Characterization of Progranulin Gene Mutations in Portuguese Patients with Frontotemporal Dementia
Abstract
1. Introduction
2. Results
3. Discussion
4. Material and Methods
4.1. Participants
4.2. Clinical and Neuropsychological Evaluations
4.3. Laboratory Determinations
4.3.1. GRN Sequencing Analysis
4.3.2. PGRN Level Determination
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Knopman, D.S.; Roberts, R.O. Estimating the Number of Persons with Frontotemporal Lobar Degeneration in the US Population. J. Mol. Neurosci. 2011, 45, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Seltman, R.E.; Matthews, B.R. Frontotemporal Lobar Degeneration. CNS Drugs 2012, 26, 841–870. [Google Scholar] [CrossRef] [PubMed]
- Logroscino, G.; Piccininni, M.; Graff, C.; Hardiman, O.; Ludolph, A.C.; Moreno, F.; Otto, M.; Remes, A.M.; Rowe, J.B.; Seelaar, H.; et al. Incidence of Syndromes Associated With Frontotemporal Lobar Degeneration in 9 European Countries. JAMA Neurol. 2023, 80, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Snowden, J.S.; Goulding, P.J.; Neary, D. Semantic dementia: A form of circumscribed cerebral atrophy. Behav. Neurol. 1989, 2, 167–182. [Google Scholar] [CrossRef]
- Hodges, J.R.; Patterson, K. Semantic dementia: A unique clinicopathological syndrome. Lancet Neurol. 2007, 6, 1004–1014. [Google Scholar] [CrossRef]
- Mesulam, M.-M. Slowly progressive aphasia without generalized dementia. Ann. Neurol. 1982, 11, 592–598. [Google Scholar] [CrossRef]
- Mesulam, M.-M. Primary progressive aphasia. Ann. Neurol. 2001, 49, 425–432. [Google Scholar] [CrossRef]
- Gorno-Tempini, M.L.; Dronkers, N.F.; Rankin, K.P.; Ogar, J.M.; Phengrasamy, L.; Rosen, H.J.; Johnson, J.K.; Weiner, M.W.; Miller, B.L. Cognition and anatomy in three variants of primary progressive aphasia. Ann. Neurol. 2004, 55, 335–346. [Google Scholar] [CrossRef]
- Johnson, J.K.; Diehl, J.; Mendez, M.F.; Neuhaus, J.; Shapira, J.S.; Forman, M.; Chute, D.J.; Roberson, E.D.; Pace-Savitsky, C.; Neumann, M.; et al. Frontotemporal lobar degeneration: Demographic characteristics of 353 patients. Arch. Neurol. 2005, 62, 925–930. [Google Scholar] [CrossRef]
- Lomen-Hoerth, C.; Anderson, T.; Miller, B. The overlap of amyotrophic lateral sclerosis and frontotemporal dementia. Neurology 2002, 59, 1077–1079. [Google Scholar] [CrossRef]
- Kertesz, A.; Martinez-Lage, P.; Davidson, W.; Munoz, D.G. The corticobasal degeneration syndrome overlaps progressive aphasia and frontotemporal dementia. Neurology 2000, 55, 1368–1375. [Google Scholar] [CrossRef] [PubMed]
- Kertesz, A.; McMonagle, P.; Blair, M.; Davidson, W.; Munoz, D.G. The evolution and pathology of frontotemporal dementia. Brain 2005, 128, 1996–2005. [Google Scholar] [CrossRef] [PubMed]
- Boeve, B.F. Links between frontotemporal lobar degeneration, corticobasal degeneration, progressive supranuclear palsy, and amyotrophic lateral sclerosis. Alzheimer Dis. Assoc. Disord. 2007, 21, S31–S38. [Google Scholar] [CrossRef] [PubMed]
- Hutton, M.; Lendon, C.L.; Rizzu, P.; Baker, M.; Froelich, S.; Houlden, H.; Pickering-Brown, S.; Chakraverty, S.; Isaacs, A.; Grover, A.; et al. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 1998, 393, 702–705. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.; Mackenzie, I.R.; Pickering-Brown, S.M.; Gass, J.; Rademakers, R.; Lindholm, C.; Snowden, J.; Adamson, J.; Sadovnick, A.D.; Rollinson, S.; et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 2006, 442, 916–919. [Google Scholar] [CrossRef]
- Cruts, M.; Gijselinck, I.; van der Zee, J.; Engelborghs, S.; Wils, H.; Pirici, D.; Rademakers, R.; Vandenberghe, R.; Dermaut, B.; Martin, J.J.; et al. Null mutations in progranulin cause ubiquitin positive frontotemporal dementia linked to chromosome 17q21. Nature 2006, 442, 920–924. [Google Scholar] [CrossRef]
- Dejesus-Hernandez, M.; Mackenzie, I.R.; Boeve, B.F.; Boxer, A.L.; Baker, M.; Rutherford, N.J.; Nicholson, A.M.; Finch, N.A.; Flynn, H.; Adamson, J.; et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 2011, 72, 245–256. [Google Scholar] [CrossRef]
- Renton, A.E.; Majounie, E.; Waite, A.; Simón-Sánchez, J.; Rollinson, S.; Gibbs, J.R.; Schymick, J.C.; Laaksovirta, H.; van Swieten, J.C.; Myllykangas, L.; et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 2011, 72, 257–268. [Google Scholar] [CrossRef]
- Moore, K.M.; Nicholas, J.; Grossman, M.; McMillan, C.T.; Irwin, D.J.; Massimo, L.; Van Deerlin, V.M.; Warren, J.D.; Fox, N.C.; Rossor, M.N.; et al. FTD Prevention Initiative. Age at symptom onset and death and disease duration in genetic frontotemporal dementia: An international retrospective cohort study. Lancet Neurol. 2020, 19, 145–156. [Google Scholar] [CrossRef]
- Gass, J.; Cannon, A.; Mackenzie, I.R.; Boeve, B.; Baker, M.; Adamson, J.; Crook, R.; Melquist, S.; Kuntz, K.; Petersen, R.; et al. Mutations in progranulin are a major cause of ubiquitin-positive frontotemporal lobar degeneration. Hum. Mol. Genet. 2006, 15, 2988–3001. [Google Scholar] [CrossRef]
- Gijselinck, I.; Van Broeckhoven, C.; Cruts, M. Granulin mutations associated with frontotemporal lobar degeneration and related disorders: An update. Hum. Mutat. 2008, 29, 1373–1386. [Google Scholar] [CrossRef] [PubMed]
- Van der Zee, J.; Le Ber, I.; Maurer-Stroh, S.; Engelborghs, S.; Gijselinck, I.; Camuzat, A.; Brouwers, N.; Vandenberghe, R.; Sleegers, K.; Hannequin, D.; et al. Mutations other than null mutations producing a pathogenic loss of progranulin in frontotemporal dementia. Hum. Mutat. 2007, 28, 416. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.R.; Baldeiras, I.; Ribeiro, M.H.; Santiago, B.; Machado, C.; Massano, J.; Guimarães, J.; Resende Oliveira, C.; Santana, I. Progranulin peripheral levels as a screening tool for the identification of subjects with progranulin mutations in a Portuguese cohort. Neurodegener. Dis. 2014, 13, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Elia, L.P.; Reisine, T.; Alijagic, A.; Finkbeiner, S. Approaches to develop therapeutics to treat frontotemporal dementia. Neuropharmacology 2020, 166, 107948. [Google Scholar] [CrossRef] [PubMed]
- Rhinn, H.; Tatton, N.; McCaughey, S.; Kurnellas, M.; Rosenthal, A. Progranulin as a therapeutic target in neurodegenerative diseases. Trends Pharmacol. Sci. 2022, 43, 641–652. [Google Scholar] [CrossRef]
- Terryn, J.; Verfaillie, C.M.; Van Damme, P. Tweaking Progranulin Expression: Therapeutic Avenues and Opportunities. Front. Mol. Neurosci. 2021, 14, 713031. [Google Scholar] [CrossRef] [PubMed]
- Bocchetta, M.; Todd, E.G.; Bouzigues, A.; Cash, D.M.; Nicholas, J.M.; Convery, R.S.; Russell, L.L.; Thomas, D.L.; Malone, I.B.; Iglesias, J.E.; et al. Structural MRI predicts clinical progression in presymptomatic genetic frontotemporal dementia: Findings from the GENetic Frontotemporal dementia Initiative cohort. Brain Commun. 2023, 5, fcad061. [Google Scholar] [CrossRef]
- Panman, J.L.; Venkatraghavan, V.; van der Ende, E.L.; Steketee, R.M.E.; Jiskoot, L.C.; Poos, J.M.; Dopper, E.G.P.; Meeter, L.H.H.; Kaat, L.D.; Rombouts, S.A.R.B.; et al. Modelling the cascade of biomarker changes in GRN-related frontotemporal dementia. J. Neurol. Neurosurg. Psychiatry 2021, 92, 494–501. [Google Scholar] [CrossRef]
- Jiskoot, L.C.; Panman, J.L.; Meeter, L.H.; Dopper, E.G.; Donker Kaat, L.; Franzen, S.; van der Ende, E.L.; van Minkelen, R.; Rombouts, S.A.R.B.; Papma, J.M.; et al. Longitudinal multimodal MRI as prognostic and diagnostic biomarker in presymptomatic familial frontotemporal dementia. Brain 2019, 142, 193–208. [Google Scholar] [CrossRef]
- Jiskoot, L.C.; Panman, J.L.; van Asseldonk, L.; Franzen, S.; Meeter, L.H.; Donker Kaat, L.; van der Ende, E.L.; Dopper, E.G.P.; Timman, R.; van Minkelen, R.; et al. Longitudinal cognitive biomarkers predicting symptom onset in presymptomatic frontotemporal dementia. J. Neurol. 2018, 265, 1381–1392. [Google Scholar] [CrossRef]
- Jin, S.C.; Pastor, P.; Cooper, B.; Cervantes, S.; Benitez, B.A.; Razquin, C.; Goate, A.; Ibero-American Alzheimer Disease Genetics Group Researchers; Cruchaga, C. Pooled-DNA sequencing identifies novel causative variants in PSEN1, GRN and MAPT in a clinical early-onset and familial Alzheimer’s disease Ibero-American cohort. Alzheimers Res. Ther. 2012, 4, 34. [Google Scholar] [CrossRef] [PubMed]
- Pires, C.; Coelho, M.; Valadas, A.; Barroso, C.; Pimentel, J.; Martins, M.; Duyckaerts, C.; de Mendonça, A.; Verdelho, A.; Miltenberger-Miltenyi, G. Phenotypic variability of familial and sporadic Progranulin p.Gln257Profs*27 mutation. J. Alzheimers Dis. 2013, 37, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Faber, I.; Prota, J.R.; Martinez, A.R.; Lopes-Cendes, I.; França, M.C., Jr. A new phenotype associated with homozygous GRN mutations: Complicated spastic paraplegia. Eur. J. Neurol. 2017, 24, e3–e4. [Google Scholar] [CrossRef] [PubMed]
- Huin, V.; Barbier, M.; Bottani, A.; Lobrinus, J.A.; Clot, F.; Lamari, F.; Chat, L.; Rucheton, B.; Fluchère, F.; Auvin, S.; et al. Homozygous GRN mutations: New phenotypes and new insights into pathological and molecular mechanisms. Brain 2020, 143, 303–319. [Google Scholar] [CrossRef] [PubMed]
- Takada, L.T.; Bahia, V.S.; Guimarães, H.C.; Costa, T.V.; Vale, T.C.; Rodriguez, R.D.; Porto, F.H.; Machado, J.C.; Beato, R.G.; Cesar, K.G.; et al. GRN and MAPT Mutations in 2 Frontotemporal Dementia Research Centers in Brazil. Alzheimer Dis. Assoc. Disord. 2016, 30, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, R.J.; Santana, I.; Bras, J.M.; Revesz, T.; Rebelo, O.; Ribeiro, M.H.; Santiago, B.; Oliveira, C.R.; Singleton, A.; Hardy, J. Novel progranulin mutation: Screening for PGRN mutations in a Portuguese series of FTD/CBS cases. Mov. Disord. 2008, 23, 1269–1273. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.R.; Macário, M.C.; Ramos, L.; Baldeiras, I.; Ribeiro, M.H.; Santana, I. Portuguese family with the co-occurrence of frontotemporal lobar degeneration and neuronal ceroid lipofuscinosis phenotypes due to progranulin gene mutation. Neurobiol. Aging 2016, 41, 200.e1–200.e5. [Google Scholar] [CrossRef]
- Taipa, R.; Tuna, A.; Damásio, J.; Pinto, P.S.; Cavaco, S.; Pereira, S.; Milterberger-Miltenyi, G.; Galimberti, D.; Melo-Pires, M. Clinical, neuropathological, and genetic characteristics of the novel IVS9+1delG GRN mutation in a patient with frontotemporal dementia. J. Alzheimers Dis. 2012, 30, 83–90. [Google Scholar] [CrossRef]
- Lima, M.; Tábuas-Pereira, M.; Duro, D.; Durães, J.; Vieira, D.; Baldeiras, I.; Almeida, M.R.; Santana, I. Neuropsychological features of progranulin-associated frontotemporal dementia: A nested case-control study. Neural Regen Res. 2021, 16, 910–915. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. ACMG Laboratory Quality Assurance Committee Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Fostinelli, S.; Ciani, M.; Zanardini, R.; Zanetti, O.; Binetti, G.; Ghidoni, R.; Benussi, L. The Heritability of Frontotemporal Lobar Degeneration: Validation of Pedigree Classification Criteria in a Northern Italy Cohort. J. Alzheimers Dis. 2018, 61, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Borroni, B.; Bonvicini, C.; Galimberti, D.; Tremolizzo, L.; Papetti, A.; Archetti, S.; Turla, M.; Alberici, A.; Agosti, C.; Premi, E.; et al. Founder effect and estimation of the age of the Progranulin Thr272fs mutation in 14 Italian pedigrees with frontotemporal lobar degeneration. Neurobiol. Aging 2011, 32, 555.e1–555.e8. [Google Scholar] [CrossRef] [PubMed]
- Moreno, F.; Indakoetxea, B.; Barandiaran, M.; Alzualde, A.; Gabilondo, A.; Estanga, A.; Ruiz, J.; Ruibal, M.; Bergareche, A.; Martí-Massó, J.F.; et al. “Frontotemporoparietal” dementia: Clinical phenotype associated with the c.709-1G>A PGRN mutation. Neurology 2009, 73, 1367–1374. [Google Scholar] [CrossRef] [PubMed]
- Moreno, F.; Alzualde, A.; Camblor, P.M.; Barandiaran, M.; Van Deerlin, V.M.; Gabilondo, A.; Martí Massó, J.F.; López de Munain, A.; Indakoetxea, B. Prion protein codon 129 polymorphism modifies age at onset of frontotemporal dementia with the C.709- 1G>A progranulin mutation. Alzheimer Dis. Assoc. Disord. 2011, 25, 93–95. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brouwers, N.; Nuytemans, K.; van der Zee, J.; Gijselinck, I.; Engelborghs, S.; Theuns, J.; Kumar-Singh, S.; Pickut, B.A.; Pals, P.; Dermaut, B.; et al. Alzheimer and Parkinson diagnoses in progranulin null mutation carriers in an extended founder family. Arch. Neurol. 2007, 64, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- Wauters, E.; Van Mossevelde, S.; Sleegers, K.; van der Zee, J.; Engelborghs, S.; Sieben, A.; Vandenberghe, R.; Philtjens, S.; Van den Broeck, M.; Peeters, K.; et al. Clinical variability and onset age modifiers in an extended Belgian GRN founder family. Neurobiol. Aging 2018, 67, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.R.; Damiano, J.; Franceschetti, S.; Carpenter, S.; Canafoglia, L.; Morbin, M.; Rossi, G.; Pareyson, D.; Mole, S.E.; Staropoli, J.F.; et al. Strikingly different clinicopathological phenotypes determined by progranulin-mutation dosage. Am. J. Hum. Genet. 2012, 90, 1102–1107. [Google Scholar] [CrossRef]
- Sellami, L.; Rucheton, B.; Ben Younes, I.; Camuzat, A.; Saracino, D.; Rinaldi, D.; Epelbaum, S.; Azuar, C.; Levy, R.; Auriacombe, S.; et al. Plasma progranulin levels for frontotemporal dementia in clinical practice: A 10-year French experience. Neurobiol. Aging 2020, 91, 167.e1–167.e9. [Google Scholar] [CrossRef]
- Le Ber, I.; van der Zee, J.; Hannequin, D.; Gijselinck, I.; Campion, D.; Puel, M.; Laquerrière, A.; De Pooter, T.; Camuzat, A.; Van den Broeck, M.; et al. Progranulin null mutations in both sporadic and familial frontotemporal dementia. Hum. Mutat. 2007, 28, 846–855. [Google Scholar] [CrossRef]
- Nalls, M.A.; Blauwendraat, C.; Sargent, L.; Vitale, D.; Leonard, H.; Iwaki, H.; Song, Y.; Bandres-Ciga, S.; Menden, K.; Faghri, F.; et al. Evidence for GRN connecting multiple neurodegenerative diseases. Brain Commun. 2021, 3, fcab095. [Google Scholar] [CrossRef]
- Vardarajan, B.N.; Reyes-Dumeyer, D.; Piriz, A.L.; Lantigua, R.A.; Medrano, M.; Rivera, D.; Jiménez-Velázquez, I.Z.; Martin, E.; Pericak-Vance, M.A.; Bush, W.; et al. Progranulin mutations in clinical and neuropathological Alzheimer’s disease. Alzheimers Dement. 2022, 18, 2458–2467. [Google Scholar] [CrossRef] [PubMed]
- Root, J.; Mendsaikhan, A.; Nandy, S.; Taylor, G.; Wang, M.; Troiano Araujo, L.; Merino, P.; Ryu, D.; Holler, C.; Thompson, B.M.; et al. Granulins rescue inflammation, lysosome dysfunction, and neuropathology in a mouse model of progranulin deficiency. bioRxiv 2023. [Google Scholar] [CrossRef]
- Neary, D.; Snowden, J.S.; Gustafson, L.; Passant, U.; Stuss, D.; Black, S.; Freedman, M.; Kertesz, A.; Robert, P.H.; Albert, M.; et al. Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology 1998, 51, 1546–1554. [Google Scholar] [CrossRef] [PubMed]
- The Lund and Manchester Groups. Clinical and neuropathological criteria for frontotemporal dementia. J. Neurol. Neurosurg. Psychiatry 1994, 57, 416–418. [Google Scholar] [CrossRef] [PubMed]
- McKhann, G.M.; Albert, M.S.; Grossman, M.; Miller, B.; Dickson, D.; Trojanowski, J.Q. Clinical and pathological diagnosis of frontotemporal dementia: Report of the Work Group on Frontotemporal Dementia and Pick’s Disease. Arch. Neurol. 2001, 58, 1803–1809. [Google Scholar] [CrossRef] [PubMed]
- Rascovsky, K.; Hodges, J.R.; Knopman, D.; Mendez, M.F.; Kramer, J.H.; Neuhaus, J.; van Swieten, J.C.; Seelaar, H.; Dopper, E.G.; Onyike, C.U.; et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011, 134, 2456–2477. [Google Scholar] [CrossRef]
- Gorno-Tempini, M.L.; Hillis, A.E.; Weintraub, S.; Kertesz, A.; Mendez, M.; Cappa, S.F.; Ogar, J.M.; Rohrer, J.D.; Black, S.; Boeve, B.F.; et al. Classification of primary progressive aphasia and its variants. Neurology 2011, 76, 1006–1014. [Google Scholar] [CrossRef]
- Freitas, S.; Simões, M.R.; Alves, L.; Santana, I. The relevance of sociodemographic and health variables on MMSE normative data. Appl. Neuropsychol. Adult. 2015, 22, 311–319. [Google Scholar] [CrossRef]
- Freitas, S.; Simões, M.R.; Alves, L.; Santana, I. Montreal Cognitive Assessment (MoCA): Normative study for the Portuguese population. J. Clin. Exp. Neuropsychol. 2011, 33, 989–996. [Google Scholar] [CrossRef]
- Guerreiro, M. Contribution of Neuropsychology for the Study of Dementia. Unpublished Doctoral Dissertation, Faculty of Medicine, Lisbon, Portugal, 1998. [Google Scholar]
- Wechsler, D. Wechsler Memory Scale; Psychological Corporation: San Antonio, TX, USA, 1945. [Google Scholar]
| FTD Patients | Family Members | p-Value | |
|---|---|---|---|
| n | 39 | 6 | |
| Age, years | 57.0 [53.0–62.0] | 38.5 [35.3–45.3] | 0.003 |
| Gender F/M | 20/19 | 3/3 | 0.625 |
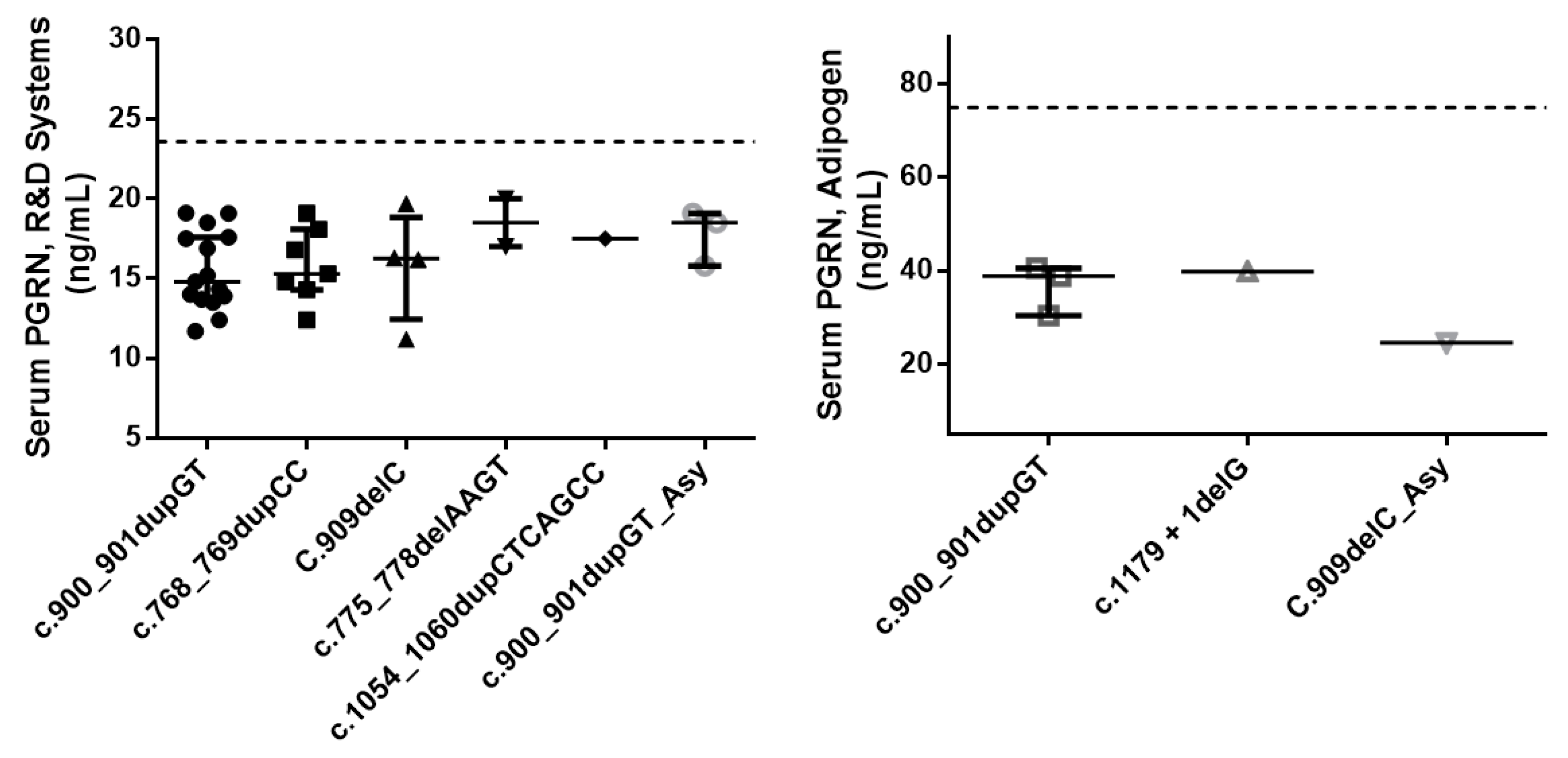
| Serum PGRN, ng/mL | |||
| R&D Systems (n = 30/3/1) | 15.9 [14.0–17.7] | 18.5 [17.2–18.8] | 0.235 |
| Adipogen (n = 4/1) | 39.3 [34.6–40.1] | 24.6 |
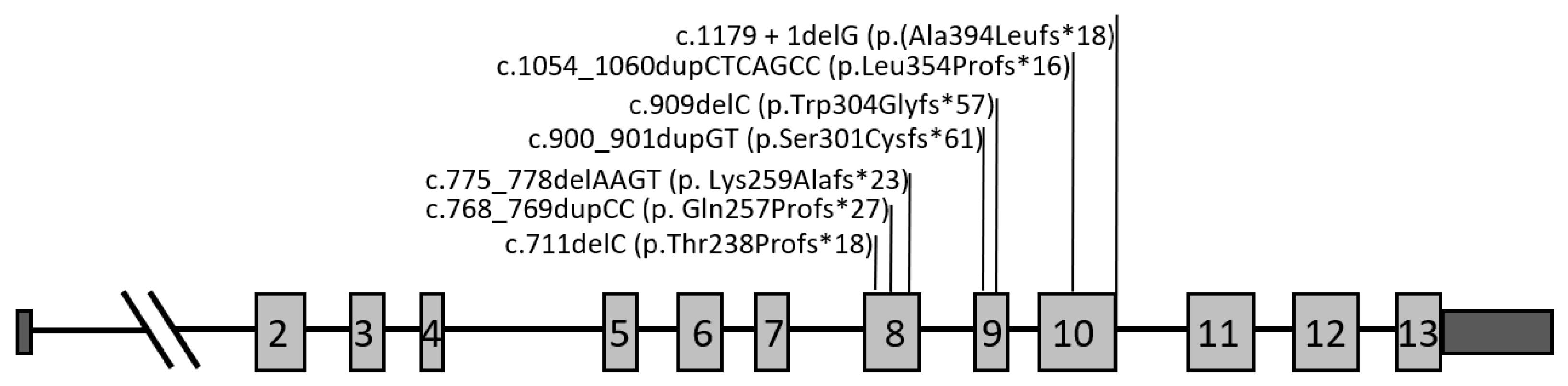
| Nucleotide Change | Predicted Protein Change | Location (Exon/Intron) | Total Number of Cases | Zygosity | Clinical Presentations | References |
|---|---|---|---|---|---|---|
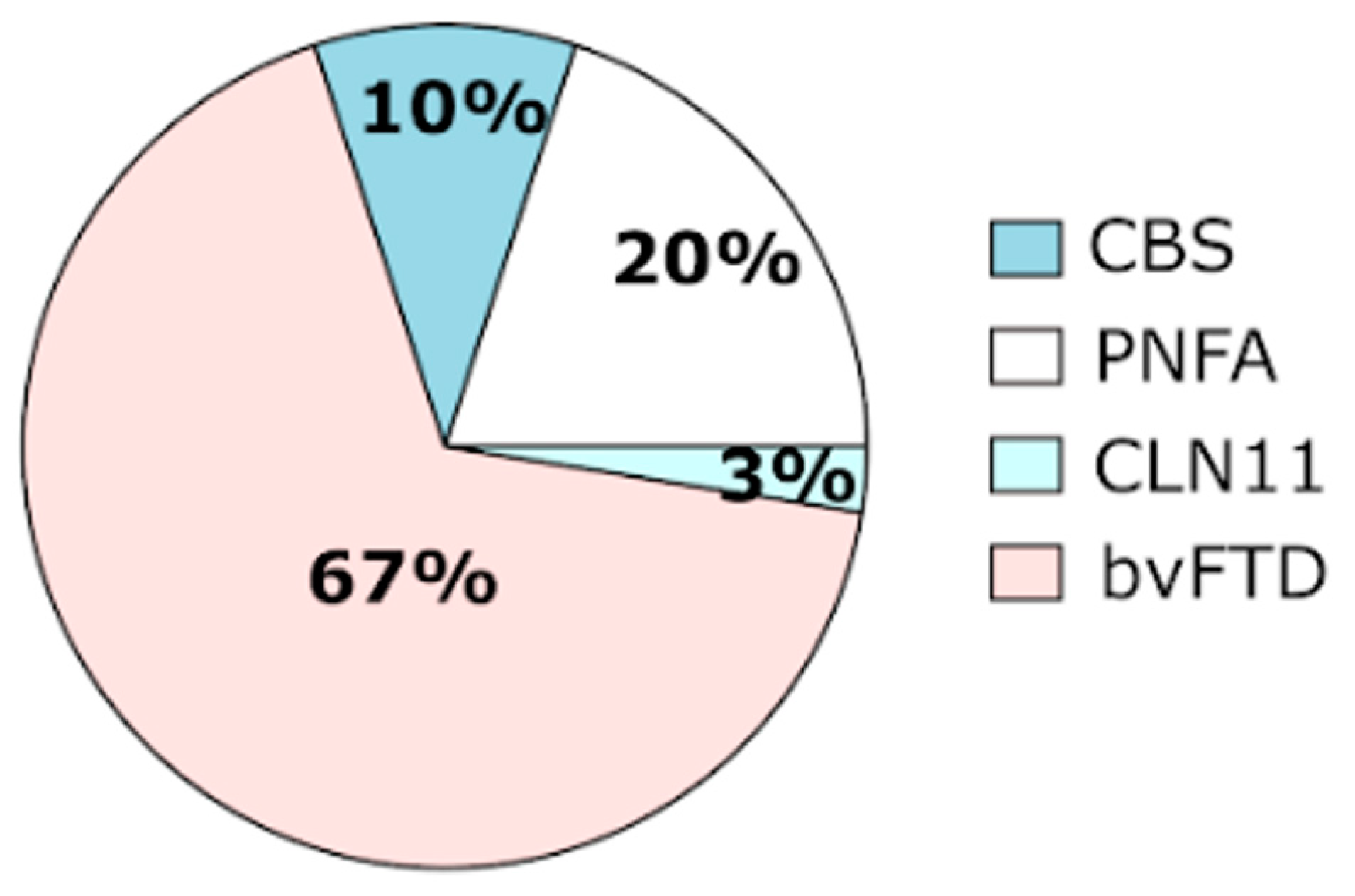
| c.711delC | p.Thr238Profs*18 | Exon 8 | 1 | Heterozygous | bvFTD | Novel |
| c.768_769dupCC | p.Gln257Profs*27 | Exon 8 | 8 | Heterozygous | bvFTD (n = 4) | [23,31,32,33,34] |
| PNFA (n = 4) | ||||||
| c.775_778delAAGT | p.Lys259Alafs*23 | Exon 8 | 2 | Heterozygous | bvFTD (n = 2) | [35] |
| c.900_901dupGT | p.Ser301Cysfs*61 | Exon 9 | 23 | Heterozygous | bvFTD (n = 12) | [23,36,37] |
| PNFA (n = 3) | ||||||
| CBS (n = 4) | ||||||
| Asymptomatic (n = 4) | ||||||
| 1 | Heterozygous | CLN11 (n = 1) | [37] | |||
| c.909delC | p.Trp304Glyfs*57 | Exon 9 | 6 | Heterozygous | bvFTD (n = 3) | [23] |
| PNFA (n = 1) | ||||||
| Asymptomatic (n = 2) | ||||||
| c.1054_1060dupCTCAGCC | p.Leu354Profs*16 | Exon 10 | 1 | Heterozygous | bvFTD | Novel |
| c.1179 + 1delG | p.(Ala394Leufs*18) | IVS10 | 4 | Heterozygous | bvFTD (n = 4) | [23,38] |
| GRN Gene | Variant | Variant Database | Disease Databases | Tools to Predict Pathogenicity | ||
| dbSNP | HGMD | ClinVar | VarSome | Franklin/Genoox | ||
| p.Thr238Profs*18 | - | - | Pathogenic | Pathogenic | Pathogenic | |
| p.Gln257Profs*27 | rs1567887004 | CI127616 DM | Pathogenic | Pathogenic | Pathogenic | |
| p.Lys259Alafs*23 | - | CD164461 DM | Pathogenic | Pathogenic | Pathogenic | |
| p.Ser301Cysfs*61 | - | CI085841 DM | - | Pathogenic | Likely pathogenic | |
| p.Trp304Glyfs*57 | rs63750366 | CD077404 DM | - | Pathogenic | Likely pathogenic | |
| p.Leu354Profs*16 | - | - | - | Likely pathogenic | Likely pathogenic | |
| p.(Ala394Leufs*18) | - | CD124917 DM | - | Likely pathogenic | Likely pathogenic | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, M.R.; Tábuas-Pereira, M.; Baldeiras, I.; Lima, M.; Durães, J.; Massano, J.; Pinto, M.; Cruto, C.; Santana, I. Characterization of Progranulin Gene Mutations in Portuguese Patients with Frontotemporal Dementia. Int. J. Mol. Sci. 2024, 25, 511. https://doi.org/10.3390/ijms25010511
Almeida MR, Tábuas-Pereira M, Baldeiras I, Lima M, Durães J, Massano J, Pinto M, Cruto C, Santana I. Characterization of Progranulin Gene Mutations in Portuguese Patients with Frontotemporal Dementia. International Journal of Molecular Sciences. 2024; 25(1):511. https://doi.org/10.3390/ijms25010511
Chicago/Turabian StyleAlmeida, Maria Rosário, Miguel Tábuas-Pereira, Inês Baldeiras, Marisa Lima, João Durães, João Massano, Madalena Pinto, Catarina Cruto, and Isabel Santana. 2024. "Characterization of Progranulin Gene Mutations in Portuguese Patients with Frontotemporal Dementia" International Journal of Molecular Sciences 25, no. 1: 511. https://doi.org/10.3390/ijms25010511
APA StyleAlmeida, M. R., Tábuas-Pereira, M., Baldeiras, I., Lima, M., Durães, J., Massano, J., Pinto, M., Cruto, C., & Santana, I. (2024). Characterization of Progranulin Gene Mutations in Portuguese Patients with Frontotemporal Dementia. International Journal of Molecular Sciences, 25(1), 511. https://doi.org/10.3390/ijms25010511






