Abstract
The COVID-19 pandemic has had a significant impact on the health and economy of the global population. Even after recovery from the disease, post-COVID-19 symptoms, such as pulmonary fibrosis, continue to be a concern. This narrative review aims to address pulmonary fibrosis (PF) from various perspectives, including the fibrotic mechanisms involved in idiopathic and COVID-19-induced pulmonary fibrosis. On the other hand, we also discuss the current therapeutic drugs in use, as well as those undergoing clinical or preclinical evaluation. Additionally, this article will address various biomarkers with usefulness for PF prediction, diagnosis, treatment, prognosis, and severity assessment in order to provide better treatment strategies for patients with this disease.
1. Introduction
Coronaviruses are a family of viruses that primarily target the human respiratory system. Historically, coronaviruses have affected humans, the most recent being severe acute respiratory syndrome (SARS-CoV) and Middle East Respiratory Syndrome (MERS-CoV), all of which pose a significant threat to public health worldwide [1]. Following the report of the first cases of a respiratory syndrome of unknown etiology in Wuhan City, Hubei Province (31 December 2019), authorities identified a novel coronavirus (SARS-CoV-2) that causes the clinical disease called coronavirus disease 19 (COVID-19) [2]. SARS-CoV-2 is a single-stranded RNA virus and has a size of 29,891 nucleotides. It also has several structural proteins: Spike (S), Envelope (E), Membrane (M), and Nucleocapsid (N) [3]. Globally, according to World Health Organization (WHO) data as of 28 December 2023, there were 773,119,173 million confirmed cases of COVID-19, including 6,990,067 deaths. In Mexico to the same date, 7,702,731 confirmed cases of COVID-19 and 334,947 deaths were reported [4,5].
1.1. Viral Transmission and Clinical Features of COVID-19 Disease
The SARS-CoV-2 virus can spread through small droplets or aerosols [6]. Sick individuals expel these particles when speaking, coughing, or sneezing. The particles can travel up to 1 m in the air before settling on environmental surfaces or the mucosa of nearby individuals [7]. The first step of SARS-CoV-2 viral infection is the recognition and binding of the receptor in host cells, then fusion with the cell membrane follows. The virus has been reported to target pulmonary epithelial cells, whereby transmission of SARS-CoV-2 occurs by binding the S protein receptor-binding domain with angiotensin-converting enzyme 2 (ACE2) receptors [8,9]. When the virus infects the host cell, its RNA genetic material is released into the cytoplasm. This process is known as uncoating [10]. Within the cytoplasm, RNA is transcribed to produce non-structural proteins (nsps) and replicate polyproteins, including RNA polymerase and RNA-dependent helicase. These form the RdRp complex, which is responsible for the transcription and replication of viral RNA. Subsequently, the viral structural proteins are translated. The proteins S, N, M, and E are processed in the membrane of the rough endoplasmic reticulum (ER) and then transported to the ER-Golgi intermediate compartment (ER-GIC), where they assemble with RNA to form viral particles. The final step is the release of the virions from the cell, in a process known as exocytosis [10,11]. The symptoms of COVID-19 are variable. The most common symptoms include fever, cough, fatigue, and headache. In severe cases, dyspnea and chest tightness may occur, which can lead to death [12,13]. The incubation period for SARS-CoV-2 is 6–7 days, while the time between illness onset and doctor visit is 4–5 days [13]. The mortality rate of the disease caused by the SARS-CoV-2 virus is lower than that of the aforementioned coronaviruses, with a fatality rate of 2–3% [14].
1.2. Post-COVID-19 Syndrome
People who manage to overcome SARS-CoV-2 infection usually present with a series of persistent symptoms; it was then that the terms prolonged COVID-19, post-COVID-19 syndrome, or long-term COVID-19 began to be used by the medical and scientific community to describe the symptoms that occur after week 12 of having started with the first symptoms [15,16]. According to various reports, the most commonly reported symptoms after COVID-19 include fatigue, dyspnea, chest pain, headache, cough, and hair loss. Some of these symptoms are associated with lung disease, such as cough, chest discomfort, decreased lung-diffusing capacity, sleep apnea, and pulmonary fibrosis (PF) [17,18]. The proportion of COVID-19 patients who remain asymptomatic is generally low. Two studies of COVID-19 survivors reported that only between 10.8% and 18.6% of patients were symptom-free after recovering from the disease [19,20]. All these reports reinforce that most of the symptoms present after post-COVID-19 infection are related to lung problems.
A meta-analysis recently revealed that one-third of individuals experience persistent fatigue, and more than one-fifth of individuals experience cognitive impairment for 12 or more weeks after being diagnosed with SARS-CoV-2. Furthermore, there is a correlation between acute illness severity, hospitalization, or longer hospital stays and post-COVID-19 symptoms or reduced quality of life [21]. Another study found that during post-COVID-19 pulmonary evaluations, the diffusing capacity for carbon monoxide (DLCO) was below 80%. Specifically, 29% of patients in the group with a scale of 4 (admitted to the hospital but requiring supplemental oxygen) and 56% of patients in the groups with scales of 5–6 (admitted to the hospital requiring high-flow nasal cannula (HFNC) and admitted to the hospital requiring extracorporeal membrane oxygenation) had DLCO below 80%. Additionally, after six months, most patients reported experiencing at least one symptom, with fatigue and muscle weakness being the most common, along with anxiety and depression [22].
2. Overview of the Respiratory System
The lungs are vital organs of the respiratory system and are responsible for exchanging gases between the environment and the bloodstream. They play a crucial role in respiration [23].
The respiratory system is divided into the respiratory tract and lung parenchyma. The airways are formed by the bronchus, which bifurcates into the trachea and then into bronchioles and alveoli (see Figure 1). The parenchyma is responsible for gas exchange [24]. Breathing involves inhaling oxygen from the environment, which then diffuses through the bloodstream via systemic circulation to produce adenosine triphosphate (ATP), the energy source for our cells. As a byproduct, we exhale carbon dioxide (CO2) [25]. The respiratory process consists of two basic stages: inspiration and expiration. The volume of air that enters and leaves the lungs during this process is known as tidal volume [26].
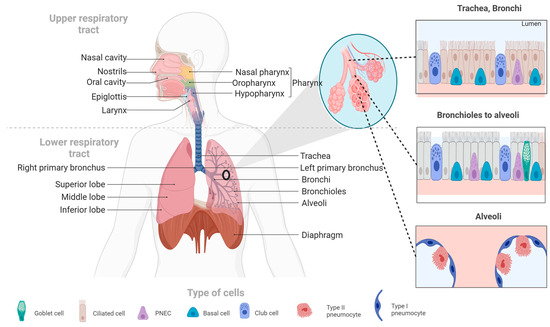
Figure 1.
The human respiratory system. This figure illustrates the main components of the human respiratory system, including the upper and lower respiratory tracts, as well as the cell types that make up the respiratory system’s tissues.
The lungs can develop various pathologies that can be divided into: (1) obstructive pulmonary disease, in which there is an alteration in breathing out and includes asthma and chronic obstructive pulmonary disease (COPD) [27]; (2) restrictive lung disease, in which the lung expansion is compromised and causes a decrease in pulmonary volumes; notably, the total lung capacity (TLC) is reduced. Some examples of restrictive lung disease are idiopathic PF (IPF), sarcoidosis, and pneumoconiosis [23,28].
3. Pulmonary Fibrosis (PF)
Interstitial lung disease (ILD) and PF are a group of lung diseases that share an inflammatory component and fibrosis of the lung parenchyma [29]. ILD has several attributed causes, including environmental exposures, microbial agents, occupational hazards, drug exposure, and autoimmune or connective tissue diseases [30]. One specific type of ILD is idiopathic pulmonary fibrosis (IPF), which is the most common type due to its unknown cause and poor prognosis [29]. IPF may be very similar to post-COVID-19 or induced PF by COVID-19 in terms of profibrotic pathophysiological mechanisms and response to treatments [31]; therefore, the term used in this review, PF, refers to any pulmonary fibrotic condition [32].
Patients with acute respiratory distress syndrome (ARDS) and pneumonia have a higher likelihood of developing pulmonary fibrosis (PF), with rates ranging from 20% to 50%, respectively. Among patients with COVID-19, 40% develop ARDS, and of those cases, 20% are severe [33]. PF is recognized as a sequela of ARDS, characterized by failure of alveolar re-epithelialization, activation of fibroblasts, and excessive deposition of collagen and other extracellular matrix (ECM) components that alter normal lung architecture [34]. The exact mechanism by which PF occurs is not completely defined; however, studies suggest that some cytokines, especially transforming growth factor β (TGF-β) and cells such as fibroblasts and myofibroblasts, in addition to angiotensin-converting enzyme 2 (ACE2), play a role in this process [35]. PF is a consequence of a severe attack on the alveolar wall of the lung [36], which is produced by the deregulation of one or more stages in the wound healing process: injury, inflammation, and repair. Here, inflammation is an essential factor in the development of PF [37]. SARS-CoV-2 can act as the initiator of alveolar wall injury (Figure 2) [38]. Fibroblasts are effector cells that participate in fibroproliferation, migrating to the site of injury by stimulating fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), TGF-β, and cytokines. In addition, fibroblast proliferation and differentiation to myofibroblasts are stimulated by endothelial growth factor (VEGF), PDGF, TGF-β, and interleukin-1 (IL-1) [39].
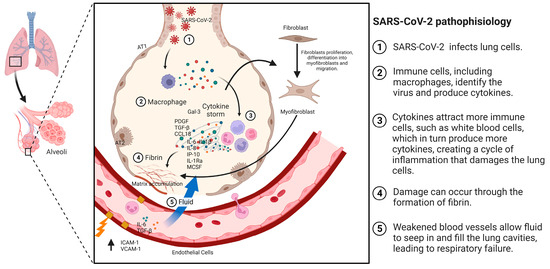
Figure 2.
Pathophysiology of PF associated with COVID-19. The mechanism of pulmonary fibrosis (PF) begins with an injury or the entry of SARS-CoV-2. This triggers the activation of macrophages and the cytokine storm phenomenon, which, in turn, activates other cells, such as fibroblasts. These fibroblasts differentiate into myofibroblasts, leading to the accumulation of extracellular matrix and the development of fibrosis.
3.1. Risk Factors for PF Associated with COVID-19
The development of PF associated with COVID-19 includes risk factors directly attributable to the patient, such as age, gender, alcohol or tobacco use, the presence of comorbidities, and genetic susceptibility. Other factors specific to the disease include the severity of the illness, the need for ICU services, mechanical ventilation, and the presence of ARDS [40,41].
3.1.1. Age and Sex
Previous reports suggest that PF is more common in men than in women, with approximately 70% of patients who develop PF being male [41]. Men are also more likely to die from COVID-19 (OR = 1.39; 95% CI: 1.31–1.47) [42]. The age of onset of PF is approximately 65 years; advanced age has been associated with the risk of developing PF (r = 0.574; p = 0.01) [34]. It has been reported that a reduction of 10% in telomere length with age was associated with a 1.35-fold increase in PF development [43].
3.1.2. Smoking and Alcoholism
Smoking has been associated with various lung diseases, such as cancer, pulmonary emphysema, and pulmonary fibrosis [44]. An increased risk of progression of PF in patients with COVID-19 has also been documented (OR = 14.5, 95% CI: 1.6–25.0) [45]. Alcohol consumption is an aggravating factor in the progression of PF and a factor associated with increased odds of ARDS (OR = 1.89; 95% CI: 1.45–2.48) [46].
3.1.3. Comorbidities
The presence of comorbidities such as diabetes mellitus (DM) and pulmonary and cardiovascular diseases is considered a risk factor for the development of PF [40]. As an example, patients with DM (a disease that affects various organs, mainly the cardiovascular system and kidneys) [47] had a 50% increased risk of developing PF [48].
3.1.4. Mechanical Ventilation and ICU Length of Stay
Patients who are admitted to the ICU and require invasive mechanical ventilation have a higher risk of developing PF [49]; compared to patients who did not require mechanical ventilation, 72% of individuals on mechanical ventilation had fibrotic abnormalities [43]. Similarly, in patients discharged after COVID-19 infection, mechanical ventilation increased the risk of PF by 4.45 (OR = 4.45; 95% CI: 1.27–15.58) [49].
3.1.5. Acute Respiratory Distress Syndrome
In COVID-19 patients who develop ARDS, 20% were reported to develop PF [50]. Similarly, in a follow-up study of 27 patients receiving mechanical ventilation for ARDS, after extubation, 85% of patients developed PF, and a relationship to the duration of pressure-controlled inverse-ratio ventilation was observed (p < 0.001) [34].
4. Mechanisms Involved in the Development of IPF
Fibroblasts synthesize collagen, fibronectin, and ECM [51]. Myofibroblasts—other cells involved in fibrosis—secrete factors such as VEGF and TGF-β, produce denser but more disorganized ECM than fibroblasts, and persist longer at the injury site [52]. One cytokine involved in tissue repair is TGF-β. Sources of TGF-β include platelet granules and macrophages. TGF-β is predominantly expressed in PF and helps stimulate the formation of ECM, collagen, fibronectin, elastic fibers, and matrix substances (Figure 2) [53].
4.1. Cells Involved in the Development of IPF
Several cells contribute to the development of PF by producing growth factors and cytokines, which contribute to the fibrotic process and pathogenesis of the disease.
4.1.1. Macrophage Activation
Macrophages are a type of cell that plays an essential role in innate immunity and inflammation [54]. There are two main macrophage phenotypes: M1, which is associated with proinflammatory responses, and M2 macrophages (M2a, M2b, M2c, and M2d), which play crucial roles in anti-inflammatory processes [55]. The transition from one phenotype to another is highly variable, and activation can be a rapid and reversible process [56]. Macrophage activation occurs through several pathways. The canonical IRF/STAT signaling pathways are activated by IFN and TLR to activate the M1 macrophage phenotype (STAT1) or to activate the M2 phenotype. IL-4 and IL-3 (STAT6) are required [57]. M1 macrophages increase the regulation of IRF5, thereby stimulating cytokines (IL-12, IL-23, TNF) involved in Th1 and Th17 responses [58]. On the other hand, it has been seen that interleukin IL-10 can activate the expression of genes mediated by STAT3, which can be associated with the M2 phenotype [59].
Two populations of macrophages have been identified in the regulation of pulmonary homeostasis: alveolar macrophages (AMs), which are located in the lumen of the airways, whose markers are CD11blow, CD11c++, and CD169+, and interstitial macrophages (IMs), which are located in the lung parenchyma, with markers CD11b+, CD11clow, CD169 [60]. Macrophage signaling pathways in PF are TGF-β/Smad, Wnt/β-catenin, and interleukin signaling [61,62,63]. In a study conducted with patients with severe COVID-19, the accumulation of CD163+ macrophages were reported, some of which co-expressed the chemokine receptor CXCR3 and complement factor C1Q. In addition, collagen deposition was reported very prominently, for which the authors propose that SARS-CoV-2 promotes genetic programs associated with fibrosis in macrophages [64].
4.1.2. Activation of Fibroblasts and its Differentiation to Myofibroblasts
Fibroblasts are mesenchymal cells whose function is homeostasis and production of ECM [65]. In the lung, fibroblasts are found in the ECM of the interstitial space, where they participate in early and late wound repair [66]. For the progression of PF, a key factor is the proliferation and differentiation of fibroblasts into myofibroblasts [67]. TGF-β 1 participates in stimulating the differentiation of fibroblasts into myofibroblasts [68]. A study found that miR-21 expression is involved in collagen production by fibroblasts or fibroblast-like cells through negative regulation of Smad7. This suggests that Smad7 could be a potential therapeutic target in PF [69].
In IPF, type 1 collagen is the most abundant and relevant collagen, as it is deposited in greater quantities in the ECM [70,71]. Fibroblasts and myofibroblasts mainly secrete this type of collagen due to the action of several cytokines and factors, such as TGF-β, in addition to the hypoxia pathway [72]. In a study conducted by Philp, C. J et al., the inhibition of the formation of crosslinks through transglutaminase 2 modified the behavior of fibroblasts by reducing adhesion and proliferation of these; it also improved the renewal of ECM, which would mean that it could be a potential therapy for IPF by reducing excess ECM at the site of injury [73]. Figure 3 displays antifibrotic drugs with evidence of their use in PF related to COVID-19 and their possible mechanisms of antifibrotic action. For instance, Nimotuzumab inhibits EGFR, thereby inhibiting the JAK/STAT pathway and preventing fibrosis from progressing or occurring [74].
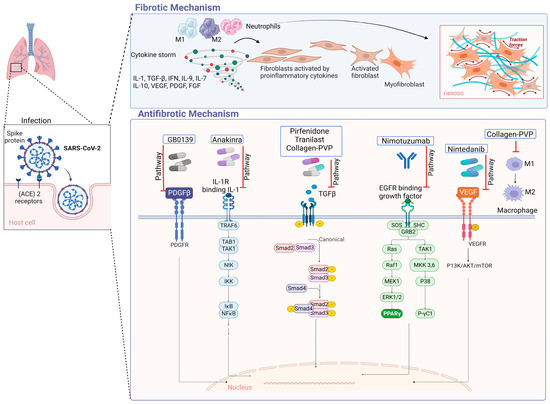
Figure 3.
Antifibrotic drugs. Figure shows a possible antifibrotic mechanism involved in the treatment of pulmonary fibrosis induced by COVID-19. Pirfenidone, Nintedanib, and collagen-polyvinylpyrrolidone have different mechanisms that may regulate PF, while Nimotuzumab, Anakinra, Tranilast, and GB0139 have more specific mechanisms of action [75,76,77,78,79,80,81].
4.2. Signaling Pathways Involved in the Development of IPF
The pathological process by which IPF develops is very complex and involves the activation of alveolar epithelial cells as well as the proliferation of fibroblasts and differentiation into myofibroblasts; likewise, various growth factors such as TGF-β intervene, leading to irreversible damage to the alveolar architecture.
4.2.1. TGF-β Signaling
TGF-β is a member of a family of polypeptides that modulate various cellular functions, the most important of which are cell proliferation, differentiation, and apoptosis [82]. There are three major inactive isoforms of TGF-β (TGF-β1, TGF-β2, TGF-β3), which are synthesized and secreted in association with latency-associated peptide (LAP) [83]. When there are normal conditions, TGF-β in its inactive conformation binds to LAP, which, through disulfide bonds, binds to latent TGF-β-binding proteins, crosslinked with the ECM [84]. TGF-β can be activated within the large latent complex (CLL) in various ways, for example, acidification, temperature variations, oxidation, proteolytic cleavage, or through interaction with integrins, which will cause TGF-β to interact with TGF-β receptors (TGF-βR), and the consequent action is to mediate downstream effects [85]. TGF-β plays an important role in the development of IPF [86,87]. Upregulation and activation of all TGF-β isoforms and receptors have been reported to be involved in the pathogenesis of IPF [88].
It is postulated that TGF-β signaling, which is a profibrotic inducer, is stimulated by the protein N of SARS-CoV. Since this protein is 90% similar to that of SARS-CoV-2, it is considered to be one of the possible mechanisms of pulmonary fibrosis associated with SARS-CoV-2 infection [89]. Another possible mechanism is that coronaviruses can induce the lowering of ACE2; therefore, there is a reduction of angiotensin II (Ang II) in the lungs. In this way, Ang II could upregulate TGF-β and connective tissue growth factor [47]. Once the lung injury occurs, TGF-β, in addition to being released by the injured epithelial and endothelial cells, is released by macrophages, fibroblasts, T cells, and monocytes, which results in a self-sustained process [85]. The canonical TGF-β signaling pathway will involve the activation of SMAD proteins that will activate transcription [90]. TGF-β, in its non-canonical pathway, can act on mitogen-activated protein kinase (MAPK), extracellular signal-regulated kinase (ERK), and N-terminal Jun N kinase (JNK), as well as RHO-associated kinase (ROCK) and AKT pathways [91]. Some of these pathways are involved in the induction of the epithelial–mesenchymal transition (EMT), which promotes the exacerbation of fibrotic lesions [92].
4.2.2. Activation of Integrins
Integrins belong to a family of proteins composed of one of 18 unique α subunits and eight β subunits that pair to create one of the 24 identified integrins [93]. They play a crucial role in maintaining cell–cell junctions, cell–EM adhesion, and signal transduction from the ECM to the intracellular compartment [94]. Integrins have been found to participate in various disease processes, including sarcoidosis and PF [95]. Recent research has demonstrated that the S protein of SARS-CoV-2 binds to integrin ⍺5β1 in vascular endothelial cells (ECs) through the RGD domain. This binding activates the expression of nuclear factor kappa β (NF-κβ), which is responsible for vascular leakage and leukocyte adhesion. These findings suggest a new mechanism by which SARS-CoV-2 affects ECs and identify integrin ⍺5β1 as a potential therapeutic target for treating COVID-19-related inflammation [96]. αVβ6 is another integrin that recognizes RGD (Arg-Gly-Asp). It is overexpressed in many aggressive epithelial tumors, as well as in pulmonary and hepatic fibrosis [97]. A study found that high levels of αVβ6 suggest the presence of fibroblast foci and can also identify a specific endotype of progressive fibrotic lung disease [98].
4.3. Growth Factors Involved in Idiopathic Pulmonary Fibrosis
Several growth factors, including PDGF, CTGF, and FGF, have been reported to play a role in the development of IPF by regulating the activity of fibroblasts.
- -
- PDGF is a mitogenic molecule that affects various connective tissue cells and other cell types [99]. This factor is composed of polypeptide chains A and B linked by a disulfide bond, which can form homo- and heterodimers [100]. PDGF is produced by several cell types, including macrophages, platelets, endothelial cells, and fibroblasts [101]. It has four isoforms that bind to two receptor tyrosine kinases (PDGFR α and β). These receptors are expressed in higher quantities in cells such as fibroblasts and myofibroblasts, where they participate in survival, proliferation, and migration [102]. PDGF works together with TGF-β to promote the release of activated alveolar macrophages and epithelial cells, which are directly involved in the self-activation cycle of fibrosis [103]. High levels of PDGF have been associated with FP, both in lung tissue and in bronchoalveolar lavage fluid, as demonstrated in various animal studies with models of PF [104]. PDGF-A and PDGF-C have also been found to be overexpressed in various cell types in a mouse model of injured lungs [105]. During pulmonary fibrosis, alveolar macrophages promote the release of PDGF, which contributes to the proliferation of alveolar myofibroblasts and fibrogenesis [106].
- -
- Fibroblast growth factors (FGFs) belong to a family of 22 members [107,108]. These can be divided into hormone-like FGFs (FGF 15/19, 21 and 23), canonical FGFs (FGF 1–10, 16–18, 20 and 22), and intracellular FGFs (FGF 11–14) [109]. FGFs are involved in several biological responses, interacting with heparan sulfate glycosaminoglycans (HPSGs). Released FGFs interact with cell surface receptor (FGFR) domains [110]; a complex of FGF, FGFR, and HPSG is formed, and thus, signaling is carried out [111]. Abnormal FGF signaling is implicated in various pathologies and is known to participate in the pathogenesis of PF [112]. The inhibition of FGF signaling by using FGFR2c decreased lung fibroblast proliferation and differentiation in vitro and in vivo in a murine model [113]. The alteration in FGFR1 and FGF1 signaling is critical for fibroblast migration in PF [114]. In SARS-CoV infection, the EGFR signaling pathway remains active even after the virus is eliminated. This pathway is believed to be responsible for the effect of FGF in the conduction to PF [115].
- -
- Connective tissue growth factor (CTGF) belongs to the IGFBP family, which is known as insulin-like growth factor-binding protein 8 (IGFBP-8) [116,117]. This 38 kDa mitogenic peptide is involved in fibrotic processes and stimulates migration, fibroblast proliferation, and ECM production [118]. CTGF is a cytokine that participates in the activation of fibroblasts; it was observed that in alveolar epithelial cells, the expression levels of CTGF were drastically reduced by inhibiting the Rho pathway. Additionally, the epithelial cells of the lung in the mice indicators of CTGF increased the activity of the promoter of CTGF when the authors treated them with bleomycin, an inducer of PF [119]. CTGF can interact with other molecules to develop its fibrotic effects, thus contributing to the generation of fibrosis; among the molecules with which it interacts, there are cytokines and growth factors such as IGF1, BMP4, BMP7, TGF-β and VEGF. This interaction with other molecules may positively or negatively alter the signaling pathways of which they are participants, resulting in changes in cell adhesion, migration, differentiation, angiogenesis, myofibroblast activation, deposition, and remodeling of ECM, which causes changes in structure and alters tissue remodeling [120].
- -
- Metalloproteinases (MMPs) belong to the family of extracellular endopeptidases, whose primary function is the degradation of ECM components, while tissue inhibitors of metalloproteinases (TIMPs) block the activity of MMPs [121]. The participation of proinflammatory cytokines affects the overexpression of MMPs, which increases its activity and thus participates in the remodeling of the airways [122]. The level of TIMPs is increased in the presence of a fibrotic process such as PF [123]. MMP-2 and MMP-9 are downregulated in severe COVID-19 patients [124]. MMP-2 has shown a correlation with mortality in patients with COVID-19, so it could be a potential prognostic predictor for patients with COVID-19 [125]. One study reported that MMP-7 correlates with clinical and functional predictors of disease severity and mortality, so it can be used to distinguish IPF from other chronic diseases [126].
5. Antifibrotic Treatments and Evidence Level
Currently, there is no drug available to cure FP. The treatment guidelines for PF (an official ATS/ERS/JRS/ALAT Clinical Practice Guideline) recommend a systemic approach that includes treating comorbidities, oxygen therapy, and pulmonary rehabilitation [127]. Although several drugs have been developed to expand the therapeutic options, Table 1 presents only those drugs that have shown favorable results in treating IPF and its association with COVID-19. These are similar to pirfenidone and nintedanib, which are FDA-approved for IPF treatment [128]. However, in studies of PF associated with COVID-19, in a case report of a 66-year-old patient with PF, pirfenidone showed favorable results: FVC values improved from 1.98 L to 2.30 L, total lung capacity (TLC) improved from 61.7% predicted to 72.3%, and carbon monoxide diffusing capacity (DLCO) improved from 30.3% predicted to 47.9% [129]. On the other hand, nintedanib was evaluated in 42 patients with COVID-19 pneumonia and was found to help improve the SpO2/FiO2 ratio (144.38 ± 118.05 vs. 55.67 ± 75.09, p = 0.006) [75].

Table 1.
Drugs with favorable results in the treatment of IPF and PF associated with COVID-19.
Other drugs with encouraging results include nimotuzumab, which was studied in 41 patients (31 severe and 10 moderate COVID-19), and it was found that nimotuzumab reduces IL-6 and PAI-1 through the EGFR pathway, which inhibits tyrosine kinase activity and may prevent fibrosis in patients with severe and moderate COVID-19 at high risk of worsening [76].
Other drugs have been used in PF and, due to their mechanisms of action, could be scaled for use in PF associated with COVID-19. These drugs are listed in Table 2. Some of these molecules are in phase II, including Pamrevlumab (FG-3019), which attenuated the progression of IPF and was well tolerated. Drugs in the preclinical stage include EW-7197, which was evaluated in a murine model of male C57BL/6 mice and Sprague-Dawley rats. It was shown to be a therapeutic agent against liver, kidney, and lung fibrosis by inhibiting TGF-β-/Smad2/3 and ROS signaling [132]. Similarly, Alamandine was evaluated in a murine model of bleomycin-induced PF in 28 Wistar rats and showed favorable results in collagen deposition and improved pulmonary ventilatory mechanics [133]. AT13387 was evaluated in a study of male C57Bl/6J mice inoculated intratracheally with 15 mg/kg of the drug. AT13387 was found to reduce alveolar inflammation, fibrosis, collagen deposition, and the development of chronic lung injury and airway hyper-responsiveness [134]. Another molecule, Piceatannol (PIC), was evaluated in a C57BL/6 mouse model of bleomycin-induced PF and was shown to attenuate bleomycin-induced collagen deposition and myofibroblast accumulation [135].

Table 2.
Molecules for the treatment of IPF and their potential use in PF due to COVID-19.
Due to the complex nature of treating PF in the context of COVID-19, scientists worldwide are proposing new molecules daily to expand the therapeutic options for this disease. Currently, several ambitious projects have been registered in this regard. Table 3 displays the studies of these drugs that have a ClinicalTrials.gov registry (consultation date: November 2023) for the therapeutic approach of IPF. These studies are in different stages, either recruitment or completed with results. For instance, the study ‘Treatment of PF Due to COVID-19 With Fuzheng Huayu’ has been completed, and the drug Fuzheng Huayu Tablet was evaluated in phase II. Another study, ‘Pirfenidone compared to placebo in post-COVID19 pulmonary fibrosis’, is still in the recruitment stage. These trials can be categorized based on their current study phase: 3 (NA Phase), 3 (Phase 1), 2 (Phase 1–2), 6 (Phase 2), 2 (Phase 2–3), 1 (Phase 3), and finally 4 (Phase 4), with Phase 2 being the most common.

Table 3.
Studies registered on ClinicalTrials.gov in order to evaluate molecules for the treatment of PF and its possible use in lung damage caused by COVID-19.
6. Biomarkers Associated with IPF, Its Progression, and Its Possible Application in PF Associated with COVID-19
The process of establishing a diagnosis of IPF requires a multidisciplinary medical team of pathologists, pulmonologists, and radiologists. First, other known causes of interstitial lung disease (ILD) are ruled out, and then a computed axial tomography scan is performed to look for concordant patterns of IPF [30]. If this cannot diagnose IPF, the next thing is to take a lung biopsy to also try to find concordant patterns of IPF [143]. It is extremely important to investigate biomarkers that may be key in diagnosing IPF. Biomarkers are objectively measured and evaluated indicators of normal physiological processes, pathological processes, or pharmacological responses to therapeutic interventions [144]. In general, biomarkers can be used for diagnosis to distinguish between diseases. They serve as a method of disease classification. They can also indicate susceptibility to a disease in healthy individuals. Prognostic biomarkers enable us to predict the disease outcome, while biomarkers linked to treatment are known as biomarkers of efficacy or treatment response [145].
Biomarkers can be found in various specimens, such as blood, sputum, fluid, or cells from bronchoalveolar lavage (BAL) samples, and even more invasive techniques such as lung tissue biopsies [144]. Biomarkers can be very useful in IPF disease, especially for early diagnosis, and even the use of biomarkers with predictive and prognostic values, as they are indicators of disease severity or progression and can be used to guide treatment, follow-up, and survival of these patients [146,147]. Figure 4 and Table 4 describe the biomarkers that have been studied in IPF over the last two years and their potential application in IPF induced by COVID-19.
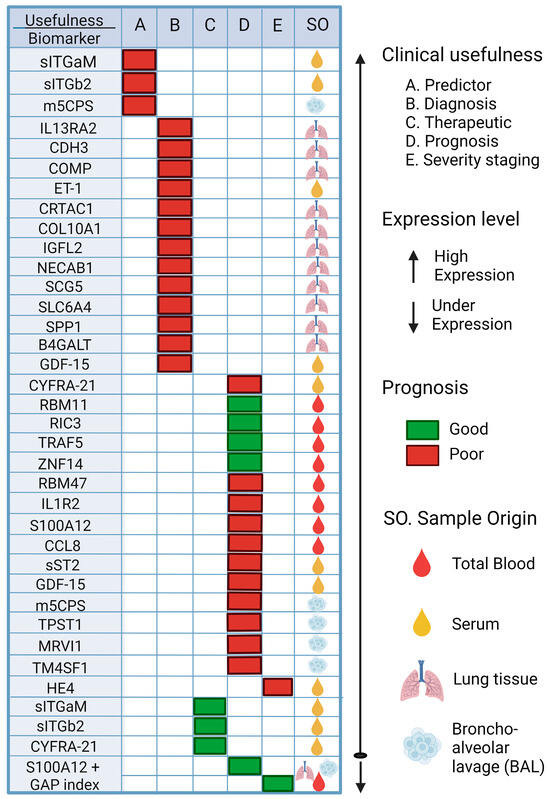
Figure 4.
Potential biomarkers rated for Predictor, Diagnostic, Therapeutic, Prognosis, and Severity of IPF. The possible biomarkers that could be used to try to make a diagnosis in time are shown, such as the use of sITGb2 or m5CPS; if they are elevated in patients, this could indicate the appearance of FP. Other potential biomarkers are also shown to predict the prognosis of patients with FP, such as the use of RBM11, which, if overexpressed, is an indicator of a good prognosis for patients, while if RBM47 is overexpressed, it is an indicator of a poor prognosis for patients. Thus, the importance of these biomarkers becomes evident for their future validation and application to FP. sITGaM (soluble integrin subunit alpha M), sITGb2 (soluble integrin subunit beta 2), m5CPS (5-methylcytosine), IL13RA2 (interleukin 13 receptor subunit alpha 2), CDH3 (cadherin 3), COMP (cartilage oligomeric matrix protein), ET-1 (endothelin-1), CRTAC1 (cartilage acid protein 1), COL10A1 (collagen type X alpha 1), IGFL2 (like family member 2), NECAB1 (N-terminal EF-hand calcium binding protein 1), SCG5 (secretogranin V), SLC6A4 (solute carrier family 6 member 4), SPP1 (secreted phosphoprotein 1), B4GALT1 (beta-1,4-galactosyltransferase), GDF-15 (growth differentiation factor 15), CYFRA21 (cytokeratin fragment 19), RBM11 (RNA-binding motif protein 11), RIC3 (resistance to inhibitors of cholinesterase 3), TRAF5 (TNF receptor-associated factor 5), ZNF14 (zinc finger protein 14), RBM47 (RNA-binding motif protein 47), IL1R2 (interleukin 1 receptor type 2), S100A12 (S100 calcium binding protein A12), CCL8 (C-C motif chemokine ligand 8), sST2 (soluble receptor form 2), TPST1 (tyrosyl protein sulfotransferase 1), MRVI1 (inositol 1,4,5-triphosphate receptor associated 1), TMF4S1 (transmembrane 4 L six family member 1), HE4 (human epididymis protein 4) [148,149,150,151,152,153,154,155,156,157,158,159,160].

Table 4.
Potential Biomarkers in IPF and their possible use in PF associated with COVID-19.
The use of these biomarkers, such as CYFRA 21-1, which can be detected in serum samples, is useful for the diagnosis and prognosis of patients with PFI; this biomarker predicted short-term progression and long-term survival (HR = 1.13 [95% CI, 0.02–1.25]; p = 0.023). RBM47 is another biomarker that can be assessed in peripheral blood and can be used to assess the progression of IPF. When its expression increases, a poor prognosis is assumed in patients with IPF (AUC at 4 years = 0.95).
Similarly, other biomarkers that have been studied, such as sITGaM and sITGb2, have been investigated for their predictive and therapeutic value in patients with COVID-19 with a value of R = 0.42, p = 0.01 [148]; high levels of these molecules have been observed in patients with COVID-19. Additionally, elevated levels of IL13RA2, CDH3, and COMP biomarkers may be useful in diagnosing IPF; the AUC for this group of three genes was reported as 0.98. Other biomarkers with prognostic value include RBM11, RIC3, TRAF5, ZNF14, and RBM47. The AUC value in the first year was low (AUC at 1 year = 0.63), but the AUC value gradually increased with time: AUC at 2 years = 0.77; AUC at 3 years = 0.85; AUC at 4 years = 0.95; this shows its predictive usefulness. The results of the survival analysis showed that high expression of RBM11, RIC3, TRAF5, and ZNF14 was associated with a good prognosis of IPF, while high expression of RBM47 was associated with a poor prognosis [154].
All of the aforementioned points underscore the necessity and utility of future research on non-invasive and efficacious biomarkers. Such molecules could offer a more holistic approach to managing patients with PF, and collectively, they have the potential to regulate and enhance the quality of life for individuals with this ailment, a condition that is likely to be further compounded by COVID-19.
7. Conclusions
PF is a disease that has multiple causes. Currently, treatment of the disease involves a multidisciplinary approach. The main therapies for patients with PF include oxygen therapy, pulmonary rehabilitation, and pharmacological therapy. Two FDA-approved drugs for PF are pirfenidone and nintedanib. The research landscape for PF remains broad, providing ample opportunities for researchers worldwide to expand the range of drugs and discover new therapeutic targets that can help prevent or cure PF. Nimotuzumab, an emerging drug, has shown promising results in treating inflammation and PF caused by COVID-19. Another drug, Treamid, has improved FVC and DLCO values in patients with pneumonia after COVID-19. However, it is important to note that none of these drugs can cure the disease.
Describing the molecular mechanisms involved in the pathology of PF is of great importance in order to develop new drugs or discover possible biomarkers for the correct management of both IPF and FP due to COVID-19 because currently, the diagnosis of PF is based on tomography and lung biopsy, which is an invasive method. Therefore, the incorporation of these new biomarkers, such as ET-1, which is elevated in the serum of IPF patients, cannot help in the differential diagnosis between IPF and ILD associated with autoimmune diseases (AD-ILD). Another biomarker, sST2, helps predict the deterioration of IPF patients and their outcome. Therefore, scientists worldwide must concentrate on developing new drugs that are more effective than current ones. Additionally, they must prioritize discovering and implementing predictive, diagnostic, severity classification, prognostic, and other biomarkers.
These tools enable us to predict patients’ life expectancy and facilitate comprehensive and personalized management of each patient with this disease to continue improving their quality of life.
Author Contributions
All authors participated in the conceptualization and editing. A.P.-F., M.L.M.-F. and I.G.-V. contributed to writing—original draft preparation. L.d.S.H.-M., E.F.G.-V. and V.F.-M. contributed to resources and review and editing. A.P.-F. and M.L.M.-F. contributed to analysis of information and table and figure construction. All authors have read and agreed to the published version of the manuscript.
Funding
Aurelio Perez-Favila wants to acknowledge the CONAHCyT doctorate scholarship, with scholarship holder number CVU 789010, through the Doctorado en Ciencias con Orientación en Medicina Molecular of Universidad Autónoma de Zacatecas.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhao, S.; Lin, Q.; Ran, J.; Musa, S.S.; Yang, G.; Wang, W.; Lou, Y.; Gao, D.; Yang, L.; He, D.; et al. Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: A data-driven analysis in the early phase of the outbreak. Int. J. Infect. Dis. 2020, 92, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Boger, B.; Fachi, M.M.; Vilhena, R.O.; Cobre, A.F.; Tonin, F.S.; Pontarolo, R. Systematic review with meta-analysis of the accuracy of diagnostic tests for COVID-19. Am. J. Infect. Control 2021, 49, 21–29. [Google Scholar] [CrossRef]
- Chan, J.F.; Kok, K.H.; Zhu, Z.; Chu, H.; To, K.K.; Yuan, S.; Yuen, K.Y. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infect. 2020, 9, 221–236. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Coronavirus (COVID-19) Dashboard; WHO: Geneva, Switzerland, 2023. [Google Scholar]
- DGE. COVID-19 Tablero México—CONAHCYT. Available online: https://datos.covid-19.conacyt.mx/ (accessed on 7 November 2023).
- Gralton, J.; Tovey, E.; McLaws, M.L.; Rawlinson, W.D. The role of particle size in aerosolised pathogen transmission: A review. J. Infect. 2011, 62, 1–13. [Google Scholar] [CrossRef]
- Fernstrom, A.; Goldblatt, M. Aerobiology and its role in the transmission of infectious diseases. J. Pathog. 2013, 2013, 493960. [Google Scholar] [CrossRef]
- Rothan, H.A.; Byrareddy, S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020, 109, 102433. [Google Scholar] [CrossRef] [PubMed]
- Singhal, T. A Review of Coronavirus Disease-2019 (COVID-19). Indian J. Pediatr. 2020, 87, 281–286. [Google Scholar] [CrossRef]
- Fehr, A.R.; Perlman, S. Coronaviruses: An overview of their replication and pathogenesis. Methods Mol. Biol. 2015, 1282, 1–23. [Google Scholar] [CrossRef]
- Emrani, J.; Ahmed, M.; Jeffers-Francis, L.; Teleha, J.C.; Mowa, N.; Newman, R.H.; Thomas, M.D. SARS-CoV-2, infection, transmission, transcription, translation, proteins, and treatment: A review. Int. J. Biol. Macromol. 2021, 193, 1249–1273. [Google Scholar] [CrossRef]
- Stokes, E.K.; Zambrano, L.D.; Anderson, K.N.; Marder, E.P.; Raz, K.M.; El Burai Felix, S.; Tie, Y.; Fullerton, K.E. Coronavirus Disease 2019 Case Surveillance—United States, January 22–May 30, 2020. MMWR Morb. Mortal Wkly. Rep. 2020, 69, 759–765. [Google Scholar] [CrossRef]
- Tian, S.; Hu, N.; Lou, J.; Chen, K.; Kang, X.; Xiang, Z.; Chen, H.; Wang, D.; Liu, N.; Liu, D.; et al. Characteristics of COVID-19 infection in Beijing. J. Infect. 2020, 80, 401–406. [Google Scholar] [CrossRef]
- Guarner, J. Three Emerging Coronaviruses in Two Decades. Am. J. Clin. Pathol. 2020, 153, 420–421. [Google Scholar] [CrossRef]
- Fernandez-de-Las-Penas, C.; Palacios-Cena, D.; Gomez-Mayordomo, V.; Cuadrado, M.L.; Florencio, L.L. Defining Post-COVID Symptoms (Post-Acute COVID, Long COVID, Persistent Post-COVID): An Integrative Classification. Int. J. Env. Res. Public Health 2021, 18, 2621. [Google Scholar] [CrossRef]
- van Kessel, S.A.M.; Olde Hartman, T.C.; Lucassen, P.; van Jaarsveld, C.H.M. Post-acute and long-COVID-19 symptoms in patients with mild diseases: A systematic review. Fam. Pr. 2022, 39, 159–167. [Google Scholar] [CrossRef]
- Cares-Marambio, K.; Montenegro-Jimenez, Y.; Torres-Castro, R.; Vera-Uribe, R.; Torralba, Y.; Alsina-Restoy, X.; Vasconcello-Castillo, L.; Vilaro, J. Prevalence of potential respiratory symptoms in survivors of hospital admission after coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. Chron Respir. Dis. 2021, 18, 14799731211002240. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 Long-term effects of COVID-19: A systematic review and meta-analysis. medRxiv 2021, 11, 16144. [Google Scholar] [CrossRef]
- Kamal, M.; Abo Omirah, M.; Hussein, A.; Saeed, H. Assessment and characterisation of post-COVID-19 manifestations. Int. J. Clin. Pr. 2021, 75, e13746. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-de-Las-Penas, C.; Palacios-Cena, D.; Gomez-Mayordomo, V.; Rodriuez-Jimenez, J.; Palacios-Cena, M.; Velasco-Arribas, M.; Guijarro, C.; de-la-Llave-Rincon, A.I.; Fuensalida-Novo, S.; Elvira-Martinez, C.M.; et al. Long-term post-COVID symptoms and associated risk factors in previously hospitalized patients: A multicenter study. J. Infect. 2021, 83, 237–279. [Google Scholar] [CrossRef] [PubMed]
- Ceban, F.; Ling, S.; Lui, L.M.W.; Lee, Y.; Gill, H.; Teopiz, K.M.; Rodrigues, N.B.; Subramaniapillai, M.; Di Vincenzo, J.D.; Cao, B.; et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav. Immun. 2022, 101, 93–135. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Haddad, M.; Sharma, S. Physiology, Lung; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Chaudhry, R.; Bordoni, B. Anatomy, Thorax, Lungs; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Brinkman, J.E.; Sharma, S. Physiology, Pulmonary; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Lofrese, J.J.; Tupper, C.; Denault, D.; Lappin, S.L. Physiology, Residual Volume; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Burney, P.; Perez-Padilla, R.; Marks, G.; Wong, G.; Bateman, E.; Jarvis, D. Chronic Lower Respiratory Tract Diseases. In Cardiovascular, Respiratory, and Related Disorders, 3rd ed.; Prabhakaran, D., Anand, S., Gaziano, T.A., Mbanya, J.C., Wu, Y., Nugent, R., Eds.; The International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA, 2017. [Google Scholar] [CrossRef]
- Martinez-Pitre, P.J.; Sabbula, B.R.; Cascella, M. Restrictive Lung Disease; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Kalchiem-Dekel, O.; Galvin, J.R.; Burke, A.P.; Atamas, S.P.; Todd, N.W. Interstitial Lung Disease and Pulmonary Fibrosis: A Practical Approach for General Medicine Physicians with Focus on the Medical History. J. Clin. Med. 2018, 7, 476. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Collard, H.R.; Egan, J.J.; Martinez, F.J.; Behr, J.; Brown, K.K.; Colby, T.V.; Cordier, J.F.; Flaherty, K.R.; Lasky, J.A.; et al. An official ATS/ERS/JRS/ALAT statement: Idiopathic pulmonary fibrosis: Evidence-based guidelines for diagnosis and management. Am. J. Respir. Crit. Care Med. 2011, 183, 788–824. [Google Scholar] [CrossRef] [PubMed]
- Patrucco, F.; Solidoro, P.; Gavelli, F.; Apostolo, D.; Bellan, M. Idiopathic Pulmonary Fibrosis and Post-COVID-19 Lung Fibrosis: Links and Risks. Microorganisms 2023, 11, 895. [Google Scholar] [CrossRef]
- Bazdyrev, E.; Rusina, P.; Panova, M.; Novikov, F.; Grishagin, I.; Nebolsin, V. Lung Fibrosis after COVID-19: Treatment Prospects. Pharmaceuticals 2021, 14, 807. [Google Scholar] [CrossRef] [PubMed]
- Rai, D.K.; Sharma, P.; Kumar, R. Post COVID 19 pulmonary fibrosis. Is it real threat? Indian J. Tuberc. 2021, 68, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Ojo, A.S.; Balogun, S.A.; Williams, O.T.; Ojo, O.S. Pulmonary Fibrosis in COVID-19 Survivors: Predictive Factors and Risk Reduction Strategies. Pulm. Med. 2020, 2020, 6175964. [Google Scholar] [CrossRef] [PubMed]
- Vaz de Paula, C.B.; Nagashima, S.; Liberalesso, V.; Collete, M.; da Silva, F.P.G.; Oricil, A.G.G.; Barbosa, G.S.; da Silva, G.V.C.; Wiedmer, D.B.; da Silva Deziderio, F.; et al. COVID-19: Immunohistochemical Analysis of TGF-beta Signaling Pathways in Pulmonary Fibrosis. Int. J. Mol. Sci. 2021, 23, 168. [Google Scholar] [CrossRef]
- Pardo, A.; Cabrera, S.; Maldonado, M.; Selman, M. Role of matrix metalloproteinases in the pathogenesis of idiopathic pulmonary fibrosis. Respir. Res. 2016, 17, 23. [Google Scholar] [CrossRef]
- Qiao, J.; Zhang, M.; Bi, J.; Wang, X.; Deng, G.; He, G.; Luan, Z.; Lv, N.; Xu, T.; Zhao, L. Pulmonary fibrosis induced by H5N1 viral infection in mice. Respir. Res. 2009, 10, 107. [Google Scholar] [CrossRef]
- Huang, W.J.; Tang, X.X. Virus infection induced pulmonary fibrosis. J. Transl. Med. 2021, 19, 496. [Google Scholar] [CrossRef]
- Shi, X.; Young, C.D.; Zhou, H.; Wang, X. Transforming Growth Factor-beta Signaling in Fibrotic Diseases and Cancer-Associated Fibroblasts. Biomolecules 2020, 10, 1666. [Google Scholar] [CrossRef]
- Duong-Quy, S.; Vo-Pham-Minh, T.; Tran-Xuan, Q.; Huynh-Anh, T.; Vo-Van, T.; Vu-Tran-Thien, Q.; Nguyen-Nhu, V. Post-COVID-19 Pulmonary Fibrosis: Facts-Challenges and Futures: A Narrative Review. Pulm. Ther. 2023, 9, 295–307. [Google Scholar] [CrossRef]
- Alrajhi, N.N. Post-COVID-19 pulmonary fibrosis: An ongoing concern. Ann. Thorac. Med. 2023, 18, 173–181. [Google Scholar] [CrossRef]
- Peckham, H.; de Gruijter, N.M.; Raine, C.; Radziszewska, A.; Ciurtin, C.; Wedderburn, L.R.; Rosser, E.C.; Webb, K.; Deakin, C.T. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat. Commun. 2020, 11, 6317. [Google Scholar] [CrossRef]
- McGroder, C.F.; Zhang, D.; Choudhury, M.A.; Salvatore, M.M.; D’Souza, B.M.; Hoffman, E.A.; Wei, Y.; Baldwin, M.R.; Garcia, C.K. Pulmonary fibrosis 4 months after COVID-19 is associated with severity of illness and blood leucocyte telomere length. Thorax 2021, 76, 1242–1245. [Google Scholar] [CrossRef] [PubMed]
- Umnuaypornlert, A.; Kanchanasurakit, S.; Lucero-Prisno, D.E.I.; Saokaew, S. Smoking and risk of negative outcomes among COVID-19 patients: A systematic review and meta-analysis. Tob. Induc. Dis. 2021, 19, 9. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Tao, Z.W.; Wang, L.; Yuan, M.L.; Liu, K.; Zhou, L.; Wei, S.; Deng, Y.; Liu, J.; Liu, H.G.; et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin. Med. J. 2020, 133, 1032–1038. [Google Scholar] [CrossRef]
- Simou, E.; Leonardi-Bee, J.; Britton, J. The Effect of Alcohol Consumption on the Risk of ARDS: A Systematic Review and Meta-Analysis. Chest 2018, 154, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Balan, I.; Yadav, S.; Matos, W.F.; Kharawala, A.; Gaddam, M.; Sarabia, N.; Koneru, S.C.; Suddapalli, S.K.; Marzban, S. Post-COVID-19 Pulmonary Fibrosis. Cureus 2022, 14, e22770. [Google Scholar] [CrossRef]
- Enomoto, T.; Usuki, J.; Azuma, A.; Nakagawa, T.; Kudoh, S. Diabetes mellitus may increase risk for idiopathic pulmonary fibrosis. Chest 2003, 123, 2007–2011. [Google Scholar] [CrossRef]
- Aul, D.R.; Gates, D.J.; Draper, D.A.; Dunleavy, D.A.; Ruickbie, D.S.; Meredith, D.H.; Walters, D.N.; van Zeller, D.C.; Taylor, D.V.; Bridgett, D.M.; et al. Complications after discharge with COVID-19 infection and risk factors associated with development of post-COVID pulmonary fibrosis. Respir. Med. 2021, 188, 106602. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Baum, J.; Duffy, H.S. Fibroblasts and myofibroblasts: What are we talking about? J. Cardiovasc. Pharmacol. 2011, 57, 376–379. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wang, L.; Wang, M.; Zhou, S.; Lu, Y.; Cui, H.; Racanelli, A.C.; Zhang, L.; Ye, T.; Ding, B.; et al. Targeting fibrosis, mechanisms and cilinical trials. Signal Transduct. Target Ther. 2022, 7, 206. [Google Scholar] [CrossRef] [PubMed]
- Rumende, C.M.; Susanto, E.C.; Sitorus, T.P. The Management of Pulmonary Fibrosis in COVID-19. Acta Med. Indones. 2021, 53, 233–241. [Google Scholar] [PubMed]
- Wen, J.H.; Li, D.Y.; Liang, S.; Yang, C.; Tang, J.X.; Liu, H.F. Macrophage autophagy in macrophage polarization, chronic inflammation and organ fibrosis. Front. Immunol. 2022, 13, 946832. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Perciani, C.T.; MacParland, S.A. Lifting the veil on macrophage diversity in tissue regeneration and fibrosis. Sci. Immunol. 2019, 4, eaaz0749. [Google Scholar] [CrossRef]
- Sica, A.; Bronte, V. Altered macrophage differentiation and immune dysfunction in tumor development. J. Clin. Investig. 2007, 117, 1155–1166. [Google Scholar] [CrossRef]
- Krausgruber, T.; Blazek, K.; Smallie, T.; Alzabin, S.; Lockstone, H.; Sahgal, N.; Hussell, T.; Feldmann, M.; Udalova, I.A. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat. Immunol. 2011, 12, 231–238. [Google Scholar] [CrossRef]
- Lang, R.; Patel, D.; Morris, J.J.; Rutschman, R.L.; Murray, P.J. Shaping gene expression in activated and resting primary macrophages by IL-10. J. Immunol. 2002, 169, 2253–2263. [Google Scholar] [CrossRef]
- Byrne, A.J.; Maher, T.M.; Lloyd, C.M. Pulmonary Macrophages: A New Therapeutic Pathway in Fibrosing Lung Disease? Trends Mol. Med. 2016, 22, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Fu, X.; Chen, X.; Han, X.; Dong, P. M2 macrophages induce EMT through the TGF-beta/Smad2 signaling pathway. Cell Biol. Int. 2017, 41, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Wang, C.; Chen, X.; Hou, J.; Xiang, Z.; Shen, Y.; Han, X. Inhibition of Wnt/beta-catenin signaling suppresses myofibroblast differentiation of lung resident mesenchymal stem cells and pulmonary fibrosis. Sci. Rep. 2018, 8, 13644. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, T.; Xu, Y.; Xu, X.; Zhu, Z.; Zhang, Y.; Xu, J.; Xu, K.; Cheng, H.; Zhang, X.; et al. Increased levels of Gab1 and Gab2 adaptor proteins skew interleukin-4 (IL-4) signaling toward M2 macrophage-driven pulmonary fibrosis in mice. J. Biol. Chem. 2017, 292, 14003–14015. [Google Scholar] [CrossRef]
- Wendisch, D.; Dietrich, O.; Mari, T.; von Stillfried, S.; Ibarra, I.L.; Mittermaier, M.; Mache, C.; Chua, R.L.; Knoll, R.; Timm, S.; et al. SARS-CoV-2 infection triggers profibrotic macrophage responses and lung fibrosis. Cell 2021, 184, 6243–6261.e6227. [Google Scholar] [CrossRef] [PubMed]
- Plikus, M.V.; Wang, X.; Sinha, S.; Forte, E.; Thompson, S.M.; Herzog, E.L.; Driskell, R.R.; Rosenthal, N.; Biernaskie, J.; Horsley, V. Fibroblasts: Origins, definitions, and functions in health and disease. Cell 2021, 184, 3852–3872. [Google Scholar] [CrossRef] [PubMed]
- White, E.S. Lung extracellular matrix and fibroblast function. Ann. Am. Thorac. Soc. 2015, 12 (Suppl. S1), S30–S33. [Google Scholar] [CrossRef] [PubMed]
- Khalil, N.; O’Connor, R. Idiopathic pulmonary fibrosis: Current understanding of the pathogenesis and the status of treatment. CMAJ 2004, 171, 153–160. [Google Scholar] [CrossRef]
- Li, X.; Bi, Z.; Liu, S.; Gao, S.; Cui, Y.; Huang, K.; Huang, M.; Mao, J.; Li, L.; Gao, J.; et al. Antifibrotic Mechanism of Cinobufagin in Bleomycin-Induced Pulmonary Fibrosis in Mice. Front. Pharmacol. 2019, 10, 1021. [Google Scholar] [CrossRef]
- Kwon, O.S.; Kim, K.T.; Lee, E.; Kim, M.; Choi, S.H.; Li, H.; Fornace, A.J., Jr.; Cho, J.H.; Lee, Y.S.; Lee, J.S.; et al. Induction of MiR-21 by Stereotactic Body Radiotherapy Contributes to the Pulmonary Fibrotic Response. PLoS ONE 2016, 11, e0154942. [Google Scholar] [CrossRef] [PubMed]
- Hewlett, J.C.; Kropski, J.A.; Blackwell, T.S. Idiopathic pulmonary fibrosis: Epithelial-mesenchymal interactions and emerging therapeutic targets. Matrix Biol. 2018, 71–72, 112–127. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Striker, L.J.; Hudson, L.D.; Striker, G.E. Extracellular matrix in normal and fibrotic human lungs. Am. Rev. Respir. Dis. 1985, 131, 281–289. [Google Scholar] [PubMed]
- Bellaye, P.S.; Shimbori, C.; Upagupta, C.; Sato, S.; Shi, W.; Gauldie, J.; Ask, K.; Kolb, M. Lysyl Oxidase-Like 1 Protein Deficiency Protects Mice from Adenoviral Transforming Growth Factor-beta1-induced Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2018, 58, 461–470. [Google Scholar] [CrossRef]
- Philp, C.J.; Siebeke, I.; Clements, D.; Miller, S.; Habgood, A.; John, A.E.; Navaratnam, V.; Hubbard, R.B.; Jenkins, G.; Johnson, S.R. Extracellular Matrix Cross-Linking Enhances Fibroblast Growth and Protects against Matrix Proteolysis in Lung Fibrosis. Am. J. Respir. Cell Mol. Biol. 2018, 58, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Abdo Cuza, A.A.; Avila, J.P.; Martinez, R.M.; Gonzalez, J.J.; Aspuro, G.P.; Gutierrez Martinez, J.A.; Suzarte, M.R.; Hernandez, D.S.; Ane-Kouri, A.L.; Ramos, T.C. Nimotuzumab for COVID-19: Case series. Immunotherapy 2021, 14, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Saiphoklang, N.; Patanayindee, P.; Ruchiwit, P. The Effect of Nintedanib in Post-COVID-19 Lung Fibrosis: An Observational Study. Crit. Care Res. Pr. 2022, 2022, 9972846. [Google Scholar] [CrossRef]
- Londres, H.D.; Armada, J.J.; Martinez, A.H.; Abdo Cuza, A.A.; Sanchez, Y.H.; Rodriguez, A.G.; Figueroa, S.S.; Llanez Gregorich, E.M.; Torres Lahera, M.L.; Peire, F.G.; et al. Blocking EGFR with nimotuzumab: A novel strategy for COVID-19 treatment. Immunotherapy 2022, 14, 521–530. [Google Scholar] [CrossRef]
- Acat, M.; Yildiz Gulhan, P.; Oner, S.; Turan, M.K. Comparison of pirfenidone and corticosteroid treatments at the COVID-19 pneumonia with the guide of artificial intelligence supported thoracic computed tomography. Int. J. Clin. Pr. 2021, 75, e14961. [Google Scholar] [CrossRef]
- Gaughan, E.E.; Quinn, T.M.; Mills, A.; Bruce, A.M.; Antonelli, J.; MacKinnon, A.C.; Aslanis, V.; Li, F.; O’Connor, R.; Boz, C.; et al. An Inhaled Galectin-3 Inhibitor in COVID-19 Pneumonitis: A Phase Ib/IIa Randomized Controlled Clinical Trial (DEFINE). Am. J. Respir. Crit. Care Med. 2023, 207, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Flores, S.; Priego-Ranero, A.; Azamar-Llamas, D.; Olvera-Prado, H.; Rivas-Redonda, K.I.; Ochoa-Hein, E.; Perez-Ortiz, A.; Rendon-Macias, M.E.; Rojas-Castaneda, E.; Urbina-Teran, S.; et al. Effect of polymerised type I collagen on hyperinflammation of adult outpatients with symptomatic COVID-19. Clin. Transl. Med. 2022, 12, e763. [Google Scholar] [CrossRef] [PubMed]
- Oshitani, N.; Watanabe, K.; Sakuma, T.; Matsuda, M.; Oyama, Y. Tranilast, an antifibrotic agent and COVID-19-induced pulmonary fibrosis. QJM 2022, 115, 249–250. [Google Scholar] [CrossRef]
- Nan, D.; Abraira-Meriel, C.; de la Roz-Fernandez, S.; Maestre-Orozco, T.; Hernandez, J.L.; Fernandez-Ayala, M. Delayed Use of the Recombinant Human IL-1 Receptor Antagonist Anakinra in Five COVID-19 Patients with Pulmonary Fibrosis and Persistent Hypoxaemia: A Preliminary Report. Eur. J. Case Rep. Intern. Med. 2021, 8, 002821. [Google Scholar] [CrossRef]
- Hyytiainen, M.; Penttinen, C.; Keski-Oja, J. Latent TGF-beta binding proteins: Extracellular matrix association and roles in TGF-beta activation. Crit. Rev. Clin. Lab. Sci. 2004, 41, 233–264. [Google Scholar] [CrossRef] [PubMed]
- Saharinen, J.; Taipale, J.; Keski-Oja, J. Association of the small latent transforming growth factor-beta with an eight cysteine repeat of its binding protein LTBP-1. EMBO J. 1996, 15, 245–253. [Google Scholar] [CrossRef]
- Annes, J.P.; Munger, J.S.; Rifkin, D.B. Making sense of latent TGFbeta activation. J. Cell Sci. 2003, 116, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Aschner, Y.; Downey, G.P. Transforming Growth Factor-beta: Master Regulator of the Respiratory System in Health and Disease. Am. J. Respir. Cell Mol. Biol. 2016, 54, 647–655. [Google Scholar] [CrossRef]
- Sheppard, D. Transforming growth factor beta: A central modulator of pulmonary and airway inflammation and fibrosis. Proc. Am. Thorac. Soc. 2006, 3, 413–417. [Google Scholar] [CrossRef]
- Xu, Y.D.; Hua, J.; Mui, A.; O’Connor, R.; Grotendorst, G.; Khalil, N. Release of biologically active TGF-beta1 by alveolar epithelial cells results in pulmonary fibrosis. Am. J. Physiol. Lung. Cell Mol. Physiol. 2003, 285, L527–L539. [Google Scholar] [CrossRef]
- Bartram, U.; Speer, C.P. The role of transforming growth factor beta in lung development and disease. Chest 2004, 125, 754–765. [Google Scholar] [CrossRef]
- Gentile, F.; Aimo, A.; Forfori, F.; Catapano, G.; Clemente, A.; Cademartiri, F.; Emdin, M.; Giannoni, A. COVID-19 and risk of pulmonary fibrosis: The importance of planning ahead. Eur. J. Prev. Cardiol. 2020, 27, 1442–1446. [Google Scholar] [CrossRef]
- Ye, Z.; Hu, Y. TGFbeta1: Gentlemanly orchestrator in idiopathic pulmonary fibrosis (Review). Int. J. Mol. Med. 2021, 48, 132. [Google Scholar] [CrossRef]
- Derynck, R.; Zhang, Y.E. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 2003, 425, 577–584. [Google Scholar] [CrossRef]
- Giacomelli, C.; Piccarducci, R.; Marchetti, L.; Romei, C.; Martini, C. Pulmonary fibrosis from molecular mechanisms to therapeutic interventions: Lessons from post-COVID-19 patients. Biochem. Pharmacol. 2021, 193, 114812. [Google Scholar] [CrossRef] [PubMed]
- Barczyk, M.; Carracedo, S.; Gullberg, D. Integrins. Cell Tissue Res. 2010, 339, 269–280. [Google Scholar] [CrossRef]
- Hynes, R.O. Integrins: Bidirectional, allosteric signaling machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef]
- Teoh, C.M.; Tan, S.S.; Tran, T. Integrins as Therapeutic Targets for Respiratory Diseases. Curr. Mol. Med. 2015, 15, 714–734. [Google Scholar] [CrossRef]
- Robles, J.P.; Zamora, M.; Adan-Castro, E.; Siqueiros-Marquez, L.; Martinez de la Escalera, G.; Clapp, C. The spike protein of SARS-CoV-2 induces endothelial inflammation through integrin alpha5beta1 and NF-kappaB signaling. J. Biol. Chem. 2022, 298, 101695. [Google Scholar] [CrossRef] [PubMed]
- Bugatti, K. alphaV beta6 Integrin: An Intriguing Target for COVID-19 and Related Diseases. Chembiochem 2021, 22, 2516–2520. [Google Scholar] [CrossRef]
- Saini, G.; Porte, J.; Weinreb, P.H.; Violette, S.M.; Wallace, W.A.; McKeever, T.M.; Jenkins, G. alphavbeta6 integrin may be a potential prognostic biomarker in interstitial lung disease. Eur. Respir. J. 2015, 46, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Raica, M.; Cimpean, A.M. Platelet-Derived Growth Factor (PDGF)/PDGF Receptors (PDGFR) Axis as Target for Antitumor and Antiangiogenic Therapy. Pharmaceuticals 2010, 3, 572–599. [Google Scholar] [CrossRef]
- Heldin, C.H.; Eriksson, U.; Ostman, A. New members of the platelet-derived growth factor family of mitogens. Arch. Biochem. Biophys. 2002, 398, 284–290. [Google Scholar] [CrossRef]
- Heldin, C.H.; Westermark, B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol. Rev. 1999, 79, 1283–1316. [Google Scholar] [CrossRef] [PubMed]
- Betsholtz, C. Biology of platelet-derived growth factors in development. Birth Defects Res. C Embryo Today 2003, 69, 272–285. [Google Scholar] [CrossRef]
- Abdollahi, A.; Li, M.; Ping, G.; Plathow, C.; Domhan, S.; Kiessling, F.; Lee, L.B.; McMahon, G.; Grone, H.J.; Lipson, K.E.; et al. Inhibition of platelet-derived growth factor signaling attenuates pulmonary fibrosis. J. Exp. Med. 2005, 201, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Bonner, J.C. Regulation of PDGF and its receptors in fibrotic diseases. Cytokine Growth Factor Rev. 2004, 15, 255–273. [Google Scholar] [CrossRef]
- Zhuo, Y.; Zhang, J.; Laboy, M.; Lasky, J.A. Modulation of PDGF-C and PDGF-D expression during bleomycin-induced lung fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2004, 286, L182–L188. [Google Scholar] [CrossRef]
- Andrae, J.; Gallini, R.; Betsholtz, C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008, 22, 1276–1312. [Google Scholar] [CrossRef]
- Prudovsky, I. Cellular Mechanisms of FGF-Stimulated Tissue Repair. Cells 2021, 10, 1830. [Google Scholar] [CrossRef]
- Jones, S.A. Physiology of FGF15/19. Adv. Exp. Med. Biol. 2012, 728, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Brooks, A.N.; Kilgour, E.; Smith, P.D. Molecular pathways: Fibroblast growth factor signaling: A new therapeutic opportunity in cancer. Clin. Cancer Res. 2012, 18, 1855–1862. [Google Scholar] [CrossRef]
- Olsen, S.K.; Garbi, M.; Zampieri, N.; Eliseenkova, A.V.; Ornitz, D.M.; Goldfarb, M.; Mohammadi, M. Fibroblast growth factor (FGF) homologous factors share structural but not functional homology with FGFs. J. Biol. Chem. 2003, 278, 34226–34236. [Google Scholar] [CrossRef]
- Beenken, A.; Mohammadi, M. The FGF family: Biology, pathophysiology and therapy. Nat. Rev. Drug Discov. 2009, 8, 235–253. [Google Scholar] [CrossRef]
- Guzy, R.D.; Li, L.; Smith, C.; Dorry, S.J.; Koo, H.Y.; Chen, L.; Ornitz, D.M. Pulmonary fibrosis requires cell-autonomous mesenchymal fibroblast growth factor (FGF) signaling. J. Biol. Chem. 2017, 292, 10364–10378. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.H.; Wang, D.D.; Zhou, Z.Y.; He, S.L.; Chen, A.A.; Wang, J. Mutant soluble ectodomain of fibroblast growth factor receptor-2 IIIc attenuates bleomycin-induced pulmonary fibrosis in mice. Biol. Pharm. Bull. 2012, 35, 731–736. [Google Scholar] [CrossRef][Green Version]
- MacKenzie, B.; Korfei, M.; Henneke, I.; Sibinska, Z.; Tian, X.; Hezel, S.; Dilai, S.; Wasnick, R.; Schneider, B.; Wilhelm, J.; et al. Increased FGF1-FGFRc expression in idiopathic pulmonary fibrosis. Respir. Res. 2015, 16, 83. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, T.; Coleman, C.M.; Frieman, M.B. Overactive Epidermal Growth Factor Receptor Signaling Leads to Increased Fibrosis after Severe Acute Respiratory Syndrome Coronavirus Infection. J. Virol. 2017, 91, e00182-17. [Google Scholar] [CrossRef]
- Kim, H.S.; Nagalla, S.R.; Oh, Y.; Wilson, E.; Roberts, C.T., Jr.; Rosenfeld, R.G. Identification of a family of low-affinity insulin-like growth factor binding proteins (IGFBPs): Characterization of connective tissue growth factor as a member of the IGFBP superfamily. Proc. Natl. Acad. Sci. USA 1997, 94, 12981–12986. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.T.; Knight, R.A.; Bloor, C.A.; Spiteri, M.A. Enhanced insulin-like growth factor binding protein-related protein 2 (Connective tissue growth factor) expression in patients with idiopathic pulmonary fibrosis and pulmonary sarcoidosis. Am. J. Respir. Cell Mol. Biol. 1999, 21, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Alkhatib, A.; Kolls, J.K.; Kondoh, Y.; Lasky, J.A. Pharmacotherapy and adjunctive treatment for idiopathic pulmonary fibrosis (IPF). J. Thorac. Dis. 2019, 11, S1740–S1754. [Google Scholar] [CrossRef]
- Yang, J.; Velikoff, M.; Canalis, E.; Horowitz, J.C.; Kim, K.K. Activated alveolar epithelial cells initiate fibrosis through autocrine and paracrine secretion of connective tissue growth factor. Am. J. Physiol. Lung Cell Mol. Physiol. 2014, 306, L786–L796. [Google Scholar] [CrossRef]
- Lipson, K.E.; Wong, C.; Teng, Y.; Spong, S. CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenesis Tissue Repair. 2012, 5, S24. [Google Scholar] [CrossRef] [PubMed]
- Cui, N.; Hu, M.; Khalil, R.A. Biochemical and Biological Attributes of Matrix Metalloproteinases. Prog. Mol. Biol. Transl. Sci. 2017, 147, 1–73. [Google Scholar] [CrossRef] [PubMed]
- Amalinei, C.; Caruntu, I.D.; Balan, R.A. Biology of metalloproteinases. Rom. J. Morphol. Embryol. 2007, 48, 323–334. [Google Scholar] [PubMed]
- Selman, M.; Ruiz, V.; Cabrera, S.; Segura, L.; Ramirez, R.; Barrios, R.; Pardo, A. TIMP-1, -2, -3, and -4 in idiopathic pulmonary fibrosis. A prevailing nondegradative lung microenvironment? Am. J. Physiol. Lung Cell Mol. Physiol. 2000, 279, L562–L574. [Google Scholar] [CrossRef] [PubMed]
- Ghezelbash, B.; Rostami, M.; Heidarvand, M.; Mafi, A.; Chegni, H.; Eskandari, N. Correlation of Expression of MMP-2, ACE2, and TMPRSS2 Genes with Lymphopenia for Mild and Severity of COVID-19. Iran J. Allergy Asthma Immunol. 2023, 22, 91–98. [Google Scholar] [CrossRef]
- Avila-Mesquita, C.D.; Couto, A.E.S.; Campos, L.C.B.; Vasconcelos, T.F.; Michelon-Barbosa, J.; Corsi, C.A.C.; Mestriner, F.; Petroski-Moraes, B.C.; Garbellini-Diab, M.J.; Couto, D.M.S.; et al. MMP-2 and MMP-9 levels in plasma are altered and associated with mortality in COVID-19 patients. Biomed. Pharmacother. 2021, 142, 112067. [Google Scholar] [CrossRef] [PubMed]
- Tzouvelekis, A.; Herazo-Maya, J.D.; Slade, M.; Chu, J.H.; Deiuliis, G.; Ryu, C.; Li, Q.; Sakamoto, K.; Ibarra, G.; Pan, H.; et al. Validation of the prognostic value of MMP-7 in idiopathic pulmonary fibrosis. Respirology 2017, 22, 486–493. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Richeldi, L.; Thomson, C.C.; Inoue, Y.; Johkoh, T.; Kreuter, M.; Lynch, D.A.; Maher, T.M.; Martinez, F.J.; et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2022, 205, e18–e47. [Google Scholar] [CrossRef]
- Raghu, G.; Selman, M. Nintedanib and pirfenidone. New antifibrotic treatments indicated for idiopathic pulmonary fibrosis offer hopes and raises questions. Am. J. Respir. Crit. Care Med. 2015, 191, 252–254. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, D.; Kong, X.; Wei, C.; LvQiu, S.; Wang, L.; Lin, Y.; Yin, Z.; Zhou, Z.; Luo, H. Case Report: Pirfenidone in the Treatment of Post-COVID-19 Pulmonary Fibrosis. Front. Med. 2022, 9, 925703. [Google Scholar] [CrossRef]
- Bazdyrev, E.; Panova, M.; Brachs, M.; Smolyarchuk, E.; Tsygankova, D.; Gofman, L.; Abdyusheva, Y.; Novikov, F. Efficacy and safety of Treamid in the rehabilitation of patients after COVID-19 pneumonia: A phase 2, randomized, double-blind, placebo-controlled trial. J. Transl. Med. 2022, 20, 506. [Google Scholar] [CrossRef]
- Cesarone, M.R.; Hu, S.; Belcaro, G.; Cornelli, U.; Feragalli, B.; Corsi, M.; Bombardelli, E.; Cotellese, R.; Hosoi, M.; Rosenkvist, L. Pycnogenol(R)-Centellicum(R) supplementation improves lung fibrosis and post-COVID-19 lung healing. Minerva. Med. 2022, 113, 135–140. [Google Scholar] [CrossRef]
- Park, S.-A.; Kim, M.-J.; Park, S.-Y.; Kim, J.-S.; Lee, S.-J.; Woo, H.A.; Kim, D.-K.; Nam, J.-S.; Sheen, Y.Y. EW-7197 inhibits hepatic, renal, and pulmonary fibrosis by blocking TGF-β/Smad and ROS signaling. Cell Mol. Life Sci. 2015, 72, 2023–2039. [Google Scholar] [CrossRef]
- Fernandes, R.S.; Dias, H.B.; de Souza Jaques, W.A.; Becker, T.; Rigatto, K. Assessment of Alamandine in Pulmonary Fibrosis and Respiratory Mechanics in Rodents. J. Renin. Angiotensin. Aldosterone Syst. 2021, 2021, 9975315. [Google Scholar] [CrossRef] [PubMed]
- Colunga Biancatelli, R.M.L.; Solopov, P.; Dimitropoulou, C.; Gregory, B.; Day, T.; Catravas, J.D. The Heat Shock Protein 90 Inhibitor, AT13387, Protects the Alveolo-Capillary Barrier and Prevents HCl-Induced Chronic Lung Injury and Pulmonary Fibrosis. Cells 2022, 11, 1046. [Google Scholar] [CrossRef]
- Sheng, H.; Lin, G.; Zhao, S.; Li, W.; Zhang, Z.; Zhang, W.; Yun, L.; Yan, X.; Hu, H. Antifibrotic Mechanism of Piceatannol in Bleomycin-Induced Pulmonary Fibrosis in Mice. Front. Pharmacol. 2022, 13, 771031. [Google Scholar] [CrossRef] [PubMed]
- Richeldi, L.; Fernandez Perez, E.R.; Costabel, U.; Albera, C.; Lederer, D.J.; Flaherty, K.R.; Ettinger, N.; Perez, R.; Scholand, M.B.; Goldin, J.; et al. Pamrevlumab, an anti-connective tissue growth factor therapy, for idiopathic pulmonary fibrosis (PRAISE): A phase 2, randomised, double-blind, placebo-controlled trial. Lancet Respir. Med. 2020, 8, 25–33. [Google Scholar] [CrossRef]
- Liu, Z.W.; Zhao, M.Y.; Su, X.L.; Ye, T.H.; Zhuang, Y.J.; Zhang, Y.; Zhang, Z.Z.; Yang, J.L.; Chen, L.J.; Long, C.F.; et al. The antifibrotic effect and mechanism of a novel tyrosine kinase inhibitor, ZSP1603, in preclinical models of pulmonary fibrosis. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 1481–1491. [Google Scholar] [CrossRef]
- Soltani Hekmat, A.; Javanmardi, K. Alamandine: Potential Protective Effects in SARS-CoV-2 Patients. J. Renin. Angiotensin. Aldosterone Syst. 2021, 2021, 6824259. [Google Scholar] [CrossRef]
- Hussein, R.M.; Arafa, E.A.; Raheem, S.A.; Mohamed, W.R. Thymol protects against bleomycin-induced pulmonary fibrosis via abrogation of oxidative stress, inflammation, and modulation of miR-29a/TGF-beta and PI3K/Akt signaling in mice. Life Sci. 2022, 314, 121256. [Google Scholar] [CrossRef]
- Ebrahimpour, A.; Ahir, M.; Wang, M.; Jegga, A.G.; Bonnen, M.D.; Eissa, N.T.; Montesi, S.B.; Raghu, G.; Ghebre, Y.T. Combination of esomeprazole and pirfenidone enhances antifibrotic efficacy in vitro and in a mouse model of TGFbeta-induced lung fibrosis. Sci. Rep. 2022, 12, 20668. [Google Scholar] [CrossRef]
- Wilkinson, A.L.; John, A.E.; Barrett, J.W.; Gower, E.; Morrison, V.S.; Man, Y.; Pun, K.T.; Roper, J.A.; Luckett, J.C.; Borthwick, L.A.; et al. Pharmacological characterisation of GSK3335103, an oral alphavbeta6 integrin small molecule RGD-mimetic inhibitor for the treatment of fibrotic disease. Eur. J. Pharmacol. 2021, 913, 174618. [Google Scholar] [CrossRef]
- Medina-De la Garza, C.E.; Salvador Flores-Torres, A.; Garcia-Hernandez, M.; de Los Angeles Castro-Corona, M. Diethylcarbamazine as potential treatment of COVID-19 lung fibrosis. Med. Hypotheses 2022, 160, 110774. [Google Scholar] [CrossRef]
- Sgalla, G.; Biffi, A.; Richeldi, L. Idiopathic pulmonary fibrosis: Diagnosis, epidemiology and natural history. Respirology 2016, 21, 427–437. [Google Scholar] [CrossRef]
- Kaarteenaho, R.; Lappi-Blanco, E. Tissue is an issue in the search for biomarkers in idiopathic pulmonary fibrosis. Fibrogenesis Tissue Repair 2015, 8, 3. [Google Scholar] [CrossRef][Green Version]
- Zhang, Y.; Kaminski, N. Biomarkers in idiopathic pulmonary fibrosis. Curr. Opin. Pulm. Med. 2012, 18, 441–446. [Google Scholar] [CrossRef]
- Guiot, J.; Moermans, C.; Henket, M.; Corhay, J.L.; Louis, R. Blood Biomarkers in Idiopathic Pulmonary Fibrosis. Lung 2017, 195, 273–280. [Google Scholar] [CrossRef]
- Drakopanagiotakis, F.; Wujak, L.; Wygrecka, M.; Markart, P. Biomarkers in idiopathic pulmonary fibrosis. Matrix Biol. 2018, 68–69, 404–421. [Google Scholar] [CrossRef]
- Siekacz, K.; Kumor-Kisielewska, A.; Milkowska-Dymanowska, J.; Pietrusinska, M.; Bartczak, K.; Majewski, S.; Stanczyk, A.; Piotrowski, W.J.; Bialas, A.J. Soluble ITGaM and ITGb2 Integrin Subunits Are Involved in Long-Term Pulmonary Complications after COVID-19 Infection. J. Clin. Med. 2023, 12, 342. [Google Scholar] [CrossRef]
- Wu, Y.; Zhong, L.; Qiu, L.; Dong, L.; Yang, L.; Chen, L. A potential three-gene-based diagnostic signature for idiopathic pulmonary fibrosis. Front. Genet 2022, 13, 985217. [Google Scholar] [CrossRef]
- Pulito-Cueto, V.; Genre, F.; Lopez-Mejias, R.; Mora-Cuesta, V.M.; Iturbe-Fernandez, D.; Portilla, V.; Sebastian Mora-Gil, M.; Ocejo-Vinyals, J.G.; Gualillo, O.; Blanco, R.; et al. Endothelin-1 as a Biomarker of Idiopathic Pulmonary Fibrosis and Interstitial Lung Disease Associated with Autoimmune Diseases. Int. J. Mol. Sci. 2023, 24, 1275. [Google Scholar] [CrossRef]
- Li, Y.; He, Y.; Chen, S.; Wang, Q.; Yang, Y.; Shen, D.; Ma, J.; Wen, Z.; Ning, S.; Chen, H. S100A12 as Biomarker of Disease Severity and Prognosis in Patients with Idiopathic Pulmonary Fibrosis. Front. Immunol. 2022, 13, 810338. [Google Scholar] [CrossRef]
- Spagnolo, P.; Oldham, J.M. On Target: CYFRA 21-1 as an Idiopathic Pulmonary Fibrosis Biomarker. Am. J. Respir. Crit. Care Med. 2022, 205, 1376–1377. [Google Scholar] [CrossRef]
- Molyneaux, P.L.; Fahy, W.A.; Byrne, A.J.; Braybrooke, R.; Saunders, P.; Toshner, R.; Albers, G.; Chua, F.; Renzoni, E.A.; Wells, A.U.; et al. CYFRA 21-1 Predicts Progression in Idiopathic Pulmonary Fibrosis: A Prospective Longitudinal Analysis of the PROFILE Cohort. Am. J. Respir. Crit. Care Med. 2022, 205, 1440–1448. [Google Scholar] [CrossRef]
- Dai, X.; Yang, Z.; Zhang, W.; Liu, S.; Zhao, Q.; Liu, T.; Chen, L.; Li, L.; Wang, Y.; Shao, R. Identification of diagnostic gene biomarkers related to immune infiltration in patients with idiopathic pulmonary fibrosis based on bioinformatics strategies. Front. Med. 2022, 9, 959010. [Google Scholar] [CrossRef]
- De Vitis, C.; D’Ascanio, M.; Sacconi, A.; Pizzirusso, D.; Salvati, V.; Mancini, M.; Scafetta, G.; Cirombella, R.; Ascenzi, F.; Bruschini, S.; et al. B4GALT1 as a New Biomarker of Idiopathic Pulmonary Fibrosis. Int. J. Mol. Sci. 2022, 23, 15040. [Google Scholar] [CrossRef]
- Zheng, J.; Dong, H.; Zhang, T.; Ning, J.; Xu, Y.; Cai, C. Development and Validation of a Novel Gene Signature for Predicting the Prognosis of Idiopathic Pulmonary Fibrosis Based on Three Epithelial-Mesenchymal Transition and Immune-Related Genes. Front. Genet 2022, 13, 865052. [Google Scholar] [CrossRef]
- Yu, Y.Z.; Gui, X.H.; Yu, M.; Huang, W.; Peng, L.Y.; Dai, J.H.; Xu, Q.Q.; Zhao, T.T.; Xie, W.P.; Xiao, Y.L.; et al. Soluble ST2 in serum predicts the prognosis of idiopathic pulmonary fibrosis: A retrospective study. Ann. Transl. Med. 2022, 10, 797. [Google Scholar] [CrossRef]
- Cao, M.; Gu, L.; Guo, L.; Liu, M.; Wang, T.; Zhang, J.; Zhang, H.; Zhang, Y.; Shi, Y.; Zhao, Y.; et al. Elevated Expression of Growth Differentiation Factor-15 Is Associated with Acute Exacerbation of Idiopathic Pulmonary Fibrosis. Front. Immunol. 2022, 13, 891448. [Google Scholar] [CrossRef]
- Huang, T.; Zhou, H.F. A Novel 5-Methylcytosine- and Immune-Related Prognostic Signature Is a Potential Marker of Idiopathic Pulmonary Fibrosis. Comput. Math Methods Med. 2022, 2022, 1685384. [Google Scholar] [CrossRef]
- Tian, M.; Meng, K.; Gao, Y.; Zhang, J.; Xie, M.; Tian, Y.; Liu, X.; Ma, M.; Cai, Y.; Wu, H.; et al. Elevated serum human epididymis protein 4 is associated with disease severity and worse survival in idiopathic pulmonary fibrosis: A cohort study. Ann. Transl. Med. 2022, 10, 992. [Google Scholar] [CrossRef]
- Wang, E.; Wang, Y.; Zhou, S.; Xia, X.; Han, R.; Fei, G.; Zeng, D.; Wang, R. Identification of three hub genes related to the prognosis of idiopathic pulmonary fibrosis using bioinformatics analysis. Int. J. Med. Sci. 2022, 19, 1417–1429. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).