1. Introduction
Despite important improvements in rehabilitation, there is no curative treatment for spinal cord injury (SCI) [
1]. The use of bone marrow-derived stem cells is currently one of the most promising experimental treatments for SCI [
2,
3,
4]. While the application of neural stem cells has the objective of replacing lost neurons and glia cells [
1,
5,
6,
7], the rationale of using mesenchymal stem cells consists mainly in modifying the endogenous response in the tissue, primarily by reducing neuroinflammation [
4,
8]. Paracrine factors and extracellular vesicles that are released from mesenchymal stem cells are expected to prevent secondary degeneration, reduce scar formation, and support regenerative plasticity after SCI [
9]. An accessible source of this type of cell is the bone marrow, which contains hematopoietic and mesenchymal stem cells. Bone marrow-derived stromal cells (bmSCs) pose no risk of tumor formation, which remains an issue with induced pluripotent stem cells [
1,
10]. The company Neuroplast BV developed a procedure to prepare human bmSCs by a negative selection procedure without cell expansion in culture (patent WO2015/059300A1). After extraction from the iliac crest, depletion of erythrocytes and lymphocytes, followed by characterization with flow cytometry and an in vitro potency assay, these cells (NeuroCells
TM) are intended for the therapy of neurological pathologies, including SCI and frontotemporal dementia. The safety and efficacy of these cells had to be tested in animal experiments. In a previous study using a rat model of SCI, we demonstrated that animals without additional immune suppression showed no negative response to the injection of human bmSCs. One intrathecal injection of two million NeuroCells at 1–2 h after SCI reduced inflammation and axonal degeneration, and had beneficial effects on motor recovery when compared to a control treatment with methylprednisolone [
11]. However, the functional improvements were small and not even significant in comparison with a different control group that received no treatment at all. With the intention of improving survival of the injected bmSCs in the acute phase after SCI, the treatment was later combined with anti-inflammatory application of tauroursodesoxycholic acid, but this failed to produce additional benefits [
12]. Thus, we reasoned that cellular treatment would be more beneficial if given when the initial inflammatory peak has passed. A clinical trial with NeuroCells is currently in progress (EudraCT 2018-000805-22), and in this case patients receive cellular treatment six to eight weeks after SCI. An additional issue of these and other related studies is that bmSCs were stored frozen and reconstituted, while a major efficacy is only claimed for fresh cells. The dose of implanted stem cells is also potentially important [
3].
The objective of the present study was to assess the safety and therapeutic benefits of a delayed intrathecal injection of bmSCs prepared by negative selection. For this we chose the contusion model of rat SCI at the thoracic level, which is the best available compromise between the demand to mimic clinical cases, practical applicability, and animal welfare (in comparison with contusion at cervical levels and the use of larger animals). Our principal focus was on detecting a meaningful functional benefit of the approach in the chronic phase after treatment. For this purpose, standard sensory–motor tests, such as sensitivity to tactile and heat stimulation, open field evaluation, and motor performance on a rotating rod were complemented with quantitative video analysis. The secondary objective was to evaluate the long-term cellular effects of bmSC treatment, such as neuronal survival and microglial and astrocyte activation.
4. Materials and Methods
4.1. Experimental Animals
The experimental protocol, surgical procedures, and postoperative care were reviewed by the ethics committee for Animal Care of the Hospital Nacional de Parapléjicos (163CEEA/2017) and approved by the regulatory authority of Castilla-la Mancha (ref. 210498, following EU directive 2010/63/EU). The study was conducted with male Wistar rats (Rattus norwegicus) from the breeding facility of the hospital, which had a body weight of 240 to 290 g at the day of surgery. Since the gain in body weight, which may affect motor performance, is different in male and female rats, mixed sex groups would have required a larger number of animals. Male rats were chosen to avoid the potential influence of changing hormone levels during the estrous cycle either at the time of SCI or at the time of treatment one week later.
Standard housing conditions consisted of a 12 h light/dark cycle, humidity 40–60%, and temperature 22 °C with
ad libitum access to food and water. A total of 68 animals with SCI entered the study, 42 in the principal investigation and 26 in an experiment to trace injected stem cells. In addition, the spinal cords of 3 rats with laminectomy only (sham) and 2 rats without surgery were processed for comparison of histological results or PCR (
Figure 1a). Sample size calculation was based on previous experience and the availability of fresh human stem cells [
12] (α = 0.05, β = 0.2, BBB score as primary outcome with expected d = 3, SD = 2, attrition 10%). Since rats had the same sex, and similar age and body weight, no randomization was necessary to allocate them to the different groups.
4.2. Surgical Procedures and Postoperative Treatment
For spinal cord injury, anesthesia consisted of 2.5% isoflurane/97.5% oxygen at 0.5 L/min for SCI with one s.c. injection of buprenorphine 0.05 mg/kg 15 min before surgery. Corneal dehydration was prevented with ophthalmic ointment (Lubrithal). Following laminectomy at thoracic level T9, a spinal cord contusion of 2 N (200 Kdyn, zero dwell time) was performed with the Infinite Horizon spinal cord impactor. The procedure was checked visually (hematoma) and by monitoring the displacement/time and force/time plots. After the operation, animals received 2 × 1.5 mL isotonic saline s.c. and antibiotic 5 mg/kg marbofloxacin (10 mg/mL, s.c.). Postoperative care, including analgesics, antibiotic treatment, and manual voiding of the bladder, was performed as described previously [
11,
12].
One week after SCI, the animals received one injection into the cisterna magna. This was done with the animals under anesthesia with one i. p. injection of ketamine 50 mg/kg combined with xylazine 5 mg/kg. For the transplantation, the anesthetized animals were positioned in a stereotactic frame, and the atlanto-occipital membrane was exposed and penetrated with a pointed scalpel blade. A Fogarty arterial embolectomy catheter (d = 0.67 mm) was then inserted 3–5 mm into the cisterna magna and the cell suspension was slowly infused with a syringe pump (150 µL/5 min). While the rat was being prepared by one researcher, a second person suspended 0.5 or 2.5 million cells for injection based on cytometric counting of cell numbers and viability. Euthanasia at the end of the study was induced by i. p. injection of 100 mg/kg sodium pentobarbital.
4.3. Experimental Groups
Animals were assigned to five experimental groups, which received the same SCI but differed in the treatment procedure (
Figure 1a). The Group
SCI-control received one injection of 150 µL saline into the cisterna magna at 7 days after SCI (7 dpo). Animals of all other treatment groups were given 150 µL cell suspension injected into the cisterna magna at 7 dpo. Group
SCI-bmSC-05 received 0.5 × 10
6 fresh bmSCs, Group
SCI-bmSC-25 received 2.5 × 10
6 fresh bmSCs, and Group
SCI-bmSC-25r received 2.5 × 10
6 bmSCs which were stored in liquid nitrogen and resuspended immediately before application. A second control, Group
SCI-ratBMC, was injected with 0.5 × 10
6 stromal cells from rat bone marrow. Cell numbers refer to live cells as determined with cytometry prior to injection. Spinal cord sections from three rats that had T9 laminectomy but underwent no contusion injury were used for comparing histological data.
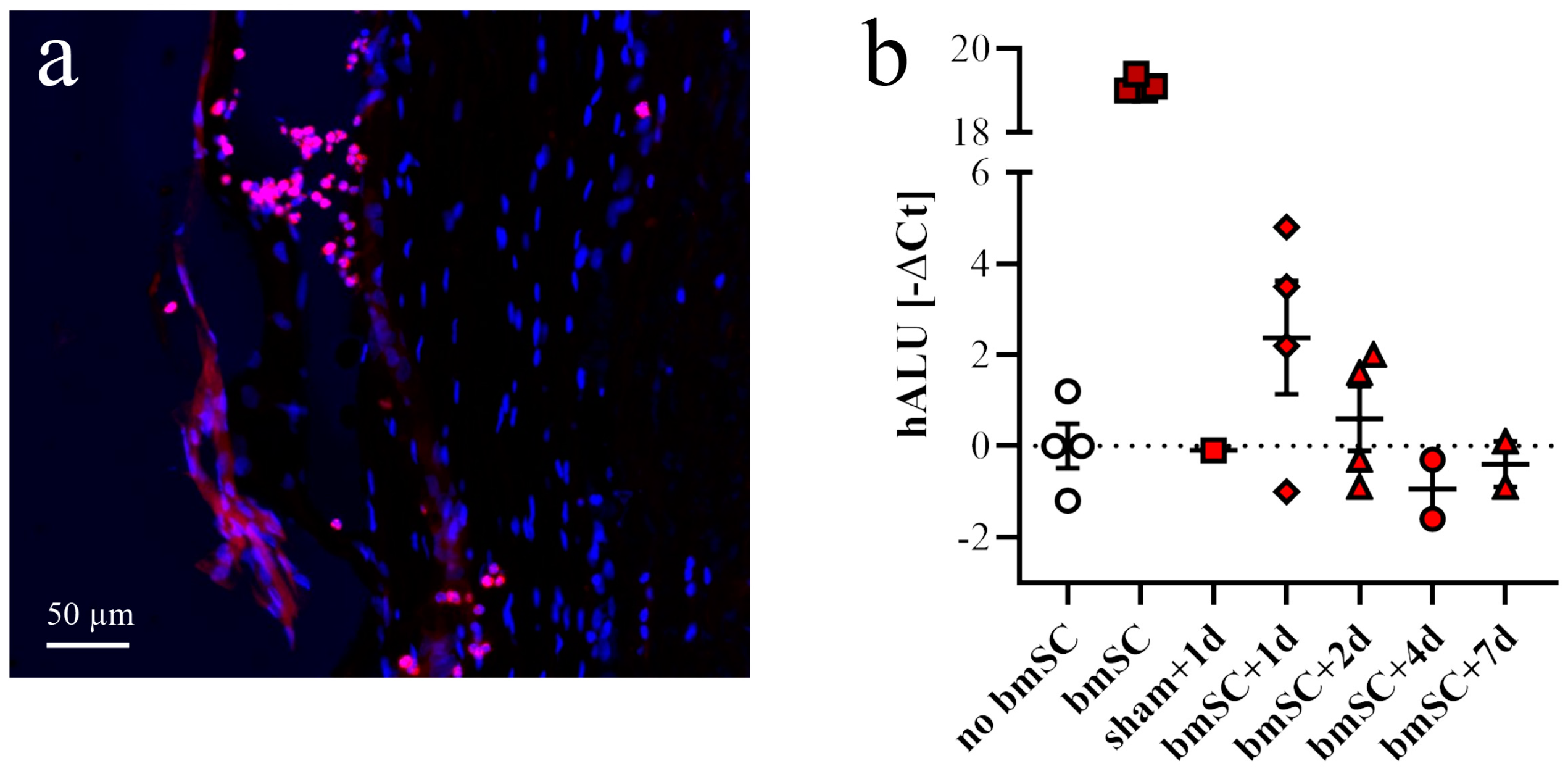
For the purpose of detecting injected cells, two additional groups of rats with SCI received one injection of 2.5 × 10
6 bmSCs (stored in liquid nitrogen and resuspended) or the same volume of saline into the cisterna magna. Animals were sacrificed 1, 2, 4, or 7 days after bmSCs injection (dpi) into the cisterna magna (
Figure 1b). Histological staining to detect implanted cells was also carried out with tissue from SCI-control and the bmSC-treated groups sacrificed after 6 weeks. The spinal cords of two rats without surgery were prepared to control for false positive PCR results.
4.4. Preparation of bmSCs
Bone marrow-derived stromal cells from human donors. Human bmSCs for SCI treatment were prepared as described previously [
11,
26]. Following bone marrow extraction from the donor, the procedure consisted of Ficoll density gradient centrifugation followed by the elimination of B-cells (CD20), T-cells (CD3), monocytes (CD14), and natural killer cells (CD56) using antibody-based cell sorting with magnetic beads under GMP conditions. Cells were not expanded by cultivation (Neuroplast BV, patent WO2015/059300A1). All procedures for collection of human bone marrow were approved by the ethics committee of Maastricht University Medical Center (METC 13-2-032). The viability and cell type composition of each batch were analyzed with flow cytometry (CD34, CD271, CD90, CD105, CD73). For the present study, bmSCs were prepared at the Neuroplast facility in Geleen, NL, and shipped in a cooled, temperature-controlled container within 24 h to the HNP Toledo, using the same procedure as followed for a clinical trial (EudraCT 2018-000805-22). One experimental group of animals received bmSCs that were cryoprotected with DMSO, frozen in liquid nitrogen, shipped on dry ice to Toledo, Spain, and then stored in liquid nitrogen until use. Cell viability (exclusion of 7-amino-actinomycin D, cytometry) was determined immediately before application in vivo.
Stromal cells from rat bone marrow. To control for effects of intrathecal cell injection, we prepared cells from the bone marrow of adult rats (ratBMC). Cells were obtained from femur and tibia bones that were aseptically harvested from male rats euthanized with an overdose of sodium pentobarbital. Bone marrow was obtained by rinsing each harvested bone with growth medium (Dulbecco’s Modified Eagle medium, DMEM; GIBCO) using a 3 mL syringe. They were separated by centrifugation at 2000 rpm at RT for 45 min on a 3 mL Ficoll gradient. The obtained cells were seeded in a 75 cm2 culture flask with 10 mL of DMEM, 20% heat-inactivated fetal bovine serum, 2 mM L-glutamine, streptomycin/penicillin (Sigma P4458, 1/100), and nonessential amino acids (Lonza 3-114E, 1/100, other cell culture reagents from GIBCO). Cells were maintained in a water-jacketed incubator at 37 °C with 5% CO2 until a monolayer of feeding cells was formed, approximately three weeks. Afterwards, a new batch was prepared using the same procedure and cultured on top of feeding cells for four passages. These ratBMC were cryopreserved with DMSO and stored in liquid nitrogen until use.
4.5. Evaluation of Locomotor Functions
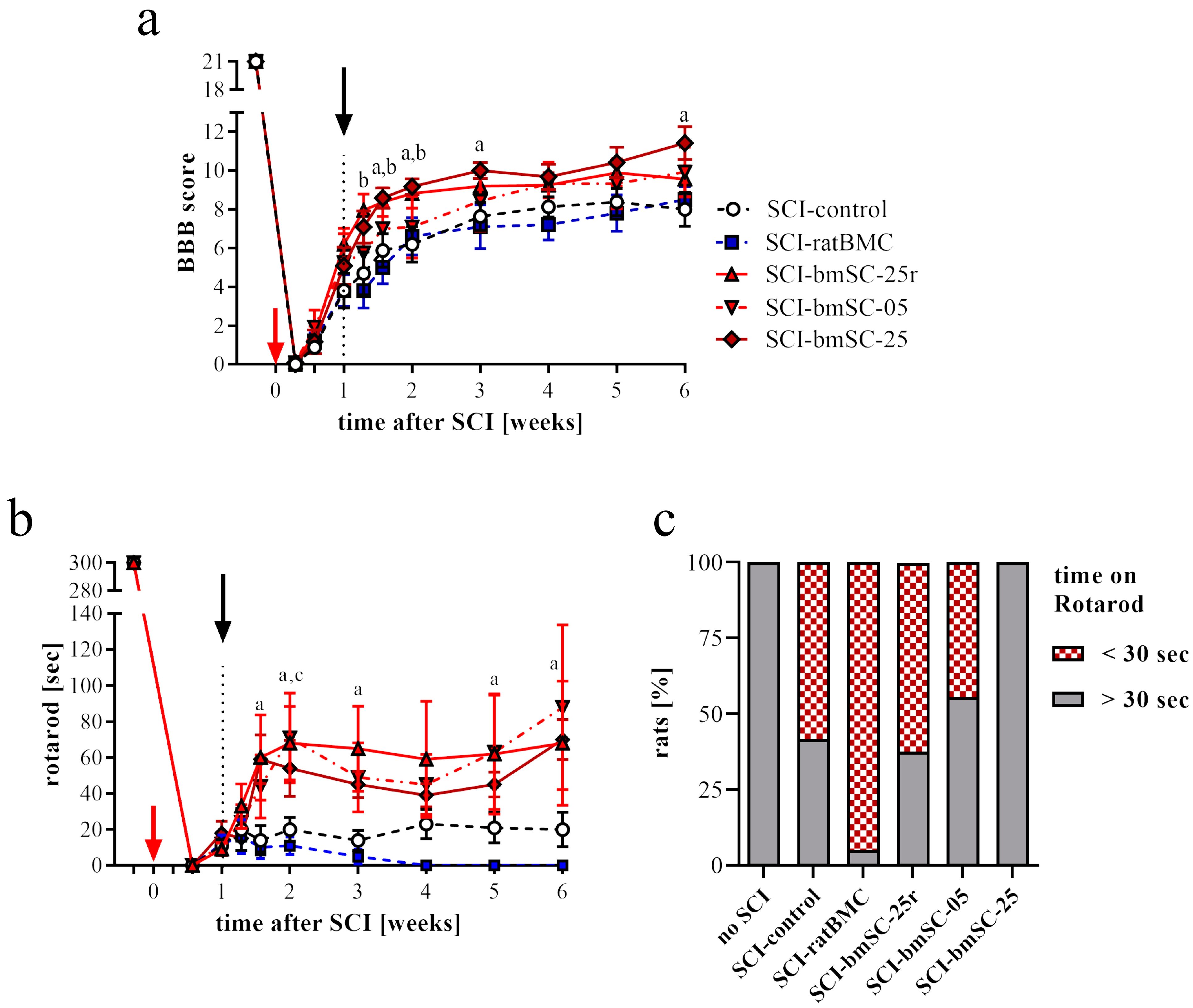
Open field assessment. Recovery of limb movements was evaluated in the open field using the Basso–Beattie–Bresnahan (BBB) locomotor function scale [
26]. This was performed before SCI surgery (baseline), then at 2 dpo, 4 dpo, 7 dpo (i.e., before treatment), at 9 dpo, 11 dpo, 14 dpo, and subsequently once per week until six weeks after SCI. At the beginning, we established a criterion of BBB ≤ 1 at 2 dpo for inclusion in the study because a higher score was considered to indicate incomplete SCI. At 7 dpo, before treatment, a negative criterion (BBB ≤ 1) was applied to exclude rats with excessive lesions. Scoring was performed independently by two investigators who were blinded with respect to the treatment of the individual animals. The average score was given if, after discussing their evaluation, both investigators assigned different ratings.
RotaRod test. Complementary to the open field assessment, the rats were subjected to the RotaRod test (Ugo Basile SRL, Gemonio, Italy). In this task, rats are positioned on a slowly rotating rod, which obliges them to use their hind legs to maintain their balance. During tests, the rotation speed was accelerated from 5 rpm to 15 rpm over a period of 5 min, and the time was recorded that the rats were able to stay on the rotating rod (two repetitions, separated by a break of ≥15 min). In six training sessions of 5 min each, at three consecutive days before SCI, all rats learned this task at a constant speed of 5 rpm of the rotating rod. After confirming at 4 dpo that none of the rats were capable of weight-supported stepping, the test was administered for the first time at 7 dpo and then once per week until the end of the study.
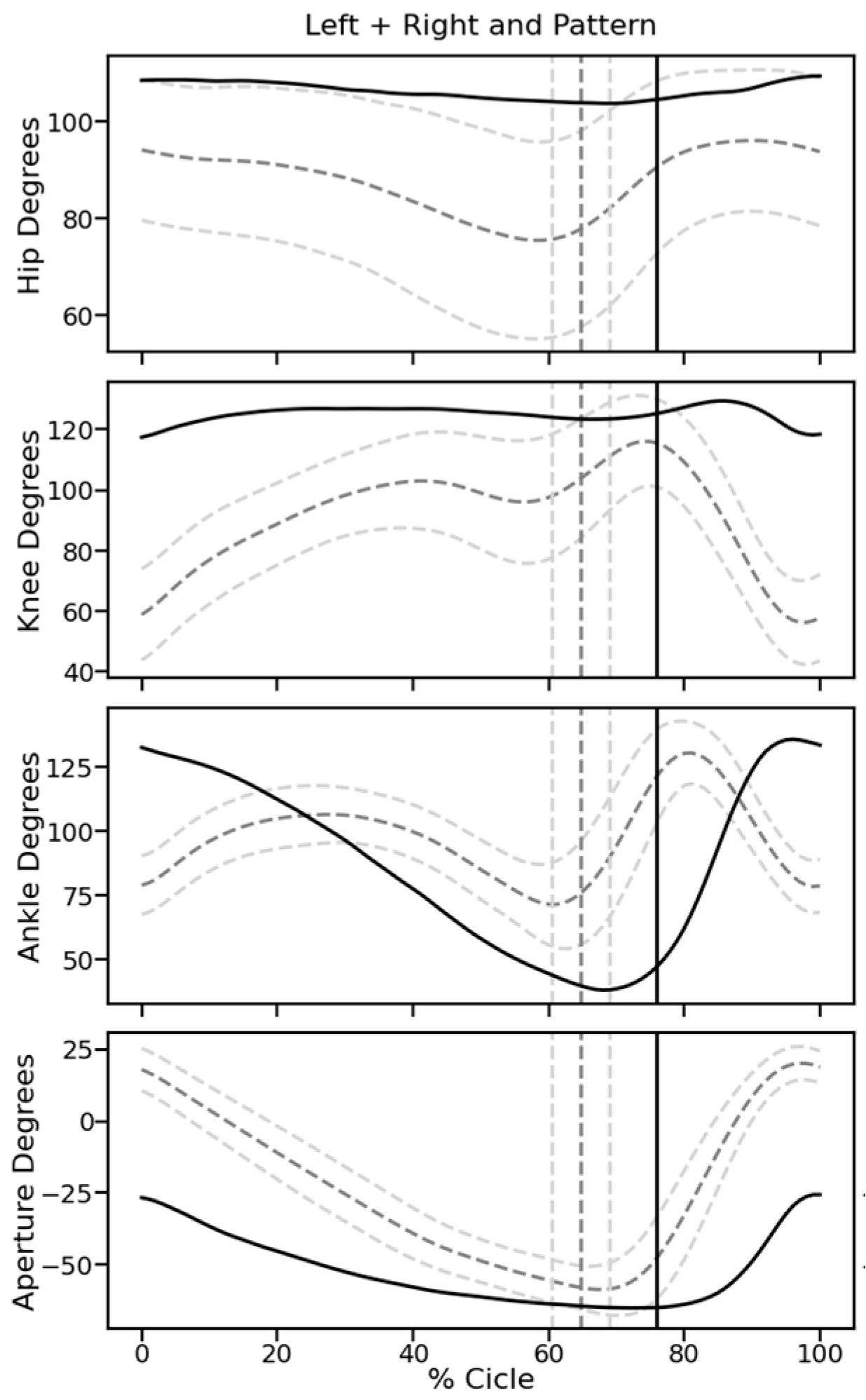
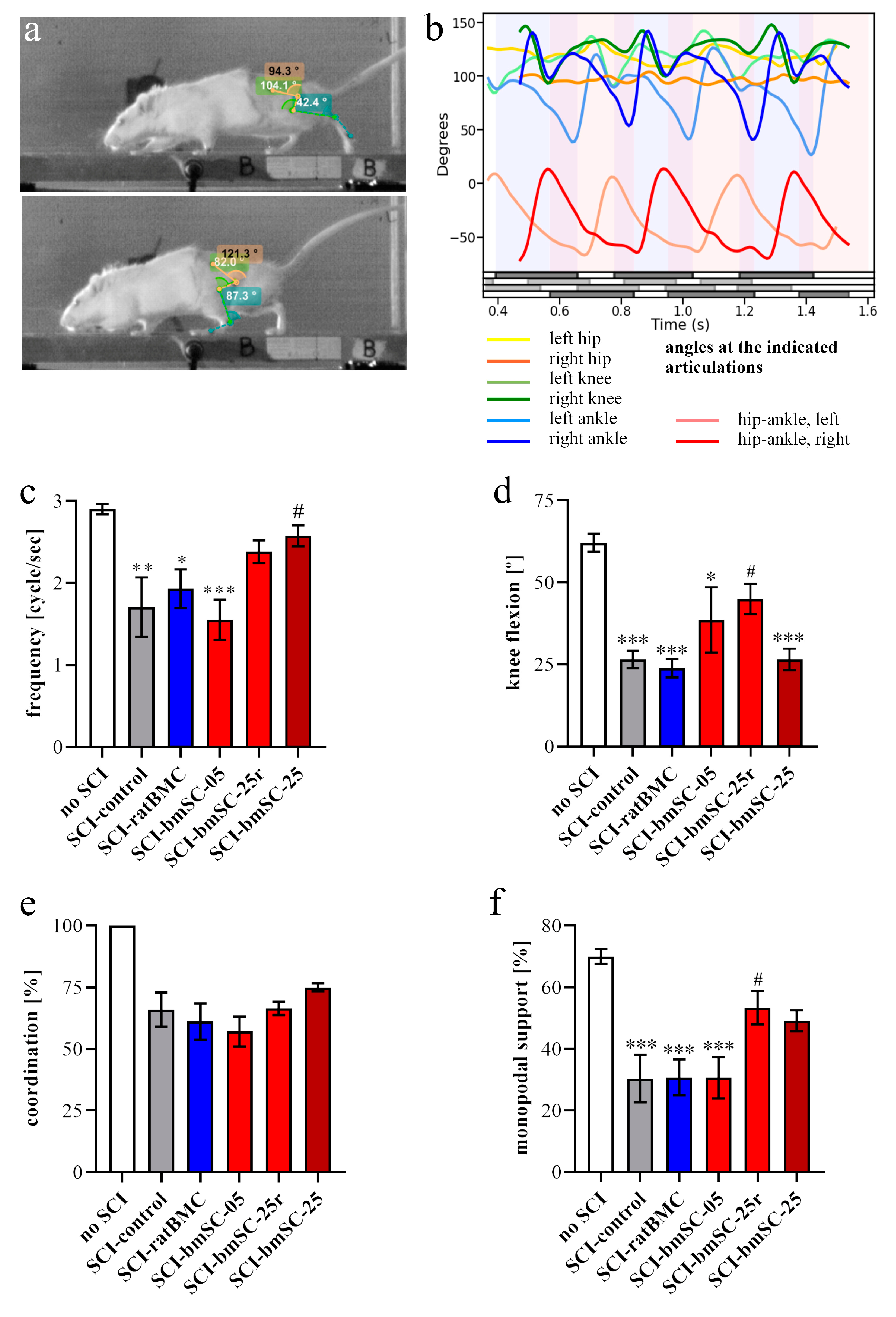
Video recordings. At 5 W, when all rats showed hind limb movements due to spontaneous or treatment-induced recovery, their motor performance was quantified with video recordings. Rats were trained to move along a 1.3 m long pathway with walls of transparent plexiglass at the sides and equipped with a dark chamber at the end. While running, the rats were being filmed by two synchronized digital cameras (MotionScope. Redlake MASD Inc., San Diego, CA, USA) at 125 fps, resolution 480 × 420 pixels, black and white. Capturing of the images was triggered externally. To later evaluate angular movements at the ankle, knee, and hip joints, black marks were painted on the shaved skin at these positions, at the end of the fifth digit, at the cervical crest, and at the ankle of the forepaws. With each rat, three successful runs were evaluated.
Kinematic analysis. For image processing we used Kinovea 0.9.5 freeware (
https://www.kinovea.org (accessed on 17 January 2022)). The times of initial contact (IC) and detachment (toe off, TO) of the paws were marked manually. Cartesian coordinates were calculated using automatic detection of the skin markers. The following data were acquired from every video frame during the rats’ movements: positions and angles of the hip, knee, and ankle of the hind limbs, as well as step length and speed (
Appendix A Figure A1). For the analysis of motor recovery after SCI, we selected the following four quantitative parameters. (1) Stepping frequency: Number of cycles (IC-TO-IC) of the hindlimbs. (2) Hindlimb mobility: Mean angle between maximal and minimal flexion of the knee joint. (3) Forelimb/hindlimb-coordination: For each cycle of the forelimb, it was determined whether there was a corresponding cycle of the ipsilateral hindlimb (IC; yes/no). (4) Monopodal support: Percent of time when only one of the hindlimbs touched the ground.
4.6. Tests of Allodynia/Hyperalgesia and Sensory Loss
Von Frey test. The response to mechanical stimulation was evaluated using a dynamic plantar aesthesiometer (von Frey test 37550; Ugo Basile, Gemonio, Italy). For each hind leg, a paw withdrawal threshold (PWT) was determined up to a maximum force of 50 g. This was performed five times with at least five-minute intervals between tests. The lowest and highest values of these readings were excluded and then the mean was calculated as the PWT.
Hargreaves method. In the same way, we determined the response to heat stimulation using an infrared beam (Hargreaves test 37570; Ugo Basile). Here, the PWT was measured as the latency [sec] until the stimulated paw was moved. The intensity of stimulation was set at 40.
Both tests were administered five weeks after SCI, when all animals were physically able to respond to the stimulation. To evaluate the responses, we established the mean PWT of five rats without SCI. A response within 2 SD above and below this threshold was considered normal. When the PWT of a limb was more than 2 SD below the mean of non-injured rats, it was considered a sign of neuropathic pain. A PWT of more than 2 SD above normal indicated a sensory deficit. All animals responded to stimulation.
4.7. Tissue Preparation and Histological Staining
Rats were sacrificed with an overdose of sodium pentobarbital followed by transcardial perfusion with PBS and 4% paraformaldehyde/PB (PFA). We dissected spinal cord segments of 2 cm length centered at the lesion site. The tissue was post-fixed for 24 h in PFA at 4 °C, stored at 4 °C in PFA for 1–3 days, then dehydrated, and embedded in paraffin (Leica EG1150H; Leica, Wetzlar, Germany). Horizontal sections of 3 μm were cut from dorsal to ventral using a Leica RM2265 microtome. They were mounted on polylysine-coated glass slides (Superfrost Plus) and stored at room temperature (RT).
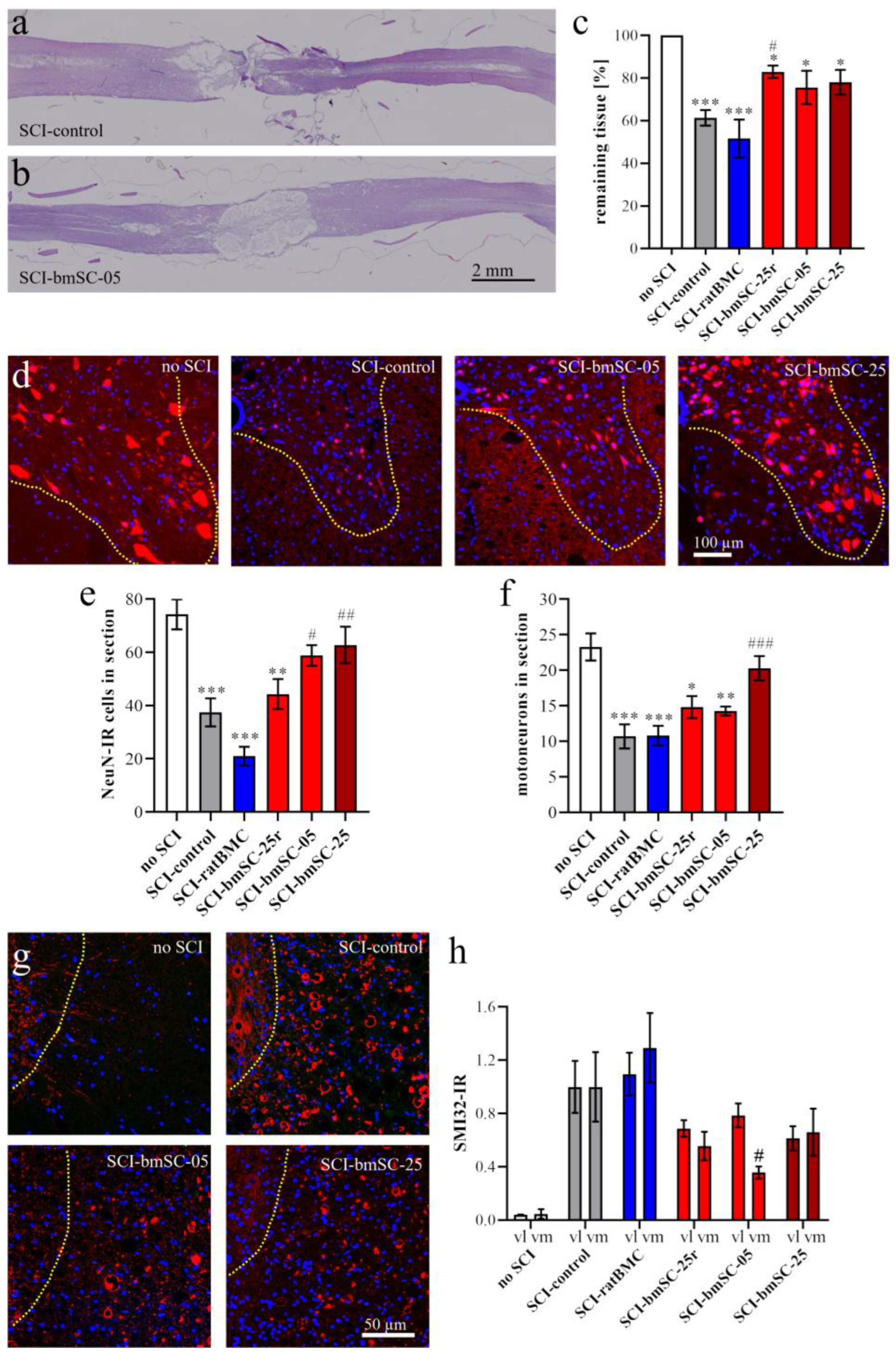
To evaluate the extent of the lesion site, one dorsal section at the horizontal level above the central gray matter was selected and stained with hematoxylin/eosin (H&E) following a standard protocol. Stained sections were again dehydrated and coverslipped with Histomount. Immunofluorescence staining (IF) was performed on the adjacent sections, all located in the dorsal spinal cord. For the assessment of neuron survival and axonal degeneration posterior to the lesion site, the remaining tissue was re-embedded in paraffin to prepare 3 μm transverse sections at 4 mm posterior to the lesion site.
4.8. Immunofluorescence Staining
For IF staining, sections were rehydrated and incubated for 30 min at 90 °C (water bath) in 10 mM Na citrate, pH 6.0, for antigen retrieval. Background staining was reduced by blocking 30 min at room temperature (RT) with 5% normal goat serum/0.1% Tween 20 in Tris-buffered saline (TBS-T). Sections were incubated with primary antibodies for 12 h at 4 °C in a humidified chamber and for 1 h at RT with fluorescence-labeled secondary antibodies using a hydrophobic marker pen and 200 μL/slide. Nuclei were stained with 10 μg/mL Hoechst-33342 for 15 min at RT. Sections were cover slipped with ImmuMount (Thermo Scientific, Alcobendas, Spain). The following primary antibodies were used in double staining experiments: polyclonal rabbit anti-GFAP (Sigma G9269; 1/500), polyclonal guinea pig anti-Iba1 (Synaptic systems 234004; 1/500), monoclonal mouse antibodies against CD68 (Serotec MCA341R1; 1/250), GFAP (BD Pharmingen 556327; 1/500), NeuN (Chemicon MAB377; 1/200), APC clone CC1 (Sigma MABC200, 1/300), and SMI32 (Covance 32R100, 1/2000). Antibodies against these epitopes were intended to visualize astrocytes and glial scar (GFAP), microglia and macrophages (Iba1), active phagocytes (CD68), neurons (NeuN), oligodendrocytes (APC clone CC1), and non-phosphorylated neurofilament in degenerating axons (SMI32).
To detect injected human bmSCs, sections from dorsal and ventral spinal cord were stained with a fluorescence-labeled mouse monoclonal antibody against human mitochondria (Merck MAB1273C3; 1/200). Secondary antibodies (for all other primary antibodies) were labeled with fluorescent dyes: goat anti-guinea pig IgG, Alexa-488 (Invitrogen A11073; 1/400), goat anti-rabbit IgG, Alexa-488 (Invitrogen A11008; 1/1000), goat anti-mouse IgG, Alexa-594 (Invitrogen A11005; 1/1000), goat anti-mouse IgG, and Alexa-488 (Jackson 115-545003; 1/1000).
4.9. Microscopy and Image Analysis
Histological and immunofluorescence staining experiments were evaluated using (1) a Leica DM5000B epifluorescence microscope (20×, 40× objectives), (2) an Olympus automated IX83 microscope (UPLXAPO 10×/0.40, 20×/0.80, 40×/0.95 objectives) equipped with
ScanR and
CellSens Dimensions software (version 0.3.2) for automatic capture and mosaic composition of multiple images, or (3) an Olympus BX61 stereotaxic microscope (2× objective), depending on the task. Exposure conditions were always kept constant for quantitative evaluation of fluorescence images. Photographs were analyzed using
QuPath Bioimage Analysis (
https://qupath.github.io/ (accessed on 10 May 2023)) and
Fiji Image-J (version 2.15.0), applying the same brightness/contrast adjustments and threshold values for each marker.
Tissue preservation. Horizontal paraffin sections through the dorsal third of the spinal cord were stained with hematoxylin and eosin (H&E, standard procedure) and photographed with the stereotaxic microscope. At 6 W, two measures were taken at (a) the anterior–posterior extension of the lesion site and (b) the remaining tissue area as a percentage of expected tissue without lesion. For this, a 1 cm segment centered on the lesion site was chosen as the ROI. The expected area of tissue without lesion was calculated using the breadths of the section at the anterior and posterior end of the ROI. The area of remaining tissue was measured using Image-J.
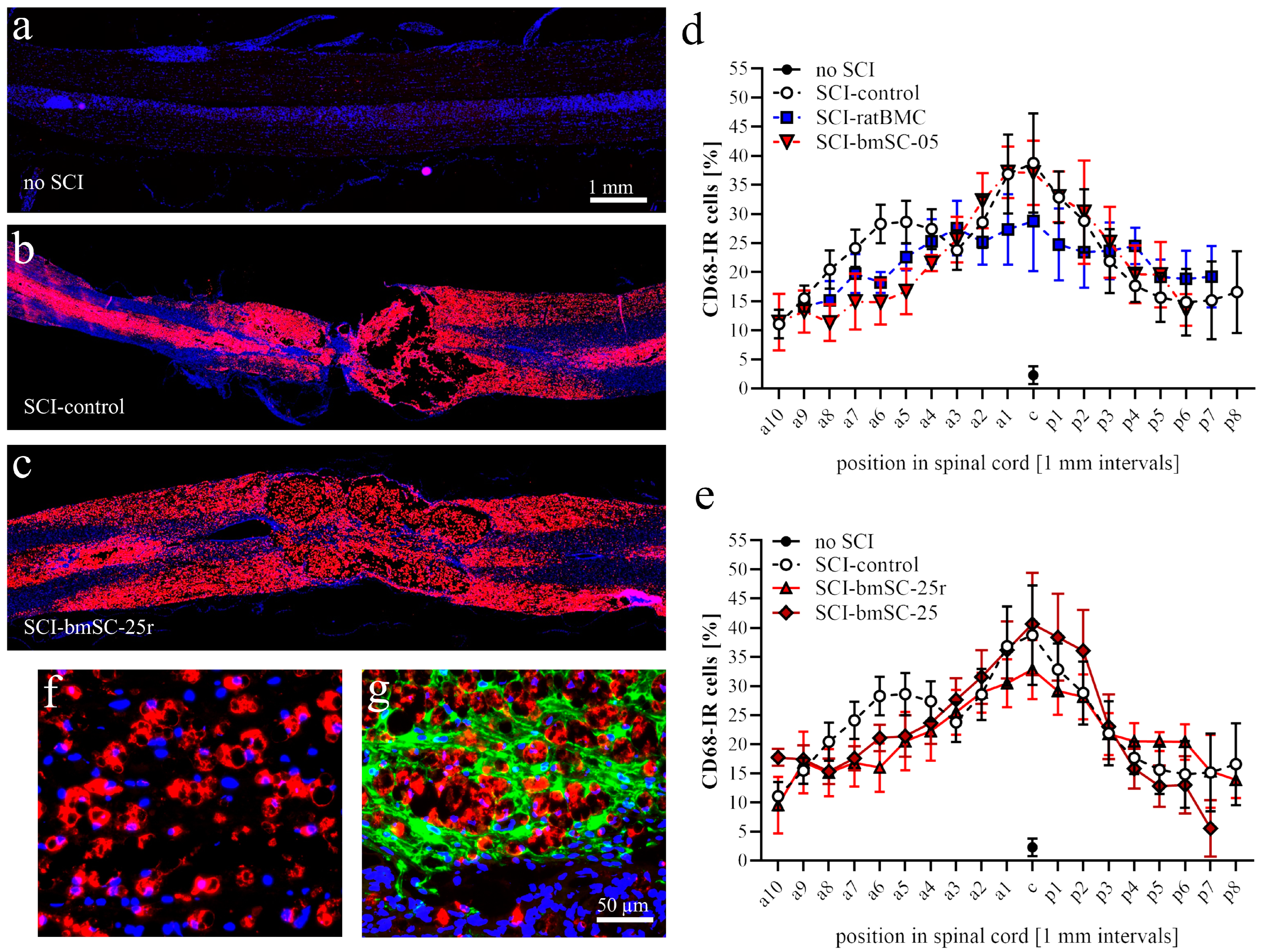
Macrophages. Using QuPath, automatic cell detection based on nuclear staining and CD68-IF was performed in dorsal spinal cord sections. We determined the percentage of CD68-positive cells in 1 mm bins from 9 mm anterior to 9 mm posterior of the lesion site. In addition, the intensity of CD68-IF was measured in the same areas (Image-J, integrated density) following background subtraction.
Microglia. Based on their morphology, Iba1-IR cells were classified as nonactivated microglia (with cellular processes, Mg0), activated microglia (large soma with few processes, MgA), phagocytes (smaller round soma without cytoplasmic extension, MΦ), or annular shaped macrophages (ring like structures surrounding empty spaces, MΦR). At positions 8 mm anterior, 4 mm anterior of lesion center, in the lesion center, and 4 mm and 8 mm posterior of the lesion center, the number of Iba1-IR cells with these morphologies was counted in ROIs with 1 mm anterior–posterior extension. Secondly, the IF intensity was measured as for CD68. In addition, a Scholl analysis was performed on Iba1-IR cells randomly selected at 4 mm posterior of the lesion center. Cells were photographed with 40× objective, and the number of cellular processes at 5 µm radial distance from the cell nucleus was counted using the concentric circles plugin for Image-J version 2.15.0 (n = 30 cells/group).
Neurons. At 4 mm posterior of the lesion center, transverse sections of the ventral half of the spinal cord were evaluated. An ROI comprising the ventral horn gray matter was analyzed with QuPath to count the total number of NeuN-positive neurons, using automatic selection parameters of cell size and IF intensity. In addition, the number of motoneurons, based on the size of NeuN-positive cells, was counted manually by two independent observers blinded to the sample conditions. SMI32-IR (integrated density) was measured in ROIs located in the ventromedial and ventrolateral white matter of these same sections.
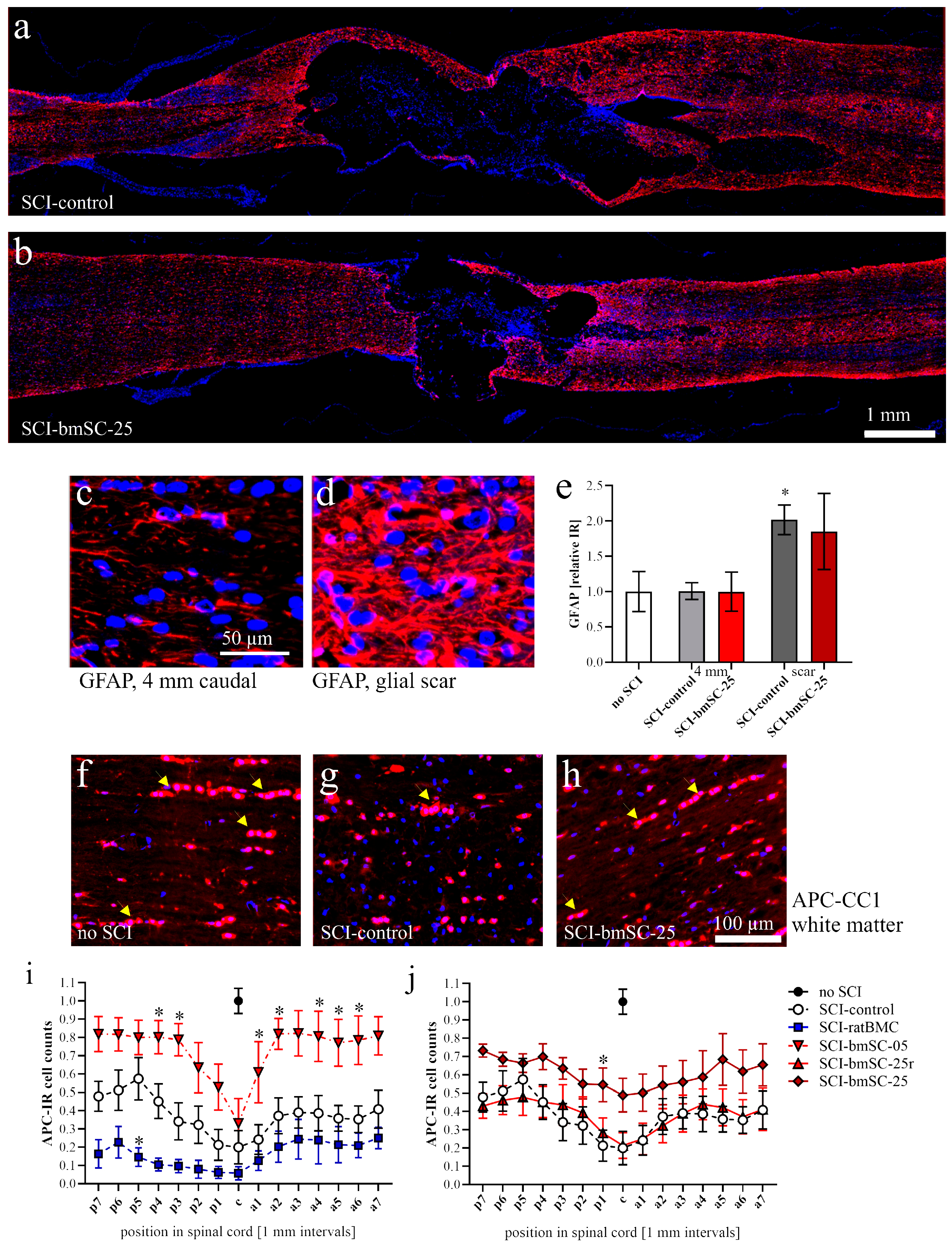
Astrocytes. The GFAP-IF signal was measured as integrated density (Image-J version 2.15.0) at positions 4 mm posterior, 4 mm anterior, and around the lesion site in a 1 mm2 (1.02 ± 0.03) ROI in three of the experimental groups (sham, SCI-control, SCI-bmSC-25).
Oligodendrocytes. The percentage of APC clone CC1-positive cells among all nucleated cells was assessed with QuPath using automatic selection parameters of cell size and IF intensity. The analysis was performed in horizontal sections of the dorsal spinal cord from 9 mm anterior to 9 mm posterior of the lesion site. White and gray matter were evaluated separately.
4.10. Detection of Human DNA in bmSCs Treated Rats
In addition to histological methods, quantitative PCR was employed to detect injected stem cells in the spinal cords of treated rats. For this, animals were sacrificed at 1, 2, 4, and 7 days after injection of bmSCs using an overdose of sodium pentobarbital. Spinal cord samples consisted of a 1 cm segment with the lesion site in the center. Of this tissue, 20 mg was homogenized in 180 µL digestion buffer (Fisher K1820-00) and the DNA was extracted according to the manufacturer’s instructions of the kit. Using the Nanodrop device, the DNA content was measured in the resulting 50 µL extract. For quantitative PCR using an ABI 7900HT sequence detection system (Applied Biosystems, Alcobendas, Spain), DNA was adjusted to 50 ng/2 µL pure water. For amplification, we used the qPCRBIO SyGreen Mix Hi-Rox (PCR Biosystems, London, UK). Each 15 µL SYBR green reaction mixture consisted of 2 µL DNA (50 ng), 7.5 µL SYBR Green PCR-mix (2×), 0.75 µL combined forward and reverse primers (10 µM each), and 4.75 µL distilled water. PCR was performed with 2 min at 95 °C, followed by 40 cycles of 15 s at 95 °C, 30 s at 60 °C, and a separate dissociation step for the melting curve. The specificity of the PCR product was confirmed by ascertaining a single melting peak in the temperature dissociation plots and electrophoresis of the products in an agarose gel (1.5%). All samples were run in duplicate. A positive detection of human DNA in the bmSCs preparation without false positive signals in spinal cord extracts from rats that received no cell treatment was confirmed using primers 5-GGTGAAACCCCGTCTCTACT-3 (forward 101 F) and 5′-GGTTCAAGCGATTCTCCTGC-3′ (reverse 206 R), directed against human Alu elements [
31].
4.11. Statistical Analysis
In the figure legends, data are presented as the mean values ± standard errors of the mean (SEM). Statistical analysis, performed with GraphPad Prism 8.0 software, consisted of one-factor or two-factor ANOVA, followed by post hoc Dunnett’s comparison tests. Unless stated otherwise, data from animals without SCI were not included in the statistical analysis. In graphical data representation, statistical significance is always indicated as follows: *, #: p < 0.05, **, ##: p < 0.01, and ***, ###: p < 0.001. The asterisks indicate differences from the group without SCI, while the pound symbol is used in comparisons with the SCI group that received control treatment. In the evaluation of tissue loss, results were tested with one-sample t-tests.