-M.; Chou, T.-Y.; Wu, C.-W. TAZ negatively regulates the novel tumor suppressor ANKRD52 and promotes PAK1 dephosphorylation in lung adenocarcinomas. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2021, 1868, 118891. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Nakagawa, Y.; Tsuji, A.; Kitagishi, Y.; Nakanishi, A.; Murai, T. Implications of PI3K/AKT/PTEN Signaling on Superoxide Dismutases Expression and in the Pathogenesis of Alzheimer’s Disease. Diseases 2018, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Tamguney, T.; Stokoe, D. New insights into PTEN. J. Cell Sci. 2007, 120, 4071–4079. [Google Scholar] [CrossRef] [PubMed]
- Moreira, N.C.D.S.; Tamarozzi, E.R.; Lima, J.E.B.D.F.; Piassi, L.D.O.; Carvalho, I.; Passos, G.A.; Sakamoto-Hojo, E.T. Novel Dual AChE and ROCK2 Inhibitor Induces Neurogenesis via PTEN/AKT Pathway in Alzheimer’s Disease Model. Int. J. Mol. Sci. 2022, 23, 14788. [Google Scholar] [CrossRef]
- Xu, X.; Shen, X.; Feng, W.; Yang, D.; Jin, L.; Wang, J.; Wang, M.; Ting, Z.; Xue, F.; Zhang, J.; et al. D-galactose induces senescence of glioblastoma cells through YAP-CDK6 pathway. Aging 2020, 12, 18501–18521. [Google Scholar] [CrossRef]
- Zhang, P.; Kishimoto, Y.; Grammatikakis, I.; Gottimukkala, K.; Cutler, R.G.; Zhang, S.; Abdelmohsen, K.; Bohr, V.A.; Misra Sen, J.; Gorospe, M.; et al. Senolytic therapy alleviates Aβ-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer’s disease model. Nat. Neurosci. 2019, 22, 719–728. [Google Scholar] [CrossRef]
- Kuhla, A.; Ludwig, S.C.; Kuhla, B.; Münch, G.; Vollmar, B. Advanced glycation end products are mitogenic signals and trigger cell cycle reentry of neurons in Alzheimer’s disease brain. Neurobiol. Aging 2015, 36, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Hoozemans, J.J.M.; Brückner, M.K.; Rozemuller, A.J.M.; Veerhuis, R.; Eikelenboom, P.; Arendt, T. Cyclin D1 and Cyclin E Are Co-Localized with Cyclo-Oxygenase 2 (COX-2) in Pyramidal Neurons in Alzheimer Disease Temporal Cortex. J. Neuropathol. Exp. Neurol. 2002, 61, 678–688. [Google Scholar] [CrossRef] [PubMed]

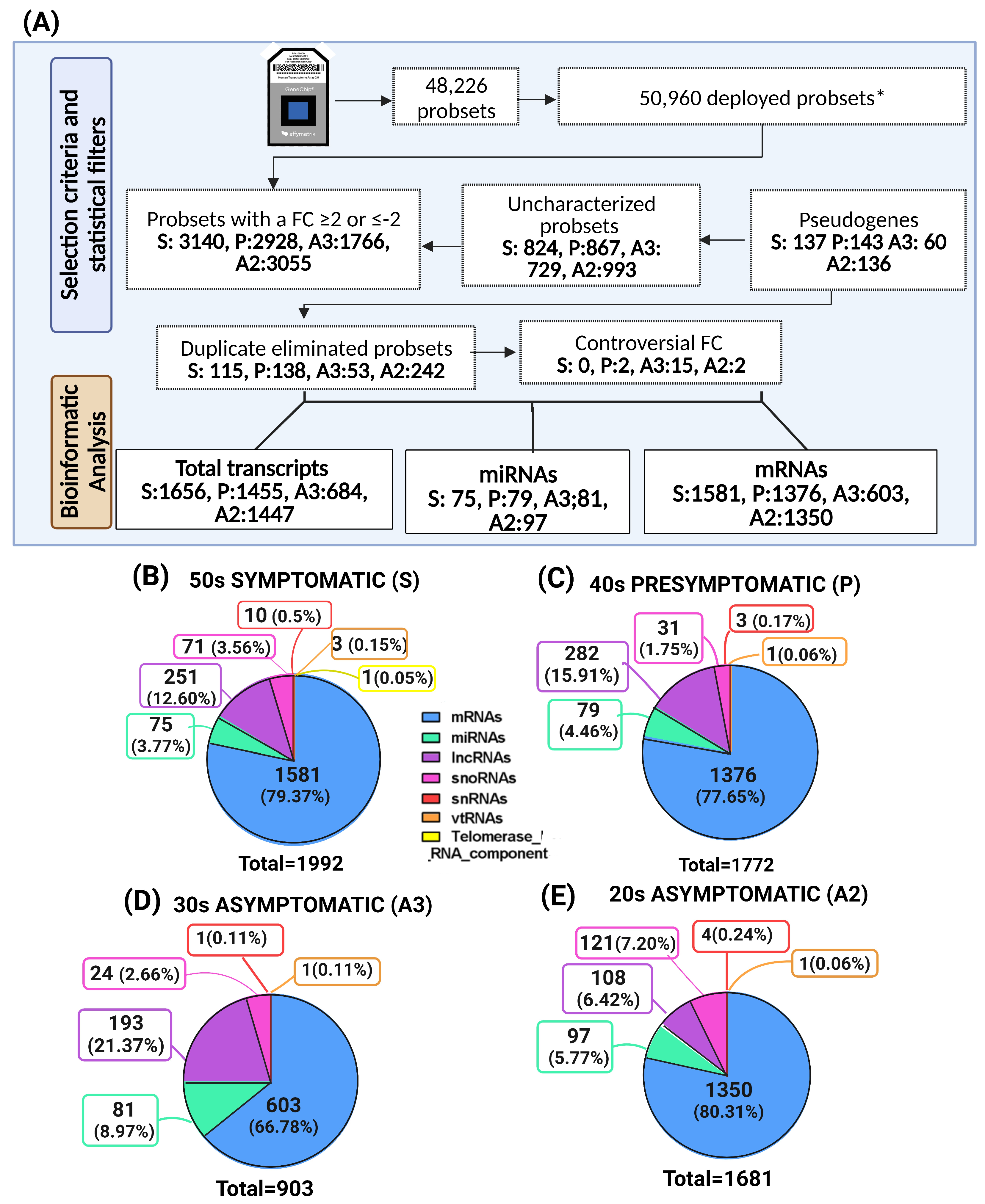
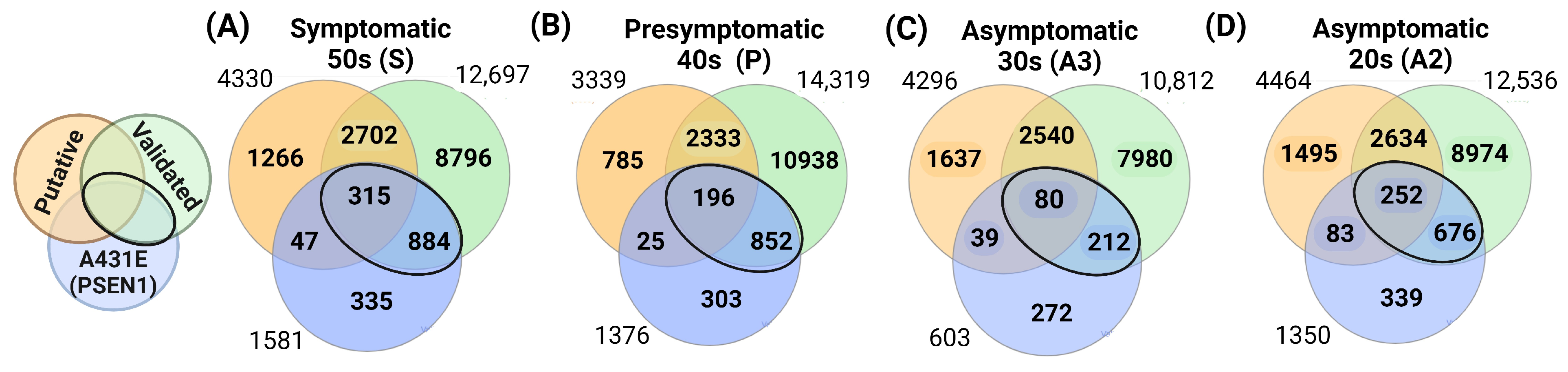


| Filtered by FC (≤2, ≥−2) | 3140 | 2928 | 1766 | 3055 |
|---|---|---|---|---|
| Symptomatic S | Presymptomatic P | Asymptomatic A3 | Asymptomatic A2 | |
| Repeated | 115 | 138 | 53 | 242 |
| Controversial FC | 0 | 2 | 15 | 2 |
| Uncharacterized | 824 | 867 | 729 | 993 |
| Pseudogenes | 137 | 143 | 60 | 136 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rochín-Hernández, L.J.; Rochín-Hernández, L.S.; Padilla-Cristerna, M.L.; Duarte-García, A.; Jiménez-Acosta, M.A.; Figueroa-Corona, M.P.; Meraz-Ríos, M.A. Mesenchymal Stem Cells from Familial Alzheimer’s Patients Express MicroRNA Differently. Int. J. Mol. Sci. 2024, 25, 1580. https://doi.org/10.3390/ijms25031580
Rochín-Hernández LJ, Rochín-Hernández LS, Padilla-Cristerna ML, Duarte-García A, Jiménez-Acosta MA, Figueroa-Corona MP, Meraz-Ríos MA. Mesenchymal Stem Cells from Familial Alzheimer’s Patients Express MicroRNA Differently. International Journal of Molecular Sciences. 2024; 25(3):1580. https://doi.org/10.3390/ijms25031580
Chicago/Turabian StyleRochín-Hernández, Lory J., Lory S. Rochín-Hernández, Mayte L. Padilla-Cristerna, Andrea Duarte-García, Miguel A. Jiménez-Acosta, María P. Figueroa-Corona, and Marco A. Meraz-Ríos. 2024. "Mesenchymal Stem Cells from Familial Alzheimer’s Patients Express MicroRNA Differently" International Journal of Molecular Sciences 25, no. 3: 1580. https://doi.org/10.3390/ijms25031580
APA StyleRochín-Hernández, L. J., Rochín-Hernández, L. S., Padilla-Cristerna, M. L., Duarte-García, A., Jiménez-Acosta, M. A., Figueroa-Corona, M. P., & Meraz-Ríos, M. A. (2024). Mesenchymal Stem Cells from Familial Alzheimer’s Patients Express MicroRNA Differently. International Journal of Molecular Sciences, 25(3), 1580. https://doi.org/10.3390/ijms25031580






